Abstract
Cerebral vasospasm and ischemic damage are important causes of mortality and morbidity in patients affected by aneurysmal subarachnoid hemorrhage (SAH). Recently, i.p. administration of recombinant human erythropoietin (r-Hu-EPO) has been shown to exert a neuroprotective effect during experimental SAH. The present study was conducted to evaluate further the effect of r-Hu-EPO administration after SAH in rabbits on neurological outcome, degree of basilar artery spasm, and magnitude of neuronal ischemic damage. Experimental animals were divided into six groups: group 1 (n = 8), control; group 2 (n = 8), control plus placebo; group 3 (n = 8), control plus r-Hu-EPO; group 4 (n = 8), SAH; group 5 (n = 8), SAH plus placebo; group 6 (n = 8), SAH plus r-Hu-EPO. r-Hu-EPO, at a dose of 1,000 units/kg, and placebo were injected i.p. starting 5 min after inducing SAH and followed by clinical and pathological assessment 72 h later. Systemic administration of r-Hu-EPO produced significant increases in cerebrospinal fluid EPO concentrations (P < 0.001), and reduced vasoconstriction of the basilar artery (P < 0.05), ischemic neuronal damage (P < 0.001), and subsequent neurological deterioration (P < 0.05). These observations suggest that r-Hu-EPO may provide an effective treatment to reduce the post-SAH morbidity.
Cerebral vasoconstriction after aneurysmal subarachnoid hemorrhage (SAH) is an important cause of cerebral ischemia, neurological disability, and premature death (1). The lack of an adequate treatment for this condition has stimulated numerous experimental and clinical studies for development of effective therapeutic strategies. Cerebral arteries respond to SAH with a biphasic contraction, the first begins minutes after the bleed, with a delayed vasospasm more than 48 h later (2, 3). Although delayed vasoconstriction has long been identified as a major complication in patients suffering from SAH (4), the clinical importance of acute vasoconstriction, a phenomenon well documented in experimental settings (5), remains to be clarified in humans. In this regard, considerable evidence has accumulated suggesting that immediate vasoconstriction produces the acute cerebral ischemia that typically follows SAH (6).
To date, several theories have been proposed to explain the occurrence of SAH-induced acute cerebral ischemia. Complications after SAH are usually attributed to luminal narrowing of the major extraparenchymal arteries. However, this mechanism cannot fully explain the diffuse brain ischemia, the phenomena of cortical inhibition, blood–brain barrier (BBB) dysfunction, and altered cerebrovascular reactivity that frequently follow SAH (7). Therefore, other mechanisms, such as cerebral microcirculatory dysfunction, may be involved as contributory causes in the pathogenic cascade after SAH (8, 9). Acute cerebral ischemia has been attributed to a decrease in cerebral perfusion pressure (CPP) (10). However, experimental and clinical studies have shown that CPP does not usually drop to the point of perfusion arrest (11, 12), suggesting that the decrease in CPP cannot fully account for the SAH-induced acute cerebral ischemia. It has been reported that acute ischemia after SAH is associated with a sudden and persistent decrease in cerebral blood flow, despite the changes in intracranial pressure and CPP (5). Recently, it has been pointed out that impairment of the NO vasodilatory pathway may play a role in acute cerebral vasoconstriction and ischemia after SAH (13, 14).
During the past several years, interest has focused on the efficacy of recombinant human erythropoietin (r-Hu-EPO) to protect against neurological injury produced in several experimental models of brain insult. In particular, studies have shown a neuroprotective effect of EPO in models of cerebral ischemia (15–18), concussive brain injury (19), experimental autoimmune encephalomyelitis, and kainate-induced seizures (19). In these experimental procedures, EPO has been administered both intrathecally or systemically, provoking controversy regarding the ability of the heavily glycosylated EPO protein to cross the BBB. Recently, it has been reported that systemic administration of r-Hu-EPO, immediately after experimental SAH, reduces the mortality rate, ameliorates functional recovery, and prevents brain ischemic damage after experimental SAH (20–23). Given these findings, and according to reports that have demonstrated that EPO enhances the NO system activity (24–26), elicits neuroprotective properties on cerebral cortical neurons from N-methyl-D-aspartate receptor-mediated glutamate toxicity (15), we further investigated the potential protective effects of r-Hu-EPO in a rabbit model of SAH.
Materials and Methods
Animals and Induction of SAH.
The procedures used in this study were based on the guidelines of the ethical committee on the care and use of laboratory animals at our institution. Male New Zealand White rabbits (n = 48) weighing 3.2–3.9 kg were anesthetized with an i.m injection of a mixture of ketamine (40 mg/kg) and xylazine (8 mg/kg) and intubated. The central ear artery was cannulated to obtain 5 ml of autologous blood that was percutaneously injected, within 1 min, into the cisterna magna. The rabbits were positioned in ventral recumbence for 15 min to allow ventral blood clot formation and then were monitored for the next 72 h.
