Abstract
Virus-host protein-protein interactions (PPIs) are fundamental to viral infections, yet high-resolution identification of their structural and molecular determinants within the native context of intact infected cells has remained an unsolved challenge. Here, we provide detailed insights into the structural interactome of herpes simplex virus 1-infected human cells by combining in-cell cross-linking mass spectrometry with the selective enrichment of newly synthesized viral proteins. In productively infected cells, we obtain 739 PPIs based on 6,194 cross-links found across intracellular compartments and at the intact host endomembrane system. These structural host-virus interactome profiling (SHVIP) data resolve PPIs to the protein domain level and augment AlphaFold-based structural modeling, facilitating detailed predictions of PPI sites within structured and intrinsically disordered regions. Importantly, SHVIP captures parts of the virus-host PPI space that are elusive to traditional interaction proteomics approaches. Validation by molecular genetics confirms that these new SHVIP identifications are genuine virus-host PPIs occurring in the complex environment of intact infected cells.
Subject terms: Protein-protein interaction networks, Viral proteins, Virus-host interactions, Mass spectrometry
Virus-host protein-protein interactions are fundamental to viral pathogenesis, yet few are structurally resolved. Here, the authors combine cross-linking mass spectrometry, bio-orthogonal labeling and AlphaFold to reveal the structural virus-host interface from intact cells infected with HSV-1.
Introduction
During an infection, viruses interact with the host’s proteome, initiating processes that lead to viral genome replication, encapsidation, and the release of new progeny. These processes are facilitated by protein-protein interactions (PPIs), forming the physical and functional interface between the viral proteome and the host’s cellular proteome.
Gaining a systematic understanding of the structural context and molecular determinants of virus-host PPIs remains a major challenge. While structural biology techniques have provided insight into the assembly of various viral protein complexes1–5, the number of solved virus-host structures outside of receptor and antibody binding is scarce. Furthermore, viral proteins frequently contain short linear motifs (SLiMs) in their intrinsically disordered regions (IDRs) for physical association with host proteins6 but viral SLiMs are typically predicted based on the amino acid composition in the primary structure, without directly considering co-evolutionary and structural relationships7. Both these limitations are potentially addressable with recently developed structure prediction algorithms, such as AlphaFold2 (AF2)-based AF-Multimer and AlphaFold3 (AF3)8,9. However, AF has been developed mainly based on structural data of multimeric complexes within one organism, with co-evolutionary constraints likely different in host-pathogen scenarios10,11. Therefore, the utility of AF tools for predicting virus-host dimers at the interactome scale remains to be established. More generally, the need for orthogonal experimental evidence to validate and improve in silico-generated models remains critical12.
A second challenge pertains to the molecular environment in which virus-host PPIs are studied. Viruses induce significant cellular changes, affecting signaling pathways13, energy metabolism14, protein subcellular localization15, and gene expression16,17. Viruses also alter the spatial organization and microenvironment of the cell, forming membrane-less organelles via liquid-liquid phase separation (LLPS)18–21 and remodeling the host membrane system to create fragile viral macromolecular assemblies22. Studying virus-host PPIs in the context of these processes requires an intact cellular environment. However, a systematic survey of virus-host PPIs in intact, natively infected cells is lacking. The reasons for this paucity are illustrated by the following three examples. First, affinity purification mass spectrometry (AP-MS), the most widely used approach for systematic virus-host PPI profiling23–33, is not applicable to intact infected cells, cannot discriminate between direct and indirect interactors, and does not provide information on the interaction contact site. Second, virus-host PPI screens based on proximity biotinylation (e.g., BioID and APEX) require genetically engineered and ectopically expressed viral proteins34,35, which may behave differently than during an actual infection. In addition, proximity biotinylation, akin to AP-MS, is unable to reveal direct interactors and interaction sites. Third, cryo electron tomography can visualize virus–host interactions at molecular resolution in native environments36, but fails to provide systematic insights across the cell and cannot reveal the identity of interacting proteins in an untargeted fashion.
These challenges can, in principle, be addressed by cross-linking mass spectrometry (XL-MS)37. XL-MS captures PPIs with the help of small organic molecules (cross-linkers) that can covalently connect pairs of specific protein residue side chains, provided that they are within reach of the cross-linker. Considering that each cross-linker has a defined maximum length, these residue-to-residue connections (cross-links), which are identified by MS, represent distance constraints that provide insights into protein conformations (through cross-links within the same protein = intra-links), PPIs (through cross-links between different proteins = inter-links), and protein interfaces (through the location of the inter-linked residues). Importantly, XL-MS is applicable to intact wild-type cells38,39. However, the sensitivity of in-cell XL-MS is rather limited, meaning that the more abundant host-host PPIs may mask virus-host PPIs.
Here, we characterize the identity, structural determinants, and spatial context of hundreds of virus-host PPIs in intact cells that are productively infected with herpes simplex virus type 1 (HSV-1), a medically relevant human herpesvirus with a complex proteome40. To achieve this, we developed Structural Host Virus Interactome Profiling (SHVIP), which utilizes the above-mentioned benefits of proteome-wide XL-MS and overcomes its sensitivity issues by combining XL-MS with selective bio-orthogonal labeling of viral proteins. The resulting HSV-1 interactome comprises known and novel PPIs supported by thousands of cross-links. Using these data as guidance for AF modeling allows resolving interaction interfaces in structured regions and predicting SLiMs within IDRs of viral proteins. By integrating our SHVIP results with affinity purification and molecular genetics, we provide the structural and molecular determinants of dozens of virus-host PPIs.
Results
Capturing the viral interactome in intact HSV-1 infected cells
In order to harness the advantages of XL-MS for the characterization of intact infected cells, we needed to enhance its sensitivity to viral proteins. Therefore, we decided to enrich viral proteins by capitalizing on the host shutoff, which is common in many viral infections41. During host shutoff, a large fraction of newly synthesized proteins in infected cells is of viral origin, which can be captured by metabolic pulse labeling with bio-orthogonal amino acids42–45.
Our SHVIP strategy presented here combines XL-MS with bio-orthogonal labeling and involves four steps (Fig. 1a). First, cells are infected with a virus that is able to replicate efficiently in a given cell line. Second, once cells are committed to a replicative viral life cycle, L-homopropargylglycine (HPG) is added to label newly synthesized—and thus predominantly viral—proteins for several hours. Third, the membrane-permeable cross-linker DSSO is added to the intact cells to covalently link lysine side chains from proteins that are in proximity with each other. Fourth, HPG-containing proteins are extracted using copper-catalyzed click chemistry together with their covalently linked interactors. Finally, the enriched proteins are digested, enriched for cross-linked peptides via strong cation exchange chromatography, and analyzed by MS.
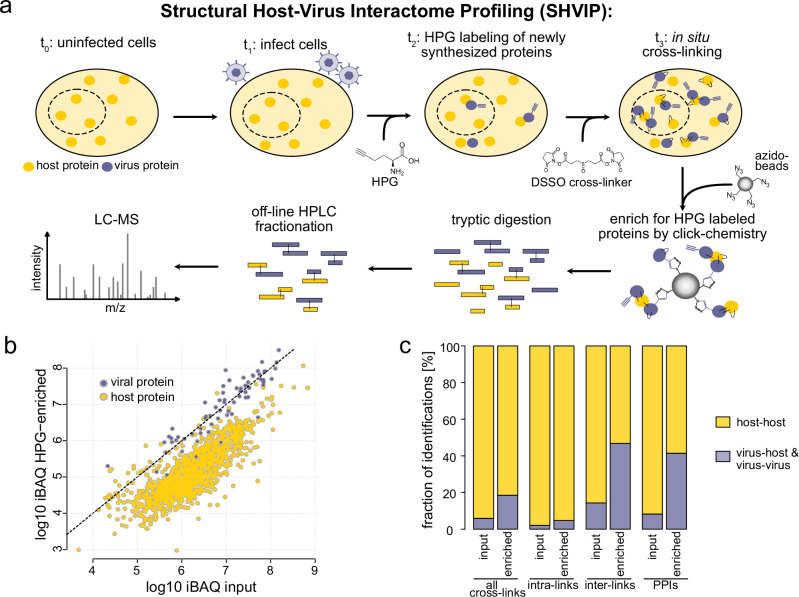
Fig. 1. SHVIP workflow and proof of concept.
a Workflow: cultured cells (t0) are infected with a virus able to induce host shutoff (t1). Once host shutoff is in place, newly synthesized proteins are labeled with L-HPG (t2), and subsequently cross-linked with DSSO (t3), and finally enriched using click chemistry. Proteins are digested into peptides, which are fractionated by off-line HPLC (in this case Strong cation exchange chromatography) and measured by LC-MS. b Abundance (measured by iBAQ) of host and viral proteins in non-cross-linked HPG-enriched and input samples. c Relative fraction of identifications involving viral proteins or only host proteins for different identification types in XL-MS data. Source data are provided.
We applied this methodology to HSV-1-infected human embryonic lung fibroblasts (HELFs). Fibroblasts support the HSV-1 lytic replication cycle, which is characterized by a temporal cascade of viral gene expression and the shutoff of host protein synthesis46. The host shutoff is achieved by a combination of transcriptional and post-transcriptional mechanisms that are installed within the first 3–6 h after infection by the concerted action of ICP4, ICP22, ICP27, and the viral endonuclease UL41 (also konwn as Vhs). After this early phase of infection, HSV-1, like all herpesviruses, has a nuclear stage of viral DNA replication and capsid assembly, and a cytoplasmic stage in which the outer tegument and membrane envelope layers of the newly forming virus particles are assembled. The peak of virus progeny production and release is typically reached around 24 h post-infection. Based on this timeline, we decided to label the cells with HPG from 7 to 24 h post-infection.
