Abstract
Hereditary hemochromatosis (HH) is a disorder of iron metabolism in which enhanced iron absorption of dietary iron causes increased iron accumulation in the liver, heart, and pancreas. Most individuals with HH are homozygous for a C282Y mutation in the HFE gene. The function of HFE protein is unknown, but it is hypothesized that it acts in association with β2-microglobulin and transferrin receptor 1 to regulate iron uptake from plasma transferrin by the duodenum, the proposed mechanism by which body iron levels are sensed. The aim of this study was to test this hypothesis by comparing clearance of transferrin-bound iron in Hfe knockout (KO) mice with that observed in C57BL/6 control mice. The mice were fed either an iron-deficient, control, or iron-loaded diet for 6 weeks to alter body iron status. The mice then were injected i.v. with 59Fe-transferrin, and blood samples were taken over 2 h to determine the plasma 59Fe turnover. After 2 h, the mice were killed and the amount of radioactivity in the duodenum, liver, and kidney was measured. In both Hfe KO and C57BL/6 mice, plasma iron turnover and iron uptake from plasma transferrin by the duodenum, liver, and kidney correlated positively with plasma iron concentration. However, duodenal iron uptake from plasma transferrin was decreased in the Hfe KO mice compared with the control mice. Despite this difference in duodenal uptake, the Hfe KO mice showed no decrease in iron uptake by the liver and kidney or alteration in the plasma iron turnover when compared with C57BL/6 mice. These data support the hypothesis that HFE regulates duodenal uptake of transferrin-bound iron from plasma, and that this mechanism of sensing body iron status, as reflected in plasma iron levels, is impaired in HH.
Hereditary hemochromatosis (HH) is a common autosomal recessive inherited disorder of iron metabolism. Most individuals with HH are homozygous for a missense (C282Y) mutation in the HFE gene that encodes a major histocompatibility class 1-like protein (1–4). One in 200 adults of Caucasian descent have this genotype (1, 2). The disease is characterized by increased absorption of dietary iron by the gastrointestinal tract and the progressive deposition of iron in the parenchymal cells, which can result in hepatic fibrosis, cirrhosis, and hepatocellular carcinoma (3).
The expression of HFE protein is widespread in many tissues, including the gastrointestinal tract. In the duodenum, HFE is localized in the perinuclear region of the enterocytes in the crypt region but not in the villus region where iron is absorbed from the lumen (5). The localization of HFE in the crypt enterocytes suggests that its function is to regulate, rather than directly participate in, iron absorption.
Dietary iron absorption is a complex process and is inversely regulated by the body iron status. Thus, iron absorption is increased with iron deficiency and decreased with iron overload. The enterocytes in the crypt region of the duodenum, which take up iron from plasma transferrin at their basolateral surfaces, migrate to the villus region where they differentiate into absorptive cells that absorb dietary iron from their luminal surfaces (6). The iron level in these differentiating cells, which is thought to be predetermined in the crypt cells, controls the expression of iron transporters such as divalent metal transporter 1 and ferroportin 1 and, subsequently, the rate of iron absorption from the lumen. In HH, dietary iron absorption is increased even in the presence of elevated plasma transferrin saturation and increased hepatic iron stores (7). This observation suggests that the regulation of iron absorption by the body iron stores is impaired, and that the duodenum is acting as if it were relatively iron-deficient, with increased expression of iron transporters leading to increased iron absorption (8, 9).
The precise function of HFE in the crypt cells is unknown. The cells express transferrin receptor 1 (TfR1) [and possibly transferrin receptor 2 (TfR2)] which mediates the uptake of transferrin-bound iron (10–12). HFE protein has been found to be associated closely with TfR1 in the crypt cells where it could influence the rate of uptake of transferrin-bound iron uptake by the crypt cells (13).
In this study, we tested the hypothesis that HFE normally participates in sensing body iron stores by regulating uptake of transferrin-bound iron from the plasma by the duodenum, and that this function is impaired in HH. Hfe knockout (KO) mice and C57BL/6 control mice were fed iron-deficient, basal, and iron-loaded diets to alter their body iron status. The mice were injected i.v. with 59Fe-transferrin, and the uptake of plasma iron by the duodenum was measured. Results indicated that iron uptake from the plasma by the duodenum of the Hfe KO mice was decreased significantly compared with that of the C57BL/6 control mice, supporting the hypothesis that HFE participates in the mechanism by which body iron levels are sensed in the duodenal crypt cells.
Materials and Methods
Animals.
A mouse model of HH was used in which the Hfe gene has been disrupted by homologous recombination, as described by Zhou et al. (14). Female Hfe KO and C57BL/6 mice (obtained from Animal Resource Centre, Murdoch, Western Australia) were fed a purified diet (AIN-93) as recommended by the American Institute of Nutrition (15) ad libitum from weaning at 3 weeks of age for 6 weeks. Hfe KO and C57BL/6 mice were divided into three groups and were fed either an iron-deficient diet (<10 mg/kg), a basal iron diet containing 35 mg/kg added iron as ferric citrate, or an iron-loaded diet supplemented with 20 g/kg carbonyl iron (Sigma). This study was approved by The University of Western Australia Animal Ethics Committee.
Plasma Iron Clearance.