Experimental Groups and Drug Administration.
The rabbits were divided into six experimental groups: group 1, control (no SAH); group 2, control plus placebo; group 3, control plus r-Hu-EPO; group 4, SAH; group 5, SAH plus placebo; group 6, SAH plus r-Hu-EPO. Each group consisted of eight animals. All doses, placebo and r-Hu-EPO, were administered i.p. starting 5 min after the induction of SAH (20–22) and repeated every 8 h for 72 h. Rabbits in groups 2 and 5 received the vehicle used for r-Hu-EPO administration (serum albumin, 2.5 mg/ml; sodium chloride, 5.84 mg/ml; sodium citrate, 5.80 mg/ml; anhydrous citric acid, 0.057 mg/ml; H2O) at the dose of 1 ml/kg body weight, as placebo (20, 21). r-Hu-EPO was given to groups 3 and 6 at a dose of 1,000 units/kg.
Neurological Evaluation.
The rabbits were observed on a flat surface and neurologically assessed with a four-point system (27). In brief, paresis of the legs or abnormal gait (circling movement or difficulty walking) were noted: grade 1, denoted no neurological deficit (normal); grade 2, a minimal or suspected neurological deficit; grade 3, a mild neurological deficit without abnormal movements; and grade 4, a severe neurological deficit with abnormal movements. The examination was conducted daily from the day after the experimental procedure by a colleague who was blinded to the animal's treatment.
Cerebrospinal Fluid (CSF) and Serum EPO Concentration Assay.
Simultaneous serum (1 ml) and CSF (1 ml) samples (1 ml) were collected at 72 h after SAH by using a 23-gauge butterfly needle inserted into the central ear artery or cisterna magna, respectively.
The EPO concentrations were determined by using an ELISA (Immuno-Biological Laboratories, Hamburg, Germany) following the manufacturer's procedure. The lower limit of detection was ≈2 milliunits/ml.
To evaluate the contamination with blood of the CSF samples, especially those from SAH animals, the hemoglobin content was measured by a photometric analysis and compared with the levels found in the serum samples.
Morphometric Analysis of the Basilar Arteries.
Seventy-two hours after SAH, rabbits were anesthetized and the central ear arteries were cannulated for monitoring blood pressure and gas level. After obtaining satisfactory respiratory parameters, all animals were killed by perfusion-fixation (28). The brains were removed and the middle third of each basilar artery was dissected out. The tissue samples were washed several times with 0.1 mol/liter PBS solution, pH 7.4, and then fixed in 1% osmium tetroxide for 1 h at room temperature. The tissues were dehydrated and placed in a mixture of propylene oxide and epoxy-resin overnight. The vessel fragments were flat-embedded the next day in 100% epoxy-resin and allowed to polymerize at 60°C for 48 h. Cross-sections of the basilar arteries, 0.5 mm thick, were obtained by using an ultra-microtome. Sections were mounted onto glass slides and stained with 0.5% toluidine blue for light microscopy. Morphometric measurement of 10 randomly selected arterial sections from each animal was performed with computer-assisted morphometry (8, 29, 30). The luminal area for each basilar artery was established in a blinded manner by calculating the average of these 10 measurements.
Histological Evaluation.
After perfusion-fixation process, the brains were cut into coronal slices at 2-mm intervals starting at the bregma and continuing posteriorly through the cerebellum. The slices were then placed in a freezing microtome and sectioned at 10–25 μm and stained with hematoxylin and eosin. Ischemic lesions were evaluated in a blinded manner by using light microscopy. The numbers of neurons with histological characteristics of abnormal structures (intracytoplasmic vacuolization, shrinkage and hyperdensity of the nuclei (pyknosis), perineuronal large halo, eosinophilic or dark, shrunken cytoplasm with indistinct neuronal processes) (31) were counted in each section. The results of neuronal counting in five randomly selected sections from each animal were averaged. Each microscopic field corresponded to ≈1.6 mm2.
Data Processing and Statistical Analysis.
Data are expressed as mean ± SEM for the given number of animals. Wilcoxon's U test was used to compare the neurological grades among the groups. Values for the EPO concentrations in the CSF and serum were analyzed by Student's t test and Pearson's correlation coefficient. Data for the cross-sectional areas were statistically compared by using ANOVA followed by Fisher's protected least significant differences test. Data obtained from the analysis of the frequency of damaged cortical neurons were compared by the Kruskal–Wallis one-way ANOVA by ranks. Statistical evaluation of blood gas values and arterial pressure was performed by ANOVA. Differences were accepted as being significant at the P < 0.05 level.
Results
Neurological Evaluation.