MS analysis of the linear (non-cross-linked) peptides in the enriched sample and an aliquot taken before HPG-enrichment (input) shows that HPG-enrichment reduces host protein abundance by a factor of 10 on average (Fig. 1b and Supplementary Data 1). Similarly, it increases the frequency of sequenced spectra of viral origin (Supplementary Fig. 1a), and expands the fraction of viral proteins contributing to the overall intensity from ~20% to ~75% (Supplementary Fig. 1b). The contribution of viral proteins to the total protein intensity from cross-linked samples was slightly lower (~60%) as additional cross-linked host proteins co-purified with the viral proteins. Importantly, SHVIP substantially increased the proportion of identified cross-links involving viral proteins (Fig. 1c). The highest increase was observed for cross-links between peptides originating from different protein sequences (inter-links), which is particularly valuable as inter-links give deeper insight into the viral PPI network. We evaluated two acquisition schemes, based on either MS2-MS3 (Experiment 1) or MS2-only (Experiment 2) fragmentation. Both the relative frequency and absolute numbers of PPIs involving viral proteins increased upon HPG enrichment (Supplementary Fig. 1c) in both experiments. Reproducibility between the two experiments (Supplementary Fig. 1d) was similar to previous XL-MS studies from complex samples38. Taken together, this demonstrates that SHVIP increases the sensitivity of viral interactome capture.46
Spatial resolution of the virus-host interactome map
In order to provide a high-coverage structural interactome of the infected cell, we analyzed the combined enriched and input samples from two experiments involving different MS-acquisition schemes. We first filtered inter- and intra-links separately at a 1% residue pair-level false discovery rate (FDR). We then focused on the most relevant PPIs and generated a subnetwork of interactions among viral proteins and their direct host interaction partners (Fig. 2a and Supplementary Data 2), yielding 739 interactions (of which 441 involve viral proteins) at a 1% separate PPI-level FDR estimated by target and decoy counts (see Methods). These data cover 46 viral proteins, representing ~68% of the viral proteins detected by MS-analysis of linear (non-cross-linked peptides) peptides from enriched samples (Supplementary Data 1). We observed cross-links for viral structural (e.g., tegument, glycoproteins) and non-structural proteins (e.g., replication machinery) as well as a few cross-links to the rigid nucleocapsid shell of the virus (Fig. 2b). Published focused biochemical studies confirm ~21.5 % of the detected PPIs from infected cells (Supplementary Data 2 and Supplementary Fig. 1e), suggesting that our network captures functionally relevant interactions. Taken together, this indicates that SHVIP sensitively captures a large part of the available viral proteome.
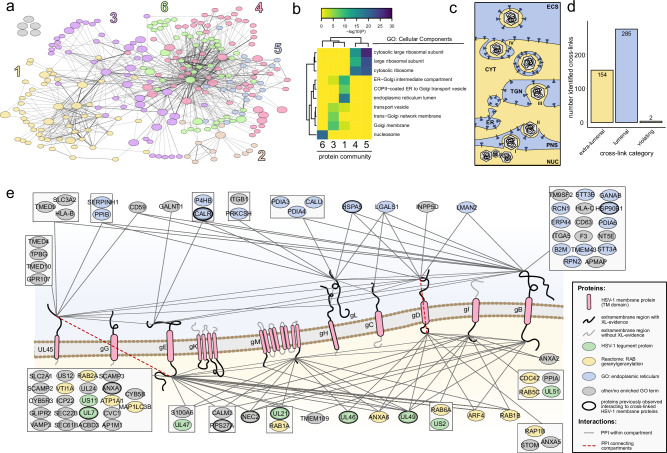
Fig. 2. The HSV-1-host protein interactome as captured by SHVIP.
a Virus-centric PPI network of HSV-1 infected cells. Host proteins (yellow) are only included when they are directly linked to viral proteins (blue). Data are filtered to 1 % separate cross-link FDR and reported at 1% inter-PPI FDR with 6194 cross-links and 254 proteins. Edge width scales with the number of identified cross-links between the two proteins. The outline color of the nodes indicates the different classes of viral proteins (glycoproteins in orange, tegument in green, capsid in red, and non-structural proteins in blue). b Inter-link (cross-link in between two different proteins) coverage for viral proteins from different classes. Source data are provided.
To assess if SHVIP captures the virus interactome across the entire host cell, we performed unsupervised clustering based on centrality indices47, dividing our network into six communities (Fig. 3a). A GO-enrichment analysis, based on cellular component annotations of host proteins, revealed distinctly enriched categories for these communities (Fig. 3b). While protein communities 4 and 5 enrich ribosomal proteins, community 6 contains many DNA binding proteins such as histones and other chromatin proteins (e.g., HMGB1, HMGB2, HMGN2). In two other communities (1 and 3), host proteins of the endomembrane system are enriched, such as proteins localizing to the endoplasmic reticulum (ER) lumen (e.g., B2M, CALR, P4HB, PDIA6, ERP44), ER-Golgi intermediate compartment or the Golgi apparatus (e.g., GALNT1, RAB6A, SCAMP2, SCAMP3). These data confirm that SHVIP can resolve virus-host PPIs across various cellular compartments.
Fig. 3. The structural interactome of HSV-1 at the host endomembrane system.
a Unsupervised community clustering47 of the structural interactome. Proteins colored according to the community numbers. The layout of the network is identical to the network layout in Fig. 2a. b GO-enrichment based on cellular component annotations of host proteins within five of the communities. Community 2 had too few host proteins to enrich any GO term. c Extra-lumenal (yellow, e.g., cytosol) and lumenal (blue, e.g., ER lumen) compartments in late stage HSV-1 infection (NUC nucleus, PNS perinuclear space, ER endoplasmatic reticulum, TGN trans-Golgi network, CYT cytosol, ECS extracellular space). d Number of cross-links connecting lumenal or extra-lumenal domains of viral transmembrane proteins and cross-linking partners with annotated domains. e Viral transmembrane proteins (red) shown in a membrane with their cytosolic tails pointing down and lumenal domains pointing up. Interaction partners with their domain-specific cross-links to either lumenal or extra-lumenal domains are grouped when they share cross-links to the same transmembrane protein domains. Color coding of host interaction partners according to GO annotations in legend. Host proteins previously observed to interact with any of the cross-linked HSV-1 membrane proteins (see also Supplementary Data 2) are highlighted by bold outlines. Host transmembrane proteins with several domain-specific links to viral transmembrane proteins are provided in Supplementary Fig. 2a. Cross-links that are in violation with the membrane topology are highlighted by red dashed edges. Source data are provided.
We were particularly interested in the PPIs at the endomembrane system. During the late stage of herpesvirus infection, cellular membranes become reorganized to allow assembly and release of mature virions22. This is regulated by a complex network of PPIs, where membranes separate the involved proteins into a lumenal (of ER, trans-Golgi, or vesicles) or extra-lumenal (cytosol, nucleoplasm, or intra-virion) population (Fig. 3c). While DSSO is able to pass the membrane, it is not able to cross-link proteins that are separated by membranes. Therefore, cross-links in this region allow evaluating whether SHVIP compromises the integrity of the cellular membranes. When evaluating cross-linking partners of viral envelope proteins at domain-level resolution (Fig. 3d), we found that almost all cross-links (99.6%) occurred between domains within either the lumenal or the extralumenal compartments, confirming that SHVIP captures interactions at a largely intact membrane system.
We mapped the domain-specific interaction partners onto HSV-1 transmembrane proteins, which validated 23 known PPIs (Fig. 3e, Supplementary Fig. 2a, and Supplementary Data 2). Specifically, we found viral tegument proteins localizing to the extra-lumenal side of the membrane and interacting with cytosolic tails of viral glycoproteins, consistent with their ascribed role in mediating secondary envelopment48–50. In addition, we observed many Rab GTPases, crucial regulators of endocytic and exocytic membrane trafficking, and related proteins at the cytosolic side. This includes not only RAB551, RAB1A/B52, and RAB653 proteins with known proviral functions, but also RAB2A, which is yet uncharacterized in HSV-1 infection (Supplementary Fig. 2b).
At the lumenal side, we observed many well-known ER-resident proteins, such as chaperones (PDIA4, PDIA6, HSP90B1) and glycosylation factors (GALNT1, GANAB), interacting with the extra-virion domains of viral glycoproteins, consistent with ER-luminal processing of viral glycoproteins. Interestingly, the viral membrane proteins gB and UL45 physically associated with cellular proteins involved in antigen presentation, such as HLA-B, HLA-C, and B2M, potentially perturbing the cellular antigen presentation pathway54. In addition, glycoproteins gH/gL and gB, core components of the viral fusion machinery, were found cross-linked to alpha and beta subunits of integrins (ITGA5, ITGB1), which are known entry receptors for HSV-155 (Supplementary Fig. 2c). We also observed cross-links between gH/gL and gB as well as the fusion-regulatory protein UL4556, supporting the idea of physical cross-talk among proteins of the HSV-1 fusion machinery57.
Similar to these viral glycoproteins, we also found other viral proteins that cause substantial membrane remodeling, such as the nuclear egress complex (NEC), composed of NEC1 and NEC2. NEC mediates the envelopment of nucleocapsids at the inner nuclear membrane, followed by their delivery to the outer nuclear membrane by membrane fusion58. The in situ PPIs of this complex (Supplementary Fig. 2d) reveal both known and novel insight into this process. First, we observed known functionally relevant interaction partners such as ICP2259 and EMD (also known as emerin)60. Second, we found the extra-lumenal domains of several viral glycoproteins associated with NEC2, previously discussed as functionally important for fusion at the outer nuclear membrane22. Third, we discovered the association of five ER-membrane proteins to the NEC (IKBIP, SEC22B, SSR3, SEC61B, and LRRC59), suggesting a yet unknown role of ER proteins during nuclear egress. Overall, our data provides detailed, domain-level insights into virus-host interactomes at the endomembrane system, relevant for glycoprotein processing, membrane trafficking, adaptive immunity, egress, and assembly.
Identifying PPIs that depend on the intact cellular environment
Having established that SHVIP provides extensive and biologically relevant data, we aimed to identify which of those PPIs critically depend on the intact cellular environment. To this end, we compared our approach to AP-MS, which is the most widely employed method for virus-host PPI profiling23–33 and requires cell lysis. We selected eight viral proteins from our network with different numbers of cross-linked interactors each. These included non-structural proteins (UL12, DBP, NEC2, and US11) as well as tegument proteins (UL47, UL48, UL49, and DUT) (see Methods). We tagged the proteins individually with an HA tag at their endogenous loci and performed anti-HA affinity purifications27, enriching the bait with its interaction partners from infected cells. As controls, we used cells infected with the parental wildtype (WT) virus, not expressing any HA-tagged protein variants (Fig. 4a, Supplementary Fig. 3a–h, and Supplementary Data 3). On average, cross-linking partners of the bait show significantly higher enrichment in the corresponding anti-HA-bait precipitate than proteins with indirect or no cross-link connection to the bait (Fig. 4b, c). At the level of individual baits, we observed significant differences in 6 out of 8 cases (Supplementary Fig. 3i–p). In total, at a stringent AP-MS log2 fold-change cut-off of 4, 42 SHVIP interactors could be validated by AP-MS (Fig. 4d). At a less stringent cut-off at log2 of 2, the number of overlapping interactors increased to 61 and remained significant (Fig. 4e). By utilizing a sliding cut-off, we observed a progressive decrease in the significance of the overlap when interactions below this threshold were considered (Fig. 4f). Thus, SHVIP reported PPIs can be validated by AP-MS and both techniques retrieve a common set of PPIs.
