Abstract
Polyinosinic-polycytidylic acid (PolyIC), a “mimic” of double-stranded viral RNA, can induce diabetes when administered to rats with RT1u, and immunization of normal H-2d mice (e.g., BALB/c) with insulin B:9–23 peptide (but not H-2b) results in the rapid induction of insulin autoantibodies. Because a mouse model of PolyIC/antigen-induced diabetes is lacking, we sought to produce insulitis and diabetes with either PolyIC and/or B:9–23 peptide immunization. Simultaneous administration of PolyIC and B:9–23 peptide to BALB/c mice (but with neither alone) induced insulitis. CD4 T lymphocytes predominated within islets, and the mice did not progress to hyperglycemia. Islets with transgene-induced expression of the costimulatory B7–1 molecule have enhanced diabetes susceptibility. Diabetes was frequently induced in B7–1 transgenic mice with H-2d in contrast to H-2b mice after PolyIC administration. Disease induction was accelerated by adding B:9–23 immunization to PolyIC. These studies demonstrate that “normal” mice have autoreactive T lymphocytes able to rapidly target islets and insulin given appropriate MHC alleles and that a peripherally administered insulin peptide (an altered peptide ligand of which is in clinical trials) can enhance specific anti-islet autoimmunity. These first PolyIC/insulin-induced murine models should provide an important tool to study the pathogenesis of type 1 diabetes with experimental autoimmune diabetes.
Induction of severe β cell destruction with permanent diabetes in a convenient murine experimental model has proven difficult to achieve. In 1956, Witebsky and colleagues reported that autoimmune thyroid disease could be induced with the administration of thyroid antigens in complete Freund's adjuvant. This seminal observation led to the characterization of autoimmune disorders of animal models and of man, and “the Rose and Witebsky” criteria for autoimmune diseases (1). Type 1A diabetes has become a prototypic immune-mediated disorder with spontaneous animal models, disease transfer with T lymphocytes, and extensive studies of children developing type 1 diabetes monitored from birth (2–6). In the 1960s, Renold, Soeldner, and colleagues produced insulitis in cows immunized with bovine insulin in complete Freund's adjuvant (7). These studies were not pursued further, in part, because of the difficulty of the animal model. Also in the 1960s Grodsky and coworkers reported the induction of insulitis in rabbits (and in a subset, diabetes) following immunization with bovine insulin in complete Freund's adjuvant (8). The insulin preparations used were not as pure as would soon be available, and subsequent studies by other investigators did not result in insulitis or diabetes.
A heat-shock protein administered to mice produced a transient “mild” (glucose <250 mg/dl) diabetes (9). Injection of autoantigens into diabetes-prone animals such as the NOD (nonobese diabetic) mice or rats with RT1u could accelerate or inhibit the timing of diabetes development (10–12) and multiple clones of T cells able to transfer diabetes into immunodeficient or young NOD mice have been derived (10, 13, 14).
Polyinosinic-polycytidylic acid (PolyIC) has been used as a viral RNA mimic to stimulate the innate immune system and in RT1u rat strains can often induce insulitis and, less often, diabetes (15), although in NOD mice PolyIC prevents diabetes (16). B7–1 is a potent costimulatory molecule that provides signals to T cells. Rat insulin promoter (RIP)-B7.1 C57BL/6 mice expressing B7–1 molecules on pancreatic β cells, however, infrequently develop insulitis or diabetes. With the Yale strain studied here, less than 3% (3 in more than 100 mice older than 10 months) developed diabetes. When these mice were backcrossed once to NOD strain or bred with human DQ8 transgenic mice, these mice developed spontaneous diabetes (17, 18). These reports demonstrate that islet B7–1 enhances susceptibility to autoimmune destruction.
