Graphical abstract

Keywords: Stem Cells, Microphysiological Systems, Organoids, Neural Organoids, Neurotoxicity
Highlights
-
•
Organoids, comprised of organ-specific precursor cells, can address the limitations of the traditional cell culture models.
-
•
Neural organoids assembled through a variety of techniques can potentially provide robust tools for neurotoxicity modeling.
-
•
Neural organoids have been used to study neurotoxicity, and have shown ability to predict some mechanisms of neurotoxicity.
Abstract
Neurotoxicity studies often depend on traditional cell-based monoculture assays and animal models, which have a number of limitations. In particular, the existing models either lack the appropriate physiological context, do not include a functional blood–brain barrier, or include cells from non-human species. The recent emergence of organoids derived from human pluripotent stem cells has provided new opportunities to understand disease etiology, discover drugs, evaluate efficacy, and evaluate toxicity in physiologically relevant contexts. Organoids comprised of organ specific precursor cells can potentially overcome the limitations of the traditional 2D cell culture and animal models in neurotoxicology studies. Here, we provide a review of recent developments of human neural organoids, and summarize their applications in neurotoxicity testing. We also discuss their several limitations, and provide a future perspective on their widespread use in neurotoxicity studies.
1. Introduction
Neurotoxicology studies evaluate the effects of exposure to any physical, chemical, and biological toxic substances on the peripheral and central nervous systems (Van Thriel, 2019, Vonberg and Blain, 2024). Neurotoxicants may cause structural and functional changes in the nervous system, leading to neurological disorders or neurodegenerative diseases (Roberts et al., 2015, Grandjean and Landrigan, 2014, Babadjouni et al., 2017). Therefore, neurotoxicity studies have become an indispensable tool in the research and development of any therapeutics intended to influence the nervous system (Crofton et al., 2022, Lopez-Suarez et al., 2022, Walker et al., 2018, Waring et al., 2015). Traditionally neurotoxicity testing of physical, chemical, and biological agents has relied on 2D cell culture assays and animal models (Forsby et al., 2009, Slotkin et al., 2006). 2D cell culture studies are limited, as they lack proper physiological context that would be present in vivo. Animal models that have been used as human surrogates have limitations that include high cost, limited throughput, and potential for interspecies differences that limit extrapolation of the results from the animal models to humans. In addition, there are many examples of human clinical trials in which animal studies did not predict acute human toxicity (Perel et al., 2007, Hackam and Redelmeier, 2006). BIA-102474-101, a drug candidate for treating medical conditions from Parkinson disease was given to five human volunteers during its first-into-human clinical trials at doses of 1/500th of the safe dose for dog studies. It caused brain hemorrhage and necrosis in all five volunteers including the death of one participant (Eddleston et al., 2016). TGN1412, a therapeutic antibody developed to treat human autoimmune disease was administered to six human volunteers at 1/500th of the dose used in animal studies during its phase I trial. It caused critical illness within minutes to all six human volunteers with long term complications (Attarwala, 2010, Suntharalingam et al., 2006). Fialuridine was developed to treat hepatitis B virus infection, but it caused death of 5 out of 15 participants from liver complications during its phase II clinical trials even after treating in a safe dose that was hundreds of times lower than that of the animal studies (McKenzie et al., 1995). These limitations have led to the development of complex, cell-based assay platforms, including human organoids (Gupta et al., 2016, Dolmetsch and Geschwind, 2011, Chen et al., 2023, Sirenko et al., 2019, Sun et al., 2021, Hou et al., 2013).
Organoids are self-assembled three-dimensional in vitro tissue constructs capable of showing real organ-like characteristics. Organoids are typically derived from stem cells in the presence of defined growth factors specific to the particular organ and it can provide more insights of developmental and disease biology (Mansour et al., 2018, Kelley and Pasca, 2021). Neural organoids derived from human embryonic stem cells (hESCs) or human induced pluripotent stem cells (iPSCs) provide a promising strategy for modeling neurological disorders and diseases (Li et al., 2024, Zheng et al., 2021, Zhang et al., 2021, Renner et al., 2020, Bergmann et al., 2018, Costamagna et al., 2021, Pas ¸ca et al., 2015, Lancaster et al., 2013). By using hESCs and iPSCs as a starting point, human neural organoids can achieve a diverse cell phenotype composition, as well as a tissue structure and function that is closer to human neural tissue when compared to conventional 2D cell cultures. Therefore, the toxicological data obtained in the human neural organoid models are likely more informative, and may be more directly relevant to human neurotoxicity (Augustyniak et al., 2019, Lee et al., 2017). Thus, human neural organoid models have received significant attention in recent years for neural toxicity research (Chhibber et al., 2020, Hogberg and Smirnova, 2022). In this review, we describe some recent developments of neural organoids, including protocols used to generate organoids and their use in evaluating neurotoxicity of various compounds. Finally, we discuss limitations of the current neural organoid models and their future perspective in neurotoxicity testing.
2. Advances in organoid fabrication techniques
Pluripotent stem cells are widely used to construct human tissue models, including organoids. Although a wide variety of techniques have been developed to form human organoids, here we generally categorize the approaches as “self-assembly”, “guided assembly”, or “bioprinting”. In self-assembly, pluripotent stem cells are placed in a supportive environment and encouraged to undergo multi-lineage differentiation over an extended timeframe. The culture conditions are defined to mimic some aspects of early human tissue development. In guided assembly, pluripotent stem cell-derived progenitor cells are intentionally placed in an environment that supports their assembly into a tissue with desired features, such as geometry, structure, and phenotypic diversity. In bioprinting, distinct cell types are placed adjacent to other cell types with a degree of spatial control at time zero, followed by dynamic changes in the structure and function of the tissues over time in culture. The following subsections discuss specific, illustrative examples of each of these organoid fabrication techniques (Table 1).
Table 1.
List of the commonly used organoid fabrication techniques for generating brain organoids.
| Sl. No. | References | Type of organoid fabrication techniques | Type of 3D matrices |
|---|---|---|---|
| 1 | Cerebral organoids model human brain development and microcephaly. Lancaster et al. 2013 (Lancaster et al., 2013) | Self-Assembly of Neural Organoids | Matrigel® |
| 2 | Organogenesis in a dish: Modeling development and disease using organoid technologies. Lancaster et al. 2014 (Lancaster and Knoblich, 2014) | Self-Assembly of Neural Organoids | Matrigel® |
| 3 | Generation of cerebral organoids from human pluripotent stem cells. Lancaster et al. 2014 (Lancaster and Knoblich, 2014) | Self-Assembly of Neural Organoids | Matrigel® |
| 4 | Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Kadoshima et al. 2013 (Kadoshima et al., 2013) | Self-Assembly of Neural Organoids | Matrigel® |
| Engineering induction of singular neural rosette emergence within hPSC-derived tissues. Knight et al. 2018 (Knight et al., 2018) | Guided Assembly of Neural Organoids | PEG Substrate | |
| 6 | Self-organization of human embryonic stem cells on micropatterns. Deglincerti et al. 2016 (Deglincerti et al., 2016) | Guided Assembly of Neural Organoids | PEG Substrate |
| 7 | Human neural tube morphogenesis in vitro by geometric constraints. Karzbrun et al. 2021 (Karzbrun et al., 2021) | Guided Assembly of Neural Organoids | Matrigel® |
| 8 | Human induced pluripotent stem cell-derived planar neural organoids assembled on synthetic hydrogels. Majumdet et al. 2024 (Majumder et al., 2024) | Guided Assembly of Neural Organoids | PEG Substrate |
| 9 | 3D bioprinting of human neural tissues with functional connectivity. Yan et al. 2024 (Yan et al., 2024) | Bio-printing of Neural Organoids | Matrigel® |
| 10 | A 3D bioprinted cortical organoid platform for modeling human brain development. Cadena et al. 2024 (Cadena et al., 2024) | Bio-printing of Neural Organoids | Matrigel® |
2.1. Self-assembly of neural organoids
Matrigel, a natural basement-membrane hydrogel produced by mouse sarcoma cells, has been widely used as an extracellular matrix for the culture and assembly of neural organoids. Using Matrigel®, Lancaster et al. established a suspension protocol for generating cerebral organoids with defined brain regions. In this suspension protocol, first embryoid bodies (EBs) were generated on day 0 from human pluripotent stem cells in a 96-well U-bottom plate. Next, the EBs were transferred to a 24-well plate on day 6 and cultured in neural induction medium to form neuroectodermal tissues, which were then transferred in droplets of Matrigel® on day 11 and grown in a cell culture dish. Finally, Matrigel® droplets were transferred to a spinning bioreactor on day 15, and culture and expansion were carried out for 3–4 weeks to form the cerebral organoids (Fig. 1A) (Lancaster et al., 2013, Lancaster and Knoblich, 2014). In another study, Lancaster et al. developed a simplified protocol that could be implemented in a standard tissue culture room to generate cerebral cortex within 2 months. This protocol has been applied to study a variety of neural disorders and diseases (Lancaster and Knoblich, 2014). Kadoshima et al. developed hESC-derived cortical neuroepithelium, which self-assembled into a multilayered structure comprised of both the neuronal zones and progenitor zones as observed in the human fetal cortex (Guo et al., 2024). The authors observed robust growth of 350 μm thick hESC-derived cortical neuroepithelium in suspension culture, and probed the self-organization process during human neo-corticogenesis. Their culture conditions allowed for growth of the cortical neuroepithelium beyond 13 weeks, as well as clear separation of cortical zones (Kadoshima et al., 2013).
