Abstract
Background
Fumarate hydratase (FH) is a key mitochondrial enzyme in the tricarboxylic acid (TCA) cycle, catalyzing the reversible hydration of fumarate to malate, thereby facilitating aerobic ATP production and maintaining metabolic homeostasis. Germline pathogenic or likely pathogenic variants in the FH gene are strongly linked to hereditary leiomyomatosis and renal cell carcinoma (HLRCC), a rare hereditary cancer syndrome characterized by cutaneous and/or uterine leiomyomas and a markedly increased risk of renal cell carcinoma (RCC). These variants span a wide spectrum of genetic alterations, including missense, nonsense, frameshift, splice-site variants, as well as large genomic deletions. However, the relationship between specific pathogenic FH variant subtypes and the risk of developing HLRCC-associated RCC remains unclear. Therefore, this study systematically reviewed the existing literatures and conducted a meta-analysis to preliminarily explore the potential role of different functional subtypes of FH variants in the development of HLRCC-associated RCC, providing a basis for future clinical risk stratification and personalized surveillance strategies.
Methods
We systematically searched 4 major electronic databases—PubMed/MEDLINE, Embase, Scopus, and Web of Science—for relevant studies. To evaluate the association between pathogenic or likely pathogenic FH variant subtypes (Missense vs. Loss-of-Function (LOF)) and the risk of HLRCC-associated RCC, we performed a fixed-effects meta-analysis based on unadjusted odds ratios (ORs). In addition, exploratory subgroup analyses were performed by geographic region (North America and Europe), histological subtype (particularly type II papillary RCC (Type II PRCC)), tumor characteristics (such as distant metastasis and clinical stage), and study design (variant/gene-first vs. phenotype-first). These stratifications were intended to assess whether clinical or methodological features might modulate the observed associations and to provide context for future hypothesis-driven research. All statistical tests were two-sided, and heterogeneity was assessed using standard metrics. Effect estimates are reported as ORs with corresponding 95% confidence intervals (CIs).
Results
Individuals harboring pathogenic or likely pathogenic FH LOF variants exhibited a significantly higher risk of developing HLRCC-associated RCC compared to those harboring missense variants (OR = 1.75, 95% CI: 1.28 to 2.38, p < 0.001). Subgroup analysis by geographic region showed a significant association in North American cohorts (OR = 1.64, 95% CI: 1.11 to 2.43, p < 0.05), while the association was not statistically significant in European cohorts (OR = 1.11, 95% CI: 0.57 to 2.17, p > 0.05). Stratification by study design further revealed a stronger association in variant-first or gene-first cohorts (OR = 1.62, 95% CI: 1.03 to 2.55, p < 0.05), while no significant association was observed in phenotype-first cohorts (OR = 1.34, 95% CI: 0.81 to 2.22, p > 0.05). Among patients diagnosed with HLRCC-associated RCC, those with LOF variants were more likely to present with advanced-stage disease at diagnosis. In contrast, patients with missense variants were more frequently associated with Type II PRCC and exhibited a higher propensity for distant metastasis.
Conclusion
This meta-analysis suggests that individuals harboring pathogenic or likely pathogenic FH LOF variants may have an approximately 1.75-fold higher risk of developing HLRCC-associated RCC compared to those with missense variants. While the pooled effect was statistically significant, subgroup analyses revealed regional and ascertainment-related differences, indicating potential underlying heterogeneity. These findings underscore the potential utility of FH variant subtypes as biomarkers for individualized risk assessment. Further prospective studies are warranted to validate these associations and guide surveillance strategies in hereditary renal cancer syndromes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40246-025-00797-8.
Introduction
The FH gene (HGNC: FH) encodes a mitochondrial enzyme that plays a pivotal role in cellular energy metabolism, catalyzing the reversible conversion of fumarate to malate within the TCA cycle [1]. Its evolutionary conservation—demonstrated by a 67% amino acid sequence homology between humans and Saccharomyces cerevisiae—highlights its nonredundant function in redox homeostasis. Disruption of FH activity leads to distinct clinical phenotypes depending on zygosity. Biallelic pathogenic variants cause autosomal recessive fumarate hydratase deficiency (OMIM 606812), a severe neurometabolic disorder characterized by infantile-onset encephalopathy, neurodevelopmental arrest, and mitochondrial dysfunction. This condition was first described by Zinn et al. in 1986, who reported the earliest known case attributable to FH deficiency [2–7]. In contrast, heterozygous pathogenic or likely pathogenic FH variants give rise to HLRCC (OMIM 605839), an autosomal dominant cancer syndrome characterized by cutaneous and uterine leiomyomas (MCUL1, OMIM 150800), as well as a significantly elevated risk of developing aggressive RCC [8]. RCC is often the sentinel malignancy in HLRCC, with an estimated lifetime risk of 10–15% and a well-documented tendency toward early metastatic dissemination [9, 10].
The oncogenicity of FH inactivation is largely attributed to intracellular fumarate accumulation, which functions as an oncometabolite by disrupting multiple cellular pathways [11–15]. These include pseudohypoxic transcriptional reprogramming via inhibition of α-ketoglutarate–dependent dioxygenases, succination of redox sensors such as KEAP1 leading to NRF2-driven chemoresistance, and impairment of homologous recombination repair, thereby promoting genomic instability [11–15]. Immunohistochemically, FH-deficient RCCs are characterized by loss of FH protein expression and accumulation of 2-succinocysteine (2SC), with distinctive cytologic features such as eosinophilic nucleoli and perinuclear halos [16, 17]. Furthermore, recent studies also implicate FH loss in pheochromocytoma/paraganglioma pathogenesis via hypoxia-inducible factors-1α (HIF-1α) stabilization [18, 19].
In 2011, Lehtonen et al. [20] reviewed the Leiden Open Variation Database (LOVD) for FH and summarized that, among 80 germline FH variants associated with HLRCC at that time, approximately 58% were missense, 18% were frameshift, and 11% were nonsense variants, most of which resulted from small insertions or deletions. However, most missense variants do not fully abolish enzymatic activity, and their clinical significance may be variable. Although an increasing number of case reports link missense variants to HLRCC-associated RCC, no consensus has yet been reached regarding the relative oncogenic risks conferred by different variant subtypes or loci [21–28]. In contrast, LOF variant are generally believed to abolish FH enzymatic activity completely. Several studies have demonstrated that LOF variants are more frequently associated with RCC in the context of HLRCC, suggesting a higher tumorigenic potential [29–31]. Nevertheless, the associated clinical phenotypes remain highly heterogeneous, implying that additional genetic or epigenetic modifiers may influence disease penetrance and severity. Therefore, clarifying genotype–phenotype correlations is critical for precision risk stratification in clinical settings.
In this study, we conducted a systematic review and meta-analysis to evaluate the association between 2 major subtypes of germline FH variants—missense and LOF—and the risk of HLRCC-associated RCC. We further investigated the geographical distribution of these variants, and analyzed clinicopathological features, including RCC histological subtypes, metastatic status, and tumor stage at diagnosis. In addition, subgroup analyses based on variant-first (genotype-driven) vs. phenotype-first (clinical presentation–driven) recruitment strategies were performed to assess the potential influence of ascertainment bias on risk estimates, with the aim of informing early diagnosis and individualized surveillance strategies for this high-risk population.
Materials and methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. The protocol was retrospectively registered on the Open Science Framework (OSF, https://osf.io/vghc3/) given that PROSPERO currently only accepts interventional reviews.
Study selection
Inclusion and exclusion criteria were defined based on the Population, Exposure, Comparison, Outcome, and Study design (PICOS) framework. The study population included individuals with germline FH variants classified as pathogenic or likely pathogenic, as determined by clinical genetic testing. Participants encompassed both clinically diagnosed HLRCC cases and asymptomatic variant-positive individuals. All included studies employed a variant-first or family-based recruitment approach rather than selecting participants based solely on RCC diagnosis, to minimize ascertainment bias. The exposure group comprised individuals with LOF FH variants. The comparison group consisted of individuals with missense variants. The primary outcome was the incidence or prevalence of HLRCC-associated RCC. Eligible studies were retrospective observational analyses that assessed RCC risk in HLRCC-affected individuals with different FH variant subtypes. Included studies applied functional or clinical criteria to classify variants and reported variant-level RCC outcomes suitable for quantitative synthesis. Studies were excluded if they were non-original reports (e.g., reviews, editorials, letters), focused exclusively on sporadic RCC or RCC unrelated to germline FH variants, lacked quantitative data on RCC risk or incidence, involved overlapping hereditary cancer syndromes (e.g., Lynch syndrome, von Hippel–Lindau disease), or presented unclear methodology or insufficient follow-up. To address potential ascertainment bias, we carefully categorized study designs. Among the 13 studies included in the meta-analysis, 9 adopted a phenotype-first design, enrolling individuals based on clinical suspicion of HLRCC (e.g., RCC, cutaneous or uterine leiomyomas) before confirming FH variant status. The remaining 4 studies followed a variant-first or gene-first approach, in which individuals were enrolled based on the presence of pathogenic or likely pathogenic FH variants identified through genetic screening and subsequently assessed for clinical manifestations. This variation in recruitment strategies may introduce differences in RCC detection rates and case composition across studies. By documenting the inclusion strategies of each study, we aimed to reduce selection bias and allow appropriate interpretation of RCC risk in different clinical and genetic contexts.
Variant classification
Variants were categorized into LOF and missense subtypes based on their predicted molecular consequences. LOF variants included nonsense, frameshift insertions or deletions, canonical splice-site variants (± 1 or 2 positions), and large deletions resulting in protein truncation or loss of function. Missense variants were defined as single nucleotide substitutions leading to amino acid changes without inducing a frameshift or premature stop codon. The pathogenicity of each variant—categorized as pathogenic or likely pathogenic (P/LP)—was determined primarily according to the classifications reported in the original studies. This study did not perform independent reclassification of the reported variants. In cases where both P/LP variants and variants of uncertain significance (VUS) were described, only individuals harboring P/LP variants were included in the analysis, while those carrying VUS were excluded. Variants with a population allele frequency greater than 1% in gnomAD were also excluded to minimize the inclusion of common polymorphisms. Furthermore, variants exclusively associated with fumarate hydratase deficiency (FHd) and lacking evidence of HLRCC phenotypes in heterozygous carriers were excluded where applicable. It should be noted that no efforts were made to re-evaluate or update the pathogenicity classification of reported VUS. Consequently, some variants classified as VUS in the original studies may have since been reclassified as pathogenic or likely pathogenic.
