Abstract
Evolutionary key innovations give organisms access to new ecological resources and cause rapid, sometimes spectacular adaptive radiation. The well known obligate pollination mutualism between yuccas and yucca moths is a major model system for studies of coevolution, and it relies on the key innovation in the moths of complex tentacles used for pollen collecting and active pollination. These structures lack apparent homology in other insects, making them a rare example of a novel limb. We performed anatomical and behavioral studies to determine their origin and found evidence of a remarkably simple mechanism. Morphological analyses of the tentacles and adjacent mouthparts in pollinators and closely related taxa showed that the tentacle appears abruptly in female pollinating yucca moths. Several morphological synapomorphies between the galeae, which constitute the characteristic lepidopteran proboscis, and the tentacle suggest that the tentacle evolved quickly through expression of the genetic template for the galea at an apical growth bud on the first segment of the maxillary palp. Behavioral data indicate that tentacle and proboscis movements are controlled by a shared hydraulic extension mechanism, thus no new mechanism was needed for tentacle function. Known developmental paths from other insects can explain the origin of this sex-specific key innovation in a few steps.
Obligate mutualisms between plants and pollinators provide some of the most apparent examples of coevolution (1, 2). A long-recognized association of this kind, between yucca moths (Prodoxidae) and yucca plants (Agavaceae), has become an important model in understanding how obligate mutualisms coevolve (3–6). In this association, established at least 40 million years ago (7), yuccas are pollinated exclusively by yucca moths, whose larvae in turn consume some of the developing yucca seeds. This has been an evolutionarily and ecologically highly successful association, with some 30–45 yucca species (8, 9) being important vegetation components throughout much of the North American deserts and semiarid regions (10).
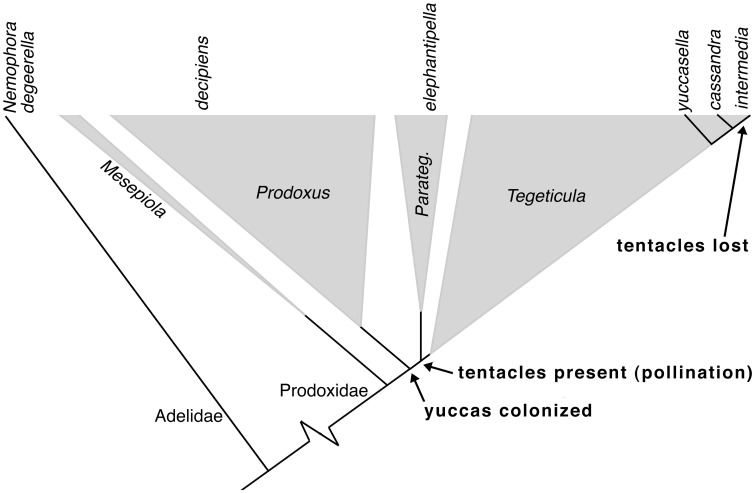
Prior analyses of the coevolution between yucca moths and yuccas have shown that the transition from antagonism to mutualism primarily involved quantitative changes in already existing traits, rather than evolutionary novelties. The one exception is the evolution of elaborate tentacular mouthparts in the yucca moths, used for handling pollen with great precision. These tentacles are an evolutionary key innovation, both in the sense that it is a truly novel trait that evolved quickly (7) (Fig. 1) and that it is linked to an adaptive radiation (11–13). Understanding how this trait evolved, then, is central to understanding the coevolutionary history of diversification and changing interactions between yuccas and yucca moths. Although reported when the relationship was first described over a century ago (14, 15), no analyses have been performed of tentacle anatomy or homology.
Figure 1.
Phylogenetic positions of the six species used in the study. Names of species used are given at top. Triangle width reflects species richness for the three yucca-feeding genera and their sister group. Spacing between prodoxid genera and the Tegeticula species triad is proportional to time, and the bottoms of triangles give deepest known radiations within each genus. The deepest split (Mesepiola vs. others) is estimated to 44.1 ± 10.6 million years ago. The internode from the Prodoxus-pollinator genera split to the pollinator genera split, along which the tentacle evolved, is so short that estimated ages of the three genera overlap, thus the uniform tentacle seen in all pollinator moths must have evolved very quickly. Data are from refs. 5 and 7, and O.P. and M. Balcázar-Lara, unpublished data.
