Abstract
YggB and MscL are the major mechanosensitive channels in Escherichia coli, and each can rescue the double knockout mutant from osmotic downshock. However, the role of MscL in wild-type bacteria is in question, not only because cells without MscL survive severe osmotic downshocks, but because 1.8 times more suction is required to gate MscL than YggB under patch clamp. Here, we extend previous evidence [Ajouz, B., Berrier, C., Garrigues, A., Besnard, M. & Ghazi, A. (1998) J. Biol. Chem. 273, 26670–26674] to show that downshock gates MscL in vivo even in the presence of YggB. We have made this determination by engineering a channel we can structurally modify in vivo (Leu-19→Cys MscL). MscLs with charges in their constrictions are known to open easily and transiently to substates and stop cell growth. In this study, we use downshock to stretch this region open to allow attachment of a charged thiosulfonate reagent MTSET+, thereby creating a toxic channel. Therefore, channel opening can be monitored by loss of colony forming units. By this measure, we find that an ≈800 mmol/kg downshock from 1,200 mmol/kg medium opens Leu-19→Cys MscL in the presence of YggB, but a downshock of only ≈400 mmol/kg is required in the absence of YggB. In parallel, Leu-19→Cys MscL, stretched open by large sustained suction in the presence of MTSET+ in voltage-clamped patches, subsequently flickers open with little suction. These observations show that MscL opening is triggered by a specific downshock, even in the presence of YggB, that YggB buffers MscL gating in vivo, and that residue 19 becomes exposed upon channel opening.
Mechanosensitive channels (MSCs) of Escherichia coli were first detected as suction-induced unitary conductances under a patch clamp (1). The genes for the two main types of MSCs, which have homologs in a large variety of bacteria (2, 3), have been cloned, expressed, and manipulated. MscL has a large conductance of ≈3 ns (4), whereas MscS has a smaller conductance of ≈1 nS that is largely the activity of the YggB protein (3). MscL protein of Mycobacterium tuberculosis has been purified, crystallized, and the crystal structure solved at 3.5 Å resolution (5). A homopentameric channel was revealed in a closed state. Each MscL subunit comprises two transmembrane helices, M1 and M2 (6), and the cytoplasmic thirds of the five M1s come together to form the constriction that closes the channel by hydrophobic interactions (ref. 5 and see Fig. 1A). Expanding the hydrophobic constriction while generating the first open substate presents the greatest energy barrier to opening (7), although there may be other gating structures (8).
Figure 1.
(A) Mesh molecular surface (except a 90° wedge) of the closed TbMscL structure derived from ref. 5 showing the region of the constricted pore (darkened center) and the location of Leu-17 of M. tuberculosis MscL and Leu-19 of E. coli MscL by analogy. (B) Structures of leucine, cysteine, and cysteine with an attached MTSET+.
A bacterium doused by rainwater must deal with the tremendous osmolality difference between the cell and the water outside (9, 10). Thus, the role of MSCs as safety valves to jettison osmolytes upon sudden osmotic downshocks was proposed at the time of their very discovery (1). Indeed, Levina et al. showed that deleting both mscL and yggB, but not either, results in bacterial lysis upon downshock (3).
However, there is a gulf between our understanding of MSCs as molecules and as functional entities in vivo. For MscL, the atomic resolution of its structure (5), computer-assisted modeling (8), biophysical calibration (7), and genetic dissection (6, 11–13) have made it a premier molecular model to study mechanosensory transduction. But the insights gained in these studies cannot be easily translated into insights in bacterial physiology. We have the following difficulties: (i) There are no electrophysiological methods to study intact bacteria during downshock; (ii) MSCs are mired in a complex tripartite envelope whose mechanical properties are not well understood; (iii) Although there is likely no osmotic pressure difference between the cytoplasm and the periplasm during steady-state growth (14, 15), the course of the rise and fall of turgor pressure during downshock, especially at the plasma membrane where MSCs reside (16), is unknown. A 1,000 mmol/kg downshock could theoretically create a turgor pressure of ≈24 atm (1 atm = 101.3 kPa) at 25°C. If presented directly to the plasma membrane at a curvature of 1-μm diameter, this pressure would create a tension of ≈600 dyne/cm. This pressure is ≈60 times the average of 10 dynes/cm that opens 50% of the MscL channels from liposomes (7) and spheroplasts (S. Sukharev, personal communication), far exceeding the lytic tension of the lipid bilayer. Obviously, the cell must have multiple mechanisms for buffering such a shock.
