Abstract
Introduction and purpose
Hypertension is one of the most common non-communicable diseases in the world and plays a significant role in the occurrence of cardiovascular complications, including coronary heart disease (CHD) and strokes. Therefore, the purpose of this study is to conduct an economic evaluation of high blood pressure screening strategies in Iran in 2020.
Method
We did an economic evaluation of 9 blood pressure screening strategies, including screening annually or every two or three years from the ages of 30, 40, or 50, using the Markov model. The Markov model was designed and implemented based on the natural history of cardiovascular disease in the 2020 TreeAge Pro software. The quality-adjusted life years and the average cost of high blood pressure screening and treatment per person were estimated from society’s perspective for the lifetime. Input data of the model were derived from published literature, expert opinion, and available data sources.
Findings
All screening interventions were more costly and more effective compared to no screening. Five strategies, including screening every three years from the age of 50, 40, and 30 years and screening every two years and annually from the age of 30, were undominated. Incremental cost-effectiveness ratios for these strategies ranged from $PPP 2,675.20 to 20,466.83. Probabilistic sensitivity analysis indicated that, at a cost-effectiveness threshold close to one times the GDP per capita, screening every two or three years from age 30 had the highest cost-effectiveness, with probabilities of 0.522 and 0.44, respectively.
Conclusion
Based on the findings of the economic evaluation, all screening strategies are more cost-effective compared to no screening, and among the screening strategies, considering about one times the GDP per capita as the cost-effectiveness threshold, a screening strategy every two years, starting at the age of 30, is the most cost-effective strategy.
Introduction
High blood pressure is one of the most common non-communicable diseases worldwide and is the main risk factor for cardiovascular diseases such as coronary artery disease, stroke, heart failure, arrhythmia, and cardiomyopathy [1]. According to studies conducted in 2010, high blood pressure was the cause of 49% of coronary heart disease cases and 62% of stroke cases [2,3]. The prevalence of high blood pressure is increasing in low and middle-income countries including Iran [4,5].
Studies conducted in different provinces of the Islamic Republic of Iran have shown a wide variety of high blood pressure prevalence [6–8]. In Iran, in 2011, about 25.6% and 39.8% of adults aged 25–70 had high blood pressure and pre-hypertension, respectively [9]. In Iran in 2020, 52.84% of men and 68.02% of women were aware of their high blood pressure (61.48% in both sexes), and among them, only 43.15% of men and 58.73% of women with high blood pressure were treated and received therapeutic drugs (52.02% in both sexes) [10].
By controlling blood pressure, heart attacks and strokes in Iran will be reduced by 25% [11,12]. Preventive interventions such as high blood pressure screening is one of the important interventions to identify people at risk and prevent high blood pressure and minimize the number of affected people and costs.
Currently, population-based screening for high blood pressure is not implemented in Iran. Although Iranian guidelines recommend hypertension screening for individuals over 30 years old [13], this program has not been fully implemented and remains limited in coverage, particularly in urban areas. Despite the increasing burden of hypertension, limited economic evidence exists on the cost-effectiveness of different screening strategies in Iran, leaving a critical gap in policy planning and implementation.
Economic evaluation is a systematic approach to identifying, measuring, valuing, and comparing the costs and outcomes of alternative programs or interventions. It helps health policymakers implement more effective health interventions. Economic evaluations help to improve the efficiency of the health system by prioritizing, rationing and optimal allocation of resources and increasing its effectiveness by improving access and equality [14].
Various studies have demonstrated the cost-effectiveness of interventions related to screening, control, and management of high blood pressure, education, and self-monitoring [15–19,20,21]. In particular, hypertension screening has been recognized as a key cost-effective strategy, both as part of a national routine program [22], and through population-based approaches [18], reinforcing the importance of early detection and timely management.
Given the shortcomings in Iran’s healthcare system—such as inadequate coverage and delays in patient identification—policymakers require reliable economic evidence to determine the most effective screening strategies. To address this gap, we utilized data from Iran’s 2019 National High Blood Pressure Control Campaign to conduct a comprehensive assessment of the costs and benefits of various hypertension screening strategies. Our study provides robust economic evidence to identify the most cost-effective screening approach. Moreover, health policymakers in Iran can leverage these findings to advocate for support from other institutions and secure financial resources for the implementation of this program, ultimately enhancing hypertension screening efforts.
Methods
This study aimed to assess the cost-effectiveness of different screening strategies for high-blood pressure using a Markov modeling approach in Iran. The Markov model simulated the progression of individuals through health states over time, allowing us to estimate the long-term costs and health outcomes associated with each screening strategy, as well as no screening strategy. The time horizon of the model was life time to capture the long-term costs and health outcomes associated with each screening strategy. The study population consisted of individuals aged 30 years or older. We adopted a societal perspective, considering direct medical and non-medical costs associated with screening, diagnosis, and treatment of high-blood pressure and its complications. Future costs and health outcomes were discounted at an annual rate of 5%.
To compile the data for this study, we utilized results from prior studies, national and international databases, outcomes from the national high blood pressure control campaign, and calculations based on these results, as well as data from Iran’s health insurance. All the data used in this research are publicly available, except for Iran’s health insurance data. Therefore, health insurance data was requested during administrative procedures through the Tehran University of Medical Sciences. The items requested by the researchers were prepared by experts from Iran’s Health Insurance Organization. The data provided was anonymized and made available to the researchers.
The ethical approval for this study was obtained from the Ethics Committee of Tehran University of Medical Sciences (Approval ID: IR.TUMS.VCR.REC.1399.380). This study is part of a doctoral dissertation. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Tehran University of Medical Sciences.
Model structure
We developed a Markov model to simulate the natural history of high-blood pressure and its associated cardiovascular disease (CVD) outcomes over time. The model comprised ten mutually exclusive health states representing different stages of hypertension and CVD events including:
Healthy.
Individuals in this state have not been diagnosed with hypertension or experienced any CVD events(including CHD or Stroke).
Normal blood pressure.
When a person is healthy and at rest, their blood pressure is less than 120/80 mmHg.
Prehypertension (borderline hypertension).
When systolic blood pressure is between 120 and 139 and/or diastolic blood pressure is between 80 and 89 mmHg.
Hypertension – stage 1.
When systolic blood pressure is between 140 and 159 and/or diastolic blood pressure is between 90 and 99 mmHg. If only one of these two values is in this range, the person is classified as having stage 1 hypertension.
Hypertension – stage 2.
When systolic blood pressure is 160 mmHg or higher and/or diastolic blood pressure is 100 mmHg or higher. At this stage, blood pressure reaches a higher level and requires serious medical intervention.
Hypertension off treatment.
Individuals diagnosed with hypertension but not receiving treatment.
Hypertension on treatment.
Individuals diagnosed with hypertension and receiving antihypertensive treatment.
Post stroke.
Individuals who have experienced a stroke event.
Post myocardial infarction.
Individuals who have experienced a myocardial infarction (heart attack).
Post unstable angina.
Individuals who have experienced unstable angina.
Post stable angina.
Individuals who have experienced stable angina.
Post transient ischemic attack.
Individuals who have experienced a transient ischemic attack (TIA or “mini-stroke”).
CVD death.
Terminal health state representing death related to CVD
Non-CVD death.
Terminal health state representing death from any cause unrelated to CVD.