Here we present anatomical data from phylogenetically pivotal moth species indicating that this complex key morphological trait for the mutualism has a surprisingly simple origin. We also use trait expression in the pollinators, their nonpollinating sister group, and derived species that have secondarily lost the tentacles to propose a possible developmental genetic basis for the trait.
The Function of the Tentacles.
The pollinating yucca moth genera Tegeticula and Parategeticula constitute a monophyletic group within the Prodoxidae (Fig. 1). Jointly they contain at least 25 extant species (5), two of which are derived nonpollinating Tegeticula species that oviposit into yucca fruit created by coexisting pollinator species (16). The sister group Prodoxus coexists with the pollinators on yuccas but feed as larvae on plant parts other than the seeds. Their radiation was thus directly facilitated by the pollinator radiation. Together these genera constitute a major adaptive radiation on yuccas, with a species diversity more than 20-fold that of their sister group, the nonpollinating seed-parasitic Mesepiola, whose larvae feed on plants in the Nolinaceae (17).
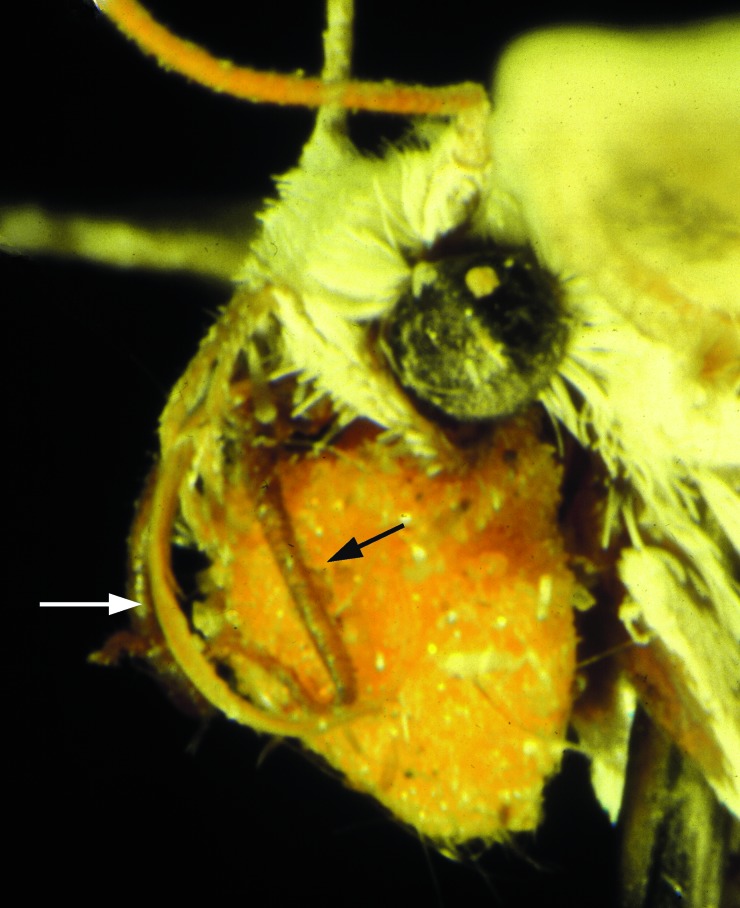
Female yucca moths possess unique tentacles on their mouthparts that are used to actively pollinate host flowers where they oviposit. The female moth gathers the glutinous pollen of yucca flowers by scraping it off the anthers with her tentacles. The pollen is immediately compacted by using tentacles and sometimes the forelegs as well, and placed as a solid batch on the concave posterioventral surface of the head (Fig. 2). The pollen mass may approach 10,000 grains and weigh up to 10% of the moth body mass (18). Prolific pollen coating maintains batch cohesion, and the tentacles are not involved in its retention. After pollen gathering, the moth seeks out flowering yucca plants where she oviposits into (Tegeticula) or near (Parategeticula) pistils. As oviposition is completed, the female flexes her tentacles and uses the apical portion to remove a small pollen load from her batch. She walks to the floral stigma and very deliberately places the pollen on it. In all but one host species, the stigmatic papillae line the interior of the hollow style, and the moth packs in the pollen with 10–20 repeated bobbing motions in the course of 3–10 sec (Movie 1, which is available as supporting information on the PNAS web site, www.pnas.org). In the single exception, the host (Hesperoyucca whipplei) has a cap-shaped stigma, and the moth pollinates by using the same dragging behavior on the stigma as is used for pollen collection on the anthers.
