Abstract
There is considerable uncertainty about the precise functional contribution of the different parts of the prefrontal cortex to mnemonic processing. Changes in regional cerebral blood flow were measured with positron emission tomography in normal human subjects exposed to abstract visual designs under various conditions. It was demonstrated that the processing of stimuli that deviate from expectations involves selectively the orbitofrontal cortex, namely the part of the frontal cortex that is preferentially linked with the limbic system. By contrast, when the subject is making an explicit decision on the contents of memory (e.g., judgments of relative stimulus familiarity), the mid-ventrolateral prefrontal cortex is involved. The mid-dorsolateral prefrontal cortex is engaged when monitoring of information within working memory is required.
Although several studies with modern functional neuroimaging techniques have reported changes in activity within the prefrontal cortex during mnemonic processing, the location of these changes within the large and heterogeneous prefrontal cortex has ranged widely, raising the question of the precise conditions under which the various prefrontal regions are involved (1). In earlier functional neuroimaging studies, we were able to demonstrate that one of the critical variables determining specific increases in activity in the mid-dorsolateral prefrontal cortex (areas 46 and 9/46) is the monitoring of multiple events within working memory, regardless of the nature of the stimulus material (for review, see ref. 1). This finding is consistent with work showing that lesions limited to this part of the prefrontal cortex in the monkey impair the ability to monitor multiple stimuli within working memory rather than the maintenance of the stimuli per se (2, 3).
The present study was aimed at a better understanding of the conditions under which activation of the mid-ventrolateral and orbital prefrontal cortex is observed. In particular, the study tested specific predictions of a theoretical model proposing that, whereas the mid-dorsolateral prefrontal cortex (areas 46 and 9/46) is critical for monitoring events in working memory, the mid-ventrolateral prefrontal cortex (areas 47/12 and 45) is critical for more basic decisions on mnemonic information, such as explicit comparison and judgment of stimuli (4). According to this model, the control processes subserved by the mid-ventrolateral prefrontal cortex would include, in the context of the processing of mnemonic information, explicit judgments concerning the relative novelty of stimuli.
With regard to the human orbitofrontal cortex, there is evidence from anatomical and lesion studies in monkeys that it may play an important basic role in the mnemonic processing of stimuli. First, the orbitofrontal cortex is massively linked with different parts of the medial temporal limbic region (5, 6), damage to which yields a severe amnesic syndrome (7–9). Second, in the monkey, bilateral lesions of the orbital frontal cortex yield a significant impairment in the habituation response to novelty (10) and on performance of the delayed non-matching-to-sample task (11, 12) which is an excellent measure of the memory impairment that follows medial temporal lobe lesions (8, 9). But what exactly might be the role of the orbitofrontal cortex in the processing of new stimuli? One possibility, which is examined here, is that some parts of the orbitofrontal cortex may be involved when the organism is faced with major deviations in the nature of the expected input. Such major deviations from expectation may or may not signal threatening situations and therefore need to be evaluated. The orbitofrontal cortex may be exercising a top-down regulating influence on such new or deviant information through its massive limbic system connections and may thus be affecting secondarily memory processing. Such a view would be consistent with the fact that electrical stimulation of the orbitofrontal cortex is known to produce several autonomic changes, such as modifications of heart rate, respiration, and gastric motility (13–15) and that, often, significant changes to incoming stimulation give rise to various autonomic changes (16, 17).
The present experiment aimed to clarify the conditions under which the orbital and the mid-ventrolateral prefrontal cortex are engaged during the processing of visual information by measuring, with positron emission tomography (PET), changes in regional cerebral blood flow (CBF) in normal human subjects.
Materials and Methods
Subjects.
Eight right-handed male volunteer subjects (mean age: 22 years, range: 19–26 years) participated in this experiment, which was approved by the Ethics Committee of the Montreal Neurological Institute.
Scanning Methods and Data Analysis.
The regional distribution of CBF, a correlate of local neuronal activity, was measured by means of the water bolus H215O method without arterial blood sampling during 60-sec scanning conditions. The subjects were scanned with a Scanditronix PC-2048 tomograph (Scanditronix Wellhofer, Bartlett, TN), which produces 15 image slices at an intrinsic resolution of 5 × 5 × 6 mm (18). Each subject also underwent a high-resolution magnetic resonance imaging (MRI) scan (64 slices, 2 mm thick) obtained with a Philips Gyroscan (1.5 T). The MRI scans were resliced so as to be in register with the PET data. Interactive three-dimensional image software was then used to establish an orthogonal coordinate frame based on the anterior–posterior commissure line as identified in the MRI image volume (18). The coordinates were used to apply a linear resampling of each matched pair of MRI and PET data sets into a standardized stereotaxic coordinate system (19). PET images were normalized for global CBF, and the mean state-dependent CBF difference image volume was obtained. This volume was converted to a t-statistic volume by dividing each voxel by the mean standard deviation in normalized regional CBF for all intracerebral voxels (20, 21). Individual MRI images were subjected to the same averaging procedure, such that composite stereotaxic image volumes, 128 × 128 × 80 voxels in extent and sampled at 1.34 × 1.72 × 1.50 mm in the x, y, and z dimensions, respectively, were obtained for t-statistic and MRI volumes. Anatomical and functional images were merged to allow (i) direct localization of t-statistic peaks, identified by an automatic peak-detection algorithm, on the MRI images and (ii) the anatomical correlation of extended zones of activation that cannot be expressed in terms of isolated peaks.