Data sources
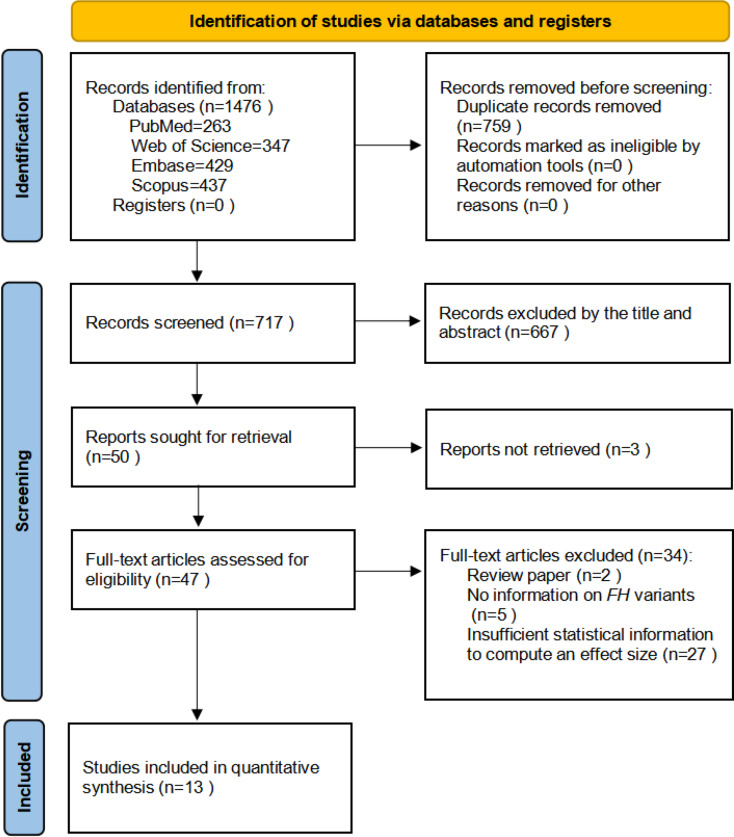
A comprehensive literature search was conducted in four major databases: PubMed/MEDLINE (1946–2025), Embase (1947–2025), Scopus (1823–2025), and Web of Science (WOS) (1991–2025). Two independent researchers (Han and Shamsnur) implemented the search using a combination of controlled vocabulary terms (e.g., MeSH in PubMed and Emtree in Embase) and free-text keywords, including “FH gene,” “Fumarate Hydratase,” “Hereditary Leiomyomatosis and Renal Cell Cancer,” “Renal Cell Carcinoma,” and “Renal Cancer Risk.” The search strategy was customized to each database’s indexing system, prioritizing MeSH terms in PubMed and Emtree in Embase. Boolean operators (AND, OR, NOT) and truncation symbols (*) were used to optimize sensitivity and precision. All search results were imported into a reference management program, and duplicate records were removed. Detailed database-specific search strategies, including keyword combinations and applied filters, are provided Supplementary Table 1. The entire process followed the PRISMA-S guidelines to ensure transparency and reproducibility, and reporting completeness (Fig. 1).
Table 1.
summary of baseline characteristics of the included studies
| Reference (year) | Country | Ascertainment basis | Tumor pathological classification | Variant types | RCCs with Variants | Variantdetection methods | LOF vs. Missense | NOS score |
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | ||||||||
| Thomas Scharnitz et al. 2023 | America | Variant-first |
Papillary renal cell carcinoma:type I,type II, mixed type Clear cell renal cell carcinoma High-grade renal cell carcinoma |
LOF(FH c.157G > T p.(Glu53Ter),FH c.301C > T p.(Arg101Ter),FH c.316del p.(Val106Ter),FH c.760C > T p.(Gln254Ter),FH c.937G > T p.(Glu313Ter),FH c.1052C > A p.(Ser351Ter),FH c.1339A > T p.(Lys447Ter),FH c.780_781del p.(Arg261AsnfsTer10),FH c.912_918del p.(Phe305LeufsTer22),FH c.1041del p.(Gly348ValfsTer9),FH c.1293del p.(Glu432LysfsTer17),FH c.1506dup p.(Pro503ThrfsTer2),FH deletion of exon 6,FH deletion of entire coding sequence,FH 5’UTR_3’UTRdel) Missense (FH c.320A > C p.(Asn107Thr),FH c.478A > G p.(Arg160Gly),FH c.560C > T p.(Ser187Leu),FH c.689A > G p.(Lys230Arg),FH c.697C > T p.(Arg233Cys),FH c.698G > A p.(Arg233His),FH c.698G > T p.(Arg233Leu),FH c.703C > T p.(His235Tyr),FH c.892G > C p.(Ala298Pro),FH c.1023T > G p.(Asp341Glu),FH c.1255T > C p.(Ser419Pro),FH c.1301G > A p.(Cys434Tyr)) |
3/32 3/25 |
Testing in CLIA-certified laboratories |
OR = 0.76(0.14 to 4.13) | 4 |
| Eric Lu et al. 2022 | America | Gene-first/Population-based | Papillary renal cell carcinoma |
LOF Missense |
29/127 20/162 |
NGS and Sanger sequencing |
OR = 2.10(1.12 to 3.93) | 6 |
| Junne Kamihara et al. 2021 | America | Variant-first |
Clear cell renal cell carcinoma Non-clear cell renal cell carcinoma Unclassified carcinoma |
LOF(FH c.301C > T p.(Arg101Ter),FH c.1-? 1530+?del,FH c.1027C > T p.(Arg343Ter),FH c.553_554insTG p.(Gln185LeufsTer18),FH c.912_918del p.(Phe305LeufsTer22),FH c.1390 + 1G > T,FH c.722_738 + 3del,FH c.1056dup p.(Leu353SerfsTer8),FH c.1041del p.(Gly348ValfsTer9),FH c.739-?_904+?del,FH c.1240A > T p.(Lys414Ter),FH c.1298dup p.(Asn433LysfsTer19),FH c.560C > G p.(Ser187Ter),FH c.1237-1G > T,FH c.568_569del p.(Thr190PhefsTer15),FH c.379-?_738+?del,FH c.201T > A p.(Tyr67Ter),FH c.353del p.(Asn118MetfsTer3),FH c.1209del p.(Phe403LeufsTer3),FH c.316del p.(Val106Ter),FH c.1370_1371insTCAC p.(Ala458HisfsTer10),FH c.139C > T p.(Gln47Ter),FH c.237dup p.(Lys80Ter),FH c.905-1G > C,FH c.905-1G > A,FH c.1293del p.(Glu432LysfsTer17),FH c.239dup p.(Ile81AspfsTer14),FH c.129_132 + 5del) Nontruncating:Missense,Deletion(FH c.698G > A p.(Arg233His),FH c.1020T > A p.(Asn340Lys),FH c.697C > T p.(Arg233Cys),FH c.700A > G p.(Thr234Ala),FH c.1093A > G p.(Ser365Gly),FH c.1301G > A p.(Cys434Tyr),FH c.689A > G p.(Lys230Arg),FH c.132G > A p.(Met44Ile),FH c.786_806del p.(Lys263_Ile269del),FH c.1120C > A p.(Pro374Thr),FH c.965T > G p.(Val322Gly),FH c.1097G > A p.(Ser366Asn),FH c.697C > G p.(Arg233Gly),FH c.1000A > C p.(Ser334Arg),FH c.892G > C p.(Ala298Pro),FH c.842C > T p.(Thr281Ile),FH c.988A > C p.(Thr330Pro),FH c.914T > C p.(Phe305Ser),FH c.557G > A p.(Ser186Asn),FH c.1202G > A p.(Gly401Glu),FH c.1255T > C p.(Ser419Pro),FH c.437G > A p.(Gly146Glu),FH c.597_599del p.(Ala200del),FH c.934T > C p.(Phe312Leu),FH c.947C > A p.(Ala316Asp),FH c.1157A > G p.(Gln386Arg),FH c.1016C > A p.(Ala339Glu),FH c.578_583del p.(Thr193_Ala194del),FH c.320A > C p.(Asn107Thr),FH c.478A > G p.(Arg160Gly)) |
20/46 25/67 |
Multi-gene panel testing | OR = 1.29(0.60 to 2.78) | 5 |
| Sánchez-Heras et al. 2020 | Spain | Phenotype-first |
Papillary renal cell carcinoma:type II,without further subclassification Clear cell renal cell carcinoma Unclassified carcinoma |
LOF(Del FH,FH Del exon 2,FH c.139C > T p.(Gln47Ter),FH c.267 + 1_267 + 10del,FH c.301C > T p.(Arg101Ter),FH c.395del p.(Leu132Ter),FH c.553del p.(Gln185ArgfsTer17),FH c.555 + 1G > A,FH c.893del p.(Ala298ValfsTer31),FH c.905-2A > G,FH c.974del p.(Ser325MetfsTer4),FH Del exon 8,FH c.1126del p.(Gln376SerfsTer5),FH Del exon 9) Missense(FH c.349G > C p.(Ala117Pro),FH c.563A > G p.(Asn188Ser),FH c.575C > T p.(Pro192Leu),FH c.697C > T p.(Arg233Cys),FH c.698G > A p.(Arg233His),FH c.703C > T p.(Arg233His),FH c.845G > T p.(Gly282Val),FH c.965T > G p.(Val322Gly),FH c.1112A > G p.(Lys371Arg),FH c.1118A > G p.(Asn373Ser),FH c.1189G > A p.(Asn373Ser),FH c.1217A > C p.(Asn406Thr),FH c.1240A > G p.(Lys414Glu)) |
6/42 13/133 |
Sanger sequencing and MLPA and NGS |
OR = 1.54(0.55 to 4.34) | 6 |
| Priya T. Bhola et al. 2018 | Canada | Phenotype-first |
Papillary renal cell carcinoma:type II Renal cell carcinoma with sarcomatoid features Tubulo-papillary renal cell carcinoma |
LOF(FH c.1111A > T p.(Lys371Ter),FH c.1263del p.(Arg421SerfsTer28),FH c.797dup p.(Met266IlefsTer6),FH c.1293del p.(Glu432LysfsTer17),FH c.1430_1437dup p.(Ser480LysfsTer6),FH c.[1104G > A;1106C > T] p.(Met368_Pro369delinsIleLeu)) Missense(FH c.965T > G p.(Val322Gly),FH c.688A > G p.(Lys230Glu),FH c.698G > T p.(Arg233Leu),FH c.706A > G p.(Thr236Ala),FH c.1103T > C p.(Met368Thr) |
4/43 1/5 |
Sanger sequencing | OR = 0.41(0.04 to 4.62) | 3 |
| Ryan P. Kopp et al. 2017 | America | Phenotype-first |
Papillary renal cell carcinoma Collecting duct renal cell carcinoma Unclassified carcinoma |
LOF(FH Partial delExon1,FH c.954_957del p.(His318GlnfsTer10),FH c.1010dup p.(Ile338AspfsTer4)) Missense(FH c.891T > A,FH c.1060G > A p.(Gly354Arg),FH c.698G > A p.(Arg233His)) |
2/3 3/5 |
Sanger sequencing | OR = 1.33(0.07 to 26.62) | 4 |
| Sanz-Ortega et al. 2013 | America | Phenotype-first | Papillary renal cell carcinoma |
LOF(FH c.1346del p.(Met449ArgfsTer5),FH c.1164del p.(Asn390ThrfsTer16),FH c.1010_1011insA p.(Ile338AspfsTer4) ,FH c.172C > T,FH SpliceDonor + 1) Missense(FH c.1187A > C,FH c.1025C > A,FH c.1126T > C,FH c.1060G > A p.(Gly354Arg),FH c.569G > A,FH c.191A > C p.(Asn64Thr),FH c.691G > C) |
