Abstract
A vivid perception of the moving form of a human figure can be obtained from a few moving light points on the joints of the body. This is known as biological motion perception. It is commonly believed that the perception of biological motion rests on image motion signals. Curiously, however, some patients with lesions to motion processing areas of the dorsal stream are severely impaired in image motion perception but can easily perceive biological motion. Here we describe a biological motion stimulus based on a limited lifetime technique that tests the perception of a moving human figure in the absence of local image motion. We find that subjects can spontaneously recognize a moving human figure in displays without local image motion. Their performance is very similar to that for classic point-light displays. We also find that tasks involving the discrimination of walking direction or the coherence of a walking figure can be performed in the absence of image motion. Thus, although image motion may generally aid processes such as segmenting figure from background, we propose that it is not the basis for the percept of biological motion. Rather, we suggest biological motion is derived from dynamic form information on body posture evolving over time.
The phenomenon of biological motion perception was demonstrated by Johannson (1). He showed that a dozen moving light points, attached to the joints of the body, suffices to create a rich perception of a moving human figure. Biological motion is a highly complex motion pattern and an extreme example of the sophistication of pattern analysis in the brain. It is also interesting because it links the perception of motion with the perception of form, two qualities that involve largely different cortical processing streams (2). Form analysis is carried out in the ventral stream whereas image motion is processed in areas of the dorsal stream. Selectivity for biological motion has been found in the superior temporal polysensory area (3, 4), which receives input from both processing streams. Patients with lesions to motion processing areas of the dorsal stream are severely impaired in image motion perception but can easily perceive biological motion (5, 6). This finding could suggest that biological motion perception does not rely on the analysis of image motion signals. Here, we test this hypothesis by using a stimulus in which we manipulate the amount of local image motion that is consistent with movement of the limbs.
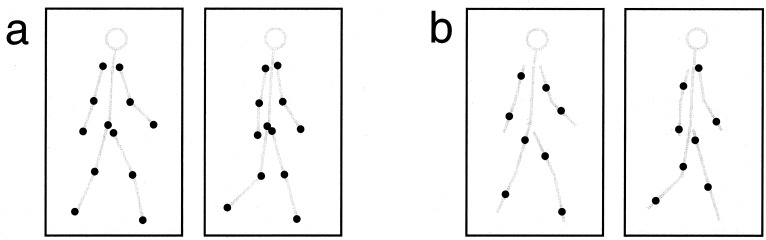
Standard biological motion stimuli (Fig. 1a and Movie 1, which is available as supporting information on the PNAS web site, www.pnas.org) consist of a frame animation of the motion of light points attached to the joints of a moving human figure (7). The moving pattern of dots in this case contains information about the position of points on the body and about the motion of these points over time. The motion signal is carried by the apparent image motion of each individual point in two successive animation frames. The stimuli we created dissociate these two sources of information. Eight light points were positioned not on the joints, but rather on the limbs at a random position between joints (Fig. 1b and Movie 2, which is available as supporting information on the PNAS web site). In every frame of the animation, each point was reallocated to another randomly selected position on the limb with every position on the line segment between the two joints having equal probability. Therefore, no individual point carried the valid image motion signal of the limb movement. But as each animation frame displays a random-dot sampling of a static posture of the body, the sequence of these static postures also carries information about the form and motion of the body. We therefore call this stimulus the sequential position walker.
Figure 1.
Standard biological motion stimuli (a) consist of a frame animation of the motion of light points attached to the joints of a moving human figure (7). In our sequential position stimulus (b) light points were positioned anywhere on the limbs and jumped to another randomly selected position for each frame.
The experiments we performed compare human perception of biological motion with and without consistent image motion signals. In the first set of experiments we tested spontaneous recognition of a moving human figure by naive observers. In the second set of experiments, we tested performance in a number of discrimination tasks.
Materials and Methods
Recognition Experiments.
Stimuli were presented on a 76-Hz monitor display and viewed binocularly from 45 cm distance. Each image consisted of eight bright dots (0.2°) projected onto a dark background. Dot positions were derived from the joint positions of a human figure walking on a treadmill (7) at 0.625 cycles/s. The duration of each animation frame in the sequence was 52 ms. The walker subtended 5 by 11° of visual angle and was centered in the middle of the screen. The starting phase in the step cycle was randomized from trial to trial. For comparison, we afterward also presented a classic walker with dots continuously presented on the joints. It simulated the same movement by a sequence of 40 frames per cycle as in ref. 7.