The statistical significance of focal changes in CBF was tested by a method based on three-dimensional Gaussian random field theory (20). For an exploratory search involving all peaks within the gray matter volume of 600 ml, the threshold for reporting a peak as significant was set at t = 4.30, corresponding to a corrected probability of P < 0.05. For predicted activation foci within the orbital, mid-ventrolateral, and mid-dorsolateral frontal cortex, the threshold for significance was set at t = 3.00, corresponding to a corrected probability of P < 0.05 based on a search region of 50 ml (21).
Testing Procedure.
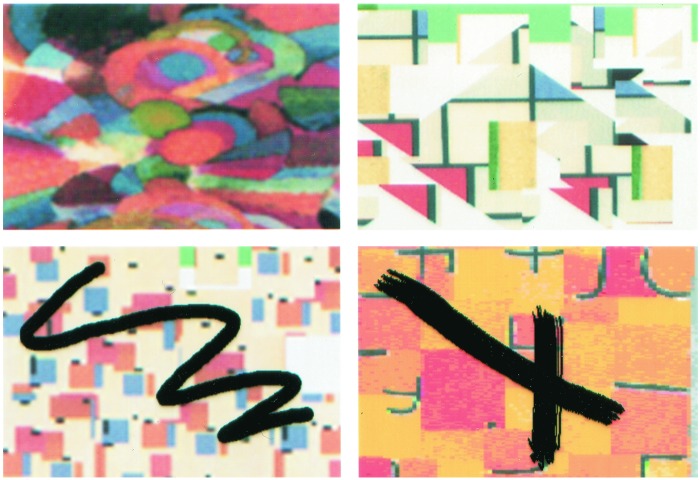
In all conditions, the stimuli were colored abstract images appearing, in pairs, on a touch-sensitive screen, and the subjects responded by touching the screen with their right index finger (Fig. 1). Thus, the mode of stimulus presentation and the way the subjects indicated their response were identical in all of the scanning conditions. We chose to use abstract visual images as the stimulus material because these stimuli cannot easily be verbalized and are therefore not likely to provoke semantic associations. Pictures of meaningful objects and scenes, with the verbalization and semantic associations that they inevitably trigger, might have set in motion several additional cognitive processes involving lateral and medial frontal areas that, in the context of the present experiment, needed to be controlled.
Figure 1.
Examples of the standard type of abstract images (Upper) used in all conditions, except in the deviant stimulation condition in which the images (Lower) were modified by the addition of material (e.g., black lines) that was clearly extraneous to them.
Control Condition.
In this condition, the subjects were simply required to view pairs of colored abstract images appearing on the screen. The subjects were instructed to view the pair of images on the screen and then to touch the screen in the space between the two stimuli to advance to the next pair. As soon as the subject touched the screen, the previous pair of stimuli disappeared and a new pair of stimuli appeared. Half of the images used in the control condition had been seen once just before scanning began and the other half were novel. In this way, the number of familiar and novel stimuli that the subjects were exposed to during the control condition was identical to that in the other experimental conditions (i.e., 50% familiar and 50% novel).
Deviant Stimulation Condition.
This condition was designed to test the hypothesis that there would be increased activity within the orbitofrontal cortex as a result of the mere inspection of significant deviations from the expected type of stimulation. The subjects were presented with pairs of abstract colored images that were different from those seen in the control condition, as well as in all of the other experimental conditions. The stimuli were produced by randomly selecting abstract images from our large set and introducing some noticeable distortion in them, such as a thick black line, so that the subjects would not fail to notice the difference from the usual set of stimuli used in all other conditions (Fig. 1). These were not changes that would evoke any emotion, but they were certainly changes that would attract attention because they were clearly perceived as not being a normal part of the designs. Apart from this change in the type of visual stimulation, all other aspects of testing in the deviant stimulation condition were identical to those of the control condition: the subjects were simply required to inspect the pair of visual abstract images presented and then to touch the screen in the space between the two stimuli to view the next pair of stimuli. Also, as in the control condition, half of the images had been seen once just before scanning.
Familiarity/Novelty Decision Condition.