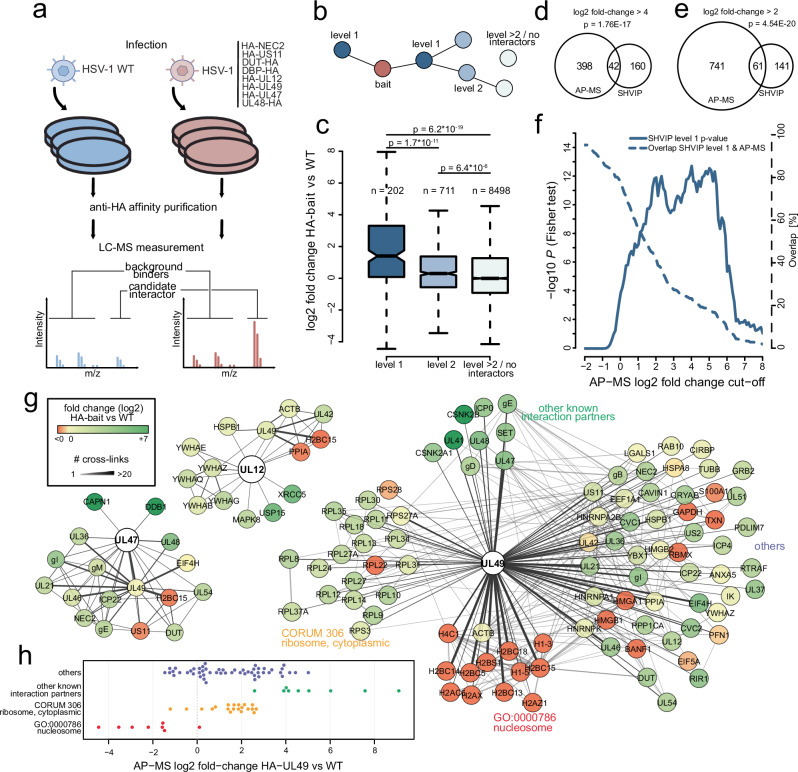
Fig. 4. SHVIP complements and extends AP-MS data.
a Workflow for AP-MS experiments. Transgenic HSV-1 viruses individually expressing the indicated HA-tagged protein variants were used to infect HELFs (n = 3 biological replicates), followed by anti-HA enrichment and LC-MS measurement. Purifications were compared to control experiments with HSV-1 wildtype (WT) not expressing any HA-tagged bait. The intensity ratio comparing HA-bait to control enrichments was computed for individual co-enriched proteins (Volcano plots for all baits are included in Supplementary Fig. 3). b For comparing to AP-MS data, proteins in the SHVIP interactome (see also Fig. 2a) were categorized into proteins cross-linked to the bait (level 1), proteins cross-linked to the bait via one other protein (level 2), or higher-order interactors and proteins not contained in the structural interactome (>level 2/no interactors). c Log2 fold-changes in the respective AP-MS experiment comparing the protein categories from (b). Boxes represent lower and upper quartiles with median marked as horizontal line. Whiskers represent 1.5 times interquartile range. Outliers (points outside the whiskers) removed for visibility. P values are based on a two-sided Wilcoxon rank-sum test. Comparison of the interactome of the 8 selected viral proteins in SHVIP and AP-MS at a higher stringency (d) and lower stringency (e) log2 fold-change cut-off applied to AP-MS data. An additional t test P value cut-off (AP-MS P values, two-sided t tests without multiple hypothesis correction, n = 3 biological replicates) of 0.01 was applied to potential AP-MS interactors. The displayed P values indicate the statistical significance of the overlap between proteins identified via AP-MS and SHVIP and are based on one-sided Fisher’s Exact test. f Comparing the overlap between SHVIP and AP-MS and its statistical significance using a sliding AP-MS fold-change cut-off. P values are based on one-sided Fisher’s Exact test. g Representative depiction of SHVIP interactomes for selected proteins. Color coding of interacting nodes according to the co-enrichment ratios with the bait in AP-MS. h AP-MS co-enrichment levels of UL49 interactors found by SHVIP. Interactors are grouped into different biological categories. Source data are provided.
Looking at individual protein examples shows that SHVIP and AP-MS capture overlapping as well as complementary parts of the interactome. For the viral proteins UL12 and UL47, almost all cross-linking partners were also found enriched in anti-HA precipitates (Fig. 4g). In the case of UL49, an abundant tegument protein with a wide variety of functions and interaction partners61, we observed a more mixed picture when comparing AP-MS and SHVIP:
Several known UL49 interaction partners, such as casein kinase 262, UL41, UL48 (also known as VP16)63, and SET (also known as TAF-I)64, were detected by XL-MS and were highly enriched in AP-MS (Fig. 4g, h). However, other biologically relevant UL49 interactors detected by XL-MS, e.g., ribosomal proteins (consistent with UL49’s known polysome association65), displayed only intermediate AP-MS co-enrichment levels. Moreover, nucleosome-associated proteins were observed as the most frequent cross-linking partners of UL49, corroborating microscopy data66–68 and in vitro experiments67,68. Surprisingly, they were not enriched in AP-MS, suggesting that the nucleosome-UL49 interaction is disrupted by cell lysis or the enrichment procedure. An explanation could be that these interactions are linked to UL49 undergoing LLPS with DNA20, a concentration-dependent process that is disturbed by cell lysis. These results suggest that, while many PPIs are identified by both SHVIP and AP-MS, those depending on the in situ environment of the infected cell are uniquely captured by SHVIP.
IDRs are enriched for virus-host contact sites
In addition to identifying interacting proteins in the infected cell, we aimed to provide structural insights into the cross-linked protein assemblies. This is possible as cross-linkers bridge a defined distance range, e.g., up to 35 Å for the DSSO cross-linker applied here. As such, our data can support the structural characterization of heterodimers from HSV-1-infected cells.
Only five inter-links (3 virus-host or virus-virus PPIs) could be mapped onto experimental structures from the Protein Data Bank (PDB) (Fig. 5a), which is largely due to the scarce structural information on multimeric HSV-1 complexes. Outside of receptor or antibody binding, only ~11 complexes have been solved, many of which only contain parts of the proteins. In order to extend insights into yet unsolved heterodimeric structures, we predicted all binary interactions in our dataset by AF-multimer69 (Fig. 5b) and categorized model quality based on the confidence score derived from predicted template modeling score (pTM) and interface pTM (ipTM)70. In general, dimers between host proteins yielded predictions with better scores than those between viral proteins only or between viral and host proteins (Fig. 5c). This is consistent with the comparably low success rates of docking predictions for viral proteins70, lower depth in the multiple sequence alignment (MSA) for viral proteins and lower expected co-evolutionary signals captured in MSA between two organisms (host and virus). In addition, when comparing AF-Multimer models to cross-links, we noticed that inter-links involving viral proteins occurred more frequently in poorly predicted regions (pLDDT <50), likely IDRs, than those between host proteins (Fig. 5d). Thus, physical contacts within ordered and disordered regions shape the structural interactome of HSV-1, with the majority of them located in IDRs.
Fig. 5. Structural insight into virus-host PPIs through SHVIP and AF-Multimer.
a Cross-links mapped onto heterodimers with experimentally solved structures. Proteins shown as bars109 or high-resolution experimental structures (gH-gL, pdb 3m1c, US12-TAP1/2, pdb 5u1d, and UL30-UL42, pdb 9enp). Note that gH-gL is an experimental structure obtained from HSV-2. Cross-links highlighted gray could not be mapped to the experimental structures because of missing coordinates. One intra-link in UL30 and UL42 each violate the distance constrain of 35 Å and is therefore colored in light red. All other plotted cross-links meet the DSSO distance constraint of 35 Å. Predicting virus-virus, virus-host, and host-host dimers of the structural interactome using AF-Multimer (b) with their respective confidence score distributions (c). d Per-residue prediction confidence (AF-Multimer pLDDT score) at the specific inter-linked lysine residue for different types of inter-links. The lower pLDDT on either lysine residue of the cross-link was used for categorization. e Distribution of inter-link distances (Cα-Cα distance of cross-linked lysines) for AF-Multimer models in different confidence score ranges. Shown are only inter-links involving lysines from protein regions with pLDDT scores > 50. Boxes represent lower and upper quartiles with median marked as horizontal line. Whiskers represent 1.5 times interquartile range. f Examples of well-predicted models agreeing with the cross-linker distance constraint. No violated cross-links were observed. See also Supplementary Fig. 4 for prediction of heterodimers using AF3. Source data are provided.
System-wide structural insights across HSV-1-host interactions
We initially focused on structural models with inter-links in well-predicted regions (pLDDT > 50). In these cases, cross-links provide orthogonal experimental evidence to validate AF-Multimer predictions. Indeed, the average distance bridged by the cross-linker decreased with increasing model confidence score (Fig. 5e), indicating that the better the model quality, the better the cross-link distances fit into the predicted structures. For models with a confidence score above 0.75 the majority of inter-links were satisfied.
In sum, we obtained 30 models (4 involving viral proteins, 26 host-host) that have good confidence scores (> 0.75) and agree with at least half of the inter-links (Supplementary Data 4 and Fig. 5e). Among those, SHVIP data supported the structural prediction for US12 with PPIA and UL42, the viral polymerase processivity subunit, binding to its cellular homolog PCNA (Fig. 5f)4. This interaction may explain why PCNA is enriched at viral DNA replication forks71.
Furthermore, a substantial number of predicted dimers had high cross-link satisfaction rates despite lower confidence scores. We obtained 31 additional models (8 involving viral proteins, 23 host-host) with confidence scores between 0.5 and 0.75, where the majority of inter-links from structured regions were satisfied (Supplementary Data 4). Among these models, we found known HSV-1 PPIs like UL30 with UL42 (confidence score: 0.66), and the ubiquitin-binding protein US272 with ubiquitin (RPS27A) (confidence score: 0.66). This demonstrates that cross-links can help prioritize realistic models when AlphaFold quality measures are not sufficient.
To test whether SHVIP is compatible with newer structure modeling tools, we additionally tested its performance using AF3, with overall similar performance to AF-Multimer (Supplementary Fig. 4a–c). In general, higher-scoring predictions aligned more closely with the distance constraints than lower-scoring ones (Supplementary Fig. 4d). Applying the same model selection criteria across both confidence categories (see above), we identified two additional models that matched at least 50% of the detected inter-links from structured regions. These include STT3A, the catalytic subunit of the ER-resident oligosaccharyltransferase complex, known to be crucial for HSV-1 glycosylation73, with glycoprotein B, and the viral membrane protein UL45 in complex with PPIB (Supplementary Fig. 4e). These findings demonstrate that SHVIP is also compatible with AF3, which may outperform AF-Multimer for certain targets.
