Abstract
Birdsong, like human speech, involves rapid, repetitive, or episodic motor patterns requiring precise coordination between respiratory, vocal organ, and vocal tract muscles. The song units or syllables of most adult songbirds exhibit a high degree of acoustic stereotypy that persists for days or months after the elimination of auditory feedback by deafening. Adult song is assumed to depend on central motor programs operating independently from immediate sensory feedback. Nothing is known, however, about the possible role of mechanoreceptive or other somatosensory feedback in the motor control of birdsong. Even in the case of human speech, the question of “how and when sensory information is used in normal speaking conditions… remains unanswered” and controversial [Smith, A. (1992) Crit. Rev. Oral Biol. Med. 3, 233–267]. We report here evidence for somatosensory modulation of ongoing song motor patterns. These patterns include the respiratory muscles that, in both birdsong and speech, provide the power for vocalization. Perturbing respiratory pressure by a brief, irregularly timed injection of air into the cranial thoracic air sac during song elicited a compensatory reduction in the electrical activity of the abdominal expiratory muscles, both in hearing and deafened adult northern cardinals (Cardinalis cardinalis). This muscle response was absent or reduced during quiet respiration, suggesting it is specifically linked to phonation. Our findings indicate that somatosensory feedback to expiratory muscles elicits compensatory adjustments that help stabilize, in real time, the subsyringeal pressure against fluctuations caused by changes in posture or physical activity.
Auditory feedback plays an essential role in vocal learning by juvenile oscine songbirds. Its elimination (1), or the disruption of sensorimotor integration (2), during certain stages of song development results in an abnormal adult song. Vocal learning is terminated by a loss of vocal plasticity in a process referred to as song crystallization. In songbirds that are age-limited learners, this occurs once during their life, in early adulthood. Other species that are open-ended learners, retain an annual seasonal vocal plasticity followed by crystallization. Even some age-limited learners require auditory feedback to maintain adult song. The crystallized song of adult zebra finches, e.g., begins to deteriorate ≈1.5–2 mo after deafening (3). Deafened, adult Bengalese finches stop singing ≈10% of their predeafened note types within 5 d after surgery and the sequence of the remaining notes within song bouts becomes less consistent (4). Adult zebra finches exposed to perturbed auditory feedback begin to sing abnormally within ≈6 wk if the syllables affected by artificial feedback vary from song to song or within 1 wk if the perturbed feedback is consistently associated with a particular syllable type (5). Analogous gradual changes also occur in the speech of adult humans who are deprived of normal auditory feedback for a period of time (e.g., see ref. 6).
Very little is known about the role of somatosensory feedback in singing. Afferent neurons from the avian vocal organ, the syrinx, were identified in the hypoglossal nerve of zebra finches (7, 8), but bilateral deafferentation of the syrinx in deafened adult birds had little effect on song. The relatively stereotyped nature of adult, crystallized song and its independence from acute auditory feedback have fostered the assumption that the maintenance of adult song may be mediated by a central motor program (1, 9), perhaps located in telencephalic song control nuclei (10, 11). Immediate real-time somatosensory feedback could be useful in fine-tuning this ongoing vocal motor program and adjusting muscle actions to varying conditions in the vocal/respiratory system. The motor program for birdsong includes activation of the abdominal expiratory muscles, which power the airflow, necessary for phonation, through the syrinx. During song, the activity of these muscles is modulated according to the temporal complexity of the song syllables (12). The amplitude of their electromyograms (EMGs) is proportional to the air sac pressure and vocal intensity (13). Unlike song, which is lateralized in the syrinx (14), the activity of expiratory muscles is not lateralized (15), although it is precisely coordinated with that of the syrinx (14, 16–18). Because the respiratory pressures that power sound production, and hence the acoustic properties of the song, may vary with changes in posture or physical activity, somatosensory feedback could provide a mechanism for monitoring syringeal airflow or pressure during phonation and for stabilizing the acoustic output despite peripheral variations. In the experiments reported here, we show that these muscles use somatosensory feedback to make real-time compensatory adjustments to unpredictable, externally applied perturbations in respiratory pressure during song.
Materials and Methods
Northern cardinals are age-limited learners whose song crystallizes between 6 and 10 mo of age (19–21). Male cardinals have repertoires of ≈8–21 different syllable types (22). Their song is composed of one to several syllable types, which are repeated in phrases. Experiments were performed on adult male northern cardinals between 9 mo and 3 yr old that had been collected as nestlings when ≈1 wk old and reared in the laboratory where they were maintained on a normal outdoor photoperiod, regularly tutored with playback of cardinal song, and exposed to live songs of other adult cardinals as well as song of several other species.
