Abstract
The rise of antimicrobial resistance (AMR) in Salmonella enterica poses a significant public health threat, particularly through the dissemination of extended-spectrum β-lactamase (ESBL) genes such as blaCTX-M-65. This study investigated the prevalence, resistance profiles, and genomic characteristics of S. enterica isolates from retail poultry products in North Carolina, collected between 2020 and 2024. Among 132 isolates representing 25 serovars, 14 were identified as multidrug-resistant (MDR) strains harboring blaCTX-M-65. Whole-genome sequencing revealed that these isolates belonged to three serovars—S. Infantis (n = 11), S. I -:r:1,5 (n = 2), and S. Senftenberg (n = 1)—with associated sequence types ST32 and ST14. Genomic analyses identified additional resistance determinants, including quinolone resistance-determining region (QRDR) mutations, and a range of mobile genetic elements, such as IncFIB(pN55391) plasmids. The genetic environment of blaCTX-M-65 was conserved, with IS1380-blaCTX-M-65-IS5 structures, highlighting its mobility potential. Phylogenetic analysis showed that isolates clustered by serovar, with strong associations to international lineages. These findings emphasize the ongoing clonal dissemination of blaCTX-M-65 and MDR Salmonella in the food supply chain, necessitating enhanced surveillance and mitigation strategies to curb the spread of resistance genes in food production environments.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-02972-2.
Keywords: Salmonella, Food chain, ESBL, Genomic surveillance, Horizontal dissemination
Introduction
The global rise of antimicrobial resistance (AMR) poses a significant threat to public health, with Salmonella enterica being a major contributor to foodborne illnesses worldwide. Among the various mechanisms driving AMR in Salmonella, the acquisition of extended-spectrum β-lactamases (ESBLs), particularly the CTX-M family, is of critical concern. The blaCTX-M-65 gene, a member of the CTX-M family, has been increasingly reported in Salmonella enterica across various serovars in food animals, underscoring its role in mediating resistance to third-generation cephalosporins, which are commonly used in clinical settings to treat severe bacterial infections.
The presence of blaCTX-M-65 in Salmonella enterica serovars such as Indiana1, Typhimurium2, Infantis3, and Senftenberg3 represents a significant public health challenge due to the potential for these strains to cause severe, difficult-to-treat infections. This gene is often located on mobile genetic elements, such as plasmids, which facilitate horizontal gene transfer between different bacteria, exacerbating the spread of resistance within both clinical and environmental settings. Understanding the prevalence, distribution, and genetic context of blaCTX-M-65 within Salmonella enterica is essential for developing effective strategies to combat the spread of ESBL-producing strains.
The National Antimicrobial Resistance Monitoring System (NARMS) program routinely monitors foodborne pathogens in retail meat products, humans, and food animals. In fact, our research group has reported the presence of foodborne pathogens, including S. enterica4, Campylobacter spp.5, and Escherichia coli6 in retail meat products. Based on our previous reports4–6, there was clear value in continuum explore whether S. enterica still circulating in retail meat products. Therefore, this study aims to investigate the prevalence of blaCTX-M-65 in various Salmonella enterica serovars isolated from retail poultry, characterize the associated resistance profiles, and assess the implications for public health.
Results
Prevalence
Among the 132 S. enterica isolates examined, 25 different serovars were identified for this regional study. Most isolates included S. Infantis (n = 34), S. Kentucky (n = 31), S. Typhimurium (n = 11), S. Hadar (n = 7), S. Senftenberg (n = 7), S. Agona (n = 7), S. Enteritidis (n = 6), S. I -:r:1,5 (n = 4), S. Newport (n = 3), S. I 4,5,12:i:- (n = 3), S. Anatum (n = 2), S. Uganda (n = 2), and S. IIIa -:z4,z23:- (n = 2). The remaining isolates were classified in 13 different serovars, including S. Reading (n = 1), S. Meleagridis (n = 1), S. Bovismorbificans (n = 1), S. Thompson (n = 1), S. Saintpaul (n = 1), S. Adelaide (n = 1), S. Tennessee (n = 1), S. London (n = 1), S. Schwarzengrund (n = 1), S. Ouakam (n = 1), S. Muenster (n = 1), S. Muenchen (n = 1), and S. Rissen (n = 1).
