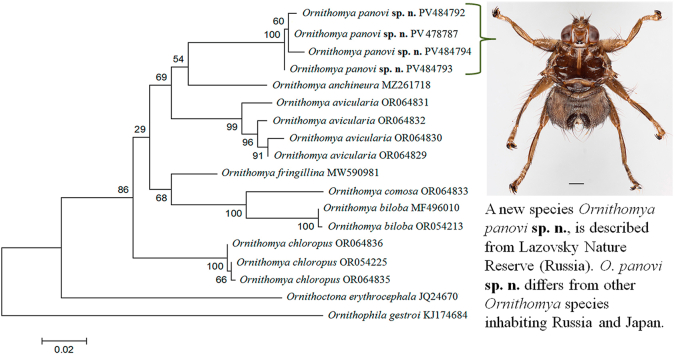
Abstract
Louse flies (Diptera: Hippoboscidae) are specialized ectoparasites of birds, influencing host health and potentially acting as vectors of pathogens. A new species of the genus Ornithomya Latreille, 1802 (Diptera: Hippoboscidae), Ornithomya panovisp. n., is described from specimens collected in Lazovsky Nature Reserve (Russia). O. panovisp. n. belongs to the avicularia species-group and is distinguished from other known Ornithomya species inhabiting Russia and Japan by several morphological features: reduction of tergite 4, wing length, ratio of costal vein sections between junctions R1 and R2+3 and between junctions R2+3 and R4+5 and arrangement of wing microtrichia as well as by genetic distances. This discovery expands our knowledge of the region's parasite biodiversity and highlights the need for continued faunistic surveys in the Russian Far East.
Keywords: Diptera, Hippoboscidae, Louse flies, New species, Ornithomya, Russia, Russian far east
Graphical abstract
Highlights
-
•
A new louse fly species is described from the Russian Far East.
-
•
The species is clearly distinguished by a unique reduction of tergite 4.
-
•
Combined morphological and molecular data confirm the species' taxonomic status.
-
•
Ornithomya panovi sp. n. belongs to the avicularia species-group.
1. Introduction
Parasitic insects play crucial roles in ecosystems by influencing host health, behavior, and population dynamics, yet many remain understudied, particularly in remote regions of Northeast Eurasia. The ectoparasite family Hippoboscidae Samouelle, 1819 includes over 200 fly species (Dick, 2006; Oboňa et al., 2019). These flies are hematophagous ectoparasites that feed on the blood of mammals and birds (Hutson, 1984). They serve as vectors for numerous pathogens, posing epidemiological risks (Bequaert, 1954; Doszhanov, 1980, 2003; Ganez et al., 2004; Farajollahi et al., 2005; Khametova et al., 2018; Peña-Espinoza et al., 2023; Wawman, 2023), and are able to transport phoretic mites (Fain, 1965a, b; Hill et al., 1967; Philips and Fain, 1991) and also feather lice (de Moya, 2019; Lee et al., 2022).
One of the largest genera, widely represented in the Palearctic, is the genus Ornithomya Latreille, 1802. All Ornithomya species exclusively parasitize birds (Maa, 1969; Doszhanov, 1980, 2003) and predominantly inhabit the temperate and middle latitudes of the Old World (Hutson, 1984). Currently this genus includes 33 living species (Dick, 2006; Nartshuk et al., 2022, 2024; Yatsuk et al., 2023, 2024a, 2024b; Matyukhin et al., 2023) and one fossil species (Maa, 1966). This species are divided into five groups, based on their morphology (Maa, 1963; Dick, 2006).
To date, 12 species of Ornithomya have been recorded within the territory of Russia and the former USSR: O. avicularia avicularia L., 1758, O. biloba Dufour, 1827; Doszhanov, 1980, 2003), O. candida Maa (1967); Yatsuk et al. (2024d), O. chloropus Bergroth, 1901, O. comosa Austen, 1930, O. fringillina Curtis, 1836; Doszhanov, 1980, 2003), O. strigilis Nartshuk, Yatsuk et Matyukhin, 2022; Nartshuk et al. (2022), O. triselevae Matyukhin, Yatsuk et Nartshuk, 2023; Matyukhin et al. (2023), O. krivolutskii Yatsuk, Matyukhin et Nartshuk, 2023; Yatsuk et al. (2023), O. nazarovi Yatsuk, Matyukhin et Nartshuk et al., 2024; Yatsuk et al. (2024a), O. delichoni Yatsuk, Matyukhin et Nartshuk et al., 2024; Nartshuk et al. (2024), O. helvipennis Yatsuk, Nartshuk et Matyukhin et al., 2023; Yatsuk et al. (2024b).