Deleting either yggB or mscL from E. coli does not affect viability after downshock, although deleting both results in lysis (3). Therefore, why have two channels evolved and been maintained if only one is sufficient? This question is more problematic for MscL because MscL opens under patch-clamp at a suction of 1.7–1.9 times that which activates MscS (11, 12), an activity largely attributed to YggB (3). Upon downshock, it seems reasonable for the rising turgor at the plasma membrane to first open YggB. This opening alone seems sufficient to deflate the bacterium (3), possibly precluding MscL's opening. However, Ajoux et al. (17) and Berrier et al. (18) have shown that MscL's presence is responsible for the release of various solutes including small proteins, implying MscL gating upon downshock in a strain that includes YggB (17, 18). Therefore, we wanted to demonstrate MscL gating and determine the threshold response for MscL opening in live bacteria upon downshock in the presence or absence of YggB.
As it is not yet possible to directly monitor MSCs' behavior during downshocks, we resorted to an indirect method to gauge the opening of MscL in vivo. In the crystal structure of MscL, the channel is closed by a hydrophobic constriction (ref. 5 and Fig. 1A). Polar or charged residues genetically engineered into this constriction result in “loose cannon” channels that gate at low or no applied suction, frequently firing as flickers and often to a substate. Bacteria harboring such channels are unable to grow, presumably because of cytoplasmic bleeding (12), and randomly generated mutations selected for this toxicity were found to cluster at this constriction (11). We reasoned that if charges can be attached to the constriction when it is loosened, they should convert the channel into a loose cannon and thereby stop growth. If so, we should be able to use growth stoppage to indicate that the constriction has been loosened and the channel opened. To this end, we made a mild substitution well within the constriction, changing the five Leu-19s into cysteines. Native MscL has no cyteines, and Leu-19 of EcoMscL is equivalent to Leu-17 of TbMscL (ref. 5 and Fig. 1A). We then added cells expressing this channel to downshock medium containing MTSET+. MTSET+ is [2(triethylammonium)ethyl] methanethiosulfonate, which specifically reacts with the sulfhydryl of cysteine (ref. 19 and Fig. 1B). A loss of colony-forming units (cfu) indicates that the Leu-19→Cys MscL channels have been opened in live bacteria, allowing MTSET+ attachment during the downshock.
Materials and Methods
Strains.
Leu-19→Cys mscL was created by megaprimer PCR and inserted into the isopropyl β-d-thiogalactoside (IPTG)-inducible pB10b as described (12). Empty, wild-type (4) or Leu-19→Cys mscL-bearing plasmids were expressed in the MscL− E. coli strain PB104 [AW405, recA(tetR) mscL∷Cm] (6), commonly used for patch-clamp studies. These plasmids also were expressed in the MscL− strain MJF367 (FRAG1, mscL∷Cm) and the MscL− YggB− strain MJF455 (FRAG1, mscL∷Cm Δ yggB) (3). MJF strains were previously used in standard bacteriological investigations and are gifts of I. Booth (Univ. of Aberdeen, U.K.). PB104 was derived from AW405 (F− gal1 gal2 ara lac xyl thr his B1− T1RT5RStrR) (20); MJF367 and MJF455 were from FRAG1 (F− gal rha thi lacZ) (21). All were of K-12 origin.
Downshock Assays.
Freshly streaked bacteria were grown overnight at 37°C in 2 ml of modified LB medium [0.5% instead of 1% NaCl (1) with 100 μg/ml ampicillin, 220 mmol/kg]. In the morning, 100 μl of the culture was diluted 1:20 into LB and outgrown at 37°C to OD650 0.5 to 1.2 and then further diluted to OD650 0.02 into 10 ml of prewarmed LB plus 500 mM NaCl. (Pregrowth was significantly slower for PB104 than the MJF strains). At OD650 of 0.12–0.17, IPTG Gold Biotechnology, St. Louis) stock was added to give 1 mM IPTG. (LBx is LB + x mM NaCl; therefore, the IPTG-containing LB + 500 mM NaCl is called LB500, 1,200 mmol/kg). After 1-hr induction, the culture was diluted 1:20 into 10 ml of various shock media in 125-ml flasks and shaken at 37°C. The isotonic shock medium was LB500 ± various concentrations of MTSET+ (Toronto Research Chemicals, Downsview, ON, Canada). The hypotonic shock medium was LB + IPTG with 0–400 mM of added NaCl (±0.5 mM MTSET+) as given. (MTSET+ 100× stock solution in water was prepared and filtered within 10 min before each shock.) After 5 min, 50–100 μl of cells was withdrawn to make tenfold serial dilutions into MTSET+-free shock media. Samples then were plated on agar with LB + 1 mM IPTG and incubated at 37°C overnight before counting cfu on triplicate plates. The downshock lasted 5 min to minimize the growth-rate differences in shock media with different amounts of added NaCl. Osmolality (mmol/kg) was determined with a Wescor Osmometer (Logan, UT).