Proinsulin/insulin (19–21) and the insulin B:9–23 peptide are studied extensively as islet autoantigens (22), and insulin was an early subject of immune response (Ir)-gene-dependent recognition, with of interest the observation that response to the B chain of insulin was H-2d restricted. H-2b mice gave a low or absent response to the B chain but responded to A chain determinants (23, 24). The sequence of the B:9–23 insulin peptide is identical in mice and humans, and T cell responses to this peptide can be demonstrated in both species. The crystal structure of B:9–23 bound to the high-risk HLA allele DQ8 was recently elucidated (25). This peptide is recognized by islet-infiltrating T cells of the NOD mouse (22, 26), and some of the clones can respond to B:9–23 presented by BALB/c antigen-presenting cells in addition to NOD antigen-presenting cells (27). In addition, administration of the B:9–23 peptide to BALB/c mice, but not C57BL/6 mice, and congenic strains with H-2d but not H-2b results in high titers of insulin autoantibodies (28). Note that the induced insulin autoantibodies react with intact insulin and cannot be absorbed with the immunizing B:9–23 insulin peptide. In this article, we describe the induction of insulitis by using the B:9–23 peptide and PolyIC in BALB/c mice and the production of diabetes with these molecules in mice with islet B7–1 expression.
Methods
Mice.
BALB/cBy or BALB/cAn mice were purchased from The Jackson Laboratory or Harlan Breeders (Indianapolis, IN). RIP-B7.1 C57BL/6 mice expressing B7–1 molecules on pancreatic β cells were generated as described (18). BALB/c mice (H-2d) were crossed with RIP-B7.1 C57BL/6 mice (H-2b) to generate F1 (H-2b/d) mice. The RIP-B7.1 transgene was detected by PCR amplification by using genomic DNA isolated from mouse tails as described (18). The B7–1+ F1 mice were further backcrossed with BALB/c mice to generate the B7–1+ backcross generation with MHC H-2d/d or H-2b/d alleles (N2). These mice were genotyped for the microsatellite maker that is linked to H-2 gene (D17Mit34) and used in this study.
The B7–1− littermates were used as control mice. The mice were housed in specific pathogen-free facilities with approved University of Colorado Health Science Center Animal Care and Use Committee protocols.
Antigens and Reagents.
Insulin B:9–23 chain (SHLVEALYLVCGERG) and tetanus toxin (TT)-peptides 830–843 (QYIKANSKFIGIFE) were synthesized and purified by reverse-phase high-performance liquid chromatography and identified by mass spectroscopy (Research Genetics, Huntsville, AL). These peptides were used as immunogens. PolyIC sodium salt was purchased from Sigma.
Disease Induction Protocol.
B:9–23 or TT peptide (100 μg/mouse) in incomplete Freund's adjuvant (IFA) was given s.c. to BALB/c mice at 4 weeks of age (day 1). Additionally, PolyIC (7.5 μg/g body weight on days 1–5 and 8–14) was administered i.p.
First-backcross generation mice having B7–1 and H-2d/d or b/d were also immunized with B:9–23 or TT peptide with PolyIC treatment in the same way.
Insulin autoantibody (IAA) expression of serum was evaluated prospectively beginning at 4 weeks of age until the development of diabetes or until 32 weeks of age. IAA were measured with a 96-well filtration plate micro IAA assay as described (29).
The blood glucose levels of the mice were monitored every week by using an Elite Glucometer (Bayer, Elkhart, IN). The mice were considered to be diabetic after two consecutive blood glucose values greater than 250 mg/dl.
Histological and Immunohistochemical Analysis.
A portion of the pancreata, thyroid, and salivary glands obtained from the mice was fixed in 10% formalin, paraffin-embedded, and stained with hematoxylin and eosin. Pancreatic sections were microscopically examined for the presence of insulitis. In brief, pancreatic frozen sections were stained by using monoclonal antibodies to mouse CD4 and CD8 (Becton Dickinson). A universal peroxidase-conjugated secondary antibody (Dako) was used as a detection system. Paraffin-embedded pancreatic sections were stained with polyclonal guinea pig anti-insulin and anti-glucacon antibodies (Linco Research Immunoassay, St. Charles, MO), followed by incubation with a peroxidase-labeled anti-guinea pig IgG secondary (Kirkegaard & Perry Laboratories). Subsequently, the sections were counterstained with hematoxylin and coverslipped.
