Abstract
Objective:
This study evaluated the hypothesis that protein concentration and mitochondrial content in gastrocnemius biopsies from patients with peripheral arterial disease (PAD) predict mortality rates.
Background:
PAD patients experience advancing myopathy characterized by mitochondrial dysfunction, myofiber degradation and fibrosis in their ischemic legs, along with increased mortality rates.
Methods:
Samples from the gastrocnemius of PAD patients were used for all analyses. Protein concentration was normalized to muscle wet weight, and citrate synthase activity (standard measure of mitochondrial content in cells) was normalized to muscle wet weight and protein concentration. Protein and citrate synthase data were grouped into tertiles and 5-year, all-cause mortality for each tertile was determined with Kaplan-Meier curves and compared by the Modified Peto test. A Cox-regression model for each variable controlled for the effects of clinical characteristics.
Results:
Of the 187 study participants, 46 died during a mean follow up of 23.0 months. Five-year mortality rate was highest for patients in the lowest tertile of protein concentration. Mortality was lowest for patients in the middle tertile of citrate synthase activity when normalized to either muscle wet weight or protein concentration. The mortality hazard ratios (HR) from the Cox analysis were statistically significant for protein concentration normalized to muscle wet weight (lowest vs middle tertile; HR=2.93; p=0.008) and citrate synthase normalized to protein concentration (lowest vs. middle tertile; HR=4.68; p=0.003; and lowest vs. highest tertile; HR=2.36; p=0.027).
Conclusion:
Survival analysis of a contemporaneous population of PAD patients identifies protein and mitochondrial content of their gastrocnemius as predictors of mortality rate.
Introduction
Epidemiological studies of Peripheral Arterial Disease (PAD) estimate that its prevalence in the general population ranges from 3% to 10% 1. In populations aged greater than 70 years, the prevalence increases to as high as 20% 1. The mortality of symptomatic PAD patients varies between studies. Published all-cause death rates vary between 3.3 % and 7.8% per year 2 and can be comparable to that of several types of cancer.
Several clinical parameters and biochemical markers have been shown to be independent predictors of mortality in the PAD population. The Ankle-brachial index (ABI) is one clinical parameter consistently correlated to mortality. The Edinburgh study examined a population of 1592 residents aged 55 to 74 years in Edinburgh, United Kingdom. At 12 years follow-up, decreasing ABI correlated with increasing all-cause mortality 3 with 63.5% of patients who had a baseline ABI of less than 0.7 being deceased compared to only 31.4% of patients with an ABI greater than 0.7 3. The Strong Heart Study, designed to investigate cardiovascular disease in American Indians, found an adjusted mortality risk of 1.69 in those patients with ABI <0.90 and a mortality risk of 1.77 in those with ABI >1.4 compared to the reference group of patients with ABIs between 0.9 and 1.4 4. The investigators found a U-shaped relationship between baseline ABI and mortality, with all-cause mortality increasing as ABI increasingly deviated from 1.04 4. Other clinical comorbidities such as diabetes mellitus, hypertension, and renal dysfunction have also been shown to be predictive of mortality in patients with PAD 1, 5.
Biochemical markers, particularly serum biomarkers, and their correlation to mortality have been extensively investigated in the cardiovascular literature, but studies in PAD patients are limited. Criqui and colleagues studying mortality in PAD patients, showed a correlation between highly sensitive C-reactive protein and mortality at 2 years but not at 6.6 years of follow-up 5. In the same study no association between mortality and several serum biomarkers (D-dimer, platelet activating factor, serum amyloid A, homocysteine, and lipoprotein-a) was found 5. Patients with ABI measurements <0.9 were recruited by Vidula et al who demonstrated that serum amyloid A level, C-reactive protein level, and D-Dimer level correlated with all-cause mortality at 4 years follow-up 6. In other studies of PAD patients, neither N-terminal pro-B-type natriuretic peptide levels 7 nor lipoprotein-associated phospholipase A2 8 correlated with mortality.
The latest data on the pathophysiology of PAD suggest that arterial stenoses and occlusions produce effort-induced cycles of ischemia and reperfusion in the affected limbs. These cycles appear to initiate a combination of mitochondrial dysfunction, oxidative damage, and inflammation in the affected limbs, which set in motion a cascade of injury to every structure in the leg including muscles, nerves, skin, and subcutaneous tissues 9–11. Accumulating injury in all tissues of the leg can lead to progressive decline in muscle structure and performance (claudication) and loss of the integrity of tissues in the ischemic limb presenting as tissue loss/gangrene. Injury to the skeletal muscle of the ischemic legs (myopathy of PAD) is the better explored component of this process in PAD limbs 10, 11. The importance of this myopathy to the overall health of PAD patients was recently demonstrated in a study showing that imaging and function-based biomarkers of this myopathy (calf muscle density measured via Computed Tomography scans, plantar flexion strength and knee extension power) correlate to the survival of PAD patients. More specifically, McDermott and colleagues demonstrated that lower calf muscle density and weaker lower plantar flexion strength and knee extension power were associated with increased mortality in PAD patients 12. However, to date there have been no studies correlating biochemical biomarkers of this myopathy to mortality in patients with PAD. Skeletal muscle protein concentration and mitochondrial content are basic biochemical biomarkers of the observed myopathy. With this study we evaluate the hypothesis that protein concentration and mitochondrial content in gastrocnemius biopsies predict the 5-year mortality rate of PAD patients.