Table 1 summarizes the neurological grade observed in each group 72 h after the experimental procedure. No neurological deficits were observed in animals of groups 1 (control), 2 (control plus placebo), and 3 (control plus r-Hu-EPO). Rabbits of groups 4 (SAH) and 5 (SAH plus placebo) showed progressive neurological deterioration after SAH. These deficits were initially observed 24 h after SAH and became most severe thereafter. The neurological status of animals treated with r-Hu-EPO (group 6) was significantly better than animals from groups 4 or 5 (P < 0.05). In particular, 24 h after the experimental procedure, of the eight animals, two presented with minimal neurological deficits (grade 2) and six with mild neurological deficits (grade 3). Thereafter, the neurological deficits gradually improved, and 72 h after SAH three animals showed minimal deficits (grade 2) and the other five were normal (grade 1).
Table 1.
Neurological deficit grade observed 72 h after SAH in rabbits
| Group | Neurological
deficit grade*
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Control | 8 | 0 | 0 | 0 |
| Control plus placebo | 8 | 0 | 0 | 0 |
| Control plus r-Hu-EPO | 8 | 0 | 0 | 0 |
| SAH | 0 | 0 | 4 | 4 |
| SAH plus placebo | 0 | 0 | 6 | 2 |
| SAH plus r-Hu-EPO** | 5 | 3 | 0 | 0 |
Neurological deficit grades are follows: 1 = no deficit; 2 = minimal or suspected deficit; 3 = mild deficit without abnormal movements; 4 = severe deficits with abnormal movements.
P < 0.05 compared with SAH and SAH plus placebo group.
CSF and Serum EPO Concentration.
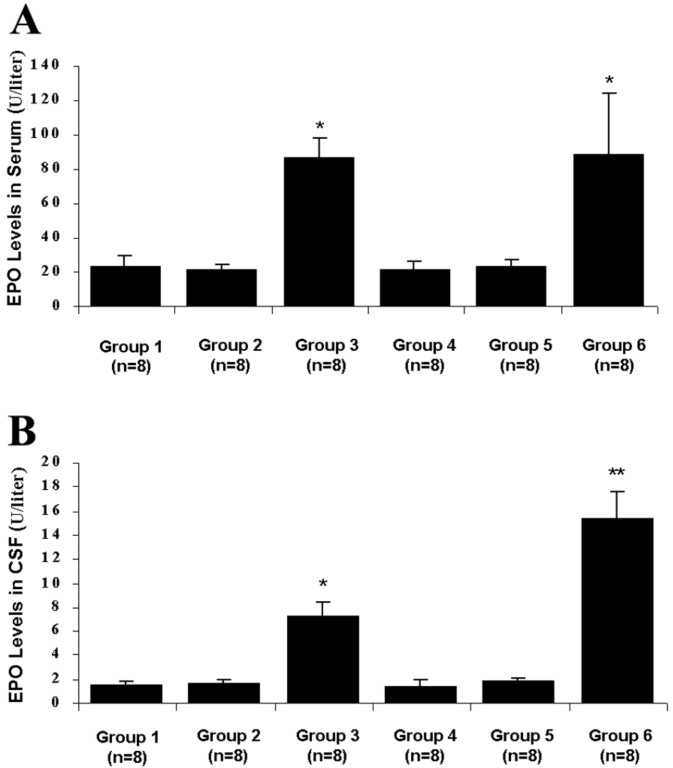
Fig. 1 shows the mean EPO concentration in the CSF and serum for each group at 72 h after SAH. The mean EPO concentration of the CSF was 1.49 ± 0.360 units/liter in group 1 (control), whereas 22.93 ± 6.29 units/liter was found in the serum. In group 2 (control plus placebo), the mean EPO value was 1.60 ± 0.399 units/liter in the CSF and 21.366 ± 3.082 units/liter in the serum. In the serum, a significant increase in the EPO concentration was found in groups 3 (control plus r-Hu-EPO) and 6 (SAH plus r-Hu-EPO) compared with the other groups (P < 0.05): the mean values observed were 86.137 ± 11.452 and 88.192 ± 35.841 units/liter, respectively. In the CSF a significant increase in EPO concentration was observed in r-Hu-EPO-treated rabbits both of group 3 (control plus r-Hu-EPO) and group 6 (SAH plus r-Hu-EPO) compared with the other groups (P < 0.001) with mean values of 7.202 ± 1.207 and 15.286 ± 2.265 units/liter, respectively, which differed significantly (P < 0.05).
Figure 1.