Mouse transferrin and albumin were prepared and labeled with iron-59, iodine-125, and iodine-131, respectively (NEN, Melbourne, Australia), as described (16). Hfe KO and C57BL/6 control mice were injected in the lateral tail vein with 30 μl of mixture containing 150 μg of 59Fe-125I-transferrin and 150 μg of 131I-albumin. Blood samples (50 μl) were collected in hematocrit tubes from the tail vein at 2, 30, 60, and 90 min. Two h after the injection, the mice were anesthetized with an i.p. injection of sodium pentobarbital. Blood was collected from the right ventricle of the heart, and the mice were perfused through the left ventricle with 10 ml of heparinized saline. The duodenum, liver, and kidneys were removed and homogenized by using a Dounce glass homogenizer (Kontes). Trichloroacetic acid (final concentration 10%) was added to an aliquot of the homogenized tissues to precipitate the protein-bound 125I and 131I. After 10 min on ice, the protein precipitate and supernatant were separated by centrifugation. Radioactivity was measured in the tissues and plasma with an LKB-Wallac 1281 Compugamma gamma counter. The amount of 59Fe in the plasma samples taken at various time intervals after the injection of radioactivity was used to calculate the rate of plasma iron clearance. The specific activity of 59Fe-transferrin was corrected for the different pool sizes of circulating transferrin-bound iron in mice fed diets containing differing levels of iron using the plasma iron concentration. The amount of 131I-albumin in the plasma and tissues was used to determine the plasma volume and the amount of plasma remaining in the tissue after perfusion. Tissue 59Fe uptake was corrected for transferrin-bound iron by measuring the amount of 125I-transferrin in the tissues (17).
Analytical Methods.
Liver non-heme iron concentration was measured by the method of Kaldor (18), and plasma iron concentration was determined by an approved method of the International Committee for Standardization in Haematology (19).
Statistical Analysis.
Results were expressed as means ± SE where appropriate. Differences between groups were determined by performing ANOVA and a Tukey's multiple comparison test by using the GraphPad PRISM program (GraphPad, San Diego). Regression analysis was performed where appropriate to determine whether there was a linear relationship between variables; analysis of co-variance was performed to determine differences in the slope and intercept between two regression analyses. Regression analysis and analysis of co-variance were performed with the Statistics Package for Social Scientists (SPSS, V.10). Statistical significance was accepted for P < 0.05.
Results
Liver Non-Heme Iron Concentration.
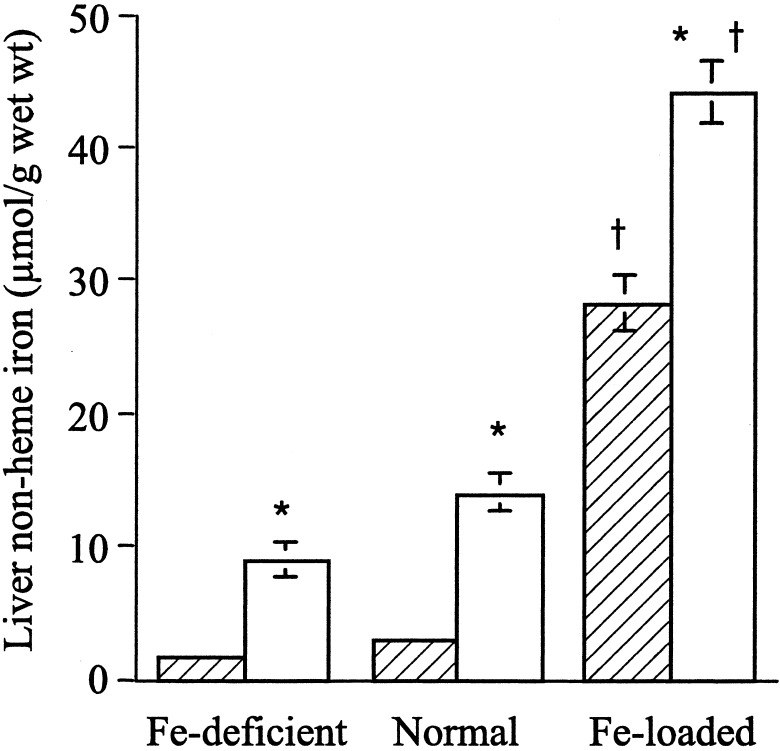
Hfe KO and C57BL/6 mice were fed either an iron-deficient, basal, or iron-loaded diet. In both the Hfe KO and C57BL/6 mice, there was a significant increase in the liver non-heme iron levels in mice fed an iron-loaded diet compared with the basal and iron-deficient diets after 6 weeks (Fig. 1). However, there was significantly more non-heme iron in the liver of the Hfe KO mice compared with the C57BL/6 control mice for all iron diets (Fig. 1).
Figure 1.
Liver non-heme iron concentration in Hfe KO (white bars) and C57BL/6 (cross-hatched bars) control mice fed an iron-deficient, normal (control) or iron-loaded diet for 6 weeks. Results are expressed as the means ± SE where n = 4–9 mice per group. Significant differences between groups: *, P < 0.001, Hfe KO vs. C57BL/6 mice; †, P < 0.001, iron-loaded diet vs. normal and iron-deficient diet.
Plasma Iron Concentration.