Some Ornithomya species exhibit extensive morphological variability, for example, in the number of scutellar setae, complicating species identification using traditional morphology alone (Doszhanov, 1980, 2003; Maa, 1967, 1969). As a result, molecular methods are increasingly employed to clarify taxonomy and resolve cryptic species boundaries within Hippoboscidae (Meiβner et al., 2020; Petersen et al., 2007a, Petersen et al., 2007b; Yatsuk et al., 2024c, 2024d).
Meiβner et al. (2020) reported molecular data on Ornithomya species from the Amur region in Russia, some of which had not been previously identified. The aim of this study is to describe a new species of Ornithomya collected from birds in the Russian Far East. This study contributes to filling existing gaps in our knowledge of louse fly biodiversity in temperate Asia.
2. Material and methods
The flies were collected during bird ringing in the Lazovsky Nature Reserve in the south of the Primorskiy Territory of Russia. The territory of the reserve is a typical mid-mountain area with average hill heights of 600–900 m above sea level. Forests cover 96 % of the territory. The climate is monsoonal, which is manifested in a pronounced change in wind directions in summer and winter. There are two climatic zones in the territory of the reserve: coastal and continental. The coldest month is January, with an average temperature of –8 °C on the coast and −13 °C in the continental part. The warmest month is August, with an average temperature of about +19 °C.
During the study in summer 2023, 111 birds from 25 species were examined. Among them 52 birds from 19 species were infested with flies. Of these birds one blue-and-white flycatcher (Cyanoptila cyanomelana (Temminck, 1829)), six grey-headed buntings (Osyris spodocephalus (Pallas, 1776)), ten yellow-rumped flycatchers (Ficedula zanthopygia (Hay, 1845)) and fourteen eurasian nuthatchs (Sitta europaea Linnaeus, 1758), were caught. Flies were collected from one C. cyanomelana, five Os. spodocephalus, six F. zanthopygia, and twelve S. europaea.
The louse fly material was preserved in 96 % ethanol. Morphological terminology follows Hutson (1984). Body length was measured using the standard method for Hippoboscidae (Doszhanov, 1980). The combined head and thorax length was measured from the anterior margin of the head, excluding the antennae, to the posterior margin of the scutellum. We follow the interpretation of Mogi et al. (2023), who regard Ornithomya avicularia aobatonis (Matsumura, 1905) as a subspecies. We base our morphological comparisons on the original description by Maa (1967).
The morphological study was conducted using an optical microscope Keyence VHX-1000 (Japan) housed at the Joint Usage Center "Instrumental Methods in Ecology" at the Institute of Ecology and Evolution, Russian Academy of Sciences. For illustration purposes (Fig. 1) images were taken with Canon EOS 90D and Canon EOS M6 Mark II cameras with a Canon EF 100 mm/2L Macro lens, stitched and processed using Helicon Focus 7 software.
Fig. 1.
Ornithomya panovisp. n. female.
A – holotype general view, dorsal side; B – holotype ventral side; C – holotype wing; D – wing drawing; E, F – examples of morphological variability of abdominal tergites, dorsal side of abdomen. Scale bars: 0.5 mm.
Total DNA was extracted from whole flies using Diatom-200 reagents (Isogen, Moscow) according to the manufacturer's instructions. Polymerase chain reaction (PCR) was performed using primers LCO1490 and HCO2198 (Folmer et al., 1994). Thermal cycling consisted of an initial denaturation step at 94 °C for 1 min, followed by 6 cycles of 1 min at 94 °C, 1 min at the annealing temperature of 45 °C and 1 min at 72 °C; followed by 40 cycles of 1 min at 94 °C, 1.5 min at the annealing temperature of 55 °C, and 4 cycles of 1.5 min at 72 °C; with a final extension at 72 °C for 6 min. The amplification product was purified using precipitation by ethyl alcohol solution with addition of 5M sodium acetate. Electrophoresis and reading of amplification product nucleotide sequences were carried out on an automatic ABI PRISM 3130 sequencer (Applied Biosystems, United States) using BigDye Terminator reagent kit 3.1 (Applied Biosystems). The study was conducted at the Joint Usage Center “Instrumental Methods in Ecology” at the IEE RAS.