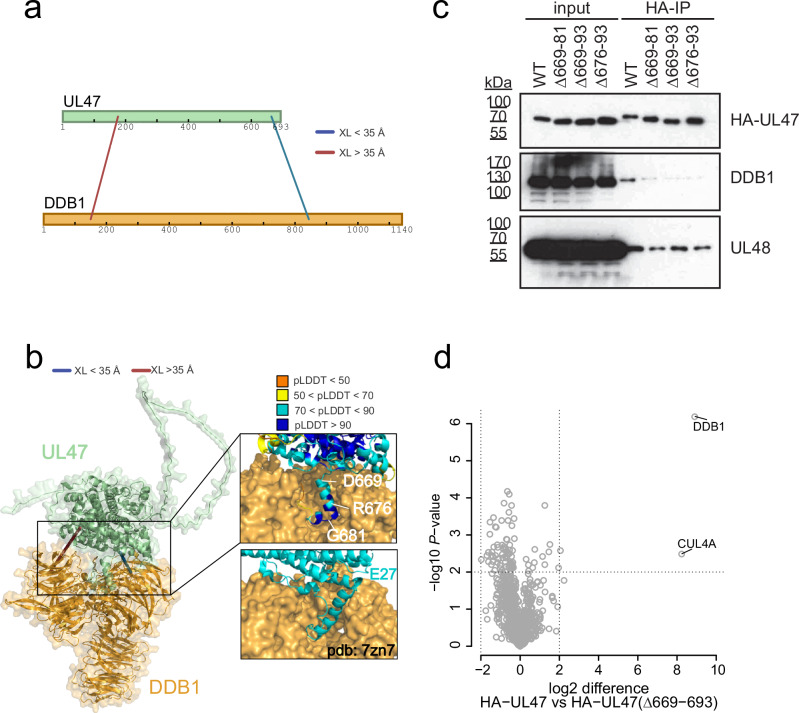
We more specifically investigated the dimer between the tegument protein UL47 and DDB1 (Fig. 6a), which was one of the best predicted models with a confidence score of 0.82 in AF-Multimer and 0.77 in AF3. DDB1 functions as an integral component of Cullin 4-RING ubiquitin ligase (CRL4) complexes74. Two UL47-DDB1 cross-links were identified, with one of them satisfying the distance constraint in the corresponding AF-Multimer model. In the model, the UL47 C-terminal region in proximity to the satisfied inter-link adopts an alpha-helical fold, in positional congruence with previously described viral DDB1 interactors, such as RCMV-E27 (Fig. 6b)75. To confirm the UL47-DDB1 interaction and assess the contribution of the UL47 C-terminal helix, we performed co-immunoprecipitation (IP) experiments. While DDB1 efficiently co-precipitated with UL47-WT, deleting the C-terminal 18 amino acids (676–693) of UL47 was sufficient to disturb this interaction (Fig. 6c). In contrast, the UL47 XL-partner UL48 could still be immunoprecipitated. Further AP-MS experiments confirmed that the UL47 C-terminal truncation leads to a strong and specific loss of DDB1-CUL4A binding (Fig. 6d), indicating that the C-terminal helix acts as a critical DDB1 interaction module.
Fig. 6. Structural determinants of the DDB1-UL47 heterodimer.
a UL47-DDB1 cross-links mapped onto sequence bars109. b DDB1-UL47 cross-links mapped onto the predicted dimer with color coding of the cross-links according to distances between Cα atoms. The inset shows the AF-Multimer prediction confidence for the C-terminal helix of UL47. Displayed below the inset is the experimentally resolved structure of E27-DDB1 (pdb 7zn7) for comparison. c HA-directed Co-IPs against different mutant and wildtype variants of UL47 from infected cells at 24 hpi (representative result of n = 2 experiments). d AP-MS experiments directly comparing the interactome of wildtype UL47 to mutant UL47 in a label-free set-up. P values are based on two-sided t tests without multiple hypothesis correction based on n = 3 biological replicates. Source data are provided.
Thus, we provide a compendium of virus-host/ virus-virus dimers, which can be validated by cross-linking distance constraints, reverse genetics, and orthogonal interaction assays.
Identifying sequence determinants for virus-host PPIs in IDRs
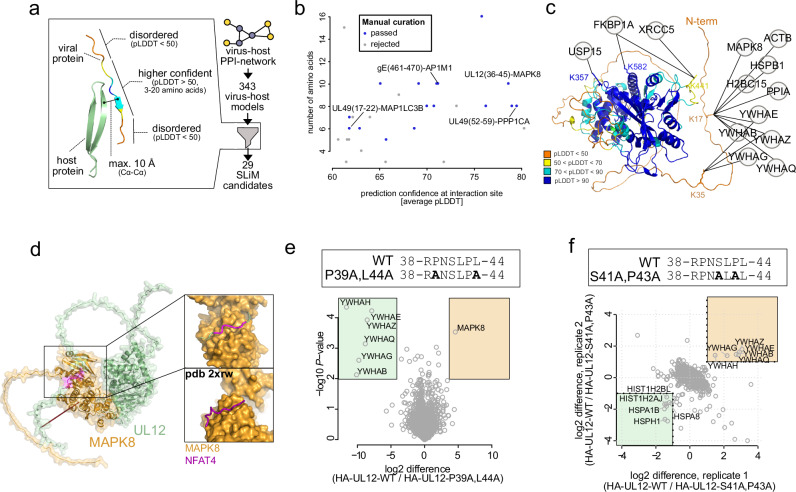
Most of our inter-links occurred in poorly predicted regions, likely IDRs. These regions are hotspots for SLiMs that viruses use to interact with host proteins6. AF-Multimer was previously used to identify SLiMs between proteins in a fragment-based approach outside of infection contexts76. To make the discovery of such interactions sites amenable for host pathogen, full protein and cross-linking scenarios, we screened all AF-Multimer models of our cross-linked virus-host PPIs for short stretches of ordered amino acids (pLDDT > 50) that are in contact with the respective binding partner and in a disordered regional context (Fig. 7a). We obtained a list of 29 candidates (Supplementary Data 5) with varying average confidence scores and amino acid length (Fig. 7b). We manually curated this list to yield 16 sites, representing a promising set of putative SLiMs mediating virus-host interactions.
Fig. 7. Identifying sequence determinants for host-virus interactions in IDRs.
a Workflow for selecting putative short linear interaction sites in IDRs from AF-Multimer models. b Comparison of the residue length for the putative interaction motif to the confidence of the predicted interface (average pLDDT of all amino acids within the selected site). c Host proteins cross-linked to UL12, depicted on the monomeric AF2 model of UL12, color coded by pLDDT. d Predicted MAPK8-UL12 dimer with a putative D-motif highlighted magenta. The inset shows the magnified region of the D-motif in UL12 interacting with the D-site in MAPK8. Shown below the inset is a solved crystal structure of MAPK8 with the D-motif containing peptide of NFAT4 (pdb 2xrw). e Mutational disruption of the D-motif in UL12 in the viral genome of HSV-1, creating P39A, L44A-UL12. Label-free comparative AP-MS of the wildtype UL12 and P39A, L44A-U12 interactomes. The p-values are based on two-sided t tests without multiple hypothesis correction of n = 3 replicates. Boxed regions highlight proteins that interact significantly stronger with P39A, L44A-UL12 (shaded green) or WT-UL12 (shaded orange), based on a cut-off at P = 0.01 and log2 fold-change of 4. f Mutational disruption of the 14-3-3 binding site in UL12 in the viral genome of HSV-1, creating S41A, P43A-UL12. SILAC-based comparative AP-MS of the wildtype UL12 to the S41A, P43A mutated UL12 interactome, with both (n = 2, label-swap) replicates depicted. Boxed regions highlight proteins that interact stronger with S41A, P43A-UL12 (shaded green) or WT-UL12 (shaded orange), based on a log2 fold-change cut-off of one in both replicates. Source data are provided.
One of the best predicted sites was observed in a short stretch of amino acids in the N-terminal disordered domain of the alkaline nuclease UL12 (amino acids 35–50) binding to MAPK8 kinase (also known as JNK1). Curiously, we observed that 14-3-3 proteins were also cross-linked to N-terminal lysines within UL12 (K35, K17) (Fig. 7c). In the MAPK8-UL12 dimer model, the disordered N-terminal domain of UL12 wraps around the MAPK8 kinase domain and binding occurs between a putative D-motif in the UL12 unstructured region and the MAPK8 docking groove (Fig. 7d)77. We therefore mutated the candidate D-motif (P39A, L44A, Fig. 7e). Comparing mutant to wildtype UL12 interactomes indicates that the mutations result in loss of MAPK8 binding but stronger association with other UL12 interactors (14-3-3 proteins).
We hypothesized that both MAPK8 and 14-3-3 may bind to the same short sequence stretch within UL12. Using a sequence-based prediction tool for 14-3-3 binding motifs78 we identified Serine 41 as best hit for 14-3-3 interaction (Supplementary Fig. 5a). Following this, we mutated critical amino acids in the viral genome (S41A, P43A) to disrupt 14-3-3 binding. We then compared wildtype to mutant in an AP-MS experiment using SILAC (stable isotope labeling of amino acids in cell culture) labeling (Supplementary Fig. 5b and Fig. 7f). Wildtype UL12 co-precipitated with 14-3-3 proteins stronger than S41A, P43A-UL12. In contrast, the mutant interactome showed enrichment of several heat-shock proteins and histones, suggesting effects on UL12 folding or stability upon loss of 14-3-3 binding. Taken together, our analysis identifies two SLiMs that co-exist on a narrow stretch of amino acid within the UL12 N-terminal disordered domain and enable UL12 to recruit MAPK8 and 14-3-3 proteins.
In addition, our list of putative SLiMs includes other compelling predictions. For example, we found the interaction of gE with the adaptor protein AP1M1, with a putative tyrosine-trafficking motif in gE (Supplementary Fig. 6a). Also, we predicted the central player in autophagy MAP1LC3B (also known as LC3-B) interacting with UL49, which has a putative LC3 interaction region (LIR)79 (Supplementary Fig. 6b). Further, we observed the interaction between UL49 and the cellular Phosphatase PP-1 (also known as PPP1CA), for which the predicted model indicated binding of an amino acid stretch in the unstructured region of UL49 to the RVXF-motif recruitment site in PP-1 (Supplementary Fig. 6c). As the corresponding amino acid sequence in UL49 suggests the presence of a non-canonical RVXF motif (Supplementary Fig. 6d), we aimed for independent validation of this binding site. Indeed, we found that mutating the motif in UL49 (R52A, F57A) reduces PP-1-UL49 binding in HA-directed AP-MS assays (Supplementary Fig. 6e, f). These findings illustrate that combining SHVIP and AF-Multimer-based structure predictions allows the discovery of critical sequence determinants of virus-host PPIs within IDRs.
Discussion
While interaction proteomics has contributed immensely to uncover the molecular processes underlying viral pathogenesis23–33,80–83, a global view of interaction contact sites within infected cells has remained elusive. The SHVIP approach introduced here addresses this shortcoming. Using SHVIP, we define the structural interactome of a complex herpesvirus in intact infected cells, revealing distinct clusters of spatially separated virus-host PPIs. Orthogonal validations, structure predictions, and molecular genetics provide insights into the structural basis of a subset of these interactions.