Fig. 1.
Three distinct approaches for developing neural organoids. (A) Schematic showing culture and expansion of neural organoids based on self-assembly of stem cells in Matrigel® suspension. In this method, hESCs or iPSCs are embedded in Matrigel® in the form of cell aggregates, which are cultured in neural induction medium to undergo neural tissue development; (Lancaster et al., 2013, Lancaster and Knoblich, 2014) (B) Schematic diagram showing culture and expansion of neural organoids based on guided assembly of stem cell-derived precursor cells on biomaterials. In this method, human iPSC-derived progenitors are seeded onto a cell-degradable biomaterial surface, often in defined geometries, for the formation of neural or neuro-vascular tissues. After varying time periods, additional iPSC-derived progenitors (e.g. microglia progenitors) or patient-derived somatic cells can be added to the assembly and neural tissue expansion can continue for multiple weeks; (Majumder et al., 2024) (C) Schematic diagram showing the use of a 3D bioprinting technique to prepare neural organoids. Here neural cells are combined with extracellular matrix biomaterials and used as “bioink” in a 3-D printing process (Maharjan et al., 2024, Yan et al., 2024, Cadena et al., 2024). This figure was created with BioRender.com.
Despite their use in a number of studies to date, neural organoids fabricated via suspension-based self-assembly have some important limitations. First, neural organoids fabricated in suspension conditions without spatial restriction or the addition of any exogenous patterning factors have typically been heterogeneous in structure, with portions that mimic multiple brain regions (Pacitti et al., 2019, Pasca, 2018, Lancaster et al., 2013, Mayhew and Singhania, 2023). The resulting heterogeneity can be beneficial when assessing the impact of environmental factors on various cell phenotypes. However, controlling the tissue development process could potentially yield tissues with more clearly defined structural and functional properties. Further, suspension culture protocols produce organoids with multiple neuroepithelial structures, with size on the order of 1 mm in diameter, and the organoids are opaque, thus limiting their analysis using traditional imaging tools (Kumar et al., 2019, Mariani et al., 2012). These limitations have provided motivation for development of guided assembly of neural organoids, wherein neural organoids are cultured with the addition of external patterning factors (e.g. constrained geometries or developmental morphogens) to produce organoids representing specific brain regions (Brawner et al., 2017, Tao and Zhang, 2016, Muguruma et al., 2015, Xiang et al., 2020, Pellegrini et al., 2020, Baldassari et al., 2020, Kelava and Lancaster, 2016).
2.2. Guided assembly of neural organoids
Over the years, researchers have used various engineering tools to guide assembly of neural organoids with particular desired properties, such as size, thickness, or geometry. Micropatterning methods offer better control of the cell shape and tissue architectures at different scales according to the micropattern geometry (D’Arcangelo and McGuigan, 2015). Knight et al. demonstrated that micropatterned culture substrates can be used to regulate the morphogenesis of neural rosette cytoarchitecture in the neural organoids. In this culture method, neural cells were grown in 2D monolayers with defined micro-scale geometry, which were allowed to grow during extended culture into 3D multilayered tissues. The authors studied efficiency of singular rosette emergence within micropatterned neuroepithelial tissues of varying sizes (circular, triangle and square), and showed that the reproducibility of singular rosette emergence can be improved by controlling neuroepithelial tissue size and geometry. This study provided a new culture platform for developing controlled neural rosette emergence within the neural organoids (Knight et al., 2018).
Standard hESC culture produces colonies of variable size and shapes (Orozco-Fuentes et al., 2019), but Deglincerti et al. reported a micropatterning method, in which hESC cells were confined to specific disk-shaped colonies. First human ESCs formed circular colonies with tightly packed cells, which further assembled into spatial patterns after treatment with bone morphogenetic protein 4 (BMP4). Immunofluorescence studies revealed expression of SOX2 protein at the center of the colonies – indicating the prospective ectoderm; and expression of BRA and SOX17 proteins at progressively larger radii of the colonies – indicating the emergence of mesoderm and endoderm. The patterning outcome was affected by the size of the colonies and cell density used in the culture. For example, smaller colonies did not show expression of SOX2 protein at the center of the colonies, and low cell density did not show the spatial organization of the colonies. These findings provide a new platform to study the mechanisms associated with cell-fate pattern formation at the onset of gastrulation (Deglincerti et al., 2016). In another tissue development model, Karzbrun et al. established a method for studying human neural tube morphogenesis in a cell culture dish. In this method, micropatterned stem cells assembled into precise 3D cell-fate patterns. Specifically, the authors used hESCs to generate neural ectoderm, which folded into a millimeter-long neural tube-like shape after stimulation with BMP4. However, no neural fold morphogenesis was observed in the absence of BMP4 treatment − suggesting the role of neural ectoderm in neural tube formation. The authors observed that neural plate width scaled linearly with the micropattern size, and non-neural ectoderm was restricted to a fixed region − suggesting that shape of the anterior–posterior axis is depended on the neural ectoderm geometry. This micropatterning method provides a new route to study of human embryonic morphogenesis and development in vitro (Karzbrun et al., 2021).
In a recent study, we reported another guided assembly approach to generate neural organoids with planar morphology. In this approach, human iPSCs-derived precursor cells were co-cultured on a cell-degradable polyethylene glycol (PEG) hydrogel surface in 96 well plates. Endothelial progenitors and pericyte progenitors were added on day 0, followed by addition of neural progenitor cells on day 7 and microglia progenitors on day 12. Neural organoid culture and expansion were continued for 28 days (Fig. 1B). We called these ‘Planar Neural Organoids (PNOs)’, since the tissue assembly into the degradable hydrogel resulted in a relatively thin (<500 μm) “planar” neural organoid geometry. We also observed that the microglia present in the PNOs responded to LPS stimulation, and treatment with anti-inflammatory drugs reduced the LPS-stimulated production of TNFα and IL-6. Our results demonstrated that the PNOs can be used as a simple and robust 3D model of neuro-inflammation (Majumder et al., 2024).
2.3. Bio-printing of neural organoids
Recently, 3-Dimensional bioprinting has made significant progress in applications that include tissue engineering and regenerative medicine (Maharjan et al., 2024). Using 3D bioprinting, a collection of cells can be placed in defined spatial locations to intentionally produce organized 3D tissue constructs (Fig. 1C). Yan et al. generated 3D bio-printed human brain organoids, in which neurons and glia formed functional connections within and between tissue layers. The printed neuronal progenitor cells differentiated into mature neurons, which formed functional neural circuits within the organoids. Similarly, the printed astrocyte progenitor cells differentiated into mature astrocytes and formed functional neuron-astrocyte networks. These designed neural tissues were formed by printing one cell layer adjacent to another cell layer horizontally and maintained in conventional cell culture. These 3D printed neural tissues were suitable for electrophysiological recording and may provide a new in vitro human model for studying neural networks (Yan et al., 2024). Cadena et al. used bioprinting to generate a tunable and reproducible gelatin methacrylate scaffold, which supported the co-culture of neural organoids and vascular cells in a custom architecture containing interconnected endothelial channels. Organoids grown in the bio-printed scaffold were healthy for up to 60 days in culture, and showed expression of genes expected for neuroectodermal differentiation (SOX2, NES, DCX, TUBB3 and MAP2). This 3D bio-printed platform may be tunable to incorporate additional ECM components, thereby creating more complex and physiologically relevant models of human brain development (Cadena et al., 2024).
3. Organoids and the prediction of neurotoxicity
Several recent studies have demonstrated the potential of human iPSC-derived neural organoids for neurotoxicity testing. One particular value of these approaches is understanding the impact of environmental chemicals on neurotoxicity, since animal models of neurotoxicity have not been highly predictive of human neurotoxicity in past studies (Crofton, 2011). In addition, there has been interest in using human organoids to study the impact of deleterious substances, such as alcohol, on neural development. Finally, the pharmaceutical industry has intensified the development of new therapies for widespread neurological diseases such as Alzheimer’s disease, Parkinson’s disease, and brain cancer, and there is strong motivation to ensure that such treatments are not neurotoxic. The following subsections will discuss illustrative examples of human organoids used to evaluate neurotoxicity in each of these application areas (Table 2).