Resistance
The MIC values ranged from 0.5 µg/mL to 512 µg/mL among 14 antibiotics tested. The majority of quinolone-resistant phenotypes (QRP) isolates presented high-level resistance (MIC ≥ 32 µg/mL) to nalidixic acid and range between 0.5 to 8 µg/mL for ciprofloxacin. Regarding broad-spectrum cephalosporin-resistant, all isolates harboring blaCTX-M-65 presented high-level resistance against ceftriaxone (MIC > 64 µg/ml). Interestingly, all strains displayed intermediate resistance against colistin.
In this study, all 132 isolates were screened for resistance to broad-spectrum cephalosporins, of these 14 isolates were considered as high priority Salmonella strains due to broad-spectrum cephalosporin resistance profiles. Then, genomic investigation was performed on 14 isolates, including high priority Salmonella strains displaying an MDR (n = 14), defined as resistant to three or more classes of antimicrobial compounds7.
While these 14 Salmonella isolates remained susceptible to carbapenems, additional genes encoding resistance to aminoglycoside [aac(3)-Iva, aadA1, aph(3')-Ia, aph(4)-Ia], sulfonamide [sul1, sul2], tetracycline [tet(A)], trimethoprim [drfA14], phenicol [floR], fosfomycin [fosA3], multidrug and metal efflux proteins [mdsA and mdsB], and quinolone [gyrA_D87Y] were confirmed by WGS as shown in Table 1.
Table 1.
Genomic features of ESBL producing Salmonella enterica serovars.
| Accession number | Serovar by WGS | Source/year | Resistance genes | Sequence type | R-type | Plasmid |
|---|---|---|---|---|---|---|
| SAMN38156057 | Infantis | Chicken Gizzard/2021 |
aac(3)-Iva, aadA1, aph(4)-Ia, blaCTX-M-65, floR, mdsA, mdsB, sul1, tet(A) gyrA_D87Y |
32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN38156058 | Infantis | Chicken Heart/2021 | aac(3)-Iva, aph(4)-Ia, blaCTX-M-65, mdsA, mdsB, tet(A), gyrA_D87Y | 32 | AMP-AXO-NAL-TET | IncFIB(pN55391) |
| SAMN38156185 | Infantis | Chicken Gizzard/2021 | aac(3)-Iva, aadA1, aph(4)-Ia, blaCTX-M-65, floR, mdsA, mdsB, sul1, tet(A), gyrA_D87Y | 32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN38156287 | Infantis | Chicken Gizzard/2021 | blaCTX-M-65, mdsA, mdsB, gyrA_D87Y | 32 | AMP-AXO-CIP-NAL | IncFIB(pN55391), Col440I, ColRNAI |
| SAMN38225586 | I -:r:1,5 | Chicken Heart/2021 |
aadA1, blaCTX-M-65, mdsA, mdsB, sul1, tet(A) gyrA_D87Y |
32 | AMP-AXO-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN38225595 | Infantis | Chicken Heart/2021 | aadA1, blaCTX-M-65, mdsA, mdsB, sul1, tet(A), gyrA_D87Y | 32 | AMP-AXO-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN23299079 | Infantis | Chicken breast/2021 | aac(3)-Iva, aadA1, aph(4)-Ia, blaCTX-M-65, floR, mdsA, mdsB, sul1, tet(A), gyrA_D87Y | 32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN23299076 | Senftenberg | ground turkey/2021 | aac(3)-IVa, aadA1, aph(3')-Ia, aph(4)-Ia, blaCTX-M-65, dfrA14, floR, mdsA, mdsB, sul1, sul2, tet(A), aph(3'')-Ib | 14 | AMP-AXO-CHL-FIS-TET-SXT | IncFIB(pN55391), IncQ1 |