Bar graphs showing the mean EPO concentration in the serum (A) and CSF (B) for each group at 72 h after SAH. (A) In the serum, a significant increase in EPO concentration was found in groups 3 (control plus r-Hu-EPO) and 6 (SAH plus r-Hu-EPO) compared with the other groups (*, P < 0, 05). No statistical differences were observed comparing group 3 with group 6. (B) In the CSF a significant increase in the EPO concentration was observed in r-Hu-EPO-treated rabbits of both group 3 (control plus r-Hu-EPO) and group 6 (SAH plus r-Hu-EPO) compared with the other groups (*, P < 0.001). The mean EPO concentration from group 6 was significantly higher than that in group 3 (**, P < 0.05).
The photometric analysis excluded any significant blood contamination in the CSF samples (data not shown).
Morphometric Analysis of the Basilar Arteries.
Table 2 shows the physiological parameters measured immediately before perfusion-fixation. Among all of the groups tested, no significant differences occurred for any of these parameters.
Table 2.
Systemic parameters measured immediately before perfusion fixation in each group
| Group | n | MABP, mm Hg | pO2, mm Hg | pCO2, mm Hg | pH |
|---|---|---|---|---|---|
| Control | 8 | 99.2 ± 2.4 | 108.3 ± 7.2 | 40.3 ± 0.7 | 7.44 ± 0.01 |
| Control plus placebo | 8 | 100.5 ± 2.3 | 115.7 ± 6.8 | 40.1 ± 0.5 | 7.46 ± 0.02 |
| Control plus r-Hu-EPO | 8 | 99.9 ± 1.4 | 110 ± 5.1 | 40 ± 0.6 | 7.47 ± 0.02 |
| SAH | 8 | 101.5 ± 4.3 | 111.2 ± 6.3 | 40 ± 0.4 | 7.49 ± 0.01 |
| SAH plus placebo | 8 | 101.4 ± 2.7 | 110.3 ± 5.4 | 39.9 ± 0.6 | 7.48 ± 0.02 |
| SAH plus r-Hu-EPO | 8 | 99.3 ± 3.4 | 110.4 ± 5.1 | 39.6 ± 0.8 | 7.46 ± 0.01 |
MABP, mean arterial blood pressure; pO2, partial oxygen pressure; pCO2, partial carbon dioxide pressure.
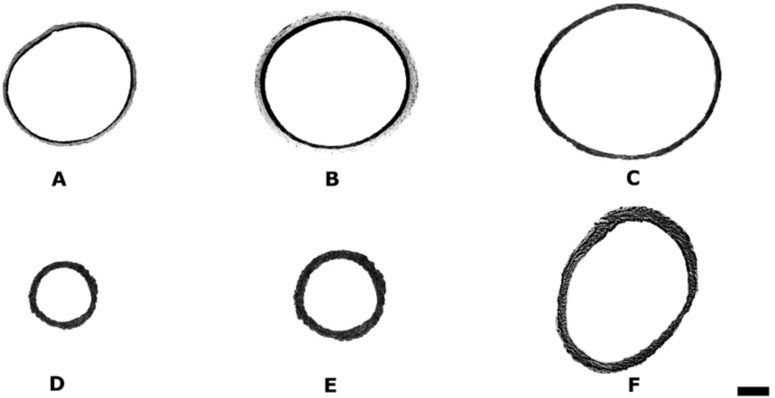
The basilar arterial cross-sectional areas of animals that did not undergo experimental SAH (groups 1, 2, and 3) were not different from each other. The mean basilar artery cross-sectional area of 0.452 ± 1.158 mm2 in the control group, was reduced to 0.086 ± 0.07 mm2 (80.7%) and 0.073 ± 0.075 mm2 (83.4%) in the SAH (group 4) and SAH plus placebo group (group 5), respectively. The histological appearance of these arteries was characterized by a marked corrugation of the internal elastic lamina and vacuolar formation within the endothelium. The cross-sectional area of arteries from SAH plus r-Hu-EPO group (group 6, 0.353 ± 0.176 mm2) demonstrated both a significant improvement of the vasoconstriction (P < 0.05) and a least prominent corrugation of the internal elastic lamina compared with groups 4 or 5. Fig. 2 illustrates representative basilar artery cross-sections from each group.
Figure 2.
Computer-generated photomicrographs illustrating the basilar artery cross-sectional area examined by light microscopy (toluidine blue staining, ×49). (A) Group 1 (control); (B) group 2 (control plus placebo); (C) group 3 (control plus r-Hu-EPO); (D) group 4 (SAH); (E) group 5 (SAH plus placebo); (F) group 6 (SAH plus r-Hu-EPO). The cross-sectional area of arteries from the SAH-plus-r-Hu-EPO group (group 6) demonstrated a significant improvement of the vasoconstriction compared with groups 4 or 5 (P < 0.05). (Calibration bar, 100 μm.)
Histological Evaluation.