Results
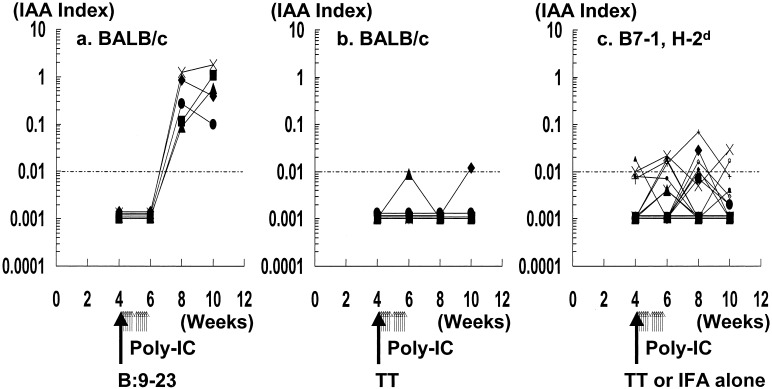
Fig. 1a illustrates the development of anti-insulin autoantibodies in BALB/c mice after s.c. injection of the B:9–23 insulin peptide in IFA with injections of PolyIC. High levels (note log scale) of IAA were rapidly induced as reported with administration of B:9–23 alone (28). In BALB/c mice receiving a control peptide and PolyIC, minimal or no induction of IAA occurred (Fig. 1b).
Figure 1.
IAA expression following peptide in IFA with PolyIC treatment in BALB/c mice. Each of five mice were s.c. injected at 4 weeks of age (day 1) with 100 μg of B:9–23 (a) or TT (b) peptide in IFA. They were also injected i.p. with 7.5 μg/g body weight of PolyIC (days 1–5 and 8–13). (c) IAA plotted for B7–1 transgenic mice with H-2d injected with PolyIC and control TT peptide in IFA or IFA alone (n = 16). Longer arrows indicate the time of immunization of peptide in IFA. Shorter arrows indicate the time of administration of PolyIC. Levels of IAA were then determined every 2 weeks.
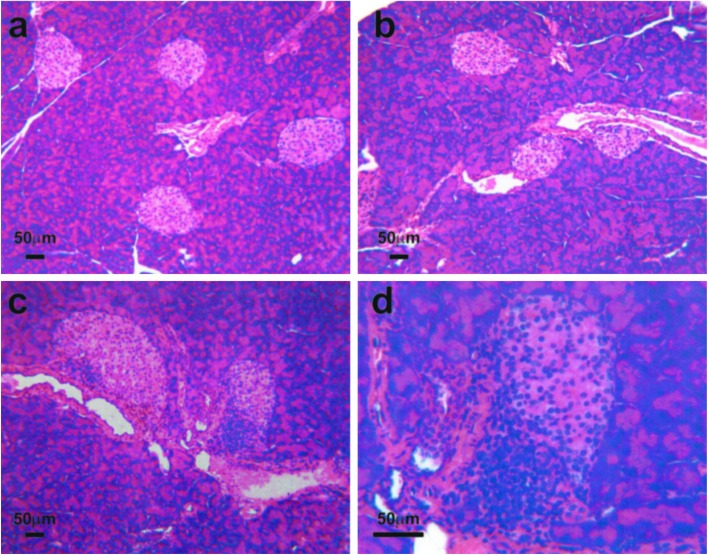
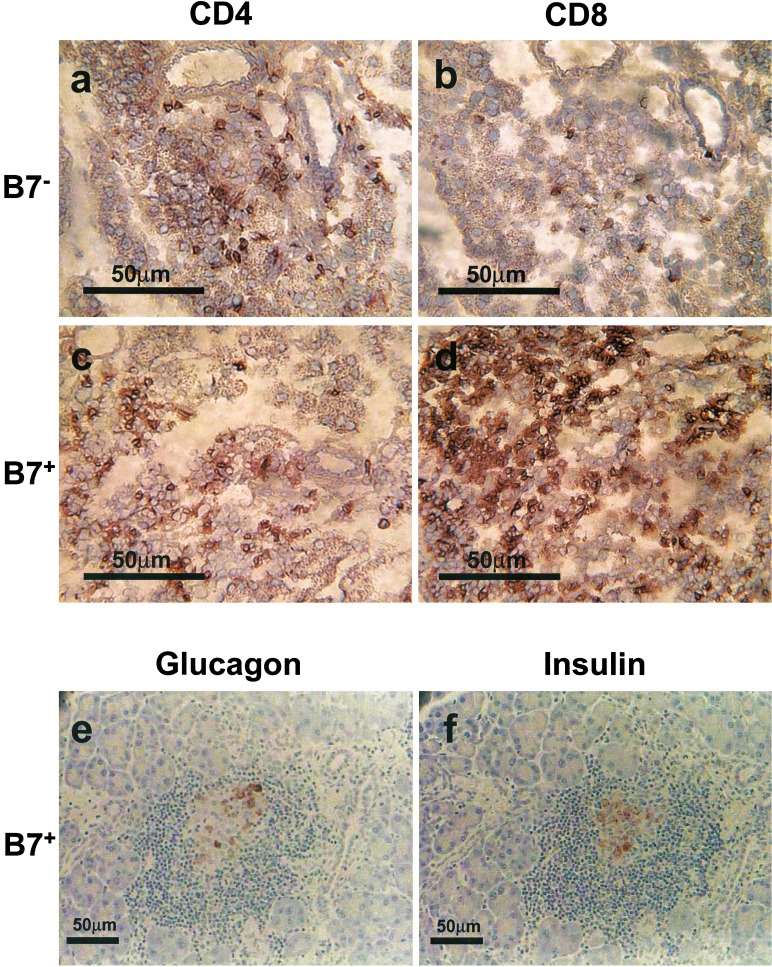
Although B:9–23 peptide in IFA induced insulin autoantibodies, insulitis was not detected (Fig. 2a), and PolyIC alone also did not induce insulitis (Fig. 2b). In contrast administration of B:9–23 with PolyIC resulted in insulitis in BALB/c mice (Fig. 2 c and d), but again without the development of diabetes. The infiltrating T lymphocytes were primarily CD4+ with scattered CD8 T cells (see Fig. 4 a and b).
Figure 2.
