Abstract
Despite the importance of aggression in the behavioral repertoire of most animals, relatively little is known of its proximate causation and control. To take advantage of modern methods of genetic analysis for studying this complex behavior, we have developed a quantitative framework for studying aggression in common laboratory strains of the fruit fly, Drosophila melanogaster. In the present study we analyze 73 experiments in which socially naive male fruit flies interacted in more than 2,000 individual agonistic interactions. This allows us to (i) generate an ethogram of the behaviors that occur during agonistic interactions; (ii) calculate descriptive statistics for these behaviors; and (iii) identify their temporal patterns by using sequence analysis. Thirty-minute paired trials between flies contained an average of 27 individual agonistic interactions, lasting a mean of 11 seconds and featuring a variety of intensity levels. Only few fights progressed to the highest intensity levels (boxing and tussling). A sequential analysis demonstrated the existence of recurrent patterns in behaviors with some similarity to those seen during courtship. Based on the patterns characterized in the present report, a detailed examination of aggressive behavior by using mutant strains and other techniques of genetic analysis becomes possible.
Aggression serves in the acquisition or defense of vital resources such as food, shelter, or access to mates in species ranging from sea anemones to humans (for reviews, see refs. 1 and 2). Despite its importance, relatively little is known of the neural and humoral mechanisms that are its proximate causes. Given the near ubiquity of aggression, it is not surprising to find aggression and territorial behavior in fruit flies (3–10), particularly among a selection of Hawaiian taxa (4, 5, 8) that have proven difficult to study in the laboratory. The existence of these behaviors in common strains of Drosophila melanogaster is not widely known, however. With powerful genetic and molecular methods available to explore fundamental biological processes, this system offers unique opportunities for the study of the structure, assembly, activation, and impact on the nervous system of a complex behavior like aggression.
In 1915, Sturtevant (3) reported on sexual selection in Drosophila and commented on the occurrence of fighting behavior. In addressing situations in which two males are courting the same female, Sturtevant wrote “in such cases they [males] may sometimes be seen to spread their wings, run at each other, and apparently butt heads. One of them soon gives up and runs away. If the other then runs at him again within the next few minutes he usually makes off without showing fight.” The latter comment suggests the existence of long-term effects resulting from a loss, but our studies thus far do not demonstrate any long-term effects.
In a study examining the effects of light on mating of ebony and light strains of D. melanogaster, Jacobs (6) reported that male flies showed what he termed “territorial behavior.” This behavior included males “charging” others, often with their wings held up and back. The charges ending up in “tussling,” in which the advancing animal pounces from any angle and tugs the wings and body of the other animal. Subsequently, Dow and von Schilcher (9) placed marked male and female flies together in a competitive situation, and demonstrated that most interactions between the flies were either aggressive or sexual in nature. The aggressive behavioral patterns these investigators reported included “wing threat” followed by quick charges, and “boxing” as dominance relationships formed among groups of male flies. The most complete studies of fighting and territorial behavior in common Drosophila species (D. melanogaster, Drosophila simulans), however, were reported by Hoffmann in 1987 (10). Following up on earlier studies (9), and using an experimental protocol that included six male flies and three mated females, the set of components that made up fighting behavior were defined, the proportions of time flies showed the different patterns were measured, and factors that influenced the outcome of fights in both species were identified (10).
The earlier studies, although important in demonstrating that D. melanogaster fight, involved complicated experimental protocols wherein multiple animals were present in the arena over an extended period of observation. Moreover, each fly in the chamber underwent different kinds of social experiences during the course of an experimental trial. Before beginning studies in which the effects of genetic manipulations on the behavior could be explored, therefore, we felt it necessary to reduce the complexity of the experimental situation by (i) establishing conditions under which only two male flies would fight; and (ii) developing a quantitative framework for measuring the behavior. Ideally, the experimental situation described here allows us to compare fighting among mutant and control animals, to determine whether incidences or characteristics of fighting behavior are altered by any genetic manipulation we perform. A quantitative analysis, similar to the one presented here, has been carried out for mating behavior in fruit flies (11).