Methods
Participant Identification
This study was approved by the Institutional Review Boards of the VA Nebraska-Western Iowa Health Care System and the University of Nebraska Medical Center. Patients evaluated and diagnosed for PAD in the vascular surgery clinic of the two institutions between April 2003 and October 2010, were identified for this study. For every patient, the diagnosis of PAD was based on medical history, physical examination, significantly decreased ankle-brachial index (ABI < 0.9) and computerized or standard arteriography demonstrating stenoses and/or occlusions in the arteries supplying the lower extremities. A total of 187 patients with PAD were enrolled. Written informed consent was obtained from each patient who elected to participate.
Comorbidities
After patient enrollment, patient characteristics and comorbidities were identified using a combination of patient interview and chart review. Age, gender, race, height, weight, and Body Mass Index (BMI) were recorded. Further, the presence of Coronary Artery Disease (CAD), hypertension, dyslipidemia, diabetes mellitus (DM), tobacco use, renal dysfunction, a family history of atherosclerotic disease, and use of statins were determined for each patient. ABI measurements were gathered by standard procedures, in the vascular laboratories at the respective institution. At the time of final analysis, an assessment of mortality status was performed using a combination of chart review and telephone interview with the patient or their family.
Muscle Specimen Collection
Muscle biopsy samples were obtained from the gastrocnemius of the more symptomatic limb based on the presentation of the patient. All muscle samples were obtained from the anteromedial aspect of the gastrocnemius belly, at a level 10 cm distal to the tibial tuberosity. All biopsies were performed by, or under the supervision of, one investigator (I.I.P.) and identical operative technique was used for muscle harvest in all patients.
Protein and Citrate Synthase Determination
Assays were performed in duplicate or triplicate in skeletal muscle homogenates using a spectrophotometer (DU Series 640; Beckman Instruments, Fullerton, CA). The total protein concentration of each specimen was measured using the Pierce bicinchoninic acid (BCA) method (Thermo Fisher Scientific Inc., Rockford, IL). Protein concentration was normalized to muscle wet weight. Next, the citrate synthase activity of each specimen was measured as the increase in absorbance from the reduction of 5,5’-dithiobis-2-nitrobenzoic acid by newly formed CoA-SH. The citrate synthase activity of each specimen was expressed as specific activity referenced to total sample wet weight (grams) and total protein (milligrams).
Statistical Analysis
Baseline characteristics between decedents and survivors were compared using the nonparametric Kruskal-Wallis test for continuous variables, and the Fisher exact test for categorical variables. Tertiles of calf muscle characteristics (Protein/muscle weight, citrate synthase/muscle weight, and citrate synthase/protein) were selected, rather than quartiles or pentiles, because the number of events (deaths) was relatively low. Similarly, because of the limited sample size and limited number of events in this study, group comparisons of the Kaplan-Meier curves was chosen as the primary analysis approach, whereas the Cox multivariate regression was our secondary analysis approach. The PROC LIFETEST procedure in SAS was used to perform the primary analyses, whereas the PROC PHREG procedure was used to perform the secondary analyses. In the primary analysis, tertiles from the Kaplan-Meier curves were compared using the Modified Peto test. In the (secondary) Cox regression models, the effects of calf muscle characteristics (Protein/muscle weight, citrate synthase/muscle weight, and citrate synthase/protein) on mortality were adjusted for the covariates age, gender, race, BMI, ankle/brachial index (ABI), smoking status (Yes/No), CAD, hypertension, dyslipidemia, family history of atherosclerotic arterial disease, DM, renal dysfunction, PAD disease stage, and statins use. All statistical analyses were performed using the statistical software SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
Deceased and surviving PAD patients did not differ with regards to age, sex, race, BMI, ABI, statin use, current tobacco use, or presence of: CAD, hypertension, dyslipidemia, renal insufficiency, or family history of atherosclerotic arterial disease. Using univariate analysis (Table 1), surviving patients had statistically different rates of Type II Diabetes Mellitus (p<0.009), frequency of claudication (p<0.0001), and frequency of critical limb ischemia (p<0.0001) compared to decedents. BMI was marginally significant at p=0.12 with decedents having a trend toward lower BMI than survivors.
