Abstract
Habitat selection behavior is an important predator-avoidance strategy for many organisms. Its particular expression is often explained as the result of a tradeoff between avoiding antagonists and acquiring resources. However, there is need for a broader perspective on this behavior, as organisms are often simultaneously involved in complex antagonistic relationships with multiple types of enemies. We show experimentally that a tradeoff between predator and parasite avoidance may be important in the evolution of habitat selection behavior in the waterflea, Daphnia magna. In this species, negatively phototactic clones suffer less from visually hunting predators by residing in deeper and darker portions of the water column during the day. However, this behavior increases the risk of parasitic infections when the Daphnia are exposed to pond sediments containing parasite spores. Positively phototactic clones, which are at a higher risk of predation, are less exposed to parasite spores in the sediment and consequently suffer less from parasitic infection. We show that the increased risk of infection remains even if the animals change their phototactic behavior on exposure to chemical cues from fish. This tradeoff highlights a substantial cost of predator-induced changes in habitat selection behavior. Tradeoffs caused by multiple enemies may explain genetic polymorphism for habitat selection behavior in many natural populations.
In the face of antagonistic interactions, habitat selection strategies in time and space are essential for the survival of many organisms. Many studies on predation have documented the ecological costs of antipredatory habitat selection behavior, such as reduced food intake, reduced competitive strength, and increased susceptibility to predation by a different kind of predator (1, 2). It has been suggested that such costs favor the evolution of inducible defenses (2) such as diel vertical migration (DVM), which is generally considered to be a predator-avoidance strategy of zooplankton (3–5). In this strategy, the zooplankton reside at greater depths during the day, thus reducing their chance of being detected by visual predators (4, 5). It has been shown that DVM can be induced in several taxa by exposing them to predator kairomones (6–8), i.e., chemical cues that indicate the presence of the predator. Phenotypic variation in induced and constitutive (not dependent on environmental stimuli for activation; ref. 9) DVM has a strong genetic component (8, 10–12), and the trait shows rapid evolutionary response to changing predator regimes (13).
Predators are not the only antagonists that the zooplankton face. Parasites (including pathogens) are common in zooplankton populations, and the fitness costs of infection are severe (14–19). Many parasites produce infective stages within the bottom sediments, where they form long-lasting spore banks (14–16). Because DVM is often so pronounced that the zooplankton reside in, at, or near the bottom sediments during the day (20, 21), we hypothesized that this behavior has costs in terms of increased exposure to parasite transmission stages.
The aim of this study was to determine experimentally whether DVM differences in zooplankton, which are known to affect their susceptibility to visually hunting fish, also affect their infection risk by parasites. This additional susceptibility would indicate that the zooplankton face a tradeoff between avoiding two types of antagonists. Using the waterflea Daphnia as a model organism, we investigated this tradeoff for both constitutive and predator-induced differences in DVM.
Methods
We tested our tradeoff hypothesis by exposing Daphnia magna clones to parasite spore banks. In a first experiment, we individually exposed replicate animals of six D. magna clones to three standardized parasite spore banks. These clones differed in phototactic behavior (constitutive daytime vertical distribution). We hypothesized that under these conditions, the more negatively phototactic clones would have a higher infection risk than the more positively phototactic clones. A second experiment was performed to verify that the interclonal differences in infection risk observed in the first experiment were due to differences in vertical distribution and not to intrinsic interclonal differences in parasite resistance. To this end, we relied on the fact that some clones can be induced to change their vertical distribution on exposure to fish kairomones.
Daphnia Clones and Parasite Spore Banks.
We selected Daphnia clones that differed in their constitutive DVM behavior as quantified by phototactic behavior (8, 22, 24). Within each of three DVM categories (positively, intermediately, and negatively phototactic behavior), we selected clones that differed in their inducible changes in behavior. Daphnia populations are often genetically adapted to local predation pressure (23), some populations showing strong and others no changes in habitat selection behavior on exposure to fish kairomones. Thus, we used three clones from each of two habitats: nonfish habitat (NFH) clones were derived from a fishless city pond (Citadelpark, Ghent, Belgium), whereas fish habitat (FH) clones were isolated from a pond that contains fish (Driehoeksvijver, Heusden, Belgium; refs. 8, 22–24). In earlier papers, the FH clones were referred to as C134 (positively phototactic), C5 (intermediately phototactic), and C39 (negatively phototactic), whereas the NFH clones were referred to as P132,85 (positively phototactic), P132,60 (intermediately phototactic), and P132,96 (negatively phototactic) (8, 22, 24). Clones differed not only in phototactic behavior but also in their response to fish kairomones: FH clones showed a strong and NFH clones a weaker induced change in phototactic behavior (refs. 8, 22–24 and unpublished data). In the present study, the clones were thus categorized according to their phototactic behavior as determined in earlier work; the differences in behavior among these clones are known to be very repeatable (see refs. 8, 22, 24).