Each cycle of the model represented a discrete time period during which individuals could transition between health states based on predefined transition probabilities. At the beginning of the model simulation, all individuals were placed in three conditions, including healthy, Hypertension off treatment, and Hypertension on treatment. At the end of each yearly cycle,
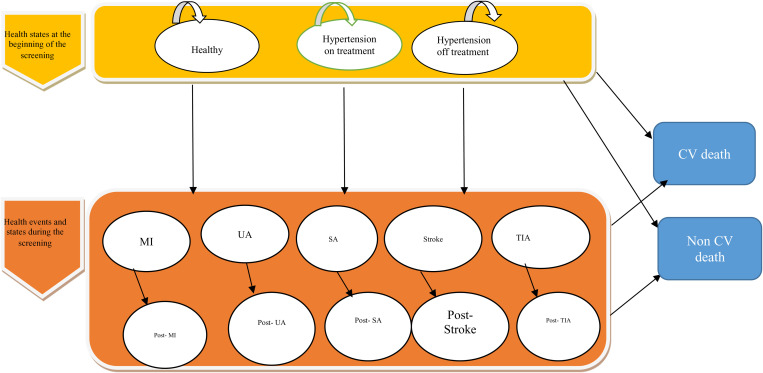
individuals in each health state may remain in their current state or suffer non-cardiovascular death. Forthermore, those in a healthy state may transition to one of the Hypertension states or encounter a coronary heart disease (CHD) or stroke event. Those in the Hypertension off treatment state, may receive a diagnosis of hypertension and commence antihypertensive treatment, or experience a CHD or stroke event. Similarly, individuals in the hypertension on-treatment state may also experience a CHD or stroke event. In the event of a CHD or stroke, individuals may either perish immediately or transition to one of the post-CVD event states. Individuals within post-CVD event states may continue in that state, undergo another CVD event, or face mortality (Fig 1).
Fig 1. Markov model structure.
MI, myocardial infarction; SA, stable angina; TIA, transient ischemic attack; UA, unstable angina.
By conducting screening, people with or at risk of hypertension are diagnosed and treated, so by conducting intervention and screening, people with undiagnosed hypertension are transferred to diagnosed and treated states and As a result, the number of CVD events and death will decreases.
Model assumptions
Transitions between health states were governed by transition probabilities derived from epidemiological studies, clinical trials, and meta-analyses.
Individuals could experience multiple CVD events over the course of the simulation.
The model assumed that individuals remained in the same health state until transitioning to another state or experiencing death.
The Markov model was designed and implemented in the 2020 TreeAge Pro software.
Screenign strategies
We evaluated several screening strategies, including:
No screening: Participants receive no systematic screening for high-blood pressure.
Annual screening from age 30–70 years
Annual screening from age 40–70 years
Annual screening from age 50–70 years
Biennial Screening from age 30–70 years
Biennial Screening from age 40–70 years
Biennial Screening from age 50–70 years
Three-yearly screening from age 30–70 years
Three-yearly screening from age 40–70 years
Three-yearly screening from age 50–70 years
Model parameters
Hypertension incidence and prevalence.
Epidemiological data concerning the incidence and prevalence of hypertension in Iran, disaggregated by age and sex, were sourced from the STEPs 2016 survey report [23]. Additionally, figures representing the proportion of individuals with hypertension who are aware of their condition and the percentage undergoing treatment, categorized by age, were obtained from STEPs 2016 survey report and prior studies conducted in Iran [24].
Cardiovascular risks and probabilities.
In this study, the probability of incidence of cardiovascular diseases in healthy people, individuals with hypertension, and those undergoing hypertension treatment was calculated separately (Table 1) using the relative risks and the incidence rates of each group [25].
Table 1. Base-case model inputs.
| Values | 95% confidence interval | references | |
|---|---|---|---|
| Probabilities of healthy states | |||
| Probability of being healthy at the beginning of the study | 0.921 | [23] | |
| Probability of screening healthy people in the screening plan(during the national campaign) | 0.563 | calculated by the researcher | |
| The probability of not screening healthy people in the screening plan (based on the national campaign). | 0.437 | calculated by the researcher | |
| Probability of transition from healthy state to CHD | Table | Appendix | [25] |
| Probability of moving from a healthy state to a false positive | 0.254 | [26] | |
| The probability of transitioning from healthy to high blood pressure | Table | Appendix | [27] |
| Probability of transition from healthy state to stroke | Table | Appendix | [25] |
| The probability of transition from a healthy state to a true negative | 0.746 | 0.479- 0.904 | [26] |
| Probability of transition from false positive to CHD event | 0.010 | 0.00090-0.019053 | [28] |
| Probability of transition from false positive to stroke event | 0.003 | 0.00030- 0.00480 | [28] |
| Probabilities of Hypertension on treatment | |||
| Probability of having high blood pressure and being treated at the beginning of the study | 0.041 | [23,27] | |
| Probability of receiving treatment for people with high blood pressure | Table | Appendix | [23,27] |
| The relative risk (RR) of transition from treated hypertension to stroke event | 0.675 | 0.633-0.717 | [28] |
| The relative risk (RR) of transition from treated hypertension to stroke event | 0.622 | 0.526- 0.717 | [28] |
| Probabilities of Hypertension off treatment | |||
| Probability of having high blood pressure and no treatment at the beginning of the study | 0.037 | [23,27] | |
| Probability of transition from untreated hypertension to CHD event | Table | Appendix | [25] |
| Probability of screening untreated patients | 0.673 | calculated by the researcher | |
| Probability of transition from untreated hypertension to stroke event | Table | Appendix | [25] |
| Probability of transitioning from untreated hypertension to false negative in screening | 0.254 | [26] | |
| The probability of not screening patients who have not been treated | 0.327 | calculated by the researcher | |
| Probability of transitioning from a true positive to a CHD event | 0.014 | 0.00171- 0.026 | [28] |
| Probability of transition from true positive to stroke event | 0.006 | 0.000702- 0.0119 | [28] |
| Probability of transition from false negative to CHD event | 0.014 | 0.00171-0.0265 | [28] |
| Probability of transition from false negative to stroke event | 0.006 | 0.00070- 0.0119 | [28] |
| Mortality and risk of cardiovascular events | |||
| Probability of non-cardiovascular death (NCV) | life table-CVD | Appendix | [20] |
| The probability of cardiovascular death (CV) | Table | Appendix | [25] |
| Probability of transition from CHD event to CHD death | 0.122 | 0.066-0.178 | [28] |
| Probability of transition from CHD event to non-fatal MI | 0.261 | 0.143-0.378 | [28] |
| Probability of transition from CHD event to non-fatal SA | 0.503 | 0.377-0.629 | [28] |
| Probability of transition from CHD event to non-fatal UA | 0.157 | 0.104-0.209 | [28] |
| The probability of transition from post UA to cardiovascular death (CV) | 0.022 | 0.0205- 0.0233 | [28] |
| Probability of transition from post stroke to CV death | 0.027 | 0.0259-0.0285 | [28] |
| Probability of transition from post SA to CV death | 0.020 | 0.0165-0.0231 | [28] |
| Probability of transition from post TIA to CV death | 0.014 | 0.011- 0.018 | [28] |
| Probability of transition from post MI to CV death | 0.027 | 0.0248- 0.0291 | [28] |
| Probability of transition from stroke event to non-fatal stroke | 0.518 | 0.517- 0.701 | [28] |
| Probability of transition from stroke event to non-fatal TIA | 0.188 | 0.134-0.361 | [28] |
| Probability of transition from stroke event to stroke death | 0.1435 | 0.122- 0.165 | [28] |
| Risk of CHD events in people with CHD compared with people without CHD | 2.4 | 1.9-2.8 | [21] |
| Probability of stroke recurrence | 0.0485 | 0.0312-0.069 | [29] |
| Screening Program Features | |||
| The probability of detecting false positive people in screening | 1 | The assumption of the study based on national campaign | |
| Probability of detecting true positive individuals without treatment in screening | 1 | The assumption of the study based on national campaign | |
| Probability of transitioning from untreated hypertension to true positive at screening | 0.746 | 0.607-0.848 | [26] |
| Probability of transition from true positive to treated status in blood pressure screening | 0.9 | The assumption of the study based on national campaign |
For this purpose, the probability of disease incidence in the general population of the country was estimated by utilizing the relationship between the incidence rate and the probability of the disease [30]. Subsequently, to estimate the probability of incidence in individuals with high blood pressure, the probability of incidence in the country’s population was multiplied by the risk of disease in individuals with high blood pressure, disaggregated by age groups.
