Abstract
Chemotherapy-free regimens have gained increasing attention as frontline treatments for B cell acute lymphoblastic leukemia (B-ALL) due to the limitations of chemotherapy. Zhang and colleagues conducted the first trial incorporating CAR-T into Ph+ B-ALL frontline treatment, demonstrating remarkable efficacy and tolerability.1
Chemotherapy-free regimens have gained increasing attention as frontline treatments for B cell acute lymphoblastic leukemia (B-ALL) due to the limitations of chemotherapy. Zhang and colleagues conducted the first trial incorporating CAR-T into Ph+ B-ALL frontline treatment, demonstrating remarkable efficacy and tolerability.
Main text
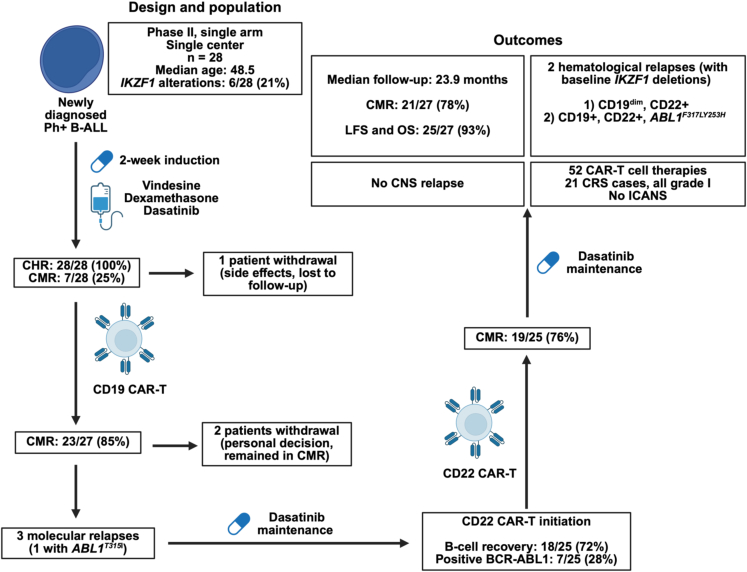
Immunotherapies, notably CD19-CAR-T and blinatumomab, have significantly improved outcomes for relapsed or refractory (r/r) B cell acute lymphoblastic leukemia (B-ALL). To enhance efficacy and reduce chemotherapy usage, the combination of tyrosine kinase inhibitors (TKIs) and blinatumomab has been investigated for frontline treatment of Ph+ B-ALL and has shown paradigm-shifting results.2 However, CAR-T therapy had not yet been investigated for treating newly diagnosed Ph+ B-ALL. The study by Zhang and colleagues is the first to incorporate cellular therapy as frontline treatment for Ph+ B-ALL. This single-center, single-arm phase 2 clinical trial enrolled 28 patients with a median age of 48.5 years (range 18–69) who underwent induction therapy with vindesine, dasatinib, and dexamethasone followed by sequential CD19-directed and, upon B cell recovery or failure to achieve complete molecular response (CMR), CD22-directed CAR-T cell therapy followed by dasatinib maintenance. The CMR rate at the end of consolidation treatment was 76%, and, with a median follow-up of 23.9 months, 2-year leukemia-free survival (LFS) and overall survival (OS) were 93%. A total of 23 cytokine release syndrome events, all grade 1, were observed over 52 CAR-T cell treatments, and no cases of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed (Figure 1).1 These outcomes are comparable to those of phase 2 trials of TKIs combined with blinatumomab for newly diagnosed Ph+ B-ALL.1,2,3,4 Of note, longer-term follow-up (median: 53 months) of frontline dasatinib + blinatumomab (GIMEMA LAL2116, D-ALBA trial) for Ph+ B-ALL has achieved an LFS of 76% and an OS of 81%.5 It will be interesting to compare longer-term survival data of sequential CAR-T cell therapy vs. blinatumomab. However, direct comparison might be challenging due to differing populations. Notably, IKZF1 alterations were seen in 21% of individuals in the study by Zhang and colleagues but in 54% of patients in the GIMEMA LAL2116, D-ALBA trial, which, in both cases, appears significantly lower than general rates of IKZF1 alterations in adult Ph+ B-ALL.6 IKZF1 alterations are associated with worse outcomes, including with cellular and immune therapies.1,2 In the study by Zhang and colleagues, the two patients that developed hematological relapses harbored baseline IKZF1 deletions.1
Figure 1.