2/6 3/9 |
LOH analysis and Sanger sequencing |
OR = 1.0(0.11 to 8.95) | 3 |
| Betty Gardie et al. 2011 | France | Phenotype-first |
Papillary renal cell carcinoma:type II Collecting duct renal cell carcinoma Sarcomatoid renal cell carcinoma |
LOF(FH c.1-?_1404+?del p.(Met1-?_X468+?del),FH c.127_128del p.(Arg43AsnfsTer12),FH c.147del p.(Phe50SerfsTer3),FH c.298del p.(Lys100SerfsTer7),FH c.666del p.(Glu224SerfsTer32),FH c.810del p.(Tyr270Ter),FH c.138 + 1_138 + 10del10,FH c.247_249 + 1delGAGGinsA,FH c.250-2A > G,FH c.1108-2A > G,FH c.172C > T p.(Arg58X),FH c.808G > T p.(Glu270X),FH c.898C > T p.(Arg300X),FH c.1371G > A p.(Trp457X),FH c.1121–1123 delTAC p.(Leu374_His375delinsTyr)) Missense(FH c.410A > G p.(His137Arg),FH c.503T > C p.(Leu168Pro),FH c.568C > T p.(Arg190Cys),FH c.575A > G p.(His192Arg),FH c.632A > G p.(Gln211Arg),FH c.806T > C p.(Phe269Ser),FH c.815T > C p.(Leu272Pro),FH c.821C > T p.(Ala274Val),FH c.869G > A p.(Cys290Tyr),FH c.989A > G p.(Asn330Ser),FH c.1028A > G p.(Gln343Arg),FH c.1060G > A p.(Gly354Arg),FH c.1265A > G p.(Tyr422Cys),FH c.220G > C p.(Ala74Pro)) |
8/71 9/74 |
qPCR and Sanger sequencing |
OR = 0.92(0.33 to 2.53) | 5 |
| Smit DL et al. 2011 | The Netherlands | Variant-first |
Papillary renal cell carcinoma:type II Wilms’ tumour |
LOF(FH c.1-?c.*100del,FH c.1234del p.(Met412Ter),FH c.157G > T p.(Glu53Ter),FH c.1210G > T p.(Glu404Ter),FH c.233del p.(Asn78fsTer85)) Missense(FH c.952C > T p.(His318Tyr),FH c.698G > A p.(Arg233His),FH c.1189G > A p.(Gly397Arg),FH c.824G > A p.(Gly275Glu),FH c.1144A > G p.(Met382Val),FH c.1002T > G p.(Ser334Arg)) |
1/9 2/26 |
qPCR/MLPA | OR = 1.50(0.12 to 18.84) | 3 |
| M-H Wei et al. 2006 | America | Phenotype-first |
Papillary renal cell carcinoma:type II Collecting duct renal cell carcinoma Renal cell carcinoma exhibits mixed features of cystic, papillary, and tubulopapillary characteristics. |
LOF(FH c.305C > G p.(Ala102Gly),FH c.172C > T,FH c.111insA,FH c.138 + 1G > C) Missense(FH c.266T > C,FH c.568C > T,FH c.569G > A,FH c.1126T > C,FH c.1187A > C,FH c.191A > C p.(Asn64Thr),FH c.349A > G,FH c.891T > A,FH c.964A > G,FH c.1025C > A) |
9/17 11/29 |
Sanger sequencing | OR = 1.84(0.55 to 6.19) | 3 |
| Badeloe S et al. 2006 |
The Netherlands Spain |
Phenotype-first | Renal cell carcinoma |
LOF(FH c.1210G > T p.(Glu404Ter),FH c.233del p.(Asn78ThrfsTer7),FH c.1238del p.(Asn415MetfsTer34),FH c.IVS3 + 1delG,FH c.IVS4 + 1G > A) Missense(FH c.424G > A p.(Gln142Lys)) |
1/25 0/2 |
qPCR and Sanger sequencing |
OR = 0.31(0.01 to 9.69) | 2 |
| Jorge R. Toro et al. 2003 |
America Canada |
Phenotype-first |
Papillary renal cell carcinoma:type II Collecting duct renal cell carcinoma Unclassified eosinophilic renal cell tumor |
LOF(FH c.288del p.(Phe96LeufsTer4),FH c.780_781del p.(Arg261AsnfsTer10),FH c.782_788del p.(Arg261LysfsTer21),FH c.1004_1005insNN p.(Met336XaafsTer2),FH c.1162del p.(Met388TrpfsTer18),FH c.1300_1307dup p.(Gly437AlafsTer15),FH c.1339del p.(Lys447SerfsTer2)) Missense(FH c.431C > T,FH c.434A > G,FH c.455T > C,FH c.560A > G,FH c.569G > A,FH c.569G > T,FH c.823C > T,FH c.836T > A p.(Val279Asp),FH c.875T > C,FH c.891T > A,FH c.968G > A,FH c.964A > G,FH c.1265A > G) |
6/22 7/58 |
qPCR and Sanger sequencing and DHPLC verification |
OR = 2.73(0.80 to 9.32) | 4 |
| N.A. Alam et al. 2003 | Britain | Phenotype-first | Low-grade collecting duct renal cell carcinoma |
LOF(FH c.10C > T p.(Gln4Ter),FH c.172C > T p.(Arg58Ter),FH c.898C > T p.(Arg300Ter),Codon17frameshift,Codon 406 frameshift,FH c.556_558del p.(Ile186del),Whole gene deletion,2bpdel codon 181) Missense(FH c.191A > C p.(Asn64Thr),FH c.220G > C p.(Ala74Pro),FH c.1060G > C p.(Gly354Arg),FH c.410A > G p.(His137Arg),FH c.425A > G p.(Gln142Arg),FH c.557T > C p.(Ile186Thr),FH c.560A > G p.(Lys187Arg),FH c.569G > A p.(Arg190His),FH c.716G > T p.(Gly239Val),FH c.934G > A p.(Glu312Lys),FH c.954C > A p.(Asn318Lys),FH c.968G > A p.(Ser323Asn),FH c.1391T > C p.(Leu464Pro)) |
0/31 1/60 |
F-SSCP and Sanger sequencing and Biochemical function detection |
OR = 0.63(0.02 to 15.91) | 4 |
*CI = confidence interval, OR = odds ratio, NOS = Newcastle-Ottawa Scale
Odds ratio for FH LOF vs FH missense variants
Due to the lack of available predicted protein data, some genotypes could not be fully annotated or described
All variants have ClinVar pathogenicity tags of P/LP or were observed in clinically confirmed HLRCC patients. Variants annotated as VUS/LB/B were excluded
Fig. 1.
Preferred reporting items for PRISMA flow diagram
Data extraction
Two independent reviewers (Han and Shamsnur) screened all titles and abstracts based on the predefined inclusion criteria. Discrepancies between reviewers were resolved through discussion, and unresolved disagreements were adjudicated by a third reviewer (Hongjing). Data were then extracted using a standardized data extraction form. Extracted variables included study characteristics (publication year, country, ascertainment basis and study design, such as retrospective observational studies or case-series), clinical and genetic features (including RCC subtype, FH variant subtype, tumor stage, and metastatic status), and quantitative outcomes (number of RCC cases in the LOF and missense variant groups, as well as effect estimates including ORs with corresponding 95% CIs. For studies with incomplete, ambiguous, or missing data, corresponding authors were contacted via email to obtain clarifications or supplementary information. All steps were performed in accordance with PRISMA 2020 standards.
Quality assessment
Two independent reviewers (Han and Shamsnur) assessed the methodological quality of the included studies using the Newcastle–Ottawa Scale (NOS), a validated tool for evaluating observational studies. The NOS evaluates studies across three domains: Selection (assessing the representativeness of participants and the appropriateness of exposure group definitions), Comparability (examining control for potential confounding variables), and Outcome, (evaluating the ascertainment of exposure and adequacy of follow-up). Studies were rated using a star-based system with a maximum score of nine. Scores of 1–3, 4–6, and 7–9 were considered indicative of low, moderate, and high quality, respectively.
Summary measures
To quantify RCC risk, unadjusted ORs were used as the primary summary measure in this meta-analysis. These were calculated from 2 × 2 contingency tables derived from raw data in each included study. No included study reported multivariable-adjusted ORs; therefore, confounding factors such as age, sex, or tumor stage could not be accounted for. As such, all pooled estimates are based on unadjusted data, and the potential influence of residual confounding should be acknowledged when interpreting the findings. This approach was applied consistently across all subgroup analyses to ensure methodological uniformity.