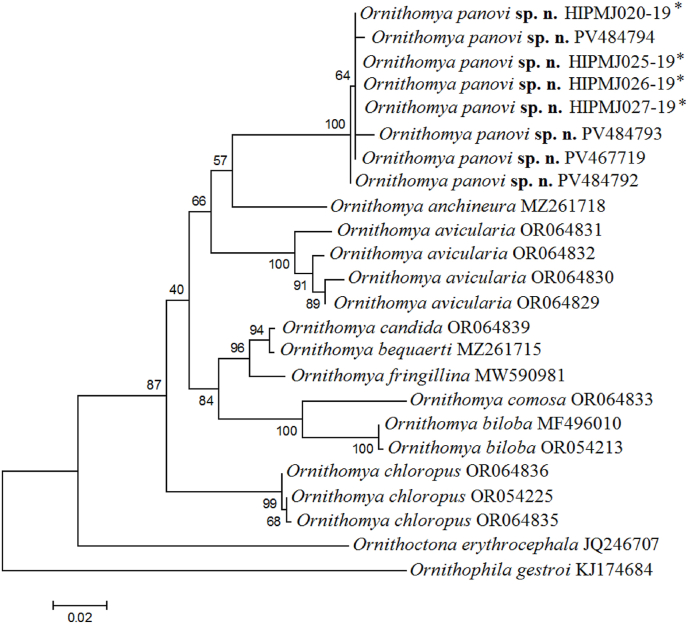
Eleven sequences of new species were obtained. NCBI (www.ncbi.nlm.nih.gov) numbers for haplotypes are presented in Table 1. The analyzed fragment consisted of 591 base pairs (bp) of the mtDNA COI gene. Other sequences were taken from the NCBI database (Table 1). Phylogenetic relationships were analyzed using the Maximum Likelihood method with the General Time Reversible (GTR) model, assuming gamma-distributed rates among sites. The analysis included 1000 bootstrap replications and was performed using the MEGA 5.1 software package (Tamura et al., 2011). Two species, Ornithoctona erythrocephala (Leach, 1817) and Ornithophila gestroi (Róndani, 1878), were used as the outgroup.
Table 1.
Sequence data.
| Species | Sequences number | Data source | Data link |
|---|---|---|---|
| Ornithomya anchineura Speiser, 1905 | MZ261718 | NCBI | Levesque-Beaudin and Sinclair (2021) |
| Ornithomya avicularia (Linnaeus, 1758) | OR064829, OR064830, OR064831, OR064832 | NCBI | Yatsuk et al. (2023) |
| Ornithomya bequaertiMaa (1969) | MZ261715 | NCBI | Levesque-Beaudin and Sinclair (2021) |
| Ornithomya biloba Dufour, 1827 | MF496010 | NCBI | Šochová et al., 2017 |
| Ornithomya biloba Dufour, 1827 | OR054213 | NCBI | Yatsuk et al. (2023) |
| Ornithomya candidaMaa (1967) | OR064839 | NCBI | Yatsuk et al. (2024d) |
| Ornithomya chloropus Bergroth, 1901 | OR054225, OR064835, OR064836 | NCBI | Yatsuk et al. (2023) |
| Ornithomya comosa Austen, 1930 | OR064833 | NCBI | Yatsuk et al. (2023) |
| Ornithomya fringillina Curtis, 1836 | MW590981 | NCBI | Lehikoinen et al. (2021) |
| Ornithomya panovisp. n. |
PV478787, PV484792, PV484794, PV484793 |
NCBI | The current study |
| Ornithomya panovisp. n. | (Barcod number AEA 1495) HIPMJ020-19, HIPMJ025-19, HIPMJ026-19, HIPMJ027-19 |
Bold System | Meiβner et al. (2020) |
| Ornithoctona erythrocephala (Leach, 1817) | JQ246707 | NCBI | Marinho et al. (2012) |
| Ornithophila gestroi (Róndani, 1878) | KJ174684 | NCBI | Gutiérrez-López et al. (2015) |
3. Results
3.1. Taxonomic summary
Ornithomya panovi Yatsuk, Matyukhin, Shokhrin, Triseleva et Nartshuk sp. n.
(Fig. 1)
Type material examined:Holotype, female: Russia, Primorskiy Terr., Lazovsky Nature Reserve, collected from blue-and-white flycatcher (Cyanoptila cyanomelana (Temminck, 1829)), July 17, 2023 (V.P. Shokhrin). The holotype in ethanol is deposited in the collection of the Zoological Institute of the Russian Academy of Sciences, St. Petersburg (inventory number INS_DIP_0001112).
Paratype ♀ female: Russia, Primorskiy Terr., Lazovsky Nature Reserve, collected from yellow-rumped flycatcher (Ficedula zanthopygia (Hay, 1845)), July 13, 2023 (V.P. Shokhrin). The paratype in ethanol is deposited in a reference collection of the A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow.
Paratypes, used to obtain molecular data.
-
♀
female: Russia, Primorskiy Terr., Lazovsky Nature Reserve, July 2020 (V.P. Shokhrin). NCBI number: PV484792.
-
♀
female: Russia, Primorskiy Terr., Lazovsky Nature Reserve, collected from wood nuthatch (Sitta europaea Linnaeus, 1758), July 17, 2023 (V.P. Shokhrin). NCBI number: PV484794.
-
♀
female: Russia, Primorskiy Terr., Lazovsky Nature Reserve, collected from wood nuthatch (Sitta europaea Linnaeus, 1758), July 18, 2023 (V.P. Shokhrin). NCBI number: PV484793.