Finally, the probability of incidence in these two groups, healthy individuals and those under treatment was also estimated by utilizing the risk of disease in each group. The risk of disease in different patient groups was extracted from prior studies [28].
Additionally, the probability of screening each individual within the target population during the national screening campaign was estimated using the results and data obtained from the national campaign.
Screening parameters.
Data related to sensitivities, and specificity of screening tests were extracted and collected from prior studies. Additionally, some of the data including participant rate in the national campaign, were calculated based on the guidelines and results of the national hypertension control campaign (Table 1).
Costs.
Costs of high blood pressure screening(For the general population), These costs were calculated using data from the National High Blood Pressure Control Campaign, following a bottom-up approach. These costs were calculated at three levels, including healthcare centers, universities, and the Ministry of Health, and for estimation three groups of costs, including human resource costs, equipment costs, and consumable costs were considered;
At the healthcare centers level, human resources costs include the salaries and wages paid to employees and screeners present at blood pressure measurement stations. Based on the number of healthcare and non-healthcare (temporary) stations and the working hours at those stations, these costs were estimated.
In addition, equipment such as laptops, blood pressure monitors, tables and chairs, and examination beds were used to measure the blood pressure of pregnant women during the implementation of the screening program. The equipment cost includes the depreciation cost of the mentioned items during the screening program period.
Consumable costs included staff meals (lunch and dinner), snacks (fruit and biscuits), disposable cups, pamphlets, posters, banners, blood pressure registration cards, the clients’ information registration lists, and the cost of printing training certificates for screeners. The average cost of each item was multiplied by the required quantity for each station per day and then multiplied by the number of days in the program and the number of stations using it. After estimating and adding up the total cost of these items, the total cost of consumables was estimated.
At the university level, costs included meetings, visits, and coordination with relevant groups, which were collected through interviews with individuals involved at this level.
At the Ministry of Health level, costs included strategic committees, executive committees, and their working groups including communications and information dissemination, treatment group, training group, support group, social participation group, disease registration and identification group, and monitoring and evaluation group. The cost of these items was estimated using the National High Blood Pressure Control campaign guidelines and interviews with the executive committee (Deputy of Health, Ministry of Health).
Costs of diseases.
In this study, data on the cost of high blood pressure and its related diseases were used. The costs of diagnosing and treating hypertension are explained in detail in the published study [31], and the cost of diagnostic tests for false positive individuals using treatment guidelines was calculated. For the costs related to heart disease and stroke, the prevalence and the number of cases of each disease using the IHME database for Global Burden of Disease were separately estimated [25], the cases of each disease were multiplied by the average cost per patient, and the total cost for each disease was calculated, considering new and previous cases, direct medical and non-medical expenses, as well as outpatient and inpatient costs.
Direct medical costs, encompassing high blood pressure diagnosis and treatment, along with costs related to selected diseases, Direct medical costs of selected diseases were calculated using health insurance data, survey [32] and a literature review [28,33,34], and Direct non-medical costs Using a literature review were calculated [33,34]. and indirect costs were calculated using the human capital approach Using the NASBOD and IHME database for Global Burden of Disease [25,35]. These costs were calculated using the bottom-up approach. then multiplied the costs for each disease category by the population-attributed fraction of high blood pressure and aggregated the costs related to high blood pressure. Data on demographic and economic data, such as population statistics, labor force employment rates, household activity rates, wage rates, and GDP per capita, were extracted from the Iran Statistics Center [36]and the World Bank [37]. These expenses have been reported along with references in Table 2.
Table 2. Estimated cost data for the model.
| Types of Expenses | Amounts (PPP(current international $)) Per case per year | 95% Confidence Interval | References |
|---|---|---|---|
| Initial cost in healthy state with screening | 1.56 | 1.06- 2.07 | calculated by the researcher |
| Initial cost of high blood pressure with screening but without treatment | 1.56 | 1.06- 2.07 | calculated by the researcher |
| Initial cost of high blood pressure treatment without screening (new cases) | 137.85 | calculated by the researcher | |
| Initial cost of high blood pressure treatment with screening (new cases) | 139.41 | calculated by the researcher | |
| Cost of high blood pressure treatment (previous cases) | 57.56 | 41.25–73.86 | calculated by the researcher |
| Average cost of high blood pressure treatment (previous and new cases) | 63.72 | 42.62-84.81 | calculated by the researcher |
| Non-fatal MI cost per cycle/year | 16,018.61 | 10,691.10 − 21,346.13 | calculated by the researcher |
| Non-fatal UA cost per cycle/year | 10,484.27 | 7,815.56 − 13,153 | calculated by the researcher |
| Non-fatal SA cost per cycle/year | 8,111.67 | 3,224.58 −12,998.75 | calculated by the researcher |
| Non-fatal stroke cost per cycle/year | 45,152.33 | 40,186 − 50,119 | calculated by the researcher |
| Non-fatal TIA cost per cycle/year | 9,077.78 | 553.45 −17,602.10 | calculated by the researcher |
| Post-MI cost in the next year (incremental) | 471.33 | 337.07 - 605.60 | calculated by the researcher |
| Post-stroke cost in the next year (incremental) | 8,618 | 2,376.4 − 14,859.7 | calculated by the researcher |
| Post-SA cost in the next year (incremental) | 121.68 | calculated by the researcher | |
| Post-TIA cost in the next year (incremental) | 237.93 | calculated by the researcher | |
| Post-UA cost in the next year (incremental) | 282.80 | calculated by the researcher | |
| Incremental treatment costs per cycle for high blood pressure without screening | 57.56 | 41.25 - 73.86 | calculated by the researcher |
| Incremental treatment costs per cycle for high blood pressure with screening | 57.56 | 41.25 - 73.86 | calculated by the researcher |
| CHD treatment cost in the year of death | 8,872.59 | calculated by the researcher | |
| Stroke treatment cost in the year of death | 32,119.09 | calculated by the researcher | |
| Cost of false positive tests in healthy screening | 212.57 | calculated by the researcher | |
| Cost of true positive tests in high blood pressure screening without treatment | 212.57 | calculated by the researcher |
In this study, all costs were adjusted to current international dollars based on the World Bank’s PPP conversion factors in 2020 (https://data.worldbank.org/indicator/PA.NUS.PPP?locations=IR).