| SAMN25653139 | I -:r:1,5 | Chicken-Mixed Parts/2021 | aac(3)-Iva, aadA1, aph(4)-Ia, blaCTX-M-65, mdsA, sul1, tet(A), mdsB, gyrA_D87Y | 32 | AMP-AXO-CIP-NAL-FIS-TET | IncFIB(pN55391), IncX1 |
| SAMN27284154 | Infantis | Chicken Thighs/2022 |
aac(3)-Iva, aadA1, aph(4)-Ia, blaCTX-M-65, dfrA14 floR, mdsA, mdsB, sul1, tet(A), gyrA_D87Y |
32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN27284156 | Infantis | Ground turkey/2022 | aac(3)-Iva, aadA1, aph(4)-Ia, blaCTX-M-65, mdsA, mdsB, sul1, tet(A), gyrA_D87Y | 32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391), IncFIB(pHCM2) |
| SAMN27284157 | Infantis | Ground turkey/2022 | aac(3)-IVa, aadA1, aph(4)-Ia, blaCTX-M-65, dfrA14, floR, fosA3, mdsA, mdsB, sul1, tet(A), gyrA_D87Y | 32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN36349286 | Infantis | Chicken Heart/2023 | aadA1, blaCTX-M-65, mdsA, mdsB, sul1, tet(A), gyrA_D87Y | 32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391) |
| SAMN37706922 | Infantis | Chicken liver/2023 | aac(3)-IVa, aadA1, aph(3')-Ia, aph(4)-Ia, blaCTX-M-65, mdsA, mdsB, sul1, tet(A), tet(A), gyrA_D87Y | 32 | AMP-AXO-CHL-CIP-NAL-FIS-TET | IncFIB(pN55391) |
Among 14 selected isolates that harbored genes conferring resistance to ESBLs, all carried the β-lactam gene blaCTX-M-65, whereas 13 isolates harbored quinolone resistance-determining region (QRDR) mutation (Table 1). These isolates harboring blaCTX-M-65 belonged to three serovars including S. Infantis (n = 11), S. I -:r:1,5 (n = 2), and S. Senftenberg (n = 1) as summarized in Table 1.
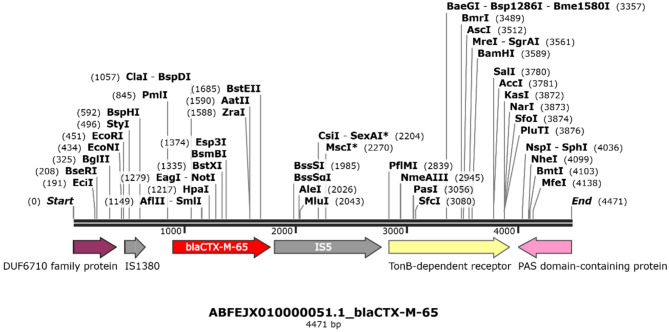
We were also able to describe the schematic representations of the genetic context surrounding blaCTX-M-65 gene. In doing so, the genetic environment was composed by IS1380-blaCTX-M-65-IS5 with 4,471 bp in size as shown in Fig. 1. While this analysis focused on a limited region surrounding blaCTX-M-65, further investigation is needed to determine the full extent of associated genetic elements. In addition, a class 1 integron was identified on a separate contig in all strains, containing the intI1 integrase gene and flanked by the resistance gene cassettes aadA1 (aminoglycoside resistance), qacE (quaternary ammonium compound resistance), and sul1 (sulfonamide resistance). On the other hand, the Salmonella Senftenberg strain harbored a class 2 integron, which contained the dfrA14 gene conferring resistance to trimethoprim.
Fig. 1.
Representative genetic context of blaCTX-M-65 of S. enterica serovars. Genes and shotgun sequences were extracted from the GenBank database. Arrows indicate the positions and directions of the genes.
Identification of international lineages among cephalosporin-resistant Salmonella serovars
Among 14 S. enterica isolates examined, two different sequence types (STs) were identified. The most frequently observed was ST32 [S. Infantis (n = 11) and S. I -:r:1,5 (n = 2)], followed by ST14 [S. Senftenberg (n = 1)]. Interestingly, S. I -:r:1,5 assigned to the ST32, which is also associated with S. Infantis.
