Abstract
Adequate spontaneous activation of tumor-specific T lymphocytes in tumor-bearing hosts is rare, despite the expression of tumor antigens that are potentially highly immunogenic. For example, failure of the immune system to raise competent responses against established tumors expressing the human adenovirus E1A-antigen allows this tumor to grow in immunocompetent mice. We show that systemic in vivo administration of agonistic anti-CD40 antibodies into tumor-bearing mice results in tumor eradication mediated by CD8+ T cells. Treatment resulted in a strong expansion and systemic accumulation of E1A-specific CTL and depended on CD40 expression on host cells, as the tumor was CD40−, and therapy failed in CD40-deficient mice. Local intratumoral administration of anti-CD40 mAb is equally effective in licensing strong, systemic CTL immunity, resulting in the clearance of distant tumor nodules. Our data indicate that the immune response after cancer–host interactions can be directed toward competence, leading to the cure of established tumors merely by delivery of a CD40-dependent “license to kill” signal.
Most solid tumors express MHC class I molecules but lack costimulatory molecules essential for appropriate CTL activation (1, 2). Therefore, presentation of tumor-derived antigens by professional antigen-presenting cells (APCs) is most likely required for optimal tumor-specific T cell induction (3–6). Such activation of naïve T cells is called cross-priming and was first demonstrated by Bevan (7). As naïve T cells are thought to recirculate within the lymphoid system, cross-presentation provides the immune system with a means to detect and respond to antigens that are expressed only in the periphery.
An important factor determining the outcome of immune responses is the level of antigen expressed in the periphery (8). In the case of relatively low levels of antigen, antigen is not presented at sufficient levels to activate naïve T cells. This situation is associated with ignorance of the antigen by the immune system. In the case of higher antigen-expression levels, antigen will be (cross-)presented in sufficient quantities to be detected by naïve T cells. In this case, antigen-recognition can either lead to tolerance or immunity (9, 10). The outcome of antigen recognition by naïve T cells, i.e., tolerance or immunity, is thought to be the consequence of the activation state of professional APCs that (cross-)present the antigen. This activation state is strongly influenced by inflammatory stimuli as well as the action of CD4+ T helper (Th) cells.
Studies on the requirement of CD4+ Th cells in cross-priming of cytotoxic T lymphocytes (CTL) showed that both Th cells and CTLs must recognize antigens presented on the same APC (11, 12). The interaction between Th cell and APC is sufficient to convert the APC to a state that allows priming of antigen-specific CTL (13, 14), which explains the observation that infusion of antigen-specific Th cells can rescue autoreactive CTL from deletion, resulting in CTL-mediated autoimmunity (15).
CD40–CD40L interactions are crucial in the delivery of T cell help for CTL priming. We have shown that vaccination with completely allogeneic tumor cells expressing a human adenovirus type 5 E1-derived model antigen (Ad5E1) results in efficient E1-specific CTL responses and protection against E1-expressing syngeneic tumors (6). CTL priming was shown to depend on CD40–CD40L interactions, as the blockade of CD40L by in vivo administration of an anti-CD40L mAb resulted in a profound inhibition of CTL priming. Similarly, no E1-specific CTLs were induced in mice lacking CD4+ Th cells. In both cases administration of an activating anti-CD40 mAb resulted in efficient restoration of E1-specific immunity, showing that CD40 signaling can replace CD4+ Th cells in priming of T helper-dependent CD8+ CTL responses (16). Together, these findings indicate that the action of T helper cells for priming of tumor-specific CTL is routed through professional APC that (cross-)present antigen by delivery of a CD40-dependent activation signal.
Despite the expression of potentially highly immunogenic tumor antigens, many tumors do not induce tumor-specific immunity. This absence of immune activation is likely caused by the fact that tumors masquerade as healthy tissues (17). Development and growth of tumors is often not accompanied by inflammatory stimuli necessary for initial APC activation, including MHC class II up-regulation, enabling optimal interaction between APC and antigen-specific Th cells. The absence of inflammatory stimuli in the case of tumor growth might lead to presentation of tumor antigens to CTL by APC that have not been alarmed to a state required for CTL priming. As a consequence, APCs draining the site of tumor growth will not be able to prime tumor-specific CTLs, allowing uncontrolled tumor growth.
Because CD40 ligation can overcome the need for CD4+ T cells in the induction of an efficient CTL response (16), we opted to investigate whether established CD40− potentially immunogenic tumors could be treated by CD40 ligation in vivo. Our data show that both systemic and local CD40 treatment leads to expansion and systemic spread of tumor-specific CTL, associated with eradication of E1A-expressing tumors.
Materials and Methods
Mice.
C57BL/6 mice were purchased from Iffa Credo. C57BL/6 Kh (B6, H-2b) mice were bred at TNO-PG (Leiden, The Netherlands). CD40−/− mice were purchased from The Jackson Laboratory. Strain 42 mice are T cell receptor (TCR) transgenic mice expressing the TCRα and β chains derived from the H-2b-restricted, Ad5E1A234–243-specific CTL clone 5 (18, 19).
Tumor Cells.
Mouse embryo cells transformed by the early region 1A of human adenovirus type 5 (Ad5E1A) plus EJ-ras (18, 20) were cultured in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Rockville, MD) supplemented with 8% (vol/vol) FCS, 50 μM 2-mercaptoethanol, glutamine, and penicillin, as described (18).