Statistical analyses
Meta-analyses were conducted using Review Manager (RevMan 5.4). For each study, log-transformed ORs and their corresponding variances were calculated. Pooled estimates with 95% CIs were derived using a fixed-effect Mantel–Haenszel model, based on the low statistical heterogeneity observed across studies (I²<50%, P > 0.10). Heterogeneity was assessed using the chi-squared (Q) test and quantified by the I² statistic. The choice of a fixed-effect model was also guided by the limited number of included studies, as random-effects models may produce unstable estimates and inflated confidence intervals under sparse data conditions. Although statistical heterogeneity was negligible, we acknowledge the potential for clinical and methodological heterogeneity—such as differences in patient selection, diagnostic criteria, and variant classification—which may not be fully captured by statistical metrics. Therefore, the pooled results should be interpreted with caution, taking into account the possibility of unmeasured sources of variation. Publication bias was assessed visually using standard funnel plots and statistically using Egger’s regression test. To estimate the potential influence of unpublished studies, the trim-and-fill method was applied. However, this method addresses only potential publication bias and does not correct for unmeasured confounding. Sensitivity analyses were performed by sequentially excluding each individual study and by removing studies with lower methodological quality (NOS score 1–3), to assess the influence of individual studies on the pooled estimates and the overall robustness of the results.
Subgroup analyses
Although no statistical heterogeneity was observed across studies (I² = 0%), exploratory subgroup analyses were conducted to assess whether specific clinicopathological or geographic factors might influence the association between FH variant types and RCC risk. Stratified analyses included geographic region (North America vs. Europe), histological subtype of HLRCC-associated RCC (particularly Type II PRCC), tumor behavior (e.g., clinical stage and presence of distant metastasis), and study design (variant/gene-first vs. phenotype-first).
Stratification by study design aimed to assess potential ascertainment bias. In variant/gene-first studies, participants were enrolled based on confirmed pathogenic or likely pathogenic germline FH variants, typically identified through multigene panel testing. Compared to phenotype-first designs, this approach may better reflect the natural history of RCC development among genetically predisposed but asymptomatic individuals. In contrast, phenotype-first studies recruited participants based on clinical features such as RCC, cutaneous leiomyomas, or uterine leiomyomas, which may enrich for more severe phenotypes and potentially overestimate RCC risk. The population-based study by Eric Lu et al., which identified FH variants through routine panel-based genetic testing in individuals without prior suspicion for HLRCC, was categorized within the variant/gene-first group. Geographic subgrouping was intended to explore potential differences in diagnostic practices, variant interpretation frameworks, and access to genetic testing, which may influence case ascertainment and clinical characterization across regions. Histological stratification focused on Type II PRCC—the most frequently reported and clinically aggressive subtype of HLRCC-associated RCC—to examine whether FH variant type may influence histopathological presentation. We also assessed whether FH variant types might be associated with tumor progression features, including clinical stage at diagnosis and metastatic status. However, due to the limited number of studies reporting these variables, these findings remain exploratory and should be interpreted with caution. It is important to note that the term “FH-deficient RCC”, as defined by the 2022 WHO classification, refers to renal tumors with either somatic or germline FH inactivation [32]. While partially overlapping with HLRCC-associated RCC, this category encompasses a broader pathological spectrum and was not used as the primary diagnostic framework in this review.
Results
A total of 263 abstracts were initially retrieved from PubMed, 429 from Embase, 437 from Scopus, and 347 from Web of Science. No relevant records were identified in the Cochrane Library. After removing duplicates, 717 unique records remained for title and abstract screening. Of these, 667 articles were excluded, and the remaining 50 full-text articles were assessed for eligibility. 3 studies were excluded due to unavailable full-texts. Among the 47 remaining articles, an additional 34 were excluded for the following reasons: review articles (n = 4), unclear definition of FH variant subtypes (n = 8), and lack of RCC incidence data (n = 22). Ultimately, 13 studies met the eligibility criteria and were included in the meta-analysis. An additional 7 studies, which lacked sufficient statistical data for effect size estimation, were included in the systematic review only. Notably, one publication contained 2 distinct study components: a variant-based analysis that met the criteria for meta-analysis, and a separate case-series of RCC patients with FH variants, which was included in the systematic review. As such, the systematic review comprised 8 studies in total, including one study partially overlapping with the meta-analysis.
Systematic review
Among the 20 studies included in the systematic review, 13 were retrospective observational studies that reported FH variant status in defined populations and examined the occurrence of RCC, while 8 were case-series based on patients already diagnosed with RCC. A summary of these studies is presented in Table 1. 9 studies adopted a phenotype-first approach, enrolling participants based on clinical features such as uterine or cutaneous leiomyomas or early-onset RCC. In several of these studies, recruitment was subsequently extended to include asymptomatic first-degree relatives of probands with confirmed FH variants, some of whom had not yet developed RCC. These individuals were included based on shared family history and molecular testing, which may have introduced variability in phenotype expression and reduced observed penetrance, particularly among those with missense variants. 4 studies followed a variant-first or gene-first design, in which individuals were identified through genetic testing and subsequently assessed for phenotypic manifestations. The classification of study design was used for subsequent subgroup analyses to explore potential sources of selection bias.
Retrospective observational studies (n = 13)
Thomas Scharnitz et al. [33] performed a retrospective chart review of 57 individuals evaluated for HLRCC at the University of Michigan between 2007 and 2020. Participants included individuals with genetically confirmed pathogenic or likely pathogenic germline FH variants, or with clinical features consistent with HLRCC and a family history of confirmed FH variant. Among these, 32 individuals harbored LOF variants, and 25 had missense variants (Table 1). In the LOF group, 3 patients were diagnosed with RCC, associated with the following FH variants: c.1506dup p.(Pro503ThrfsTer2), c.912_918del p.(Phe305LeufsTer22), and a complete deletion of the coding region. In the missense group, 3 RCC cases were also observed, associated with c.697 C > T p.(Arg233Cys), c.698G > A p.(Arg233His), and c.320 A > C p.(Asn107Thr). The unadjusted OR was 0.76 (95% CI: 0.14 to 4.13), indicating no statistically significant difference in RCC risk between individuals with LOF versus missense variants. However, the small number of RCC events and wide confidence intervals suggest limited statistical power to detect meaningful differences.
Eric Lu et al. [34] conducted a large-scale analysis involving 120,061 individuals who underwent hereditary cancer panel testing at a CLIA-certified commercial laboratory (Table 1). Among them, 302 individuals were identified with autosomal dominant FH pathogenic or likely pathogenic variants spanning 69 distinct variant types, and 492 individuals harbored autosomal recessive variants associated with FHd, involving 11 distinct alterations. The analysis focused exclusively on individuals with autosomal dominant variants associated with HLRCC phenotypes. Among 127 individuals harboring LOF variants, 29 were diagnosed with RCC, compared to 20 RCC cases among 162 individuals with missense variants. The resulting OR was 2.10 (95% CI: 1.12 to 3.93), indicating a statistically significant association between LOF variants and increased RCC risk. These results support the hypothesis that LOF variants may confer a higher predisposition to HLRCC-associated RCC than missense variants, reinforcing their potential utility in clinical risk stratification.
Junne Kamihara et al. [35] performed a retrospective analysis of individuals identified with germline FH pathogenic or likely pathogenic variants through multigene panel testing at a single diagnostic laboratory (Table 1). FH variants were categorized functionally as either truncating (including nonsense, frameshift, canonical splice-site, large deletions, and predicted disruptive missense changes) or non-truncating (such as synonymous substitutions and in-frame insertions/deletions). Truncating variants were operationally defined as canonical LOF variants, while most non-truncating variants—especially missense changes—may retain partial function but exhibit variable pathogenic potential. In total, 46 individuals harbored truncating variants, and 67 harbored non-truncating missense variants. RCC was diagnosed in 20 individuals (43.47%) with LOF variants and in 25 individuals (37.31%) with missense variants. The calculated OR was 1.29 (95% CI: 0.60 to 2.78), suggesting no statistically significant difference in RCC risk between these 2 variant categories. These findings highlight the complexity of variant interpretation and suggest that not all LOF variants confer uniformly elevated cancer risk relative to missense alterations.
Sánchez-Heras et al. [36] conducted a multicenter retrospective study in Spain involving 197 individuals with clinical suspicion of HLRCC who underwent germline FH testing between 2009 and 2019. Of these, 175 (88.8%) were found to harbor pathogenic or likely pathogenic FH variants (Table 1). Among them, 42 individuals harbored LOF variants, and 6 of these were diagnosed with RCC. The identified LOF variants included 2 cases with FH c.395delT, and one case each involving an exon 2 deletion, FH c.974delG, an exon 8 deletion, and an exon 9 deletion. The remaining 133 individuals harbored missense variants, including FH c.698G > A, FH c.845G > T, and FH c.1240 A > G; notably, 10 RCC cases were associated with the recurrent missense variant FH c.1118 A > G. The calculated OR comparing RCC risk between LOF and missense variant groups was 1.54 (95% CI: 0.55 to 4.34), indicating no statistically significant difference. These findings suggest that in this clinically ascertained cohort, FH LOF variants were not associated with a substantially higher risk of RCC compared to missense variants, although variant-specific effects cannot be ruled out.
Priya T. Bhola et al. [37] retrospectively reviewed clinical and genetic records from 69 individuals evaluated for HLRCC at a Canadian genetics center between 2004 and 2016, ultimately including 48 individuals with confirmed pathogenic or likely pathogenic FH variants in the final analysis (Table 1). Of these, 43 harbored LOF variants, and 4 were diagnosed with RCC. The associated variants included c.1430_1437dup, p.(Ser480LysfsTer6); c.797dupT, p.(Met266IlefsTer6); c.1111 A > T, p.(Lys371Ter); and c.1293delA, p.(Glu432LysfsTer17). Among 5 individuals with missense variants, only 1 RCC case was identified, involving FH c.965T > G, p.(Val322Gly). The OR for RCC risk comparing LOF and missense variant groups was 0.41 (95% CI: 0.04 to 4.62). Although no significant association was found, the wide confidence interval reflects the limited number of missense variant cases, reducing the statistical power to detect meaningful differences.
Ryan P. Kopp et al. [38] analyzed a small cohort of 9 individuals with germline FH variants who underwent clinical evaluation at a U.S. cancer center between 2008 and 2013 (Table 1). After excluding one individual due to incomplete clinical records, 8 participants were included in the final analysis. Among the 3 individuals with LOF variants, 2 were diagnosed with RCC—associated with FH c.954_957del, p.(Cys318LeufsTer5), and FH c.1010dup, p.(Ile338AspfsTer4), the latter with a reported family history of RCC. Among the 5 individuals with missense variants, 3 RCC cases were documented, including two with FH c.891T > A and FH c.1060G > A (both with a family history of RCC), and one with FH c.698G > A, p.(Arg233His), without a reported family history. The calculated OR was 1.33 (95% CI: 0.07 to 26.62), showing no statistically significant difference between the two variant groups. However, the wide confidence interval underscores the extremely limited sample size and the need for cautious interpretation.