Phototactic behavior was quantified following a standard procedure by using a small-scale experimental setup. The setup consisted of a small transparent plastic column (25-cm height, 5-cm internal cross section) filled with aged tap water and placed in a darkened room at 20 ± 1°C. During an experiment, the column was illuminated from above. At 1-min intervals during an experimental period of 10 min, the position of the test animals was recorded. For that purpose, the column was externally divided into an upper compartment of 12-cm height (U), a lower compartment of 3-cm height (L), and a middle compartment of 10-cm height (M). The phototactic behavior of the test population was determined by the phototactic index I = (U − L)/(U + M + L), calculated for the second 5 min of the experiment. This phototactic index can range from +1 for extremely positively phototactic behavior to −1 for extremely negatively phototactic behavior. For further information on the experimental details used to quantify the clones' phototactic behavior, the reader is referred to refs. 8, 13, 22–24.
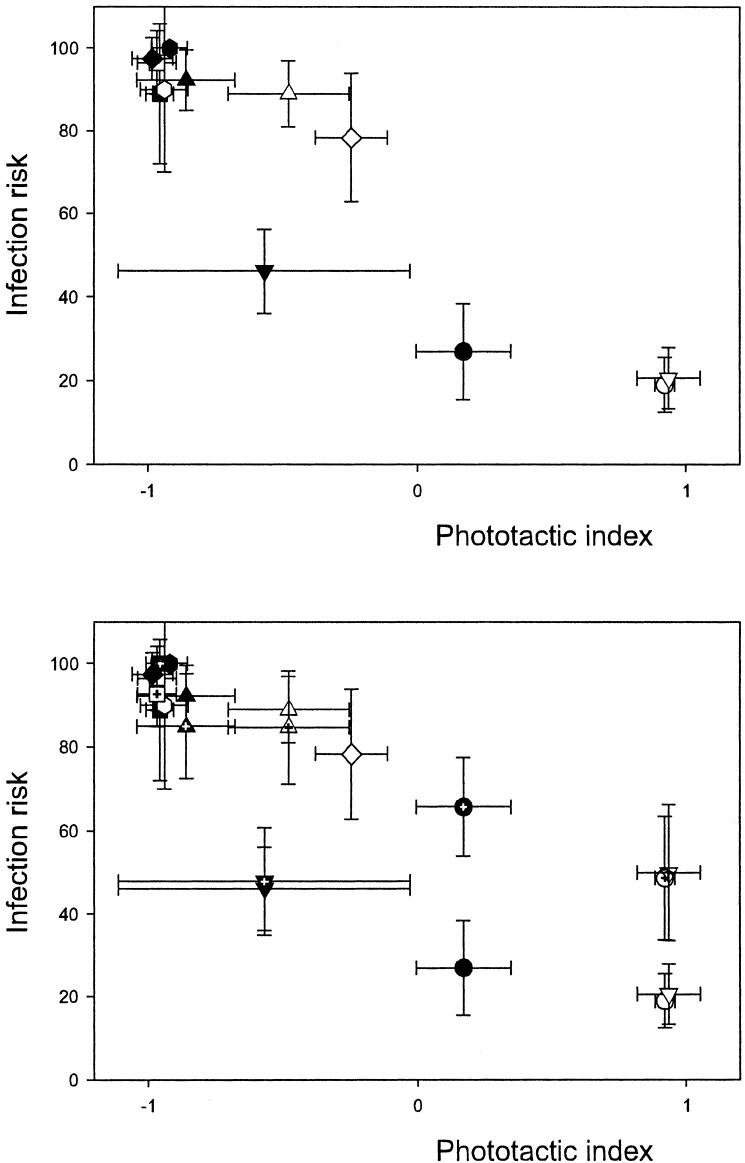
The phototactic behavior of the clones was determined in the absence and presence of fish kairomones. In the fish kairomones treatment, we replaced 20% of the medium daily with tap water conditioned by planktivorous fish (Leuciscus idus) for 24 h. The final concentration of fish kairomones corresponded to one fish (8 cm) per 100 liters. In the treatment without fish kairomones, the same amount of medium was refreshed by aged (24 h) tap water (see ref. 23). Animals were exposed to both treatments for at least one generation before experimentation. Four replicate experiments were carried out for each treatment × clone combination; all experiments were done on independent groups of experimental animals. For each experiment, a group of 10 adult females carrying their second egg clutch (second adult instar) was used. The phototactic behavior of the six clones in the absence and presence of fish kairomones is shown in Fig. 1. The FH clones showed a strong shift to more negatively phototactic behavior in the presence of fish kairomones, whereas the NFH clones showed a more modest change on exposure to fish kairomones (Fig. 1).