XL-MS integrates well with AF to inform structural predictions at interactome scale84–86 delivering residue-residue connections as corroborative evidence to purely in silico analyses. Enhancing interactome-wide AF modeling with SHVIP is particularly valuable from a virological point of view because (1) AF-only predictions of dimers involving viral proteins have generally lower quality than those of host proteins70 (Figs. 5 and 2). Experimental structures of host-virus protein complexes are scarce for HSV-1 and many other viruses. Augmenting AF with XL-MS data proved to be effective to cross-validate generally lower-scoring host-virus protein complex predictions and thus identify convincing structures, which are, in this case, potentially linked to DNA replication, immune evasion, and signaling. For instance, we found a C-terminal helix in the tegument protein UL47 that interacts with the CRL4 adapter unit DDB1 (Fig. 6) in a manner that is typical for DCAF-type substrate receptors of CRL4. It will be interesting to see whether UL47 acts in analogy to other viral DCAF mimics75 and exploits CRL4 to target antiviral host proteins for proteasomal degradation. To arrive at even more confident predictions, it will be promising to improve structure modeling, as e.g., through approaches that incorporate cross-linking distance constraints87 or massive sampling strategies88,89 in the future.
SHVIP can capture weak and context-sensitive PPIs because it capitalizes on protein cross-linking within intact cells. Such interactions may be disrupted during cell lyses and therefore elusive to AP-MS. While SHVIP allows characterizing viral proteins that are challenging to study outside their cellular context (such as proteins undergoing LLPS90 or transmembrane proteins91), the sensitivity of XL-MS workflows depends a lot on the abundance and structural complexity of the specific protein. Although our approach could enrich viral proteins, it does not eliminate identification bias originated from protein abundances. When interpreting XL-MS data, it is therefore critical to keep in mind that interactions for abundant proteins are more likely to be covered compared to low-abundant proteins. This abundance bias is reduced in AP-MS, which is because detecting linear peptides and focusing on interactors of one individual protein may increase sensitivity. Beyond sensitivity considerations, AP-MS is primarily based on protein affinity, whereas XL-MS is driven by protein proximity. These methodological differences likely explain why both techniques provide overlapping information while also offering a substantial degree of complementarity (Fig. 4). XL-MS captures a snapshot of the in situ occurring interactome that preserves the context of higher-tier interactors. It is therefore better able to capture PPIs only existing in specific subcellular contexts, such as LLPS-based condensates. For instance, we identified UL49 as the most connected viral protein in the XL-MS-based interaction network, which is in agreement with previous findings that UL49 interacts with a variety of viral and cellular proteins61,63,64,92, such as histones66–68. It is possible that UL49 achieves the organization of many PPIs through its ability to undergo LLPS20.
We observed that cross-links involving viral proteins are enriched in IDRs (Fig. 5d), recognized hotspots for SLiMs frequently exploited by viruses to manipulate host processes6. Based on XL-supported heterodimers and their corresponding AF-Multimer models, we propose 16 potential SLiMs within IDRs (Fig. 6b). We validated the non-canonical RVXF-motif in the UL49 protein as a SLiM for binding PP-1 phosphatase. In analogy to HCMV-UL3293, UL49-PP-1 interaction may be important to regulate the tegument phosphorylation state94, facilitating virion assembly. Furthermore, we identified two co-existing SLiMs within the disordered segment of alkaline nuclease UL12 responsible for competitive binding of MAPK8 and 14-3-3 proteins. These interactions might constitute a regulatory switch, allowing HSV-1 to link the viral replication machinery to MAPK8, a potent effector of cellular stress signaling that is crucial for reactivating HSV-1 from latency95. These examples show that canonical and non-canonical interaction motifs are discoverable by integrating SHVIP and AF-Multimer. The distinctive advantage of this approach lies in its incorporation of structural context and co-evolutionary relationships, enabling a significant improvement over sequence-based methods for predicting SLiMs7.
The methodology is, in principle, applicable to study native cells infected with any virus inducing host shutoff41. This includes medically or veterinary relevant viruses such as influenza A viruses42,96, poxviruses97, picornaviruses98, flaviviruses99, or asfarviruses45. Also, it does not require upfront genetic work, which makes it readily applicable to emerging viruses and viruses that do not tolerate epitope tagging in their genome. However, it is important to consider that HPG-labeling requires methionine starvation, impacting cell viability100 and viral titers101, particularly when employed for prolonged periods. This may, in the future, be mitigated by adopting starvation-free protocols100 for SHVIP.
In summary, SHVIP offers various conceptual advantages for virus-host PPI profiling. Our data on a complex and medically relevant herpesvirus deliver a blueprint for the structural characterization of PPIs within their intact environment during viral infections. The method unlocks an opportunity for the in-depth characterization of viral gene functions during cellular infections.
Methods
Cells and viruses
Human embryonic lung fibroblasts (HELFs) were maintained according to established protocols102, using Eagle’s minimum essential medium (EMEM) supplemented with Earle’s balanced salt solution, 25 mM HEPES, 1 mM sodium pyruvate, 2 mM L-alanyl-L-glutamine, nonessential amino acids, 0.75 ‰ (w/v) sodium bicarbonate, 50 µg/ml gentamicin and 10 % fetal bovine serum (FBS) and used for preparation of viral stocks. Recombinant HSV-1 strain 17103 was used for all experiments. Infectious virus titerss were determined by immunotitration and indicated as immediate early (IE)-forming units (IU) per mL. In brief, HELFs were infected with serial dilutions of cell-free virus stocks. At 8 hpi, the number of infected cells was determined by flow cytometry of IE antigen ICP4 expression, using a 1:200 dilution of the mouse anti-ICP4 antibody clone H943 from Santa Cruz Biotechnology (sc-69809) and an appropriate fluorescently labeled anti-mouse IgG antibody. Viral mutants were created by traceless bacterial artificial chromosome mutagenesis according to established protocols104. Mutations were verified by Sanger sequencing and PCR. See Supplementary Data 6 for a list of mutagenesis primers. For all infection experiments, a multiplicity of infection of 5 IU/cell was used.
Stable isotope labeling in cell culture
HELFs were cultured for at least five passages in SILAC-DMEM medium supplemented with 10 % (v/v) dialyzed fetal bovine serum (FBS, Pan-Biotech), 1 × Glutamax (Gibco), 1 × non-essential amino acids (Gibco), 100 Units/mL Penicillin (Gibco), and 100 µg/mL Streptomycin (Gibco). For heavy labeling medium was supplemented with 0.8 mmol/L L-[13C6,15N2]-Lysine (Lys8) and 0.4 mmol/L L-[13C6,15N4]-Arginine (Arg10). For light labeling medium was supplemented with natural Lysine and Arginine to the same concentrations, respectively.
Co-immunoprecipitation
Cells were harvested at 24 h post infection and extracted by freezing-thawing in immunoprecipitation buffer, containing 50 mM Tris–Cl pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM NaF, 10 mM β-glycerophosphate, 0.5 mM Na3VO4, 10 mM N-ethylmaleimide, 0.5% Nonidet P-40 (NP-40), 10% glycerol, 1 mM dithiothreitol (DTT), 0.3 μM aprotinin, 23 μM leupeptin, 1 μM pepstatin, 0.1 mM Pefabloc. HA-UL47-containing protein complexes were immunoprecipitated by incubating extracts with HA-specific antibodies and protein G-coupled Sepharose beads (4 Fast Flow, GE Healthcare). The sepharose-bound proteins were analyzed by standard immunoblotting for the presence of HA-UL47, DDB1, and UL48. The following antibodies were used: anti-DDB1 (rabbit polyclonal, Bethyl Laboratories, A300-462A), anti-HA (rat clone 3F10, Roche, ROAHAHA), anti-UL48 (mouse clone 1–21, Santa Cruz Biotechnology, sc-7545).
Affinity-purification mass spectrometry
Eight viral baits were selected from our network for subsequent orthogonal validation by AP-MS. We excluded viral glycoproteins, capsid or capsid-associated proteins, and proteins with fewer than two PPIs. From the remaining shortlist, we selected three viral proteins with few PPIs (<10 PPIs: UL48, DBP, DUT), three with moderate number of PPIs (>10 and <50 PPIs: UL12, NEC2, UL47), and two highly inter-linked proteins (UL49, US11). AP-MS was carried out according to established protocols27. Label-free experiments were performed in triplicates, and SILAC-based experiments were performed in label-swap duplicates. In all cases, cells were harvested 24 h after infection with wild-type or mutant virus. One fully confluent 15 cm dish of HELFs per replicate and condition was used. Following washing with PBS, cells were lysed by a 30 min incubation in lysis buffer: 25 mM Tris-HCl (pH 7.4), 125 mM NaCl, 1 mM MgCl2, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 5% glycerol, 1 mM DTT, 0.3 μM aprotinin, 23 μM leupeptin, 1 µM pepstatin, 0.1 mM Pefabloc. Lysates were cleared by centrifugation, and the cleared lysates were incubated with anti-HA magnetic microbeads (Miltenyi) for 60 min before being applied to µMACS microcolumns (Miltenyi). We used lysis buffer for the first washing step, lysis buffer without detergent for the second, and 25 mM Tris-HCl (pH 7.4) for the final washing step. Protein material was eluted in a total volume of 0.2 mL 8 M guanidine hydrochloride at 95 °C and precipitated from the eluates by adding 1.8 mL LiChrosolv ethanol and 1 µL GlycoBlue. After incubation at 4 °C overnight, samples were centrifuged for 1 h at 4 °C, and ethanol was decanted before samples were subjected to sample preparation.
SHVIP sample preparation
Cells were infected at a multiplicity of infection of 5 IU/cell, using standard protocols. At 7 h post infection, cells were washed twice with pre-warmed PBS before the L-HPG-labeling medium was added, consisting of 500 µM HPG (Click Chemistry Tools), methionine-free DMEM (Gibco ref.), and 10% dialyzed fetal bovine serum (Pan Biotech). Cells were washed in PBS and harvested by scraping at 24 hpi before being cross-linked with 5 mM DSSO in a 1:1 (vol/vol) PBS/cell suspension for 1 h at room temperature under constant shaking. Afterwards, cells were lysed in a buffer containing 200 mM Tris (pH 8.0), 4% 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate, 1 M NaCl, 8 M urea, and protease inhibitors. Cell lysates were incubated for 30 min at 4 °C. Genomic DNA in the samples was digested by addition of Benzonase. Afterwards, cell lysates were sonicated in a Bioruptor Pico (Diagenode) with 10 cycles of 30 s sonication pulses, followed by 30 s without sonication. Samples were centrifuged at 10,000 × g for 10 min, and the resulting pellet was discarded. Approximately 5% of the sample volume was saved as input, while the remainder was subjected to enrichment of labeled proteins.