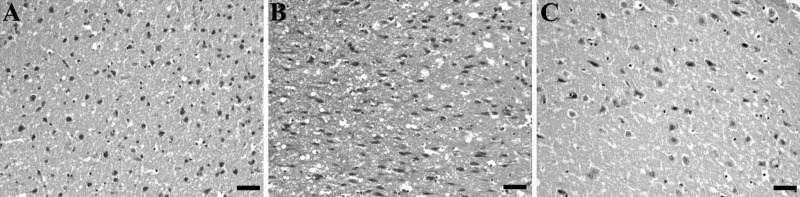
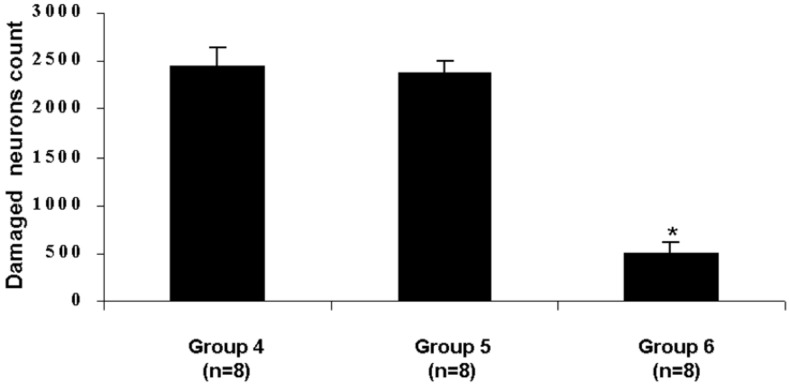
Brain sections obtained from SAH (group 4) and SAH plus placebo (group 5) animals were characterized by a high frequency of necrotic cortical neurons in the cerebral cortex (Fig. 3 A and B). Necrotic cortical neuronal counts from SAH plus r-Hu-EPO group (group 6) demonstrated a significant decrease in the frequency of necrotic neurons (Fig. 3C) compared with SAH (group 4) and SAH plus placebo groups (group 5) (P < 0.001). Fig. 4 summarizes the differences in total necrotic cortical neurons in the SAH, SAH plus placebo, and SAH plus r-Hu-EPO groups.
Figure 3.
Photomicrographs of brain sections at 72 h after SAH. (hematoxylin and eosin, ×920.) Brain sections (piriform cortex) obtained from animals of group 4 (SAH) (A) and 5 (SAH plus placebo) (B) are characterized by a high frequency of necrotic cortical neurons. Section from animals of group 6 (SAH plus r-Hu-EPO) (C) contains few damaged neurons compared with SAH and SAH-plus-placebo groups (P < 0.001). (Calibration bar, 0.1 mm.)
Figure 4.
Graph showing the mean necrotic cortical neurons number in the SAH (group 4), SAH plus placebo (group 5), and SAH plus r-Hu-EPO (group 6) groups. Animals treated with r-Hu-EPO (group 6) presented with a significant decrease in the frequency of necrotic neurons compared with SAH (group 4) and SAH plus placebo groups (group 5) (*, P < 0.001).
Discussion
The results of this study strongly support a neuroprotective activity of r-Hu-EPO when administered systemically after the induction of experimental SAH. The i.p. administration of r-Hu-EPO significantly reduced the vasoconstriction of the basilar artery in SAH-plus-r-Hu-EPO-treated animals compared with other animals that underwent SAH. The analysis of cortical neurons with ischemia-induced damage showed that damaged cortical-cell counts obtained from brain sections of the SAH-receiving r-Hu-EPO group presented a significant decrease in the frequency of necrotic neurons compared with the SAH (group 4) and SAH plus placebo (group 5) groups.
Intracranial arterial spasm has been recognized as a detrimental clinical entity for more than 40 years, but the etiology and pathogenesis of such a symptomatic cerebral vasospasm are still not well understood. Despite numerous attempts at prevention and treatment, cerebral vasospasm is still associated with significant mortality and an unfavorable outcome (1). An early, short-lived phase may occur immediately after SAH, and a subsequent phase that is prolonged or chronic (2, 3). The delayed vasospasm, seen on angiogram in 40–70% of patients with SAH (32) in the second week after hemorrhage, seems to be most important clinically. The acute vasoconstriction, before considered to be a laboratory observation, recently has been strongly suspected also in humans (4, 5). Both phases of vasospasm are considered to result from an abnormal constriction of the muscular layers of the cerebral vessels, and both have been considered the main cause of cerebral ischemia after SAH (33). However, whether the two phases are independent or interactive with respect to the clinical course has not been settled. On the basis of this evidence, an increasing number of investigators have recently focused their attention in assessing the efficacy of drugs administered a few minutes after the induction of experimental SAH with a view to prevent brain ischemia (13, 34, 35).
The ischemic brain injury that often follows SAH is mediated mainly by glutamate (14). By using intracerebral microdialysis, studies on SAH in humans have demonstrated variable increases in extracellular glutamate concentrations (36). Such an occurrence has been confirmed in experimental models in which measurements of extracellular glutamate levels in cortical regions at various times have provided an indication of the severity and duration of ischemia during SAH (37).