Table 2.
List of the brain organoids and neural tissue constructs used for neurotoxicity testing.
| Sl. No. | References | Type of 3D culture | Applications |
|---|---|---|---|
| 1 | Human induced pluripotent stem cell derived neuronal cells cultured on chemically-defined hydrogels for sensitive in vitro detection of botulinum neurotoxin. Pellett et al. 2015 (Pellett et al., 2015) | Neural Tissue Constructs | To study neurotoxicity of Botulinum neurotoxin (BoNT). |
| 2 | Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Schwartz et al. 2015 (Schwartz et al., 2015) | Neural Tissue Constructs | To evaluate drug and chemical safety. |
| 3 | Neurovascular organotypic culture models using induced pluripotent stem cells to assess adverse chemical exposure outcomes. Nguyen et al. 2019 (Nguyen et al., 2019) | Neural Tissue Constructs | To investigate the impact of chemicals on neurovascular vessel integrity. |
| 4 | Assessment of drug-induced toxicity biomarkers in the brain microphysiological system (MPS) using targeted and untargeted molecular profiling. Mina et al. 2019 (Mina et al., 2019) | Neural Tissue Constructs | To study drug-induced neurotoxicity. |
| 5 | Engineered perineural vascular plexus for modeling developmental toxicity. Kaushik et al. 2020 (Kaushik et al., 2020) | Neural Tissue Constructs | To study xenobiotics induced pathological responses in a developing nervous system. |
| 6 | Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Qian et al. 2016 (Qian et al., 2016) | Brain Organoids | To evaluate neurotoxicity of bisphenol A and other similar types of neurotoxicants. |
| 7 | Engineering brain organoids to probe impaired neurogenesis induced by cadmium. Yin et al. 2018 (Yin et al., 2018) | Brain Organoids | To investigate neural dysfunctions caused by cadmium (Cd) exposure. |
| 8 | PM2.5 induces developmental neurotoxicity in cortical organoids. Han et al. 2024 (Han et al., 2024) | Brain Organoids | To study particulate matter (PM), specifically PM2.5-induced neurotoxicity. |
| 9 | Human-induced pluripotent stem cell-derived neural organoids as a novel in vitro platform for developmental neurotoxicity assessment. Hongen et al. 2024 (Hongen et al., 2024) | Brain Organoids | To study chemical (rotenone)-induced neurotoxicity. |
| 10 | Modeling alcohol-induced neurotoxicity using human induced pluripotent stem cell-derived three-dimensional cerebral organoids. Arzua et al. 2020 (Arzua et al., 2020) | Brain Organoids | To study alcohol-induced neurotoxicity. |
| 11 | Probing impaired neurogenesis in human brain organoids exposed to alcohol. Zhu et al. 2017 (Zhu et al., 2017) | Brain Organoids | To study the effects of alcohol exposure on fetal brain development. |
| 12 | Human cerebral organoids as a therapeutic drug screening model for Creutzfeldt–Jakob disease. Groveman et al. 2021 (Groveman et al., 2021) | Brain Organoids | To screen drugs for neurodegenerative Creutzfeldt–Jakob disease |
| 13 | Human iNSC-derived brain organoid model of lysosomal storage disorder in Niemann-Pick disease type C. Lee and co-workers. 2020 (Lee et al., 2020) | Brain Organoids | To test drugs for Niemann-Pick disease type C. |
| 14 | Neurotoxicity of phenylalanine on human iPSC-derived cerebral organoids. Kim et al. 2022 (Kim et al., 2022) | Brain Organoids | To study neurotoxicity of phenylalanine in the developing human brain. |
| 15 | Induced pluripotent stem cell-derived brain organoids as potential human model system for chemotherapy induced CNS toxicity. Scholz et al. 2022 (Scholz et al., 2022) | Brain Organoids | To investigate paclitaxel induced toxicity in the central nervous system. |
3.1. Neurotoxicity testing of environmental chemicals
Environmental chemicals such as pesticides, microplastics, and pharmaceutical waste are increasing at alarming rates, and are detectable in the air, food, and water (Mishra et al., 2023). Human exposure to these environmental chemicals may result in severe nervous system disorders (Babadjouni et al., 2017). Therefore, it is necessary to develop new testing methods for neurotoxicity studies of environmental chemicals. Several recent studies have shown the potential of both the 3D neural tissue constructs and neural organoids in neurotoxicity testing of environmental chemicals. In one early study, Pellett et al. established a human iPSC-derived 3D neural tissue construct model for neurotoxicity screening of Botulinum neurotoxin (BoNT), which is known to prevent neurotransmitter release leading to neuromuscular junction diseases. In this neurotoxicity assay, active botulinum neurotoxin A1 (BoNT/A1) was chosen as a reference toxin for validating the human iPSC-derived neuronal cell model. Botulinum neurotoxin was evaluated by assessing the sensitivity of human iPSC-derived neurons to active BoNT/A1, and the measured sensitivity was comparable to the approved in vivo mouse bioassay (Pellett et al., 2015). In 2015, Schwartz et al. developed a human ESC-derived neural organoid model that, for the first time, included an endothelial network and microglia (Fig. 2) (Schwartz et al., 2015). The authors cultured human ESC-derived endothelial cells, neural progenitor cells, mesenchymal stem cells, and microglia on chemically defined PEG hydrogels to encourage cellular interactions present within the developing brain. A machine learning method was employed to create a predictive model based on changes in global gene expression in neural constructs exposed to a training set of 60 different compounds, including both non-toxic chemicals and known toxicants. The model was then exposed to 10 unknown compounds, and was able to correctly classify 9 of the 10 compounds as either toxic or non-toxic in a blinded trial. This combined strategy illustrates the significance of human cell-based assays for predictive toxicology and could be beneficial for both drug and chemical safety evaluations. It is noteworthy that the one incorrectly identified compound in the Schwartz et al. work was a non-toxic compound that the model identified as toxic (i.e., a false positive) (Schwartz et al., 2015). However, the false positive compound actually is toxic to neurons, but does not cross the blood brain barrier in vivo, so it is not typically classified as a neurotoxicant. Thus, the false positive in this case demonstrates a need to build a functional human brain vasculature into emerging brain organoid models, perhaps using technologies analogous to the above mentioned bio-printed model (Cadena et al., 2021).
Fig. 2.
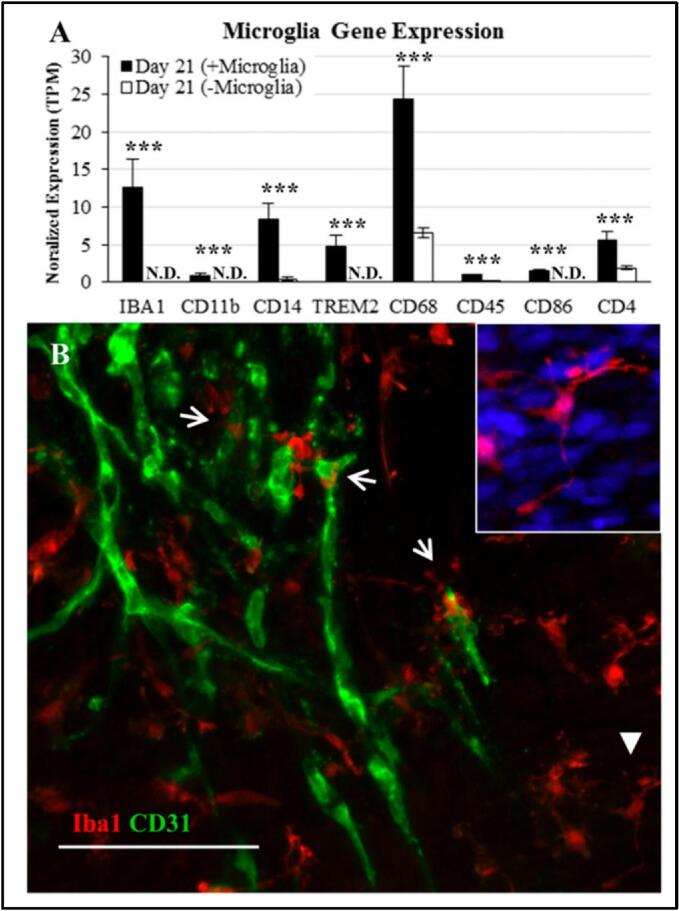
Endothelial network and microglia incorporation into the neural constructs. (A) Gene expression for the neural constructs formed in the Schwartz et al. manuscript (Schwartz et al., 2015) with or without microglia (N.D., not detected). (B) Expression of Iba1 (microglia marker, red) and CD31 (endothelial cell marker, green) for a day 21 neural construct formed in the Schwartz et al. manuscript (Schwartz et al., 2015) (Scale bar, 100 μm.). Closed arrows indicate ramified morphologies of microglia, and open arrows indicate capillary tubules. [Adapted with permission from Reference (Schwartz et al., 2015)]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In another advanced co-culture study, Nguyen et al. created a neurovascular tissue construct model using human iPSC-derived endothelial cells and astrocytes to investigate the impact of chemicals on neurovascular vessel integrity. The researchers tested 38 vascular-disrupting chemicals in this neurovascular model and observed distinct morphological changes for each chemical. More specifically, these changes included cells forming small disconnected structures and adopting rounded, non-adhesive morphologies. The researchers also noted greater sensitivity and consistency in chemical detection for the PEG hydrogel-derived neurovascular model compared to equivalent Matrigel® based neurovascular units (Nguyen et al., 2019).