Plasmid incompatibility group
The most common plasmid incompatibility group identified in our collection was IncFIB(pN55391). In addition, we identified several other plasmids including IncQ1, IncX1, Col440I, ColRNAI and IncFIB(pHCM2) as shown in Table 1. Genomic prediction confirmed that the incQ1 plasmid harbored three AMR genes including aph(3'')-Ib, tet(A), and sul2.
Phylogenetic and evolutionary dynamics of S. enterica isolates
The 14 strains were distributed across six phylogenetic clusters (Fig. 2), all grouping with strains previously isolated in the USA, suggesting a potential regional lineage. Each serovar formed a distinct monophyletic clade, with bootstrap values greater than 99, indicating high confidence in the phylogenetic structure. This clustering suggests that these strains may have evolved within specific epidemiological niches, potentially contributing to the persistence and dissemination of blaCTX-M-65 within the USA’s food production system.
Fig. 2.
Maximum-likelihood phylogenetic tree of 53 S. enterica strains from different sources, countries, and years. The phylogeny is rooted at midpoint.
Discussion
The emergence and dissemination of Salmonella enterica serovars harboring the blaCTX-M-65 gene and displaying multidrug-resistant (MDR) profiles has been documented worldwide and pose a significant threat to public health1–4. In this study, we identified 132 S. enterica isolates from various chicken parts, including the heart, gizzard, breast, thighs, and liver, as well as ground turkey, collected between 2020 and 2024. These isolates reflect a snapshot of the prevalence of antimicrobial resistance in retail poultry, a critical reservoir for zoonotic transmission of resistant Salmonella strains. These 132 S. enterica isolates, encompassing 25 different serovars, with S. Infantis and S. Kentucky being the most prevalent. Among these, 14 isolates were of particular concern due to their resistance to broad-spectrum cephalosporins, confirmed to carry the blaCTX-M-65 gene. This finding highlights the continued spread of ESBL-producing Salmonella strains, which complicates treatment options in both human clinical and veterinary settings.
The high prevalence of S. Infantis (n = 34) in our collection, and particularly its association with blaCTX-M-65 in 11 isolates, is consistent with global reports that link this serovar to multidrug resistance and the presence of blaCTX-M genes1–4. The presence of ST32 among S. Infantis isolates, a sequence type frequently associated with this serovar, further supports the notion that certain lineages of S. Infantis are adept at acquiring and disseminating resistance genes via mobile genetic elements, such as plasmids. Sequence type (ST) can influence the propensity of bacterial strains to acquire and disseminate resistance genes through plasmids due to factors such as plasmid-host compatibility, recombination frequency, and ecological adaptation. In the case of ST32, previous studies have reported a high prevalence of multidrug resistance, suggesting a history of successful horizontal gene transfer events. Additionally, certain sequence types may have genetic traits, such as specific recombination systems or regulatory elements, that facilitate plasmid acquisition and stability. To further support this, comparative genomic analysis of ST32 strains could reveal potential mechanisms contributing to their adaptation in acquiring plasmid-borne resistance genes. Interestingly, the ST32 sequence type was also observed in two isolates of S. I -:r:1,5, suggesting that this serovar may share a common evolutionary pathway with S. Infantis in acquiring antimicrobial resistance traits.
Although S. Senftenberg was less prevalent in our collection (n = 1), the detection of blaCTX-M-65 in this isolate and its assignment to ST14 underscores the adaptability of resistance genes across different Salmonella serovars. This highlights the importance of continuous surveillance, especially in understudied or less prevalent serovars, to prevent the silent spread of critical resistance genes such as blaCTX-M-65.
Whole-genome sequencing (WGS) identified the presence of additional resistance determinants, including genes encoding resistance to aminoglycosides [aac(3)-Iva, aadA1, aph(3')-Ia], sulfonamides [sul1, sul2], tetracyclines [tet(A)], and quinolones [QRDR mutation gyrA_D87Y]. These findings suggest that MDR Salmonella strains not only harbor β-lactam resistance but also present broad resistance profiles that could further complicate treatment strategies. Additionally, the presence of metal efflux genes [mdsA and mdsB] suggests potential environmental survival advantages, which could enhance persistence in food production environments, thus increasing the risk of human exposure8.