Table 1 –
Clinical Characteristics and Biochemical Muscle Descriptors
| All (N=187) | Decedents (N=46) | Survivors (N=141) | P-value | |
|---|---|---|---|---|
| Age (years) | 64.18 (8.48) | 65.00 (9.18) | 63.92 (8.26) | 0.70 |
| Male (%) | 97.33 | 100 | 96.45 | 0.34 |
| African American (%) | 7.49 | 8.7 | 7.09 | 0.75 |
| BMI (kg/m2) | 26.89 (6.05) | 25.81 (5.60) | 27.24 (6.17) | 0.12 |
| ABI | 0.51 (0.40) | 0.59 (0.62) | 0.49 (0.29) | 0.97 |
| Current Smoker (%) | 55.61 | 60.87 | 53.9 | 0.49 |
| CAD (%) | 42.78 | 52.17 | 39.72 | 0.17 |
| HTN (%) | 79.14 | 82.61 | 78.01 | 0.68 |
| Dyslipidemia (%) | 63.64 | 63.04 | 63.83 | 0.99 |
| Family History (%) | 29.41 | 28.26 | 29.79 | 0.99 |
| DM (%) | 39.57 | 56.52 | 34.04 | 0.009 |
| Renal Failure (%) | 14.44 | 13.04 | 14.89 | 0.99 |
| Claudicants (%) | 53.16 | 22.5 | 61.33 | <0.0001 |
| CLI (%) | 46.84 | 77.5 | 38.67 | <0.0001 |
| Statin use (%) | 67.91 | 56.52 | 71.63 | 0.069 |
| Protein/muscle wet weight (mg/100 g) | 100.59 (30.87) | 79.17 (23.36) | 107.57(29.84) | 0.0001 |
| Citrate Synthase/ muscle wet weight (units/100 g) | 14.56 (5.92) | 12.22 (4.88) | 15.49 (6.02) | 0.0019 |
| Citrate Synthase/ protein (units/mg) | 149.41(49.16) | 159.89 (57.50) | 145.99 (45.82) | 0.088 |
Mean (SD) or %; P-values from the nonparametric Kruskal-Wallis test or the Fisher Exact test
Kaplan-Meier survival plots of tertiles for protein content normalized over muscle wet weight, citrate synthase content normalized over muscle wet weight, and citrate synthase content normalized over protein are presented in Figures 1, 2, and 3, respectively. Table 2 provides a breakdown of the lowest, middle, and highest tertile values for protein/muscle wet weight, citrate synthase/muscle wet weight, and citrate synthase /protein.
Figure 1 –
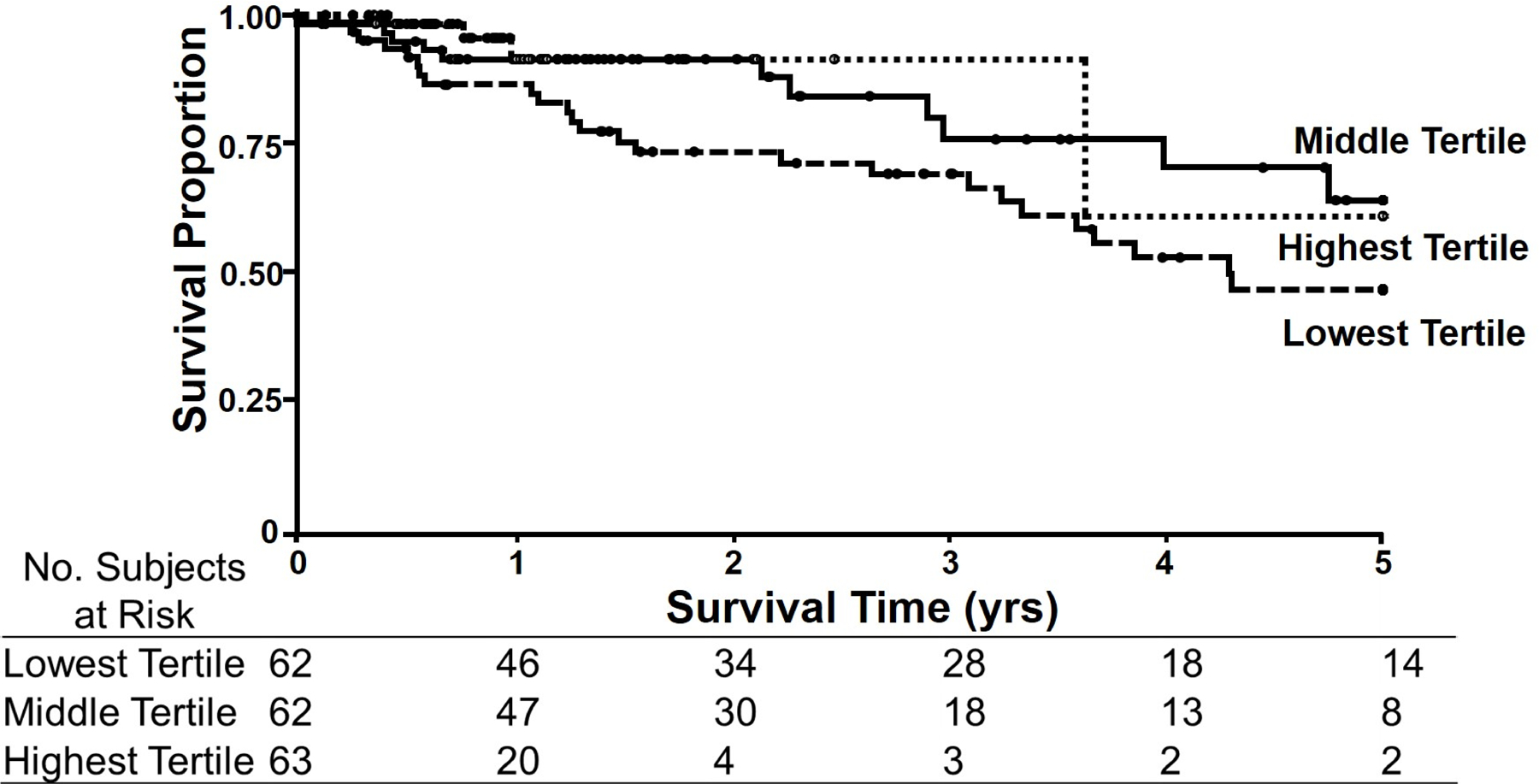
Kaplan-Meier Curves for protein concentration in gastrocnemius biopsies from PAD patients. Protein concentration was determined as protein content (mg) per muscle wet weight (g). Patients in the lowest (dashed line) tertile of protein concentration in their gastrocnemius had the worst survival with 5-year mortality of 57.0%. Patients in either the middle (continuous line) or highest (squared dot line) tertile for protein concentrations in their gastrocnemius had the best survival with 5-year mortality of 34.8% and 37.5%, respectively.