This condition was designed to test the hypothesis that the mid-ventrolateral prefrontal cortex would be engaged when an active explicit decision was made regarding the relative familiarity of stimuli, as opposed to passively viewing novel and familiar stimuli. Just before scanning, the subjects saw novel abstract designs. During scanning, the subjects saw pairs of abstract images, one of which had been seen before scanning and the other of which was novel. The subjects were required to make an explicit decision as to which one of the two stimuli was the novel one and to touch it in order that the next pair should be presented. Note that the number of familiar and novel stimuli that the subjects were exposed to in the control and the present condition was the same, the only difference being that an explicit judgment was now required of the subject.
Monitoring Condition.
In this condition, we wished to include an active decision with regard to the relative familiarity of stimuli that is hypothesized to involve the mid-ventrolateral frontal cortex plus a monitoring requirement of events in working memory that was previously shown to depend on the mid-dorsolateral frontal cortex. During scanning, the subjects again saw pairs of abstract images and were required to touch one of these stimuli to advance to the next pair. The subjects were told that some of the pairs of stimuli would recur and, when this happened, they should touch the stimulus that they had not touched before. Half of the pairs involved stimuli that were repetitions and the other half were novel. Thus, during scanning the subjects were required to decide that certain pairs of stimuli were novel and proceed to select one of the stimuli and that other pairs of stimuli were recurring and to touch the stimulus that they had not touched before. Note that because half of the pairs of stimuli were recurring and, in these cases, the subjects had to touch the stimulus that had not been touched when the pair was first presented, the subjects had to keep track of (i.e., monitor) their earlier choices. This need to monitor one's selections is the fundamental requirement leading to severe impairments on the self-ordered tasks in patients with frontal excisions (22) and monkeys with excisions limited to the mid-dorsolateral prefrontal cortex (2, 3).
Results
Deviant Stimulation Condition Compared with the Control Condition.
As is well known, the mere inspection of novel stimuli results in their automatic encoding. As expected, therefore, recognition performance in the tests administered at the end of the PET scanning session for the stimuli seen in the control and the deviant conditions was very high (96% for the deviant stimuli and 92% for the control stimuli), demonstrating that the subjects had processed those stimuli although they had not been instructed to do so.
The question whether there would be significant and selective modulations of activity within the human orbital frontal cortex related to the mere inspection of stimuli that deviated from those expected was addressed by subtracting activity in the control condition from that in the deviant stimulation condition. This subtraction revealed significant increases in activity within the right orbitofrontal cortex (Table 1; Fig. 2). It is interesting to note that the significant negative peaks (i.e., less activity in the deviant stimulation condition relative to the control condition) were also restricted to the orbitofrontal cortex and that no changes in activity (positive or negative) were observed in any other part of the frontal cortex.
Table 1.
Deviant stimulation condition compared with the control condition
| Stereotaxic coordinates
|
t statistic | Brain area | ||
|---|---|---|---|---|
| x | y | z | ||
| Deviant stimulation minus control | ||||
| Right hemisphere | ||||
| 31 | 49 | −15 | 3.50 | Orbital frontal cortex (area 11) |
| 21 | 24 | −21 | 3.14 | Orbital frontal cortex (area 13) |
| Control minus deviant stimulation | ||||
| Left hemisphere | ||||
| −8 | 42 | −20 | 3.59 | Orbital frontal cortex (area 14) |
| −36 | 22 | −12 | 3.51 | Orbital frontal cortex (area 13) |
| Right hemisphere | ||||
| 8 | 39 | −14 | 3.37 | Orbital frontal cortex (area 14) |
Peaks of statistically significant changes in normalized CBF (see text). The stereotaxic coordinates, in this and the other tables, are expressed in mm. x, Medial-to-lateral distance relative to the midline (positive = right); y, anterior–posterior distance relative to the anterior commissure (positive = anterior); z, superior–inferior distance relative to the anterior commissure–posterior commissure line (positive = superior).
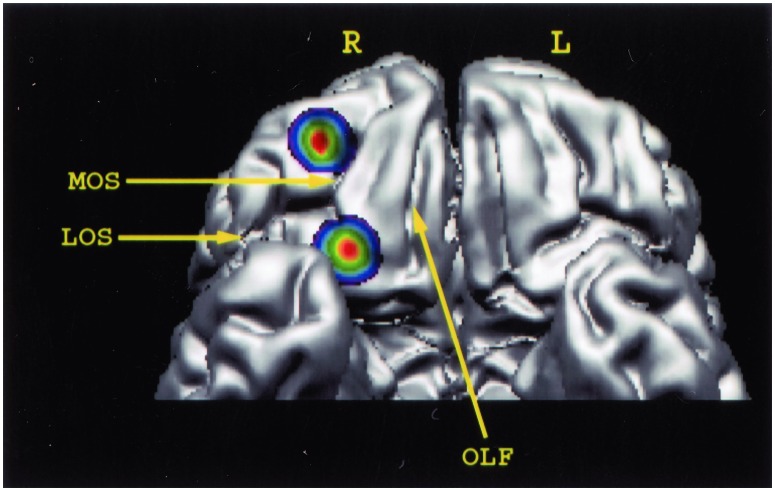
Figure 2.