-
♀
2 females: Russia, Primorskiy Terr., Lazovsky Nature Reserve, collected from yellow-rumped flycatcher (Ficedula zanthopygia (Hay, 1845)) and from black-faced bunting (Ocyris spodocephala (Pallas, 1776)), July 17, 2023 (V.P. Shokhrin). ♀ 2 females: Russia, Primorskiy Terr., Lazovsky Nature Reserve, collected from wood nuthatch (Sitta europaea Linnaeus, 1758), July 15, 2023 (V.P. Shokhrin). ♀ female: Russia, Primorskiy Terr., Lazovsky Nature Reserve, collected from wood nuthatch (Sitta europaea Linnaeus, 1758), July 13, 2023 (V.P. Shokhrin). ♀ 2 females and ♂1 male: Russia, Primorskiy Terr., Lazovsky Nature Reserve (V.P. Shokhrin). NCBI number of their haplotype: PV478787.
Type host: The new species was collected on Cyanoptila cyanomelana (Temminck, 1829) – insectivorous bird from Southeast Asia Passeriformes.
Etymology: The new species is named in honor of Yevgeniy Nikolaevich Panov, doctor of biological sciences, Chief Researcher at the A. N. Severtsov Institute of Ecology and Evolution, who studied the birds of the Southern Primorye.
3.2. Description
Head and thorax length combined 2.5 mm.
Head with posterior part located between humeral tubercles and slightly covering anterior margin of thorax. Eye one-fourth as wide as head. Ocelli separated from each other by one width of ocellus. Inner orbits slightly widened posteriorly. Width of inner orbit almost equal to one-third of mediovertex width. Length of mediovertex equal to half of head length. 4 long orbital setae present in the center and 4 orbital setae – near antennae. Posterior margin of lunula rounded. Lunula horns located between antennae, clearly separated from lunula. Anterior margin of lunula horns notched. Palpus equal in length to second antennal segment. Antennae brown. Ventral side of head light.
Mesonotum light brown. Humeral tubercles approximately cone-shaped, protruding anterolaterally. Longitudinal, transversal and scuto-scutellar sutures clearly visible. Transversal suture interrupted in middle; longitudinal suture not reaching scuto-scutellar suture. Setae of mesonotum: 5 long and approximately 10 short humeral setae, approximately 12 black mesopleural setae, 1 of them long, 1 long and 3 short black notopleural setae, 2 long and 2 short postalar setae, 1 long and 1 short prescutellar setae. Setae of scutellum: thin short setae forming fringe on its posterior margin; 8–10 thin setae forming row on its anterior margin; under them in center of scutellum present short row of light setae; 8–10 long black setae forming transverse row along posterior margin of scutellum; row of light setae present above them. Ventral side of thorax light.
Wing length 5.0–5.3 mm. Wing with full venation, with three transverse and seven longitudinal veins. Costa interrupted before juncture with Sc; longitudinal veins R1, R2+3 and R4+5 connecting with costa at acute angle. Section on costa between juncture of R1 and R2+3 almost 1.3 to section between juncture of R2+3 and R4+5. The transverse vein between cells 2bc and 1m mostly unpigmented. Costa and basicosta covered with hairs. Microtrichia covering most of cell 3r, excepting small bare areas in cell base and near vien R4+5 and bare stripes along viens R4+5 and M1+2, and forming 3 stripes in cell 1m. Some paratypes have microtrichia stripe in cell 2m. Wing membrane light and transparent.
Legs light. Femora strong. Claws bifid. Empodium and paired pulvilli not reduced.
Abdomen covered with short setae. Tergite 1 + 2 with straight posterior margin. Tergite 3 approximately one fourth – one sixth as wide as abdomen. Tergite 5 approximately one sixth – one eighth as wide as abdomen. Tergite 4 is absent completely or reduced to a point. Tergite 6 divided into two oval sclerites, each with 2–4 strong black setae.
4. Discussion
4.1. Comparison
O. panovisp. n. differs from all other species of the genus Ornithomya due to the almost complete reduction of tergite 4 making it easily identifiable. Additionally, other Ornithomya species inhabiting Russia, north of Japan and Kuril Islands differ from the new species by the following features.