Patch-Clamp Assays.
Preparation of giant spheroplasts, recording pipettes, pipette fillant, back-filling procedure, patch formation, current amplification and filtration, and the presentation of MTSET+ were as described (12, 22). Frozen spheroplast aliquots were thawed for the day's use. After prolonged storage at −20°C, some spheroplasts yielded patches that had channel activities during seal formation. They were excluded from analysis. A series of suctions, each lasting several seconds, was applied to the patch. Although the absolute pressures needed to open MscS (including YggB) and MscL depend on the geometry of the particular patch, the activities of MscS served as an internal calibration for the behavior of MscL in the same patch (4, 6).
Results
MTSET+ Dependence of Downshock-Induced Death.
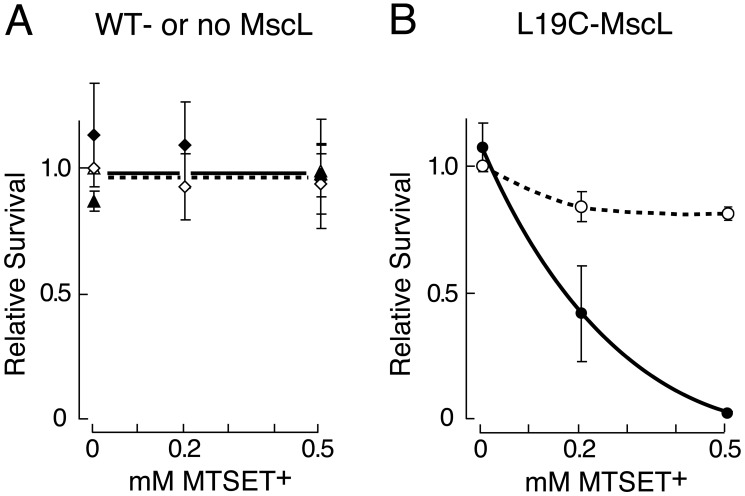
Given our previous use of E. coli strain PB104 (mscLΔ yggB+ in the AW405 background) for mutational and patch-clamp analyses (2, 6, 11, 12, 22), we first used this strain to investigate MTSET+ dependence of downshock-induced death. For an isoosmotic shock, cells are diluted 1:20 from LB500 growth medium into fresh LB500 for 5 min. For a downshock, cells are diluted 1:20 from LB500 into LB, i.e., from 1,200 to 220 mmol/kg medium. Both conditions are tested with and without MTSET+. For PB104 cells expressing the wild-type mscL plasmid or the empty plasmid, neither the downshock nor the MTSET+ had any significant effect (Fig. 2A). On the other hand, PB104 cells expressing Leu-19→Cys mscL suffered a large loss of cfu after the downshock but not after the isoosmotic transfer; this loss occurred only in the presence of MTSET+ (Fig. 2B). Ninety-eight percent of cfu-expressing Leu-19→Cys mscL are lost upon downshock in the presence of 0.5 mM MTSET+. This response depends upon the MTSET+ concentration so that 0.2 mM MTSET+ has an intermediate effect (Fig. 2B). For simplicity, we refer to “removing cfu” as “killing” below.
Figure 2.