Sanz-Ortega et al. [39] examined RCC risk among 19 female individuals from a family with confirmed HLRCC syndrome. 4 participants neither exhibited pathogenic or likely pathogenic FH variants nor developed RCC and were excluded from the genetic risk analysis. Among the remaining 15 participants, 6 were found to possess LOF variants and 9 had missense variants (Table 1). RCC was diagnosed in two individuals with LOF variants—FH c.172 C > T and FH c.1346del, p.(Met449ArgfsTer5)—and in 3 individuals with missense variants—FH c.569G > A, FH c.1187 A > C, and FH c.1025 C > A. The unadjusted OR was 1.00 (95% CI: 0.11 to 8.95), indicating no statistically significant difference in RCC risk between the 2 variant subtypes. However, the wide confidence interval reflects the limited statistical power due to the small cohort size.
Gardie et al. [40] evaluated a multicenter cohort comprising 129 individuals from 56 families with clinical features of HLRCC. Among 145 individuals with confirmed FH variants, 71 exhibited LOF variants and 74 exhibited missense variants (Table 1). RCC was identified in 8 individuals from the LOF group and 9 individuals from the missense group. The unadjusted OR was 0.92 (95% CI: 0.33 to 2.53), suggesting no statistically significant association between variant subtype and RCC risk. This finding highlights the heterogeneous phenotypic expression of FH-related disease and the likely involvement of additional modifying factors beyond variant type alone.
Smit DL et al. [41] collected clinical and genealogical data from 33 apparently unrelated probands undergoing genetic counseling in the Netherlands, including 29 Dutch individuals and 4 from other European countries. Among these families, pathogenic or likely pathogenic FH variants were identified in 14 kindreds (Table 1). 3 RCC cases were documented across these variant-positive kindreds: 1 associated with an LOF variant (FH c.233del, p.(Asn78ThrfsTer7)) and 2 with missense variants (FH c.1189G > A, p.(Gly397Arg) and FH c.698G > A, p.(Arg233His)). RCC was diagnosed in 1 of 9 individuals with LOF variants and in 2 of 26 individuals with missense variants. The unadjusted OR was 1.50 (95% CI: 0.12 to 18.84), suggesting no significant difference in RCC risk by variant subtype. The wide confidence interval underscores substantial statistical uncertainty, likely due to the small number of RCC events and possible low penetrance of RCC in certain FH variant-positive families.
M.-H. Wei et al. [42] investigated 21 families diagnosed with HLRCC and identified 46 individuals with confirmed germline FH variants through comprehensive molecular screening (Table 1). Among them, 17 individuals were found to harbor LOF variants, and 29 were identified with missense variants. 9 RCC cases were observed among the LOF group (52.94%), while 11 cases (37.93%) were observed among individuals with missense variants. The calculated unadjusted OR comparing RCC risk between LOF and missense variant groups was 1.84 (95% CI: 0.55 to 6.19), indicating no statistically significant difference. Although not conclusive, this trend suggests a possible increase in RCC penetrance in individuals with LOF variants, meriting further investigation in larger and more diverse populations.
Badeloe S et al. [43] conducted a clinical and genetic evaluation involving 6 index patients diagnosed with cutaneous leiomyomatosis and their family members across Dutch and Spanish lineages. Among 27 symptomatic relatives with confirmed FH variants, 2 individuals were identified with the FH c.424G > A (p.Gln142Lys) missense variant, while the remaining 25 individuals had LOF variants, including p.(Glu404Ter), p.(Asn78ThrfsTer7), p.(Asn415MetfsTer34), and 2 canonical splice site alterations (Table 1). Only 1 RCC case was reported, occurring in an individual with the p.(Asn78ThrfsTer7) variant. The unadjusted OR for RCC risk comparing LOF and missense variant types was 0.31 (95% CI: 0.01 to 9.69), reflecting no statistically significant association. The extremely small number of RCC cases and broad confidence interval limit the interpretability of these findings.
Jorge R. Toro et al. [44] analyzed 31 families with germline FH variants recruited via the American Academy of Dermatology. Pedigree-based segregation analysis was used to infer variant status in affected relatives. A total of 80 variant-positive individuals were included in the analysis: 22 inferred to harbor LOF variants and 58 inferred to harbor missense variants (Table 1). 13 RCC cases were identified—6 in the LOF group and 7 in the missense group. The resulting unadjusted OR was 2.73 (95% CI: 0.80 to 9.32), which did not reach statistical significance. The reliance on inferred, rather than directly confirmed, variant status and the modest sample size introduce uncertainty but suggest a potential trend warranting further study.
N.A. Alam et al. [45] evaluated 132 individuals across 46 families, identifying 35 kindreds with confirmed germline FH variants. Due to incomplete clinical or genetic data, only 91 individuals were included in the final analysis (Table 1). The study noted geographic clustering of specific variants (e.g., p.(Asn64Thr) in White British families, p.(Arg190His) in Spanish individuals). Despite the relatively large sample, only a single RCC case was observed, associated with the p.(Asn318Lys) missense variant. The unadjusted OR was 0.63 (95% CI: 0.02 to 15.91), indicating no statistically significant association. The rarity of RCC in this cohort, combined with the wide confidence interval, highlights the potential for modifying effects such as ethnicity or environmental exposures on disease expressivity.
The 13 retrospective studies included in this review were conducted in varied settings, including academic centers in North America and Europe and commercial genetic testing laboratories. Study populations included individuals with clinical HLRCC diagnoses, those identified through genetic testing for pathogenic or likely pathogenic FH variants, and family members enrolled via cascade screening. In terms of RCC risk, most studies did not observe a statistically significant difference between individuals with LOF versus missense variants, although one large population-based analysis did report a significantly elevated RCC risk among those with LOF variants. A few studies noted trends toward more advanced-stage disease or earlier onset in LOF variant carriers, but these findings were not consistently replicated due to small sample sizes, low event rates, and wide confidence intervals. Differences in study design, variant interpretation, and follow-up contribute to methodological heterogeneity. Overall, evidence linking FH variant function to RCC risk in HLRCC remains limited and inconsistent. Larger, prospective studies with standardized classification are needed to clarify genotype–phenotype correlations and guide risk stratification.
Case-series studies (n = 8)
Several case series have provided additional insights into the variant spectrum observed in patients with RCC and confirmed FH deficiency. These studies adopted a “RCC-first” design, in which individuals were initially diagnosed with RCC and subsequently tested for FH variants. Yanfei Yu et al. [46] reported 3 unrelated young female RCC patients, each found to harbor a germline FH variant. Two individuals had missense variants, both associated with highly aggressive tumor behavior, while the third carried a LOF variant. Ja Young Seo et al. [47] analyzed 13 RCC cases and identified 7 with FH variants—5 LOF and 2 missense variants. Using the MSK-IMPACT platform, Lijing Zhang et al. [48] retrospectively screened 7,571 RCC patients at Memorial Sloan Kettering Cancer Center and identified 11 FH variant-positive cases, including 7 LOF and 4 missense variants. This study also noted a potential ethnic bias, with most FH-altered RCC cases occurring in individuals of White or Ashkenazi Jewish ancestry. Mitsuko Furuya et al. [49] described a cohort of 10 RCC patients with FH alterations (4 LOF and 6 missense), mostly male, with a median age of 45 years. Hubert D. Lau et al. [50] reported 32 RCC cases across centers in the U.S. and Australia, 14 of whom had FH variants (6 LOF, 8 missense). Ying-Bei Chen et al. [51] described 9 RCC cases with FH alterations (4 LOF, 5 missense), and Taru A. Koski et al. [52] analyzed 11 Finnish HLRCC cases with confirmed germline FH alterations (7 LOF, 4 missense). Additionally, Gardie et al. [40] reported 10 cases of Type PRCCII, evenly split between LOF and missense variant types (n = 5 each).
These studies provide supportive, though non-comparative, data suggesting both LOF and missense FH variants may contribute to the pathogenesis of HLRCC-associated RCC. However, due to the absence of control groups, they are best classified as descriptive case series and cannot yield direct risk estimates.
Meta-analyses
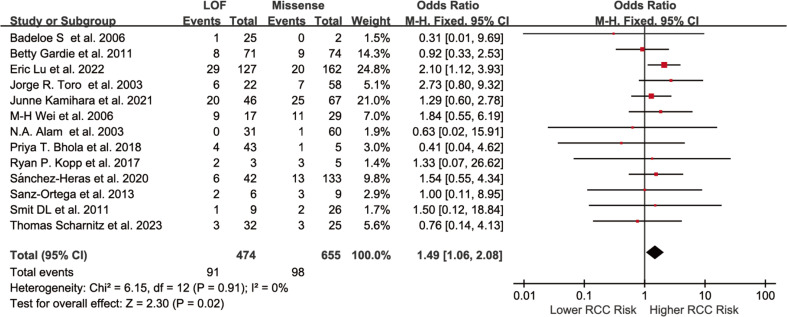
Among the 20 studies included in this systematic review, 13 were eligible for quantitative synthesis. The remaining 8 case-series lacked sufficient data for effect size estimation and were included in the narrative review only. Among the 13 meta-analyzed studies, 5 were of low quality (NOS 1–3) and 8 of moderate quality (NOS 4–6) (Table 1). The pooled analysis showed that individuals with FH LOF variants had a significantly higher risk of developing HLRCC-associated RCC compared to those with missense variants (OR = 1.49, 95% CI: 1.06 to 2.08) (Fig. 2).
Fig. 2.