Figure 1.
Infection risk versus phototactic index in treatments with (solid symbols) and without fish kairomones (empty symbols). (Upper) Only stable treatment. (Lower) Stable and sediment suspension treatment (symbols marked with a cross). ○, NFH positively; ▵, NFH intermediately; □, NFH negatively; ▿, FH positively; ◊, FH intermediately; , FH negatively phototactic clone. In Lower, the FH intermediately and negatively phototactic clone in sediment suspension treatment are not shown. Note that for a given clone, phototactic behavior is the same in the stable and the sediment suspension treatment. The categories “positively,” “intermediately,” and “negatively” phototactic behavior refer to the behavior in the absence of fish kairomones.
We exposed the Daphnia to parasite spore-containing mud, collected from three shallow ponds in Heverlee, Belgium (OM1, -2, and -3; ref. 25; only OM2 was used in the second experiment). We placed 30 ml of mud in each vial and allowed it to settle before Daphnia were added. Exposure to these spore banks lead mainly to infection by Pasteuria ramosa, a bacterial endoparasite of Daphnia (26). Pasteuria is found in high prevalences in field studies and reduces host lifetime reproductive success by more than 90% (19, 26). Infection starts when the host ingests spores by filter feeding. The bacterium grows in the body cavity of its host, eventually filling it with millions of spores. Infection can then be easily recognized, as the parasite spores give a reddish–brownish shine to the host. The parasite has to kill its host for transmission, as spores are released only from the decaying cadaver (19, 26, 27).
Experimental Design and Culture Conditions.
Daphnia were kept under standardized culture conditions and fed daily with the alga Scenedesmus acutus. Maternal effects were controlled for by keeping lineages separate for two generations before an experiment (27). In the first experiment, 30 individuals from each clone were used. Ten replicate individuals were exposed to each of the three spore banks, with each individual incubated separately. Animals were fed 25 × 103 Scenedesmus cells/ml daily at a temperature of 19 ± 1°C and a light/dark cycle of 16:8 h. During the experiment, offspring were removed daily. After 21 days, the Daphnia were squashed on microscopic slides, covered with a coverslip, and checked for parasite transmission stages by using phase contrast microscopy with ×400 magnification.
In the second experiment, we used mixed populations of the three clones derived from one habitat, but with different phototactic behavior. This mixing resulted in a treatment with either the three FH clones or the three NFH clones. We used a factorial design to combine the presence and absence of sediment suspension and the presence and absence of fish kairomones. In the sediment suspension treatment, the experimental jars (0.5 liter) were rotated on their horizontal axis at five turns per minute during 5 min per hour. This suspension treatment stirred up parts of the sediment and spores for the purpose of increasing the encounter rates of Daphnia with parasite spores. Although sediment suspension in this treatment was not so strong as to homogeneously distribute the sediment with the spores in the medium, it did increase the animals' contact with the spores, particularly for those animals that might otherwise hardly be exposed, i.e., the positively phototactic animals. The fish kairomones treatment was included, because fish kairomones cause a change in habitat selection behavior without any change in host genotype. In the fish kairomones and the control treatment, we replaced 20 ml of the water every day with freshly made fish-conditioned water and nonconditioned aged (24 h) tap water, respectively. Medium with fish kairomones was prepared by allowing fish (Leuciscus idus, Teleostei, Cyprinidae; 15 ± 2 cm) to condition the medium for 24 h (temperature: 19 ± 1°C). Every 3 days, fish were fed standard commercial fish food. The feeding was done in a separate aquarium to avoid contaminating the medium with fish food substances. The presumed final concentration of fish kairomones in the experimental jars was kept high (1 fish for 100 liters) to ensure a maximal response in phototactic behavior.