Surgery and Data Recording.
A few days before an experiment, a timed-release (15 mg over 3 wk) pellet of testosterone propionate was implanted s.c. in a singing adult to increase the frequency of singing. The bird was anesthetized for surgery by inhalation of isoflurane. A pair of stainless steel wire recording electrodes (0.025 mm diameter, insulated except at the tip) were implanted in the abdominal expiratory muscles, primarily the M. obliquus externus abdominus as described by Wild et al. (23). Two silastic cannulae (ID 1.02 mm, OD 2.16 mm, length 6–8 cm) were inserted through the abdominal wall, one on each side of the midline, just posterior to the last rib so that they extended a few millimeters into the left and right cranial thoracic air sacs, respectively. In some experiments, a microbead thermistor (Thermometrics, Edison, NJ, BB05JA202N) also was placed in each primary bronchus, under Chloropent anesthesia (3.8–4.0 μl⋅g−1; recipe courtesy of Fort Dodge, Fort Dodge, IA), to measure airflow through each side of the syrinx as described by Suthers (24).
After surgery, the bird was returned to its home cage where it was free to move about on the end of a tether composed of fine wires from the EMG electrodes, pressure transducer and thermistors, and a small plastic tube for air injection. One cannula monitored air sac pressure and was connected to a miniature piezoresistive pressure transducer (Fujikura model FPM-02PG, Marietta, Ga) on the bird's backpack. The other cannula was connected to a pressure pulse generator (Parker Hannifin, Fairfield, NJ, Picospritzer II) by a length of inelastic plastic tubing (OD 1.8 mm, ID 0.8 mm). A small one-way valve on the bird's back prevented air from flowing out through the cannula when air sac pressure was above atmospheric pressure. When the picospritzer was triggered, a puff of compressed air was injected into one cranial thoracic air sac. Air injection was irregularly timed so it could not be anticipated by the bird. The timing and amplitude of the air puff entering the air sac was monitored by a heated microbead thermistor (Thermometrics, BB07PA302N) mounted in the air injection cannula ≈14 cm from the air sac. The air pulse reached the thermistor 1 ms before arriving in the air sac, but the data are not corrected for this delay. The injected pulse of air was adjusted to be shorter in duration than most song syllables and with enough amplitude to produce a slight transient increase in cranial thoracic air sac pressure. Air sacs on opposite sides of the body are connected through the interclavicular air sac, allowing a pressure change on one side to be transmitted to the contralateral side.
Two birds were deafened by bilateral removal of the cochlea according to the method described by Konishi (25).
Data were transmitted by fine wires to microconnectors on a backpack and then through the top of the cage, by the tether, to signal conditioning and recording instruments. Muscle potentials were differentially amplified (Dagan Instruments, Minneapolis, model EX4–400 or Princeton Applied Research model 113) and bandpass filtered (200–3,000 Hz, Dagan EX4–400 and Krohn-Hite model 3550, Avon, MA). The thermistors monitoring air injection and bronchial airflow were heated to a constant temperature by a feedback circuit (Hector Engineering, Elletsville, IN). The current required to maintain this temperature was converted to a voltage that was nonlinearly proportional to the rate of airflow. The bird's song was picked up by a microphone (Audio-Technica, model AT835b, Stow, OH) mounted in front of the cage. All data were recorded digitally on separate channels of a data recorder (Metrum-Datatape, RSR-512, Littleton, CO).
Data Analysis.
Recorded data were reproduced at one-half speed and converted into “Signal” (Engineering Design, Belmont, MA) files with a real-time digitization rate of 40 kHz (Data Translation 2821-G; Marlboro, MA) per channel. EMGs were full-wave rectified (ENV command in Signal with time constant of 0.1 ms) and smoothed with a smoothing width of 10 ms for amplitude measurement. Because expiratory muscle EMG, air sac pressure, and sound intensity typically increase with each successive syllable during the early part of a phrase (e.g., Fig. 1 and see Fig. 4a), each test syllable, which could occur in different parts of a phrase, was matched by a control syllable of the same type occupying the same position in the phrase.