Mina et al. utilized a brain micro-physiological system based tissue construct model to study molecular signatures of both neurotoxic and non-neurotoxic compounds. This micro-physiological system, comprised of human ESC-derived neural progenitor cells was treated with either the neurotoxic drug bortezomib or the non-neurotoxic drug tamoxifen for 14 days. Neurotoxicity was assessed by monitoring the level of the biomarker N-acetyl aspartic acid (NAA) associated with neuronal function. The authors observed a high level of NAA release just two days after bortezomib treatment, indicating cellular dysfunction. This brain micro-physiological system could serve as a valuable platform for drug-induced neurotoxicity studies (Mina et al., 2019).
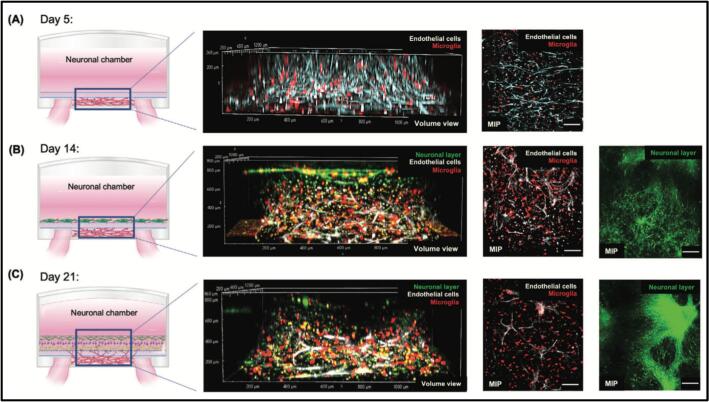
Kaushik et al. used synthetic hydrogels and human ESC-derived cells in a novel device geometry to create a perineural vascular plexus (PNVP) neural tissue construct model for studying neurotoxicity. The researchers treated the PNVP model with putative neurotoxicants and investigated their influence on PNVP formation and integration. The authors observed that developmental toxicant teratogens reduced 3D migration of endothelial cells and microglia, and decreased the production of vascular endothelial growth factor-A (VEGF-A) in the culture. By measuring 3D cell migration, vascular network disruption, and cytotoxicity of neurotoxicants, the PNVP model provided a simple and robust tool to make assessments of developmental toxicity. Thus, the PNVP model could be useful for physiologically relevant predictions of neurotoxicity in a developing nervous system (Fig. 3) (Kaushik et al., 2020).
Fig. 3.
Schematic diagram showing time-course development of a perineural vascular plexus (PNVP) tissue construct model. A) The formation of vascular network was observed at day 5 using live Cy5 dye-stained ECs. B) The development of the neuronal layer was observed at day 14 using live Cy5 dye-stained ECs and CellTracker Green-stained NPCs. C) The maturation of PNVP model was observed at day 21 using live Cy5 dye stained ECs, and CellTracker Green-stained NPCs. Scale bars: 100 μm. [Adapted with permission from Reference (Kaushik et al., 2020)]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Using a miniaturized spinning bioreactor, Qian et al. developed forebrain-specific organoids from human iPSCs. The researchers used this forebrain organoid model to test Bisphenol A, a commonly found chemical in many plastic products. They observed that bisphenol A treatment reduced the thickness of the ventricular zone in the forebrain organoids. The researchers also observed that higher concentrations of bisphenol A treatment reduced the proliferation of the neural progenitor cells. This forebrain-specific organoid model could be helpful for the quantitative evaluation of neurotoxicity of bisphenol A and other similar types of neurotoxicants (Qian et al., 2016). Recently, Yin et al. developed a brain organoid model on a micropillar chip to investigate neural dysfunctions caused by cadmium (Cd) exposure. The researchers performed immunostaining and real-time PCR assays to study the effect of Cd exposure in the brain organoids and observed an increase in cell death and skewed neural differentiation, suggesting impaired neurogenesis in the developing brain. Additionally, they observed a higher rate of cell death in the brain organoid even 10 days after the Cd exposure was removed– indicating long-term toxic effect of Cd in human brain. This work offers a robust platform for investigating abnormal neurogenesis induced by various toxic agents including Cd (Yin et al., 2018).
Han et al. developed 3D cortical organoids to investigate the mechanisms of particulate matter (PM), specifically PM2.5-induced neurotoxicity. Treatment with PM2.5 caused neuronal apoptosis and disrupted neural differentiation in the organoids, indicating the impact of PM2.5 on neurodevelopment. PM2.5 exposure also induced abnormalities in mitochondrial complex I functionality, as revealed in the transcriptomic analysis (Han et al., 2024). In 2024, Hongen et al. constructed a human iPSC-derived forebrain organoid model to predict chemical-induced neurotoxicity (Hongen et al., 2024). This study assessed the neurotoxicity of rotenone, which is a plant-derived pesticide known to affect the developing nervous system (Li et al., 2005). Immunofluorescence studies showed formation of neural networks in the control group after 29 days, whereas these networks were not completely developed in the rotenone-treated group. RNA-seq analysis also revealed that rotenone treatment significantly reduced the expression of 78 genes associated with neurodevelopment. This model displayed various neurodevelopmental features as observed in previously published organoid models. In particular, this forebrain-type brain organoid model serves as a better alternative to the traditional heterogeneous brain organoids for studying neurotoxicity, since this model was able to mimic the neurodevelopmental process occurring in the brain (Hogberg and Smirnova, 2022, Chesnut et al., 2021, Kim and Chang, 2023).
3.2. Neurotoxicity testing of alcohol exposure
Alcohol abuse can lead to impairment of cognitive function, hepatic failure, intoxication, and brain trauma (Brust, 2010, Harper, 2007, Mendes et al., 2023). Prenatal alcohol exposure can result in physical, behavioral, and cognitive deficits (Zhang et al., 2018, O’Connor and Paley, 2009, Maier and West, 2001, Muggli et al., 2024). Numerous studies have shown that alcohol exposure during pregnancy can cause nervous system disorders (Pamies et al., 2018, Kim et al., 2014, Kim et al., 2016). Neural organoids derived from neural precursor cells offer an efficient and reliable platform to screen for the effects of alcohol exposure on neurodevelopment (Sandström et al., 2017). For example, Arzua et al. developed a human cerebral organoid model to assess the toxic effects of alcohol exposure on the neuropathology phenotypes (Arzua et al., 2020). The authors observed that the apoptotic effects of alcohol exposure on cerebral organoids depended on alcohol concentration. More specifically, they found that astrocytes were less vulnerable to alcohol-induced apoptosis than neurons differentiated from the neural progenitor cells in the cerebral organoids.
Alcohol consumption during pregnancy can seriously affect fetal brain development, leading to fetal alcohol syndrome (Hepper et al., 2012, Maier and West, 2001, Lees et al., 2020). Zhu et al. developed a human iPSC-derived ‘brain organoids-on-a-chip’ model to study the effects of alcohol exposure on fetal brain development. The researchers found that alcohol exposure caused attenuated neurite outgrowth and skewed neural maturation in the brain organoids. They performed transcriptome analysis and identified a series of neural development related genes including RSPO2, GSX2, GPR39, VSX2 and SIX6, which were found to be altered in the organoids with alcohol exposure. These results provide essential insights into the underlying genetic mechanisms associated with prenatal alcohol exposure (Zhu et al., 2017).