HPG-labeled proteins were enriched using copper(I)-catalyzed azide-alkyne cycloaddition, also known as click reaction, using the Click-&-Go protein enrichment kit (Click Chemistry Tools) according to the manufacturer’s protocol. In brief, samples were incubated overnight rotating together with picolyl-azide conjugated agarose beads and a copper(I)-ion containing catalyst solution. Protein-conjugated agarose beads were centrifuged at 1000 × g for 1 min, and the supernatants containing unbound, unlabeled cellular proteins were discarded. Afterwards, disulfide protein bridges were reduced by adding 10 mM DTT and alkylated by adding 40 mM chloroacetamide (CAA). Subsequently, agarose beads were transferred to 0.8 mL Pierce centrifuge columns (Thermo Scientific). Beads were subjected to a five-step washing protocol employing 10 times 500 µl of the following washing buffers: (i) 1 % w/v SDS, 250 mM NaCl, 5 mM EDTA in 100 mM Tris (pH 8.0); (ii) 8 M urea in 100 mM Tris (pH 8.0); (iii) 80 % v/v acetonitrile (ACN) in water; (iv) 5 % v/v ACN in 50 mM triethylammonium bicarbonate (TEAB); (v) 2 M urea plus 5 % v/v ACN in 50 mM TEAB. After the last washing step, beads were resuspended in the final washing buffer. To release the captured proteins from the beads, proteins were digested by addition of trypsin and lysyl endopeptidase C (Lys-C). Samples were incubated at 37 °C overnight shaking. The peptide-containing supernatants were recovered by 5 min centrifugation at 500 × g, and proteolytic digestion was stopped by addition of 1% formic acid (FA). Peptides were desalted using Sep-Pak C8 1cc vacuum cartridges (Waters) according to manufacturer’s protocol. Proteins from input samples were extracted using methanol-chloroform precipitation. Precipitated proteins were resuspended in a buffer containing 1% w/v SDC, 5 mM Tris-(2-carboxyethyl)phosphin (TCEP), 40 mM CAA in 50 mM TEAB (pH 8.0). The following digestion by trypsin and Lys-C was conducted as described above.
Off-line fractionation
Desalted peptides were further fractionated by strong cation exchange (SCX) chromatography using an Agilent 1260 Infinity II HPLC system equipped with a PolySULFOETHYL A column (PolyLC). Peptides were separated using a 95 min gradient ranging from 100% Buffer A (20% ACN in water) to a mixture of 20% Buffer A and 80% Buffer B (5 M NaCl in 20% ACN and water), collecting 45 s fractions. Fractions were desalted using C8 stage tips105 and dried by speed vacuum. Dried samples were stored at −20 °C before LC-MS measurement.
AlphaFold predictions
Hetero-dimers were predicted by AF-Multimer v2.38 using the default protocol and database sequence search methods. pTM, ipTM, confidence scores (0.8*ipTM+0.2*pTM), and predicted alignment errors were extracted from.pkl files. Of each dimeric prediction, we selected the model with the highest AlphaFold confidence score and highest cross-link agreement for further analysis. Predicted alignment errors were plotted using Python. Cross-links were plotted on predicted structures using the bio3D package in R Studio. Models with at least half of cross-links in-range (<35 Å), taking into account only links from well-structured regions (pLDDT > 50), were included in Supplementary Data 4. The AF2 model of HSV-1-UL12 was downloaded from https://www.bosse-lab.org/herpesfolds/.
Hetero-dimeric PPIs were additionally predicted by AlphaFold3 v.3.0.19 on a local workstation utilizing an NVIDIA RTX A6000. Structure prediction was done in a Docker environment with default parameters for protein-protein sequences, generating 5 predicted models through 1 random seed; model parameters were provided by Google Deepmind.
To identify interaction sites within disordered regions, we developed a two-step algorithm in Python 3.10 utilizing Biopython106. For each predicted dimeric structure (all ranks), residues of a chain are extracted if their corresponding C-alpha coordinates exhibit a Euclidean distance of less than 10 Å to C-alpha coordinates of the other chain. Following this, we investigated these stretches of residues for their pLDDT scores. As a pLDDT below 50 is a predictor of disorder107, consecutive residues with a motif length of three to up to 20 amino acids are extracted if each of them exhibits a pLDDT above 50 and if they are adjacent to residues with a pLDDT below 50. We then excluded motifs with an average pLDDT below 60 to retain higher confidence predictions.
Structural images were generated using PyMOL (Schrödinger, LLC, version 2.4).
LC-MS of XL-MS data
Cross-linked peptides were measured on an Orbitrap Fusion Lumos Tribrid system (Thermo Fisher Scientific) equipped with a FAIMS Pro Duo interface (Thermo Fisher Scientific) operating with Xcalibur 4.6 and Tune 4.0. For this, ~1 µg of sample was loaded with an online-connected Ultimate 3000 RSLC nano LC system (Thermo Fisher Scientific) onto a 50 cm analytical, in-house packed reverse-phase column (Poroshell 120 EC-C18, 2.7 µm, Agilent Technologies) and separated with a 180 min or 120 min gradient going from 0.1% w/v FA in water (buffer A) to 0.1% w/v FA in 80% v/v ACN (buffer B) at a flow rate of 250 nl/min. FAIMS compensation voltages were alternated between −50, −60 and −75 V.
We conducted two experiments with different MS acquisition schemes (MS2-MS3 and MS2-only) to provide evidence of the performance of the workflow when using different acquisition schemes. For the SHVIP experiment with MS2-only acquisition scheme, cross-linked peptides were detected using a stepped-higher energy collision-induced dissociation (HCD)-MS2 method, where for MS1, Orbitrap resolution was set to 120,000 with a scan range of 375–1600 m/z and mass range set to Normal. Standard parameters were used for automatic gain control (AGC), and 50 ms of injection time was used. Precursors of charge states 4-8 were selected with an isolation window of 1.6 m/z and fragmented by stepped-HCD with the energies 21%, 27% and 33%. Dynamic exclusion was enabled with an exclusion period of 60 s. Cycle time in between master scans was set to 2 s. For MS2, Orbitrap resolution was set to 60,000 with mass range set to Normal and scan range to Auto. Maximum of injection time was adjusted to 118 ms, and AGC target to 200%.
For the SHVIP experiment with the MS2-MS3 acquisition scheme, cross-linked peptides were detected by a stepped HCD-MS2-collision-induced dissociation (CID)-MS3 method. MS1 and MS2 scan parameters were the same as for the stepped-HCD-MS2-only method. MS3 scans were triggered when DSSO signature peaks with a mass difference of 31.9721 mass units were detected. The two most intense reporter peaks with charge states 2–6 were selected as precursors for MS3 with an isolation window of 2 m/z and fragmented by CID with a collision energy of 35% and an activation time of 10 ms. MS3 scans were acquired in the Ion Trap with scan rate set to Rapid, 100 ms injection time, AGC target set to 200%, mass range set to Normal, and scan range set to Auto.
LC-MS of bottom-up data
Bottom-up proteomic samples were measured on an Orbitrap Fusion Tribrid instrument (Fusion), an Orbitrap Fusion Lumos instrument (Lumos), an Orbitrap Elite instrument (Elite) (all Thermo Fisher Scientific) connected online to an Ultimate 3000 RSLC nano LC system (Thermo Fisher Scientific) or an Orbitrap Exploris 480 (Exploris) connected online to a Vanquish neo UHPLC system (Thermo Fisher Scientific). Peptides were separated on an in-house packed 50 cm analytical, reverse-phase column (Poroshell 120 EC-C18, 2.7 µm, Agilent Technologies) with 120 min or 180 min gradients at a flow rate of 250 nl/min.
Methods run on Fusion or Lumos were measured with a HCD-MS2 method with MS1 scans acquired in the Orbitrap with the resolution set to 120,000, while MS2 spectra were acquired in the Ion Trap. Cycle time in between master scans was set to 1 s, and dynamic exclusion was set to 40 s. Intensity threshold was set to 1E04 for Fusion and to 5E03 for Lumos, and maximum injection time to 50 ms, while AGC target was kept at standard settings. Precursors of charge state 2–4 were selected for fragmentation with a precursor isolation window of 1.6 m/z and fragmented with the HCD energy of 30 %.
For measurement on Exploris, peptides were measured with an HCD-MS2 method with MS1 scans acquired in the Orbitrap with the resolution set to 120,000. Cycle time in between master scans was set to 2 s, and dynamic exclusion was set to 40 s. Intensity threshold was set to 1E04, and maximum injection time was set to Auto. AGC target was set to 300%. Precursors of charge state 2–4 were selected for fragmentation with a precursor isolation window of 1.6 m/z and fragmented with the HCD energy of 30%. MS2 spectra were acquired in the Orbitrap with a resolution set to 15,000, with AGC target set to Standard and maximum injection time set to Auto.
Measurements run on Elite were acquired using a CID-MS2 method. MS1 scans were acquired in the Orbitrap with resolution set to 120,000 and mass range from 350 to 1500 m/z. Dynamic exclusion was enabled with a duration of 60 s. Top 15 precursors of charge states 2 and 3 were selected with an isolation window of 1 m/z for fragmentation by CID. Normalized collision energy of 35%, activation time of 10 ms, and activation Q of 0.25 were used for CID fragmentation. MS2 spectra were acquired in the Ion trap with mass range set to Normal and scan rate set to Rapid.
Cross-link data analysis
Peak lists (.mgf files) were generated in Proteome Discoverer (v.2.1) to convert .raw files into .mgf files containing HCD-MS2 or HCD-MS2-MS3 data, respectively. The .mgf files were searched using a stand-alone search engine based on XlinkX v.2.0108 with the following settings: MS ion mass tolerance, 10 ppm; MS2 ion mass tolerance, 20 ppm; fixed modification, Cys carbamidomethylation; variable modification, Met oxidation; enzymatic digestion, trypsin; allowed number of missed cleavages, 3; DSSO cross-linker, 158.0038 Da (short arm, 54.0106 Da; long arm, 85.9824 Da). Reaction sites at lysine residues and protein N-termini were considered. Spectra were searched against a concatenated target-decoy database generated on the basis of the virus and host proteome determined by bottom-up proteomics. Raw files from both experiments (MS2-only and MS2-MS3 acquisition) were searched separately, results were then combined, and the FDR at 1% was applied at the level of residue-residue connections, separately for intra- and inter-links on the basis of a target-decoy competition strategy using randomized decoys. Following this, all cross-links were retained when they matched to a viral protein or to host protein with a viral interaction partner (virus-centric network). This network still contains host-host cross-links between host proteins that are also linked to viral proteins. The PPI-level FDR in this set of PPIs was assessed based on the ratio of decoys to targets in the virus-centric network. The resulting network was clustered with the edge-betweenness algorithm47 using in-house R scripts and the edge.betweenness.community function from igraph package. Clusters and networks were visualized in Cytoscape v3.7.2 or using xiNET109. The comparison of PPI and cross-link identifications between input and enriched samples is based on individual searches as described above, but with a naive 1 % FDR (that is, FDR filtering applied on the combined set of intra- and inter-links).