Figure 2 –
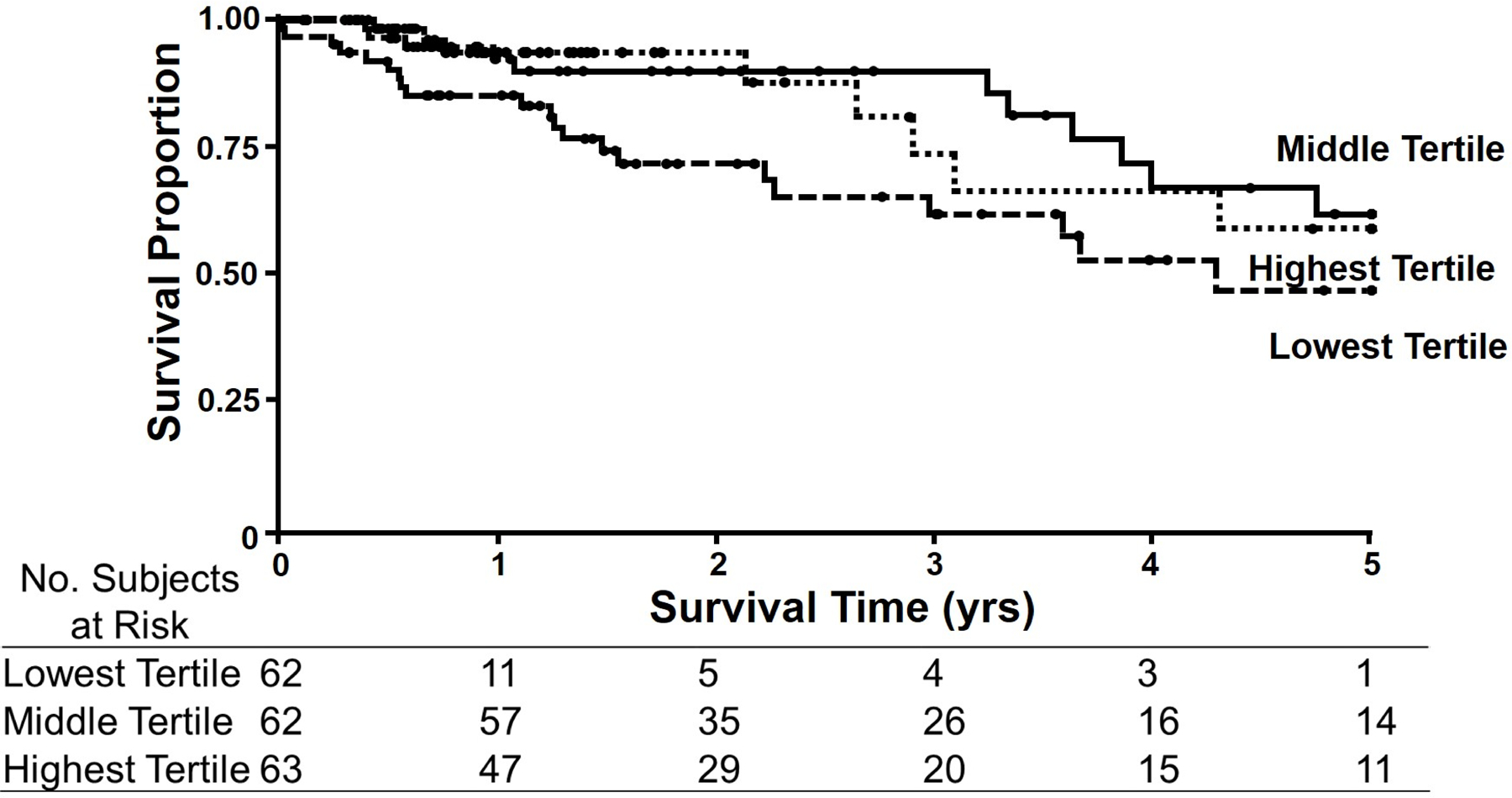
Kaplan-Meier Curves for Citrate Synthase activity normalized to muscle wet weight in gastrocnemius biopsies from PAD patients. Citrate Synthase activity was normalized to muscle wet weight (g). Patients in the middle (continuous line) tertile had the best survival with a 5-year mortality of 33.3%. This was followed by the highest (squared dot line) tertile with a 5-year mortality rate of 44.2%. Mortality at 5 years was highest (50.1%) in those patients in the lowest (dashed line) tertile.
Figure 3 –

Kaplan-Meier Curves for Citrate Synthase activity normalized to protein content in muscle biopsies from PAD patients. Citrate Synthase activity was normalized to protein content (mg). Patients in the middle (continuous line) tertile had the best survival with a 5-year mortality of 23.2%. This was followed by the highest (squared dot line) tertile with a 5-year mortality rate of 44.2%. Mortality at 5 years was highest (64.0%) in those patients in the lowest (dashed line) tertile.