3.3. Neurotoxicity testing of therapeutic candidates
Neurotoxicity of therapeutic candidates causes 10 % of all drug withdrawals during clinical studies (Staflin et al., 2016). Therefore, neurotoxicity testing has become an indispensable tool in the development of new drugs and therapeutics. Neurotoxicity testing is typically performed in preclinical studies using animal models before first-in-human clinical trials (Sanfeliu et al., 2024). Several reports have suggested that animal models are poor predictors of drug toxicity in humans (Atkins et al., 2020, Neziri et al., 2024, Van Norman, 2019). Organoid technologies offer unique opportunities to assess neurotoxicity of therapeutic candidates because they have features that mimic human neural tissues (Tang et al., 2022). Recently, Groveman et al. studied a human iPSC-derived cerebral organoid as a therapeutic drug screening model for neurodegenerative Creutzfeldt–Jakob disease. The researchers tested an anti-prion compound, pentosan polysulfate, and demonstrated that human cerebral organoids can be a suitable model to identify therapies for Creutzfeldt–Jakob disease. The authors observed that pentosan polysulfate reduced prion seeding activity and accumulation when compared to the control treatment (Groveman et al., 2021). Lee and co-workers developed an organoid model for testing drugs for Niemann-Pick disease type C, which is a rare neurodegenerative disease. The researchers prepared organoids using induced neural stem cells derived from the fibroblasts of Niemann-Pick disease type C affected patients. The researchers observed that the organoids treated with valproic acid, a histone deacetylase inhibitor, showed an increase in neuronal expressions. This study revealed that valproic acid is critical to enhance autophagy and restore neuronal differentiation in the Niemann-Pick disease type C organoids (Lee et al., 2020). In another study, Kim et al. utilized human iPSC-derived cerebral organoids to study the neurotoxicity of a high dose of phenylalanine, which causes the genetic metabolic disorder phenylketonuria. Cerebral organoids showed induction of apoptosis and alterations in organoid size, including lower thickness of the cortical rosettes upon treatment with phenylalanine for 5 days. RNA-seq analyses revealed apoptosis related gene expression for the phenylalanine-treated organoids. These findings indicated a possible effect of phenylalanine exposure in the developing human brain (Kim et al., 2022). Recently, Scholz et al. used human iPSC-derived brain organoids to investigate paclitaxel induced toxicity in the central nervous system. Paclitaxel treatment showed dose dependent apoptosis induction and neurotoxicity in the brain organoids. Researchers also observed paclitaxel treatment showed degradation of neuronal calcium sensor one protein (NCS-1). These findings suggest that brain organoids may be useful as a preclinical model to investigate chemotherapy induced neurotoxicity (Scholz et al., 2022). Taken together, these results show the potential of neural organoid models for screening neurotoxicity of therapeutic candidates.
4. Limitations and future perspectives
Several recent studies have proven that neural organoids have potential in neurotoxicant screening. However, there are many challenges that limit their application (Kim and Chang, 2023, Urrestizala-Arenaza et al., 2024, Li et al., 2024). The variability of current neural organoids is a major limiting factor for application in neurotoxicity testing (Sandoval et al., 2024). However, recent use of synthetic matrices such as PEG hydrogels in place of the traditionally used Matrigel® has improved the reproducibility of organoids. Another important limitation is the lack of functional vasculature with intact blood–brain-barrier in the neural organoids. Although some neural organoids have been assembled to include microcapillary networks, the formed networks are not fully functional (Pham et al., 2018, Qian et al., 2019). Recent studies showed that transplanting organoids into animals may enable the host vasculature to grow into the organoid graft, which may offer a promising strategy for achieving vascularization of human neural tissues (Mansour et al., 2018, Daviaud et al., 2018, Pham et al., 2018, Werschler et al., 2024, Matsui et al., 2021, Wörsdörfer et al., 2019, Zhao et al., 2021, Boutom et al., 2024). Recently, Cakir et al. established a method to generate functional vasculature-like networks in human cortical organoids. In this study, hESCs were engineered to express transcription factor ETS variant 2, which induced endothelial differentiation in the hESCs. The researchers observed the formation of CD31 endothelial tubes in human cortical organoids at day 30, while control organoids lacked the formation of such vascular tubes (Cakir et al., 2019). Ham et al. developed a human vascularized cerebral organoid with blood vessels induced by VEGF. VEGF treatment enhanced differentiation of the endothelial cells in the cell aggregates, which formed the resulting cerebral organoids with vascular structures expressing CD31 and genes associated with the formation of the blood–brain barrier. VEGF treatment also upregulated genes associated with vasculogenesis. These results demonstrated that ETV-2 induction and VEGF treatment are promising tools to prepare vascularized neural organoids (Ham et al., 2020).
Long-term survival and related neural tissue maturation are other limitations of existing neural organoid technologies. Recently, Stefano Giandomenico and co-authors developed a novel air–liquid interface culture technique, in which nutrient-rich culture medium ‘below’ and oxygen in the air ‘above’ improved neuronal survival and axon outgrowth in the neural organoids. The researchers observed formation of thick axon tracts with long-range projection within the organoids. This air–liquid interface culture technique helped to maintain nutrients in the culture to produce more mature neural organoids, which remained viable over a period of one year (Giandomenico et al., 2019).
5. Concluding remarks
Neural organoids derived from human ESCs or PSCs in near physiological conditions provide a robust tool for neurotoxicity studies of any physical, chemical and biological toxic substances in vitro. In contrast to 2D cell cultures, organoid cultures have been reported to mimic some of the complex structural and functional properties of the human brain. Therefore, one can hypothesize that the toxicological data obtained in the neural organoid models may be more informative, and more predictive of human neurotoxicity. Future developments could focus on including more well-defined and mature cell phenotypes, and using bioengineering approaches to construct more complex neural organoids with mature, functional vasculature and biomimetic immune components.
CRediT authorship contribution statement
Joydeb Majumder: Methodology, Visualization, Writing – original draft, Writing – review & editing. William L. Murphy: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The author(s) declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: W. L. Murphy is a co-founder and shareholder in Stem Pharm, Inc., which is focused on commercial applications of neural organoids.
Acknowledgments
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the US Environmental Protection Agency (STAR Grant no. 83573701), the US National Institutes of Health (award nos 1U01TR002383, R01HL093282, 1R01NS109427, 1R43ES029898, and 1R43ES029897) and the US National Science Foundation (award nos. EEC1648035 and DMR 170179).
Footnotes
This article is part of a special issue entitled: ‘Stem cells for discovery and mechanistic toxicology’ published in Current Research in Toxicology.
Data availability
Data will be made available on request.