Utility and quality of life weights.
Data related to utility or quality of life were extracted and collected from prior studies and is fully reported in Table 3.
Table 3. Data of utility and quality of life of the model.
| The quality (utility) of life | Values | 95% confidence interval | references |
|---|---|---|---|
| Quality of life in a state of healthy | 1 | ||
| Quality of life in the state hypertension off treatment in no screening | 0.946 | 0.97-0.922 | [38] |
| Quality of life in the state of hypertension off treatment in screening | 0.946 | 0.97-0.922 | [38] |
| Quality of life in the state of hypertension on treatment in no screening | 1 | [28] | |
| Quality of life in the state of hypertension on treatment in screening | 1 | [28] | |
| Quality of life in the state post-MI | 0.760 | 0.894-0.626 | [28] |
| Quality of life in the state post-stroke | 0.629 | 0.935-0.323 | [28] |
| Quality of life in the state post SA | 0.808 | 0.942-0.674 | [28] |
| Quality of life in the state post-TIA | 1 | [28] | |
| Quality of life in the state post-UA | 0.770 | 0.904-0.636 | [28] |
After completing all the health states in the model, the model design process is nearly finished. Consequently, all initial health states and subsequent health states have been fully designed and model parameters were inserted. The cycles of the model were repeated for 70 periods (total life), each with a duration of one year in the Tree Age 2020 software. The simplified structure of the transition states in the Markov model is shown in Fig 1 [39].
In this model, after the target population is categorized into Healthy, Hypertension on Treatment, and Hypertension Off Treatment states, individuals either remain in their respective health states or eventually transition to a disease event over the time horizon (including myocardial infarction, unstable angina, stable angina, stroke, transient ischemic attack, post-event conditions, or death). In each case, the probability of remaining in a particular state is determined by transition probabilities.
According to Fig 2, when healthy individuals are not screened and develop hypertension, they either receive treatment (“On treatment”) or remain untreated (“Off treatment”). Each individual transitions into one of these states based on their condition, and this cycle continues over time.
Fig 2. Health states of healthy individuals in the model.
In any screening program, two types of tests are involved: a screening test and a diagnostic test. Initially, individuals undergo screening, and those who test positive proceed to a confirmatory diagnostic test. Since the sensitivity and specificity of the screening test are not 100%, false positives and false negatives may occur.
In our study, we assumed that the diagnostic test has 100% sensitivity and specificity. Consequently, individuals with false-positive results in the screening test are correctly identified as healthy by the diagnostic test. However, those with false-negative results remain undiagnosed until the next screening round.
Model implementation and extraction of findings
To implement the model, first the collected and calculated data were entered into the model.Then, the model’s output was evaluated for accuracy and conformity with reality using Markov Coherence in software, and any necessary changes were made to run the model. Afterward, the model was run to extract maps and cost-effectiveness ratios of screening strategies and other desired findings.
In this study, the Plane and cost-effectiveness ratio (incremental) of screening strategies using the Markov model in TreeAge software were extracted. By extracting the cost-effectiveness Plane, the cost-effectiveness frontier and dominated and undominated strategies were identified, as well as the willingness to pay chart and its intersection with the cost-effectiveness frontier. By extracting the incremental cost-effectiveness ratio of screening strategies, the status of each strategy was determined relative to no screening, relative to its previous best strategy, and relative to the cost-effectiveness threshold.
Also, the quantity of QALYs gained from identifying and preventing individuals from developing high blood pressure, coronary heart disease, ischemic stroke, hemorrhagic stroke, and their complications by implementing blood pressure screening strategies was estimated using the results of the Markov model. The value of each QALY was estimated based on the results of Jahanbin et al.‘s study, which was 1.35 times the GDP per capita [40]. This amount is less than the estimated threshold for cancer interventions [41]. Additionally, the GDP per capita was extracted from World Bank reports [42].
The benefits of these strategies include the monetary value of saved life-years (the number of QALYs gained), and to calculate the net benefit, we subtracted the costs of each strategy from these benefits. The costs of the program include the intervention costs and the costs of the disease and its complications.
Sensitivity analysis
In this study, various sensitivity analyses were conducted to test the strength of the assumptions of the model and data sources. First, a one-way sensitivity analysis was performed between important parameters that had the most significant impact on the results in each screening strategy.
The variables included in this analysis were the “Quality of life after stable angina, quality of life after unstable angina, quality of life after myocardial infarction (MI), the discount rate of outcomes, cost of stroke in the next year, quality of life after stroke, the discount rate of costs, probability of transition from healthy to true negative in screening, probability of transition from stroke to non-fatal transient ischemic attack, probability of transition from high blood pressure without Treatment to true positive in screening, probability of stroke recurrence and coronary heart disease, probability of transition from stroke event to nonfatal TIA, and probability of transition from CHD to CHD-related death”. The sensitivity analysis involved three scenarios: one with base values, another with minimum possible values for parameters, and a third with maximum possible values. This approach allowed for an evaluation of how results responded to variations at both ends of these key variables, providing insights into the model’s sensitivity across different scenarios.
Furthermore, a probabilistic sensitivity analysis was used to quantify the uncertainty around the point estimates of input parameters. A probability distribution was defined for each input parameter of the model. The model was then run multiple times (1000 iterations), each time a value for each input parameter was randomly sampled from its corresponding probability distribution. The average costs and QALYs were calculated using these sampled values. The probability distribution in the analysis was based on error estimates from prior studies. The results of the probabilistic sensitivity analysis (PSA) were presented in cost-effectiveness acceptability curves, scatter plots, and cost-effectiveness acceptability curves for willingness-to-pay thresholds.
Results
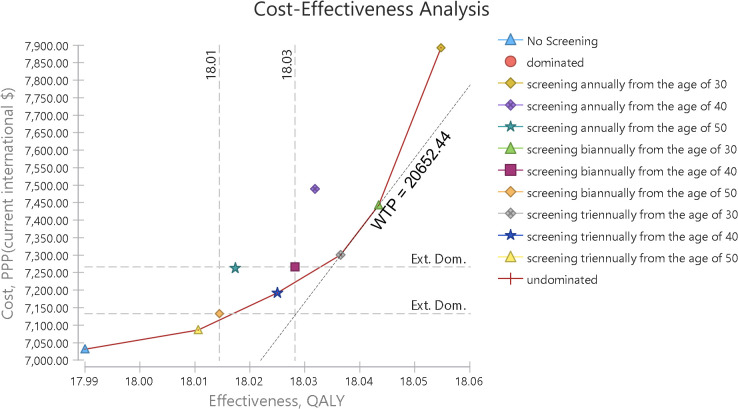
As indicated in Fig 3, all screening strategies have higher costs and effectiveness compared to no screening. Undominated strategies are connected to each other by a line that forms the cost-effectiveness frontier.
Fig 3. Cost-effectiveness plane of screening strategies.
The cost-effectiveness frontier and out-of-frontier strategies are evident in Fig 3, and as shown in this graph, among the 9 different strategies and non-screening, five interventions are undominated, while the annual screening strategies starting at age 40 and age 50 are absolutely dominated, and the biennial screening strategies at both age 40 and 50 are Extended dominated.