Materials and Methods
Several variables were explored in an attempt to attract two male flies to the food surface and to induce them to fight. These included the size and shape of the arena, temperature, quality of food, presence of a female, and illumination of the surface. The conditions that favored the occurrence of fighting behavior were as follows.
The Arena.
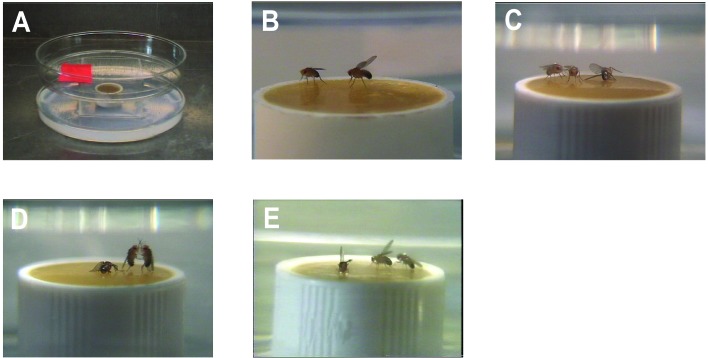
A square chamber was constructed by gluing halves of four glass microscope slides (3.75 cm × 2.5 cm × 1 mm thick) together at their edges. This chamber was optically clear, allowing good video images to be obtained. The square chamber was placed on a Petri dish (10-cm diameter) containing a layer of agarose (≈5-mm thick) and a food cup (1.5 cm × 1 cm, width/height) containing Jazz Mix (Applied Scientific) and a drop of apple juice was placed in the center of the chamber. A lid was constructed from a plastic Petri dish cover (10-cm diameter) with small holes for ventilation and a larger hole for insertion of male flies by aspiration. Finally, a piece of black filter paper with a hole allowing light to fall mainly on the food cup surface was placed in the upper Petri dish (see Fig. 1A). The temperature was maintained at 25°C, which also was the temperature at which the flies were reared.
Figure 1.
Experimental chamber and components of fruit fly fighting. (A) The chamber used in all fights. The black filter paper with a cutout limiting light to the food dish surface is not illustrated here. (B) “Wing threat” seen during the progression of fights. No decapitated female is seen in this frame. (C) High-level “fencing” in which animals push against the opponent with the forelegs. (D) “Boxing,” a high-intensity component that is rarely seen in fights. Animals stand on their hind legs and strike at each other with their forelegs. (E) “Defensive wing threat” during “chase” by the winner of an encounter. This is one of the patterns seen during “retreat” of the fly losing an encounter.
Animals.
The Canton-S laboratory strain of D. melanogaster was used in all studies. Flies were reared on Jazz mix at 25°C under a 12:12 h day/night schedule. Newly emerged male flies were freshly collected each day, anesthetized with CO2, painted on the thorax with a dot of white or yellow acrylic paint for identification, and isolated in small test tubes containing food. Flies were paired randomly and tested for fighting behavior after periods of isolation of up to 1 week. As reported by others, we observed no fighting behavior for the first day after emergence of adults (6, 9, 10), but reliable fighting could be observed after 3 days of isolation.
Experimental Protocol.
A drop of fresh apple juice was added to the surface of food cups and allowed to dry into the food. Mated females were obtained from mixed male/female culture tubes, decapitated, and placed on the food surface to aid in attracting the males. Although male flies would fight without the female present, there were fewer encounters and it was more difficult to attract males to the food surface. On rare occasions, males would attempt to copulate with the decapitated female. Two randomly selected males were removed from isolation chambers by aspiration, and placed in the arena through the hole in the top, which then was covered to prevent their escape. Within 20 min, both males were on the food surface. Videotaping with a digital video camera (Sony Digital 8 Handycam, DCR TR7000) began when both males were in the arena, and continued for 30 min after both were on the food surface, after which the experiment was terminated.