There were 152 (4 treatments × 2 populations × 19 replicate jars) experimental jars, each containing a set of 15 individuals (three clones with different behaviors × five individuals from each clone). The Daphnia were fed 375 × 103 algal cells/ml daily. Offspring were removed daily, and after 30 days, the Daphnia were checked for infection. As we used mixed populations, we used cellulose acetate electrophoresis (28) to identify each Daphnia individual to clone.
Statistical Analyses.
In the first experiment, infection risk was calculated by using the number of infected Daphnia of all replicate individuals from the same clone that were confronted with a particular spore bank. For each spore bank, a separate Pearson correlation was performed on the means of the infection risks and the phototactic indices of the different clones. Phototactic index was log-transformed [log(X + 1.2)], and infection risk was angular transformed {arcsin[sqrt(infection risk/100)]}. The first experiment was further analyzed by using a categorical model (Procedure CATMOD, SAS Institute, Cary, NC; ref. 29; response variable: infected versus noninfected), incorporating “spore bank,” “habitat,” and “phototactic behavior” as the main effects. As our design yielded only one value for each clone in each combination of spore bank, habitat, and phototactic behavior, we could not analyze interaction terms.
In the second experiment, infection risk was derived by using the number of infected individuals on the remaining individuals from the initial five Daphnia of each clone in one jar. We used Pearson correlations to analyze the relationship between risk of infection (angular transformed) and behavior (log-transformed) in the absence and presence of fish kairomones, and we also carried out a repeated-measures two-way ANOVA to analyze the effect of phototactic behavior (phototaxis), habitat, and kairomone treatment on the infection risk of both sets of clones in the stable treatment. Phototactic behavior was taken as a repeated measure, because clones with different phototactic behavior were pooled together in the same experimental jars. We carried out a repeated-measures two-way ANOVA on the infection results of the three investigated NFH clones, with kairomones and sediment suspension treatment as main effect. The intermediately and negatively phototactic FH clones in the sediment suspension treatment showed high mortality (64.2 and 84.2%, respectively, which may have been related to the high infection rates combined with reduced vigor because of exposure to suspended sediments). Therefore, we could not perform a two-way ANOVA on the infection results of these clones with behavior as a repeated measure. The mortality levels of the positively phototactic FH clone were acceptable (20.8%). We thus performed a three-way ANOVA, in which we analyzed the effects of fish kairomones and sediment suspension treatment on the infection risks of the positively phototactic clones in both habitats: FH and NFH (habitat taken as third main effect).
Results
In the first experiment, the analysis of the infection patterns confirmed our hypothesis that phototactic behavior influences the likelihood of contracting disease (infection risk) (Table 1). The more positively phototactic a clone was, the less likely it was to be infected by the parasite Pasteuria ramosa. We also observed that the origin of the spore banks had a significant effect (Table 1), possibly reflecting different spore concentrations in the sediments and/or different genotypes of parasites. There was no overall impact of the habitat from which the Daphnia clones were isolated.
Table 1.
Infection risks of clones exposed to standardized spore banks
| Phototaxis | Spore bank
|
Average | ||
|---|---|---|---|---|
| OM1 | OM2 | OM3 | ||
| Positive | 0 | 18.7 | 10 | 9.6 |
| Intermediate | 41.3 | 59.7 | 0 | 33.7 |
| Negative | 21.3 | 100 | 55.6 | 59 |
| Average | 20.9 | 59.5 | 21.8 | |
The phototactic behavior (phototaxis) of the six clones is categorized as positive, intermediate, and negative (in the absence of fish kairomones). Spore banks are referred to as the ponds from which the spore banks were isolated (OMI, -2, -3, Heverlee, Belgium, ref. 25). The cell entries give the infection risk (percentage of infected D. magna) in each combination. Average infection risks are given for each row (right column) and each column (bottom of table). For each combination of behavior and phototaxis the average of infection risk of two clones, one NFH clone and one FH clone, is given. Pearson correlation between mean infection risk and mean phototactic index of the different clones, exposed to three different spore banks is for OM1: r = −0.25, P < 0.05; for OM2: r = −0.89, P < 0.05; and for OM3: r = −0.78, P < 0.05. Results of Maximum-likelihood ANOVA of categorical model analysis: intercept: DF = 1, χ2 = 20.15, P < 0.0001; spore bank: DF = 2, χ2 = 24.33, P < 0.0001; habitat: DF = 1, χ2 = 0.26, P < 0.6079; behavior: DF = 2, χ2 = 22.36, P < 0.0001; likelihood ratio: DF = 12, χ2 = 44.15, P < 0.0001.