In addition, in Fig 4, the cost-effectiveness plane is shown along with the willingness-to-pay curve. The willingness-to-pay curve shows the slope of the willingness to pay, which is tangent to the optimal strategy. The tangency of the willingness-to-pay slope with any strategy indicates the optimality or the high probability of cost-effectiveness of that intervention, and it helps in selecting a cost-effective strategy. Of course, only the cost-effective frontier strategies can be optimal choices. In this graph, the willingness-to-pay slope is tangent with the strategy of screening every two years from the age of 30. In Table 4, the costs and incremental benefits of all screening strategies compared to no screening have been reported. According to this table, all screening strategies have higher costs and greater effectiveness compared to no screening.
Fig 4. Cost-effectiveness plane and willingness to pay.
Table 4. Incremental costs and benefits of screening strategies compared to no screening.
| Screening strategies | Average cost per person (PPP(current international $)) | Incremental cost (PPP(current international $)) | Effectiveness/qualy | incremental effectiveness | Incremental cost-effectiveness ratio (PPP(current international $)) | The state of each strategy |
|---|---|---|---|---|---|---|
| No screening | 7,030.78 | 17.99 | undominated | |||
| Screening every three years from the age of 50 | 7,085.80 | 55.01 | 18.01 | 0.02 | 2,675.20 | undominated |
| Screening every two years from the age of 50 | 7,133.10 | 102.32 | 18.01 | 0.02 | 4,189.08 | Ext. dominated |
| Screening every three years from the age of 40 | 7,192.20 | 161.42 | 18.03 | 0.03 | 4,612.59 | undominated |
| Screening every two years from the age of 40 | 7,266.44 | 235.65 | 18.03 | 0.04 | 6,166.56 | Ext. dominated |
| Annual screening from the age of 50 | 7,262.90 | 232.12 | 18.02 | 0.03 | 8,493.02 | abs. dominated |
| Screening every three years from the age of 30 | 7,301.06 | 270.28 | 18.04 | 0.05 | 5,813.10 | undominated |
| Screening every two years from the age of 30 | 7,442.80 | 412.02 | 18.04 | 0.05 | 7,712.71 | undominated |
| Annual screening from the age of 40 | 7,489.57 | 458.79 | 18.03 | 0.04 | 10,965.49 | abs. dominated |
| Annual screening from the age of 30 | 7,892.55 | 861.77 | 18.05 | 0.06 | 13,311.10 | Undominated |
Additionally, in Table 5, based on the results of the Markov model, the incremental cost-effectiveness ratios (ICERs) of undominated screening strategies compared to their previous best strategy have been compared and ranked. In this table, four dominated strategies have been eliminated, and only the undominant strategies have been ranked in order of increasing effectiveness. After eliminating the dominated strategies, five strategies remained, including screening every three years from ages 50, 40, and 30, and screening every two years and annually from age 30. The ICERs of these strategies, compared to their own previous best strategies, were $ PPP 2,675.20, 7,373.41, 9,466.41, 20,466.83, and 39,731.03, respectively.
Table 5. Costs and benefits of undominated screening strategies.
| Screening strategies | Average cost per person (PPP(current international $)) | Incremental cost (PPP(current international $)) | Effectiveness/qualy | incremental effectiveness | Incremental cost-effectiveness ratio (PPP(current international $)) |
|---|---|---|---|---|---|
| No screening | 7,030.78 | 17.99 | |||
| Screening every three years from the age of 50 | 7,085.80 | 55.01 | 18.01 | 0.02 | 2,675.20 |
| Screening every three years from the age of 40 | 7,192.20 | 106.41 | 18.03 | 0.01 | 7,373.41 |
| Screening every three years from the age of 30 | 7,301.06 | 108.86 | 18.04 | 0.01 | 9,466.41 |
| Screening every two years from the age of 30 | 7,442.80 | 141.74 | 18.04 | 0.01 | 20,466.83 |
| Annual screening from the age of 30 | 7,892.55 | 449.75 | 18.05 | 0.01 | 39,731.03 |
Furthermore, by implementing screening interventions, in all strategies, a certain amount of gained quality-adjusted life years (QALYs) has been added to each individual throughout their remaining lifetime compared to no screening. In Table 4, the number of gained QALYs per individual for each blood pressure screening strategy compared to no screening has been reported, and in Table 5, the gained QALYs compared to the previous best strategy have been reported for undominated cases.
Sensitivity analysis
We performed various sensitivity analyses to test the robustness of the model results. First, we conducted a univariate sensitivity analysis between key model parameters (including discount rates for costs and outcomes, quality of life, and probabilities) (Table 6), and the results of this sensitivity analysis were presented using tornado diagrams (Appendix). The title, baseline values, and the range of selected variables for the sensitivity analysis of different screening strategies are presented in the following table.
Table 6. Selected variables for one-way sensitivity analysis.
| Variables | Amounts (costs in PPP(current international $)) |
|---|---|
| Cost of post stroke in following year | 8,618 (95%CI: 2,376.4 − 14,859.7) |
| Discount rate for costs | 0.05 (0.03-0.07) |
| Discount rate for outcomes | 0.05(0.03-0.07) |
| Probability of Stroke Recurrence | 0.05(0.031-0.069) |
| Quality of life in the state post MI | 0.76(95%CI:626–894) |
| Quality of life in the state post stroke | 0.63(95%CI:323–935) |
| Quality of life in the state post SA | 0.81(95%CI:0.674–0.942) |
| Quality of life in the state post UA | 0.77(95%CI:0.634–0.904) |
| Quality of life in the state hypertension off treatment | 0.946(95%CI:0.922–0.97) |
| relative risk for CHD events in those with established CHD compared with CHD-free | 2.40(1.9-2.8) |
| Trasition probability from healthy to true negetive in screening | 0.75(95%CI:0.479–0.904) |
| Trasition probability from hypertention off treatment to true positive in screening | 0.75 (95%CI:0.607–0.848) |
| Transition probability from Stroke event to non fatal TIA | 0.19(0.134-0.361) |
| Transition probability from chd event to CHD death | 0.122(0.066-0.178) |
According to the deterministic sensitivity analysis results, in most strategies, the estimated outcomes were more sensitive to changes in several variables, including “Probability of transition from healthy to true negative in screening (specificity of the screening test), and discount rates for outcomes and costs.” In this model, probabilistic sensitivity analysis (PSA) was also used to account for uncertainty around the point estimates of input parameters. The PSA results showed very small differences between the estimated average costs and qalys (effectiveness) and their values in the base case analysis, indicating the stability of the results. The PSA results were presented using cost-effectiveness acceptability curves, acceptability at the willingness-to-pay Curve, and cost-effectiveness scatter plots.