Tumor Experiments.
E1A-expressing tumor cells (1 × 107) were injected s.c. into 7 to 13-week-old male mice in 200 μl of PBS. Tumor size was measured twice weekly with calipers in three dimensions. Treatment was started 20–30 days after tumor inoculation, when palpable tumors were present. Mice were killed when tumor size exceeded 1 cm3 to avoid unnecessary suffering.
Antibody Treatments.
The FGK-45 hybridoma cells producing a stimulatory anti-CD40 Ab were provided by A. Rolink (Basel Institute for Immunology, Basel, Switzerland) (21). Mice received 100 μg of the anti-CD40 mAb given either i.v. (day 0, 1, and 2 of treatment) in 200 μl of PBS or intratumorally, s.c. or i.m. (day 0 and 3 of treatment) in 40 μl of PBS. As a control, mAb mice received 100 μg of rat-IgG specific for human CD40 (6E9; ref. 22) in the same volume of PBS. Depletion of CD4+, CD8+, and natural killer (NK) cells was started at the same day as the anti-CD40 treatment. Depleting antibodies [GK1.5 (23), 2.43 (24) and PK136 (25), respectively] were injected i.p. at day 0, 2, and 4 of treatment and then twice weekly (100 μg in 200 μl of PBS per injection) until the end of the experiment. Ten days after the start of depletion, depletion of the T cell subsets was confirmed by FACS-analysis by using the RM4–4 and 53–6.7 antibodies (PharMingen), which do not compete with the depleting antibodies for binding to CD4 and CD8, respectively. Depletion of NK1.1 cells was determined by cytotoxicity against NK-sensitive YAC-1 target cells in a standard 5 h 51Cr-release assay.
Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) Labeling and Adoptive Transfer.
Single-cell suspensions were made from spleen and peripheral lymph nodes from TCR-transgenic mice expressing a T cell receptor recognizing the Ad5E1A-peptide (strain 42 mice). Erythrocytes were depleted by ammonium chloride treatment (2 min on ice). Cells were washed once in cold medium and once in cold PBS, after which they were resuspended in PBS at 1 × 107 cells per ml and incubated with 0.5 μM CFSE (Molecular Probes) for 30 min at 37°C. FCS was added to a concentration of 5% FCS, and the cells were washed in PBS. TCR-transgenic CD8+ T cells (4 × 106) were injected in the tail veins of tumor-bearing mice in 200 μl of PBS.
Flow Cytometry.
Tumors were removed, cut into small pieces, and treated with collagenase (400 units/ml) for 15 min at 37°C. Living cells were subsequently isolated by performing a Ficoll-gradient. Single-cell suspensions of spleen and lymph nodes were prepared by mechanical disruption. Blood samples were depleted of erythrocytes by ammonium chloride treatment for 5 min at room temperature.
Cells were stained with directly allophycocyanin-conjugated monoclonal antibody against CD8 (PharMingen) and phycoerythrin-conjugated E1A234–243-loaded H-2 Db tetramers. Data acquisition and analysis was done on a Becton Dickinson FACScan with CELLQUEST software.
Statistical Analysis.
A log-rank test was used to determine the significance of the differences in survival between groups.
Results
E1A Peptide Is Naturally Presented in Tumor-Draining Lymph Node.
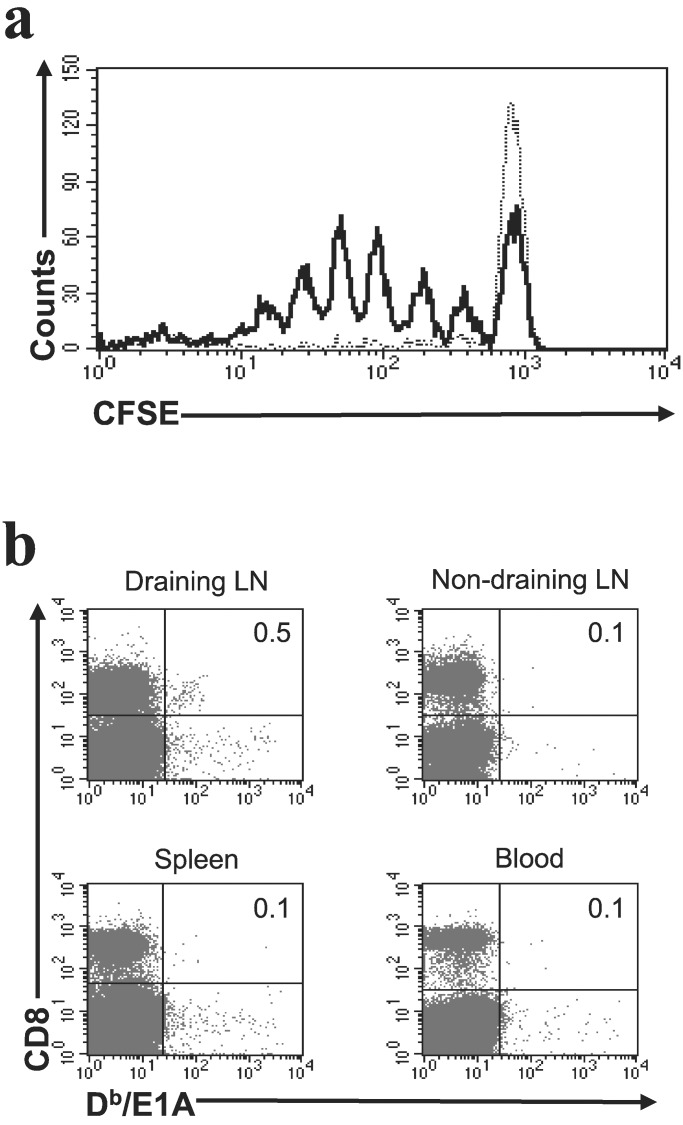
Ad5E1A-transformed tumor cells derived from C57BL/6 Kh mice harbor an E1A-encoded CTL epitope, which is presented in the context of H-2Db. Despite the presence of this highly antigenic CTL epitope (19), E1A-transformed tumor cells readily form progressively growing tumors when injected s.c. This finding prompted us to investigate whether the E1A-peptide is presented to the immune system in vivo. Therefore, we injected tumor-bearing mice with CFSE-labeled spleen cells derived from TCR-transgenic mice expressing a TCR specific for the E1A-peptide (strain 42 mice). Three days later, lymph nodes and spleen were taken, and cell division of CFSE-labeled cells was monitored. As shown in Fig. 1a, cell division was observed only in the tumor-draining lymph node, whereas no division of the transgenic T cells was observed in the spleen or nondraining lymph node. These data indicate that the E1A-tumor antigen is presented in the lymph node that drains the site of tumor growth.