In the second experiment, parasite infection risk increased significantly with deeper daytime distribution in both the kairomones treatment (Pearson correlation coefficient: −0.92, P < 0.05) and in the control (Pearson correlation coefficient: −0.91, P < 0.05). The interaction between kairomones treatment and habitat on infection risk was shown to be significant (Table 2), with infection levels being higher in the kairomones treatment for the FH clones than for the NFH clones (Fig. 1). This pattern of increased infection risk parallels the pronounced shifts to more negatively phototactic behavior, which were also more strongly expressed in the FH clones than in the NFH clones (Fig. 1). As expected, the positively phototactic FH and NFH clones, which were behaviorally protected from infections, showed a significant increase in infection risk in the sediment suspension treatment (Fig. 1 Lower, Table 3). Because the intermediately and negatively phototactic NFH clones already showed very high infection risks without sediment suspension, no further increase was expected. Thus, an analysis of infection risk in the NFH clones showed a significant sediment suspension × behavior interaction (Table 4). In the three-way ANOVA on the positively phototactic clones from both habitats (FH and NFH), there was a significant three-way interaction term (Table 3). This was due to an interaction effect between the sediment suspension and the fish kairomones treatment in the FH clone but not in the NFH clone. Whereas infection levels in the NFH clone increased in the sediment treatment for both individuals that were cultured in the absence or presence of fish kairomones, the FH clones showed increased infection levels only in the animals that were not exposed to fish kairomones (Fig. 1 Lower).
Table 2.
ANOVA on the infection risk of all the NFH and FH clones
| Factors | MS | DF | F | P |
|---|---|---|---|---|
| Habitat* | 0.01 | 1 | 0.002 | 0.962 |
| Kairomones* | 0.54 | 1 | 3.593 | 0.071 |
| Habitat × kairomones* | 0.66 | 1 | 4.409 | 0.047 |
| Phototaxis† | 5.86 | 2 | 56.53 | <0.0001 |
| Habitat × phototaxis† | 0.12 | 2 | 1.198 | 0.311 |
| Kairomones × phototaxis† | 0.10 | 2 | 0.948 | 0.395 |
| Kairomones × phototaxis × habitat† | 0.02 | 2 | 0.215 | 0.807 |
| Error* | 0.15 | 22 | ||
| Error† | 0.10 | 44 |
Repeated-measures two-way ANOVA testing for the effect of population, fish kairomones, and phototactic behavior (phototaxis) on the infection risk of three NFH and three FH clones that differ in phototactic behavior (second experiment). MS, mean square; DF, degree of freedom; F, F value of the F distribution in ANOVA.
and
are used to link the MS and DF of the errors with the appropriate factors.
Table 3.
ANOVA on the infection risk of the positively phototactic NFH and FH clones
| Factors | MS | DF | F | P |
|---|---|---|---|---|
| Kairomones | 0.87 | 1 | 8.192 | 0.005 |
| Sediment suspension | 2.99 | 1 | 28.06 | <0.0001 |
| Kairomones × sediment suspension | 0.04 | 1 | 3.697 | 0.0568 |
| Habitat | 0.01 | 1 | 0.0364 | 0.8492 |
| Kairomones × habitat | 0.01 | 1 | 0.0585 | 0.8093 |
| Sediment suspension × habitat | 0.09 | 1 | 0.8556 | 0.3568 |
| Sediment suspension × habitat × kairomones | 0.57 | 1 | 5.3313 | 0.0223 |
| Error | 0.11 | 120 |
Three-way ANOVA testing for the effect of sediment suspension, fish kairomones, and phototactic behavior on the infection risk of the positively NFH and FH clones in the second experiment. For an explanation of MS, DF, and F, see Table 2 legend.
Table 4.