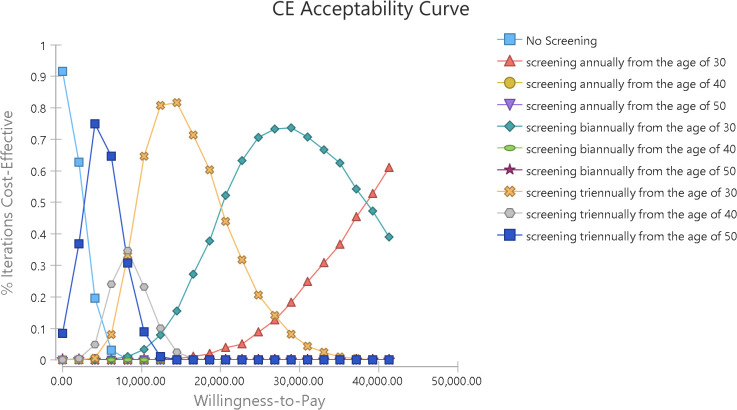
The cost-effectiveness acceptability curve is a commonly used output for communicating the results of probabilistic sensitivity analysis in cost-effectiveness models. The acceptability curve shows the relative cost-effectiveness as a function of the willingness-to-pay threshold. For each willingness-to-pay value, the curve uses net benefits to determine the percentage of simulation iterations that are deemed appropriate for each strategy. As the willingness-to-pay threshold increases, the percentage of iterations for more effective strategies increases (Briefly, this curve indicates the probability of cost-effectiveness at any point within a range or domain of willingness-to-pay). Like a sensitivity analysis, the acceptability curve requires a wide range of values for willingness-to-pay (ICER threshold).
Furthermore, Fig 5 displays the cost-effectiveness acceptability curve that shows the probability of cost-effectiveness at different willingness-to-pay thresholds. In other words, this graph shows the probability of each strategy being cost-effective at the specified threshold.
Fig 5. Cost-effectiveness acceptance curve.
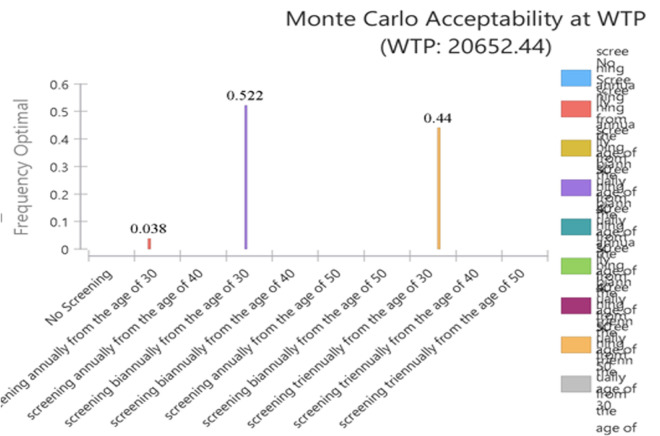
According to Fig 6, at a threshold of 1.35 times GDP per capita ($ PPP 20,652.44), the probability of cost-effectiveness for screening every two and three years from age 30 was 0.522 and 0.44, respectively, and the probability of cost-effectiveness for annual blood pressure screening from age 30 was 0.038, while the probability of cost-effectiveness for other strategies was almost zero.
Fig 6. Acceptability at willingness-to-pay threshold in probabilistic sensitivity analysis.
Similarly, Fig 4 demonstrates that the “Screening every two years from the age of 30” strategy is identified as the optimal choice, as the willingness-to-pay (WTP) threshold is tangent to its curve. This alignment indicates a high probability of cost-effectiveness.
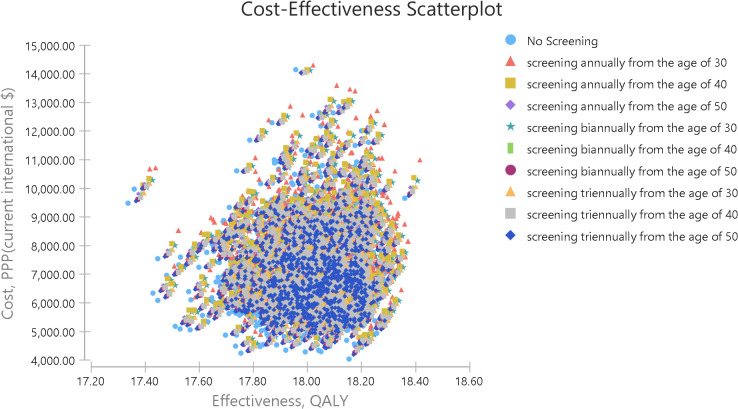
The cost-effectiveness plane, which plots each strategy’s average cost and effectiveness, can naturally expand to a scatter plot for simulation. The scatter plot uses the cost-effectiveness plane to plot pairs of costs and effects separately for each model recalculation. all points of each strategy have a specific color (Fig 7).
Fig 7. Cost-effectiveness scatter plot.
Discussion
In this study we analyzed the economic evaluation of nine blood pressure screening strategies, and the cost-effectiveness ratios and other results of the screening strategies were reported in two groups. In one group, an incremental cost-effectiveness ratio was reported for the screening strategies compared to no screening, and all screening strategies had an incremental cost-effectiveness ratio less than the threshold or willingness-to-pay (($ PPP 20,652.44). However, four strategies, including annual screening from age 40 and 50 due to higher costs and lower effectiveness (absolutely dominated), and screening every two years from age 40 and 50 because of a higher incremental cost-effectiveness ratio compared to other interventions, were dominated.
The second group comprises five strategies, excluding dominated cases. Four of these involve screening every three years at ages 30, 40, and 50, and every two years from age 30, with an incremental cost-effectiveness ratio ranging from $PPP 2,675.20 to $PPP 20,466.83 per Quality Adjusted Life Year (QALY) gained, which is less than the threshold cost-effectiveness ratio (($ PPP 20,652.44). And the last strategy is annual screening from age 30, which had an incremental cost-effectiveness ratio greater than the threshold.
According to a study conducted in Korea in 2021, the cost per Quality Adjusted Life Year (QALY) with hypertension screening strategies was less than the incremental cost-effectiveness ratio threshold (approximately 30.5 million KRW) compared to no screening. Strategies, including the first screening with confirmatory examination every three years for adults over 40 (10.2 million KRW), every two years (13.2 million KRW), or annually (19.9 million KRW), were deemed cost-effective. The most cost-effective strategy involved a first screening with a second confirmatory examination at age 40 or older every three years [15].
Additionally, in the study conducted in Vietnam, screening scenarios varied in intervals (one-time, annually, biennially) and initiation ages (35, 45, or 55 years), considering treatment coverage. Over a 10-year and lifetime horizon, probabilistic sensitivity analysis addressed parameter uncertainty, with a decision-making threshold set at three times the per capita gross domestic product (GDP).
According to the screening results, for men starting screening at the age of 55, all screening scenarios were likely to be cost-effective. For women starting screening at the age of 55, the one-time screening had a 90% chance of being cost-effective. Over a lifetime horizon, the cost per quality-adjusted life year (QALY) obtained was less than the threshold of 15,883 international dollars in all screening scenarios among men. Similar results were observed for women starting screening at the age of 55. Additionally, if biennial screening with an increase in treatment coverage to 20% is considered or even with only biennial screening, starting screening in women at the age of 45 is likely to be cost-effective [19].
Routine blood pressure screening in adolescents is effective, but population-based interventions with broader accessibility for preventing cardiovascular diseases may offer greater cost-effectiveness and efficiency. It is recommended to select and implement interventions based on their cost-effectiveness ratio, considering community conditions and feasibility [43].
Screening potentially reduces diagnosis and treatment time and can be cost-effective if it is linked to primary healthcare through health centers for providing treatment to patients identified through screening [17].
Integrating high blood pressure screening into routine medical examinations and health insurance coverage is deemed appropriate from a health economics perspective.Preventing cardiovascular diseases through high blood pressure screening is cost-effective. Screening strategy should be based on age, gender, and screening intervals [18]. A comprehensive approach to care and prevention, facilitated by government support, subsidies, and an active health network operating at different service levels, has the potential to be cost-effective in a screening program [29].