Bottom-up proteomics data analysis
AP-MS experiments were designed as triplicate experiments comparing anti-HA APs in lysates containing the transgene to control purifications of the wildtype strain, or in case of mutation of short linear interaction motifs, comparing HA-tagged wildtype protein containing virus against the HA-tagged mutant protein containing virus. The set-up was label-free, and control experiments were performed and measured in parallel. Raw files were analyzed using MaxQuant v.2.0.3.0110, with standard parameters and match between runs, iBAQ, and LFQ enabled. The HSV-1 reference proteome UP00009294 and Uniprot reference proteome of human protein sequences (downloaded 2020) was used. FDR cut-offs were set at 1% at PSM, protein, and modification site levels. The proteinGroups.txt file was used for subsequent analysis with potential contaminants, reverse database hits, and proteins only identified by a modification site removed. LFQ intensities were log2 transformed and, when appropriate, missing values were imputed on the basis of a normal distribution shrunk by a factor of 0.3 and downshifted by 1.8 standard deviations only when a protein was quantified in all three replicates of either experiment or control. Log2 fold-changes and p-values from a t test were calculated based on these values. The significance of overlap between AP-MS and XL-MS was calculated using phyper function in R.
SILAC-quantified AP-MS experiments were conducted in duplicates, comparing HA-tagged wild-type target protein containing virus with HA-tagged mutant target protein containing virus. Raw files were analyzed using MaxQuant v. 1.6.2.6. Quantification was based on MaxQuant normalized SILAC ratios using two parameter groups in the analysis for light and heavy (Lys8 and Arg10) labeled proteins, respectively. Remaining parameters were kept as described above, and the re-quantify option was enabled. The proteinGroups.txt file was used for data evaluation in R with log2-transformed SILAC ratios. Proteins enriched or depleted over a log2 ratio of 1 in both replicates were considered as hits.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The authors thank Beate Sodeik (MHH, Hannover) for providing us with recombinant HSV-1 strain 17 and Philip Lössl (Absea Biotechnology, Berlin) for critically reviewing and editing the manuscript. Funding was provided by the Deutsche Forschungsgemeinschaft (DFG) grant BO 5917/1-1 (B.B.), Leibniz-Wettbewerb (K284/2019) (F.L.). Financial support to A.E. was obtained from the Swedish Research Council for Natural Science, grant No. VR-2016-06301 and Swedish E-science Research Centre, and from Knut and Alice Wallenberg Foundation. Computational resources to A.E. were obtained by the Swedish National Infrastructure for Computing via grants: SNIC 2021/5-297, SNIC 2021/6-197, Berzelius-2021-29, and Berzelius-2022-106.
Author contributions
B.B. conceptualized the project. B.B., L.M., I.G., J.R., L.W., and F.L. developed the methodology. B.B., L.M., I.G., B.V., J.R., A.E., and L.W. conducted the investigations. B.B., L.M., I.G., and L.W. conducted formal analysis. I.G., B.V., L.W., A.E., and F.L. procured resources. B.B. and L.M. performed visualization. B.B. and L.M. curated the data. B.B., L.W., and F.L. acquired funding. B.B., L.W., and F.L. administered the project. B.B., L.W., and F.L. supervised the project. B.B. and L.M. wrote the original draft. B.B., L.W., and F.L. reviewed and edited the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE111 partner repository with the dataset identifier PXD047422. AlphaFold models are available via figshare under: 10.6084/m9.figshare.24639279.v2 (AF2.3) or 10.6084/m9.figshare.29064041.v1 (AF3). A summary on the performed experiments and raw files is available in Supplementary Data 7. Source data are provided with this paper.
Competing interests
F.L. is a scientific advisor to Absea Biotechnology and vantAI. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Boris Bogdanow, Lars Mühlberg.
Contributor Information
Boris Bogdanow, Email: boris.bogdanow@charite.de.
Lüder Wiebusch, Email: lueder.wiebusch@charite.de.
Fan Liu, Email: fliu@fmp-berlin.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-61618-z.
References
- 1.Connolly, S. A., Jardetzky, T. S. & Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol.19, 110–121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdary, T. K. et al. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol.17, 882–888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai, X. & Zhou, Z. H. Structure of the herpes simplex virus 1 capsid with associated tegument protein complexes. Science360, eaao7298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuccola, H. J., Filman, D. J., Coen, D. M. & Hogle, J. M. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol. Cell5, 267–278 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Fan, H. et al. Structures of influenza A virus RNA polymerase offer insight into viral genome replication. Nature573, 287–290 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonetti, L., Nilsson, J., McInerney, G., Ivarsson, Y. & Davey, N. E. SLiM-binding pockets: an attractive target for broad-spectrum antivirals. Trends Biochem. Sci.48, 420–427 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Hagai, T., Azia, A., Babu, M. M. & Andino, R. Use of host-like peptide motifs in viral proteins is a prevalent strategy in host-virus interactions. Cell Rep.7, 1729–1739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, R. et al. Protein complex prediction with AlphaFold-multimer. bioRxiv 2021.10.04.46303410.1101/2021.10.04.463034 (2022).
- 9.Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature10.1038/s41586-024-07487-w (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa Marrero, M., Jänes, J., Baptista, D. & Beltrao, P. Integrating large-scale protein structure prediction into human genetics research. Annu. Rev. Genomics Hum. Genet.25, 123–140 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Bryant, P. Deep learning for protein complex structure prediction. Curr. Opin. Struct. Biol.79, 102529 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Terwilliger, T. C. et al. AlphaFold predictions are valuable hypotheses and accelerate but do not replace experimental structure determination. Nat. Methods21, 110–116 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arend, K. C. et al. Kinome profiling identifies druggable targets for novel human cytomegalovirus (HCMV) antivirals. Mol. Cell. Proteom.16, S263–S276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vastag, L., Koyuncu, E., Grady, S. L., Shenk, T. E. & Rabinowitz, J. D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog.7, e1002124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jean Beltran, P. M., Mathias, R. A. & Cristea, I. M. A portrait of the human organelle proteome in space and time during cytomegalovirus infection. Cell Syst.3, 361–373.e6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soh, T. K. et al. Temporal proteomic analysis of herpes simplex virus 1 infection reveals cell-surface remodeling via pUL56-mediated GOPC degradation. Cell Rep.33, 108235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weekes, M. P. et al. Quantitative temporal viromics: an approach to investigate host-pathogen interaction. Cell157, 1460–1472 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etibor, T. A., Yamauchi, Y. & Amorim, M. J. Liquid biomolecular condensates and viral lifecycles: review and perspectives. Viruses13, 366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosse, J. B. & Brune, W. Viral dew: phase separation and the formation of viral replication compartments. PLoS Pathog.19, e1011145 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu, G. et al. Viral tegument proteins restrict cGAS-DNA phase separation to mediate immune evasion. Mol. Cell81, 2823–2837.e9 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Charman, M. et al. A viral biomolecular condensate coordinates assembly of progeny particles. Nature616, 332–338 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Johnson, D. C. & Baines, J. D. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol.9, 382–394 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature583, 459–468 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batra, J. et al. Protein interaction mapping identifies rbbp6 as a negative regulator of Ebola virus replication. Cell175, 1917–1930.e13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah, P. S. et al. Comparative flavivirus-host protein interaction mapping reveals mechanisms of dengue and Zika virus pathogenesis. Cell175, 1931–1945.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scaturro, P. et al. An orthogonal proteomic survey uncovers novel Zika virus host factors. Nature561, 253–257 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Bogdanow, B. et al. Cross-regulation of viral kinases with cyclin A secures shutoff of host DNA synthesis. Nat. Commun.11, 4845 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo, Y. et al. HIV–host interactome revealed directly from infected cells. Nat. Microbiol.1, 16068 (2016). [DOI] [PMC free article] [PubMed]
- 29.Yiu, S. P. T. et al. An Epstein-Barr virus protein interaction map reveals NLRP3 inflammasome evasion via MAVS UFMylation. Mol. Cell83, 2367–2386.e15 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobre, L. V. et al. Human cytomegalovirus interactome analysis identifies degradation hubs, domain associations and viral protein functions. Elife8, e49894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozenblatt-Rosen, O. et al. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature487, 491–495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pichlmair, A. et al. Viral immune modulators perturb the human molecular network by common and unique strategies. Nature487, 486–490 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Cristea, I. M. et al. Tracking and elucidating alphavirus-host protein interactions. J. Biol. Chem.281, 30269–30278 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Liu, X. et al. SARS-CoV-2-host proteome interactions for antiviral drug discovery. Mol. Syst. Biol.17, e10396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen, Z. et al. Interactomes of SARS-CoV-2 and human coronaviruses reveal host factors potentially affecting pathogenesis. EMBO J.40, e107776 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeev-Ben-Mordehai, T., Hagen, C. & Grünewald, K. A cool hybrid approach to the herpesvirus ‘life’ cycle. Curr. Opin. Virol.5, 42–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Reilly, F. J. & Rappsilber, J. Cross-linking mass spectrometry: methods and applications in structural, molecular and systems biology. Nat. Struct. Mol. Biol.25, 1000–1008 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Wheat, A. et al. Protein interaction landscapes revealed by advanced in vivo cross-linking-mass spectrometry. Proc. Natl. Acad. Sci. USA118, e2023360118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang, P.-L. et al. A membrane-permeable and immobilized metal affinity chromatography (IMAC) enrichable cross-linking reagent to advance in vivo cross-linking mass spectrometry. Angew. Chem. Int. Ed. Engl.61, e202113937 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whisnant, A. W. et al. Integrative functional genomics decodes herpes simplex virus 1. Nat. Commun.11, 2038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern-Ginossar, N., Thompson, S. R., Mathews, M. B. & Mohr, I. Translational control in virus-infected cells. Cold Spring Harb. Perspect. Biol.11, a033001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogdanow, B. et al. The dynamic proteome of influenza A virus infection identifies M segment splicing as a host range determinant. Nat. Commun.10, 5518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogdanow, B., Katsimani, N., Liu, F. & Selbach, M. Combining metabolic pulse labeling and quantitative proteomics to monitor protein synthesis upon viral infection. Methods Mol. Biol.2610, 149–165 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Kato, A. et al. Identification of a herpes simplex virus 1 gene encoding neurovirulence factor by chemical proteomics. Nat. Commun.11, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wöhnke, E., Klupp, B. G., Blome, S., Mettenleiter, T. C. & Karger, A. Mass-spectrometric evaluation of the African swine fever virus-induced host shutoff using dynamic stable isotope labeling with amino acids in cell culture (SILAC). Viruses15, 1283 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedel, C. C. et al. Dissecting herpes simplex virus 1-induced host shutoff at the RNA level. J. Virol.95, e01399–20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Girvan, M. & Newman, M. E. J. Community structure in social and biological networks. Proc. Natl. Acad. Sci. USA99, 7821–7826 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turcotte, S., Letellier, J. & Lippé, R. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol.79, 8847–8860 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugimoto, K. et al. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triply fluorescent herpes simplex virus 1. J. Virol.82, 5198–5211 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alconada, A., Bauer, U., Sodeik, B. & Hoflack, B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol.73, 377–387 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollinshead, M. et al. Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J.31, 4204–4220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zenner, H. L., Yoshimura, S.-I., Barr, F. A. & Crump, C. M. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J. Virol.85, 8012–8021 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johns, H. L., Gonzalez-Lopez, C., Sayers, C. L., Hollinshead, M. & Elliott, G. Rab6-dependent post-Golgi trafficking of HSV1 envelope proteins to sites of virus envelopment. Traffic15, 157–178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neumann, J., Eis-Hübinger, A. M. & Koch, N. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J. Immunol.171, 3075–3083 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Campadelli-Fiume, G., Collins-McMillen, D., Gianni, T. & Yurochko, A. D. Integrins as herpesvirus receptors and mediators of the host signalosome. Annu. Rev. Virol.3, 215–236 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Haanes, E. J., Nelson, C. M., Soule, C. L. & Goodman, J. L. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J. Virol.68, 5825–5834 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avitabile, E., Forghieri, C. & Campadelli-Fiume, G. Cross-talk among the glycoproteins involved in herpes simplex virus entry and fusion: the interaction between gB and gH/gL does not necessarily require gD. J. Virol.83, 10752–10760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hagen, C. et al. Structural basis of vesicle formation at the inner nuclear membrane. Cell163, 1692–1701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruzuru, Y. et al. Role of herpes simplex virus 1 immediate early protein ICP22 in viral nuclear egress. J. Virol.88, 7445–7454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leach, N. et al. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J. Virol.81, 10792–10803 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, L. et al. Alphaherpesvirus major tegument protein VP22: its precise function in the viral life cycle. Front. Microbiol.11, 1908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elliott, G., O’Reilly, D. & O’Hare, P. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology226, 140–145 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Taddeo, B., Sciortino, M. T., Zhang, W. & Roizman, B. Interaction of herpes simplex virus RNase with VP16 and VP22 is required for the accumulation of the protein but not for accumulation of mRNA. Proc. Natl. Acad. Sci. USA104, 12163–12168 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Leeuwen, H. et al. Herpes simplex virus type 1 tegument protein VP22 interacts with TAF-I proteins and inhibits nucleosome assembly but not regulation of histone acetylation by INHAT. J. Gen. Virol.84, 2501–2510 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Vink, E. I., Andrews, J., Duffy, C. & Mohr, I. Preventing translational inhibition from ribosomal protein insufficiency by a herpes simplex virus–encoded ribosome-associated protein. In Proc. Natl. Acad. Sci. USA118, e2025546118 (2021). [DOI] [PMC free article] [PubMed]
- 66.López, M. R., Schlegel, E. F. M., Wintersteller, S. & Blaho, J. A. The major tegument structural protein VP22 targets areas of dispersed nucleolin and marginalized chromatin during productive herpes simplex virus 1 infection. Virus Res.136, 175–188 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trapp-Fragnet, L. et al. Cell cycle modulation by Marek’s disease virus: the tegument protein VP22 triggers S-phase arrest and DNA damage in proliferating cells. PLoS ONE9, e100004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren, X., Harms, J. S. & Splitter, G. A. Bovine herpesvirus 1 tegument protein VP22 interacts with histones, and the carboxyl terminus of VP22 is required for nuclear localization. J. Virol.75, 8251–8258 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bryant, P., Pozzati, G. & Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun.13, 1265 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dembowski, J. A., Dremel, S. E. & DeLuca, N. A. Replication-coupled recruitment of viral and cellular factors to herpes simplex virus type 1 replication forks for the maintenance and expression of viral genomes. PLoS Pathog.13, e1006166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang, M.-H. et al. The US2 gene product of herpes simplex virus 2 is a membrane-associated ubiquitin-interacting protein. J. Virol.87, 9590–9603 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu, H., Cherepanova, N. A., Gilmore, R., Contessa, J. N. & Lehrman, M. A. Targeting STT3A-oligosaccharyltransferase with NGI-1 causes herpes simplex virus 1 dysfunction. FASEB J.33, 6801–6812 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson, S. & Xiong, Y. Targeting protein ubiquitylation: DDB1 takes its RING off. Nat. Cell Biol.11, 379–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le-Trilling, V. T. K. et al. Structural mechanism of CRL4-instructed STAT2 degradation via a novel cytomegaloviral DCAF receptor. EMBO J.42, e112351 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee, C. Y. et al. Systematic discovery of protein interaction interfaces using AlphaFold and experimental validation. Mol. Syst. Biol.20, 75–97 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garai, Á et al. Specificity of linear motifs that bind to a common mitogen-activated protein kinase docking groove. Sci. Signal.5, ra74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madeira, F. et al. 14-3-3-Pred: improved methods to predict 14-3-3-binding phosphopeptides. Bioinformatics31, 2276–2283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Birgisdottir, ÅB., Lamark, T. & Johansen, T. The LIR motif - crucial for selective autophagy. J. Cell Sci.126, 3237–3247 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Richards, A. L., Eckhardt, M. & Krogan, N. J. Mass spectrometry-based protein–protein interaction networks for the study of human diseases. Mol. Syst. Biol.17, e8792 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greco, T. M., Diner, B. A. & Cristea, I. M. The impact of mass spectrometry-based proteomics on fundamental discoveries in virology. Annu. Rev. Virol.1, 581–604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashimoto, Y., Sheng, X., Murray-Nerger, L. A. & Cristea, I. M. Temporal dynamics of protein complex formation and dissociation during human cytomegalovirus infection. Nat. Commun.11, 806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Justice, J. L. et al. Systematic profiling of protein complex dynamics reveals DNA-PK phosphorylation of IFI16 en route to herpesvirus immunity. Sci. Adv.7, eabg6680 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartolec, T. K. et al. Cross-linking mass spectrometry discovers, evaluates, and corroborates structures and protein-protein interactions in the human cell. Proc. Natl. Acad. Sci. USA120, e2219418120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bogdanow, B. et al. Redesigning error control in cross-linking mass spectrometry enables more robust and sensitive protein-protein interaction studies. Mol Syst Biol21, 90–106 (2025). [DOI] [PMC free article] [PubMed]
- 86.O’Reilly, F. J. et al. Protein complexes in cells by AI-assisted structural proteomics. Mol. Syst. Biol.19, e11544 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kosinski, J. Improving AlphaFold 3 structural modeling by incorporating explicit crosslinks. bioRxiv10.1101/2024.12.03.626671 (2024).
- 88.Raouraoua, N. et al. MassiveFold: unveiling AlphaFold’s hidden potential with optimized and parallelized massive sampling. Nat. Comput. Sci.4, 824–828 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallner, B. Improved multimer prediction using massive sampling with AlphaFold in CASP15. Proteins91, 1734–1746 (2023). [DOI] [PubMed] [Google Scholar]
- 90.Wühr, M., Keber, F., Nguyen, T. & Brangwynne, C. Evidence for widespread cytoplasmic structuring into mesoscopic condensates. FASEB J.36, 346–352 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pankow, S. et al. Deep interactome profiling of membrane proteins by co-interacting protein identification technology. Nat. Protoc.11, 2515–2528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maringer, K., Stylianou, J. & Elliott, G. A network of protein interactions around the herpes simplex virus tegument protein VP22. J. Virol.86, 12971–12982 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bogdanow, B. et al. Spatially resolved protein map of intact human cytomegalovirus virions. Nat. Microbiol.8, 1732–1747 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morrison, E. E., Wang, Y. F. & Meredith, D. M. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol.72, 7108–7114 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cliffe, A. R. et al. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe18, 649–658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kotova, I. et al. Snapshot of in-cell protein contact sites reveals new host factors and hijacking of paraspeckles during influenza A virus infection. bioRxiv10.1101/2025.03.09.642134 (2025).
- 97.Dhungel, P., Cantu, F. M., Molina, J. A. & Yang, Z. Vaccinia virus as a master of host shutoff induction: targeting processes of the central dogma and beyond. Pathogens9, 400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marissen, W. E., Gradi, A., Sonenberg, N. & Lloyd, R. E. Cleavage of eukaryotic translation initiation factor 4GII correlates with translation inhibition during apoptosis. Cell Death Differ.7, 1234–1243 (2000). [DOI] [PubMed] [Google Scholar]
- 99.Roth, H. et al. Flavivirus infection uncouples translation suppression from cellular stress responses. MBio8, e00488–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ignacio, B. J. et al. THRONCAT: metabolic labeling of newly synthesized proteins using a bioorthogonal threonine analog. Nat. Commun.14, 3367 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serwa, R. A. et al. Analysis of a fully infectious bio-orthogonally modified human virus reveals novel features of virus cell entry. PLoS Pathog.15, e1007956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zydek, M., Hagemeier, C. & Wiebusch, L. Cyclin-dependent kinase activity controls the onset of the HCMV lytic cycle. PLoS Pathog.6, e1001096 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Snijder, B. et al. Single-cell analysis of population context advances RNAi screening at multiple levels. Mol. Syst. Biol.8, 579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tischer, B. K., Smith, G. A. & Osterrieder, N. En passant mutagenesis: a two step markerless red recombination system. Methods Mol. Biol.634, 421–430 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using stagetips. Nat. Protoc.2, 1896–1906 (2007). [DOI] [PubMed] [Google Scholar]
- 106.Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics25, 1422–1423 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature596, 590–596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu, F., Rijkers, D. T. S., Post, H. & Heck, A. J. R. Proteome-wide profiling of protein assemblies by cross-linking mass spectrometry. Nat. Methods12, 1179–1184 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Combe, C. W., Fischer, L. & Rappsilber, J. xiNET: cross-link network maps with residue resolution. Mol. Cell. Proteom.14, 1137–1147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol.26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res.50, D543–D552 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE111 partner repository with the dataset identifier PXD047422. AlphaFold models are available via figshare under: 10.6084/m9.figshare.24639279.v2 (AF2.3) or 10.6084/m9.figshare.29064041.v1 (AF3). A summary on the performed experiments and raw files is available in Supplementary Data 7. Source data are provided with this paper.