Table 2 –
Tertile Breakdown for Biochemical Muscle Descriptors
| Variable | Tertile | Number of patients | Mean | Standard Deviation | Range |
|---|---|---|---|---|---|
| Protein/muscle wet weight (mg/100 g) | lowest | 62 | 66.7 | 16.2 | (20.7, 86.2) |
| middle | 62 | 100.4 | 8.2 | (87.1, 115.3) | |
| highest | 63 | 134.1 | 15.5 | (116.4, 187.5) | |
| Citrate Synthase/ muscle wet weight (units/100 g) | lowest | 62 | 8.6 | 2.2 | (1.2, 11.4) |
| middle | 62 | 14.2 | 1.6 | (11.5, 16.8) | |
| highest | 63 | 21.2 | 4.3 | (16.8, 34.6) | |
| Citrate Synthase/ protein (units/mg) | lowest | 62 | 98.4 | 18.6 | (38.8, 123.0) |
| middle | 62 | 145.2 | 12 | (123.5, 163.5) | |
| highest | 63 | 204.3 | 33.1 | (164.0, 315.6) |
For protein concentration normalized to muscle weight, the patients in the lowest tertile of protein concentration in their gastrocnemius had the worst survival. At 5 years, the mortality in this group was 57.0%. Patients in either the middle or highest tertile for protein concentrations in their gastrocnemius had the best survival with 5-year mortality of 34.8% and 37.5%, respectively. The lowest tertile was statistically different from both the middle tertile (p=0.032) and the highest tertile (p=0.009) using the modified Peto test (Table 3).
Table 3 –
5 year Mortality
| Lowest tertile | Middle tertile | Highest tertile | P-value1 | |
|---|---|---|---|---|
| Protein/muscle wet weight (mg/100 g) | 57.0%*, ‡ | 34.8%* | 37.5%‡ | * 0.032 ‡ 0.009 |
| Citrate Synthase/ muscle wet weight (units/100 g) | 50.1%* | 33.3%* | 44.2% | * 0.041 |
| Citrate Synthase/ protein (units/mg) | 64.0%* | 23.2%* | 44.2% | * 0.017 |
All comparisons performed using the Modified Peto test
When citrate synthase level was normalized to the wet weight of the muscle sample, the patients with citrate synthase level in the middle tertile had the lowest mortality (33.3%) at 5 years, pointing at a quadratic (or, U-shaped) trend. This was statistically different from the 5 year-mortality of the patient group that had citrate synthase concentration in the lowest tertile, which was 50.1%. At 5 years, these two groups were statistically different using the modified Peto test (p=0.041, Table 3). The 5 year-mortality of the patient group that had citrate synthase concentration in the highest tertile, was also higher (44.2%) than the middle tertile (33.3%) but the difference did not reach statistical significance (p=0.11, Table 3).
Citrate synthase level was also expressed relative to total protein in the muscle sample. The mortality pattern was similar to the citrate synthase/muscle wet weight data, that is, the patients with citrate synthase levels in the middle tertile once again had the best survival (5 year mortality of 23.2%), pointing at a quadratic (or, U-shaped) trend. Conversely, the mortality rate was approximately 64.0% at 5 years for the patients in the lowest tertile, and 44.2% for the patients in the highest tertile (Table 3). The middle and lowest tertiles were again found to be statistically different using the modified Peto test (p=0.017, Table 3).
Finally, in our secondary analyses, all parameters were put into a multivariate Cox regression model, controlling for all the demographics described previously in Table 1. Three clinical parameters were predictive of mortality in our model: BMI (Hazard Ratio, HR = 0.92; p = 0.005), presence of DM (HR = 2.50; p = 0.017), and presence of CAD (HR = 2.08; p = 0.035). The rest of the parameters including age, sex, race, ABI, statin use, current tobacco use, or presence of: hypertension, dyslipidemia, renal insufficiency, or family history of atherosclerotic arterial disease, clinical presentation of PAD (claudication versus CLI) and use of statin were not predictive of mortality in our cohort. Protein concentration normalized to muscle weight, and citrate synthase normalized to protein concentration were the two biochemical parameters which reached statistical significance in this model. For protein concentration, hazard ratios for the lowest, middle, and highest (reference) tertiles were, respectively, 2.93, 1.07, and 1.00. The lowest tertile of protein concentration had the highest likelihood of death and was statistically different from the middle tertile (p=0.008, Table 4). For citrate synthase normalized to protein concentration, hazard ratios for the lowest, middle (reference), and highest tertiles were, respectively, 4.96, 1.00, and 1.98. The middle tertile compared to the lowest tertile was significantly different at p=0.003 (Table 4). Similarly, the highest tertile was statistically different compared to the lowest tertile at p=0.027 (Table 4). Our data demonstrate that, over the 5-year follow-up, PAD patients that are in the middle tertile for mitochondrial density in their gastrocnemius are almost five times more likely to survive than those patients in the lowest tertile and two times more likely to survive than those patients in the highest tertile.