In the present study, the net present value and incremental net present value of all blood pressure screening interventions for each individual with a 5% discount rate were positive.For calculating the net benefit, the value of the QALY gained in each screening strategy was determined. To do this, after identifying the average QALY gained per individual in each strategy, it was multiplied by the assigned value of each QALY.
The incremental net benefits of screening every two and three years from age 30 was significantly higher than the other strategies, amounting to $PPP 691and $PPP 690, respectively, while in the strategy of annual screening from the age of 30, it was $PPP475.
The study also revealed positive base and annual return on investment (ROI) for all screening interventions. The base ROI was higher due to not considering the compound effect, which could create a significant difference over time. The longer the time period, the greater the difference between the approximate annual ROI (base), which is calculated by dividing ROI by the study period, and the annualized return on investment.
A study in Ghana assessed the cost-benefit of hypertension screening and treatment by community health workers. The analysis, based on screening 25,000 individuals above the age of 30, revealed that with a diuretic treatment regimen and a 30% long-term adherence rate, the intervention could prevent 29 deaths (equivalent to 505 years of life), 11.1 cases of heart disease, 0.1 cases of stroke, and 1.6 cases of heart failure over ten years. The estimated benefits amounted to 7.1 million ¢GH (1.6 million dollars) at an 8% discount rate, with intervention costs of 2.2 million ¢GH (0.5 million dollars), resulting in a benefit-to-cost ratio of 3.3 [44].
These findings align with the present study’s results, suggesting that screening interventions can be implemented with higher effectiveness and efficiency, or as part of multi-interventional preventive programs for managing and treating high blood pressure [40,41]. Considering existing healthcare networks and infrastructure, such interventions not only identify undetected individuals through health systems but also raise awareness about the disease, especially among those at risk. With a cohesive healthcare referral system, diagnosing and treating individuals becomes more feasible, making preventive interventions like blood pressure screening highly effective.
In the annual screening strategy for individuals over 30, the average cost per person screened, diagnosed with high blood pressure, or suspected of having high blood pressure, was $PPP1.56, $PPP5.56, and $PPP46.80, respectively. Treating each diagnosed patient incurred a cost of $PPP137.85, while the net benefit of screening strategies compared to no screening ranged from approximately $PPP581 to $PPP780.65. The budgetary impact analysis projected a total cost of $PPP 438,799,413.74 for high blood pressure screening, diagnosis, and treatment interventions for individuals over 30 in the first year.
Considering the budgetary impact of screening interventions compared to the consequences resulting from untreated hypertension, prioritizing screening and treatment interventions for patients with high blood pressure under the leadership of the Ministry of Health could yield significant benefits. The study’s results are based on the economic evaluation of high blood pressure screening, and it suggests that programs conducted with broad participation from national institutions and media, particularly radio and television, not only directly benefit patients by identifying them through screening but also create awareness and sensitivity among the public, encouraging active participation in diagnostic and treatment processes. These additional benefits were not evaluated in the study.
The study demonstrates that screening and treatment strategies for hypertension, when implemented within a comprehensive and integrated framework, can be cost-effective, especially at a willingness-to-pay threshold of 1.35 times the GDP per capita per Quality-Adjusted Life Year (QALY), equivalent to $PPP 20,652.44 per QALY. This approach yields significant benefits for both the population and the government. Probabilistic sensitivity analysis indicates that biennial and triennial screening from age 30 have the highest probabilities of cost-effectiveness at this threshold, with probabilities of 0.522 and 0.44, respectively. Annual screening from age 30 also has increasing cost-effectiveness probabilities as the willingness-to-pay threshold rises.
In the present study, probabilistic sensitivity analysis demonstrates very low uncertainty in the base case analysis, indicating that the study findings are robust to a large extent.
Limitations
The screening costs provided in the study lack some necessary information such as the expenses associated with building or renting healthcare centers and the cost of some services. Furthermore, some costs were derived from previous studies, which may differ from current patient costs.
The Markov model was designed based on the assumption that the follow-up and treatment of suspected and diagnosed individuals align with the screening program guidelines. However, in reality, not all eligible individuals may receive complete follow-up and treatment. While the study acknowledges this limitation and adjusts the model accordingly, its conclusions still rely on adherence to screening guidelines.
Conclusion
High blood pressure is one of the most dangerous and very costly chronic diseases, but it is preventable. Preventive interventions like blood pressure screening can be the optimal and cost-effective strategies for controlling its economic burden. The study results identify screening every two and three years from the age of 30 as the optimal strategy, with probabilities of cost-effectiveness at 0.522 and 0.44, respectively.
Early and universal measures are deemed necessary to swiftly prevent and treat high blood pressure. Failure to address this health issue may lead to increased mortality, reduced life expectancy, and a substantial economic burden on both individuals and the government.
Policymakers and health system planners are urged to create favorable conditions for various preventive interventions. This includes initiatives such as education and awareness campaigns to encourage regular blood pressure measurement and maintaining blood pressure at optimal levels. Implementing these measures is essential for reducing the prevalence of high blood pressure and improving overall public health outcomes.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This study is a part of the doctoral dissertation of health economics. We hereby thank and appreciate the Tehran University of Medical Sciences for its approval and support.
Data Availability
All relevant data are within the manuscript and its Supporting information files
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Girouard H. Hypertension and the brain as an end-organ target. Springer. 2016. [Google Scholar]
- 2.He FJ, MacGregor GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. 2010;52(5):363–82. doi: 10.1016/j.pcad.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 3.Boon CS, Taylor CL, Henney JE. Strategies to reduce sodium intake in the United States. National Academies Press. 2010. [PubMed] [Google Scholar]
- 4.Ibrahim MM, Damasceno A. Hypertension in developing countries. Lancet. 2012;380(9841):611–9. doi: 10.1016/S0140-6736(12)60861-7 [DOI] [PubMed] [Google Scholar]
- 5.Haghdoust A, Sadeghirad B, Rezazadeh KM. Epidemiology and heterogeneity of hypertension in Iran: a systematic review. 2008. [PubMed]
- 6.Malekzadeh MM, Etemadi A, Kamangar F, Khademi H, Golozar A, Islami F, et al. Prevalence, awareness and risk factors of hypertension in a large cohort of Iranian adult population. J Hypertens. 2013;31(7):1364–71; discussion 1371. doi: 10.1097/HJH.0b013e3283613053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Namayandeh SM, Sadr S, Ansari Z, Rafiei M. A cross-sectional study of the prevalence of coronary artery disease traditional risk factors in Yazd urban population, Yazd healthy heart project. 2011.