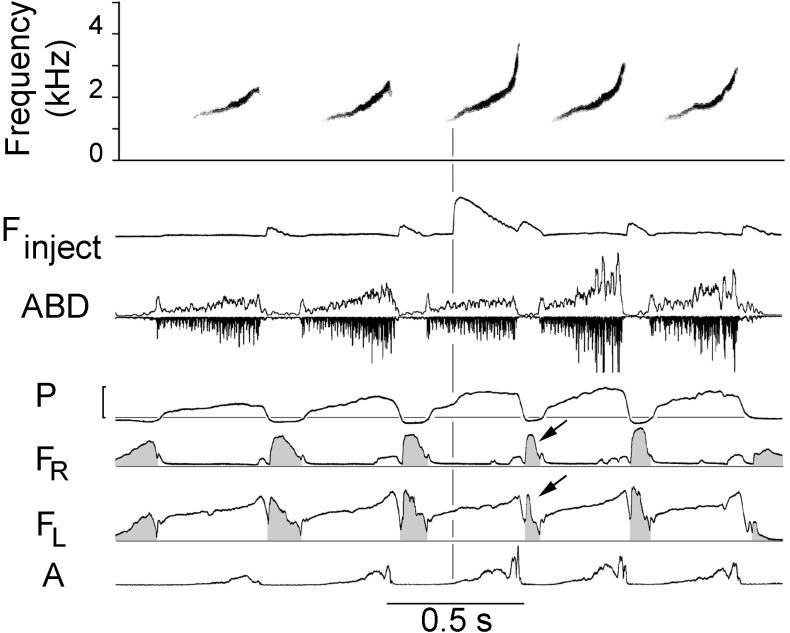
Figure 1.
Song phrase composed of relatively long syllables sung at a low repetition rate of ≈2 sec−1. A puff of air was injected into the air sac during the third syllable, resulting in a compensatory reduction in the amplitude of the EMG in the abdominal expiratory muscle. Note the upward inflection in air sac pressure produced by the injection. The following inspiration, minibreath, (arrows) is also smaller than normal, presumably because of the added volume of air. Most of this syllable type is produced in the left syrinx, as indicated by the absence of airflow through the right syrinx, which remains closed until just before the end of the syllable. The right side opens briefly during the terminal high frequency portion of the syllable when it may generate sounds above ≈3.5 kHz. Finject, rate of flow through the injection cannula from the picospritzer. The large upward deflection indicates the time course of the injected air puff. Some air also flows through the cannula during each inspiration, indicated by small deflections when air sac pressure is negative. ABD, EMG of abdominal expiratory muscle shown rectified (time constant 0.1 ms) and integrated (time constant 5 ms) (upward) and rectified (downward); P, subsyringeal air sac pressure (bracket = 10 cm H2O); FR and FL, rate of airflow through the right and left sides of the syrinx, respectively. Direction of airflow is indicated by air sac pressure. Inspiratory airflow is shaded. A, rectified (time constant 0.1 ms) and integrated (time constant 1 ms) amplitude of vocalization. Horizontal lines indicate ambient pressure and zero airflow.
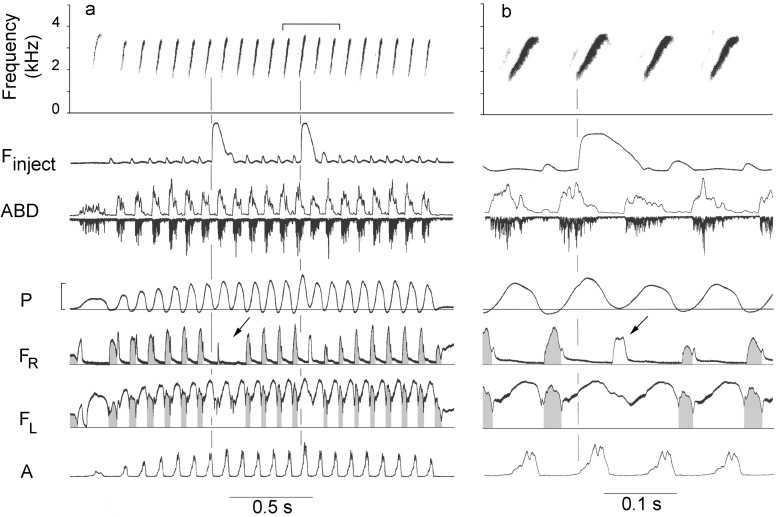
Figure 4.