Figure 1.
(a) The presence of the E1A-derived CTL epitope is detected in the tumor-draining lymph node only. CFSE-labeled E1A-specific transgenic T cells (4 × 106) were injected i.v. into mice bearing an Ad5E1A-expressing tumor. Three days later, the spleen, tumor-draining lymph node, and a nondraining lymph node were taken and analyzed for division of E1A-specific CTL by FACS. The results obtained from tumor-draining lymph node (solid black line) and spleen (dotted line) are shown and are representative of 12 mice. Results were comparable for anti-CD40-treated and -untreated mice. (b) Endogenous E1A-specific CTL can be detected in tumor-draining lymph nodes. Mice bearing an Ad5E1A-expressing tumor were killed 35 days after tumor inoculation. FACS analysis was performed on blood, spleen, and lymph nodes. In over 30% of mice (n = 23), E1A-specific CTL were detected in tumor-draining lymph nodes only, whereas no E1A-specific CTL were observed in the other animals of this group (not shown). Numbers in the upper right quadrants represent the percentage of Db/E1A tetramer-positive cells of the total pool of CD8+ cells.
Endogenous E1A-Specific CTL Can Be Detected in Tumor-Draining Lymph Nodes.
The data presented above show that naive E1A-specific TCR-transgenic CTLs are activated in lymph nodes draining the site of tumor growth but do not address the question whether endogenous CTLs are activated as well. As CTL-mediated immunity directed against E1A is dominated by CTL specific for the E1A-derived CTL epitope SGPSNTPPEI (19), we analyzed CTL immunity against this epitope by using E1A/Db tetramers. In ≈30% of animals, E1A-specific CTL were detectable in tumor-draining lymph nodes (Fig. 1b), whereas no E1A-specific CTLs could be observed in the remaining 70% of tumor-bearing animals (not shown). Together, these date indicate that the E1A-epitope is presented at a sufficient level for detection by E1A-specific CTL. Nonetheless, no effective CTL response is present, as no E1A-specific CTL are found in the majority of tumor-bearing animals 35 days after tumor inoculation. In the animals that harbor tumor-specific CTL, tumor growth is still progressive, reflecting the situation found in, for example, melanoma patients in which melanoma-specific CTLs are present.
Ad5E1A-Expressing Tumors Are Eradicated by Systemic CD40 Activation.