Survival of mscLΔ yggB+ PB104 bacteria (of AW405 background) expressing different pB10b-based plasmids in the presence of various MTSET+ upon downshock from LB500 to LB (filled symbols, continuous line) or after an LB500-to-LB500 isoosmotic transfer (open symbols, broken line). (Means ± SD are given. Unity for relative survival is the value after isoosmotic transfer, no MTSET+.) Before transfer into shock solution, ≈2 × 107 cfu/ml were present based upon cfu determined after 1:20 dilution into isoosmotic medium. (A) The empty plasmid (▴, n = 3) determinations only at 0 and 0.5 mM MTSET+, the wild-type mscL plasmid (♦, n = 4), or (B) the Leu-19→Cys mscL plasmid (○, n = 3).
Approximately 800 mmol/kg Downshock and MTSET+ Kill Bacteria Harboring Leu-19→Cys MscL.
We estimated the severity of the downshock needed to kill Leu-19→Cys mscL-expressing PB104 bacteria in the presence of 0.5 mM MTSET+ as an indirect gauge of the downshock that opens their MscL channel(s) in vivo. An MTSET+-specific death is clearly evident upon downshock from 1,200 to 420 mmol/kg or lower osmolality medium (to LB100 or LB <100) (Fig. 3A).
Figure 3.
Survival of mscLΔ yggB+ bacteria of two different genetic backgrounds expressing the same Leu-19→Cys mscL plasmid after graded osmotic downshocks from LB500 to LB with added NaCl given in the presence (filled symbols, continuous line) or absence (open symbols, broken line) of 0.5 mM MTSET+. (Means ± SD given. Unity for relative survival is value after isoosmotic transfer, no MTSET+. cfu/ml before shock is in parentheses.) (A) PB104 bacteria in AW405 background (⋄, n = 4, 1 × 107 cfu/ml). (B) MJF367 bacteria in FRAG1 background (○, n = 3, 1 × 108 cfu/ml).
Because the role of MSCs in vivo has been explored in bacteria of the FRAG1 background (3), we investigated MTSET+-induced death of Leu-19→Cys-mscL-transformed bacteria in this genetic background as well. As shown in Fig. 3B, Leu-19→Cys mscL-expressing MJF367 bacteria (mscLΔ yggB+ in the FRAG1 background) also suffer a major loss of viability when downshocked from 1,200 to 420 mmol/kg or lower osmolality medium (to LB100 or LB <100). However some death is evident even with a 1,200 to 610 mmol/kg downshock (to LB200). The similar MTSET+-specific killing in both E. coli strains indicates that Leu-19→Cys MscL channels in most of the cells are apparently opened by an ≈800 mmol/kg downshock. In both cases, YggB is present. Note that the response differences between the two bacterial strains of different genetic backgrounds (PB104 and MJF367) are largely unrelated to MTSET+ (Fig. 3 A vs. B).
Approximately 400 mmol/kg Downshock and MTSET+ Kill Bacteria Harboring Leu-19→Cys MscL When YggB Is Absent.
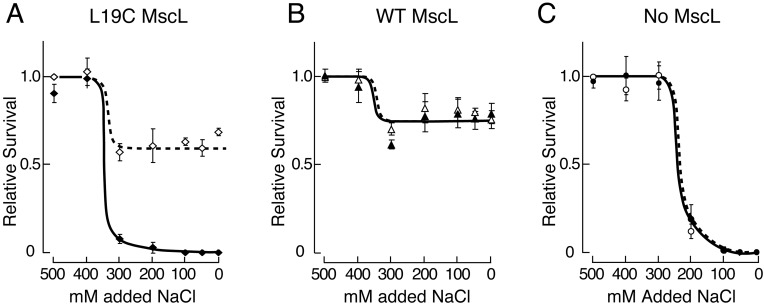
In the above experiments, MscL opens in a membrane that contains YggB, the channel that opens at lower stretch force under patch clamp. We also wished to estimate the downshock needed to open MscL when there is no YggB. Therefore, the above experiments were repeated by expressing the Leu-19→Cys mscL transgene in MJF455 bacteria (mscLΔ yggBΔ in the FRAG1 background; ref. 3). Fig. 4A shows that a downshock of ≈400 mmol/kg into LB300 (800 mmol/kg) kills most of the bacteria when 0.5 mM MTSET+ is present. This finding indicates that Leu-19→Cys MscL opened in the absence of YggB upon this ≈400 mmol/kg downshock. Comparing Figs. 3B and 4A, it is apparent that Leu-19→Cys-MscL opens at significantly milder downshocks when YggB is absent. Note that in the absence of both MscL and YggB, bacteria are killed by a downshock into LB200 (420 mmol/kg) (Fig. 4C), which is entirely consistent with results of Levina et al. (3), even though we are using an LB-based medium that includes ampicillin and we are shocking for only 5 min (3).