Forest plot of the association between individuals with FH LOF variants and those with missense variants in relation to the risk of HLRCC-associated RCC (unadjusted ORs). The squares represent the risk estimates for individual studies; horizontal lines indicate the 95% CIs; the diamonds represent the pooled estimate with corresponding 95% CI. A fixed-effect model was used, and all statistical tests were two-sided. Heterogeneity test: χ² = 6.15, df = 12 (P = 0.91); I² = 0.0%
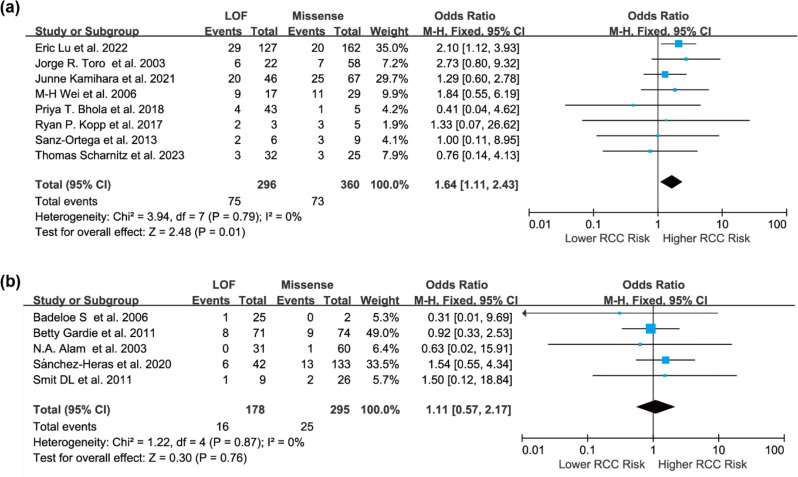
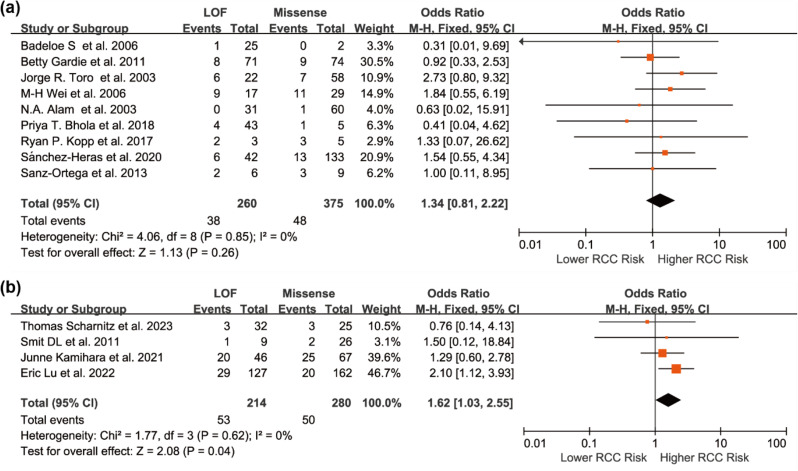
To explore potential sources of variation in risk estimates, subgroup analyses were conducted. Given possible differences in testing practices, variant interpretation, and population background, studies were stratified by geographic region. The association remained significant in the North American subgroup (OR = 1.64, 95% CI: 1.11 to 2.43), but not in European studies (OR = 1.11, 95% CI: 0.57 to 2.17), likely due to limited statistical power (Fig. 3). Another stratification based on ascertainment strategy showed a stronger association in variant-/gene-first studies (OR = 1.62, 95% CI: 1.03 to 2.55) than in phenotype-first studies (OR = 1.34, 95% CI: 0.81 to 2.22), suggesting study design may influence risk estimates (Fig. 4).
Fig. 3.
Forest plots of the association between individuals with FH LOF variants and those with missense variants in relation to the risk of HLRCC-associated RCC in different geographic regions, presented as unadjusted ORs: (a) North America; (b) Europe. Squares represent the risk estimates for individual studies, and horizontal lines indicate the corresponding 95% CIs. Diamonds represent the pooled ORs with their 95% CIs. A fixed-effect model was applied, and all statistical tests were two-sided. Heterogeneity tests: (a) χ² = 3.94, df = 7 (P = 0.79); I² = 0.0%. (b) χ² = 1.22, df = 4 (P = 0.87); I² = 0.0%
Fig. 4.
Forest plots of unadjusted ORs comparing FH LOF and missense variants in HLRCC-associated RCC, stratified by study design: (a) Phenotype-first studies; (b) Variant-/gene-first studies. Squares represent the effect estimates from individual studies, with horizontal lines indicating the 95% CIs. Diamonds indicate the pooled estimates and corresponding 95% CIs. A fixed-effect model was applied, and all statistical tests were two-sided. Heterogeneity tests: (a) χ²=4.06, df = 8 (P = 0.85); I² = 0.0%. (b) χ²=1.77, df = 3 (P = 0.62); I²=0.0%
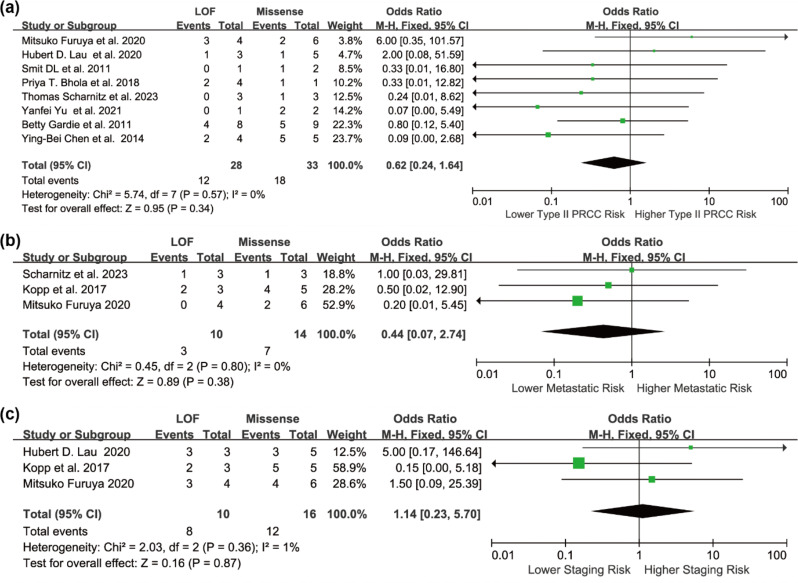
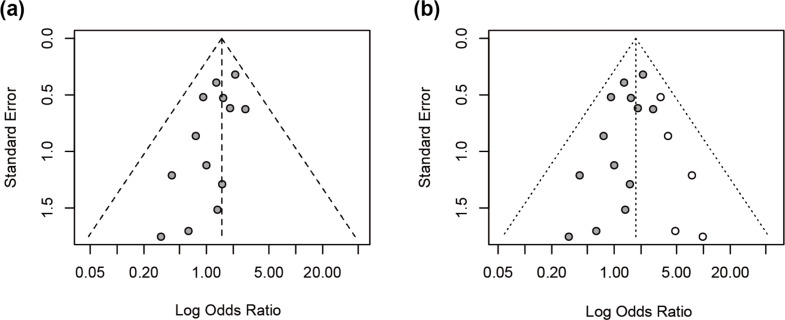
Three additional subgroup analyses were performed among RCC cases. Patients with LOF variants showed a lower, though non-significant, risk of type II papillary RCC (OR = 0.44, 95% CI: 0.24 to 1.64), and a lower tendency toward metastasis (OR = 0.44, 95% CI: 0.07 to 2.74). Conversely, they had a higher likelihood of advanced-stage disease at diagnosis (OR = 1.14, 95% CI: 0.23 to 5.70) (Fig. 5). Although none of these associations reached statistical significance, the directionality of effect may reflect underlying phenotypic differences between variant types. Despite no statistical heterogeneity (I²=0%), differences in study design, variant classification, and follow-up duration likely introduced unmeasured clinical or methodological heterogeneity. Visual inspection of the funnel plot suggested slight asymmetry, and Egger’s test confirmed potential publication bias (p = 0.0431). After applying the trim-and-fill method to account for publication bias, the imputed pooled OR was 1.75 (95% CI: 1.28 to 2.38), supporting the consistency of the association despite potential reporting bias. (Fig. 6).
Fig. 5.
Forest plots illustrating the association between FH LOF variants and missense variants among patients with HLRCC-associated RCC, presented as unadjusted ORs, for the following outcomes: (a) Risk of type II papillary RCC; (b) Risk of distant metastasis; (c) Risk of advanced-stage disease. Squares represent the effect estimates from individual studies, with horizontal lines indicating the 95% CIs. Diamonds indicate the pooled estimates and corresponding 95% CIs. A fixed-effect model was applied, and all statistical tests were two-sided. Heterogeneity tests: (a) χ² =5.74, df = 7 (P = 0.57); I²=0.0%. (b) χ²=0.45, df = 2 (P = 0.80); I²=0.0%. (c) χ²=2.03, df = 2 (P = 0.36); I²=1.0%
Fig. 6.
(a) Funnel plot of the standard error versus the log-transformed odds ratio for the overall meta-analysis assessing the association between FH LOF and missense variants with HLRCC-associated RCC. (b) Funnel plot after applying the trim-and-fill method to assess potential publication bias
Sensitivity analyses indicated that the findings were somewhat dependent on specific studies. Removing any single study did not substantially alter the effect direction, but exclusion of either the Lu or Toro study rendered the association non-significant, suggesting these studies contributed substantially to the observed effect. After removing all low-quality studies, the pooled OR remained significant (OR=1.53, 95% CI: 1.07 to 2.21) (Table 2), indicating overall robustness of the findings.
Table 2.
Influence of individual studies on the pooled estimate
Discussion
Our meta-analysis found that individuals harboring FH LOF variants had a significantly higher risk of developing HLRCC-associated RCC compared to those with missense variants (OR = 1.49, 95% CI: 1.06 to 2.08). This trend was consistently observed across both North American and European populations, with a stronger association in the North American subgroup (OR = 1.64, 95% CI: 1.11 to 2.43), potentially reflecting broader adoption of multigene panel testing, more consistent variant interpretation standards, and heightened clinical awareness in that region.
Although statistical heterogeneity was minimal (I²=0%), we performed multiple subgroup analyses to explore potential clinical and methodological sources of variability. In analyses stratified by RCC histology, clinical stage, and metastasis status, individuals with LOF variants were less likely to present with Type II PRCC or distant metastasis, but more frequently diagnosed with advanced-stage tumors. These findings suggest that FH variant subtypes may not only influence RCC risk but also shape divergent tumor progression trajectories through distinct biological mechanisms. However, given the limited number of studies contributing to these subgroup analyses, these findings should be interpreted cautiously and viewed as preliminary signals warranting further validation.
Previous studies on HLRCC-associated RCC have primarily focused on missense variants, which are more frequently reported in FH [23, 27, 53]. This emphasis may have contributed to the prevailing perception that missense variants are more closely associated with RCC development. Importantly, although most missense variants retain partial enzymatic function, several—particularly those affecting residues critical to catalytic function or tetramerization (e.g., Arg190, Arg233)—can severely disrupt protein integrity, stability, or subcellular localization. Some may exert dominant-negative effects. In our analysis, the higher proportion of Type II PRCC and metastatic disease in individuals with missense variants may reflect unique tumor biology associated with this variant class. Still, due to small subgroup sizes, these trends should be considered hypothesis-generating.