References
- Arzua T., Yan Y.S., Jiang C.S., Logan S., Allison R.L., Wells C., Kumar S.N., Schafer R., Bai X.W. Modeling alcohol-induced neurotoxicity using human induced pluripotent stem cell-derived three-dimensional cerebral organoids. Transl Psychiat. 2020;10(1):347. doi: 10.1038/s41398-020-01029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J.T., George G.C., Hess K., Marcelo-Lewis K.L., Yuan Y., Borthakur G., Khozin S., LoRusso P., Hong D.S. Pre-clinical animal models are poor predictors of human toxicities in phase 1 oncology clinical trials. Br. J. Cancer. 2020;123:1496–1501. doi: 10.1038/s41416-020-01033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attarwala H. TGN1412: from discovery to disaster. J. Young Pharm. 2010;2:332–336. doi: 10.4103/0975-1483.66810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustyniak J., Bertero A., Coccini T., Baderna D., Buzanska L., Caloni F. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. 2019;39:1610. doi: 10.1002/jat.3815. [DOI] [PubMed] [Google Scholar]
- Babadjouni R.M., Hodis D.M., Radwanski R., Durazo R., Patel A., Liu Q., et al. Clinical effects of air pollution on the central nervous system; a review. J. Clin. Neurosci. 2017;43:16–24. doi: 10.1016/j.jocn.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babadjouni R.M., Hodis D.M., Radwanski R., Durazo R., Patel A., Liu Q., Mack W.J. Clinical effects of air pollution on the central nervous system; a review. J. Clin. Neurosci. 2017;43:16–24. doi: 10.1016/j.jocn.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassari S., Musante I., Iacomino M., Zara F., Salpietro V., Scudieri P. Brain organoids as model systems for genetic neurodevelopmental disorders. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.590119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S., Lawler S.E., Qu Y., Fadzen C.M., Wolfe J.M., Regan M.S., Pentelute B.L., Agar N.Y.R., Cho C.F. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018;13:2827–2843. doi: 10.1038/s41596-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutom S.M., Silva T.P., Palecek S.P., Shusta E.V., Fernandes T.G., Ashton R.S. Central nervous system vascularization in human embryos and neural organoids. Cell Rep. 2024;43(12) doi: 10.1016/j.celrep.2024.115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner A.T., Xu R., Liu D., Jiang P. Generating CNS organoids from human induced pluripotent stem cells for modeling neurological disorders. Int. J. Physiol. Pathophysiol. Pharmacol. 2017;9(3):101–111. [PMC free article] [PubMed] [Google Scholar]
- Brust J.C. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int. J. Environ. Res. Public Health. 2010;7(4):1540–1557. doi: 10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena M., Ning L., King A., Hwang B., Jin L., Serpooshan V., Sloan S.A. 3D bioprinting of neural tissues. Adv. Healthc. Mater. 2021;10(15) doi: 10.1002/adhm.202001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena M.A., Sing A., Taylor K., Jin L., Ning L., Salar A.M., Singh Y., Lanjewar S.N., Tomov M.L., Serpooshan V., Sloan S.A. A 3D bioprinted cortical organoid platform for modeling human brain development. Adv. Healthc. Mater. 2024;13(27) doi: 10.1002/adhm.202401603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B., Xiang Y., Tanaka Y., Kural M.H., Parent M., Kang Y.J., et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bury L.A., Chen F., Aldinger K.A., Miranda H.C., Wynshaw-Boris A. Generation of advanced cerebellar organoids for neurogenesis and neuronal network development. Hum. Mol. Genet. 2023;32(18):2832–2841. doi: 10.1093/hmg/ddad110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut M., Hartung T., Hogberg H., Pamies D. Human oligodendrocytes and myelin in vitro to evaluate developmental neurotoxicity. Int. J. Mol. Sci. 2021;22:7929. doi: 10.3390/ijms22157929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhibber T., Bagchi S., Lahooti B., Verma A., Al-Ahmad A., Paul M.K., Pendyala G., Jayant R.D. CNS organoids: an innovative tool for neurological disease modeling and drug neurotoxicity screening. Drug Discov. 2020;25(2):456–465. doi: 10.1016/j.drudis.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costamagna G., Comi G.P., Corti S. Advancing drug discovery for neurological disorders using iPSC-derived neural organoids. Int. J. Mol. Sci. 2021;22:2659. doi: 10.3390/ijms22052659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton K.M., et al. Developmental neurotoxicity testing: Recommendations for developing alternative methods for the screening and prioritization of chemicals. ALTEX. 2011;28(1):9–15. [PubMed] [Google Scholar]
- Crofton K.M., Bassan A., Behl M., Chushak Y.G., Fritsche E., Gearhart J.M., Marty M.S., Mumtaz M., Pavan M., Ruiz P., Sachana M., Selvam R., Shafer T.J., Stavitskaya L., Szabo D.T., Szabo S.T., Tice R.R., Wilson D., Woolley D., Myatt G.J. Current status and future directions for a neurotoxicity hazard assessment framework that integrates in silico approaches. Comput. Toxicol. 2022;22 doi: 10.1016/j.comtox.2022.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo E., McGuigan A.P. Micropatterning strategies to engineer controlled cell and tissue architecture in vitro. Biotechniques. 2015;58(1):13–23. doi: 10.2144/000114245. [DOI] [PubMed] [Google Scholar]
- Daviaud N., Friedel R.H., Zou H. Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. eNeuro. 2018;5 doi: 10.1523/ENEURO.0219-18.2018. ENEURO.219–ENEURO.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglincerti A., Etoc F., Guerra M., Martyn I., Metzger J., Ruzo A., Simunovic M., Yoney A., Brivanlou A.H., Siggia E., Warmflash A. Self-organization of human embryonic stem cells on micropatterns. Nat. Protoc. 2016;11:2223–2232. doi: 10.1038/nprot.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch R., Geschwind D.H. The human brain in a dish: the promise of iPSC-derived neurons. Cell. 2011;145(6):831–834. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M., Cohen A.F., Webb D.J. Implications of the BIA-102474-101 study for review of first-into-human clinical trials. Br. J. Clin. Pharmacol. 2016;81(4):582–586. doi: 10.1111/bcp.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsby A., Bal-Price A.K., Camins A., Coecke S., Fabre N., Gustafsson H., Honegger P., Kinsner-Ovaskainen A., Pallas M., Rimbau V., Rodríguez-Farré E., Suñol C., Vericat J.A., Zurich M.G. Neuronal in vitro models for the estimation of acute systemic toxicity. Toxicol. In Vitro. 2009;23(8):1564–1569. doi: 10.1016/j.tiv.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Giandomenico S.L., Mierau S.B., Gibbons G.M., Wenger L.M.D., Masullo L., Sit T., Sutcliffe M., Boulanger J., Tripodi M., Derivery E., Paulsen O., Lakatos A., Lancaster M.A. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 2019;22(4):669–679. doi: 10.1038/s41593-019-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Landrigan P.J. Neuro-behavioral effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groveman B.R., Ferreira N.C., Foliaki S.T., et al. Human cerebral organoids as a therapeutic drug screening model for Creutzfeldt–Jakob disease. Sci. Rep. 2021;11:5165. doi: 10.1038/s41598-021-84689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Lee T., Sun J., Sun J., Cai W., Yang Q., Sun T. Molecular lineages and spatial distributions of subplate neurons in the human fetal cerebral cortex. Adv. Sci. 2024;11 doi: 10.1002/advs.202407137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Liu J.R., Patel B., Solomon D.E., Vaidya B., Gupta V. Microfluidics-based 3D cell culture models: utility in novel drug discovery and delivery research. Bioeng. Transl. Med. 2016;1:63–81. doi: 10.1002/btm2.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam D.G., Redelmeier D.A. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- Ham O., Jin Y.B., Kim J., Lee M.O. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2020;521:84–90. doi: 10.1016/j.bbrc.2019.10.079. [DOI] [PubMed] [Google Scholar]
- Han Y., Yu Z., Chen Y., Guo X., Liu Y., Zhang H., Li Z., Chen L. PM2.5 induces developmental neurotoxicity in cortical organoids. Environ. Pollut. 2024;361 doi: 10.1016/j.envpol.2024.124913. [DOI] [PubMed] [Google Scholar]
- Harper C. The neurotoxicity of alcohol. Hum. Exp. Toxicol. 2007;26:251–257. doi: 10.1177/0960327107070499. [DOI] [PubMed] [Google Scholar]