(a) Two air puffs, each containing ≈0.14 ml, injected during a trilled phrase of short syllables repeated at a rate of 11 sec−1. The first air puff is delivered during a minibreath between syllables and reduces the normally negative inspiratory pressure to nearly zero so that there is almost no inspiratory airflow during the next two minibreaths (arrow). The temporal pattern of the respiratory cycle is unaffected. The second air puff is delivered at the beginning of a syllable. The expiratory muscle EMG has already peaked and it is difficult to tell whether it is affected by the increased pressure. Air sac pressure is still high during the next syllable, however, and the EMG is reduced. (b) Expanded view of second air puff in a. Note that, although the air puff is delivered at the onset of the second syllable, the pressure increase, visible as a slight inflection in the pressure cycle, occurs in the last one-half of the burst in expiratory muscle EMG activity. Pressure remains positive during the next inspiratory interval between syllables so that airflow during this “minibreath” (arrow) is in an expiratory direction. Air injection continues during the first one-half of the next burst of EMG activity, which is reduced in amplitude. See legend of Fig. 1. Pressure scale = 10 cm H2O.
For each syllable tested, the mean EMG amplitude was computed for each successive sample point starting at the EMG onset. The amplitude of the mean control EMG was then subtracted from that of each experimental EMG at the same syllable position to obtain the amplitude difference between control and experimental EMGs. These experimental difference EMGs were moved backward or forward in time to align the onset of the air puff to its median onset time relative to the start of each EMG. This temporal displacement was typically limited to several ms, by selecting trials in which there was only a small variation in the time of the air pulse, and did not exceed 25% of the syllable duration. The mean experimental difference EMG was then calculated. The latency of the EMG response was estimated by measuring the time from the onset of the air injection to the point when the SEM control EMG and the mean experimental difference EMG no longer overlapped. The amount by which the air injection changed the amplitude of the EMG was determined by comparing the mean amplitude of the experimental EMG, measured between the end of the latent period to the time when amplitude returned to the control value, to the mean amplitude of corresponding segments of the control EMGs at the same syllable position. The paired t test (two tailed) ± SE was used for statistical analysis unless otherwise indicated.
Care was taken to identify possible artifacts. The computer programs used for analysis were checked against manual calculations for accuracy. The most likely source of artifacts arises from the temporal translation of the EMGs necessary to time-align the onset of the air injection in different trials. For this reason, the amount of translation was minimized as described above, but some syllable types—for which the onset time of the EMG could not be accurately determined or in which the envelope of the rectified EMG was characterized by large, rapid amplitude modulation—could not be reliably analyzed and were excluded.
Results
Effect on Abdominal Expiratory Muscle EMG.