Figure 2.
Head of female Tegeticula carnerosanella with yucca pollen load. Black arrow = left tentacle; white arrow = proboscis.
Materials and Methods
Male and female moths of six species were included in the study (Fig. 1). Two outgroup taxa were used to determine the basal condition. Nemophora degeerella (Adelidae) is a basal member of the superfamily Incurvarioidea, which includes Prodoxidae. Prodoxus decipiens represents the pollinator sister group, thus being a close relative of the common ancestor of the tentacle-bearing moths of Tegeticula and Parategeticula. A clade of three Tegeticula species was used, including the pollinators Tegeticula yuccasella and Tegeticula cassandra, and the derived nonpollinator Tegeticula intermedia. T. intermedia recently diverged from T. cassandra (5), thus providing information on loss of the tentacles. A single representative of the smaller pollinator genus Parategeticula, Parategeticula elephantipella, was included to cover the phylogenetic range of species with tentacles. Sample data are provided in Table 1.
Table 1.
Species used and geographic origins of samples for the analyses
| Species | Locality |
|---|---|
| Adelidae | |
| Nemophora degeerella (L.) (2, 2) | Austria: Vienna, Satzberg |
| Prodoxidae | |
| Prodoxus decipiens Riley (2, 2) | U.S.: Texas, Jefferson |
| P. elephantipella Pellmyr & Balcázar-Lara (0, 1) | México: Veracruz, near Huatusco |
| T. yuccasella (Riley) (4, 4) | U.S.: Nashville, TN |
| T. cassandra Pellmyr (5, 5) | U.S.: Florida, Lake Placid |
| T. intermedia (Riley) (6, 6) | U.S.: Florida, Torreya State Park |
Number of individuals (male, female) used for the anatomical analyses are given in parentheses.
Live moths of all Tegeticula, Prodoxus, and Nemophora samples were placed for 24 h in alcoholic Bouins' fixative and then transferred to permanent storage in 70% ethanol. For Parategeticula, internal anatomy was analyzed in a female moth stored directly in 70% ethanol, and two dried male and female individuals were examined. Studies of external anatomy were performed on dehydrated and gold-coated heads by using scanning electron microscopy. Internal anatomy was examined by using serial semithin sections of specimens embedded in epoxy resin.
Tentacle size in T. intermedia was compared by scoring relative length on a linear scale from 0 to 10, where 0 was no trace and 10 represented the longest rudiment, which was one-half of the length in the sister species T. cassandra. Ten fixed individuals of each sex were used for the comparison.
Movement patterns in tentacles, palps, and proboscis during pollen collection, pollination, and drinking were observed to determine whether extension mechanisms are shared between tentacles and other parts. Fourteen female T. yuccasella and T. cassandra gathered in the field were placed individually in glass vials with a Yucca filamentosa flower. As moths became active at late dusk, we observed mouthpart movements through a ×10 lens under red narrow-bandwidth illumination (peak 626 nm) that did not affect moth behavior on the flowers.
Results
Tentacle Anatomy.