Experimental studies have recently shown a potent neuroprotective effect of EPO during cerebral ischemia. Morishida and coworkers (15) have demonstrated that in cultured neurons EPO prevents the glutamate-induced cell death in a dose-dependent manner. Sadamoto et al. (16) have shown that EPO ameliorates place-navigation disability, cortical infarction and thalamic degeneration in rats after undergoing a permanent occlusion of the middle cerebral artery. Sakanaka et al. (17) have reported, by using a lateral ventricular infusion of soluble EPO-R, that EPO ameliorates neuron survival in a model of cerebral ischemia in the gerbil. Koshimura et al. (25) have suggested that EPO stimulates neuronal and functional viability, mitogen-actived protein kinase activity, dopamine release, and NO synthesis. Bernaudin et al. (18) have shown the neuroprotective action of intraventricular-injected EPO in focal permanent cerebral ischemia in mice. We have reported that systemic administration of r-Hu-EPO, immediately after experimental SAH, reduces the mortality rate, ameliorates functional recovery, and prevents brain ischemic damage (20–23). Very recently, Brines et al. (19) have demonstrated the ability of systemically administered r-Hu-EPO to exert a neuroprotective effect in animal models of focal brain ischemia, concussive brain injury, experimental autoimmune encephalomyelitis, and kainate-induced seizure.
The neuroprotective effect of r-Hu-EPO in this model is in agreement with reports showing similar effects of EPO during cerebral ischemia (15–18). Several possible mechanisms exist through which EPO could achieve this protective effect. It has been suggested that, during ischemia, EPO protects neurons from glutamate toxicity by activation of Ca2+ channels (15), increases the activity of antioxidant enzymes in neurons (17), modulates the angiogenesis in the ischemic brain, thus improving blood flow and tissue oxygenation in the border zone of the ischemic area (38), and protects endothelial cells from apoptotic cell death (18). On the other hand, according to the ability of EPO in preventing cerebral vasospasm, rather than a direct action on cerebral parenchyma, one possible mechanism could be a direct effect of EPO on the vascular endothelium, with secondary benefits neurologically. In this role, peripherally available EPO could interact with the luminal EPO-R preventing constriction. This hypothesis is not surprising because it has been reported that brain vascular endothelial cells express two forms of EPO-R mRNA (38). Accordingly, EPO could act directly on cerebral arteries by binding with its receptor, exerting a competence activity in the control of the cerebrovascular tone. Finally, one might also speculate that EPO could prevent vasospasm after SAH by enhancing the endothelial release of NO during the early stage of SAH. Previous investigations have demonstrated an acute decrease in cerebral NO levels after SAH (13) and a significant improvement of NO system activity after administration of r-Hu-EPO (24–26). Although further studies are required to clarify the action of EPO in the central nervous system both in physiological and pathological conditions, we propose that EPO acts in multiple ways involving, at least, both direct neuroprotection and antivasospastic properties to protect the brain from the damage consequent to SAH.
The modality by which r-Hu-EPO acts in the central nervous system across the BBB remains a matter of controversy. According to the literature, EPO has shown a neuroprotective action both by intrathecal or systemic administration. Knowledge of the mechanisms by which peptides and proteins are transported across the BBB is still limited. At present five mechanisms have been proposed: transmembrane diffusion, carrier-mediated transport, fluid-phase endocytosis, nonspecific adsorptive endocytosis, and receptor-mediated adsorptive endocytosis (39–42). On the other hand, it has been reported that only by a leakage of the BBB can systemically administered EPO cross from the periphery into the central nervous system (43). However, Brines et al. (19) have recently suggested that, after systemic administration, r-Hu-EPO may be transported across the BBB by a specific receptor-mediated mechanism. These authors, by administrating biotinylated r-Hu-EPO, demonstrated an active translocation of peripheral EPO across the BBB in the absence of any neural insult. Our results are in agreement with such a hypothesis. In our study, an increase in EPO concentration in the CSF was detected in all animals treated with r-Hu-EPO. In animals of group 3 (control plus r-Hu-EPO) no SAH was induced, therefore the increase in EPO level in the CSF could be explained by the presence of a specific translocation mechanism across the intact BBB. In animals of group 6 (SAH plus r-Hu-EPO) the increase in EPO concentration was more evident and statistically significant compared with the untreated animals and animals of group 3 (control plus r-Hu-EPO). Based on the evidence that SAH is followed by an impairment of the BBB, our findings suggest that systemically administered r-Hu-EPO effectively increases the EPO concentration in the CSF in the presence of both normal and abnormal BBB integrity.