The plasmid analysis showed that the most common incompatibility group identified was IncFIB(pN55391), which has been associated with the spread of resistance genes, particularly in ESBL-producing Enterobacterales9. The detection of plasmids such as IncQ1 and IncX1 further emphasizes the role of plasmids in the horizontal transfer of resistance genes. This reinforces the notion that plasmid-mediated gene transfer plays a pivotal role in the dissemination of blaCTX-M-65 among Salmonella enterica serovars, contributing to the global issue of antibiotic resistance.
The schematic representation of the genetic context surrounding the blaCTX-M-65 gene revealed conserved structures such as IS1380 and IS5 that could serve as targets for future intervention strategies. By understanding the genetic elements facilitating the mobility of resistance genes, such as insertion sequences and transposons, more precise control measures can be developed to limit the spread of ESBL-producing pathogens.
Investigating the genomic diversity among Salmonella isolates is valuable from an epidemiological perspective. Our analysis revealed that S. enterica serovar and sequence type were the primary factors driving the clustering, as isolates typically grouped by serovar rather than by resistance profile, year, source, or geographic location. However, given the limited sample size, further studies with larger datasets are needed to confirm these findings and assess their broader epidemiological significance. Phylogenetic analysis revealed that S. Infantis isolates clustered tightly, forming a highly supported monophyletic clade, which may indicate clonal expansion. These strains were all collected between 2020 and 2024 from a variety of sources, suggesting the possibility of a common source or sustained circulation within a specific sector of the poultry production chain. Such clustering warrants further surveillance to trace potential dissemination routes across the food supply chain.
In summary, the findings from this study underscore the importance of routine surveillance for MDR Salmonella enterica serovars, particularly those harboring blaCTX-M-65. The detection of this gene across multiple serovars suggests that the spread of blaCTX-M-65 is not restricted to a single lineage or serovar, but rather is a widespread phenomenon. This study highlights the need for comprehensive strategies, including genomic monitoring and the prudent use of antibiotics, to curb the spread of resistance in food production settings. In addition to surveillance and antimicrobial stewardship, consumer education on proper food handling, cooking, and hygiene practices is essential to reducing the risk of Salmonella infections. Public health initiatives should emphasize safe food preparation, including thorough cooking of poultry products and preventing cross-contamination in home kitchens. To our knowledge, this is among the first studies to report chromosomal integration of blaCTX-M-65 in S. Infantis isolates recovered from U.S. retail meat, which differs from earlier reports where the gene was primarily plasmid-borne10. This genomic configuration may affect the mobility and persistence of resistance and highlights the need for continued genomic monitoring.
Methods
Data collection
The Salmonella data collection was conducted in accordance with the NARMS surveillance and laboratory protocols in North Carolina11. The timeframe of this study was from January 2020 through December 2024. During this time, retail meat samples, totaling 46 packages of fresh (never frozen) poultry products, were collected twice a month from a variety of grocery stores throughout North Carolina. The FDA pre-assigned grocery store sampling locations using the chain store guide https://www.chainstoreguide.com/ to identify all grocery stores within a 50 mile radius from the NCSU Molecular Epidemiology Laboratory based on zip codes. The FDA divided the sampling sites geographically into quadrants and used a random number generator to randomly assign the order to be sampled. The FDA randomized list of grocery stores is updated for each year12. Caution was taken during sample collection to select a variety of brands and products from multiple sources.