Table 4 –
Cox Regression Model Analysis
| Lowest tertile HR | Middle tertile HR | Highest tertile HR | P-value | |
|---|---|---|---|---|
| Protein/muscle wet weight (mg/100 g) | 2.93*, ‡ | 1.07* | 1.00 ‡ (Reference) | * 0.008 ‡ 0.075 |
| Citrate Synthase/ protein (units/mg) | 4.68*, ‡ | 1.00* (Reference) | 1.98‡ | * 0.0033 ‡ 0.0270 |
HR=Hazard Ratio;
Discussion
Previous studies from our and other laboratories have established that a myopathy is present in the ischemic lower limbs of patients with PAD and it is associated with a variety of molecular and cellular abnormalities including myofiber degeneration, oxidative stress, mitochondrial dysfunction, and inflammation 9–11, 13–17. In this study we chose to evaluate two basic markers of skeletal muscle biochemistry; protein concentration and mitochondrial content (citrate synthase) as possible predictors of mortality in PAD patients. Our data demonstrate that both protein concentration and mitochondrial content in the gastrocnemius of PAD patients correlate with mortality rate. PAD patients with relatively low protein concentration in their gastrocnemius have the highest mortality. Furthermore, mortality is lowest for patients in the middle tertile of citrate synthase activity when normalized to both muscle wet weight and protein concentration.
Several studies have correlated clinical parameters in a PAD patient’s history and clinical presentation with mortality. Such parameters include the presence of CLI, Diabetes Mellitus, hypertension, renal dysfunction and abnormal ABI 3, 5, 18–20. These studies were completed with cohorts of several hundred to thousands of patients. In our study, using a multivariate Cox regression model, the clinical parameters that were found to be predictive of mortality were BMI, presence of DM and presence of CAD. Presence of CLI, hypertension, renal dysfunction and abnormal ABI were not statistically significant predictors in the multivariate Cox model, however, these parameters may achieve significance in a similar study with a larger sample size. Given the sample size limitations, our findings demonstrate the quality and clinical relevance of basic biochemical indices (protein concentration and mitochondrial density) in the ischemic gastrocnemius as predictors of mortality in PAD patients. Serum biomarkers such as highly sensitive C-reactive protein, serum amyloid A, and D-dimer have also been found to correlate with mortality in PAD patients 5, 6. However, no mortality association was found with other serum markers including homocysteine, lipoprotein-a, N-terminal pro-B-type natriuretic peptide, platelet activating factor, and lipoprotein-associated phospholipase A2 5–8. This is the first study to correlate skeletal muscle biochemical characteristics, specifically protein concentration and citrate synthase amount, with mortality. Our findings are in agreement with those of McDermott et al. In their study 12, they investigated the relationship between calf muscle density, area and percent fat (measured by Computerized Tomographic scanning), as well as knee extension power, knee extension isometric strength and plantar flexion isometric strength with mortality in PAD patients. All measurements were performed in the legs of 434 participants with PAD, defined in this particular study as individuals with an ABI < 0.90. After adjusting for co-variates, lower calf muscle density and weaker lower plantar flexion strength and knee extension power were associated with higher all-cause mortality hazard ratios12. The authors concluded that some of these calf muscle markers may represent the burden of atherosclerotic disease and its effect on the end organ, the muscle, and the overall health of the PAD patient.
There is a lack of studies reporting skeletal muscle protein concentration in healthy adults and the range of normal has not been established 21. Our data demonstrate a range of skeletal muscle protein concentration exists in patients with PAD and that its level can predict mortality rate in this patient population. Specifically, in our cohort, PAD patients with the lowest protein concentration in their gastrocnemius had more than 50% mortality. As humans age the protein synthesis and protein concentration in their skeletal muscle, as well as their muscle mass and muscle function decrease, a phenomenon known as sarcopenia 22, 23. Some patients have a greater degree of sarcopenia than others, and this reflects and/or leads to greater likelihood of functional impairment and disability 23, 24. Similar findings connecting poor calf skeletal muscle characteristics with decreased physical activity and functional decline have been demonstrated by McDermott and colleagues for patients with PAD 25–27. Furthermore, a number of studies have recently demonstrated that sarcopenia is also associated with mortality rates. A recent study by the European Working Group on Sarcopenia in Older People, the ilSIRENTE trial, identified an increased mortality rate in elderly patients (age >80) with sarcopenia compared to those without, even after adjusting for possible confounders 28. This study defined patients with sarcopenia as those with a gait speed of less than 0.8 m/s or low grip strength (<30kg in men and <20kg in women) plus mid-arm muscle circumference in the lower one third of all subjects 28. Using this same definition, Arango-Lopera et al. confirmed higher mortality in a separate group of elderly patients with sarcopenia 29. A different study used dual energy X-ray absorptiometry to determine muscle and fat mass in elderly individuals as an objective measurement of sarcopenia 30. Low amount of appendicular (limb) fat-free mass, one of the sarcopenia markers in this study, was associated with increased mortality 30. Finally, a study evaluating the correlation between body composition and all-cause mortality demonstrated that higher lean body mass, examined using bioelectrical impedance analyses, is predictive of improved survival 31. PAD is a disease of the elderly and the addition of ischemic myopathy to the sarcopenia of aging likely leads to greater reduction of skeletal muscle mass and function. We speculate, therefore, that low protein concentration in PAD calf muscles may reflect the progressive decline of the cardiovascular and neuromuscular systems of the PAD patient and it likely is one of the reasons why the patients with lowest protein in their calf muscle are at an increased likelihood of death.