In conclusion, our results confirm the positive effects exerted by r-Hu-EPO in treating the post-SAH pathogenic cascade, because r-Hu-EPO improved the vasospasm, brain ischemic damage, and consequently, the neurological outcome in this experimental setting. The present findings extend the work of our previous studies (20–23) in several ways. First, we have demonstrated that systemic administration of r-Hu-EPO can significantly attenuate SAH-induced cerebral vasospasm and ischemia in a well-characterized and reproducible experimental model. Second, we have documented that the neuroprotective effect of r-Hu-EPO is evident even 72 h later the induction of SAH. Finally, we have observed an increase in EPO concentration in the CSF in the presence of both disturbed and normal BBB. Such an observation strongly supports an effective clinical use of EPO after the onset of SAH.
Acknowledgments
We are grateful to Dr. Michael L. Brines (The Kenneth S. Warren Institute, Kitchawan, NY) for helpful suggestions and assistance in preparing the manuscript.
Abbreviations
- SAH
subarachnoid hemorrhage
- EPO
erythropoietin
- r-Hu-EPO
recombinant human EPO
- CSF
cerebrospinal fluid
- BBB
blood–brain barrier
References
- 1.Broderick J P, Brott T G, Duldner J E, Tomsick T, Leach A. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 2.Jakowski A, Crockard A, Burnstack G, Russel R, Kristek F. J Cereb Blood Flow Metab. 1990;10:835–839. doi: 10.1038/jcbfm.1990.140. [DOI] [PubMed] [Google Scholar]
- 3.Delgado T J, Brismar J, Svendgaard N A. Stroke. 1985;16:595–602. doi: 10.1161/01.str.16.4.595. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins RH. Neurosurgery. 1980;6:189–210. doi: 10.1227/00006123-198002000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Bederson J B, Levy A L, Ding W H, Kahn R, DiPerna C A, Jenkins A L, Vallabhakosyula P. Neurosurgery. 1998;42:352–360. doi: 10.1097/00006123-199802000-00091. [DOI] [PubMed] [Google Scholar]
- 6.Stoltenburg-Didinger G, Schwarz K. In: Stroke and Microcirculation. Cervos-Navarro J, Ferst R, editors. New York: Raven; 1987. pp. 471–480. [Google Scholar]
- 7.Smith B. J Neurol Neurosurg Psychiatr. 1963;26:535–539. doi: 10.1136/jnnp.26.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiernsperger N, Schulz U, Gygax P. Stroke. 1981;12:624–627. doi: 10.1161/01.str.12.5.624. [DOI] [PubMed] [Google Scholar]
- 9.Nagai H, Katsumata T, Ohya M. Neurochirurgia. 1976;19:135–144. doi: 10.1055/s-0028-1090403. [DOI] [PubMed] [Google Scholar]
- 10.Nornes H. Acta Neurochir (Wien) 1978;41:39–48. doi: 10.1007/BF01809135. [DOI] [PubMed] [Google Scholar]
- 11.McCormick P W, McCormick J, Zabramski J M, Spetzler R F. J Neurosurg. 1994;80:710–715. doi: 10.3171/jns.1994.80.4.0710. [DOI] [PubMed] [Google Scholar]
- 12.Dorsh N W, Branston N M, Symon L. Neurol Res. 1989;11:201–204. doi: 10.1080/01616412.1989.11739893. [DOI] [PubMed] [Google Scholar]
- 13.Sehba F, Schwartz A Y, Chereshnev I, Bederson J. J Cereb Blood Flow Metab. 2000;20:604–611. doi: 10.1097/00004647-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Benveniste H, Drejer J, Schousboe A, Diemer N H. J Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 15.Morishida E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 16.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 17.Sakanaka M, Wen T C, Masuda S, Morishita E, Nagao M, Sasaki R. Proc Natl Acad Sci USA. 1998;95:4653–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernaudin M, Marti H H, Roussel S, Divoux D, Nouvelot A, MacKenzie E T, Petit E A. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Brines M L, Ghezzi P, Keenan S, Agnello D, de Lanerolle N C, Cerami C, Itri L M, Cerami A. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buemi M, Grasso G, Corica F, Calapai G, Salpietro F M, Casuscelli T, Sfacteria A, Aloisi C, Alafaci C, Sturiale, et al. Eur J Pharmacol. 2000;392:31–34. doi: 10.1016/s0014-2999(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 21.Alafaci C, Salpietro F M, Grasso G, Sfacteria A, Passalacqua M, Morabito A, Tripodo E, Calapai G, Buemi M, Tomasello F. Eur J Pharmacol. 2000;406:219–225. doi: 10.1016/s0014-2999(00)00691-9. [DOI] [PubMed] [Google Scholar]