Salmonella strains and serotyping
During a national surveillance study, 132 nontyphoidal Salmonella enterica (NTS) isolates recovered over a 5-year period (2020 to 2024), from the poultry production chain, in North Carolina, have been subjected to antimicrobial resistance screening. Specifically, in this study, we have focused on high-priority S. enterica isolates (n = 14) harboring extended-spectrum β-lactamase (ESBL) resistance genes (Table 1). Bacterial isolation and serotyping were performed as previously reported4. Briefly, the retail meat samples were stored at 4 °C and processed within 96 h of purchase. To begin processing, 50 g of meat was aseptically transferred into sterile Whirl–Pak® bags containing 250 ml of buffered peptone water (BPW). The samples were then shaken at 200 rpm for 15 min using a mechanical shaker. Following agitation, the bags were incubated at 35 °C for 24 h. After incubation, 0.1 ml of the BPW rinse from each sample was transferred into a test tube containing 10 ml of Rappaport–Vassiliadis (RVR10) medium and incubated in a water bath at 42 °C for 20–24 h4. The resulting RVR10 cultures were briefly vortexed and streaked onto XLT-4 agar plates, which were incubated at 35 °C for 18–24 h4. The plates were examined for colonies with typical Salmonella morphology. A single presumptive Salmonella colony from each plate was selected and streaked onto blood agar, followed by incubation at 35 °C for 24 h. The blood agar plates were then inspected for purity, and Salmonella identification was confirmed using standard biochemical methods, including API and MALDI-TOF4. In addition, the isolates were serotyped in silico using default settings in SeqSero 1.2 (http://www.genomicepidemiology.org/).
Whole-genome sequencing analysis
DNA extraction of the isolates were performed using a commercial kit (QiAmp tissue, Qiagen, Germany) according to manufacturer’s recommendation. Genomic DNA of the isolates were sequenced at a 300-bp paired-end-read using the Nextera XT library preparation kit on the MiSeq platform (Illumina, San Diego, CA). FastQ files generated were uploaded into CLC Genomics Workbench (CLC Bio, Qiagen, Aarhus, Denmark), to check the quality of the sequences and ensure the non-contamination of the reads. Subsequently, de novo assembly were performed using the same software. The assemblies were analyzed for antimicrobial resistance genes, plasmids, and sequence typing using default settings (90% identity, 60% coverage) of ResFinder 4.1, PlasmidFinder 2.1, and MLST 2.0 databases, respectively, available at the Center for Genome Epidemiology (http://www.genomicepidemiology.org/). The genetic context of the blaCTX-M-65 gene was analyzed by annotating the genomes using the PATRIC (Pathosystems Resource Integration Center) platform. The surrounding genetic environment was further examined using SnapGene to visualize the genomic context and identify adjacent resistance genes, mobile genetic elements, and potential insertion sequences.
Antimicrobial susceptibility testing
The isolates were characterized phenotypically by broth microdilution using Sensititre® Gram Negative Plate (Trek Diagnostic Systems, OH), which comprise antimicrobials used in human and veterinary medicine such as cefoxitin (FOX), ceftriaxone (AXO), amoxicillin/clavulanic acid 2:1 ratio (AUG2), ceftiofur (XNL), ampicillin (AMP), nalidixic acid (NAL), ciprofloxacin (CIP), chloramphenicol (CHL), tetracycline (TET), gentamicin (GEN), sulfisoxazole (FIS), trimethoprim/sulfamethoxazole (SXT), streptomycin (STR), and azithromycin (AZI). Results were interpreted as per the Clinical and Laboratory Standards Institute (CLSI, 2023)13 the VET01S14.
Phylogenetic and evolutionary dynamics of S. enterica isolates
In order to investigate the genomic similarity among S. enterica strains, a maximum likelihood phylogenetic tree was performed by using default settings of CSI Phylogeny 1.4 and uploading the raw sequencing reads of fifty-three S. enterica strains harboring blaCTX-M-65 originating from different sources, countries, and years into CSI Phylogeny 1.4 (https://cge.food.dtu.dk/services/CSIPhylogeny/). Whole-genome sequences were extracted from NCBI. The resulting phylogeny was visualized and annotated using iTol version 6 (https://itol.embl.de).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the USDA and FDA CVM NARMS scientists for conducting whole genome sequencing of Salmonella isolates.
Author contributions
DFMM was responsible for analyzing the data and drafting the main manuscript text EH- was responsible for sample collection, processing, and manuscript editing LBH was responsible for the generation of whole genome sequencing data and in the analysis ST was responsible for securing the funding, data analysis, and manuscript editing.