ANOVA on the infection risk of all NFH clones
| Factors | MS | DF | F | P |
|---|---|---|---|---|
| Kairomones* | 0.04 | 1 | 0.262 | 0.612 |
| Sediment suspension* | 0.27 | 1 | 1.655 | 0.207 |
| Kairomones × sediment suspension* | 0.09 | 1 | 0.554 | 0.462 |
| Phototaxis† | 6.33 | 2 | 53.12 | <0.0001 |
| Kairomones × phototaxis† | 0.19 | 2 | 1.612 | 0.207 |
| Sediment suspension × phototaxis† | 0.43 | 2 | 3.597 | 0.033 |
| Sediment suspension × phototaxis × kairomones† | 0.22 | 2 | 1.862 | 0.164 |
| Error* | 0.16 | 31 | ||
| Error† | 0.12 | 62 |
Repeated-measures two-way ANOVA testing for the effect of sediment suspension, fish kairomones, and phototactic behavior on the infection risk of the NFH clones in the second experiment. For details on MS, DF, F,
, and †, see Table 2 legend.
Discussion
Our results provide clear evidence that migrating zooplankton face a tradeoff between reducing the risk of predation by visual hunting predators (mainly fish) and reducing the risk of infection by parasites and pathogens. Although it has been shown that both predators and parasites have a dramatic impact on fitness and population dynamics of Daphnia (1, 11, 17, 26), we further show here that habitat selection behavior may play a central role in balancing the risks of both threats. Whereas the results of our first experiment could also be interpreted as reflecting interclonal differences in parasite susceptibility, the results of the fish kairomones and sediment suspension treatment in our second experiment effectively rule out this explanation. In the fish kairomones treatment, we observed an increase in infection risk that parallels a given clone's change in vertical distribution, clearly indicating that encounter rates are the key factor. Similarly, increasing the encounter rate of positively phototactic clones in the sediment suspension treatment also yielded increased infection rates. DVM thus influences the zooplankton's encounter rates not only with predators but also with parasites, and both encounter rates tend to be negatively correlated with each other. This tradeoff reveals an important and hitherto unappreciated ecological cost for both constitutive and induced predator avoidance behavior in zooplankton, one that may explain the often strikingly high levels of genetic polymorphism reported for this habitat selection behavior (5, 11–13). Parasitic infections may, in addition to other known costs (e.g., reduced food intake and lower growth rates associated with residing at lower temperatures), also explain why DVM is a conditional response in many populations and species, being induced by the presence of the predator itself (2, 5, 6, 8, 10). It may further explain why there are several alternative inducible predator-avoidance and escape mechanisms exhibited by Daphnia clones in various combinations (e.g., change in size at maturity, hiding in macrophyte beds, DVM; ref. 12), because the costs associated with different defense mechanisms may be of a different nature. For example, our results indicate that in a shallow pond and with high prevalence of parasites, diel horizontal migration into macrophyte beds to escape from fish predation may be less costly than hiding in the sediments.
Tradeoffs between predator and parasite defenses are expected to be common, and previous studies have reported tradeoffs between particular predator-defense responses and parasite-specific resistance mechanisms (30, 31). In this study, we document a tradeoff in a multiple antagonist environment mediated by encounter rates with parasites and predators. With respect to host–parasite coevolution, the relationship between habitat selection behavior and infection risk may result in the Daphnia's maintenance of genetic variation for resistance against parasites (18, 27). This variation can be maintained because the selection pressure to evolve resistance is higher for negatively phototactic clones than for the positively phototactic clones, as the latter clones are behaviorally protected against infection. As we focused on the spore bank present in sediments, one may argue that our results hold only for zooplankton inhabiting shallow lakes or exhibiting very strong DVM behavior with a daytime residence close to the sediments. However, even in deep lakes, it is conceivable that parasite spores accumulate to some extent in the metalimnion because of the density gradient associated with the thermocline. Because many zooplankton populations exhibiting DVM in deep lakes reside in or below the metalimnion during the day, the tradeoff described here may operate also in these systems.
Acknowledgments
We thank Lies Neys and Annelies Cappan for practical assistance and Steven Declerck, Karl Cottenie, Tine Huyse, Joost Vanoverbeke, and Tom Little for discussions and advice. We gratefully acknowledge constructive comments by two anonymous referees. Support for this research was provided by the Flemish Institute of Scientific Research in Industry (IWT) (to E.D.), by the KULeuven Research Fund and the National Fund for Scientific Research–Flanders (to L.D.M.), and by the Swiss Nationalfonds (to D.E.).