Study design, treatment strategy, and outcomes
IKZF1 alterations therefore remain a critical biomarker in the era of cellular and immune therapies for B-ALL. One of the factors explaining worse outcomes with loss of IKZF1 might be control of IKAROS on expression of CD19 and CD22. Indeed, chemogenetic approaches have shown a dose-relationship response between IKAROS expression and CD19 and CD22 surface levels.7 However, this is unlikely to be the sole determinant of worse outcomes associated with IKZF1 alterations, as CD19+/CD22+ relapses can also occur in that setting, as shown in the study by Zhang and colleagues.1 Nevertheless, antigen loss remains one of the major mechanisms of relapses after CAR-T cell therapy. In that regard, sequential CD19/CD22 CAR-T, while seemingly not leading to a substantial increase in CMR rates (Figure 1), might decrease rates of relapses associated with antigen loss. Administration of CD22-directed CAR-T cell therapy in patients who relapsed after CD19-directed immunotherapy has shown promising results, with an OS of 52.6% with a median follow-up of 38 months. Interestingly, among the six patients who were refractory to CD22-targeting inotuzumab treatment, four reached hematological remission after CD22-CAR-T infusion, highlighting the potential superior efficacy of CAR-T cell over antibody therapy under certain circumstances.8 Similarly, a phase 2 trial has investigated sequential infusion of CD19- and CD22-directed CAR-T cells for childhood r/r B-ALL, reporting a 1-year LFS of 80%.9 It remains to be determined whether upfront sequential CAR-T cell therapy is a superior approach. A key argument in that favor is that heavily pretreated patients might have compromised T cell quality, thus hampering the CAR-T cell manufacturing and treatment process. In addition, sequential CAR-T cell therapy could be safer in the frontline than in the r/r setting, as CD19/CD22 sequential treatment led very high rates of CRS (80% grade 1–2, 15% grade 3) and ICANS (32% grade 1–2, 4% grade 3, 1% grade 4) compared to a 40% rate of CRS (all grade 1) and no ICANS in the frontline setting.1,9 Many variables might account for these major differences in safety signals, including different patient populations (adult vs. pediatric), prior lines of therapy with r/r B-ALL, and the likely better tumor debulking with frontline induction therapy. On a similar note, one notable advantage of CAR-T cell over antibody therapy is its potential for prolonged persistence and durable remissions, although more mature data will be required to see whether this translates to improved long-term outcomes. An important variable in the study by Zhang and colleagues is also the concurrent use of dasatinib, with pre-clinical studies showing that dasatinib might reduce cytotoxicity but prevent exhaustion and improve persistence of CAR-T cells.10
Overall, this innovative clinical trial sheds light on frontline CAR-T cell therapy for Ph+ B-ALL in an era where chemotherapy-free regimens, notably with TKIs plus blinatumomab, are becoming increasingly established as standards of care. While blinatumomab has the benefit of being an off-the-shelf approach, CAR-T has several advantages, including persistence, possibility of overcoming antigen loss through sequential CD19- and CD22-targeting, and better central nervous system penetrance. Moreover, if effective, frontline CAR-T cell therapy could significantly shorten the treatment duration, in which a single CAR-T infusion could potentially replace four to five cycles of blinatumomab. Notably, the findings from this study also support the potential application of frontline CAR-T to treat Ph− B-ALL. However, more mature data and larger trials are required to further evaluate this strategy. Combination therapy with ponatinib could also be considered, notably to prevent relapses with the ABL1T315 mutation.