Histopathology of insulitis in BALB/c mice. Hematoxylin/eosin staining of pancreatic islets. (a) Insulin peptide B:9–23 immunization alone (low magnification): no insulitis. (b) Tetanus toxin immunization with PolyIC (low magnification): no insulitis. (c) B:9–23 immunization with PolyIC (low magnification): with induced insulitis. (d) B:9–23 immunization with PolyIC (high magnification) with induced insulitis. Five mice in each group were examined for histopathology of insulitis in BALB/c mice. All mice examined showed the same results for the presence of insulitis.
Figure 4.
Immunohistochemistry of pancreatic sections of B7–1− BALB/c mouse (a and b) and B7–1+ diabetic mouse having H-2d (c–f). Mice received immunization with B:9–23 peptide and PolyIC treatments and were killed for the examination of islets histology at 13 weeks of age. (a and c) CD4+ cells. (b and d) CD8+ cells. Islet-infiltrating cells in B7–1− mice were primarily CD4+ with fewer CD8 T cells. Islet infiltrating cells in B7–1+ mice contained a marked CD8 T cell infiltration with fewer CD4 T cells. e is stained for glucagon-producing cells and f for insulin. Relatively few remaining β cells were observed with approximately an equal number of α cells.
We crossed BALB/c mice with RIP-B7.1 C57BL/6 mice (RIP-B7.1, a transgene that results in β cell expression of the B7–1 costimulatory molecule). Crossing to BALB/c results in mice with the major histocompatibility complex from BALB/c (H-2d) able to generate insulin autoantibodies after B:9–23 immunization (28). These B7–1 mice with H-2d did not spontaneously develop insulitis or diabetes (0 of 11). These B7–1 mice with H-2d were immunized at 4 weeks of age with B:9–23 peptide in IFA concomitant with PolyIC injections.