Based on our findings, citrate synthase can also be used to predict mortality. As discussed with protein concentration, the normal range for citrate synthase activity has not been established 32, 33. Citrate synthase is a mitochondrial matrix enzyme that catalyzes the first step of the Krebs cycle (condensation of oxaloacetate and the acetyl group of acetyl coenzyme-A) and is the most commonly used marker of mitochondrial content in cells 10. PAD patients in the mid-range of mitochondrial content have greater survival than those outside of this range. Based on our findings we speculate that in PAD patients, the extremes of mitochondrial content reflect a decline in the functional capacity of the mitochondrial energy-providing system, deterioration of the limb, and overall health of the PAD patient. It is possible that increasing mitochondrial content is the product of a switch in the phenotype of ischemic myofibers from fast-twitch, type II myofibers (low mitochondrial content) to slow-twitch, type I myofibers (high in mitochondrial content). This phenotype switch has been previously demonstrated by several groups and appears to be a marker of ischemic damage on the PAD muscle34–37. Another possibility is that the abnormally increased mitochondrial content reflects accumulation of dysfunctional organelles 15, 38 possibly secondary to increased mitochondrial damage and impaired mitochondrial catabolism in the ischemic muscle. On the other hand, decreasing mitochondrial content in PAD muscle may also represent degeneration of the mitochondrial population 39 which may be the result of a combination of a decrease in mitochondrial biogenesis 40, increase in mitophagy of damaged organelles and the overall decrease of the proteome 41 of some PAD patients. In both cases the dysfunctional organelles have a reduced capacity for supporting the needs of muscle for energy, an increased susceptibility to apoptosis, and an increase in generation of reactive oxygen species 10, 11, 15, 42, 43.
This is a descriptive study and therefore its principal limitation is that it cannot identify cause and effect linkages between gastrocnemius protein or mitochondrial content and mortality in PAD patients. Instead, the study demonstrated that the two markers are predictors of mortality in PAD patients, and consequently, identified the myopathy of PAD as a potential correlate to the overall health state of patients with PAD. Furthermore, our study population is fairly homogeneous, composed of primarily Caucasian males. This relative homogeneity may limit the generalizability of our findings to other populations. Additionally, the duration these patients were followed was relatively short for survival analysis, with a mean follow-up of 23.0 months. We are continuing to follow these patients to strengthen our results and increase our duration of follow-up. In this study we chose to evaluate two basic markers of skeletal muscle biochemistry; protein concentration and mitochondrial content (citrate synthase activity) as possible predictors of mortality in PAD patients. In the future more advanced markers reflecting the complexity of adaptation and damage suffered by the ischemic muscle proteome (such as levels of regulatory proteins, contractile elements, complexes of the electron transport chain, oxidatively damaged proteins, inflammatory biomarkers and cellular stress proteins) can be also evaluated as potential predictors of morbidity and mortality in this patient population. Studies of muscle from PAD limbs are crucial for our understanding of PAD pathophysiology but are difficult to be generalized mainly due to the need for obtaining a muscle biopsy. In the future, the value of surrogate markers of muscle damage such as urinary creatinine, dual-energy X-ray absorptiometry scans, as well as Computerized Tomographic and Magnetic resonance scans of the lower extremities may play a role in the measurement of skeletal muscle deterioration in PAD patients. Alterations in the neuromuscular system of the lower limbs are of central importance in the pathogenesis of PAD and future work will be critical for improving our knowledge of the mechanisms operating in the legs of PAD patients and their effects on the overall health of the patient with PAD.
Conclusions
Myopathy is a recognized component of PAD pathogenesis. Our data demonstrate that protein and mitochondrial content, two basic biochemical parameters of this myopathy, are predictors of mortality in PAD patients. Future studies are needed to determine whether interventions such as exercise therapy, revascularization, medications with neural and muscular trophic effects and medications that can optimize mitochondrial function can treat the biochemical abnormalities in PAD limbs and improve the overall health and lifespan of the patient with PAD.
Funding Sources
This work was supported by NIH grants K08HL079967 and R01AG034995 and by a W.J. von Liebig Award from the American Vascular Association and by the Charles and Mary Heider Fund for Excellence in Vascular Surgery. Furthermore, this material is the result of work supported with resources and the use of facilities at the VA Nebraska-Western Iowa Health Care System.