Three-dimensional reconstruction of the human orbital frontal lobe to illustrate the location of the two peaks of increased activity in the right orbitofrontal cortex resulting from the deviant stimulation minus the control condition comparison. A comment must be made regarding our definition of the architectonic areas of the orbitofrontal cortex. Brodmann did not parcellate the orbitofrontal cortex in detail. A more detailed recent parcellation divided the human orbitofrontal cortex into various areas corresponding to those of the macaque monkey (23). According to this parcellation (23), the rostral peak of activity, which lies between the rostral parts of the medial and the lateral orbital sulci, is located in area 11. The caudal activity peak, which lies along the caudal part of the medial orbital sulcus, is located in medial area 13. L, left hemisphere; LOS, lateral orbital sulcus; MOS, medial orbital sulcus; OLF, olfactory sulcus; R, right hemisphere.
There were two foci of activity changes within the orbitofrontal cortex. The rostral one was bounded by the medial and lateral orbital sulci and was lying in front of the transverse orbital sulcus. Architectonic studies have shown that this region of the orbital frontal cortex contains area 11 in both humans and monkeys (23). The caudal focus lies in the region occupied by area 13. CBF in these two right orbitofrontal foci was correlated with CBF in the rest of the brain to find out with which other brain loci these two areas interacted. Activity in the caudal orbitofrontal focus (area 13) was positively correlated with activity in the right mid-ventrolateral frontal cortex (area 47/12; x = 47, y = 27, z = −7.5, t = 5.08), the right rostral orbitofrontal cortex (area 11; x = 25, y = 49, z = −15, t = 4.37), the right anterior inferotemporal cortex (area 20; x = 58, y = −35, z = −21, t = 4.18), as well as the left caudal orbitofrontal cortex (area 13; x = −21, y = 20, z = −13.5, t = 4.14) and the left ventrolateral frontal cortex (x = −46, y = 27, z = 3, t = 4.31). Activity in the rostral orbitofrontal cortex (area 11) was positively correlated with that in the right ventrolateral frontal cortex (area 47/12; x = 47, y = 25, z = −7.5, t = 4.65) and the right anterior (x = 17, y = 27, z = 21, t = 4.04) and posterior (x = 11, y = −50, z = 19.5, t = 3.67) cingulate cortex.
Familiarity/Novelty Decision Condition Compared with the Control Condition.
The second major question explored in the present investigation was whether there would be significant increases in the mid-ventrolateral prefrontal cortex when explicit judgments of the relative novelty/familiarity of the stimuli were made. This question was tested by subtracting activation in the control condition from that in the familiarity/novelty decision condition. This subtraction revealed significantly greater activity in the mid-ventrolateral prefrontal cortex, and this activity change was more extensive in the right hemisphere (Table 2 and Fig. 3). It was located in area 47/12, as shown by the fact that the activation extended within the horizontal ramus of the Sylvian fissure and, ventrally, it did not extend medial to the lateral orbital sulcus (23). Thus, the present findings with PET provided strong confirmation of the hypothesis that the mid-ventrolateral frontal cortex, unlike the orbitofrontal cortex, plays a major role when explicit judgments concerning mnemonic information must be made. Activity in the right mid-ventrolateral frontal focus (x = 50, y = 46, z = −14) was negatively correlated with the right inferior temporal gyrus (x = 44, y = −49, z = −5, t = 3.51).
Table 2.
Familiarity/novelty decision condition compared with the control condition
| Stereotaxic
coordinates
|
t statistic | Brain area | ||
|---|---|---|---|---|
| x | y | z | ||
| Familiarity/novelty decision minus control | ||||
| Left hemisphere | ||||
| −28 | 27 | 3 | 3.44 | Mid-ventrolateral frontal cortex (area 47/12 and 45 sulcal) |
| Right hemisphere | ||||
| 39 | 27 | −6 | 4.25 | Mid-ventrolateral frontal cortex (area 47/12) |
| 50 | 46 | −14 | 4.40 | Mid-ventrolateral frontal cortex (area 47/12) |
| 32 | 24 | 3 | 4.70 | Mid-ventrolateral frontal cortex (area 45 sulcal) |
| 32 | −66 | 48 | 5.26 | Posterior parietal cortex (area 7) |
| 8 | −2 | 5 | 5.42 | Thalamus (anterior nucleus) |
| 23 | −88 | −9 | 5.46 | Ventral prestriate cortex (area 18) |
| Bilateral | ||||
| 0 | 36 | 36 | 5.38 | Anterior paracingulate cortex (area 32) |
| 1 | −19 | −9 | 4.94 | Subthalamic region |
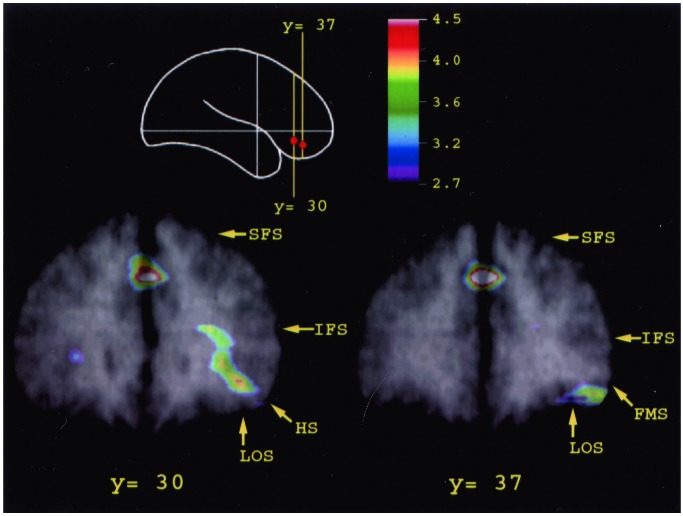
Figure 3.