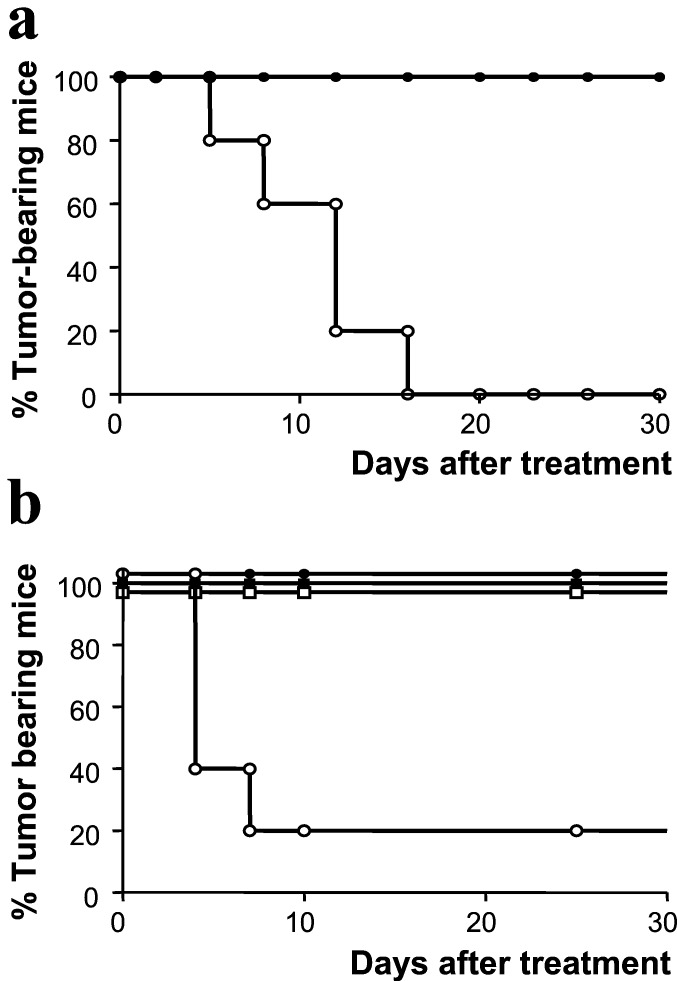
The data described above indicate that the presentation of the E1A-epitope is apparently not sufficient to induce an efficient immune response capable of mediating tumor rejection. As CD40–CD40L interactions have been shown to be crucial for the induction of antitumor immunity, we wished to study whether systemic CD40 triggering could mount an immune response capable of eradicating established tumors. Therefore, we injected E1A-transformed tumor cells into naïve C57BL/6 mice. Twenty days later, at a time when all mice had developed palpable tumors, these mice were treated by i.v. injection of an agonistic anti-CD40 antibody. As depicted in Fig. 2a, this treatment resulted in the disappearance of the tumors, whereas in control-treated mice, the tumors grew progressively, despite the presence of small numbers of CD8+ CTL reactive with the E1A-tumor antigen in at least a proportion of the animals (Fig. 1b). Disappearance of the tumors after treatment with the anti-CD40 antibody was not caused by a direct effect of the antibody on the tumor cells, as these were CD40− (data not shown). Furthermore, anti-CD40 treatment was not effective in CD40 knockout mice (Fig. 2b), indicating that CD40 expression on host cells was required for successful treatment.
Figure 2.
(a) CD40 ligation in vivo leads to the elimination of established tumors. Mice were injected s.c. with Ad5E1A-expressing tumor cells. At the time that mice had developed palpable tumors, mice were injected i.v. with a CD40-activating mAb (○) or with a control mAb (●). (b) Anti-CD40 ligation in vivo does not have an antitumor effect in CD40-deficient mice. C57BL/6 mice (●) and BL6/CD40−/− mice (□) were injected s.c. with Ad5E1A-expressing tumor cells. At the time that mice had developed palpable tumors, mice were injected i.v. with a CD40-activating mAb (open symbols) or control mAb (closed symbols).
CD8+ T Cells Are Important for Tumor Eradication.
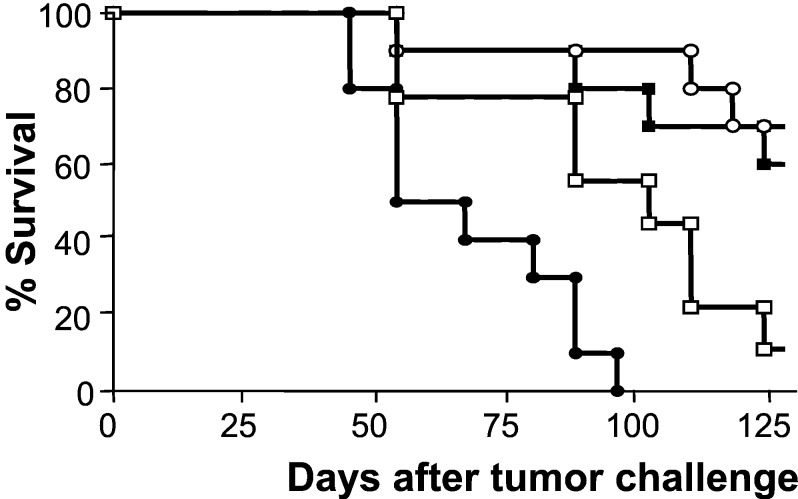
To determine which cells are responsible for tumor clearance after CD40 ligation, we investigated the role of NK cells, CD4+ T cells, and CD8+ T cells in tumor eradication. The respective role of each of these cell types was determined by depletion of the specific cell type in tumor-bearing mice at the time anti-CD40 treatment was started. As depicted in Fig. 3, mice depleted of CD4+ T cells were still able to resolve the tumor. Also, depletion of NK cells did not result in less efficient tumor eradication (not shown). In contrast, anti-CD40-treated mice depleted of CD8+ T cells lost the ability to eradicate the s.c. growing tumor.
Figure 3.
CD8+ T cells but not CD4+ T cells are crucial for tumor eradication after in vivo CD40 ligation. Mice were injected with Ad5E1A-expressing tumor cells. At the time that mice had developed palpable tumors, treatment was started. Mice received no treatment (□), i.v. anti-CD40 mAb-treatment (○), or a combination of anti-CD40 mAb with a CD8-depleting (●) or a CD4-depleting Ab (■). To avoid unnecessary suffering, mice were killed when the size of the tumors exceeded 1 cm3. Untreated vs. anti-CD40, P < 0.01; anti-CD40 vs. anti-CD40 + CD4-depletion, P = 0.75; anti-CD40 vs. anti-CD40 + CD8-depletion, P ≪ 0.01 (log-rank test).