Analysis of Behaviors.
As in other species, fly fights involve a series of interactions (encounters) during which animals approach, interact, and separate repeatedly within the 30-min observation period. The end of an interaction is defined by using pauses in activity and distance between flies. If flies paused for more than 2 s and/or were separated by more than two body lengths with no pursuit seen, that was considered the end of an interaction. Videotapes were analyzed by using an iMAC-DV computer, and the following measures were scored separately for each encounter in the chamber: duration of encounter, order and intensity levels of behaviors, highest intensity level reached during encounter, who initiated, its outcome, and the time until the beginning of the next encounter. All average values are reported (±SEM).
Statistical Methods.
Single-order Markov chains (12) tested for the existence of nonrandom temporal associations between behavioral patterns. Toward this goal, we constructed transition matrices by tabulating all instances in which one behavioral pattern led to another. To evaluate whether certain transitions were more likely to occur than others, likelihood-ratio tests (G statistics) were applied. In the cases in which the overall matrix showed significance, cell-wise examinations (Freeman–Tukey deviates) were performed to identify the cells that had contributed to the overall significance. To examine whether differences in social status were associated with the use of different behavioral strategies, separate matrices were constructed and compared for individuals who became the eventual winners and the eventual losers (Mantel Matrix procedures, ref. 13). All such analyses were performed by using a collection of public domain Java Applets for the analysis of behavioral data (available at http://caspar.bgsu.edu/∼software/java/).
Results and Discussion
In preliminary experiments, we noted that female flies showed only limited fighting. Most often they pushed off with their legs when another animal approached (low level fencing), and on rare occasions would head-butt another fly (but see ref. 14 for a recent study examining factors that influence aggression in females). Subsequent experiments, therefore, only used male flies. In an attempt to provide a comprehensive characterization of the behavior, 75 fights between pairs of 3-day-old male flies were performed, of which 73 provided useful data. Only two of the trials featured no fighting behavior. We observed a total of 2,074 encounters between flies. Table 1 identifies and defines the components we observed in fly fighting behavior under these experimental conditions. The qualitative aspects of the behavioral analysis observed and scored in this simplified experimental design were consistent with those described previously (6, 9, 10). Instances of these patterns are shown on Fig. 1 B–E. On average, 27 ± 1.9 encounters (range 3 to 78; n = 73 trials) were observed for a 30 min observation period lasting 11 ± 0.5 s (range 1 to 279 s; n = 2,074 encounters). The encounter duration is similar to that reported by Hoffmann (10.1 s, in ref. 10) for a more complex social scenario.
Table 1.
Ethogram of offensive and defensive actions of male flies during agonistic encounters
| Component | Description |
|---|---|
| Offensive actions | |
| Approach | One fly lowers body, then advances in the direction of the other |
| Low-Level fencing | Both flies extend one leg and tap opponent's leg |
| Wing threat | One fly quickly raises both wings to a 45° angle towards opponent |
| High-level fencing | One or both flies face each other, extend leg forward and push opponent |
| Chasing | One fly runs after the other |
| Lunging | One fly rears up on hind legs and snaps down on the other |
| Holding | One fly grasps the opponent with forelegs and tries to immobilize |
| Boxing | Both flies rear up on hind legs and strike the opponent with forelegs |
| Tussling | Both flies tumble over each other, sometimes leaving food surface |
| Defensive actions | |
| Walk away | Loser turns and retreats slowly from advance of winner |
| Defensive wing threat | Loser flicks wings at 45° angle while facing away from opponent |
| Run away/being chased | Loser runs away quickly from advance of winner |
| Fly away | Loser flies off food surface |
Within each category the order of the components is roughly in increasing levels of intensity.