Each syllable in cardinal song is accompanied by a burst of activity in the EMG of the abdominal expiratory muscles, which begins ≈12 ms before the onset of sound production. The response of expiratory muscles to air injection is greatest if the air puff occurs early in the motor program producing the syllable.
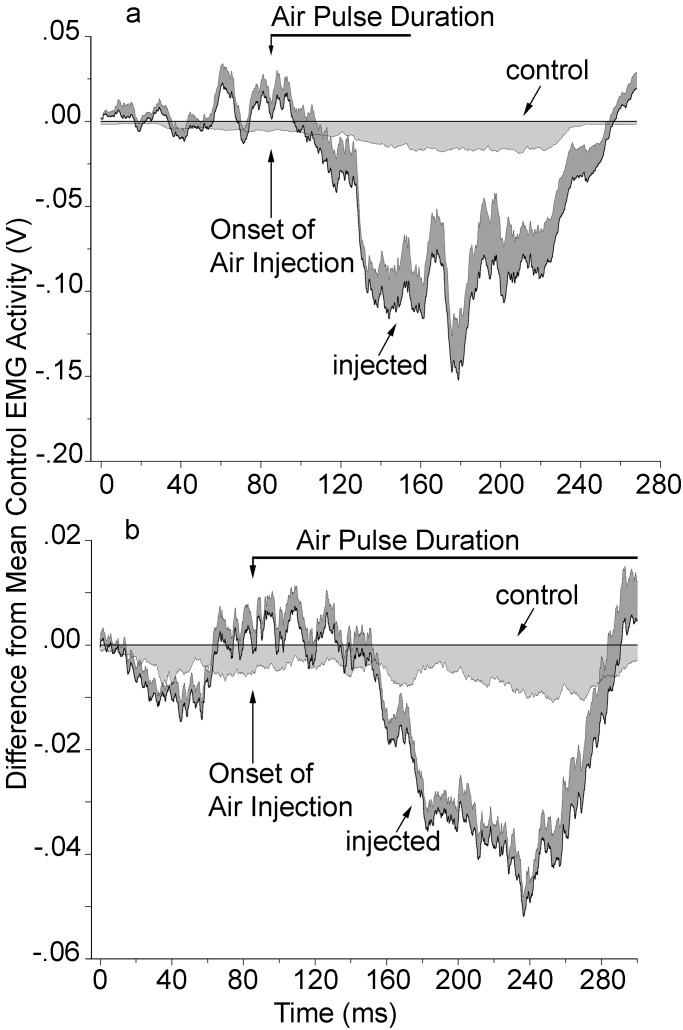
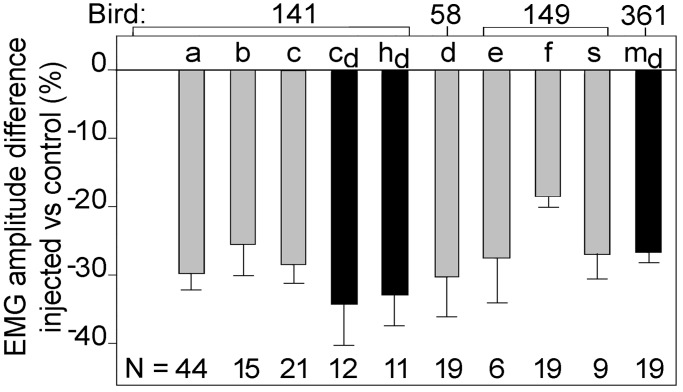
The injection of air into the cranial thoracic air sac (Fig. 1) during the first half of the EMG elicited a transient reduction in its amplitude (Fig. 2). The latency of this response, as estimated graphically from plots of the difference between the amplitude of the control and experimental EMG (e.g., Fig. 2a), is between ≈35 and 70 ms for seven syllable types of three hearing birds. The mean reduction in EMG amplitude for each of these syllables (Fig. 3; syllables a, b, c, d, e, f, and s) ranged from 18.6% to 30.3% (mean of all syllables = 23.9%). There was no consistent effect of air injection on the duration of the expiratory EMG.
Figure 2.
Relative mean amplitudes of abdominal expiratory muscle EMG during syllables with air injection (injected) compared to same syllable without air injection (control). (a) Bird 141 with hearing intact (syllable b). (b) Same bird after deafening (syllable h). See Materials and Methods. Shading indicates 1 SE. Air puff duration includes period of exponential decay after initial peak (see Fig. 1).
Figure 3.
Reduction in the mean amplitude of the abdominal expiratory muscle EMG after injection of air into the cranial thoracic air sac during song for each of the seven syllable types in hearing birds (gray) and three syllables in deaf birds (black). The mean rectified amplitude of the EMG during the interval from the end of the latent period (see Materials and Methods) after air injection until the control and experimental SEs again overlap (Fig. 2) is compared to the same segment of EMG in control syllables occupying the same position in the phrase as the experimental syllables. Mean ± SE (syllables sung by hearing birds: a, b, c, d, e, and s, P < 0.0001; f, P = 0.013; syllables sung by deaf birds: cd, hd, and md, P < 0.001; paired t test)
Air injected during the last half of a syllable typically elicited a smaller effect on the abdominal expiratory EMG amplitude, even though the EMG continued well past the latent period. In four different syllable types from three birds, the effect of air injection near the beginning of the EMG was compared to that of injection early in the second one-half of the same syllable type. These mid-syllable EMG injections produced the following changes in EMG amplitude relative to the uninjected controls: Syllable a, +1.1% ± 3.6, n = 15, P = 0.82; b, −5.9% ± 5.5, n = 8, P = 0.39; d, −14.2% ± 3.3, n = 14, P = 0.01; and s, −14.2% ± 2.0, n = 5, P = 0.02. Only in syllables d and s was there a significant reduction of the EMG, but this reduction was approximately one-half that produced by a similar injection early in the EMG (Fig. 3). The mean reduction in EMG amplitude for these four syllables is 28.2% ± 3.3 for early air injections and 8.3% ± 3.7 for late injections.
Auditory Feedback Is Not Required.
Deafening two birds by bilateral removal of their cochleae did not abolish the alteration in EMGs after air injection. In both deaf birds, air injection during the first part of a syllable was followed by a mean reduction in EMG amplitude of 31.3 ± 4.1% (range 26.7–34.3%; Fig. 3), indicating a dependence on nonauditory, presumably somatosensory, feedback. There was no detectable difference in the estimated response latency between deaf and hearing birds (Fig. 2).