- 8.Tabrizi JS, Sadeghi-Bazargani H, Farahbakhsh M, Nikniaz L, Nikniaz Z. Prevalence and associated factors of prehypertension and hypertension in Iranian population: The Lifestyle Promotion Project (LPP). PLoS One. 2016;11(10):e0165264. doi: 10.1371/journal.pone.0165264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteghamati A, Etemad K, Koohpayehzadeh J, Abbasi M, Meysamie A, Khajeh E. Awareness, treatment and control of pre-hypertension and hypertension among adults in Iran. 2016. [PubMed]
- 10.Non-Communicable Diseases Research Center. Hypertension awareness and treatment. Available from: https://vizit.tums.ac.ir/panel/steps-2020/fa/main.html#/forestLocation. 2020. [Google Scholar]
- 11.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagherikholenjani F, Shahidi S, Khosravi A, Mansouri A, Ashoorion V, Sarrafzadegan N, et al. Update of the clinical guideline for hypertension diagnosis and treatment in Iran. Clin Hypertens. 2024;30(1):13. doi: 10.1186/s40885-024-00269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosadeghrad AM, Jaafaripooyan E, Zamandi M. Economic evaluation of health interventions: a critical review. Iran J Public Health. 2022;51(10):2159–70. doi: 10.18502/ijph.v51i10.10975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HY, Lee SW, Kim HC, Ihm SH, Park SH, Kim TH. Cost-effectiveness analysis of hypertension screening in the Korea national health screening program. Korean Circulation J. 2020;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53(6S2):S121–30. doi: 10.1016/j.amepre.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nugent R, Brower E, Cravioto A, Koehlmoos T. A cost-benefit analysis of a National Hypertension Treatment Program in Bangladesh. Prev Med. 2017;105S:S56–61. doi: 10.1016/j.ypmed.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 18.Kaur G, Chauhan AS, Prinja S, Teerawattananon Y, Muniyandi M, Rastogi A, et al. Cost-effectiveness of population-based screening for diabetes and hypertension in India: an economic modelling study. Lancet Public Health. 2022;7(1):e65–73. doi: 10.1016/S2468-2667(21)00199-7 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T-P-L, Wright EP, Nguyen T-T, Schuiling-Veninga CCM, Bijlsma MJ, Nguyen T-B-Y, et al. Cost-effectiveness analysis of screening for and managing identified hypertension for cardiovascular disease prevention in Vietnam. PLoS One. 2016;11(5):e0155699. doi: 10.1371/journal.pone.0155699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. GHO: GHE life tables - probability of dying between ages x and x. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-life-tables-nqx-probability-of-dying-between-ages-x-and-x-n. [Google Scholar]
- 21.Briffa TG, Hobbs MS, Tonkin A, Sanfilippo FM, Hickling S, Ridout SC, et al. Population trends of recurrent coronary heart disease event rates remain high. Circ Cardiovasc Qual Outcomes. 2011;4(1):107–13. doi: 10.1161/CIRCOUTCOMES.110.957944 [DOI] [PubMed] [Google Scholar]
- 22.Lee HY, Lee SW, Kim HC, Ihm SH, Park SH, Kim TH. Cost-effectiveness analysis of hypertension screening in the Korea National Health Screening Program. Korean Circ J. 2021;51(7):610–22. doi: 10.4070/kcj.2021.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamandi M. Estimation of the economic burden of hypertension and the cost-benefit analysis of the national high blood pressure control campaign in Iran in 2020. Teheran University of Medical Sciences. 2022. [Google Scholar]
- 24.Najafipour H, Nasri HR, Rostamzadeh F, Amirzadeh R, Shadkam M, Mirzazadeh A. Prevalence and incidence of pre-hypertension and hypertension (awareness/control) in Iran: findings from Kerman coronary artery diseases risk factors study 2 (KERCADRS). Journal of Human Hypertension. 2020;:1–12. [DOI] [PubMed] [Google Scholar]
- 25.GBD results. Available from: https://vizhub.healthdata.org/gbd-results/. [Google Scholar]
- 26.Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FDR, Deeks JJ, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. doi: 10.1136/bmj.d3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najafipour H, Nasri HR, Rostamzadeh F, Amirzadeh R, Shadkam M, Mirzazadeh A. Prevalence and incidence of pre-hypertension and hypertension (awareness/control) in Iran: findings from Kerman coronary artery diseases risk factors study 2 (KERCADRS). J Hum Hypertens. 2022;36(5):461–72. doi: 10.1038/s41371-020-00392-5 [DOI] [PubMed] [Google Scholar]
- 28.Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FR. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. The Lancet. 2011;378(9798):1219–30. [DOI] [PubMed] [Google Scholar]
- 29.Hankey GJ. Secondary stroke prevention. Lancet Neurol. 2014;13(2):178–94. doi: 10.1016/S1474-4422(13)70255-2 [DOI] [PubMed] [Google Scholar]
- 30.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25(1):3–6. doi: 10.2165/00019053-200725010-00002 [DOI] [PubMed] [Google Scholar]
- 31.Zamandi M, Daroudi R, Sari AA. Direct costs of hypertension treatment in Iran. Iran J Public Health. 2023;52(9):1973–83. doi: 10.18502/ijph.v52i9.13579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazemi Z. Estimating and forecasting the health and cost effects of the main diseases attributed to air pollution in the metropolis of Tehran using mathematical modeling and micro simulation. Tehran university of medical science. 2023. [Google Scholar]
- 33.Movahed MS, Barghazan SH, Adel A, Rezapour A. Economic burden of stroke in Iran: a population-based study. Value Health Reg Issues. 2021;24:77–81. doi: 10.1016/j.vhri.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Raghfar H, Sargazi N, Mehraban S, Akbarzadeh MA, Vaez Mahdavi MR, Vahdati Manesh Z. The economic burden of coronary heart disease in Iran: a bottom-up approach in 2014. J Ardabil Univ Med Sci. 2018;18(3):341–56. [Google Scholar]
- 35.Non-Communicable Diseases Research Center, Nasbod Survey. Available from: https://vizit.report/panel/nasbod/fa/main.html#/treemap. 2016. [Google Scholar]
- 36.Statistical Center of Iran. Statistical Center of Iran. 2020. Available from: http://www.amar.org.ir. [Google Scholar]
- 37.World bank. 2020. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CN?Locations=IR. [Google Scholar]
- 38.Akbari Sari A, Karimi F, Emrani Z, Zeraati H, Olyaeemanesh A, Daroudi R. The impact of common chronic conditions on health-related quality of life: a general population survey in Iran using EQ-5D-5L. Cost Eff Resour Alloc. 2021;19(1):28. doi: 10.1186/s12962-021-00282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng H-M, Pearson A, Sung S-H, Yu W-C, Chen C-H, Karnon J. Cost-effectiveness of noninvasive central blood pressure monitoring in the diagnosis of hypertension. Am J Hypertens. 2015;28(5):604–14. doi: 10.1093/ajh/hpu214 [DOI] [PubMed] [Google Scholar]
- 40.Jahanbin S-F, Yusefzadeh H, Nabilou B, Alinia C. Value of willingness to pay for a QALY gained in Iran; a modified chained-approach. BMC Health Serv Res. 2021;21(1):1339. doi: 10.1186/s12913-021-07344-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safari H, Poder T, Afshari S, Nahvijou A, Arab-Zozani M, Moradi N. Determination of a cost-effectiveness threshold for cancer interventions in a middle-income Country. Front Oncol. 2022;6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Bank. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CN?locations=IR. 2020. [Google Scholar]
- 43.Wang YC, Cheung AM, Bibbins-Domingo K, Prosser LA, Cook NR, Goldman L, et al. Effectiveness and cost-effectiveness of blood pressure screening in adolescents in the United States. J Pediatr. 2011;158(2):257–64.e1-7. doi: 10.1016/j.jpeds.2010.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asmah EE, Andoh FK, Razvi S, Wong B. Cost-benefit analysis of community health worker-led screening and treatment for hypertension in Ghana. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files