One prominent feature in D. melanogaster fighting is the existence of strong initiator effects. Thus, the fly that instigated the first encounter was more likely to emerge as the eventual winner of the fight. Moreover, the higher the intensity level with which that fly started the fight, the greater were its chances of winning. For example, if a fly initiated with a slow approach, its chances of ultimately becoming the winner were 3 to 1 (614 wins vs. 206 losses), whereas fight initiation at higher intensities increased the likelihood of winning to 16 to 1 (50 wins vs. 3 losses). A fly that had won an earlier encounter was also 7 times more likely to win the following one. These factors combine to create a stable dominance relationship between opponents in the pair.
A close association existed between encounter duration and its maximum intensity: when high intensity levels were seen (boxing and tussling) encounters lasted 4–5 times longer than if only low levels were observed (approach, low-level fencing, and wing threats) (25.2 ± 3.9 s, n = 37; vs. 6 ± 0.33 s, n = 633). The time to the next encounter only showed a small dependence on the intensity level reached in the previous one, taking slightly longer when the interactions went to the highest intensity levels, boxing and tussling (73.6 ± 28.8 s, n = 37, vs. 44.2 ± 2.2 s, n = 1,965).
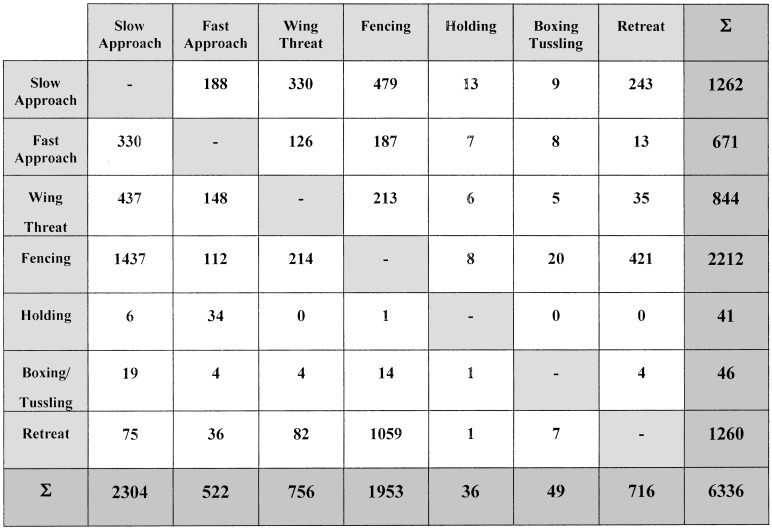
A combined transition matrix was constructed (Fig. 2) to examine the temporal structure of fly fighting behavior in greater detail. For this analysis, we grouped the 13 behavioral components shown on Table 1 in the following way. All defensive actions, including walking or running away from the opponent, flicking wings in a defensive wing threat, or flying off the food surface, were grouped together as retreat. Offensive actions were placed into distinct categories involving: (i) actions by one fly in which there was limited or no direct contact with the opponent [slow approach, fast approach (including lunge), and wing threat]; and (ii) actions involving both flies showing high or low intensities of direct physical contact between the pairs of flies (fencing, holding, boxing and tussling). Within the groupings, some behavioral components listed separately on Table 1 were combined either because there were too few instances for a statistical analysis (e.g., boxing and tussling), or because these behaviors were linked too closely to be easily divided (e.g., boxing and tussling, high and low level fencing).
Figure 2.
Transition matrix. This matrix summarizes how often each agonistic behavior (y axis) was followed by any other behavior (x axis). By comparing these frequencies with a null distribution from a model of independence, we identified those transitions that occurred more or less frequently than predicted by chance (see Fig. 3).
Initially, separate analyses of temporal structure were performed for eventual winners and eventual losers. Data were combined, however, when these matrices proved homogeneous by using matrix correlations (Mantel's Z, ref. 13). The analysis of behavioral sequences used transition matrices for first order Markov chains (Figs. 2 and 3). The fundamental characteristics of fruit fly agonistic behavior closely match predictions based on game theoretical considerations (15–18). The temporal structure of fighting presumably allows individuals to acquire increasingly detailed information concerning the opponent's strength and fighting abilities as described by assessment strategies. No obvious differences in fighting separate eventual winners from losers.