Merged PET and MRI sections illustrating the average CBF increase for all subjects in mid-ventrolateral prefrontal area 47/12 observed in the familiarity/novelty decision minus the control comparison. Architectonic studies have shown that this area extends along the sulcus frontomarginalis lateralis (FMS) as far as the lateral orbital sulcus (LOS) and, caudally, continues along the banks of the horizontal sulcus (23). The location of the activity peaks respects remarkably well the conclusions of the architectonic studies. The schematic outline of the brain indicates the level of the coronal sections. HS, horizontal sulcus; IFS, inferior frontal sulcus; FMS, sulcus frontomarginalis lateralis; LOS, lateral orbital sulcus; SFS, superior frontal sulcus.
A further test of the hypothesis that the mid-ventrolateral frontal cortex is involved when explicit judgments of novelty/familiarity are made was obtained when activation in the deviant information condition was subtracted from the familiarity/novelty decision condition. Significant increases in activity were again observed in the right and left mid-ventrolateral prefrontal cortex (x = 31, y = 20, z = −12, t = 5.18; x = 43, y = 46, z = −11, t = 5.15; x = 38, y = 30, z = −17, t = 4.04; x = −25, y = 18, z = −11, t = 4.35; x = −29, y = 24, z = 8, t = 4.44).
Monitoring Condition Compared with the Control Condition.
The monitoring condition minus the control condition comparison revealed four peaks of increased activity in the right prefrontal cortex (Table 3; Fig. 4). Two of these peaks were located in the mid-dorsolateral prefrontal cortex (areas 46 and 9/46), and the other two peaks were in mid-ventrolateral prefrontal cortex (area 47/12 and the junction 47/12 with 45). The activation in the mid-dorsolateral prefrontal cortex replicates earlier findings of activity in this region of the prefrontal cortex whenever monitoring within working memory is required (24). The important finding now is that, because the comparison is with a basic control task that does not require any active judgment, the ventrolateral prefrontal cortex also shows greater activity reflecting the role of this cortical region in such judgments. CBF in the right mid-dorsolateral prefrontal focus (x = 42, y = 36, z = 27) was positively correlated with that in the right retrosplenial cortex (x = 13, y = −44, z = 22, t = 3.18).
Table 3.