- 22.Grasso G. J Neurosurg Sci. 2001;45:7–14. [PubMed] [Google Scholar]
- 23.Grasso G, Passalacqua M, Sfacteria A, Conti A, Morabito A, Mazzullo G, De Vico G, Buemi M, Macrì B, Tomasello F. J Neurosurg. 2002;96:565–570. doi: 10.3171/jns.2002.96.3.0565. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee D, Rodriguez M, Nag M, Adamson JW. Kidney Int. 2000;57:1895–1904. doi: 10.1046/j.1523-1755.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- 25.Koshimura K, Murakami Y, Sohmiya M, Tanaka J, Kato Y. J Neurochem. 1999;72:2565–2572. doi: 10.1046/j.1471-4159.1999.0722565.x. [DOI] [PubMed] [Google Scholar]
- 26.Migliori M, Taccola D, Panichi V, De Pietro S, Andreini B, Di Benedetto A, Filippi C, Palla R, Giovannini L. Kidney Blood Press Res. 1999;22:140–145. doi: 10.1159/000025920. [DOI] [PubMed] [Google Scholar]
- 27.Endo S, Branson P J, Alksne J F. Stroke. 1988;19:1420–1425. doi: 10.1161/01.str.19.11.1420. [DOI] [PubMed] [Google Scholar]
- 28.Johshita H, Kassell N F, Sasaki T, Nakagomi T, Ogawa H. Surg Neurol. 1992;37:106–114. doi: 10.1016/0090-3019(92)90185-p. [DOI] [PubMed] [Google Scholar]
- 29.Hart M N. Stroke. 1980;11:653–655. doi: 10.1161/01.str.11.6.653. [DOI] [PubMed] [Google Scholar]
- 30.Arthur A S, Fergus A H, Lanzino G, Mathys J, Kassell N F, Lee K S. Neurosurgery. 1997;41:1385–1392. doi: 10.1097/00006123-199712000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Garcia J H, Lossinsky A S, Kauffman F C, Conger K A. Acta Neuropathol. 1978;43:85–95. doi: 10.1007/BF00685002. [DOI] [PubMed] [Google Scholar]
- 32.Zabramski J M, Hamilton M G. In: Neurovascular Surgery. Carter L P, Spetzler R F, editors. New York: McGraw-Hill; 1995. pp. 583–602. [Google Scholar]
- 33.Weir B, Grace M, Rothberg C. J Neurosurg. 1978;48:173–178. doi: 10.3171/jns.1978.48.2.0173. [DOI] [PubMed] [Google Scholar]
- 34.Pluta R M, Oldfield E H, Boock R J. J Neurosurg. 1997;87:746–751. doi: 10.3171/jns.1997.87.5.0746. [DOI] [PubMed] [Google Scholar]
- 35.Cuevas P, Carceller F, Nieto I, Giménnez-Gallego G. Surg Neurol. 1998;49:176–180. doi: 10.1016/s0090-3019(97)00167-5. [DOI] [PubMed] [Google Scholar]
- 36.Persson L, Valtysson J, Enblad P, Warme P, Cesarini K, Lewen A, Hillered A. J Neurosurg. 1996;84:606–616. doi: 10.3171/jns.1996.84.4.0606. [DOI] [PubMed] [Google Scholar]
- 37.Shimada N, Graf R, Rosner G, Wakayama A, George C P, Heiss W D. J Cereb Blood Flow Metab. 1989;9:603–606. doi: 10.1038/jcbfm.1989.86. [DOI] [PubMed] [Google Scholar]
- 38.Yamaji R, Okada T, Moriya T, Naito M, Tsuruo T, Miyatake K, Nakano Y. Eur J Biochem. 1996;239:494–500. doi: 10.1111/j.1432-1033.1996.0494u.x. [DOI] [PubMed] [Google Scholar]
- 39.Broadwell R D. Acta Neuropathol. 1989;79:117–128. doi: 10.1007/BF00294368. [DOI] [PubMed] [Google Scholar]
- 40.Banks W A, Kastin A J, Barrera C M, Maness L M. Brain Res Bull. 1991;27:819–823. doi: 10.1016/0361-9230(91)90215-6. [DOI] [PubMed] [Google Scholar]
- 41.Banks W A, Audus K L, Davis T P. Peptides. 1992;13:1289–1294. doi: 10.1016/0196-9781(92)90037-4. [DOI] [PubMed] [Google Scholar]
- 42.Van Bree J B, De Boer A G, Danhof M, Breimer D D. Pharm World Sci. 1993;19:2–9. doi: 10.1007/BF02116163. [DOI] [PubMed] [Google Scholar]
- 43.Marti H H, Gassmann M, Wenger R H, Kvietikova I, Morganti-Kossmann M C, Kossmann T, Trentz O, Bauer C. Kidney Int. 1997;51:416–418. doi: 10.1038/ki.1997.55. [DOI] [PubMed] [Google Scholar]