Figure 4.
Survival of mscLΔ yggBΔ bacteria (in FRAG1 background) expressing various pB10b-based plasmids after graded osmotic downshocks from LB500 to LB with added NaCl in the presence (filled symbols, continuous line) or absence (open symbols, broken line) of 0.5 mM MTSET+. (Means ± SD given. Unity for relative survival is value after isoosmotic transfer, no MTSET+. cfu/ml before shock is in parentheses). (A) Expressing Leu-19→Cys mscL plasmid (♦, n = 5, 2 × 108 cfu/ml). (B) Wild-type plasmid (▵, n = 3, 2 × 108 cfu/ml). (C) Empty plasmid (○, n = 3, 1 × 108 cfu/ml).
Interestingly, more than half the MJF455 cells expressing wild-type or Leu-19→Cys mscL survived the shock to LB in the absence of MTSET+ (Figs. 4 A and B), whereas most of these cells are killed when both MscL and YggB are absent (ref. 3 and Fig. 4C). Here, plasmid-expressed wild-type MscL channels (23) and Leu-19→Cys MscL channels partially rescued the double knockout, undoubtedly by opening. Because MTSET+ abolishes rescue by Leu-19→Cys mscL and not by wildtype mscL (Fig. 4A), cell death must be caused by the binding of MTSET+ to Cys-19 in channels that have exposed this residue during gating. Notably, Leu-19→Cys MscL and apparently wild-type MscL openings are slightly detrimental to the cell population shocked into LB300 in the absence of YggB and MTSET+ (compare Figs. 4 A and B with C). These results are specific to a YggB− background and suggest that both the Leu-19→Cys MscL and wild-type MscL channels open at a similar downshock in vivo (compare Figs. 3B and 4).
MTSET+ Attacks Leu-19→Cys MscL After It Has Been Opened Under a Patch-Clamp.
Inside-out patches excised from Leu-19→Cys mscL-expressing PB104 spheroplasts were examined. Leu-19→Cys MscL apparently activates at a threshold higher than that of the wild-type MscL (12, 22). Activities were registered at strong suctions but could not be interpreted quantitatively with confidence, because these suctions often break the patches. Possibly, the high threshold is not caused by disulfide bridges among the five cysteines in the pentamer, because the addition of 5 mM DTT to the solution bathing the cytoplasmic side of the patch did not reduce the threshold. However, after 1 mM MTSET+ is applied to the bath, flickering MscL activity can be discerned (arrows, Fig. 5Ai), but only after prolonged application of strong suction. This suction is more than twice the negative pressure needed to activate all of the smaller MS conductances, including MscS in the same patch (Fig. 5A, arrowheads and the plateau; MscS conductance is largely the activities of the YggB channels; ref. 3). Once the Leu-19→Cys MscL activities appear, they can be observed riding on the MscS activities (Fig. 5Aii, arrowhead) in subsequent episodes with mild or even no suction applied to the same patch. Unlike the wild-type MscL activities that can easily be resolved as flat-top unitary currents at our rate of filtration (5 kHz; ref. 4), these Leu-19→Cys MscL activities rapidly flicker, usually not reaching the full-open level. This flickering behavior is typical of that observed previously in MscLs with genetically placed charges in the hydrophobic constriction (11, 12, 22). Because of the rapid kinetics, some highly transient channel openings are presumably filtered out by our recording system.
Figure 5.
Behavior of Leu-19→Cys MscL under a patch clamp upon the presentation of MTSET+. Inside-out patches were excised from PB104 spheroplasts expressing Leu-19→Cys MscL. After seal formation (with no MTSET+ present), repeated suction pulses usually activated MscS only (not shown). (A) MTSET+ presented from the bath (presumed cytoplasmic side). (i) A large suction (lower trace) eventually produced flickering activities (solid arrows, Leu-19→Cys MscL) but only after the suction was so high that it had activated all of the MscS in the patch (arrowheads and plateau). (ii) A subsequent episode of the same patch. Typical of five experiments. (B) MTSET+ presented from the pipette (presumed periplasmic side) by back filling. The tip of the pipette was filled with the pipette solution supplemented with 0.3 M sucrose (without MTSET+). (i) For ≈20 min after backfill in the absence of suction, there was no activation. Upon suction, flickers were eventually observed (arrows) but, again, only at suctions that activated all MscS (arrowheads, plateau). (ii) A subsequent episode of the same patch, typical of five experiments.