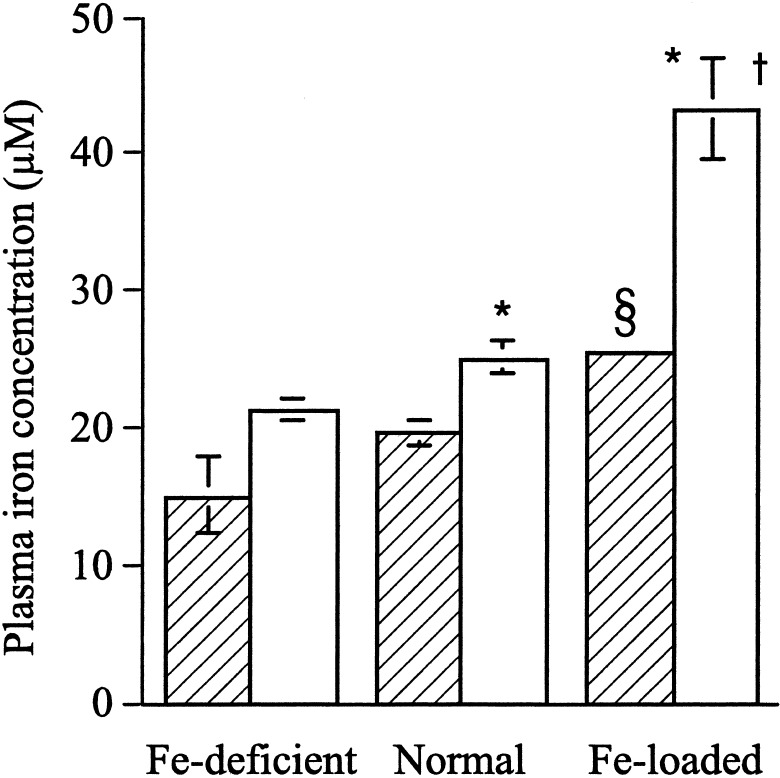
Plasma iron concentrations increased with dietary iron loading in both C57BL/6 and Hfe KO mice (Fig. 2). Hfe KO mice have significantly higher plasma iron concentrations than C57BL/6 mice fed control and iron-loaded diets, but not those fed the iron-deficient diet.
Figure 2.
Plasma iron concentration in Hfe KO (white bars) and C57BL/6 (cross-hatched bars) mice fed an iron-deficient, normal, or iron-loaded diet for 6 weeks. The results are given as means ± SE where n = 4–9 mice per group. Significant differences: *, P < 0.05 Hfe KO vs. C57BL/6 mice; †, P < 0.001, Hfe KO mice iron-loaded diet vs. normal and iron-deficient diet; §, P < 0.05 C57BL/6 mice iron-loaded diet vs. normal and iron-deficient diet.
Transferrin-Bound Iron Uptake by the Duodenum.
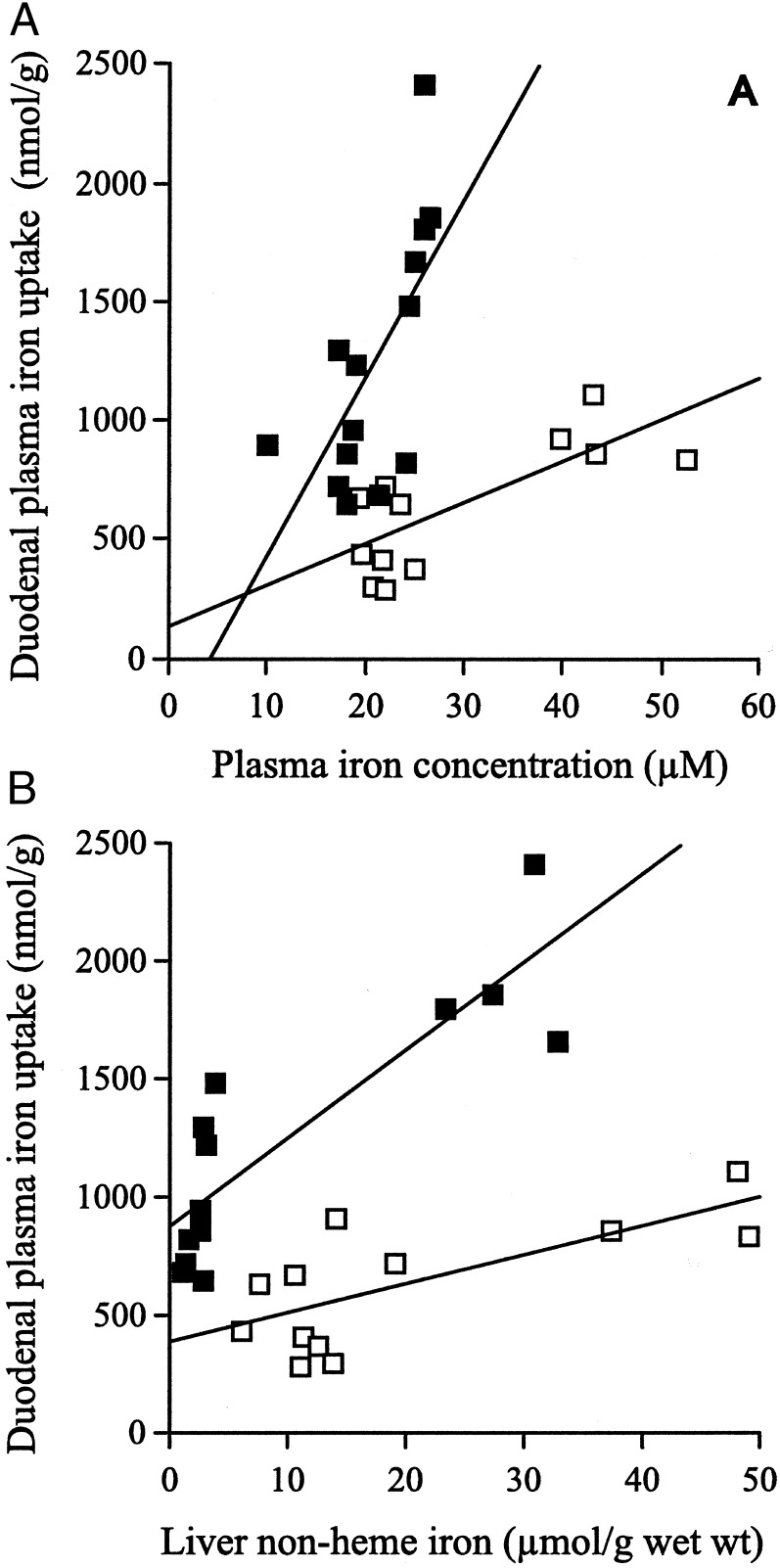
To determine the uptake of plasma transferrin-bound iron by the duodenum, the Hfe KO and C57BL/6 mice were given an i.v. injection of 59Fe-transferrin, and the amount of 59Fe in the duodenum was measured after 2 h. There was an increase in the uptake of plasma transferrin-bound iron by the duodenum with increasing plasma iron concentrations in both Hfe KO and control mice. The uptake of plasma transferrin-bound iron by the duodenum in both the Hfe KO and C57BL/6 control mice was correlated linearly with the plasma iron levels (Hfe KO, r2 = 0.57, P = 0.004; C57BL/6, r2 = 0.44, P = 0.009; Fig. 3A) However, there was a significant difference in the amount of uptake of plasma transferrin-bound iron by the duodenum in the Hfe KO and C57BL/6 control mice.