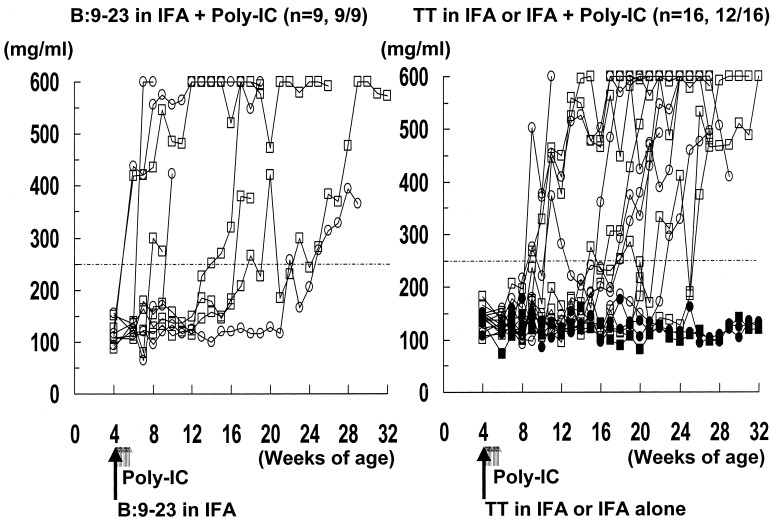
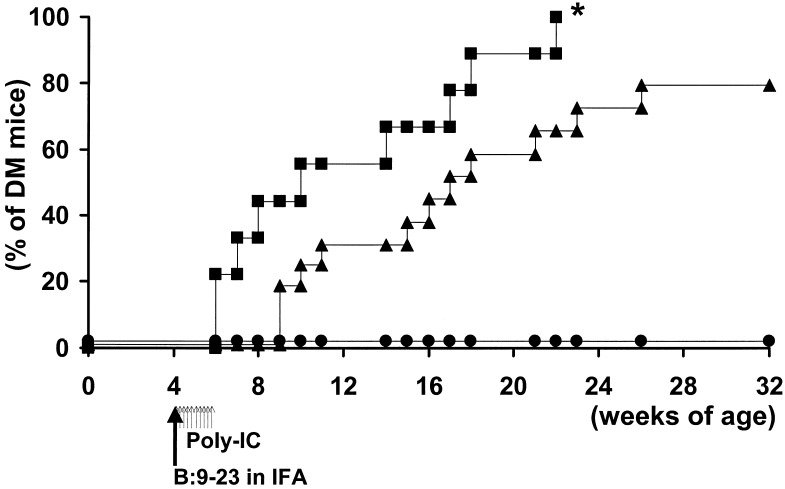
Fig. 3 illustrates blood glucose levels in the B7–1, H-2d mice. Even PolyIC without insulin B:9–23 peptide immunization (Fig. 3 Right) produced hyperglycemia in a subset of mice. As illustrated in Fig. 5, B:9–23 peptide immunization combined with PolyIC treatment (Fig. 3 Left) accelerated diabetes compared with treatment with PolyIC without B:9–23 (P < 0.05). Most of the mice (5 of 9) in the group treated with B:9–23 concomitant with PolyIC developed diabetes by 10 weeks of age, and some of these mice developed diabetes as early as 2 weeks after immunization.
Figure 3.
Blood glucose levels in B7–1 mice with H-2d (d/d or d/b). These mice were s.c. injected with 100 μg of peptide in IFA or just IFA at 4 weeks of age (day 1). They were also injected i.p. with 7.5 μg/g body weight of PolyIC (days 1–5 and 8–13). Longer arrows indicate the time of immunization of peptide in IFA. Shorter arrows indicate the time of administration of PolyIC. Blood glucose was measured weekly. The mice were considered diabetic after two consecutive blood glucose values above 250 mg/dl. Closed squares (H-2d/b) and circles (H-2d/d) indicate nondiabetic mice. Open squares (H-2d/b) and circles (H-2d/d) indicate diabetic mice. (Left) B:9–23 in IFA + PolyIC. (Right) Tetanus toxin (TT) in IFA or IFA alone + PolyIC.
Figure 5.
The induction of diabetes in PolyIC-treated H-2d, B7–1 mice immunized with insulin peptide B:9–23 in IFA, or IFA alone, or IFA with control tetanus toxin (TT) peptide. The solid line with closed squares represents B:9–23 in IFA. The solid line with closed triangles represents TT in IFA or IFA alone; the solid line with closed circles represents untreated mice. Longer arrows indicate the time of immunization of peptide in IFA. Shorter arrows indicate the time of administration of PolyIC. *, P < 0.05 by log-rank test when compared with TT or nonimmunized group.
Diabetes was induced in mice with the MHC genotype H-2d/d and H-2d/b (Fig. 3 Left; 4 of 4 H-2d/d mice became diabetic and 5 of 5 H-2d/b mice, Fig. 3 Right; 6 of 8 H-2d/d mice and 6 of 8 H-2d/b mice became diabetic). Of 16 H-2d, B7–1 mice treated with PolyIC, without B:9–23, 9 developed low levels of insulin autoantibodies (Fig. 1c).