- Hepper P.G., Dornan J.C., Lynch C. Fetal brain function in response to maternal alcohol consumption: early evidence of damage. Alcohol. Clin. Exp. Res. 2012;36(12):2168–2175. doi: 10.1111/j.1530-0277.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg H.T., Smirnova L. The future of 3D brain cultures in developmental neurotoxicity testing. Front. Toxicol. 2022;4 doi: 10.3389/ftox.2022.808620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongen T., Sakai K., Ito T., Qin X.Y., Sone H. Human-induced pluripotent stem cell-derived neural organoids as a novel in vitro platform for developmental neurotoxicity assessment. Int. J. Mol. Sci. 2024;25:12523. doi: 10.3390/ijms252312523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Zhang J., Schwartz M.P., Stewart R., Page C.D., Murphy W.L., Thomson J.A. A human pluripotent stem cell platform for assessing developmental neural toxicity screening. Stem Cell Res. Ther. 2013;1(Suppl. 1):S12. doi: 10.1186/scrt373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M., Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA. 2013;110(50):20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzbrun E., Khankhel A.H., Megale H.C., Glasauer S.M.K., Wyle Y., Britton G., Warmflash A., Kosik K.S., Siggia E.D., Shraiman B.I., Streichan S.J. Human neural tube morphogenesis in vitro by geometric constraints. Nature. 2021;599(7884):268–272. doi: 10.1038/s41586-021-04026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik G., Gupta K., Harms V., Torr E., Evans J., Johnson H.J., Soref C., Acevedo-Acevedo S., Antosiewicz-Bourget J., Mamott D., Uhl P., Johnson B.P., Palecek S.P., Beebe D.J., Thomson J.A., Daly W.T., Murphy W.L. Engineered perineural vascular plexus for modeling developmental toxicity. Adv. Healthcare Mater. 2020;9 doi: 10.1002/adhm.202000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava I., Lancaster M.A. Stem cell models of human brain development. Cell Stem Cell. 2016;18:736–748. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Kelley K.W., Pasca S.P. Human brain organogenesis: Toward a cellular understanding of development and disease. Cell. 2021;185:42–61. doi: 10.1016/j.cell.2021.10.003. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Chang M.Y. Application of human brain organoids-opportunities and challenges in modeling human brain development and neurodevelopmental diseases. Int. J. Mol. Sci. 2023;24:12528. doi: 10.3390/ijms241512528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Duan L., Tu T.G., Elie O., Kim Y., Mathiyakom N., Elashoff D., Kim Y. Molecular effect of ethanol during neural differentiation of human embryonic stem cells in vitro. Genom. Data. 2014;2:139–143. doi: 10.1016/j.gdata.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee S., Lee J., Park J.C., Kim K.H., Ko J.M., Park S.H., Kim S.K., Mook-Jung I., Lee J.Y. Neurotoxicity of phenylalanine on human iPSC-derived cerebral organoids. Mol. Genet. Metab. 2022;136(2):132–144. doi: 10.1016/j.ymgme.2022.04.005. [DOI] [PubMed] [Google Scholar]
- Kim Y.Y., Roubal I., Lee Y.S., Kim J.S., Hoang M., Mathiyakom N., Kim Y. Alcohol-induced molecular dysregulation in human embryonic stem cell-derived neural precursor cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight G.T., Lundin B.F., Iyer N., Ashton L.M., Sethares W.A., Willett R.M., Ashton R.S. Engineering induction of singular neural rosette emergence within hPSC-derived tissues. Elife. 2018;29(7) doi: 10.7554/eLife.37549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.V., Er P.X., Lawlor K.T., Motazedian A., Scurr M., Ghobrial I., Combes A.N., Zappia L., Oshlack A., Stanley E.G., Little M.H. Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development. 2019;146(5) doi: 10.1242/dev.172361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194) doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014;9(10):2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.T., Bendriem R.M., Wu W.W., Shen R.F. 3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017;24:59. doi: 10.1186/s12929-017-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Shin N., Kook M.G., Kong D., Kim N.G., Choi S.W., Kang K.S. Human iNSC-derived brain organoid model of lysosomal storage disorder in Niemann-pick disease type C. Cell Death Dis. 2020;11(12):1059. doi: 10.1038/s41419-020-03262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B., Mewton L., Jacobus J., Valadez E.A., Stapinski L.A., Teesson M., Tapert S.F., Squeglia L.M. Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the adolescent brain cognitive development study. Am. J. Psychiatry. 2020;177(11):1060–1072. doi: 10.1176/appi.ajp.2020.20010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.R., Men S.H., Wang Z.Y., Liu C., Zhou G.R., Yan Z.G. The application of human-derived cell lines in neurotoxicity studies of environmental pollutants. Sci. Total Environ. 2024;912 doi: 10.1016/j.scitotenv.2023.168839. [DOI] [PubMed] [Google Scholar]
- Li J., Spletter M.L., Johnson D.A., Wright L.S., Svendsen C.N., Johnson J.A. Rotenone-induced caspase 9/3-independent and -dependent cell death in undifferentiated and differentiated human neural stem cells. J. Neurochem. 2005;92:462–476. doi: 10.1111/j.1471-4159.2004.02872.x. [DOI] [PubMed] [Google Scholar]
- Li M., Yuan Y., Hou Z., Hao S., Jin L., Wang B. Human brain organoid: trends, evolution, and remaining challenges. Neural Regen. Res. 2024;19(11):2387–2399. doi: 10.4103/1673-5374.390972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Suarez L., Al Awabdh S., Coumoul X., Chauvet C. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: focus on organic pollutants. Neuro Toxicol. 2022;92:131–155. doi: 10.1016/j.neuro.2022.07.008. [DOI] [PubMed] [Google Scholar]
- Maharjan S., Ma C., Singh B., Kang H., Orive G., Yao J., Shrike Z.Y. Advanced 3D imaging and organoid bioprinting for biomedical research and therapeutic applications. Adv. Drug Deliv. Rev. 2024;208 doi: 10.1016/j.addr.2024.115237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.E., West J.R. Drinking patterns and alcohol-related birth defects. Alcohol Res. Health. 2001;25(3):168–174. [PMC free article] [PubMed] [Google Scholar]
- Maier S.E., West J.R. Drinking patterns and alcohol-related birth defects. Alcohol Res. Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- Majumder J., Torr E.E., Aisenbrey E.A., Lebakken C.S., Favreau P.F., Richards W.D., Yin Y., Chang Q., Murphy W.L. Human induced pluripotent stem cell-derived planar neural organoids assembled on synthetic hydrogels. J Tissue Eng. 2024;15 doi: 10.1177/20417314241230633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A.A., Goncalves J.T., Bloyd C.W., Li H., Fernandes S., Quang D., Johnston S., Parylak S.L., Jin X., Gage F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018;36(5):432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A.A., Gonçalves J.T., Bloyd C.W., Li H., Fernandes S., Quang D., Johnston S., Parylak S.L., Jin X., Gage F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J., Simonini M.V., Palejev D., Tomasini L., Coppola G., Szekely A.M., Horvath T.L., Vaccarino F.M. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2012;109(31):12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T.K., Tsuru Y., Hasegawa K., Kuwako K.I. Vascularization of human brain organoids. Stem Cells. 2021;39(8):1017–1024. doi: 10.1002/stem.3368. [DOI] [PubMed] [Google Scholar]
- Mayhew C.N., Singhania R. A review of protocols for brain organoids and applications for disease modeling. STAR Protoc. 2023;4(1) doi: 10.1016/j.xpro.2022.101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie R., Fried M.W., Sallie R., Conjeevaram H., Di Bisceglie A.M., Park Y., Savarese B., Kleiner D., Tsokos M., Luciano C., et al. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N. Engl. J. Med. 1995;333:1099–1105. doi: 10.1056/NEJM199510263331702. [DOI] [PubMed] [Google Scholar]
- Mendes P.F.S., Baia-da-Silva D.C., Melo W.W.P., Bittencourt L.O., Souza-Rodrigues R.D., Fernandes L.M.P., Maia C.D.S.F., Lima R.R. Neurotoxicology of alcohol: a bibliometric and science mapping analysis. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1209616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina S.G., Alaybeyoglu B., Murphy W.L., Thomson J.A., Stokes C.L., Cirit M. Assessment of drug-induced toxicity biomarkers in the brain microphysiological system (MPS) using targeted and untargeted molecular profiling. Front. Big Data. 2019;2:23. doi: 10.3389/fdata.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R.K., Mentha S.S., Misra Y., Dwivedi N. Emerging pollutants of severe environmental concern in water and wastewater: a comprehensive review on current developments and future research. Water-Energy Nexus. 2023;6:74–95. ISSN 2588-9125. [Google Scholar]
- Muggli E., Halliday J., Hearps S., Nguyen T.N., Penington A., Thompson D.K., Spittle A., Forster D.A., Lewis S., Elliott E.J., Anderson P.J. Low to moderate prenatal alcohol exposure and neurodevelopment in a prospective cohort of early school aged children. Sci. Rep. 2024;14:7302. doi: 10.1038/s41598-024-57938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma K., Nishiyama A., Kawakami H., Hashimoto K., Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10:537–550. doi: 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Neziri S., Köseoğlu A.E., Deniz Köseoğlu G., Özgültekin B., Özgentürk N.Ö. Animal models in neuroscience with alternative approaches: evolutionary, biomedical, and ethical perspectives. Animal Model. Exp. Med. 2024;7(6):868–880. doi: 10.1002/ame2.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen E.H., Dombroe M.J., Fisk D.L., Daly W.T., Sorenson C.M., Murphy W.L., Sheibani N. Neurovascular organotypic culture models using induced pluripotent stem cells to assess adverse chemical exposure outcomes. Appl. In Vitro Toxicol. 2019;5(2):92–110. doi: 10.1089/aivt.2018.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M.J., Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev. Disabil. Res. Rev. 2009;15:225–234. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- Orozco-Fuentes S., Neganova I., Wadkin L.E., Baggaley A.W., Barrio R.A., Lako M., Shukurov A., Parker N.G. Quantification of the morphological characteristics of hESC colonies. Sci. Rep. 2019;9:17569. doi: 10.1038/s41598-019-53719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitti D., Privolizzi R., Bax B.E. Organs to cells and cells to Organoids: the evolution of in vitro central nervous system modelling. Front. Cell. Neurosci. 2019;13:129. doi: 10.3389/fncel.2019.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamies D., Block K., Lau P., Gribaldo L., Pardo C.A., Barreras P., Smirnova L., Wiersma D., Zhao L., Harris G., Hartung T., Hogberg H.T. Rotenone exerts developmental neurotoxicity in a human brain spheroid model. Toxicol. Appl. Pharmacol. 2018;354:101–114. doi: 10.1016/j.taap.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pas ¸ca A.M., Sloan S.A., Clarke L.E., Tian Y., Pas ¸ca S.P. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca S.P. The rise of three-dimensional human brain cultures. Nature. 2018;553:437–445. doi: 10.1038/nature25032. [DOI] [PubMed] [Google Scholar]