Figure 3.
Plasma transferrin-bound iron uptake by the duodenum in Hfe KO (□) and C57BL/6 (■) mice. Results are plotted in relation to plasma iron concentration (A) or liver non-heme iron concentration (B). There was a positive correlation of duodenal uptake of plasma transferrin-bound iron by the Hfe KO and C57BL/6 control mice with the plasma iron levels (Hfe KO, r2 = 0.57, P = 0.004; C57BL/6, r2 = 0.44, P = 0.009) and liver non-heme iron levels (Hfe KO, r2 = 0.51, P = 0.009; C57BL/6, r2 = 0.75, P = 0.001). There was a significant difference in the slope (P = 0.012) but not in the value of the intercepts (P = 0.37) of the regression lines for plasma transferrin-bound iron uptake with increasing plasma iron levels for Hfe KO vs. the C57BL/6 control mice. There was a significant difference between both the slope (P = 0.002) and intercept (P = 0.003) of the regression lines for plasma transferrin-bound iron uptake with increasing liver non-heme iron levels for Hfe KO vs. the control mice.
This difference in uptake depended on the plasma iron concentration. In fact, at low plasma iron concentrations, plasma transferrin-bound iron uptake was similar in the Hfe KO and control mice. However, as the plasma iron concentration increased, plasma transferrin-bound iron uptake in the Hfe KO mice increased less than in the control mice. Analysis of co-variance was performed on the two regression lines for duodenal uptake vs. plasma iron concentration for Hfe KO and control mice (Fig. 3A); it demonstrated a significant difference in the slope of the two regression lines (P = 0.012) but no significant difference in the value of the intercepts (P = 0.37).
Increased uptake of plasma transferrin-bound iron by the duodenum correlated with increasing liver non-heme iron levels in both Hfe KO and control mice. Regression analysis indicated that there was a correlation between duodenal uptake of plasma transferrin-bound iron and the liver non-heme concentration in the Hfe KO and control mice (Hfe KO, r2 = 0.51, P = 0.009; C57BL/6, r2 = 0.75, P = 0.001; Fig. 3B). Furthermore, there was significantly lower uptake of transferrin-bound iron from plasma by the duodenum of the Hfe KO mice compared with the control mice. As