Abbreviations
- DVM
diel vertical migration
- NFH
nonfish habitat
- FH
fish habitat
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sih A. In: Predation, Direct and Indirect Impacts on Aquatic Communities. Kerfoot C W, Sih A, editors. Hanover, NH: Univ. Press of New England; 1987. pp. 203–224. [Google Scholar]
- 2.Tollrian R, Harvell C D. In: The Ecology and Evolution of Inducible Defenses. Harvell C D, Tollrian R, editors. Princeton: Princeton Univ. Press; 1999. pp. 306–321. [Google Scholar]
- 3.Stich H B, Lampert W. Nature (London) 1981;293:396–398. [Google Scholar]
- 4.Lampert W. Funct Ecol. 1989;3:21–27. [Google Scholar]
- 5.De Meester L, Dawidowicz P, Van Gool E, Loose C J. In: The Ecology and Evolution of Inducible Defenses. Harvell C D, Tollrian R, editors. Princeton: Princeton Univ. Press; 1999. pp. 160–176. [Google Scholar]
- 6.Neill W E. Nature (London) 1990;345:524–526. [Google Scholar]
- 7.Ringelberg J. J Plankton Res. 1991;13:83–89. [Google Scholar]
- 8.De Meester L. Ecology. 1993;74:1467–1474. [Google Scholar]
- 9.Harvell C D. Quart Rev Biol. 1990;65:323–340. doi: 10.1086/416841. [DOI] [PubMed] [Google Scholar]
- 10.Neill W E. Nature (London) 1992;356:54–57. [Google Scholar]
- 11.De Meester L, Weider L J, Tollrian R. Nature (London) 1995;378:483–485. [Google Scholar]
- 12.Boersma M, Spaak P, De Meester L. Am Nat. 1998;152:237–248. doi: 10.1086/286164. [DOI] [PubMed] [Google Scholar]
- 13.Cousyn C, De Meester L, Colbourne J K, Brendonck L, Verschuren D, Volckaert F. Proc Natl Acad Sci USA. 2001;98:6256–6260. doi: 10.1073/pnas.111606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green J. Trans Zool Soc London. 1974;32:417–515. [Google Scholar]
- 15.Ebert D. J Anim Ecol. 1995;64:361–369. [Google Scholar]
- 16.Ebert D, Payne R J H, Weisser W W. In: Vertical Food Web Interactions: Evolutionary Patterns and Driving Forces. Dettner K, Bauer G, Völkl W, editors. Heidelberg: Springer; 1997. pp. 91–111. [Google Scholar]
- 17.Stirnadel H A, Ebert D. J Anim Ecol. 1997;66:212–222. [Google Scholar]
- 18.Little T J, Ebert D. J Anim Ecol. 1999;68:134–149. [Google Scholar]
- 19.Ebert D, Lipsitch M, Mangin K L. Am Nat. 2000;156:459–477. doi: 10.1086/303404. [DOI] [PubMed] [Google Scholar]
- 20.Hart R C, Allanson B R. Freshwater Biol. 1976;6:183–198. [Google Scholar]
- 21.DeStasio B T. Bull Mar Sci. 1993;53:44–64. [Google Scholar]
- 22.De Meester L. Arch Hydrobiol Beih. 1993;39:137–155. [Google Scholar]
- 23.De Meester L. Evolution (Lawrence, Kans) 1996;50:1293–1298. doi: 10.1111/j.1558-5646.1996.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 24.De Meester L. Hydrobiologia. 1991;225:217–227. [Google Scholar]
- 25.De Meester L, Cousyn C. Hydrobiologia. 1997;360:169–175. [Google Scholar]
- 26.Ebert D, Rainey P, Embley T M, Scholz D. Philos Trans R Soc London B. 1996;351:1689–1701. [Google Scholar]
- 27.Carius H J, Little T J, Ebert D. Evolution (Lawrence, KS) 2001;55:1146–1152. [Google Scholar]
- 28.Hebert P D N, Beaton M J. Methodologies for Allozyme Analysis Using Cellulose Acetate Electrophoresis. Beaumont, TX: Helena Laboratories; 1993. [Google Scholar]
- 29.SAS Institute. SAS/STAT. Cary, NC: SAS Institute; 1990. , Ver. 6. [Google Scholar]
- 30.Thompson J N. The Coevolutionary Process. Chicago: Univ. Chicago Press; 1994. [Google Scholar]
- 31.Rigby M C, Jokela J. Proc R Soc London Ser B. 2000;267:171–176. doi: 10.1098/rspb.2000.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]