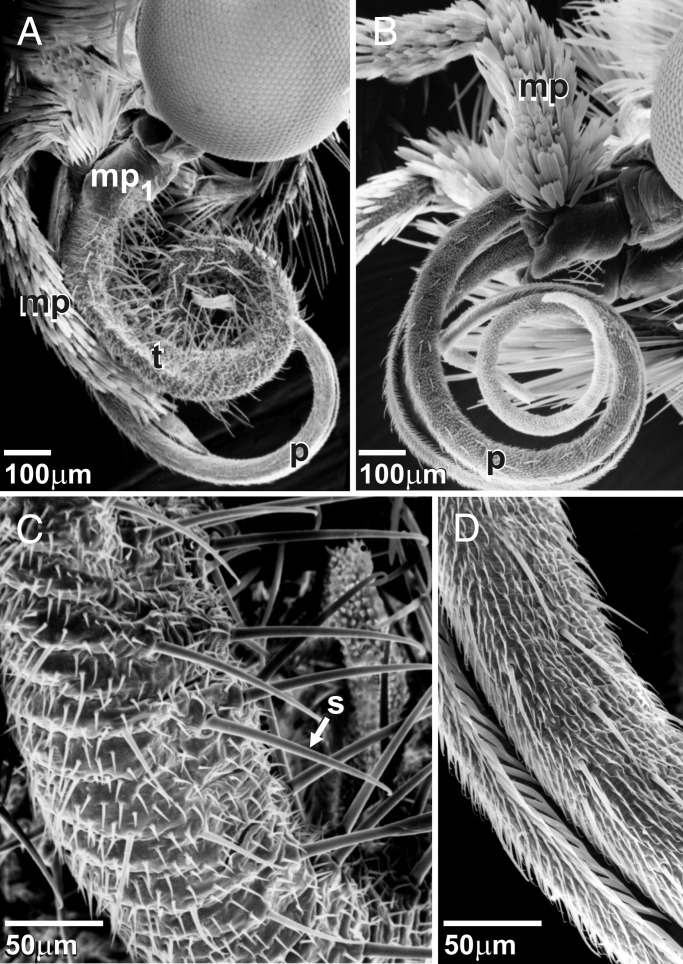
The tentacle emerges frontally on the first maxillary palp segment and is found only in females of the pollinating species of Tegeticula and Parategeticula (Fig. 3). Males possess a small cuticle elevation in the same area (Fig. 3B). No traces of a tentacle were found in either Prodoxus or Nemophora. No variation in tentacle morphology was found among the three analyzed pollinator species. The nonsegmented tentacle is 2.4 ± 0.23 mm-long (n = 10) in T. yuccasella, about 100 μm in cross section at midpoint, and it tapers toward the tip. During rest, the tentacle is coiled under the head when the female does not carry pollen (Fig. 3) and is wrapped around the pollen load when one is present (Fig. 2). The surface (Fig. 3C) is annulated, densely covered with microtrichia, and has numerous trichoid sensilla with a hooked tip. These sensilla are located primarily on ventral and lateral sides but appear on all sides near the apex.
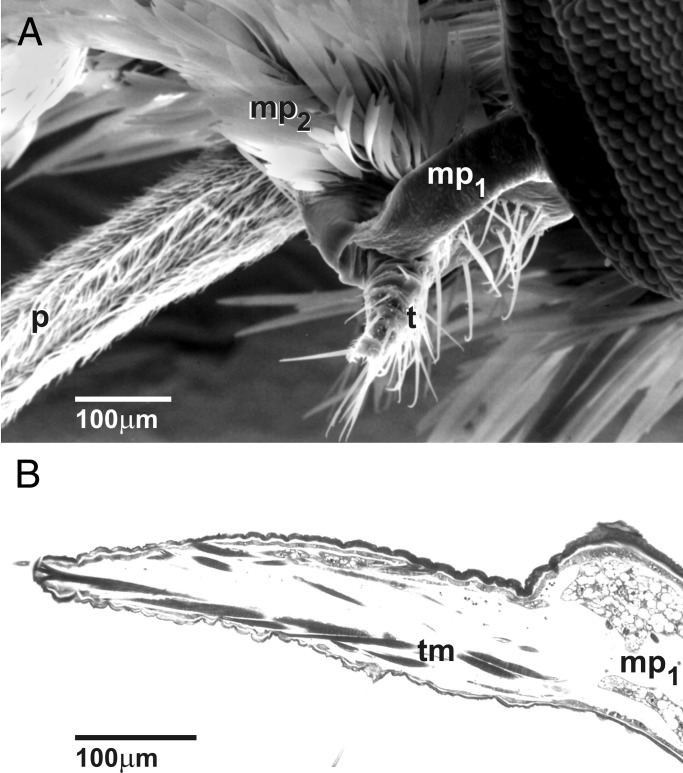
Figure 3.