Because even PolyIC with IFA or TT in IFA could induce diabetes, we have checked in B7–1+, H-2d mice further whether B:9–23 immunization alone (without PolyIC) could induce diabetes. Three of 3 mice with B:9–23 alone expressed high titer of IAA (IAA peak, 0.717, 0.596, and 0.965, respectively), and 2 of 3 mice developed diabetes by 20 weeks of age (diabetic age; 11 and 17 weeks of age). To evaluate the effect of PolyIC itself in B7–1 mice without control immunization, PolyIC alone (without IFA or TT in IFA) was administered. As expected, 3 of 4 mice with PolyIC alone developed diabetes by 28 weeks of age (diabetic age; 13, 21, and 28 weeks of age). None of the mice (0 of 7) developed diabetes without the B7–1 transgene even when given PolyIC and the B:9–23 peptide.
No inflammatory infiltration was observed in either the thyroid or salivary gland in PolyIC-treated B7–1, H-2d mice with B:9–23 immunization (not shown). Severe insulitis was observed in PolyIC-treated B7–1, H-2d mice with or without B:9–23 immunization. Fig. 4d illustrates the marked infiltration by CD8 T lymphocytes into the islets of a diabetic B7–1, H-2d mouse that was immunized with B:9–23 and PolyIC treatment. Marked destruction of islet β cells occurred (Fig. 4 e is stained for glucagon and f for insulin). In normal mice, islets contain ≈5-fold more β cells as compared with α cells, whereas in these diabetic mice relatively few β cells remain, with approximately an equal number of α cells, consistent with selective β cell destruction.
We also studied induction of diabetes in H-2b mice with islet B7–1 after PolyIC with or without B:9–23. PolyIC alone induced diabetes in one H-2b/b mouse with islet B7–1 (1 of 6; 6 weeks of age). PolyIC plus B:9–23 peptide also only induced diabetes in one mouse (1 of 6; 20 weeks of age) with the diabetic mouse having a marked cellular infiltration of CD4, CD8, and B cells (not shown).
Discussion
Our prior studies indicate that immunization with the B:9–23 peptide but not other insulin peptides (A:1–15, A:7–21, B:1–15, B:16–30) induce insulin autoantibodies in mice with H-2g7 (e.g., NOD) or H-2d (e.g., BALB/c, B10D2H-2d) (28). The study of congenic strains indicates that this autoantibody response maps to the MHC and not to background genes with H-2b unable to support autoantibody induction after immunization with insulin peptide B:9–23 (28). In that the autoantibodies generated react with intact insulin and cannot be absorbed with the B:9–23 insulin peptide, normal BALB/c mice presumably have T cells able to respond to the B:9–23 peptide and activate B lymphocytes able to produce IAA.
The combination of B:9–23 peptide and PolyIC, but neither alone, induced insulitis in BALB/c, but did not induce diabetes. The insulitis was composed predominantly of CD4 T cells. The RIP-B7.1 transgene was originally bred onto C57BL/6 (H-2b/b) mice. Diabetes was induced in a small percentage of the mice lacking H-2d with administration of PolyIC alone, or with administration of B:9–23 peptide plus PolyIC. In contrast, B7–1, H-2d mice were readily susceptible to the induction of diabetes. H-2d mice were highly inducible with just PolyIC administration or insulin B:9–23 peptide immunization, and disease induction was enhanced with PolyIC plus B:9–23. Disease, as well as autoantibody induction, was influenced by MHC alleles (e.g., H-2b vs. H-2d mice). At present the pathogenic mechanisms determining the differences between H-2b and H-2d mice are not defined but are likely to relate to differences in peptide presentation, thymic deletion, and/or immunoregulation. Exploration of these mechanisms would benefit from class II B:9–23 peptide tetramers that are being pursued.
In the current study, diabetes depends on islet expression of B7–1. The most obvious histologic difference between mice with and without the B7–1 transgene is the marked islet infiltrate of CD8 T cells with B7–1, and it is likely that CD8 T cells similar to those described by Wong and coworkers reacting with insulin peptides contribute to β cell destruction (30). The lesions within the pancreas are limited to islets, and diabetes can develop within 2 weeks of immunization.