In the above experiment, a buffer bathes the excised inside-out patch, and the MTSET+ in the buffer presumably attacks the channel from the cytoplasmic side. We also examined the effect of applying MTSET+ from the presumed periplasmic side by backfilling the recording pipettes with 1 mM MTSET+. The patch showed only MscS activities even upon strong suctions for long periods (not shown). After ≈20 min of incubation, a suction often finally elicited the Leu-19→Cys MscL activities (arrows, Fig. 5Bi), but the suction, again, had to be large enough to activate all of the MscSs in the patch (arrowhead and plateau, Fig. 5Bi). Once these were observed, as before, little suction was required for their appearance in subsequent episodes in the same patch (Fig. 5Bii). We interpret these findings to indicate that, under tension, residue 19 eventually becomes accessible to MTSET+ from either side of the membrane, consistent with the tension having opened an aqueous path lined by the five Cys-19 residues.
Discussion
We found MTSET+-dependent killing in bacteria-expressing Leu-19→Cys mscL, demonstrating MscL gating after certain osmotic downshocks in vivo (Figs. 2B, 3, and 4). MscL's residue 19s are modeled to be within the constriction in the closed state but exposed to water upon opening (8, 24). Here, we found that downshock apparently stretches the Leu-19→Cys MscL channel open, allowing MTSET+ attachment (Fig. 1) and converting the channel into a toxic leak. This conclusion is corroborated by the patch-clamp observation (Fig. 5 Ai and Bi) that severe suction is required to give MTSET+ access to Cys-19. The channel subsequently opens more easily, behaving like one with charges embedded in its hydrophobic constriction (refs. 11, 12, and 22 and Fig. 5 Aii and Bii). In contrast, in vivo controls indicate that the killing effects are not caused by the entry of MTSET+ into the cell through an MscL channel, nor is it caused by the loss of a wild-type channel (Fig. 2A). Therefore, our work also confirms the inaccessibility of residue 19 in the closed state and its subsequent exposure upon opening.
Because this downshock- and MTSET+-dependent toxicity appears in bacteria that have both Leu-19→Cys MscL and YggB (Figs. 2B and 3), we extended the evidence for MscL's function in vivo (3, 17, 18) by monitoring downshock-dependent Leu-19→Cys MscL opening in vivo, even in the presence of YggB. However, YggB buffered the shock to Leu-19→Cys MscL because a more powerful downshock (by approximately 400 mmol/kg) was required to kill 90% of the Leu-19→Cys MscL-harboring cells when YggB was present (compare Figs. 3B and 4A). As mentioned in Results, the pattern of partial rescue of the double mutant by either Leu-19→Cys MscL or wild-type MscL (Fig. 4 A and B) are further evidence of channel opening at a downshock threshold, which coincides with the MTSET+-specific killing of cells harboring Leu-19→Cys MscL upon downshock (Fig. 4).
Our conclusions are subject to several caveats:
- 1.
We wish to know whether the wild-type MscL opens upon downshock, but only the Leu-19→Cys MscL can be tested by our method. However, Leu-19→Cys MscL has a higher threshold than normal and requires MTSET+ to lower the gating threshold (Fig. 5). Thus, Leu-19→Cys MscL may provide a test more stringent than does wild-type MscL.
- 2.
Leu-19→Cys MscL is able to rescue MJF455 (which is MscL−YggB−) upon downshock in the absence of MTSET+ (Fig. 4A). Thus, it must open in vivo, but suctions that open this stiff channel in vitro frequently disrupt the gigaohm seal of the patch. On the other hand, necessary signal filtration may obscure any fleeting openings at lower suctions. Additionally, differences in chemical milieu, membrane voltage, or reducing potential may alter the behavior of Leu-19→Cys MscL in the patch relative to its behavior in vivo, although channel behavior does not change with 5 mM DTT applied to the cytoplasmic side of the patch (see Results). Mycobacterium tuberculosis MscL, also a stiff channel, cannot rescue MJF455 upon downshock (23); but Leu-19→Cys MscL is able to effect partial rescue (Fig. 4A), as does another stiff channel G22A MscL (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). Therefore, a high-gating threshold alone does not reliably predict an inability to rescue. This paradox may be because of inaccuracies in determining the gating threshold at high range, as discussed above. In addition, factors such as channel kinetics and/or substate behavior may contribute to the ability of a channel to rescue MJF455 upon downshock.