In the ascertainment-strategy subgroup analysis, studies employing a variant-first/gene-first design—where individuals were enrolled based on confirmed pathogenic or likely pathogenic germline FH variants—demonstrated a stronger association between LOF variants and RCC risk (OR = 1.62, 95% CI: 1.03 to 2.55). In contrast, phenotype-first studies, which recruited individuals based on clinical features suggestive of HLRCC, showed a weaker and non-significant association (OR = 1.34, 95% CI: 0.81 to 2.22). This disparity may reflect differences in case composition and detection bias. Variant-first studies often included a broader spectrum of individuals with pathogenic or likely pathogenic variants—particularly those with milder phenotypes—resulting in lower observed RCC penetrance and amplifying the risk differential between variant classes. In contrast, phenotype-first studies frequently enriched for RCC cases by design, thereby diminishing the apparent magnitude of LOF-related risk elevation. Additionally, considerable variability was observed across studies in terms of pedigree inclusion strategies—some limited analyses to individuals with cutaneous or uterine manifestations (narrow-spectrum pedigrees), while others incorporated broader family members regardless of phenotype (expanded pedigrees). These inconsistencies in case definition and recruitment likely introduced further challenges in comparing RCC risk across cohorts, and may have contributed to discrepancies in apparent variant penetrance.
In this meta-analysis, we employed a function-based variant classification system informed by enzymatic assays, structural modeling, and ACMG criteria. LOF variants included truncating, splice-site, frameshift, and large deletion variants expected to abolish enzymatic activity, leading to fumarate accumulation. Excess fumarate promotes tumorigenesis via stabilization of HIFs (pseudohypoxia), activation of NRF2 signaling, epigenetic reprogramming, and mitochondrial dysfunction [29, 54–58]. In contrast, many missense variants retain partial function; however, those located in key structural or functional regions may lead to substantial disruption. Notably, a subset of missense variants may, in fact, result in complete LOF but were grouped as “missense” based on sequence consequence rather than confirmed functional assessment. This inherent limitation in available data may blur the boundary between variant classes and attenuate observed differences in cancer risk. We excluded individuals with biallelic or compound heterozygous FH variants, who present with fumarate hydratase deficiency, a distinct autosomal recessive metabolic disorder unrelated to the HLRCC–RCC spectrum. Likewise, the WHO 2022 classification of “FH-deficient RCC”—which includes tumors with either germline or somatic inactivation of FH—was not used as our primary histologic framework, as it does not directly map onto the clinical definition of HLRCC [32].
Interestingly, the Spanish cohort (Sánchez-Heras et al. [59]) exhibited a high frequency of the FH c.1118 A > G (p.Asn373Ser) variant with a relatively low RCC detection rate, suggesting a potential founder effect. This population-specific phenomenon may lead to decoupling between variant frequency and clinical penetrance, thus limiting the generalizability of pooled risk estimates. Sensitivity analyses showed that the pooled effect estimate lost statistical significance when individual studies—such as those by Lu et al. or Toro et al.—were excluded. This suggests the overall association may be sensitive to specific data sources. Moreover, several included studies lacked granular data on histological subtype, tumor stage, or follow-up duration, constraining our ability to draw firm conclusions about clinical progression patterns.
From a clinical perspective, if confirmed, the differences in cancer risk between LOF and missense variants could inform tailored management strategies for FH variant carriers. Individuals harboring higher-risk LOF variants may benefit from earlier initiation of surveillance, increased screening frequency, and the use of more sensitive imaging modalities such as MRI rather than ultrasound. Stratifying clinical management according to variant subtype could help optimize cancer prevention and early detection in this population. A further limitation of this study is the limited geographic diversity of the included cohorts. Most of the available data are derived from North American and European populations, with minimal representation from Asian, African, or other ethnic groups. This lack of diversity restricts the generalizability of our findings and highlights the need for more inclusive studies across diverse populations. In addition, we relied on the pathogenicity classifications reported in the original studies and did not independently reassess VUS. Some variants originally classified as VUS may have since been reclassified as pathogenic or likely pathogenic. The exclusion of these variants could result in an underestimation of RCC risk associated with FH variants, introducing a potential source of bias. Finally, although an analysis limited to asymptomatic relatives identified through family screening—excluding probands diagnosed with RCC—would provide a clearer estimate of true RCC penetrance, such an approach was not feasible in this meta-analysis. Most studies did not clearly distinguish probands from relatives, nor did they provide separate data for unaffected carriers. This limitation constrains our ability to assess baseline RCC risk independent of clinical presentation.
In summary, while our findings support a potential functional link between FH variant subtype and RCC risk in HLRCC, the results should be interpreted in light of residual confounding, heterogeneity in study design, and functional misclassification. Larger, prospective, and harmonized studies—including rigorous functional validation of variants—are essential to confirm and expand these observations. Ultimately, functional subclassification of FH variants may offer meaningful stratification for risk assessment in HLRCC, analogous to the role of BRCA1/2 variant characterization in hereditary breast and ovarian cancer [60–63]. Such an approach may facilitate individualized screening, genetic counseling, and early intervention strategies in families affected by FH-related hereditary kidney cancer.
Conclusion
Unlike previous studies that primarily provided descriptive insights, this systematic review and meta-analysis offers quantitative evidence suggesting that individuals with FH LOF variants may have a moderately increased risk—approximately 1.75-fold—of developing HLRCC-associated renal cell carcinoma compared to those with missense variants. While acknowledging the limitations inherent to the available data, these findings provide important preliminary support for the role of variant functional class in modulating cancer risk, underscoring the potential value of FH variant stratification in future risk assessment and clinical decision-making.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks my friends.
Author contributions
Han Wang: Writing– review & editing, Writing– original draft. Shamsnur Rehim: Writing– review & editing. Hongjing Wang: Writing– review & editing, Conceptualization.
Funding information
This research received no funding.
Data availability
The studies included in this meta-analysis were identified through systematic searches of public databases including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/), Scopus (https://www.scopus.com/), and Web of Science (https://www.webofscience.com/). All data used are available from the published articles cited in the references.
Declarations
Ethics, consent to participate, and consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jarvik GP, Evans J. Mastering genomic terminology. Genet Med. 2017;19(5):491–2. 10.1038/gim.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinn AB, Kerr DS, Hoppel CL. Fumarase deficiency: A new cause of mitochondrial encephalomyopathy. N Engl J Med. 1986;315(8):469–75. 10.1056/NEJM198608213150801. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeron T, Chretien D, Poggi-Bach J, et al. Mutation of the fumarase gene in two siblings with progressive encephalopathy and fumarase deficiency. J Clin Invest. 1994;93(6):2514–8. 10.1172/JCI117261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gellera C, Uziel G, Rimoldi M, et al. Fumarase deficiency is an autosomal recessive encephalopathy affecting both the mitochondrial and the cytosolic enzymes. Neurology. 1990;40(3part1):495–495. 10.1212/WNL.40.3_Part_1.495. [DOI] [PubMed] [Google Scholar]
- 5.Remes AM, Hiltunen JK, Rantala H, Leisti J, Ruokonen A. Fumarase deficiency: two siblings with enlarged cerebral ventricles and polyhydramnios in utero. Pediatrics. 1992;89(4):730–4. 10.1542/peds.89.4.730. [PubMed] [Google Scholar]
- 6.Ottolenghi C, Hubert L, Allanore Y, et al. Clinical and biochemical heterogeneity associated with fumarase deficiency. Hum Mutat. 2011;32(9):1046–52. 10.1002/humu.21534. [DOI] [PubMed] [Google Scholar]
- 7.Bayley JP, Launonen V, Tomlinson IP. The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet. 2008;9(1):20. 10.1186/1471-2350-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson IPM, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30(4):406–10. 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 9.Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13(4):637–44. 10.1007/s10689-014-9735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller M, Ferlicot S, Guillaud-Bataille M, et al. Reassessing the clinical spectrum associated with hereditary leiomyomatosis and renal cell carcinoma syndrome in French mutation carriers. Clin Genet. 2017;92(6):606–15. 10.1111/cge.13014. [DOI] [PubMed] [Google Scholar]
- 11.Panarsky R, Crooks DR, Lane AN, et al. Fumarate hydratase-deficient renal cell carcinoma cells respond to asparagine by activation of the unfolded protein response and stimulation of the hexosamine biosynthetic pathway. Cancer Metab. 2020;8:7. 10.1186/s40170-020-00214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer IM, Hornick JL, Bovée JVMG. The role of metabolic enzymes in mesenchymal tumors and tumor syndromes: genetics, pathology, and molecular mechanisms. Lab Invest. 2018;98(4):414–26. 10.1038/s41374-017-0003-6. [DOI] [PubMed] [Google Scholar]
- 13.Podkalicka P, Mucha O, Kruczek S, et al. Synthetically lethal interactions of Heme Oxygenase-1 and fumarate hydratase genes. Biomolecules. 2020;10(1):143. 10.3390/biom10010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu IS, Maksim NJ, Amouzougan EA, Gallion BW, Raviele ALJ, Ooi A. Sustained NRF2 activation in hereditary leiomyomatosis and renal cell cancer (HLRCC) and in hereditary tyrosinemia type 1 (HT1). Biochem Soc Trans. 2015;43(4):650–6. 10.1042/BST20150041. [DOI] [PubMed] [Google Scholar]