-
–
O. avicularia avicularia in head and thorax length combined (3.0–3.5 mm), wing length (5.5–7.0 mm) and ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (two times) (Doszhanov, 1980, 2003);
-
–
O. avicularia aobatonis in postoccipital seta line on ventral side of head (long), wing length (5.3–6.5 mm) and ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (two times) (Maa, 1967);
-
‒
O. biloba in palpus length (palpus longer than antennae), number of prescutellar setae (4–6) and arrangement of wing microtrichia (microtrichia cover almost entirely cells 3r and 1m and almost half of cell 2m) (Hutson,1984; Doszhanov, 1980, 2003);
-
–
O. candida in number of long scutellum setae (4) and ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (1.5 times);
-
–
O. chloropus in number of scutellar setae (6), color of ventral side of head (light with brown triangles on sides) and color of ventral side of thorax (light with brown diamond-shaped spots);
-
–
O. comosa in color of ventral side of head (dark brown), number of long scutellar setae (10–12) and arrangement of wing microtrichia (microtrichia cover all wing cells);
-
‒
O. fringillina in wing length (3.5–4.5 mm), number of long scutellar setae (4) and arrangement of wing microtrichia (microtrichia form 1 stripe in cell 2m) (Doszhanov, 1980, 2003);
-
‒
O. krivolutskii in number of scutellar setae (presence of 4 black long setae above 6 strong setae row along posterior margin of scutellum), ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (two times), head and thorax length combined (3 mm), wing length (4 mm) and arrangement of wing microtrichia (microtrichia cover most of cell 3r, 1m and distal part of cell 2m) (Yatsuk et al., 2023);
-
‒
O. strigilis in head and thorax length combined (4.3 mm), wing length (7.5–8 mm) and in ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (two times) (Nartshuk et al., 2022);
-
‒
O. triselevae in ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (two times), wing length (5.8–6.0 mm) and number of long scutellar setae (4) (Matyukhin et al., 2023).
-
‒
O. nazarovi in number of scutellar setae (6 black long setae forming along posterior margin) and arrangement of wing microtrichia (microtrichia almost completely cover cells 3r and 1m) (Yatsuk et al., 2024a)
-
‒
O. delichoni in ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (two times), color of ventral side of head (brown), color of ventral side of thorax (light with brown triangles on sides) and arrangement of wing microtrichia (microtrichia covering most of cells 3r and 1m and short part of cell 2m) (Nartshuk et al., 2024)
-
‒
O. helvipennis in ratio of section of costa between junctions of R1 and R2+3 to section between the junctions of R2+3 and R4+5 (two times), arrangement of wing microtrichia (microtrichia cover all wing cells) and color of ventral side of thorax (brown) (Yatsuk et al., 2024b).
O. panovisp. n. belongs to the avicularia species-group along with other species O. alpicola Maa, 1975, O. anchineuria Speiser, 1905, O. apelta Maa (1969), O. avicularia, O. bequaerti Maa (1969), O. candida, O. chloropus, O. fringillina, O. fuscipennis Bigot, 1885, O. gigantea Bear and Friedberg, 1995, O. marginalis Maa, 1964, O. medinalis Maa, 1975, O. opposita Walker, 1849, O. papillosa Maa, 1964, O. parva Macquart, 1843; Maa (1963); Dick (2006).
4.2. Molecular data
Pairwise genetic distance analysis (Table 2) showed that haplotypes from Muraviovka Park (Amur Oblast, Russia), listed in BOLD Systems and attributed by Meiβner et al. (2020) to O. avicularia aobatonis, are nearly identical (0.3 % difference) to O. panovi sp. n. We believe this identification was a misassignment, as the morphological traits in the figure provided by Meiβner et al. (2020) correspond closely with those of O. panovi sp. n., notably, the absence of tergite 4, a costal vein ratio of ∼1.3:1, and wing length of 5.2 mm. Within the genus Ornithomya the genetic distance between individual species mostly ranges from 5.5 % between O. biloba and O. comosa to 9.8 % between O. avicularia and O. comosa (Table 2). The distance between O. panovi sp. n. and its closest species O. anchinuera is 6.6–6.8 %, aligning with the genetic distance between other species within the studied genus and family (Petersen et al., 2007a, Petersen et al., 2007b; Yatsuk et al., 2024e).
Table 2.
Distances between species (%).
| Ornithomya species | panovi sp. n. (from Bold System) | panovi sp. n. (from current study) | avicularia | anchineura | fringillina | biloba | comosa | chloropus | candida |
|---|---|---|---|---|---|---|---|---|---|
| panovisp. n. (from current study) | 0.3 | ||||||||
| avicularia | 7.2 | 7.4 | |||||||
| anchineura | 6.6 | 6.8 | 6.7 | ||||||
| fringillina | 7.6 | 7.8 | 6.6 | 6.3 | |||||
| biloba | 8.9 | 9.2 | 8.5 | 8.9 | 6.9 | ||||
| comosa | 9.5 | 9.7 | 9.8 | 9.5 | 7.1 | 5.5 | |||
| chloropus | 8.0 | 8.2 | 7.5 | 7.7 | 6.3 | 9.5 | 9.4 | ||
| candida | 7.5 | 7.6 | 6.4 | 6.4 | 2.0 | 6.2 | 7.1 | 6.5 | |
| bequaerti | 7.3 | 7.5 | 6.2 | 6.4 | 2.0 | 5.8 | 6.8 | 6.5 | 0.3 |
Among the 11 sequences of O. panovi sp. n. generated in this study and four sequences from BOLD Systems, we identified four haplotypes. Phylogenetic analysis (Fig. 2) indicates two species groups within the genus Ornithomya. One group includes O. panovi sp. n., O. avicularia, and O. anchinuera, while the second group comprises O. bequaerti, O. biloba, O. candida, O. comosa, and O. fringillina. The species O. chloropus stands apart. The observed divergence of louse fly species is consistent with the phylogenetic framework proposed for the family Hippoboscidae (Yatsuk et al., 2024d). Unfortunately, molecular data are currently unavailable for several species known from the Russian Far East (O. strigilis, O. triselevae, O. krivolutskii, O. nazarovi, O. delichoni, and O. helvipennis), limiting their inclusion in the present phylogenetic analysis. Future sequencing efforts targeting these species will be essential for resolving their taxonomic placement and for achieving a more complete understanding of Ornithomya diversity in this understudied region.