the liver non-heme iron concentration increased, duodenal uptake of plasma transferrin-bound iron in the Hfe KO mice increased more slowly than in the control mice. Analysis of co-variance was undertaken on the two regression lines for duodenal uptake vs. liver non-heme iron concentration in Hfe KO and C57BL/6 mice. There was a significant difference between both the slope (P = 0.002) and intercept (P = 0.003) of the two regression lines.
Uptake of Transferrin-Bound Iron by the Liver and Kidney.
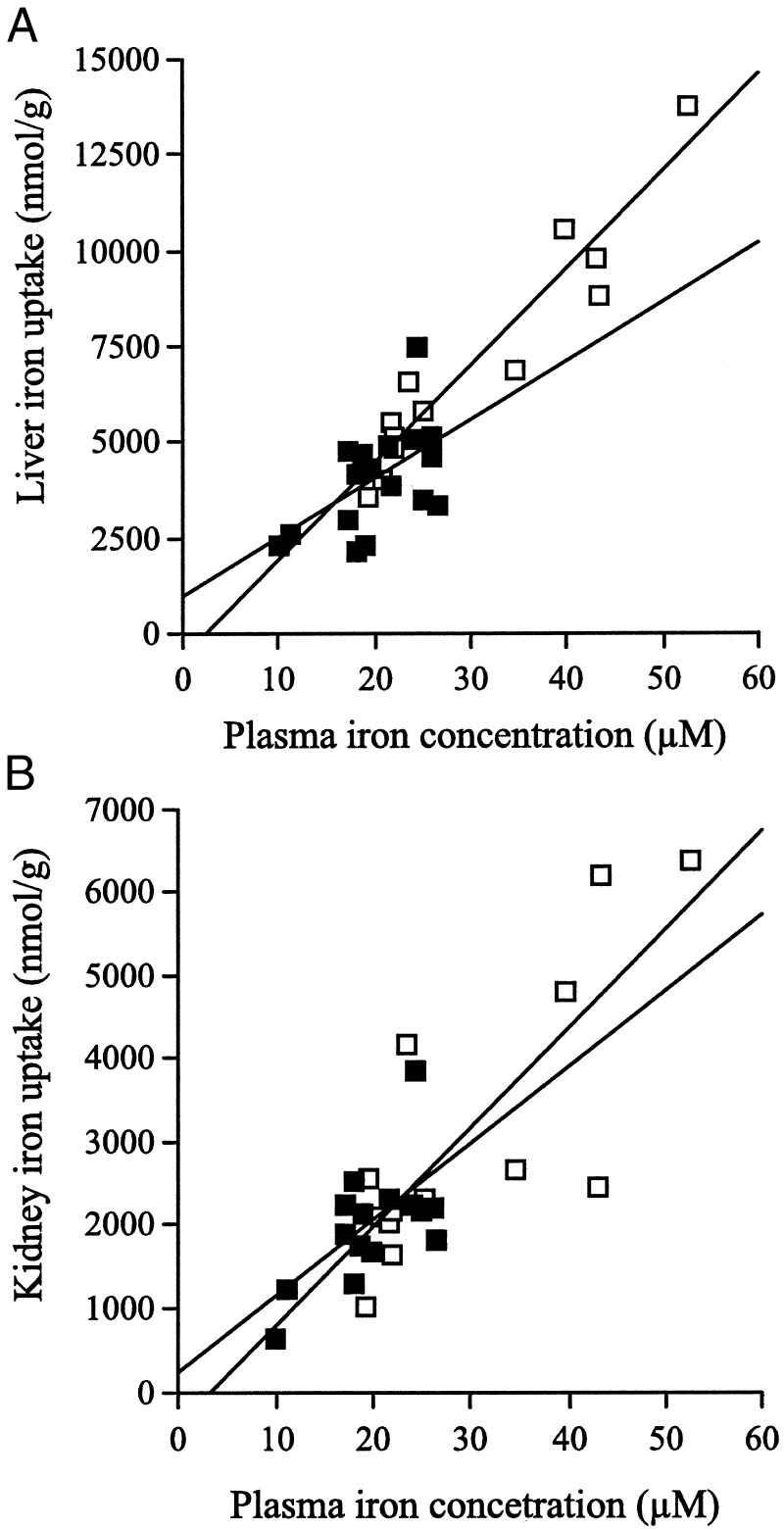
The uptake of 59Fe-transferrin by the liver and kidney in the Hfe KO and control mice also depended on the plasma iron concentration. There was a positive correlation between the plasma iron concentration and hepatic iron uptake in the Hfe KO and C57BL/6 mice (Hfe KO, r2 = 0.91, P = 0.001; C57BL/6, r2 = 0.30, P = 0.02; Fig. 4A), and this was also true for uptake by kidney (Hfe KO, r2 = 0.61, P = 0.002; C57BL/6, r2 = 0.40, P = 0.012; Fig. 4B). The uptake of iron by the liver was approximately two-fold greater than by the kidney at any given plasma iron concentration in both types of mice. However, analysis of co-variance showed there was no significant difference in the amount of iron taken up by the liver or the kidney with increasing plasma iron concentrations in the Hfe KO vs. the C57BL/6 mice. (Liver: slope, P = 0.11; intercept, P = 0.28. Kidney: slope, P = 0.61; intercept, P = 0.61; Figs. 4A and 4B).
Figure 4.
Transferrin-bound iron uptake by the liver (A) and kidney (B) in Hfe KO (□) and C57BL/6 (■) mice. There was a positive correlation between the plasma iron concentration and the plasma transferrin-bound iron uptake by the liver (Hfe KO, r2 = 0.91, P = 0.001; C57BL/6, r2 = 0.30, P = 0.02) and kidney (Hfe KO, r2 = 0.61, P = 0.002; C57BL/6, r2 = 0.40, P = 0.012) in Hfe KO and control mice. There was no significant difference in the regression lines for the uptake of plasma transferrin-bound iron by the liver or the kidney with increasing plasma iron concentrations in the Hfe KO vs. the C57BL/6 mice. (Liver: slope, P = 0.11, intercept, P = 0.28; kidney: slope, P = 0.61, intercept, P = 0.61).
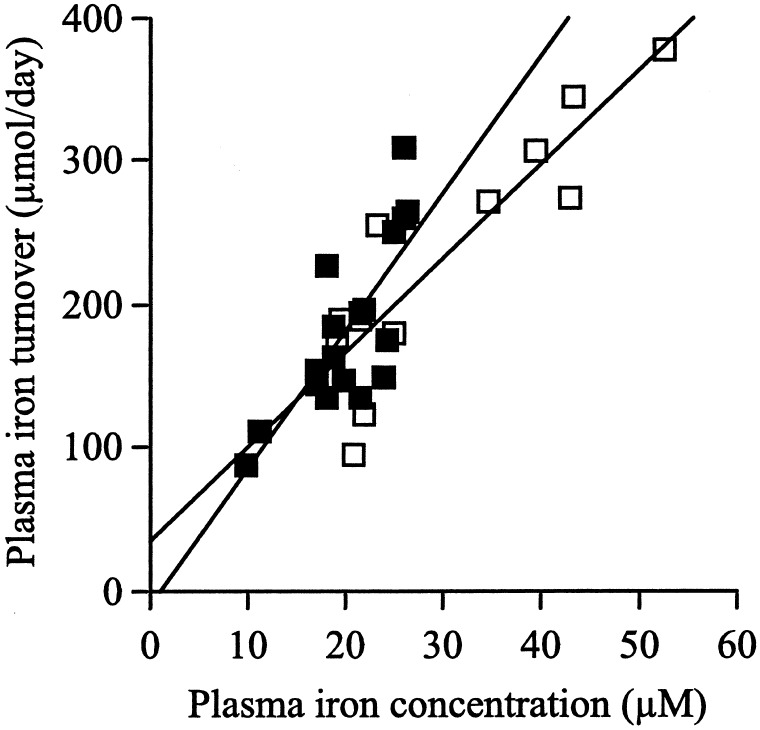
Plasma Iron Turnover.