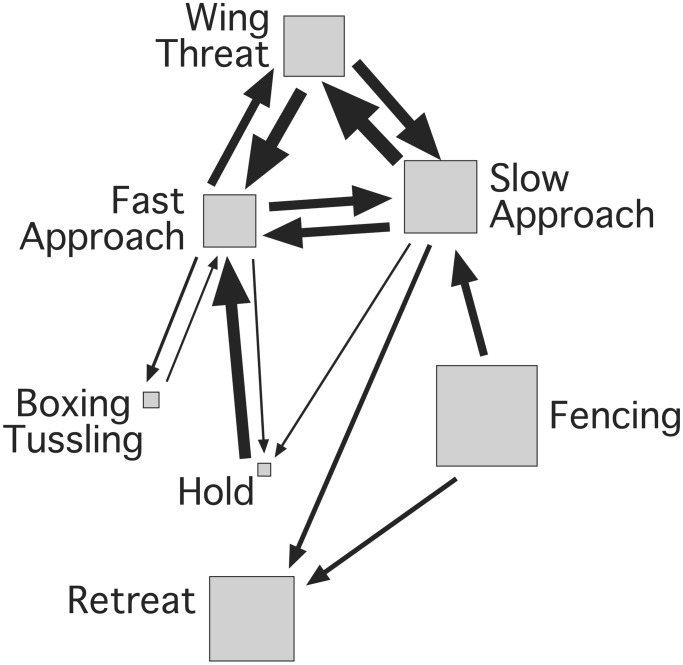
Figure 3.
Analysis of fighting behavior in D. melanogaster. The figure represents a first-order Markov chain analysis of a combined transition matrix constructed from 9,031 behavior patterns during 2,074 encounters in 73 fights between pairs of flies. The size of bounding boxes around the behaviors is proportional to the relative frequency of occurrence of a particular behavioral pattern. Sequences (or chains) of behaviors are depicted as arrows when transitions occur significantly more often than predicted by chance. The size of the arrows indicates the degree to which particular transitions are over-represented.
The existence of a complex, highly structured behavior, which can be easily elicited, makes fly fighting behavior suitable for quantitative analysis. A summary of our observations follows. (i) The behavior during fight initiation is a powerful predictor of eventual success, and it allows us to obtain an unbiased estimate of the initial aggressive state of individual flies. (ii) Fig. 3 shows that during much of the time that flies interact with each other, no direct contact takes place. Instead, flies cycle through three distinct behavioral patterns that form a loop: slow approach, fast approach, and wing threat. A similar analysis of mating behavior demonstrates the existence of two loops that dominate the behavioral pattern (11). Loops that involve chase and approach figure prominently in both mating and fighting behavior, but additional components of the behavior like wing threat, or higher intensity patterns like boxing and tussling, clearly differentiate the pattern of agonistic behavior from that of mating behavior. Fencing, the pattern that occupies the greatest proportion of fighting time, also leads into the loop of slow approach, fast approach, and wing threat. (iii) In some instances, high-intensity fighting with physical contact emerges out of instances of fast approach. These branches go toward boxing, tussling, and holding, which are relatively rare events. (iv) Retreat comes suddenly with no prominent paths leading to it. This finding indicates that the decision to give up may follow any of the listed behaviors. Flies therefore appear to face few behavioral constraints in selecting retreat. Such a decision may come when the opponent is recognized as either stronger or more willing to invest energy in the fight. (v) Losing flies continue to re-engage the opponent in encounters. This behavior is different from what is seen in fights between animals bearing dangerous weapons, like lobsters and crayfish, where losers refuse to fight with winners for extended periods of time (19, 20).