- 15.Crooks DR, Maio N, Lang M, et al. Mitochondrial DNA alterations underlie an irreversible shift to aerobic Glycolysis in fumarate hydratase-deficient renal cancer. Sci Signal. 2021;14(664):eabc4436. 10.1126/scisignal.abc4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merino MJ, Torres-Cabala C, Pinto P, Marston Linehan W. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31(10):1578. 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 17.McMurtry V, Mahlow J, Coleman JF, et al. Morphologic characteristics and mutational analysis of fumarate hydratase deficient kidney and smooth muscle tumors. Am J Clin Pathol. 2023;159(2):164–71. 10.1093/ajcp/aqac148. [DOI] [PubMed] [Google Scholar]
- 18.Clark GR, Sciacovelli M, Gaude E, et al. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metabolism. 2014;99(10):E2046–50. 10.1210/jc.2014-1659. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23(9):2440–6. 10.1093/hmg/ddt639. [DOI] [PubMed] [Google Scholar]
- 20.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer. 2011;10(2):397–411. 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 21.Damaso E, Heras ABS, Esteve Y, et al. Hereditary leiomyomatosis and renal cell carcinoma: identification and characterization of a new Spanish founder mutation in the FH gene. Eur J Hum Genet. 2022;30(SUPPL 1):401–2. 10.1038/s41431-021-01026-1. [Google Scholar]
- 22.Yu Y, Zheng M, Zhu W, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): case series and review of the literature. Urol Oncol. 2021;39(11):791.e9-791.e16. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Xu Y, Wang C, et al. A missense mutation c.1132G > A in fumarate hydratase (FH) leads to hereditary leiomyomatosis and renal cell Cancer (HLRCC) syndrome and insights into clinical management in uterine leiomyomata. Genes. 2023;14(3):744. 10.3390/genes14030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan HH, Ruan DD, Wu M, et al. Clinical phenotype and genetic function analysis of a rare family with hereditary leiomyomatosis and renal cell carcinoma complicated with Birt–Hogg–Dubé syndrome. J Med Genet. 2023;60(12):1210–4. 10.1136/jmg-2023-109328. [DOI] [PubMed] [Google Scholar]
- 25.Arenas Valencia C, Rodríguez López ML, Cardona Barreto AY, Garavito Rodríguez E, Arteaga Díaz CE. Hereditary leiomyomatosis and renal cell cancer syndrome: identification and clinical characterization of a novel mutation in the FH gene in a Colombian family. Fam Cancer. 2017;16(1):117–22. 10.1007/s10689-016-9922-4. [DOI] [PubMed] [Google Scholar]
- 26.Raymond VM, Herron CM, Giordano TJ, Gruber SB. Familial renal cancer as an indicator of hereditary leiomyomatosis and renal cell cancer syndrome. Fam Cancer. 2012;11(1):115–21. 10.1007/s10689-011-9485-3. [DOI] [PubMed] [Google Scholar]
- 27.ALAM N A, OLPIN S, ROWAN A, et al. Missense mutations in fumarate hydratase in multiple cutaneous and uterine leiomyomatosis and renal cell cancer. J Mol Diagn. 2005;7(4):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CHAN I, WONG T, MARTINEZ-MIR A, et al. Familial multiple cutaneous and uterine leiomyomas associated with papillary renal cell cancer. Clin Exp Dermatol. 2005;30(1):75–8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Li L, Zhang Y, Zeng C. Hereditary leiomyomatosis and renal cell cancer: recent insights into mechanisms and systemic treatment. Front Oncol. 2021;11:686556. 10.3389/fonc.2021.686556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner AK, Tulchiner G, Seeber A, Siska PJ, Thurnher M, Pichler R. Targeting strategies in the treatment of fumarate hydratase deficient renal cell carcinoma. Front Oncol. 2022;12:906014. 10.3389/fonc.2022.906014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kancherla P, Daneshvar M, Sager RA, Mollapour M, Bratslavsky G. Fumarate hydratase as a therapeutic target in renal cancer. Expert Opin Ther Targets. 2020;24(9):923–36. 10.1080/14728222.2020.1804862. [DOI] [PubMed] [Google Scholar]
- 32.Goswami PR, Singh G, Patel T, Dave R. The WHO 2022 Classification of Renal Neoplasms (5th Edition): Salient Updates. Cureus. 16(4):e58470. 10.7759/cureus.58470 [DOI] [PMC free article] [PubMed]
- 33.SCHARNITZ T, NAKAMURA M. The spectrum of clinical and genetic findings in hereditary leiomyomatosis and renal cell cancer (HLRCC) with relevance to patient outcomes: a retrospective study from a large academic tertiary referral center. Am J Cancer Res. 2023;13(1):236–. [PMC free article] [PubMed] [Google Scholar]
- 34.LU E, HATCHELL K E, NIELSEN S M, et al. Fumarate hydratase variant prevalence and manifestations among individuals receiving germline testing. Cancer. 2022;128(4):675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KAMIHARA J, HORTON C, TIAN Y, et al. Different fumarate hydratase gene variants are associated with distinct Cancer phenotypes. Jco Precision Oncol. 2021;5:1568–78. [DOI] [PubMed] [Google Scholar]
- 36.SáNCHEZ-HERAS A B CASTILLEJOA, GARCíA-DíAZ JD et al. Hereditary leiomyomatosis and renal cell Cancer syndrome in spain: clinical and genetic characterization. Cancers (Basel), 2020, 12(11). [DOI] [PMC free article] [PubMed]
- 37.BHOLA P T, GILPIN C, SMITH A, et al. A retrospective review of 48 individuals, including 12 families, molecularly diagnosed with hereditary leiomyomatosis and renal cell cancer (HLRCC). Fam Cancer. 2018;17(4):615–20. [DOI] [PubMed] [Google Scholar]
- 38.KOPP R, STRATTON K. GLOGOWSKI E, Utility of prospective pathologic evaluation to inform clinical genetic testing for hereditary leiomyomatosis and renal cell carcinoma. J Urol, 2014, 1): e381. [DOI] [PubMed]
- 39.SANZ-ORTEGA J, VOCKE C, STRATTON P, et al. Morphologic and molecular characteristics of uterine leiomyomas in hereditary leiomyomatosis and renal cancer (HLRCC) syndrome. Am J Surg Pathol. 2013;37(1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardie B, Remenieras A, Kattygnarath D, et al. Novel FH mutations in families with hereditary leiomyomatosis renal cell cancer (HLRCC) and in patients with isolated type 2 papillary renal cell carcinoma. J Med Genet. 2011;48(4):226. 10.1136/jmg.2010.085068. [DOI] [PubMed] [Google Scholar]
- 41.Smit DL, Mensenkamp AR, Badeloe S, et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet. 2011;79(1):49–59. [DOI] [PubMed] [Google Scholar]
- 42.Wei MH, Toure O, Glenn GM, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badeloe S, Van Geel M, Van Steensel MAM, et al. Diffuse and segmental variants of cutaneous leiomyomatosis: novel mutations in the fumarate hydratase gene and review of the literature. Exp Dermatol. 2006;15(9):735–41. [DOI] [PubMed] [Google Scholar]
- 44.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73(1):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alam NA, Rowan AJ, Wortham NC, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12(11):1241–52. [DOI] [PubMed] [Google Scholar]
- 46.Yu YF, Zheng MM, Zhu W, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): case series and review of the literature. Volume 39. Urologic Oncology-Seminars and Original Investigations; 2021. 11. [DOI] [PubMed]
- 47.Seo JY, Ahn JY, Keam B, et al. Genotypic and phenotypic characteristics of hereditary leiomyomatosis and renal cell Cancer syndrome in Korean patients. Annals Lab Med. 2021;41(2):207–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Walsh MF, Jairam S, et al. Fumarate hydratase FH c.1431_1433dupAAA (p.Lys477dup) variant is not associated with cancer including renal cell carcinoma. Hum Mutat. 2020;41(1):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furuya M, Iribe Y, Nagashima Y, et al. Clinicopathological and molecular features of hereditary leiomyomatosis and renal cell cancer-associated renal cell carcinomas. J Clin Pathol. 2020;73(12):819–25. [DOI] [PubMed] [Google Scholar]
- 50.Lau HD, Chan E, Fan AC, et al. A clinicopathologic and molecular analysis of fumarate Hydratase-deficient renal cell carcinoma in 32 patients. Am J Surg Pathol. 2020;44(1):98–110. [DOI] [PubMed] [Google Scholar]
- 51.Chen YB, Brannon AR, Toubaji A, et al. Hereditary leiomyomatosis and reenal cell carcinoma syndrome-associated renal Cancer recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol. 2014;38(5):627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koski TA, Lehtonen HJ, Jee KJ, et al. Array comparative genomic hybridization identifies a distinct DNA copy number profile in renal cell cancer associated with hereditary leiomyomatosis and renal cell cancer. Genes Chromosomes Cancer. 2009;48(7):544–51. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Du R, Han L, Yang R, Li Y. A new missense mutation c.1240A > G in fumarate hydratase gene leads to uterine leiomyoma associated hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome in Chinese. Transl Oncol. 2024;45:101963. 10.1016/j.tranon.2024.101963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valcarcel-Jimenez L, Rogerson C, Yong C, et al. HIRA loss transforms FH-deficient cells. Sci Adv. 2022;8(42):eabq8297. 10.1126/sciadv.abq8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogerson C, Sciacovelli M, Maddalena LA, et al. FOXA2 controls the anti-oxidant response in FH-deficient cells. Cell Rep. 2023;42(7):112751. 10.1016/j.celrep.2023.112751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frezza C, Zheng L, Folger O, et al. Haem Oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477(7363):225–8. 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- 57.Bardella C, Olivero M, Lorenzato A, et al. Cells lacking the fumarase tumor suppressor are protected from apoptosis through a hypoxia-inducible factor-independent, AMPK-dependent mechanism. Mol Cell Biol. 2012;32(15):3081–94. 10.1128/MCB.06160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zyla RE, Hodgson A, BHOLA P T, GILPIN C, SMITH A et al. A retrospective review of 48 individuals, including 12 families, molecularly diagnosed with hereditary leiomyomatosis and renal cell cancer (HLRCC). Fam Cancer, 2018, 17(4): 615– 20. J Clin Pathol. 2021;74(10):615–619. 10.1136/jclinpath-2021-207830 [DOI] [PubMed]
- 59.Sanchez-Heras AB, Damaso E, Castillejo A et al. Genetic and clinical characterization of a novel FH founder mutation in families with hereditary leiomyomatosis and renal cell cancer syndrome. Orphanet J Rare Dis, 2024, 19(1) (no pagination). [DOI] [PMC free article] [PubMed]
- 60.Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107(12):2005–9. 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121(2):269–75. 10.1002/cncr.29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moran A, O’Hara C, Khan S, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11(2):235–42. 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 63.Mocci E, Milne RL, Méndez-Villamil EY, et al. Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer Epidemiol Biomarkers Prev. 2013;22(5):803–11. 10.1158/1055-9965.EPI-12-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The studies included in this meta-analysis were identified through systematic searches of public databases including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/), Scopus (https://www.scopus.com/), and Web of Science (https://www.webofscience.com/). All data used are available from the published articles cited in the references.