(A) Mouthparts of female T. yuccasella, lateral view. Tentacle (t) originates from first segment of maxillary palp (mp1); tentacle coiled laterally from the proboscis (p); distal segments of the maxillary palp (mp) covered with scales. (B) Male of T. yuccasella with maxillary palp (mp) showing a minor frontal elevation on first segment instead of a tentacle; proboscis (p) coiled under the head. (C) Tentacle surface of T. yuccasella with microtrichiated surface and prominent trichoid sensilla (s) with hooked tips. (D) Proboscis surface of female T. yuccasella with microtrichiated surface and trichoid sensilla with straight tips.
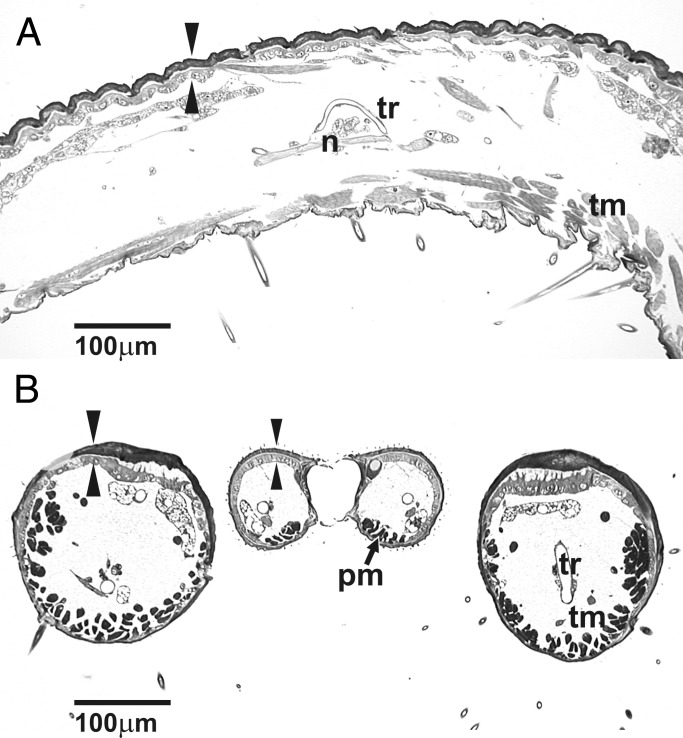
The most prominent internal feature of the tentacle of pollinators (Fig. 4) is longitudinal musculature consisting of several hundred minute muscles distributed mostly along the ventrolateral walls, although some fibers have an oblique dorsolateral course (Fig. 4B). These muscles permit recoiling and possibly some degree of lateral movement of the tentacle. They are quite distinct from muscles attached to the base of the second maxillary palp segment in all studied species, where they serve as flexors and extensors. Other features of the tentacle include a thickened dorsal epidermis and cuticle, nerves, and a single longitudinal trachea (Fig. 4).
Figure 4.
(A) Internal anatomy of the tentacle of female T. yuccasella in longitudinal section shows longitudinal tentacle musculature (tm), thickened dorsal epidermis and cuticle (arrowheads), a nerve (n), and a trachea (tr). (B) Cross section through tentacles (left and right sections) and proboscis, the latter of which is composed of the two galeae (Center), in female T. yuccasella. Shared internal features include proboscis muscles (pm) and tentacle muscles (tm) in ventral lumen, thickened dorsal epidermis and cuticle (arrowheads), nerve, and trachea (tr).
In the derived nonpollinator T. intermedia, tentacle rudiments are present in both sexes (Fig. 4B), and there is no significant difference in length between them (t test, P > 0.12). Microtrichiation and a reduced number of hooked sensilla are still present (Fig. 5). A reduced number of muscles are present regardless of length of the rudiment (Fig. 5B). Tentacles are thus sexually monomorphic in the nonpollinators but dimorphic in their pollinating ancestor.
Figure 5.
(A) Tentacle rudiment (t) on first maxillary palp segment (mp1) of female T. intermedia; second maxillary palp segment (mp2) with scales; microtrichiated proboscis (p). (B) Tentacle rudiment of female T. intermedia in longitudinal section emerging from first maxillary palp segment (mp1) contains tentacle musculature (tm).
Comparison with the Proboscis.