- Pellegrini L., Bonfio C., Chadwick J., Begum F., Skehel M., Lancaster M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science. 2020;369 doi: 10.1126/science.aaz5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S., Schwartz M.P., Tepp W.H., Josephson R., Scherf J.M., Pier C.L., Thomson J.A., Murphy W.L., Johnson E.A. Human induced pluripotent stem cell derived neuronal cells cultured on chemically-defined hydrogels for sensitive in vitro detection of botulinum neurotoxin. Sci. Rep. 2015;5:14566. doi: 10.1038/srep14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perel P., Roberts I., Sena E., et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334:197–203. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham M.T., Pollock K.M., Rose M.D., Cary W.A., Stewart H.R., Zhou P., Nolta J.A., Waldau B. Generation of human vascularized brain organoids. Neuroreport. 2018;29(7):588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham M.T., Pollock K.M., Rose M.D., Cary W.A., Stewart H.R., Zhou P., Nolta J.A., Wal-dau B. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Nguyen H.N., Song M.M., Hadiono C., Ogden S.C., Hammack C., Yao B., Hamersky G.R., Jacob F., Zhong C., Yoon K.J., Jeang W., Lin L., Li Y., Thakor J., Berg D.A., Zhang C., Kang E., Chickering M., Nauen D., Ho C.Y., Wen Z., Christian K.M., Shi P.Y., Maher B.J., Wu H., Jin P., Tang H., Song H., Ming G.L. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X.Y., Song H.J., Ming G.L. Brain organoids: advances, applications and challenges. Development. 2019;146(8) doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner H., Grabos M., Becker K.J., Kagermeier T.E., Wu J., Otto M., Peischard S., Zeuschner D., TsyTsyura Y., Disse P., Klingauf J., Leidel S.A., Seebohm G., Schöler H.R., Bruder J.M. A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids. Elife. 2020;9 doi: 10.7554/eLife.52904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.A., Aschner M., Calligaro D., Guilarte T.R., Hanig J.P., Herr D.W., Hudzik T.J., Jeromin A., Kallman M.J., Liachenko S., Lynch J.J., 3rd, Miller D.B., Moser V.C., O'Callaghan J.P., Slikker W., Jr., Paule M.G. Translational biomarkers of neurotoxicity: a health and environmental sciences institute perspective on the way forward. Toxicol. Sci. 2015;148(2):332–340. doi: 10.1093/toxsci/kfv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval S.O., Cappuccio G., Kruth K., Osenberg S., Khalil S.M., Méndez-Albelo N.M., Padmanabhan K., Wang D., Niciu M.J., Bhattacharyya A., Stein J.L., Sousa A.M.M., Waxman E.A., Buttermore E.D., Whye D., Sirois C.L., Cross-IDDRC Human Stem Cell Consortium, Williams A., Maletic-Savatic M., Zhao X. Rigor and reproducibility in human brain organoid research: where we are and where we need to go. Stem Cell Rep. 2024;19(6):796–816. doi: 10.1016/j.stemcr.2024.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström J., Eggermann E., Charvet I., Roux A., Toni N., Greggio C., Broyer A., Monnet-Tschudi F., Stoppini L. Development and characterization of a human embryonic stem cell- derived 3D neural tissue model for neurotoxicity testing. Toxicol. In Vitro. 2017;38:124–135. doi: 10.1016/j.tiv.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C., Bartra C., Suñol C., Rodríguez-Farré E. New insights in animal models of neurotoxicity-induced neurodegeneration. Front. Neurosci. 2024;17 doi: 10.3389/fnins.2023.1248727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz S., Lewis K., Saulich F., Endres M., Boehmerle W., Huehnchen P. Induced pluripotent stem cell-derived brain organoids as potential human model system for chemotherapy induced CNS toxicity. Front. Mol. Biosci. 2022;9 doi: 10.3389/fmolb.2022.1006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.P., Hou Z., Propson N.E., Zhang J., Engstrom C.J., Santos Costa V., Jiang P., Nguyen B.K., Bolin J.M., Daly W., Wang Y., Stewart R., Page C.D., Murphy W.L., Thomson J.A. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc. Natl. Acad. Sci. USA. 2015;112(40):12516–12521. doi: 10.1073/pnas.1516645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko O., Parham F., Dea S., Sodhi N., Biesmans S., Mora-Castilla S., Ryan K., Behl M., Chandy G., Crittenden C., Vargas-Hurlston S., Guicherit O., Gordon R., Zanella F., Carromeu C. Functional and mechanistic neurotoxicity profiling using human iPSC-derived neural 3D cultures. Toxicol. Sci. 2019;167:58–76. doi: 10.1093/toxsci/kfy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T.A., Levin E.D., Seidler F.J. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ. Health Perspect. 2006;114(5):746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staflin K., Misner D., Dambach D. Utilization of in vitro neurotoxicity models in pre‐clinical toxicity assessment. Stem Cells Toxicol. Med. 2016:155–178. [Google Scholar]
- Sun N., Meng X., Liu Y., Song D., Jiang C., Cai J. Applications of brain organoids in neurodevelopment and neurological diseases. J. Biomed. Sci. 2021;28:30. doi: 10.1186/s12929-021-00728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G., Perry M.R., Ward S., et al. Cytokine storm in phase 1 trial of the anti-CD28 monoclonal antibody TGN1312. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Tang X.Y., Wu S., Wang D., et al. Human organoids in basic research and clinical applications. Sig. Transduct. Target Ther. 2022;7:168. doi: 10.1038/s41392-022-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Zhang S.C. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell. 2016;19:573–586. doi: 10.1016/j.stem.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrestizala-Arenaza N., Cerchio S., Cavaliere F., Magliaro C. Limitations of human brain organoids to study neurodegenerative diseases: a manual to survive. Front. Cell. Neurosci. 2024;18 doi: 10.3389/fncel.2024.1419526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman G. Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? J. Am. Coll. Cardiol. Basic Trans. Sci. 2019;4(7):845–854. doi: 10.1016/j.jacbts.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Thriel C. Neurotoxicology: an update on epidemiology, mechanisms, and pathology. Acta Neuropathol. 2019;138:339–341. doi: 10.1007/s00401-019-02051-7. [DOI] [PubMed] [Google Scholar]
- Vonberg F.W., Blain P.G. Neurotoxicology: a clinical system- based review. Pract. Neurol. 2024;24:357–368. doi: 10.1136/pn-2023-003983. [DOI] [PubMed] [Google Scholar]
- Walker A.L., Imam S.Z., Roberts R.A. Drug discovery and development: Biomarkers of neurotoxicity and neurodegeneration. Exp. Biol. Med. 2018;243:1037–1045. doi: 10.1177/1535370218801309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M.J., Arrowsmith J., Leach A.R., Leeson P.D., Mandrell S., Owen R.M., et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015;14(7):475–486. doi: 10.1038/nrd4609. [DOI] [PubMed] [Google Scholar]
- Werschler N., Quintard C., Nguyen S., Penninger J. Engineering next generation vascularized organoids. Atherosclerosis. 2024;398 doi: 10.1016/j.atherosclerosis.2024.118529. [DOI] [PubMed] [Google Scholar]
- Wörsdörfer P., Dalda N., Kern A., et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019;9:15663. doi: 10.1038/s41598-019-52204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Cakir B., Park I.H. Deconstructing and reconstructing the human brain with regionally specified brain organoids. Semin. Cell Dev. Biol. 2020;111:40–51. doi: 10.1016/j.semcdb.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Li X., Gao Y., Bhattacharyya A., Zhao X., Zhang S.-C. 3D bioprinting of human neural tissues with functional connectivity. Cell Stem Cell. 2024;31:260–274.e7. doi: 10.1016/j.stem.2023.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F.C., Zhu Y.J., Wang Y.Q., Qin J.H. Engineering brain Organoids to probe impaired neurogenesis induced by cadmium. ACS Biomater Sci. Eng. 2018;4(5):1908–1915. doi: 10.1021/acsbiomaterials.8b00160. [DOI] [PubMed] [Google Scholar]
- Zhang X.L., Hashimoto J.G., Guizzetti M. Developmental neurotoxicity of alcohol: effects and mechanisms of ethanol on the developing brain. Adv. Neurotoxicol. 2018;2:115–144. [Google Scholar]
- Zhang D.Y., Song H., Ming G.L. Modeling neurological disorders using brain organoids. Semin. Cell Dev. Biol. 2021;111:4–14. doi: 10.1016/j.semcdb.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Xu Z., Xiao L., Shi T., Xiao H., Wang Y., Li Y., Xue F., Zeng W. Review on the vascularization of organoids and organoids-on-a-chip. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.637048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Zhang F., Xu S., Wu L. Advances in neural organoid systems and their application in neurotoxicity testing of environmental chemicals. Genes Environ. 2021;43:39. doi: 10.1186/s41021-021-00214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Wang L., Yin F., Yu Y., Wang Y., Shepard M.J., et al. Probing impaired neurogenesis in human brain organoids exposed to alcohol. Integr. Biol. (Camb) 2017;9(12):968–978. doi: 10.1039/c7ib00105c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.