These results indicate that CD8+ T cells play a crucial role in eradication of these tumors after CD40 ligation in vivo.
Tumor-Specific CTL Immunity Is Enhanced after CD40 Activation.
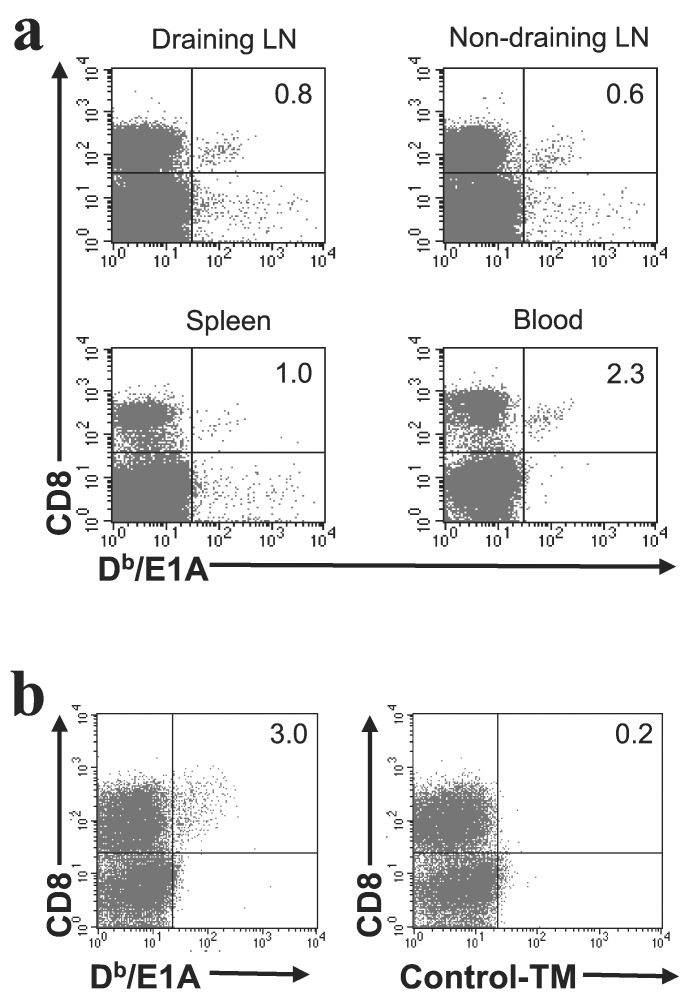
The observation that CD8+ CTL are important for tumor clearance prompted us to investigate whether tumor-specific CTL immunity could be visualized after CD40 injection. For this purpose, tumor-bearing mice treated i.v. with anti-CD40 mAb were killed 10 days later to perform FACS analysis on cell populations derived from blood, spleen, and lymph nodes.
As outlined above, no E1A-specific CTLs were detected in 70% of untreated animals, whereas E1A-specific CTLs were detectable in the lymph nodes that drained the site of tumor growth in the other 30% of tumor-bearing mice. E1A-specific CTLs were not observed in blood, spleen, or nondraining lymph nodes of any of the untreated mice (Fig. 1b). Anti-CD40 treatment resulted in a dramatic increase in E1A-specific CTL in both the tumor-draining lymph node as well as in nondraining lymph nodes, spleen, and blood (Fig. 4a). Moreover, a further enrichment of tumor-specific CTL in anti-CD40 treated animals was observed in tumor tissue (Fig. 4b).
Figure 4.
In vivo CD40 activation leads to systemic spread of tumor-specific CTL. Mice were injected with Ad5E1A-expressing tumor cells. At the time that mice had developed palpable tumors, they were treated intravenously with anti-CD40 mAb. Ten days after anti-CD40 injection, mice were killed. (a) FACS analysis was performed on blood, spleen, and lymph nodes. In all mice, E1A-specific CTL were readily detectable (n = 29). (b) Tumor infiltration of E1A-specific CTL was determined by FACS analysis. (a and b) Numbers in the upper right corner represent percentage of Db/E1A tetramer-positive cells of the total pool of CD8+ cells.
Together, these findings show that CD40 activation leads to a strong expansion, systemic spread, as well as enrichment of tumor-specific CTL infiltrating the tumor, emphasizing the potent effects of CD40 treatment on the tumor-specific CTL response.
Local Injection of Anti-CD40 Antibodies Leads to Systemic Antitumor Immunity.
As described above, i.v. injection of an anti-CD40 antibody induces an adequate antitumor immune response. However, upon further experimentation, we found that systemic injection of the anti-CD40 antibody, but not a control antibody, two weeks after start of treatment resulted in severe side effects. All mice receiving the anti-CD40 mAb two weeks after the first injection developed a shock syndrome that was fatal in 50% of cases. These side effects were not observed when the antibody was given s.c. or intratumorally (data not shown). These observations led us to investigate whether such local treatment also leads to therapeutic immunity. Therefore, groups of mice bearing E1A-expressing tumors in both flanks or in the right flank only were injected with agonistic anti-CD40 mAb or a control antibody into one tumor site only.