Fig. 2.
Reconstructed phylogenetic tree of the Ornithomya species with the representatives of the genera Ornithophila and Ornithoctona as an outgroups.
Maximum likelihood bootstrap support is shown in the nods; scale bar depicts the genetic distance between haplotypes.
∗ – specimens, that were previously erroneously identified as O. avicularia aobatonis.
Thus, the newly described species, O. panovi sp. n., differs from all known Ornithomya species both morphologically and genetically. This species is likely one of the most widespread species of this genus in the Russian Far East. O. panovi sp. n. is, to our knowledge, the first described species in Ornithomya in which tergite 4 is entirely absent or reduced to a minute point.
CRediT authorship contribution statement
Aleksandra Yatsuk: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Data curation, Conceptualization. Emilia Nartshuk: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Tatiana Triseleva: Writing – review & editing, Writing – original draft, Methodology, Data curation. Valeriy Shokhrin: Writing – review & editing, Writing – original draft, Methodology. Oleg Tolstenkov: Writing – review & editing, Writing – original draft, Conceptualization. Andrey Safonkin: Writing – review & editing, Writing – original draft, Methodology. Alexandr Matyukhin: Writing – review & editing, Writing – original draft, Methodology, Conceptualization.
Statements and declarations
The authors declare no competing interests.
This research was not conducted on vertebrates. Furthermore, hippoboscid flies are not a protected species and no ethical approval or research permits were required.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank our colleagues from the Joint Usage Center “Instrumental methods in ecology” at the IEE RAS for their comprehensive assistance and support. We also thank Sergei A. Shchedrin (Moscow, Russia) for taking pictures of specimen. The work was performed as part of the State Research Projects of the A.N. Severtsov Institute of Ecology and Evolution (number 1022061500172-3-1.6.19) for A.A. Yatsuk, A.V. Matyukhin and of Zoological Institute project (number 125012901042-9) for E.P. Nartshuk.
References
- Bequaert J.C. The hippoboscidae or louse-flies (diptera) of mammals and birds. Part II. Taxonomy, evolution and revision of American genera and species. Entomol. Am. 1954;34:1–232. [Google Scholar]
- de Moya R.S. Implications of a dating analysis of hippoboscoidea (diptera) for the origins of phoresis in feather lice (psocodea: phthiraptera: Philopteridae) Insect Systematic. Divers. 2019;3(4):1–5. doi: 10.1093/isd/ixz008. [DOI] [Google Scholar]
- Dick C.W. Department of Zoology, Field Museum of Natural History; Chicago: 2006. Checklist of World Hippoboscidae (Diptera: Hippoboscoidea) [Google Scholar]
- Doszhanov T.N. Nauka; Alma-Ata: 1980. Mukhi-Krovososki (Diptera, Hippoboscidae) Kazakhstana (Louse Flies (Diptera, Hippoboscidae) of Kazakhstan) [Google Scholar]
- Doszhanov T.N. Nauka; Almaty: 2003. Mukhi-Krovososki (Diptera, Hippoboscidae) Palearktiki (Louse Flies (Diptera, Hippoboscidae) of the Palaearctic) [Google Scholar]
- Fain A. Paleis der Academien; Brussel: 1965. A Review of the Family Epidermoptidae Trouessart Parasitic on the Skin of Birds (Acarina: Sarcoptiformes). Part 1. [Google Scholar]
- Fain A. Paleis der Academien; Brussel: 1965. A Review of the Family Epidermoptidae Trouessart Parasitic on the Skin of Birds (Acarina: Sarcoptiformes). Part 2. [Google Scholar]
- Farajollahi A., Crans V.J., Nickerson D., Bryant P., Wolf B., Glaser F., Andreadis T.G. Detection of west nile virus RNA from the louse fly icosta Americana (diptera: Hippoboscidae) J. Am. Mosq. Control Assoc. 2005;21(4):474. doi: 10.2987/8756-971X(2006)21(474:DOWNVR)2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3(5):294–299. [PubMed] [Google Scholar]