Monitoring condition compared with the control condition
| Stereotaxic
coordinates
|
t statistic | Brain area | ||
|---|---|---|---|---|
| x | y | z | ||
| Monitoring minus control | ||||
| Right hemisphere | ||||
| 29 | 27 | 5 | 4.09 | Mid-ventrolateral frontal cortex (area 47/12 and 45 sulcal) |
| 48 | 41 | −12 | 3.43 | Mid-ventrolateral frontal cortex (area 47/12) |
| 42 | 36 | 27 | 3.19 | Mid-dorsolateral frontal cortex (area 46) |
| 46 | 20 | 36 | 3.23 | Mid-dorsolateral frontal cortex (area 9/46) |
| 32 | −66 | 47 | 5.77 | Posterior parietal cortex (area 7) |
| 7 | −9 | 5 | 4.46 | Thalamus (dorsomedial nucleus) |
| Bilateral | ||||
| 1 | 34 | 38 | 4.91 | Anterior paracingulate cortex (area 32) |
| 1 | −13 | −3 | 4.88 | Subthalamic region |
| Control minus monitoring | ||||
| Left hemisphere | ||||
| −5 | 56 | −14 | 4.27 | Orbital frontal cortex (rostromedial area 14) |
| −17 | 48 | −6 | 3.79 | Orbital frontal cortex (area 11) |
| −19 | 10 | −17 | 3.29 | Orbital frontal cortex (area 13) |
| Right hemisphere | ||||
| 16 | 13 | −15 | 2.72 | Orbital frontal cortex (area 13) |
| 4 | 25 | 0 | 3.27 | Subcallosal cortex (septal region) |
| 63 | −28 | 0 | 3.82 | Middle temporal cortex (area 21) |
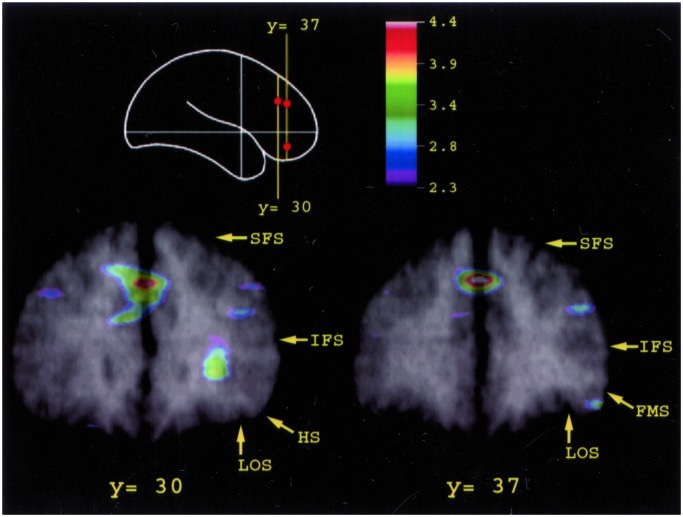
Figure 4.
Merged PET and MRI sections illustrating the average CBF increase for all subjects in the mid-dorsolateral prefrontal area 9/46 on the middle frontal gyrus above the inferior frontal sulcus (IFS) and in mid-ventrolateral prefrontal area 47/12 observed in the monitoring minus the control comparison. The schematic outline of the brain indicates the level of the coronal sections. Abbreviations as in Fig. 3.
A direct comparison of the familiarity/novelty decision and the monitoring conditions by subtraction showed that, relative to the monitoring condition, there was greater activity within the mid-ventrolateral prefrontal cortex (area 47/12) in the familiarity/novelty decision condition (x = 39, y = 34, z = −8, t = 4.55; x = 35, y = 20, z = −14, t = 4.19). By contrast, relative to the familiarity/novelty decision condition, there was greater activity within the mid-dorsolateral prefrontal cortex (areas 9/46 and 46) in the monitoring condition (x = 21, y = 34, z = 45, t = 4.34; x = 35, y = 44, z = 31.5, t = 2.70).
Discussion
The present study attempted to shed light on the precise conditions under which the orbital, the mid-ventrolateral, and the mid-dorsolateral prefrontal cortical regions are involved in the processing of abstract visual stimuli. As expected, given the nonverbal nature of the stimulus material, most of the activity changes occurred within the right frontal cortex, but different frontal regions exhibited modulations in activity depending on the task.
Orbitofrontal Cortex.
Relative to the inspection of standard abstract designs (control condition), there was a selective increase in activity within the right orbitofrontal region when the subjects inspected abstract designs that had been modified in some manner (deviant stimulation condition) (Table 1 and Fig. 2). Note that no decision was required of the subjects during the inspection of the stimuli and, therefore, the modulation in activity in the orbitofrontal cortex reflects the brain response set in motion by the mere inspection of stimuli that deviate in a major way from expectation. These focal activity changes were observed in area 11 and area 13 of the orbitofrontal cortex, and it is of considerable interest that both these regions receive major visual inputs from the anterior inferotemporal region (5, 6). Furthermore, activity in the anterior inferotemporal region was correlated with activity in area 13, which was in turn correlated with that in area 11, suggesting functional interactions in this circuit during the processing of deviant information.
The changes in activity in the orbitofrontal cortex observed in the present PET study are consistent with the findings of a recent single-neuron recording study in the monkey (25). Many neurons in orbitofrontal areas 11 and 13, which responded when the monkey viewed familiar pictures that had particular behavioral significance for the animal, increased their firing rate when the monkey was faced with novel pictures. Furthermore, there was a response to novel stimuli by certain other orbitofrontal neurons, the activity of which reflected particular reward expectations, and their firing rate was modified as the animal's reward expectations regarding these novel stimuli (as indexed by behavioral reactions) were modified. Thus, the responses of orbitofrontal neurons reflected changes in the expectations of the behavioral significance of stimuli.