The plasma iron turnover in the Hfe KO and C57BL/6 mice was calculated from the rate of clearance of 59Fe from the plasma over 2 h. The plasma iron turnover increased in a linear manner with increasing plasma iron concentrations in both the Hfe KO and C57BL/6 mice (Hfe KO, r2 = 0.79, P = 0.001; C57BL/6, r2 = 0.60, P = 0.001). There was no significant difference in the plasma iron turnover with increasing plasma iron concentrations in the Hfe KO vs. the control mice (analysis of co-variance: slope, P = 0.20; intercept, P = 0.40; Fig. 5).
Figure 5.
Plasma iron turnover in Hfe KO (□) and C57BL/6 control (■) mice fed an iron-deficient, normal, or iron-loaded diet. Plasma iron turnover was correlated linearly with the plasma iron concentrations for both the Hfe KO and C57BL/6 mice (Hfe KO, r2 = 0.79, P = 0.001; C57BL/6, r2 = 0.60, P = 0.001). There was no significant difference in the plasma iron turnover with increasing plasma iron concentrations in the Hfe KO vs. the control mice (slope, P = 0.20; intercept, P = 0.40).
Discussion
In this study, we demonstrated that there was a significant impairment in the uptake of transferrin-bound iron by the duodenum in the Hfe KO mice as compared with the control mice, which became more obvious as iron stores increased. This finding indicates that in the absence of Hfe, iron uptake by the duodenum still reflects iron status, but this process is less sensitive to the body iron stores. This inhibitory effect was specific to the duodenum, as no significant differences were observed in the uptake of transferrin-bound iron by the liver or kidney in the Hfe KO mice compared with control mice, regardless of the plasma iron concentration. Also, there was no overall change in the plasma iron turnover in the Hfe KO compared with the control mice. The plasma iron turnover reflects mainly the rate of clearance of iron by the bone marrow for erythropoiesis. This result indicates that iron uptake by the erythroid cells was not regulated by HFE or altered in the Hfe KO model of HH.
Impaired uptake of transferrin-bound iron by the duodenum of the Hfe KO mice would lead to a relatively iron-deficient duodenum. This observation is consistent with findings from studies in HH patients that have shown that the enterocytes have increased iron-regulatory protein activity and reduced ferritin levels (20, 21). Furthermore, expression of both iron transporters, divalent metal transporter 1 and ferroportin 1, is up-regulated in HH patients (22, 23). In the Hfe KO mice, divalent metal transporter 1 mRNA and protein were reported to be increased (24, 25). However, these observations were not reproduced by a study using another Hfe KO mouse model or the β2-microglobulin (β2M) KO mouse model of iron overload (26).
Although we did not measure dietary iron absorption in this study, two other studies (25, 27) have shown that the absorption of luminal iron by the duodenum was increased in the Hfe KO mice compared with the control mice. This finding could be explained by the results of the present study showing impaired uptake of transferrin-bound iron from plasma by the cells in the crypt region of the duodenum in Hfe KO mice compared with that seen in the control mice. The reduced uptake of iron would lead to relatively decreased iron levels in duodenal crypt cells that do not reflect the high plasma iron levels in the Hfe KO mice. In turn, as the crypt cells migrate to the villus region of the duodenum and undergo differentiation into absorptive cells, the absorption of dietary iron from the intestinal lumen would be inappropriately up-regulated in the Hfe KO mice.
Macrophages have some similarities to the enterocytes from the crypt region of the duodenum in that both types of cells express high levels of HFE and are relatively iron-deficient in HH. A recent study of macrophages by Montosi et al. (28) obtained results qualitatively similar to ours and showed that transferrin-bound iron uptake by macrophages from HH patients was decreased.
The mechanism involved in the HFE regulation of transferrin-bound iron uptake by the crypt cells of the duodenum and the macrophages is unknown. Wild-type HFE is associated closely with β2M, and TfR1 and is expressed on the cell surface and in the TfR1-containing endosomes (29–33). A number of experiments have been undertaken in which wild-type HFE was overexpressed in cultured cells and the overexpressed wild-type HFE decreased transferrin-bound iron uptake (32–37). However, Waheed et al. (37) recently reported that when both wild-type HFE and β2M were overexpressed in Chinese hamster ovary (CHO) cells, the cellular uptake of transferrin-bound iron was increased, which was attributed to an increased recycling rate of TfR1 through the cell. This stimulation of uptake of transferrin-bound iron by HFE was seen only if β2M also was overexpressed in the CHO cells. If the function of HFE expressed in CHO cells provides a model for HFE's function in the duodenal crypt cells, as Waheed et al. (37) proposed, then a stable wild-type HFE-β2M complex would be necessary to enhance TfR1-mediated cellular uptake of transferrin-bound iron by duodenal crypt cells. C282Y HFE does not associate with either β2M or TfR1, and its cell surface expression is greatly reduced (29, 30). Thus, it is unlikely that C282Y HFE could enhance cellular uptake of transferrin-bound iron by duodenal crypt cells. The relative decrease of transferrin-bound iron uptake compared with wild-type HFE in CHO cells is consistent with our observations of decreased transferrin-bound iron uptake by the duodenum in the Hfe KO mice and by macrophages from HH patients (28).
For many years, it has been proposed that there is another factor—termed a “stores regulator”—in the plasma that controls whole-body iron homeostasis and responds to changes in body iron stores (38). A number of candidates for this stores regulator have been suggested, such as transferrin-bound iron, serum ferritin, serum TfR1, and the recently identified hepcidin (39–42). Hepcidin is a hepatic peptide which is secreted into the blood; in its absence, iron overload with characteristics similar to HH develops (42). Hepcidin has been proposed by Nicolas et al. (42) to be the stores regulator in plasma that reflects hepatic iron levels and interacts with the HFE-β2M-TfR1 complex in the duodenum to regulate iron uptake. In the presence of C282Y HFE, as in HH or animal models of HH such as the Hfe and β2M KO mice, this interaction may be impaired, contributing to the decreased transferrin-bound iron uptake and to the incorrect sensing of body iron stores by the duodenum.