The galea is an appendage that emerges next to the multisegmented maxillary palp from a shared basal maxillary plate. In Lepidoptera, the two galeae zip together after adult emergence to form the highly distinctive proboscis that is used for drinking (Fig. 3 B and D). The morphological analyses revealed a striking number of shared specializations between the tentacle and proboscis (Fig. 3 C and D). Synapomorphies between the tentacle and proboscis include nonarticulation and coiling ability, dorsally thickened epidermis and cuticle, fluted sensory bristles, and similarly arranged intrinsic longitudinal muscles. Modifications in the tentacle from the proboscis include increased thickness and increased number of muscle fibers, absence of a medial food groove, and hooked tips of the sensilla. No common features with the maxillary palp were found apart from the surface microtrichia.
Mouthpart Movements.
Behavior observations showed that the proboscis was extended whenever the tentacles were, indicating a shared hydraulic mechanism for proboscis and tentacle extension. During feeding, the proboscis was straight and in line with the body axis, with the tip touching the substrate. Meanwhile the tentacles, which are not used during feeding, were partly inflated so that they formed a semicircle in females without pollen loads. During pollen collection and pollination, tentacles were fully inflated and nearly straight, whereas the nonparticipating proboscis was even more inflated than when feeding, being straight and raised above the body axis so that it did not touch the ground. When a pollen load was present, both proboscis and tentacles were already partly uncoiled as they wrapped around the pollen. In such circumstances, concerted tentacle movement with the proboscis was reduced relative to individuals without a pollen load. Regardless of pollen status of a moth, the terminal maxillary palp segment also moved forward and backward in unison with tentacles and proboscis, as predicted from a shared hydraulic mechanism.
Discussion
The tentacular appendages of the maxillary palp in the female yucca moths are a rare example of a complex key innovation that emerged in one clade without any homologous structure in related taxa. A considerable number of morphological synapomorphies are shared between the tentacle and galea, suggesting that the genetic template for the galea is fundamental in producing the tentacle as well. They include the rare condition of a nonarticulate and coilable arthropod limb, similarly arranged novel musculature, dorsally thickened epidermis and cuticle, and specific sensilla. Both structures arise from the same basal maxillary part, the stipes tube, which has been functionally interpreted to be a hemolymph pump in the higher, glossate Lepidoptera (19, 20). The behavior data indicate that the hydraulic extension mechanism is shared as well, as tentacle extension simultaneously led to proboscis extension and movement of the terminal maxillary palp segments. The lesser movement of tentacles as the proboscis is extended for drinking is expected for two reasons. First, the larger volume of the tentacle creates slower extension when pressure is adjusted for proboscis extension. Second, when the moth carries a pollen load the tentacle is already partly uncoiled without internal liquid pressure as it wraps around the pollen, and influx will cause only further extension as it reaches beyond the uncoiling.
Modifications in the tentacle from the galea are modest and mostly quantitative. The tentacle has a greater diameter than the galea, and the number of ventrolateral muscle fibers is much increased. The bristle-like sensilla are clustered in the ventral region, are larger, and have gained the distinctive terminal hook. They aid in handling the pollen and seem to evolve easily among pollen-collecting insects. Similar hooked-tip sensilla have evolved independently at least 11 times among more than 50 bee species that collect pollen from flowers whose anthers are concealed in narrow tubes (21–24). In bees, the hooked hairs appear on mouthparts or foretarsi, depending on what body part a particular species uses for pollen-gathering. Finally, a medial flattened side of the galea that forms the central food canal of the proboscis is lost.
Genetic Basis of the Tentacle.
From a morphological perspective, it is striking that such a complex structure as the tentacle has evolved without any homologous features in the related prodoxid moths. The simplest explanation for the shared specializations between the tentacle and galea is that a shared developmental pathway is involved. Although the galea is basal to all insects (25, 26), this maxillary appendage is highly modified in most Lepidoptera. The most basal clades (Micropterigoidea, Agathiphagoidea, and Heterobathmioidea) have a minute protrusion, but the clade Glossata, which constitutes about 99.9% of all described Lepidoptera, is characterized by elongated and connected galeae that form the distinctive proboscis (20, 27). Whereas the earliest proboscides lacked intrinsic musculature for tight coiling, this trait evolved well before the origin of the Prodoxidae (28–30). The yucca moths have a relatively short proboscis consisting of loosely connected galeae; they are splayed apart on wet surfaces of yucca flowers, allowing liquids such as water and nectar to be imbibed by capillary force (31).