The main goals of these experiments were (i) to find conditions under which fighting behavior between D. melanogaster males is expressed as a robust event; and (ii) to provide a quantitative characterization of the behavior. The construction of transition matrices that describe this behavior should now allow us to search for genetically or pharmacologically induced alterations in these behavioral characteristics. Indeed, certain mutations already have been seen to impact on aspects of fighting behavior in reports from other laboratories (6, 21–23). The experimental paradigm offers a context in which the effects of selected mutations on fighting behavior can be measured. Moreover, if changes in gene expression accompany changes in social status in flies, by pooling groups of animals and by using gene chips or other technologies, it also should allow examination of the consequences of winning and losing fights at the level of changes in gene expression.
Acknowledgments
We thank Drs. Martin Heisenberg and Björn Brembs for information about preliminary experiments they carried out on fighting behavior in D. melanogaster, and Drs. Kathleen Siwicki and Stefan Thor for supplying us with the strains of flies used in these studies and for instruction on the rearing and handling of flies. This work was supported by a starter grant from the Mind, Brain and Behavior Program at Harvard University (to E.A.K. and Dr. Stefan Thor), by Research Experience for Undergraduates awards from the National Science Foundation (to N.M.B., A.Y.L., and S.C.), by a Harvard College scholarship (to A.Y.L.), by National Science Foundation Research Grants NSF-IBN-9874608 and NSF-DBI-0070334 and National Institutes of Health Grant NIH-MH62557-01 (to R.H.), and by National Institute of General Medical Science Grants NIH-NIGMS and R21-GM65595 (to E.A.K.).
References
- 1.Ayre D J, Grosberg R K. Am Nat. 1995;146:427–453. [Google Scholar]
- 2.Loeber R, Hay D. Ann Rev Psychol. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- 3.Sturtevant H. J Anim Behav. 1915;5:351–366. [Google Scholar]
- 4.Spieth H T. Evol Biol. 1968;2:157–193. [Google Scholar]
- 5.Spieth H T. Annu Rev Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs M E. Ecology. 1960;41:182–188. [Google Scholar]
- 7.Price D K, Boake C R B. J Insect Behav. 1995;8:595–616. [Google Scholar]
- 8.Boake C R B, Price D K, Andreadis D K. Heredity. 1998;80:642–650. doi: 10.1046/j.1365-2540.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- 9.Dow M A, von Schilcher F. Nature (London) 1975;254:511–512. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann A. Anim Behav. 1987;35:807–818. [Google Scholar]
- 11.Markow T A, Hanson S J. Proc Natl Acad Sci USA. 1981;78:430–434. doi: 10.1073/pnas.78.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottman J M, Roy A K. Sequential Analysis: A Guide to Behavioral Researchers. Cambridge, U.K.: Cambridge Press; 1990. [Google Scholar]
- 13.Schnell G D, Watt D J, Douglas M E. Anim Behav. 1985;33:239–253. [Google Scholar]
- 14.Ueda A, Kidokoro Y. Physiol Entomol. 2002;27:1–8. [Google Scholar]
- 15.Maynard-Smith J, Price G R. Nature (London) 1973;246:15–18. [Google Scholar]
- 16.Parker G A, Rubenstein D I. Anim Behav. 1981;29:221–240. [Google Scholar]
- 17.Enquist M, Leimar O. J Theor Biol. 1983;102:387–410. [Google Scholar]
- 18.Austad S N. Trends Ecol Evol. 1989;4:2–3. [Google Scholar]
- 19.Huber R, Kravitz E A. Brain Behav Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- 20.Bruski C A, Dunham D W. Behavior. 1987;103:83–107. [Google Scholar]
- 21.Lee G, Hall J C. Behav Gen. 2000;30:263–275. doi: 10.1023/a:1026541215546. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs M E. Behav Gen. 1978;8:487–502. doi: 10.1007/BF01067478. [DOI] [PubMed] [Google Scholar]
- 23. Baier, A., Wittek, B. & Brembs, B. (2002) J. Exp. Biol., in press. [DOI] [PubMed]