- 3.
The constriction of Leu-19→Cys MscL may be stretched to a point that allows MTSET+ entry while the channel is not “open” to pass osmolytes. Sukharev et al. (8, 24) proposed that the ultimate gate of MscL is formed by the N-terminal peptides, not seen in the crystal structure. This model accounts for the finding that much of the MscL deformation and mechanical work performed occur between the closed and first open substate as the constriction is expanded and previously buried hydrophobic residues are exposed to water (7). Thus, even by this model, the downshock- and MTSET+-specific toxicity indicates that the Leu-19→Cys MscL has been severely deformed, likely close to its first open substate, by the downshock.
- 4.
A drop in cfu unrelated to MTSET+ also was observed in Fig. 3A (downshock to LB400 or LB300). This finding showed that PB104 (mscLΔ yggB+ in the AW405 background) is less well equipped to handle these relatively slight osmotic shocks than a more acute osmotic shock to LB. In contrast, MJF367 (mscLΔ yggB+ in the FRAG1 background) did not show this deficiency (Fig. 3B). These observations suggest that PB104 and MJF367 may have genetic differences with regard to regulating the response to relatively small osmotic shocks. Nonetheless, our major conclusions are drawn from the clear MTSET+-dependent and Cys-19 specific effects during downshock and not from these other effects.
- 5.
Because bacteria survive downshocks without MscL (4), MscL's role in the presence of YggB could be argued previously by its conservation in a large variety of bacteria (2, 13), and, by its presence, allowing solute extrusion upon downshock (17, 18). Here, we demonstrate MscL gating in vivo and define the threshold downshock that gates MscL in vivo even in the presence of YggB, but we leave unanswered what selective advantage MscL actually confers on the wild-type bacteria.
The innocuous effect of MTSET+ in the growth medium of cells harboring Leu-19→Cys MscL channels (Figs. 2B and 3A) supports the idea that for most, if not all of the cells, the plasma membrane is not under the tension required to stretch open Leu-19→Cys MscL during this exposure. One must acknowledge many caveats not discussed here that might make MscL harder to gate in the live cell. However, based solely upon in vitro patch-clamp data, one can calculate a pressure that hypothetically could gate half of the MscLs in vivo. Previous experimental data in liposomes (7) and spheroplasts (S. Sukharev, personal communication) show that an average of ≈10 dyne/cm of tension is required. Assuming a spherical cell volume with a 1-μm diameter, ≈0.5 atm of pressure will gate 50% of wild-type MscL channels; with a 2-μm diameter, half of that pressure would be required. This pressure translates respectively into a 20–10 mM difference in solute concentration across the plasma membrane to hypothetically open MscLs, assuming no additional in vivo support (1, 7). Whatever the actual difference required to gate Leu-19→Cys MscL in the cell, it has apparently not been reached in the absence of downshock (Fig. 2B and refs. 14 and 15).
In contrast, immediately upon downshock, one would expect some water and small solutes to evacuate the periplasm through porins, although solutes larger than 600 Da would be trapped inside (25). As the periplasm became hypoosmotic relative to the cytoplasm, a tension would develop in the plasma membrane to open at least YggB. This scenario is without kinetic detail so far. However, the present study has added two pieces to the puzzle. (i) At a critical downshock, the disequilibrium between the periplasm and cytoplasm rises faster than the ability of YggB channels to relieve the pressure. Therefore, YggB is not able to prevent the tension on the plasma membrane from continuing to mount to the point where MscL opens (Fig. 3). (ii) MscL opens at milder shocks when there is no YggB (Fig. 4A vs. 3B). This result indicates that YggB indeed opens at a lower tension than MscL in live bacteria, as predicted from patch-clamp analyses. Apparently, YggB opening in the FRAG1 background is enough to combat downshock to LB300, so that Leu-19→Cys MscL opening does not occur (Fig. 3B). When there is no YggB, the tension apparently rises above the Leu-19→Cys and wild-type MscL thresholds even at this shock (Figs. 4 A and B). Thus, YggB acts as a buffer for Leu-19→Cys MscL in vivo, and because wild-type MscL may gate more easily than Leu-19→Cys MscL, YggB most likely acts as a buffer for wild-type MscL as well. This investigation is an attempt to bring the genetics, biochemistry, and biophysics of mechanosensitive channels to bear on bacterial physiology. We hope that our findings will stimulate further studies and discussion on membrane tension during growth, as well as bacteria's rapid and transient osmotic defense.