The method of reallocating dots after each frame occasionally might make points appear to jump to a new location rather than to disappear and reappear, thus leading to spurious motion signals. These spurious motion vectors are unlikely to provide valid motion signals for perception of biological motion. We analyzed the distribution of jump vectors that connect nearest points in successive frames. Comparison of their distribution to that of the true motion vectors that would be expected if points remained at their limb position showed that only 1% of the jumps remained within 10% range (Weber's fraction) of the true motion vector. Thus, these jump vectors can be considered as noise to motion detectors.
In the recognition experiments, subjects were asked to verbally report their percept. The stimulus was presented as long as the subjects needed to feel sure about their answer. Each subject saw the sequential position walker only once and always before the display of the classic walker. Afterward, we asked them about prior experience with point-light walker displays and confirmed that they had not seen this stimulus before. We collected data from 91 naive subjects, 17–51 years of age.
Discrimination Experiments.
A parametric analysis on the perception of the sequential position walker was out carried in three additional experiments.
The first experiment tested discrimination ability in two tasks. In the direction task, subjects discriminated between a leftward or rightward walking stimulus. In the coherence task, subjects discriminated between a walking figure whose upper and lower body was facing and walking in the same (coherent) or in opposite directions (incoherent). For each task, both alternatives were presented with equal probability and repeated 20 times. The dots disappeared after 1-s stimulus presentation, whereupon subjects had to indicate their choice. Before each trial, a central white dot was shown for 0.5 s. Six subjects, including the authors, participated.
In the second experiment, lifetime and number of dots was varied in the direction discrimination task. Each frame contained one, two, four, or eight dots. Each dot stayed at one limb position for one, two, four, or eight frames before it was reallocated. Initial values were chosen at random to prevent a simultaneous refresh of all dots. Instead of reallocating a dot on the same limb, the range of possible new positions was spread out evenly over the four limb segments. This process reduced response variation across trials in case of few visible dots, because some joints (i.e., the ankles) are known to carry more useful information than others (8). Each condition was repeated 10 times. Stimulus duration was 1.6 s (one complete step cycle). Three subjects, including the authors, participated.
In the third experiment, the influence of lifetime was tested in the presence of background noise. Noise consisted of dots that were reallocated after each frame at a random position within a window of 10 by 12.5° centered on the eight-point walker. Using a one-up, two-down staircase, with stimuli presented interleaved, the number of noise dots at which subjects discriminated direction 70.7% correct was determined. Stimulus duration was 1.6 s (one complete step cycle). Three subjects, including the authors, participated.
Results and Discussion
Recognition of Sequential Position Walker vs. Classic Walker.
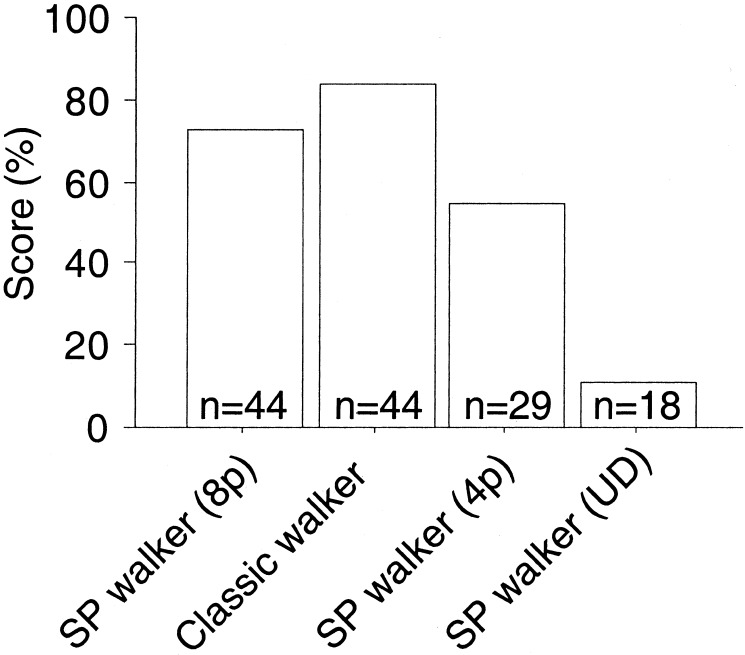
A single still image from the biological motion sequence is not sufficient to induce the percept of a human figure. But a sequence of frames presented in succession gave the clear impression of a walking person that appeared to carry a flickering arrangement of dots on the body. We showed this stimulus to 44 uninformed observers, asking them to verbally describe what they saw. As Fig. 2 illustrates, 73% spontaneously described the stimulus as a walking person. When the classic biological motion stimulus was shown to the same subjects afterward, 84% recognized it. Thus, although the sequential position stimulus contained fewer dots in each frame than the classic stimulus, and although biological motion stimuli in which dots are placed on the limbs rather than on the joints are somewhat harder to recognize (9), almost as many subjects recognized the walking figure in the sequential position stimulus as those in the classic case. Because residual motion may be present in the sequential positional walker if points revisit the same location on the limb again, we also presented a combination in which the number of points per frame was reduced to four and each point was reallocated to a different limb in each frame. Still, more than half of all observers (55% of 29 additional subjects) were able to recognize the walker. Furthermore, we found that when the sequential position stimulus was presented upside-down, almost all (16 of 18 subjects) failed to recognize a walking figure, an effect specific to biological motion perception found for classic point-light displays as well. These findings suggest that biological motion perception does not need local image motion signals.