- Ganez A.Y., Baker I.K., Lindsay R., Dibernardo A., McKeever K., Hunter B. West nile virus outbreak in North American owls, Ontario, 2002. Emerg. Infect. Dis. 2004;10(12):2135–2142. doi: 10.3201/eid1012.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-López R., Martínez-de la Puente J., Gangoso L., Soriguer R.C., Figuerola J. Comparison of manual and semi-automatic DNA extraction protocols for the barcoding characterization of hematophagous louse flies (diptera: Hippoboscidae) J. Vector Ecol. 2015;40(1):11–15. doi: 10.1111/jvec.12127. [DOI] [PubMed] [Google Scholar]
- Hill D.S., Wilson N., Corbet G.B. Mites associated with British species of ornithomya (diptera: Hippoboscidae) J. Med. Entomol. 1967;4(2):102–122. doi: 10.1093/jmedent/4.2.102. [DOI] [PubMed] [Google Scholar]
- Hutson A.M. vol. 10. Royal Entomological Society of; London, London: 1984. Handbooks for the identification of British insects. (Part 7. Hippoboscidae and Nycteribiidae (Keds, flat-flies and bat-flies)). [Google Scholar]
- Khametova A.P., Pichurina N.L., Zabashta M.V., Romanova L.V., Orekhov I.V., Borodina T.N., Adamenko V.I., Zabashta A.V. Biocenotic structure of natural focus of borreliosis in the Rostov region. Med. Parazitol. Parazit. Bolezni. 2018;4:33–39. doi: 10.33092/0025-8326mp2018.4.33-39. [DOI] [Google Scholar]
- Lee L., Tan D.J.X., Oboňa J., Gustafsson D.R., Ang Y., Meier R. Hitchhiking into the future on a fly: toward a better understanding of phoresy and avian louse evolution (phthiraptera) by screening bird carcasses for phoretic lice on hippoboscid flies (diptera) Syst. Entomol. 2022;47(3):420–429. doi: 10.1111/syen.12539. [DOI] [Google Scholar]
- Lehikoinen A., Pohjola P., Valkama J., Mutanen M., Pohjoismäki J.L.O. Promiscuous specialists: host specificity patterns among generalist louse flies. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0247698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque-Beaudin V., Sinclair B.J. Louse fly (diptera, hippoboscidae) associations with raptors in southern Canada, with new north American and European records. Int. J. Parasitol. Parasites Wildl. 2021;16:168–174. doi: 10.1016/j.ijppaw.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa T.C. Genera and species of hippoboscidae (diptera): types, synonymy, habitats and natural groupings. Pac. Insects Monogr. 1963;6:1–186. [Google Scholar]
- Maa T.C. Redescription of the fossil Ornithomya rottensis (statz) Pac. Insects Monogr. 1966;10:3–9. [Google Scholar]
- Maa T.C. A synopsis of diptera pupipara of Japan. Pac. Insects. 1967;9(4):727–760. [Google Scholar]
- Maa T.C. A revised checklist and concise host index of hippoboscidae (diptera). Pac. Insects Monogr. 1969;20:261–299. [Google Scholar]
- Marinho M.A., Junqueira A.C., Paulo D.F., Esposito M.C., Villet M.H., Azeredo-Espin A.M. Molecular phylogenetics of oestroidea (diptera: calyptratae) with emphasis on calliphoridae: insights into the inter-familial relationships and additional evidence for paraphyly among blowflies. Mol. Phylogenet. Evol. 2012;65(3):840–854. doi: 10.1016/j.ympev.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Matyukhin A.V., Yatsuk A.A., Red’kin Ya A., Smirnov P.A., Nartshuk E.P. A new species of the genus Ornithomya latreille (diptera, hippoboscidae) from iturup (kuril islands) Entomol. Rev. 2023;103(4):450–454. doi: 10.1134/S0013873823040061. [DOI] [Google Scholar]
- Meißner B.R., Rodríguez-Vera F., Hawlitschek O., Heim W., Jentzsch M. Incidence of louse flies on birds from the Russian far east (diptera: Hippoboscidae) and investigation of the status of some taxa by DNA barcoding. Russ. Entomol. J. 2020;29(3):327–335. doi: 10.15298/rusentj.29.3.14. [DOI] [Google Scholar]
- Mogi M., Kim H.-C., Klein T.A. Notes on diptera pupipara of the eastern palaearctic region 1. The taxonomic status of species and subspecies of hippoboscidae and nycteribiidae described from temperate Japan and the Republic of Korea. Med. Entomol. Zool. 2023;74(1):1–5. doi: 10.7601/mez.74.1. [DOI] [Google Scholar]