Information about galea developmental genetics is limited but informative in developing a hypothesis for tentacle development. The growth bud of the galea shows expression of Distal-less (Dll), a homeodomain transcription factor characteristic of and required for the development of distal limb structures (32–34) in a broad taxonomic range of insects (35, 36). Galea development is usually sexually monomorphic, including in the yucca moths. In contrast, functional tentacles are sexually dimorphic in pollinating yucca moths, but sexually monomorphic partial expression of maxillary tentacle rudiments in nonpollinating T. intermedia is evidence that the developmental template is present in both sexes of pollinators but repressed in males.
A simple explanation for the observed patterns holds that the basic tentacle is an appendage using the genetic template for the galea. Dll is expressed in all maxillary palp segments (35, 36), and the tentacle would require protracted expression at an apical bud on the first palp segment. Limitation to expression in females could be determined by as little as a single gene, such as bab, known to integrate homeotic and other pathways to cause sexual dimorphism in Drosophila (37). A simple genetic basis is consistent with the rapid reversal to sexual monomorphism in the derived nonpollinators, but could also be explained by a more complex trait with at least one major-effect gene. In contrast, the variable degree of rudiment expression in nonpollinators, ranging from a blunt point to a short tentacle with some intrinsic musculature, indicates slow functional loss through mutations in different parts of the tentacle template. This hypothesis is consistent with life history reconstruction of the nonpollinating yucca moths, which suggests loss of pollination behavior was secondary to a shift from ovipositing in flowers to ovipositing in fruit (5), making the structures and behaviors associated with pollination redundant and subject to gradual loss through absence of purifying selection.
In conclusion, the morphological data strongly indicate that the unique tentacles of female yucca moths originated through expression in a novel site of the genetic template for the elongated galeae of Lepidoptera. Prior work on the interaction has suggested that the tentacles are the only truly novel morphological trait in these moths, and the present findings show that acquisition of this complex trait and its mechanism of movement may have been evolutionarily simple. Meanwhile, the behavioral component of pollination is likely derived from a common probing behavior for nectar in floral tubes in more basal prodoxids (4) and on the wet stigmas of yuccas, thus a passive, less efficient pollination mechanism may have preceded the origin of the tentacles and active pollination in the yucca moths. Given the significance of these organisms in studies of coevolutionary processes, coupled with the rarity of evolution of new limbs, it should be important to test the explicit predictions about patterns of gene expression that derive from this hypothesis about tentacle origin.
Supplementary Material
Acknowledgments
We dedicate this paper to the memory of Ebbe Schmidt Nielsen, who contributed greatly to our understanding of basal evolution of Lepidoptera. We thank Kari Segraves and Manuel Balcázar-Lara for help gathering specimens, Ursula Hannappel and Alexander Pernstich for the preparation of the semithin sections, and Jim Kane for guidance on bee sensilla. Florida State Parks provided permission to gather samples in Torreya State Park. Behavior experiments were performed at Archbold Biological Station. This work was supported by the National Geographic Society, the National Science Foundation, and the Austria Science Fund (P 13944).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Herre E A. In: Levels of Selection in Evolution. Keller L, editor. Princeton: Princeton Univ. Press; 1999. pp. 209–237. [Google Scholar]