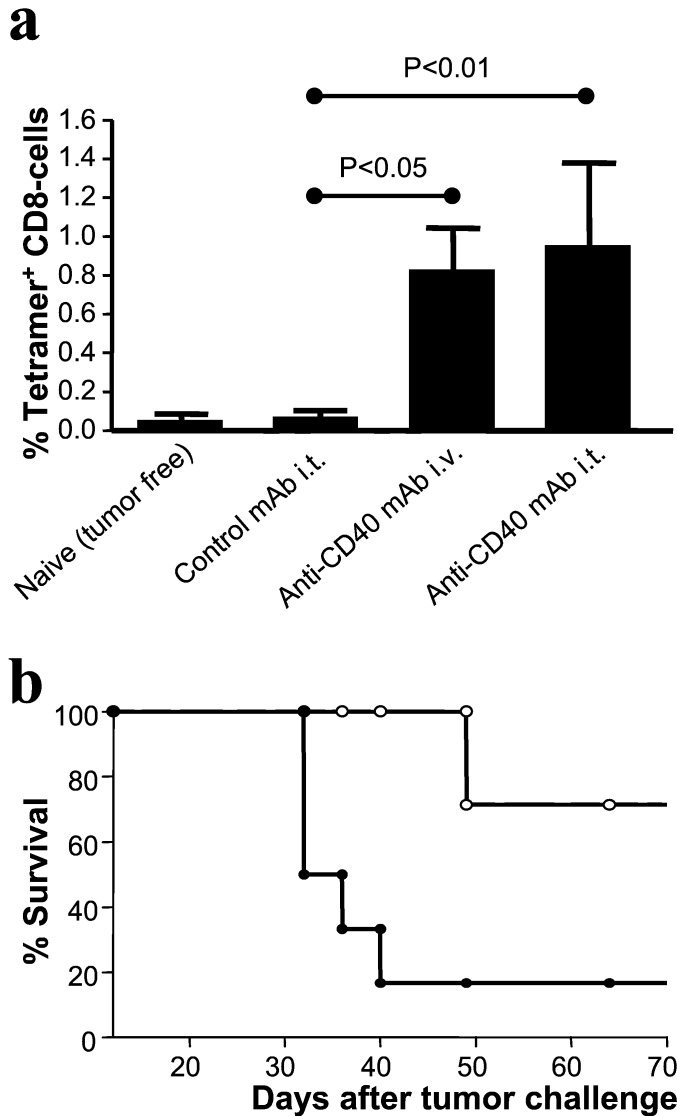
In Fig. 5a, it is shown that the intratumoral injection of anti-CD40 mAb leads to the appearance of E1A-specific CTL in peripheral blood, comparable to the number in i.v.-treated animals. More importantly, mice receiving anti-CD40 mAb were able to clear the E1A-expressing tumor in both the treated as well as the untreated flank (Fig. 5b).
Figure 5.
Local CD40 activation causes systemic antitumor immunity. Mice were injected with Ad5E1A-expressing tumor cells in both flanks. At the time that palpable tumors had developed in both flanks, anti-CD40 or control mAb was given intratumorally in the right tumor. (a) Ten days after treatment, blood samples were taken and analyzed by FACS to determine the presence of E1A-specific CTL. Bars represent the number of E1A-specific CD8+ T cells as a percentage of the total pool of CD8+ cells (means + SEM, n = 7). (b) Survival curve of anti-CD40-treated (○) or control-treated (●) animals. To avoid unnecessary suffering, mice were killed when the size of one of the tumors exceeded 1 cm3. Log-rank test, P < 0,05.
Likewise, local s.c. as well as i.m. injection of the anti-CD40 mAb resulted in systemic CTL induction and tumor clearance (data not shown).
Thus, local treatment of primary established tumors with CD40-activating agents can lead to the induction of systemic antitumor immunity and rejection of tumors at distant sites. Furthermore, mice that had rejected E1A-expressing tumors after anti-CD40 treatment were capable of rejecting a subsequent tumor challenge for at least 80 days after treatment (data not shown).
Discussion
To develop immunological strategies for the eradication of tumors, in-depth understanding of the ways in which such therapies operate is clearly required. In this study, we show that CD40 ligation in vivo can lead to the clearance of established CD40− tumors. Tumor eradication was strongly associated with the emergence of tumor-specific CTL in peripheral blood, spleen, as well as tumor and depended on CD40 expression by host cells.
Recently, it has been reported that CD40 stimulation has an adverse effect on CTL immunity in tumor-bearing mice (26). Although the reasons for this observation are not known, it could be that the timing of anti-CD40 treatment is an important parameter influencing the outcome of treatment. CD40 ligation leads to maturation of APC, which is associated with a reduced ability for antigen uptake and presentation. Therefore, CD40 ligation relatively shortly after tumor inoculation could lead to the maturation of APCs that have not yet acquired sufficient antigen to prime CTL.