In an earlier PET study, we showed a selective increase of activity within orbitofrontal area 11 in the right hemisphere when the subjects were simply inspecting novel abstract designs as compared with familiar ones (26). In the present study, the degree of familiarity of the stimuli to the subject, in terms of previous exposure, was perfectly matched between the control and deviant stimulation conditions (see Materials and Methods). The difference in the deviant condition derived from changes in the stimuli that made them look inconsistent with the expected standard type of abstract designs that appeared in all of the other conditions. Thus, it appears that orbitofrontal area 11 and area 13 are engaged whenever a noticeable change in the stimulation is introduced. Significant deviations from the expected type of stimulation must be evaluated with regard to their potential positive or negative implications for the organism and the orbitofrontal cortex with its strong and preferential connections with several limbic structures would be in an ideal position to regulate further information processing in these structures. The present argument is consistent with earlier work in both monkey (27) and human (28) subjects, suggesting that the orbital frontal cortex is critically involved in the regulation of the emotional/motivational states of the organism and that orbitofrontal neuronal activity reflects changes in expectations of the significance of stimuli (25).
A recent PET study reported increased activity in the caudal orbital frontal cortex during the performance of a recognition task that required differentiation between currently relevant items from previously relevant but currently irrelevant items (29). The activity increases were focused on the caudomedial orbitofrontal cortex around the gyrus rectus, whereas increases in activity in our study occurred in caudal and rostral orbital frontal cortex lateral to the gyrus rectus. In fact, the activity in the medial orbitofrontal cortex (gyrus rectus) decreased in our study. The caudomedial orbitofrontal cortex is distinguished from other orbitofrontal areas by its strong input from the subiculum (6), raising the possibility that it may play a closer role in hippocampal related processing. This major anatomical difference between the medial (gyrus rectus) and the more lateral orbitofrontal cortex suggests functional differences between these two regions.
Mid-Ventrolateral Prefrontal Cortex.
The second major hypothesis investigated in the present experiment was that the mid-ventrolateral prefrontal cortex might be critically involved in control processes that underlie the capacity to make explicit decisions (e.g., judgments) on the content of information held in memory (6). In the familiarity/novelty decision condition, the subjects made explicit judgments of whether stimuli had been seen just before the scanning. Comparison of activity between the familiarity/novelty decision and the control condition revealed a selective increase in activity in the mid-ventrolateral frontal cortex (Table 2 and Fig. 3). This activity change was more extensive in the right hemisphere and was located in area 47/12. It is important here to note that the amount of novel and familiar material to which the subject was exposed in the familiarity/novelty decision and the control conditions was exactly the same (i.e., 50% novel and 50% familiar stimuli). Thus, the only difference between the two conditions lay in the fact that in the control condition the subject simply viewed a pair of stimuli on the screen and pressed to view the next pair of stimuli, whereas in the familiarity/novelty decision condition, the subject made an explicit judgment of which one of the two stimuli was seen before and which one was novel. Thus, the present findings provide strong evidence that the mid-ventrolateral prefrontal cortex, unlike the orbitofrontal cortex, plays a major role when explicit judgments concerning mnemonic information must be made.
The present findings are consistent with earlier functional neuroimaging work that demonstrated increases in activity within the ventrolateral region in visual delayed matching-to-sample tasks (30), but suggest that it is the explicit decision on mnemonic information that engages the ventrolateral prefrontal cortex rather than memory per se. This conclusion is reinforced by a study in the monkey (31) demonstrating that lesions of the ventrolateral prefrontal cortex led to an initial impairment on the delayed non-matching-to-sample test requiring a choice between familiar and novel objects but, when the monkeys recovered their ability to perform the test, increases in the number of stimuli to be remembered or the delays during which the information had to be maintained did not affect performance. By contrast, the monkeys with orbitofrontal lesions had severe and long-lasting deficits. In view of the present PET findings, it could be argued that, whereas lesions of the orbitofrontal cortex impaired a basic brain response to the novelty of stimuli and thus led to long-lasting impairments, lesions of the ventrolateral prefrontal cortex led to a loss of the capacity to make an explicit judgment on the relative familiarity of stimuli. Initially, this loss resulted in impaired performance on the delayed non-matching-to-sample task, but with continued training the monkeys with ventrolateral prefrontal lesions adopted an implicit solution of the task based on the attraction exerted by novelty and the consistent reward provided whenever the novel stimulus was chosen. Monkeys with orbital frontal lesions, however, had a more fundamental impairment emanating from their loss of an appropriate response to the novelty of stimuli and therefore continued to be impaired. This conclusion is consistent also with the results of another study in the monkey (32), which showed an initial impairment on simultaneous matching-to-sample after lesions of the ventrolateral prefrontal cortex, but once the principle was acquired there was no impairment when delays were introduced.
Mid-Dorsolateral Prefrontal Region.
It is important to note the absence of activation within the mid-dorsolateral prefrontal cortex in the two conditions discussed above. This lack of activation is consistent with work in nonhuman primates demonstrating that lesions of the dorsolateral prefrontal cortex do not affect performance on recognition memory tasks that require discrimination between familiar and novel stimuli (2, 11, 12). By contrast, there is considerable support from work in the monkey (2, 3) that lesions confined to the mid-dorsolateral prefrontal region give rise to a selective impairment on working memory tasks in which multiple events must be monitored. Functional neuroimaging studies have provided data in agreement with this view by demonstrating increases in activity in the mid-dorsolateral prefrontal region whenever information in working memory must be monitored (e.g., ref. 24; for review, see ref. 1).