- Nartshuk E.P., Yatsuk A.A., Matyukhin A.V., Shokhrin V.P. A new species of the genus ornithomya (diptera: Hippoboscidae) from the far east. Zoosyst. Ross. 2022;31(2):190–194. doi: 10.31610/zsr/2022.31.2.190. [DOI] [Google Scholar]
- Nartshuk E.P., Matyukhin A.V., Markovets M.Yu, Yatsuk A.A. Description of a new Ornithomya latreille, 1802 (diptera: Hippoboscidae) species with a key to all species of this genus. Proc. Zoological Inst. RAS. 2024;328(4):640–657. doi: 10.31610/trudyzin/2024.328.4.640. [DOI] [Google Scholar]
- Oboňa J., Sychra O., Greš S., Heřman P., Manko P., Roháček J., Šestáková A., Šlapák J., Hromada M. A revised annotated checklist of louse flies (diptera, hippoboscidae) from Slovakia. ZooKeys. 2019;862:129–152. doi: 10.3897/zookeys.862.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Espinoza M., Em D., Shahi-Barogh B., Berer D., Duscher G.G., van der Vloedt L., Glawischnig W., Rehbein S., Harl J., Unterköfler M.S., Fuehrer H.-P. Molecular pathogen screening of louse flies (diptera: Hippoboscidae) from domestic and wild ruminants in Austria. Parasit. Vectors. 2023;16:179. doi: 10.1186/s13071-023-05810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen F.T., Damgaard J., Meier R. DNA taxonomy: how many DNA sequences are needed for solving a taxonomic problem? The case of two parapatric species of louse flies (diptera: hippoboscidae: ornithomya latreille, 1802) Arthropod Syst. Phylogeny. 2007;65(2):119–125. doi: 10.3897/asp.65.e31670. [DOI] [Google Scholar]
- Petersen F.T., Meier R., Kutty S.N., Wiegmann B.M. The phylogeny and evolution of host choice in the hippoboscoidea (diptera) as reconstructed using four molecular markers. Mol. Phylogenet. Evol. 2007;45(1):111–122. doi: 10.1016/j.ympev.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Philips J.R., Fain A. Acarine symbionts louse flies (diptera: Hippoboscidae) Acarologia. 1991;32(4):377–384. [Google Scholar]
- Šochová E., Husník F., Nováková E., Halajian A., Hypša V. Arsenophonus and sodalis replacements shape evolution of symbiosis in louse flies. PeerJ. 2017;5 doi: 10.7717/peerj.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maxi-mum parsimony methods. Mol. Biol. Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawman D.C. Ornithomya biloba, Pseudolynchia garzettae and Pseudolynchia canariensis (diptera: Hippoboscidae): three new United Kingdom colonists and potential disease vectors. Med. Vet. Entomol. 2023:1–12. doi: 10.1111/mve.12703. [DOI] [PubMed] [Google Scholar]
- Yatsuk A.A., Matyukhin A.V., Shapoval A.P., Nartshuk E.P. A new species of Ornithomya latreille, 1802 (diptera: Hippoboscidae) from the curonian spit (russia) Caucasian Entomol. Bull. 2023;19(1):101–104. doi: 10.5281/zenodo.8367753. [DOI] [Google Scholar]
- Yatsuk A.A., Nartshuk E.P., Red'kin Y.A., Smirnov P.A., Matyukhin A.V. Description of a new Ornithomya latreille, 1802 species (diptera: Hippoboscidae) from simushir island, Russia. Caucasian Entomol. Bull. 2024;20(2):255–258. doi: 10.5281/zenodo.14414744. [DOI] [Google Scholar]
- Yatsuk A.A., Nartshuk E.P., Matyukhin A.V. Description of a new louse fly species of the genus Ornithomya latreille, 1802 (diptera: Hippoboscidae) from Irkutsk, Russia. Caucasian Entomol. Bull. 2024;20(1):83–87. doi: 10.5281/zenodo.10894260. [DOI] [Google Scholar]
- Yatsuk A.A., Triseleva T.A., Matyukhin A.V., Nartshuk E.P. New data on classification of hippoboscidae (diptera): Genus lipoptena nitzsch, 1818. Euroasian Entomol. J. 2024;23(6):353–359. doi: 10.15298/euroasentj.23.06.12. [DOI] [Google Scholar]
- Yatsuk A.A., Triseleva T.A., Narchuk E.P., Matyukhin A.V., Safonkin A.F. Morphology of the wings and attachment apparatus in the evolution of the family hippoboscidae (diptera) Integr. Zool. 2024;19:941–954. doi: 10.1111/1749-4877.12786. [DOI] [PubMed] [Google Scholar]
- Yatsuk A.A., Triseleva T.A., Matyukhin A.V., Safonkin A.F., Narchuk E.P. Two cases of introducing Lipoptena fortisetosa maa (diptera: Hippoboscidae) into Europe through different deer species. J. Nat. Hist. 2024;58:1787–1801. doi: 10.1080/00222933.2024.2395905. [DOI] [Google Scholar]