Funding
ST Grant #1U01FD007145-01 Funder name: National Antimicrobial Resistance Monitoring System URL: https://www.cdc.gov/narms/index.html. Whole-genome sequencing work is supported by the National Institutes of Health/Food and Drug Administration under award #5U 18FD006194-02. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Data availability
Sequence data that support the findings of this manuscript have been deposited in the National Center for Biotechnology Information database under the project accession PRJNA292661.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bai, L. et al. Prevalence of salmonella isolates from chicken and pig slaughterhouses and emergence of ciprofloxacin and cefotaxime co-resistant S. enterica Serovar Indiana in Henan, China. PLoS ONE, 10, e0144532 (2015). 10.1371/journal.pone.0144532 [DOI] [PMC free article] [PubMed]
- 2.Ali, M. S. et al. Antimicrobial resistance profiles and molecular characteristics of extended-spectrum β-lactamase-producing salmonella enterica serovar typhimurium isolates from food animals during 2010–2021 in South Korea. Foodborne Pathog. Dis.21, 634–642. 10.1089/fpd.2023.0128 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Li, C. et al. The spread of pESI-mediated extended-spectrum cephalosporin resistance in Salmonella serovars-Infantis, Senftenberg, and Alachua isolated from food animal sources in the United States. PLoS ONE19, e0299354. 10.1371/journal.pone.0299354 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull, D. M., Harrell, E., Harden, L. & Thakur, S. Multidrug resistance and virulence genes carried by mobile genomic elements in Salmonella enterica isolated from live food animals, processed, and retail meat in North Carolina, 2018–2019. Int. J. Food Microbiol.378, 109821. 10.1016/j.ijfoodmicro.2022.109821 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Hull, D. M., Harrel, E., Harden, L. & Thakur, S. Detection of resistance and virulence plasmids in Campylobacter coli and Campylobacter jejuni isolated from North Carolina food animal production, 2018–2019. Food Microbiol.116, 104348. 10.1016/j.fm.2023.104348 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Aworh, M. K. et al. Characteristics of antimicrobial resistance in Escherichia coli isolated from retail meat products in North Carolina. PLoS ONE19, e0294099. 10.1371/journal.pone.0294099 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect.18, 268–281. 10.1111/j.1469-0691.2011.03570.x (2012). [DOI] [PubMed] [Google Scholar]
- 8.Song, S., Hwang, S., Lee, S., Ha, N. C. & Lee, K. Interaction mediated by the putative tip regions of MdsA and MdsC in the formation of a Salmonella-specific tripartite efflux pump. PLoS ONE9(6), e100881 (2014). 10.1371/journal.pone.0100881 [DOI] [PMC free article] [PubMed]
- 9.M’ikanatha, N. M., Yin, X., Boktor, S. W., Dettinger, L. A. & Tewari, D. Integrated surveillance for antimicrobial resistance in Salmonella from clinical and retail meat sources reveals genetically related isolates harboring quinolone- and ceftriaxone-resistant determinants. Open Forum Infect. Dis.8, ofab213 (2021). 10.1093/ofid/ofab213 [DOI] [PMC free article] [PubMed]
- 10.Burnett, E. et al. Whole-genome sequencing reveals the presence of the blaCTX-M-65 gene in extended-spectrum β-lactamase-producing and multi-drug-resistant clones of salmonella serovar infantis isolated from broiler chicken environments in the Galapagos Islands. Antibiotics (Basel, Switzerland)10(3), 267. 10.3390/antibiotics10030267 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration (FDA). National Antimicrobial Resistance Monitoring System (NARMS) Retail Meat Surveillance Laboratory Protocol. In: U.S. Health and Human Services. editor. p. 8. (2019).
- 12.Food and Drug Administration. Human Health Services. NARMS Methods: FDA, (2020) [cited 2020 1 Aug]. https://www.fda.gov/media/101741/download
- 13.CLSI. Performance standards for antimicrobial susceptibility testing. 33rd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute (2023).
- 14.CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 6th ed. CLSI supplement VET01S. Clinical and Laboratory Standards Institute (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data that support the findings of this manuscript have been deposited in the National Center for Biotechnology Information database under the project accession PRJNA292661.