At present several human trials are underway to prevent autoimmune β cell destruction by using islet antigens, including a study with an altered peptide ligand of the B:9–23 peptide. A major difficulty of such trials is our current inability to measure accurately either an induced protective or pathogenic T cell immune response. As illustrated by the current study, the possibility exists with an insulin self-peptide of accelerating a pathogenic T cell response. Having an experimental autoimmune diabetes model (similar to experimental autoimmune encephalitis models) should aid in the discovery of surrogate markers of such pathogenic immune responses. In addition it is of interest that PolyIC or immunization with insulin peptides in the periphery (relative to pancreas) can so easily induce insulitis.
PolyIC is a potent inducer of IFN-α and activator of toll receptors. The effect of PolyIC can depend on the dose and timing of administration. For example, low-dose PolyIC treatment prevented diabetes in the diabetes prone BioBreeding (BB) rat (31), whereas a high dose of polyIC accelerated diabetes (32). PolyIC protected NOD mice (16). The difference between the effect of PolyIC in NOD mice and in our model may be related to the multiple genetic differences between the two strains including the iddm loci of the NOD strain. It is likely that PolyIC contributes to the induction of insulitis through its effects on the innate immune system. PolyIC is often used as a viral RNA mimic. One can hypothesize that a viral infection with or without a peptide mimicking insulin (or other antigens), with the activation of innate immunity could provide the stimulus for autoimmune β cell destruction in a genetically susceptible host.
As illustrated by this study, immune reactivity can proceed to diabetes despite transient administration of the environmental factor. With the ability now to identify children on the basis of HLA typing with more than a 50% risk of activating anti-islet autoimmunity in the first 2 years of life (33), it should be possible to search for such contributing viral infections. At present we do not know whether other peptides of insulin or peptides of other islet antigens in model systems can similarly accelerate or create insulitis/diabetes. A wealth of murine knockout and transgenic strains is available (34), and these strains can now be combined with the above experimentally induced diabetes models to understand disease pathogenesis better.
Acknowledgments
We thank Ning-Yuan Chen for technical assistance. The Histology Core at the University of Colorado Health Sciences Center Diabetes Endocrine Research Center also aided this study. This study was supported by National Institutes of Health Grants DK-55969 and P30-DK-57516, the Juvenile Diabetes Foundation, and the Children's Diabetes Foundation. H.M. and L.W. are supported by the Postdoctoral Fellowship and Career Development Award, respectively, from the Juvenile Diabetes Research Foundation. N.A. was supported by an American Diabetes Association mentor-based fellowship. E.L. is supported by National Institutes of Health Training Grant T32 AI07365. S.W. is a Wellcome Trust Senior Fellow in Clinical Sciences.
Abbreviations
- NOD
nonobese diabetic
- RIP
rat promoter insulin
- TT
tetanus toxin
- IAA
insulin autoantibody
- IFA
incomplete Freund's adjuvant
- PolyIC
polyinosinic-polycytidylic acid
References
- 1.Rose N R, Bona C. Immunol Today. 1993;14:426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 2.Mordes J P, Greiner D L, Rossini A A. In: Diabetes Mellitus: A Fundamental and Clinical Text. LeRoith D, Taylor S I, Olefsky J M, editors. Philadelphia: Lippincott; 1996. pp. 349–360. [Google Scholar]
- 3.Salomon B, Lenschow D J, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone J A. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 4.Bach J F. In: Contemporary Endocrinology: Autoimmune Endocrinopathies. Volpe R, editor. Totowa, NJ: Humana; 1999. pp. 293–307. [Google Scholar]