Supplementary Material
Acknowledgments
We thank W. J. Haynes, Y. Saimi and D. S. Cayley (University of Wisconsin) for helpful discussions and S. Sukharev (University of Maryland) for sharing data before publication. We also thank N. Stokes and I. Booth (University of Aberdeen, U.K.) for the use of their strains, MJF455 and MJF367. This work was supported by National Institutes of Health Grant 47856 (to C.K.). K.Y. was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- MSC
mechanosensitive channels
- cfu
colony-forming units
References
- 1.Martinac B, Buechner M, Delcour A H, Adler J, Kung C. Proc Natl Acad Sci USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moe P C, Blount P, Kung C. Mol Microbiol. 1998;28:583–592. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- 3.Levina N, Totemeyer S, Stokes N R, Louis P, Jones M A, Booth I R. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukharev S I, Blount P, Martinac B, Blattner F R, Kung C. Nature (London) 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 5.Chang G, Spencer R H, Lee A T, Barclay M T, Rees D C. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 6.Blount P, Sukharev S I, Moe P C, Schroeder M J, Guy H R, Kung C. EMBO J. 1996;15:4798–4805. [PMC free article] [PubMed] [Google Scholar]
- 7.Sukharev S I, Sigurdson W J, Kung C, Sachs F. J Gen Physiol. 1999;113:525–540. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukharev S, Betanzos M, Chiang C S, Guy H R. Nature (London) 2001;409:720–724. doi: 10.1038/35055559. [DOI] [PubMed] [Google Scholar]
- 9.Britten R J, McClure F T. Bacteriol Rev. 1962;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrier C, Coulombe A, Szabo I, Zoratti M, Ghazi A. Eur J Biochem. 1992;206:559–565. doi: 10.1111/j.1432-1033.1992.tb16960.x. [DOI] [PubMed] [Google Scholar]
- 11.Ou X, Blount P, Hoffman R J, Kung C. Proc Natl Acad Sci USA. 1998;95:11471–11475. doi: 10.1073/pnas.95.19.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Biophys J. 1999;77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer J A, Elmore D E, Lester H A, Dougherty D A. J Biol Chem. 2000;275:22238–22244. doi: 10.1074/jbc.M003056200. [DOI] [PubMed] [Google Scholar]
- 14.Stock J B, Rauch B, Roseman S. J Biol Chem. 1977;252:7850–7861. [PubMed] [Google Scholar]
- 15.Cayley D S, Guttman H J, Record M T., Jr Biophys J. 2000;78:1748–1764. doi: 10.1016/s0006-3495(00)76726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrier C, Coulombe A, Houssin C, Ghazi A. FEBS Lett. 1989;259:27–32. doi: 10.1016/0014-5793(89)81486-3. [DOI] [PubMed] [Google Scholar]
- 17.Ajoux B, Berrier C, Garrigues A, Besnard M, Ghazi A. J Biol Chem. 1998;273:26670–26674. doi: 10.1074/jbc.273.41.26670. [DOI] [PubMed] [Google Scholar]
- 18.Berrier C, Garrigues A, Richarme G, Ghazi A. J Bacteriol. 2000;182:248–251. doi: 10.1128/jb.182.1.248-251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlin A, Akabas M H. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong J B, Adler J, Dahl M M. J Bacteriol. 1967;93:390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhoads D B, Waters F B, Epstein W. J Gen Physiol. 1976;67:325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura K, Batiza A, Kung C. Biophys J. 2001;80:2198–2206. doi: 10.1016/S0006-3495(01)76192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moe P C, Levin G, Blount P. J Biol Chem. 2000;275:31121–31127. doi: 10.1074/jbc.M002971200. [DOI] [PubMed] [Google Scholar]
- 24.Sukharev S, Durell S R, Guy H R. Biophys J. 2001;81:917–936. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakae T. Biochem Biophys Res Commun. 1976;71:877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.