Declaration of interests
J.H.P. received consulting fees from Adaptive Biotechnologies, Affyimmune Therapeutics, Allogene Therapeutics, Amgen, Artiva Biotherapeutics, Ascentage, Autolus, Beigene, Bright Pharmaceutical Services Inc., Caribou Bioscience, Curocell, Kite, Galapagos, Iovance, Jazz Pharmaceuticals, Medpace, Pfizer, Servier, Sobi, Synthekine, and Takeda. M.M. is a scientific advisory board member of Liangzhu Laboratory and the Versiti Blood Research Institute.
References
- 1.Zhang M., Fu S., Feng J., Hong R., Wei G., Zhao H., Zhao M., Xu H., Cui J., Huang S., et al. Dasatinib and CAR T-Cell Therapy in Newly Diagnosed Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia: A Nonrandomized Clinical Trial. JAMA Oncol. 2025 doi: 10.1001/jamaoncol.2025.0674. https://jamanetwork.com/journals/jamaoncology/fullarticle/2832720 Published online April 17, 2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foà R., Bassan R., Vitale A., Elia L., Piciocchi A., Puzzolo M.C., Canichella M., Viero P., Ferrara F., Lunghi M., et al. Dasatinib–Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020;383:1613–1623. doi: 10.1056/NEJMoa2016272. [DOI] [PubMed] [Google Scholar]
- 3.Advani A.S., Moseley A., O’Dwyer K.M., Wood B.L., Park J., Wieduwilt M., Jeyakumar D., Yaghmour G., Atallah E.L., Gerds A.T., et al. Dasatinib/prednisone induction followed by blinatumomab/dasatinib in Ph+ acute lymphoblastic leukemia. Blood Adv. 2023;7:1279–1285. doi: 10.1182/bloodadvances.2022008216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabbour E., Short N.J., Jain N., Huang X., Montalban-Bravo G., Banerjee P., Rezvani K., Jiang X., Kim K.H., Kanagal-Shamanna R., et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet. Haematol. 2023;10:e24–e34. doi: 10.1016/S2352-3026(22)00319-2. [DOI] [PubMed] [Google Scholar]
- 5.Foà R., Bassan R., Elia L., Piciocchi A., Soddu S., Messina M., Ferrara F., Lunghi M., Mulè A., Bonifacio M., et al. Long-Term Results of the Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL. J Clin Oncol Off J Am Soc Clin Oncol. 2024;42:881–885. doi: 10.1200/JCO.23.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullighan C.G., Miller C.B., Radtke I., Phillips L.A., Dalton J., Ma J., White D., Hughes T.P., Le Beau M.M., Pui C.-H., et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 7.Domizi P., Sarno J., Jager A., Merchant M., Pacheco K.Z.B., Yamada-Hunter S.A., Rotiroti M.C., Liu Y., Baskar R., Reynolds W.D., et al. IKAROS levels are associated with antigen escape in CD19- and CD22-targeted therapies for B-cell malignancies. Nat. Commun. 2025;16:3800. doi: 10.1038/s41467-025-58868-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers R.M., DiNofia A.M., Li Y., Diorio C., Liu H., Wertheim G., Fraietta J.A., Gonzalez V., Plesa G., Siegel D.L., et al. CD22-targeted chimeric antigen receptor-modified T cells for children and adults with relapse of B-cell acute lymphoblastic leukemia after CD19-directed immunotherapy. J. Immunother. Cancer. 2025;13 doi: 10.1136/jitc-2025-011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan J., Tang K., Luo Y., Seery S., Tan Y., Deng B., Liu F., Xu X., Ling Z., Song W., et al. Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2023;24:1229–1241. doi: 10.1016/S1470-2045(23)00436-9. [DOI] [PubMed] [Google Scholar]
- 10.Weber E.W., Lynn R.C., Sotillo E., Lattin J., Xu P., Mackall C.L. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3:711–717. doi: 10.1182/bloodadvances.2018028720. [DOI] [PMC free article] [PubMed] [Google Scholar]