- 5.Atkinson M A, Eisenbarth G S. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 6.Wucherpfennig K W, Eisenbarth G S. Nat Immunol. 2001;2:1–3. doi: 10.1038/ni0901-767. [DOI] [PubMed] [Google Scholar]
- 7.LeCompte P M, Steinke J, Soeldner J S, Renold A E. Diabetes. 1966;15:586–596. doi: 10.2337/diab.15.8.586. [DOI] [PubMed] [Google Scholar]
- 8.Grodsky G M, Feldman R, Toreson W E, Lee J C. Diabetes. 1966;15:579–585. doi: 10.2337/diab.15.8.579. [DOI] [PubMed] [Google Scholar]
- 9.Elias D, Markowits D, Reshef T, Van der Zee R, Coen I R. Proc Natl Acad Sci USA. 1991;88:3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zekzer D, Wong F S, Ayalon O, Millet I, Altieri M, Shintani S, Solimena M, Sherwin R S. J Clin Invest. 1998;101:68–73. doi: 10.1172/JCI119878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerling I C, Serreze D V, Christianson S W, Leiter E H. Diabetes. 1992;41:1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 12.Heath V L, Hutchings P, Fowell D J, Cooke A, Mason D W. Diabetes. 1999;48:2157–2165. doi: 10.2337/diabetes.48.11.2157. [DOI] [PubMed] [Google Scholar]
- 13.Haskins K, Wegmann D. Diabetes. 1996;45:1299–1305. doi: 10.2337/diab.45.10.1299. [DOI] [PubMed] [Google Scholar]
- 14.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Nature (London) 2000;406:739–742. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 15.Ellerman K E, Like A A. Diabetologia. 2000;43:890–898. doi: 10.1007/s001250051466. [DOI] [PubMed] [Google Scholar]
- 16.Serreze D, Hamaguchi H, Leiter E H. J Autoimmun. 1989;2:759–776. doi: 10.1016/0896-8411(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 17.Wong S, Guerder S, Visintin I, Reich E-P, Swenson K E, Flavell R A, Janeway C A. Diabetes. 1995;44:326–329. doi: 10.2337/diab.44.3.326. [DOI] [PubMed] [Google Scholar]
- 18.Wen L, Wong F S, Tang J, Chen N Y, Altieri M, David C, Flavell R, Sherwin R. J Exp Med. 2000;191:97–104. doi: 10.1084/jem.191.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z J, Davidson L, Eisenbarth G, Weiner H L. Proc Natl Acad Sci USA. 1991;88:10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muir A, Peck A, Clare-Salzler M, Song Y-H, Cornelius J, Luchetta R, Krischer J, Maclaren N. J Clin Invest. 1995;95:628–634. doi: 10.1172/JCI117707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong F S, Karttunen J, Dumont C, Wen L, Visintin I, Pilip I M, Shastri N, Pamer E G, Janeway C A J. Nat Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 22.Wegmann D R, Norbury-Glaser M, Daniel D. Eur J Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 23.Keck K. Nature (London) 1975;254:78–79. doi: 10.1038/254078a0. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal A S. Immunol Rev. 1978;40:135–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee K H, Wucherpfennig K W, Wiley D C. Nat Immunol. 2001;2:501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 26.Daniel D, Wegmann D R. Proc Natl Acad Sci USA. 1996;93:956–960. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abiru N, Wegmann D, Kawasaki E, Gottlieb P, Simone E, Eisenbarth G S. J Autoimmun. 2000;14:231–237. doi: 10.1006/jaut.2000.0369. [DOI] [PubMed] [Google Scholar]
- 28.Abiru N, Maniatis A K, Yu L, Miao D, Moriyama H, Wegmann D, Eisenbarth G S. Diabetes. 2001;50:1274–1281. doi: 10.2337/diabetes.50.6.1274. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Robles D T, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth G S. Proc Natl Acad Sci USA. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong F S, Visintin I, Wen L, Flavell R A, Janeway C A. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobel D O, Goyal D, Ahvazi B, Yoon J W, Chung J H, Bagg A, Harlan D M. J Autoimmun. 1998;11:343–352. doi: 10.1006/jaut.1998.0203. [DOI] [PubMed] [Google Scholar]
- 32.Ewel C H, Sobel D O, Zeligs B J, Bellanti J A. Diabetes. 1992;41:1016–1021. doi: 10.2337/diab.41.8.1016. [DOI] [PubMed] [Google Scholar]
- 33. Robles, D. T., Eisenbarth, G. S., Wang, T., Erlich, H. A., Bugawan, T. L., Babu, S. R., Barriga, K., Norris, J., Hoffman, M., Klingensmith, G., et al. (2002) Clin. Immunol., in press. [DOI] [PubMed]
- 34.Grewal I S, Flavell R A. Lab Invest. 1997;76:3–10. [PubMed] [Google Scholar]