Figure 2.
Percentage of subjects that recognized a walking human figure from the classic point-light walker, the sequential position (SP) walker (8p), the sequential position walker with four points per frame (4p), and an upside-down display of the sequential position walker (UD). n indicates the number of subjects for that experiment.
Discrimination Performance with Sequential Position Stimuli.
With our stimulus, we also investigated properties of biological motion perception by using two-alternative forched choice discrimination tasks. We found that subjects were able to determine the figure's walking direction 100% correctly for 1-s stimuli. Furthermore, in displays in which upper and lower body presented walking in opposite directions (8), subjects were able to distinguish a veridical walker from an incoherent walker (82% correct).
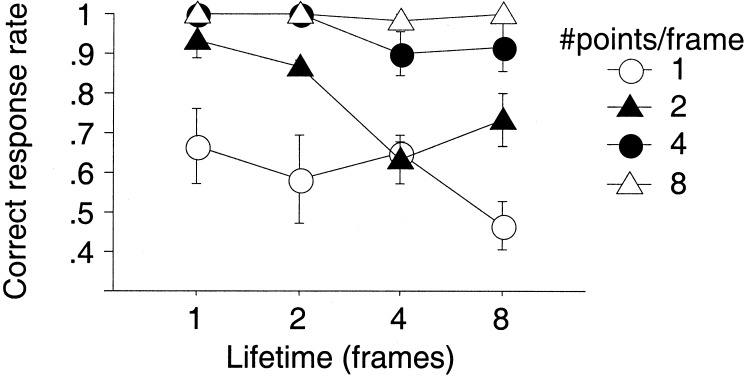
Having shown that several of the classic properties of biological motion perception can be reproduced with the sequential position walker, we analyzed the contribution of motion signals more systematically. To this end, we varied the lifetime of the individual dots for different numbers of visible dots. With prolonged lifetime, each dot remains on the same body position for more frames and therefore will carry more valid image motion information. If image motion signals contribute to biological motion perception, we would expect performance to increase. As illustrated by Fig. 3, however, longer lifetimes did not improve subjects' discrimination of walking direction for any number of visible dots. Rather, we observed a slight decrease in the correct response rate. A possible explanation for this drop in performance might be a loss of form information, because together with prolonged lifetime fewer dots are reallocated, resulting in less of the body being drawn out over time. This explanation, and the fact that performance strongly increases with number of dots (see also ref. 10) would suggest that form analysis plays a dominant role in the perception of biological motion. These findings corroborate the previous results from the spontaneous recognition experiment, suggesting that biological motion perception does not rely on local image motion.
Figure 3.
Rate of correctly discriminated walking direction as function of lifetime (in 52-ms long increments) for different number of stimulus points. Data are collapsed over walking direction and represent the average over three subjects. Error bars are ± 1 SE.
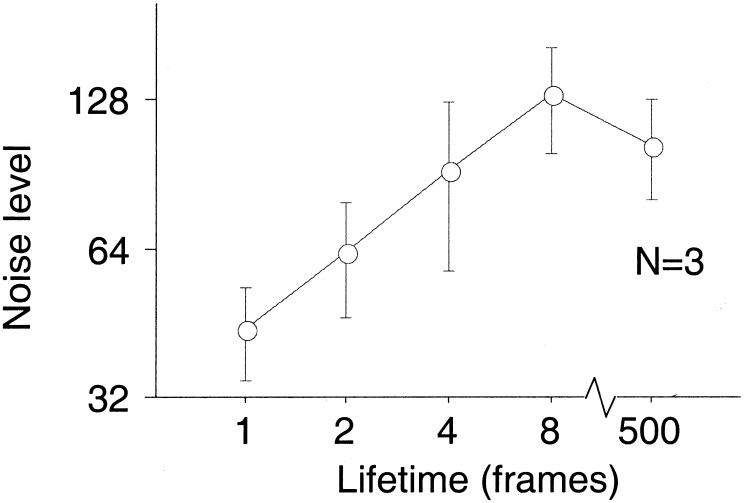
Do image motion signals contribute to biological motion perception as well, even though they are not required? Given the flickering arrangement of the sequential position walker, we expected that image motion might aid the perception of biological motion in the presence of dynamic noise. In a further experiment, we therefore investigated direction discrimination in the presence of randomly distributed noise dots. There was a clear effect of lifetime on the tolerance to the superimposed noise (Fig. 4). This result shows that motion signals become available at longer lifetimes and contribute to performance in this task.