Footnotes
Disclosures
NONE
References
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45 Suppl S:S5–67. [DOI] [PubMed] [Google Scholar]
- 2.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001; 344:1608–21. [DOI] [PubMed] [Google Scholar]
- 3.Lee AJ, Price JF, Russell MJ, et al. Improved prediction of fatal myocardial infarction using the ankle brachial index in addition to conventional risk factors: the Edinburgh Artery Study. Circulation 2004; 110:3075–80. [DOI] [PubMed] [Google Scholar]
- 4.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004; 109:733–9. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Ho LA, Denenberg JO, et al. Biomarkers in peripheral arterial disease patients and near- and longer-term mortality. J Vasc Surg 2010; 52:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidula H, Tian L, Liu K, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med 2008; 148:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shadman R, Allison MA, Criqui MH. Glomerular filtration rate and N-terminal pro-brain natriuretic peptide as predictors of cardiovascular mortality in vascular patients. J Am Coll Cardiol 2007; 49:2172–81. [DOI] [PubMed] [Google Scholar]
- 8.Allison MA, Denenberg JO, Nelson JJ, et al. The association between lipoprotein-associated phospholipase A2 and cardiovascular disease and total mortality in vascular medicine patients. J Vasc Surg 2007; 46:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makris KI, Nella AA, Zhu Z, et al. Mitochondriopathy of peripheral arterial disease. Vascular 2007; 15:336–43. [DOI] [PubMed] [Google Scholar]
- 10.Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg 2007; 41:481–9. [DOI] [PubMed] [Google Scholar]
- 11.Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg 2008; 42:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott MM, Liu K, Tian L, et al. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. J Am Coll Cardiol 2012; 59:1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Signorelli SS, Mazzarino MC, Spandidos DA, et al. Proinflammatory circulating molecules in peripheral arterial disease. Int J Mol Med 2007; 20:279–86. [PubMed] [Google Scholar]
- 14.Marbini A, Gemignani F, Scoditti U, et al. Abnormal muscle mitochondria in ischemic claudication. Acta Neurol Belg 1986; 86:304–10. [PubMed] [Google Scholar]
- 15.Kemp GJ. Mitochondrial dysfunction in chronic ischemia and peripheral vascular disease. Mitochondrion 2004; 4:629–40. [DOI] [PubMed] [Google Scholar]
- 16.Brevetti G, Giugliano G, Brevetti L, et al. Inflammation in peripheral artery disease. Circulation 2010; 122:1862–75. [DOI] [PubMed] [Google Scholar]
- 17.Tisi PV, Shearman CP. Biochemical and inflammatory changes in the exercising claudicant. Vasc Med 1998; 3:189–98. [DOI] [PubMed] [Google Scholar]
- 18.Fowkes FG, Housley E, Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991; 20:384–92. [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326:381–6. [DOI] [PubMed] [Google Scholar]
- 20.Haus JM, Carrithers JA, Trappe SW, et al. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 2007; 103:2068–76. [DOI] [PubMed] [Google Scholar]
- 21.Trappe S, Gallagher P, Harber M, et al. Single muscle fibre contractile properties in young and old men and women. J Physiol 2003; 552:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malafarina V, Uriz-Otano F, Iniesta R, et al. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas 2012; 71:109–14. [DOI] [PubMed] [Google Scholar]
- 23.Evans NS, Liu K, Criqui MH, et al. Associations of calf skeletal muscle characteristics and peripheral nerve function with self-perceived physical functioning and walking ability in persons with peripheral artery disease. Vasc Med 2011; 16:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MM, Ferrucci L, Guralnik J, et al. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation 2009; 120:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raval Z, Liu K, Tian L, et al. Higher body mass index is associated with more adverse changes in calf muscle characteristics in peripheral arterial disease. J Vasc Surg 2012; 55:1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Guralnik JM, Ferrucci L, et al. Physical activity, walking exercise, and calf skeletal muscle characteristics in patients with peripheral arterial disease. J Vasc Surg 2007; 46:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013. [DOI] [PubMed] [Google Scholar]
- 28.Arango-Lopera VE, Arroyo P, Gutierrez-Robledo LM, et al. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging 2013; 17:259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunout D, de la Maza MP, Barrera G, et al. Association between sarcopenia and mortality in healthy older people. Australas J Ageing 2011; 30:89–92. [DOI] [PubMed] [Google Scholar]
- 30.Han SS, Kim KW, Kim KI, et al. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. J Am Geriatr Soc 2010; 58:312–7. [DOI] [PubMed] [Google Scholar]
- 31.Pipinos II, Judge AR, Zhu Z, et al. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med 2006; 41:262–9. [DOI] [PubMed] [Google Scholar]
- 32.Bhat HK, Hiatt WR, Hoppel CL, et al. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation 1999; 99:807–12. [DOI] [PubMed] [Google Scholar]
- 33.Hedberg B, Angquist KA, Henriksson-Larsen K, et al. Fibre loss and distribution in skeletal muscle from patients with severe peripheral arterial insufficiency. Eur J Vasc Surg 1989; 3:315–22. [DOI] [PubMed] [Google Scholar]
- 34.Hedberg B, Angquist KA, Sjostrom M. Peripheral arterial insufficiency and the fine structure of the gastrocnemius muscle. Int Angiol 1988; 7:50–9. [PubMed] [Google Scholar]
- 35.Regensteiner JG, Hargarten ME, Rutherford RB, et al. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology 1993; 44:1–10. [DOI] [PubMed] [Google Scholar]
- 36.Steinacker JM, Opitz-Gress A, Baur S, et al. Expression of myosin heavy chain isoforms in skeletal muscle of patients with peripheral arterial occlusive disease. J Vasc Surg 2000; 31:443–9. [PubMed] [Google Scholar]
- 37.Dirks AJ, Hofer T, Marzetti E, et al. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 2006; 5:179–95. [DOI] [PubMed] [Google Scholar]
- 38.van Bilsen M, Smeets PJ, Gilde AJ, et al. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res 2004; 61:218–26. [DOI] [PubMed] [Google Scholar]
- 39.Sheng B, Wang X, Su B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 2012; 120:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang JH, Hood DA. Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life 2009; 61:201–14. [DOI] [PubMed] [Google Scholar]
- 41.Chabi B, Ljubicic V, Menzies KJ, et al. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 2008; 7:2–12. [DOI] [PubMed] [Google Scholar]
- 42.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 2000; 526:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