The lack of activity in the mid-dorsolateral prefrontal region in the familiarity/novelty decision condition of the present study was therefore expected. In the familiarity/novelty decision condition, the decision of which stimulus is novel and which one is familiar need be based only on the judgment of the two stimuli that are currently on the screen. In other words, each decision is independent of previous decisions and therefore there is no need to keep track of (i.e., monitor) earlier decisions. This interpretation was tested in the monitoring condition, in which several of the pairs of stimuli were presented more than once and, when faced with such pairs, the subjects had to touch the stimulus that had not been selected before. Thus, during scanning, the subjects were required to monitor carefully their earlier choices. Comparison of the monitoring condition with the control condition revealed increased activity in the mid-dorsolateral prefrontal cortex (Table 3; Fig. 4), consistent with several earlier functional neuroimaging studies (see ref. 1) and work in the monkey (2, 3) that demonstrated the critical involvement of this cortical region to the monitoring of information in working memory.
Acknowledgments
This work was supported by a grant from the Canadian Institutes for Health Research.
Abbreviations
- PET
positron emission tomography
- CBF
cerebral blood flow
References
- 1.Petrides M. In: Brain Mapping: The Systems. Toga A W, Mazziotta J C, editors. San Diego: Academic; 2000. pp. 159–176. [Google Scholar]
- 2.Petrides M. Proc R Soc London Ser B. 1991;246:293–298. doi: 10.1098/rspb.1991.0157. [DOI] [PubMed] [Google Scholar]
- 3.Petrides M. J Neurosci. 2000;20:7496–7503. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrides M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 9. Amsterdam: Elsevier; 1994. pp. 59–82. [Google Scholar]
- 5.Barbas H. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael S T, Price J L. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 7.Milner B. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 8.Mishkin M. Philos Trans R Soc London B. 1982;298:85–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- 9.Squire L R, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 10.Butter C M. Science. 1964;144:313–315. doi: 10.1126/science.144.3616.313. [DOI] [PubMed] [Google Scholar]
- 11.Bachevalier J, Mishkin M. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- 12.Meunier M, Bachevalier J, Mishkin M. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 13.Delgado J M R, Livingston R B. J Neurophysiol. 1948;11:39–55. doi: 10.1152/jn.1948.11.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Livingston R B, Chapman W P, Livingston K E, Kraintz L. Res Publ Assoc Res Nerv Ment Dis. 1948;27:421–432. [PubMed] [Google Scholar]
- 15.Hall R E, Livingston R B, Bloor C M. J Neurosurg. 1977;46:638–647. [PubMed] [Google Scholar]
- 16.Sokolov E N, Vinogradova O S, editors. Neuronal Mechanisms of the Orienting Reflex. Hillsdale, NJ: Erlbaum; 1975. [Google Scholar]
- 17.Zimney G H, Schwabe L W. Psychophysiology. 1965;2:103–115. doi: 10.1111/j.1469-8986.1965.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 18.Evans A C, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. NeuroImage. 1992;1:43–63. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- 19.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 20.Worsley K J, Evans A C, Marrett S, Neelin P. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 21.Worsley K J, Marrett S, Neelin P, Vandal A C, Friston K J, Evans A C. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Petrides M, Milner B. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 23.Petrides M, Pandya D N. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 9. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]
- 24.Petrides M, Alivisatos B, Evans A C, Meyer E. Proc Natl Acad Sci USA. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tremblay L, Schultz W. J Neurophysiol. 2000;83:1877–1855. doi: 10.1152/jn.2000.83.4.1877. [DOI] [PubMed] [Google Scholar]
- 26.Frey S, Petrides M. Proc Natl Acad Sci USA. 2000;97:8723–8727. doi: 10.1073/pnas.140543497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolls E T. Philos Trans R Soc London B. 1996;351:1433–1444. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- 28.Bechara A, Damasio H, Damasio A R. Cereb Cortex. 2000;10:1047–3211. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 29.Schnider A, Treyer V, Buck A. J Neurosci. 2000;20:5880–5884. doi: 10.1523/JNEUROSCI.20-15-05880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haxby J V, Ungerleider L G, Horwitz B, Maisog J M, Rapoport S I, Grady C L. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalska D M, Bachevalier J, Mishkin M. Neuropsychologia. 1991;29:583–600. doi: 10.1016/0028-3932(91)90012-w. [DOI] [PubMed] [Google Scholar]
- 32.Rushworth M F S, Nixon P D, Eacott M J, Passingham R E. J Neurosci. 1997;17:4829–4838. doi: 10.1523/JNEUROSCI.17-12-04829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]