Systemic anti-CD40 triggering has been used successfully in the treatment of mice with B cell lymphomas (27) or mice bearing lung metastases after infusion of tumor-specific TCR-transgenic CD4+ T cells (28). In these cases, the mode of action was not extensively studied, and it was not clear, for example, whether direct effects of the anti-CD40 mAb on the CD40+ tumors contributed to the treatment. CD40 ligation of B cell leukemias results in B cell activation and up-regulation of costimulatory molecules, which could, in turn, enhance T cell activity. CD40 ligation also can result in direct growth inhibition (29) and apoptosis (30) of CD40+ tumor cells, which would affect tumor growth and survival. Likewise, induction of tumor cell death may enhance (re)presentation of tumor antigens, resulting in pronounced T cell activation. We consider it unlikely that these possibilities contribute to our findings. We have shown that CD40 ligation in vivo can mediate strong antitumor effects when established CD40− tumors are the subject of treatment. NK cells do not seem to play a crucial role, because anti-CD40 therapy was still effective in mice depleted for NK cells. CD8+ T cells, but not CD4+ T cells, are crucially involved in tumor eradication. The induction of these CD8+ T cells depended on CD40 expression on host cells, as therapy failed in CD40 knockout mice. This observation, together with the finding that the E1A-peptide is readily presented to tumor-specific CTL in tumor-draining lymph nodes (Fig. 1a) and the fact that murine CD8+ T cells are CD40−, indicates that CD40+ cells, capable of antigen presentation to naïve T cells in the T cell areas of (draining) lymph nodes, are the major target of agonistic anti-CD40. In a model involving a tumor cell vaccine consisting of completely allogeneic E1-expressing tumor cells, we have shown (16) that B cells are not required for CTL priming. Because cells belonging to the dendritic cell (DC) lineage are CD40+ and are able to present antigen to naïve T cells but are only qualified to prime CTL responses after receiving a proper activation stimulus (13, 14), we consider it likely that the cellular target of the anti-CD40 mAb is a DC. CD40 triggering of DC not only results in their activation required for proper CTL priming, but also leads to their migration from the periphery to draining lymph nodes (31). In this way, anti-CD40 treatment may not only contribute to CTL induction by activation of DC but also by enhancing the number of antigen-presenting DC in tumor-draining lymph nodes.
The demonstration that the primary target of the anti-CD40 mAb is of host origin is consistent with several reports demonstrating the importance of host APC in shaping the antitumor immune response (4, 6, 16, 28, 32, 33) but are seemingly in disagreement with a recent study (34) showing that induction of tumor-specific CTL depends on sufficient tumor cells reaching secondary lymphatic organs. In this study, it was shown that direct intrasplenic injection of cells expressing a strong viral CTL epitope led to the induction of CTL in the apparent absence of cross-presentation of antigen. Clearly, the circumstances under which direct or indirect priming of CTL predominates require further investigation, but it would be interesting to study whether CD40 ligation also would enhance CTL immunity in case tumor cells are injected directly into lymphoid organs.
CTL reactive to E1A/Db-tetramers could be detected in approximately 30% of tumor-bearing mice. Nonetheless, this CTL response is clearly not able to mediate tumor-eradication, as only a few mice (less than 5%) spontaneously rejected their tumors. Apparently, this CTL response does not fully develop, as evidenced from the fact that CTLs are only detectable in the draining lymph node and are not present in sufficient amounts outside these nodes to destroy the tumor target tissue. This observation is an interesting one, especially in light of the recent findings that a brief encounter with antigen is sufficient for naïve CD8+ T cells to initiate a program that will carry them through multiple cycles, allowing development of cytotoxic effector function (35). Apparently, antigen recognition in vivo as such is not sufficient to induce effective antitumor CTL responses, as CD40 signaling dramatically enhanced CTL responses and migration. It is tempting to speculate that the initial encounter with antigen can lead to different programs, both beginning with cell division but ultimately ending in a different outcome, depending on the activation status of APC. CD40 activation changes the molecular make-up of APC dramatically, not only leading to expression of the full costimulatory potential but also to the expression of other molecules implicated in T cell activation, such as 4–1BB ligand, which is thought to be important for CTL expansion and survival (36–39). Thus, our results indicate that tumor-specific CTL can clonally expand after antigen recognition in tumor-bearing hosts, but that additional signals such as those provided by CD40 ligation of professional APCs are clearly required for the induction of an effective CTL response.
Our study demonstrates that local injection of anti-CD40 can result in a strong systemic antitumor effect, because intratumoral injection into a single tumor also resulted in the eradication of other, distant tumors in the absence of specific vaccination. The fact that local injection of the mAb also is capable of eliciting a systemic antitumor immune response (Fig. 5a) is important, because we found that a subsequent i.v. injection of the mAb two weeks after the first i.v. injections led to a shock-like syndrome in all mice, resulting in 50% mortality, probably by induction of a cytokine burst of CD40+ cells. This toxic effect was not observed after multiple intratumoral injections of anti-CD40 mAb. Likewise, local s.c. or i.m. injections resulted in a systemic tumor-specific CTL response (data not shown). Together, these findings indicate that local anti-CD40 treatment is to be preferred over systemic injection.
The finding that tumor-bearing animals can be cured by local injection of a CD40 mAb provides a rationale to apply CD40-stimulating agents in a clinical setting without the need of insight in the tumor antigens that are involved. Employment of CD40-stimulating agents seems particularly attractive in an adjuvant setting because intratumoral injection can lead to systemic immunity capable of eradicating (micro)metastases. For example, colon carcinoma, melanoma, and cervical carcinoma are known to harbor several tumor antigens. In a setting in which CD40-stimulating agents are injected 1 week before surgery, the immune system could be (re)activated, allowing early and optimal detection of potential metastases. Also, the induction of CD8+ tumor-specific CTL migration from local tumor-draining lymph nodes by anti-CD40 treatment would allow surgical removal of such nodes without the dangerous side effect of removing the main, if not only, source of tumoricidal CTL precursors. In addition, the formation of an effective antitumor response in case of a new tumor challenge after rejection of a first tumor, indicates that immunologic memory has developed by the treatment with the anti-CD40 mAb. This development might prevent relapse of tumors also in the clinical setting.