A number of studies have examined TfR1 expression in the duodenum. TfR1 expression is high in the crypt relative to the villus region, consistent with the crypt cells undergoing rapid proliferation and having a high requirement for iron. The level of expression in the crypt is not altered by the iron status of the body, allowing the uptake of transferrin-bound iron to vary with transferrin saturation and reflect body iron levels (10, 43–46). Impairment of this uptake process as a consequence of mutations in HFE is the proposed defect in HH. Recently, a cohort of Italian patients with a mutation in the TfR2 gene (termed HFE3) were reported to develop iron overload (47), suggesting that TfR2, a recently described second transferrin receptor, plays a role in the regulation of iron homeostasis. The mechanism by which loss of function of TfR2 leads to iron storage remains to be established. West et al. (48) have reported that, unlike TfR1, TfR2 does not form a stable complex with HFE-β2M). Thus, the mechanism by which it influences iron absorption may not involve the pathway of iron sensing in which HFE-β2M participates.
In conclusion, our study provides direct evidence to support the hypothesis that HFE regulates the uptake of transferrin-bound iron from the plasma by the duodenum. Disruption of this HFE-dependent regulation of transferrin-bound iron uptake by the crypt cells in HH would decrease the iron uptake by the crypt cells, so that intracellular iron levels would not correctly reflect the high plasma iron levels found in HH. As a result, the differentiating enterocytes would be programmed to absorb iron inappropriately.
Acknowledgments
We thank Hugh Barrett for his assistance with the statistical analysis. This research was supported by grants from the National Health and Medical Research Council of Australia (to D.T., J.K.O., and E.H.M.) and by National Institutes of Health Grants DK53405, DK40163, and GM34182 (to W.S.S.).
Abbreviations
- HH
hereditary hemochromatosis
- KO
knockout
- TfR1
transferrin receptor 1
- TfR2
transferrin receptor 2
- β2M
β2-microglobulin
References
- 1.Olynyk J K, Cullen D J, Aquilia S, Rossi E, Summerville L, Powell L W. N Engl J Med. 1999;341:718–724. doi: 10.1056/NEJM199909023411002. [DOI] [PubMed] [Google Scholar]
- 2.Merryweather-Clarke A T, Pointon J J, Shearman J D, Robson K J. J Med Genet. 1997;34:275–278. doi: 10.1136/jmg.34.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon B R, Tavill A S. In: Hepatology: A Textbook of Liver Disease. Zakim D, Boyer T D, editors. Philadelphia: Saunders; 1996. pp. 1439–1472. [Google Scholar]
- 4.Feder J N, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy D A, Basava A, Dormishian F, Domingo R, Jr, Ellis M C, Fullan A, et al. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 5.Parkkila S, Waheed A, Britton R S, Feder J N, Tsuchihashi Z, Schatzman R C, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 1997;94:2534–2539. doi: 10.1073/pnas.94.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad M E, Crosby W H. Blood. 1963;22:406–415. [PubMed] [Google Scholar]
- 7.McLaren G D, Nathanson M H, Jacobs A, Trevett D, Thomson W. J Lab Clin Med. 1991;117:390–401. [PubMed] [Google Scholar]
- 8.Parkkila S, Niemela O, Britton R S, Fleming R E, Waheed A, Bacon B R, Sly W S. Gastroenterology. 2001;121:1489–1496. doi: 10.1053/gast.2001.29617. [DOI] [PubMed] [Google Scholar]
- 9.Roy C N, Enns C A. Blood. 2000;96:4020–4027. [PubMed] [Google Scholar]
- 10.Anderson G J, Powell L W, Halliday J W. Gastroenterology. 1990;98:576–585. doi: 10.1016/0016-5085(90)90276-7. [DOI] [PubMed] [Google Scholar]
- 11.Fleming R E, Migas M C, Holden C C, Waheed A, Britton R S, Tomatsu S, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 2000;97:2214–2219. doi: 10.1073/pnas.040548097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawabata H, Yang R, Hirama T, Vuong P T, Kawano S, Gombart A F, Koeffler H P. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 13.Waheed A, Parkkila S, Saarnio J, Fleming R E, Zhou X Y, Tomatsu S, Britton R S, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 1999;96:1579–1584. doi: 10.1073/pnas.96.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X Y, Tomatsu S, Fleming R E, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt E M, Ruddy D A, Prass C E, et al. Proc Natl Acad Sci USA. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves P G, Nielsen F H, Fahey G C., Jr J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 16.Yeoh G C, Morgan E H. Cell Differ. 1979;8:331–343. doi: 10.1016/0045-6039(79)90008-3. [DOI] [PubMed] [Google Scholar]
- 17.Taylor E M, Morgan E H. Brain Res Dev Brain Res. 1990;55:35–42. doi: 10.1016/0165-3806(90)90103-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaldor I. Aust J Exp Biol. 1954;32:795–800. doi: 10.1038/icb.1954.82. [DOI] [PubMed] [Google Scholar]