Figure 4.
Noise tolerance as function of lifetime for direction discrimination of the sequential position walker. Noise tolerance is expressed as the number of noise dots at which 70.7% correct is reached. Data are collapsed over walking direction and represent the average over 3 subjects. Error bars are ± 1 SE.
In summary, the discrimination experiments allow three conclusions. First, subjects are able to perform classic point-light tasks with the sequential position stimulus. Second, performance in general does not improve with increased image motion signal. Third, performance in the presence of background noise benefits from the availability of motion signals. We suggest that the role of motion signals in the presence of noise lies mainly in a better segregation of stimulus points from the background. This would also explain results in support of low-level motion contributions obtained in previous experiments using background noise (8, 15). A role for motion mainly in segregation is also consistent with the observation that the “motion-blind” patient LM loses her ability to see biological motion as soon as the stimulus is embedded in noise (5).
Motion-from-Form Instead of Form-from-Motion.
We have shown that biological motion perception is possible from stimuli that contain only sequential position cues rather than motion signals from the joints. Traditional accounts of biological motion perception (1, 8, 9) as well as many computational models (refs. 11–13, but see also ref. 14 for a different view) have put the emphasis on the analysis of motion signals. Motion-based models should be severely impaired with the sequential position stimulus. We propose that biological motion perception may instead progress by an analysis of sequential posture information, obtained from position signals of points on the body. This might be accomplished by dynamic form templates that accumulate the evidence for human form over time, while allowing for a dynamic change in the shape of the body. Such a mechanism would involve primarily form analysis.
Supplementary Material
Acknowledgments
We offer special thanks to Karsten Georg for collecting data. This work was supported by Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie Biofuture Price.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Johansson G. Percep Psychophys. 1973;14:201–211. [Google Scholar]
- 2.Ungerleider L G, Mishkin M. In: The Analysis of Visual Behavior (Visual System) Ingle D J, Goodale M A, Mansfield R J, editors. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- 3.Oram M W, Perrett D I. J Cognit Neurosci. 1994;6:99–116. doi: 10.1162/jocn.1994.6.2.99. [DOI] [PubMed] [Google Scholar]
- 4.Grossman E D, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. J Cognit Neurosci. 2000;12:711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- 5.Vaina L, Lemay M, Bienfang D C, Choi A Y, Nakayama K. Visual Neurosci. 1990;5:353–369. doi: 10.1017/s0952523800000444. [DOI] [PubMed] [Google Scholar]
- 6.McLeod P, Dittrich W, Driver J, Perret D, Zihl J. Vis Cognit. 1996;3:363–391. [Google Scholar]
- 7.Cutting J E. Behav Res Methods Instrumentation. 1978;10:91–94. [Google Scholar]
- 8.Mather G, Radford K, West S. Philos Trans R Soc London B. 1992;249:149–155. doi: 10.1098/rspb.1992.0097. [DOI] [PubMed] [Google Scholar]
- 9.Cutting J E. J Exp Psychol Hum Percept Perform. 1981;7:71–87. [Google Scholar]
- 10.Neri P, Morrone M C, Burr D C. Nature (London) 1998;395:894–896. doi: 10.1038/27661. [DOI] [PubMed] [Google Scholar]
- 11.Rashid R F. IEEE Trans Patt Anal Mach Intell. 1980;6:574–581. [Google Scholar]
- 12.Hoffman D D, Flinchbaugh B E. Biol Cybern. 1982;42:195–204. doi: 10.1007/BF00340076. [DOI] [PubMed] [Google Scholar]
- 13.Webb J A, Aggarwal J K. Comp Vis Image Understand. 1982;19:107–130. [Google Scholar]
- 14.Giese M A, Poggio T. Int J Comput Vision. 2000;38:59–73. [Google Scholar]
- 15.Thornton I, Pinto J, Shiffrar M. Cognit Neuropsychol. 1998;15:535–552. doi: 10.1080/026432998381014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.