- 2.Thompson J N. Science. 1999;284:2116–2118. doi: 10.1126/science.284.5423.2116. [DOI] [PubMed] [Google Scholar]
- 3.Bogler D J, Neff J L, Simpson B B. Proc Natl Acad Sci USA. 1995;92:6864–6867. doi: 10.1073/pnas.92.15.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellmyr O, Thompson J N, Brown J, Harrison R G. Am Nat. 1996;148:827–847. [Google Scholar]
- 5.Pellmyr O, Leebens-Mack J. Am Nat. 2000;156:S62–S76. doi: 10.1086/303416. [DOI] [PubMed] [Google Scholar]
- 6.Bronstein J L. Ecol Lett. 2001;4:277–287. [Google Scholar]
- 7.Pellmyr O, Leebens-Mack J. Proc Natl Acad Sci USA. 1999;96:9178–9183. doi: 10.1073/pnas.96.16.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reveal J L. In: Intermountain Flora. Cronquist A, Holmgren A H, Holmgren N H, Reveal J L, Holmgren P K, editors. Vol. 6. New York: New York Botanical Garden Press; 1977. pp. 527–536. [Google Scholar]
- 9.Clary K H. Ph.D. thesis. Austin, TX: Univ. of Texas; 1997. [Google Scholar]
- 10.Brown D E, editor. Biotic Communities: Southwestern United States and Northwestern Mexico. Salt Lake City: Univ. of Utah Press; 1994. [Google Scholar]
- 11.Simpson G G. The Major Features of Evolution. New York: Columbia Univ. Press; 1953. [Google Scholar]
- 12.Heard S B, Hauser D L. Hist Biol. 1995;10:151–173. [Google Scholar]
- 13.Schluter D. The Ecology of Adaptive Radiation. Oxford: Oxford Univ. Press; 2000. [Google Scholar]
- 14.Riley C V. Nature (London) 1872;6:444. [Google Scholar]
- 15.Riley C V. Missouri St Entomol Annu Rep. 1873;5:150–160. [Google Scholar]
- 16.Pellmyr O, Leebens-Mack J, Huth C J. Nature (London) 1996;380:155–156. doi: 10.1038/380155a0. [DOI] [PubMed] [Google Scholar]
- 17.Frack D C. M.S. thesis. Pomona, CA: California State Polytechnic University; 1982. [Google Scholar]
- 18.Pellmyr O. Ecology. 1997;78:1655–1660. [Google Scholar]
- 19.Bänziger H. Mitt Schweiz Entomol Ges. 1971;43:225–239. [Google Scholar]
- 20.Krenn H W. Zoomorphology. 1990;110:105–114. [Google Scholar]
- 21.Müller A. Entomol Gener. 1995;20:43–57. [Google Scholar]
- 22.Alves dos Santos I, Wittmann D. Plant Syst Evol. 2000;223:127–137. [Google Scholar]
- 23.Michener C D. The Bees of the World. Baltimore: Johns Hopkins Univ. Press; 2000. [Google Scholar]
- 24.Thorp R W. Plant Syst Evol. 2000;222:211–223. [Google Scholar]
- 25.Snodgrass R E. Principles of Insect Morphology. New York: McGraw–Hill; 1935. [Google Scholar]
- 26.Chapman R F. The Insects: Structure and Function. Cambridge, U.K.: Cambridge Univ. Press; 1998. [Google Scholar]
- 27.Kristensen N P, Skalski A W. In: Lepidoptera: Moths and Butterflies 1. Handbook of Zoology. Kristensen N P, editor. Berlin: de Gruyter; 1998. pp. 7–25. [Google Scholar]
- 28.Kristensen N P. Ent Medd. 1968;36:239–293. [Google Scholar]
- 29.Nielsen E S, Kristensen N P. Invert Taxon. 1996;10:1199–1302. [Google Scholar]
- 30.Krenn H W, Kristensen N P. Zool Anz. 2000;239:179–196. [Google Scholar]
- 31.Pellmyr O. Syst Entomol. 1999;24:243–271. [Google Scholar]
- 32.Cohen S, Bronner M, Kuttner F, Jurgens G, Jackle H. Nature (London) 1989;338:432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- 33.Panganiban G, Irvine S M, Lowe C, Roehl H, Corley L S, Sherbon B, Grenier J, Fallon J F, Kimble J, Walker M, et al. Proc Natl Acad Sci USA. 1997;94:5162–5166. doi: 10.1073/pnas.94.10.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abzhanov A, Kaufman T. Dev Biol. 2000;227:673–689. doi: 10.1006/dbio.2000.9904. [DOI] [PubMed] [Google Scholar]
- 35.Popadic A, Panganiban G, Rusch D, Shear W A, Kaufman T C. Dev Genes Evol. 1998;208:142–150. doi: 10.1007/s004270050165. [DOI] [PubMed] [Google Scholar]
- 36.Scholtz G, Mittmann B, Gerberding M. Int J Dev Biol. 1998;42:801–810. [PubMed] [Google Scholar]
- 37.Kopp A, Duncan I, Carroll S B. Nature (London) 2000;408:553–559. doi: 10.1038/35046017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.