- 19.International Committee For Standardisation in Hematology. J Clin Pathol. 1971;24:334–335. [Google Scholar]
- 20.Pietrangelo A, Casalgrandi G, Quaglino D, Gualdi R, Conte D, Milani S, Montosi G, Cesarini L, Ventura E, Cairo G. Gastroenterology. 1995;108:208–217. doi: 10.1016/0016-5085(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 21.Cairo G, Recalcati S, Montosi G, Castrusini E, Conte D, Pietrangelo A. Blood. 1997;89:2546–2553. [PubMed] [Google Scholar]
- 22.Zoller H, Pietrangelo A, Vogel W, Weiss G. Lancet. 1999;353:2120–2123. doi: 10.1016/S0140-6736(98)11179-0. [DOI] [PubMed] [Google Scholar]
- 23.Zoller H, Koch R O, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile D J, Vogel W, Weiss G. Gastroenterology. 2001;120:1412–1419. doi: 10.1053/gast.2001.24033. [DOI] [PubMed] [Google Scholar]
- 24.Fleming R E, Migas M C, Zhou X, Jiang J, Britton R S, Brunt E M, Tomatsu S, Waheed A, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 1999;96:3143–3148. doi: 10.1073/pnas.96.6.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths W J, Sly W S, Cox T M. Gastroenterology. 2001;120:1420–1429. doi: 10.1053/gast.2001.24050. [DOI] [PubMed] [Google Scholar]
- 26.Canonne-Hergaux F, Levy J E, Fleming M D, Montross L K, Andrews N C, Gros P. Blood. 2001;97:1138–1140. doi: 10.1182/blood.v97.4.1138. [DOI] [PubMed] [Google Scholar]
- 27.Bahram S, Gilfillan S, Kuhn L C, Moret R, Schulze J B, Lebeau A, Schumann K. Proc Natl Acad Sci USA. 1999;96:13312–13317. doi: 10.1073/pnas.96.23.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montosi G, Paglia P, Garuti C, Guzman C A, Bastin J M, Colombo M P, Pietrangelo A. Blood. 2000;96:1125–1129. [PubMed] [Google Scholar]
- 29.Feder J N, Tsuchihashi Z, Irrinki A, Lee V K, Mapa F A, Morikang E, Prass C E, Starnes S M, Wolff R K, Parkkila S, et al. J Biol Chem. 1997;272:14025–14028. doi: 10.1074/jbc.272.22.14025. [DOI] [PubMed] [Google Scholar]
- 30.Waheed A, Parkkila S, Zhou X Y, Tomatsu S, Tsuchihashi Z, Feder J N, Schatzman R C, Britton R S, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 1997;94:12384–12389. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkkila S, Waheed A, Britton R S, Bacon B R, Zhou X Y, Tomatsu S, Fleming R E, Sly W S. Proc Natl Acad Sci USA. 1997;94:13198–13202. doi: 10.1073/pnas.94.24.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross C N, Irrinki A, Feder J N, Enns C A. J Biol Chem. 1998;273:22068–22074. doi: 10.1074/jbc.273.34.22068. [DOI] [PubMed] [Google Scholar]
- 33.Ramalingam T S, West A P, Jr, Lebron J A, Nangiana J S, Hogan T H, Enns C A, Bjorkman P J. Nat Cell Biol. 2000;2:953–957. doi: 10.1038/35046611. [DOI] [PubMed] [Google Scholar]
- 34.Roy C N, Penny D M, Feder J N, Enns C A. J Biol Chem. 1999;274:9022–9028. doi: 10.1074/jbc.274.13.9022. [DOI] [PubMed] [Google Scholar]
- 35.Salter-Cid L, Brunmark A, Li Y, Leturcq D, Peterson P A, Jackson M R, Yang Y. Proc Natl Acad Sci USA. 1999;96:5434–5439. doi: 10.1073/pnas.96.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikuta K, Fujimoto Y, Suzuki Y, Tanaka K, Saito H, Ohhira M, Sasaki K, Kohgo Y. Biochim Biophys Acta. 2000;1496:221–231. doi: 10.1016/s0167-4889(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 37.Waheed A, Grubb J H, Zhou X Y, Tomatsu S, Fleming R E, Costaldi M E, Britton R S, Bacon B R, Sly W S. Proc Natl Acad Sci USA. 2002;99:3117–3122. doi: 10.1073/pnas.042701499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finch C. Blood. 1994;84:1697–1702. [PubMed] [Google Scholar]
- 39.Taylor P, Martinez-Torres C, Leets I, Ramirez J, Garcia-Casal M N, Layrisse M. J Nutr. 1988;118:1110–1115. doi: 10.1093/jn/118.9.1110. [DOI] [PubMed] [Google Scholar]
- 40.Raja K B, Pountney D J, Simpson R J, Peters T J. Blood. 1999;94:3185–3192. [PubMed] [Google Scholar]
- 41.Cook J D, Dassenko S, Skikne B S. Br J Haematol. 1990;75:603–609. doi: 10.1111/j.1365-2141.1990.tb07806.x. [DOI] [PubMed] [Google Scholar]
- 42.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Proc Natl Acad Sci USA. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oates P S, Thomas C, Freitas E, Callow M J, Morgan E H. Am J Physiol. 2000;278:G930–G936. doi: 10.1152/ajpgi.2000.278.6.G930. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee D, Flanagan P R, Cluett J, Valberg L S. Gastroenterology. 1986;91:861–869. doi: 10.1016/0016-5085(86)90687-6. [DOI] [PubMed] [Google Scholar]
- 45.Lombard M, Bomford A B, Polson R J, Bellingham A J, Williams R. Gastroenterology. 1990;98:976–984. doi: 10.1016/0016-5085(90)90022-s. [DOI] [PubMed] [Google Scholar]
- 46.Oates P S, Thomas C, Morgan E H. Pflügers Arch. 2000;440:116–124. doi: 10.1007/s004240000256. [DOI] [PubMed] [Google Scholar]
- 47.Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P. Nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 48.West A P, Jr, Bennett M J, Sellers V M, Andrews N C, Enns C A, Bjorkman P J. J Biol Chem. 2000;275:38135–38138. doi: 10.1074/jbc.C000664200. [DOI] [PubMed] [Google Scholar]