Acknowledgments
We thank Michel Mulders for biotechnical help. This work was supported by European Community Grant QZK 3-T-1999-00064 and by Dutch Cancer Foundation Grants RUL 99-2025 and RUL 97-1450. The research of R.E.M.T. has been made possible by a fellowship from the Royal Academy of Arts and Sciences.
Abbreviations
- APC
antigen-presenting cell
- Th cell
helper T cell
- CTL
cytotoxic T lymphocyte
- TCR
T cell receptor
- Ad5E1
E1-region of adenovirus type 5
- NK cell
natural killer cell
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cell
References
- 1.Allison J P. Curr Opin Immunol. 1994;6:414–419. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 2.Townsend S E, Allison J P. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 3.Seung S, Urban J L, Schreiber H. J Exp Med. 1993;178:933–940. doi: 10.1084/jem.178.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 5.Wu T C, Huang A Y, Jaffee E M, Levitsky H I, Pardoll D M. J Exp Med. 1995;182:1415–1421. doi: 10.1084/jem.182.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toes R E, Blom R J, van der Voort E, Offringa R, Melief C J, Kast W M. Cancer Res. 1996;56:3782–3787. [PubMed] [Google Scholar]
- 7.Bevan M J. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurts C, Sutherland R M, Davey G, Li M, Lew A M, Blanas E, Carbone F R, Miller J F, Heath W R. Proc Natl Acad Sci USA. 1999;96:12703–12707. doi: 10.1073/pnas.96.22.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurts C, Heath W R, Carbone F R, Allison J, Miller J F, Kosaka H. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurts C, Kosaka H, Carbone F R, Miller J F, Heath W R. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett S R, Carbone F R, Karamalis F, Miller J F, Heath W R. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husmann L A, Bevan M J. Ann NY Acad Sci. 1988;532:158–169. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- 13.Schuurhuis D H, Laban S, Toes R E, Ricciardi-Castagnoli P, Kleijmeer M J, van der Voort E I, Rea D, Offringa R, Geuze H J, Melief C J, Ossendorp F. J Exp Med. 2000;192:145–150. doi: 10.1084/jem.192.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridge J P, Di Rosa F, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 15.Kurts C, Carbone F R, Barnden M, Blanas E, Allison J, Heath W R, Miller J F. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 17.Melief C J, Toes R E, Medema J P, van der Burg S H, Ossendorp F, Offringa R. Adv Immunol. 2000;75:235–282. doi: 10.1016/s0065-2776(00)75006-1. [DOI] [PubMed] [Google Scholar]
- 18.Toes R E, Offringa R, Blom R J, Melief C J, Kast W M. Proc Natl Acad Sci USA. 1996;93:7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kast W M, Offringa R, Peters P J, Voordouw A C, Meloen R H, van der Eb A J, Melief C J. Cell. 1989;59:603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 20.Medema J P, de Jong J, van Hall T, Melief C J, Offringa R. J Exp Med. 1999;190:1033–1038. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolink A, Melchers F, Andersson J. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 22.Mittler R S, Bailey T S, Klussman K, Trailsmith M D, Hoffmann M K. J Exp Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 24.Sarmiento M, Glasebrook A L, Fitch F W. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 25.Koo G C, Dumont F J, Tutt M, Hackett J, Jr, Kumar V. J Immunol. 1986;137:3742–3747. [PubMed] [Google Scholar]
- 26.Kedl R M, Jordan M, Potter T, Kappler J, Marrack P, Dow S. Proc Natl Acad Sci USA. 2001;98:10811–10816. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.French R R, Chan H T, Tutt A L, Glennie M J. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 28.Sotomayor E M, Borrello I, Tubb E, Rattis F M, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky H I. Nat Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 29.Hirano A, Longo D L, Taub D D, Ferris D K, Young L S, Eliopoulos A G, Agathanggelou A, Cullen N, Macartney J, Fanslow W C, Murphy W J. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- 30.von Leoprechting A, van der Bruggen P, Pahl H L, Aruffo A, Simon J C. Cancer Res. 1999;59:1287–1294. [PubMed] [Google Scholar]
- 31.Moodycliffe A M, Shreedhar V, Ullrich S E, Walterscheid J, Bucana C, Kripke M L, Flores-Romo L. J Exp Med. 2000;191:2011–2020. doi: 10.1084/jem.191.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Nature (London) 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 33.Sotomayor E M, Borrello I, Rattis F M, Cuenca A G, Abrams J, Staveley-O'Carroll K, Levitsky H I. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 34.Ochsenbein A F, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel R M. Nature (London) 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 35.van Stipdonk M J, Lemmens E E, Schoenberger S P. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi C, Mittler R S, Vella A T. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 37.Cannons J L, Lau P, Ghumman B, DeBenedette M A, Yagita H, Okumura K, Watts T H. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 38.Shuford W W, Klussman K, Tritchler D D, Loo D T, Chalupny J, Siadak A W, Brown T J, Emswiler J, Raecho H, Larsen C P, et al. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurtado J C, Kim Y J, Kwon B S. J Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]