Effect on Expiratory Airflow During Song.
The partial relaxation of the expiratory muscles in response to air injection stabilizes syringeal airflow during phonation, but this response depends on the time during the syllable when air sac pressure is perturbed. Bronchial thermistors were placed in two birds to measure the rate of airflow through each side of the syrinx. When air was injected during the first half of one syllable (two syllable types; n = 13 and 14), the mean amplitude of the abdominal EMG decreased 23.4% (P = 0.0001) and 27.7% (P = 0.0002), respectively, in each bird, and there was no significant change in the rate of expiratory airflow through either the left (P = 0.1332) or right (P = 0.0777) bronchus. In one bird there was no measurable effect on the mean air sac pressure (P = 0.1479) or on the amplitude of the vocalization (P = 0.1044). In the other bird the mean air sac pressure increased 8.6% and the amplitude of the vocalization increased ≈1.7 dB.
When a similar volume of air was injected during the last half of the same syllable type (n = 14 and 15), there was no significant change in the mean amplitude of the abdominal EMG in either bird (see above) and the mean air sac pressure increased 19.3% (P = 0.014) and 23.9% (P < 0.0001), respectively, in each bird. The rate of airflow through the left side of the syrinx did not change but that through the right side increased 15.6% (P < 0.01) and the amplitude of the acoustic waveform increased 3.2 dB (P < 0.01). These results suggest that, as the song syllable progresses, air injection has a reduced effect on the song motor program.
Inspiratory Airflow Is Not Stabilized.
During the inspiratory minibreaths between syllables, abdominal expiratory muscles have no detectable EMG and thoracic inspiratory muscles are active (23). We have not recorded from inspiratory muscles during air injection so we do not know whether they respond to pressure perturbations, but when at high syllable repetition rates an injected air puff included both a phonatory expiration and its adjacent minibreath, the rate of inspiratory airflow (Fig. 4, FR) varied according to the altered air sac pressure. Thus inspiration between syllables was greatly reduced, completely eliminated (Fig. 4a, arrow) or replaced by an expiration (Fig. 4b, arrow). This finding is in contrast to expiratory airflow, which remains nearly constant during sound production despite the air injection (Fig. 4, FL).
Effect on Syllable Repetition Rate.
Air injection during phonation affected the magnitude of the motor output to expiratory muscles but had little effect on its temporal pattern. Except at the highest repetition rates in which there was no inspiration between syllables, the tempo of a song was set by the period of the respiratory cycle. Air injection at the beginning of a syllable in a trill (Fig. 4) increased the duration of that syllable ≈17% from a mean of 40–47 ms (P = 0.026; n = 7 trills), but this increase was largely compensated for by an approximately equivalent reduction in the following intersyllable interval (from a mean of 56 ms in control song to 47 ms in experimental song; P = 0.024). There is therefore only a slight change in the instantaneous syllable repetition rate (from ≈10.5 to 10.2 syllables⋅s−1; P = 0.003). Each syllable type in cardinal song is sung at a characteristic tempo that may be regulated by oscillator circuits operating independently of feedback from externally imposed perturbations (at least for the short durations tested here) in respiratory pressure or in the velocity or direction of syringeal airflow.
Quiet Respiration.
Injection of a similar volume of air into the air sac during the first half of the expiratory phase of silent respiration in deaf cardinal 141 did not elicit a significant change in the abdominal expiratory muscle EMG compared to the EMG during either the immediately preceding or subsequent uninjected expiration (mean injected EMG = 0.0319 ± 0.0014; control before = 0.0352 ± 0.0008, injected 9.4% < control, P = 0.06, n = 22; control after = 0.0345 ± 0.0008, injected 7.5% < control, P = 0.12, n = 22). During silent respiration, however, the recorded EMG is of very low amplitude with a poor signal-to-noise ratio (inspiration noise = 0.0242 ± 0.0008; n = 12; mean expiration/mean inspiration = 1.45) that limits the reliability of the measurement. Sometimes air injection appeared to be followed by a small decrease in EMG amplitude, but this reduction was never consistent enough or large enough to reach statistical significance. This expiratory reflex thus appears to be greatly reduced or absent during quiet respiration.
Discussion
Somatosensory Feedback Stabilizes Vocal Output in Real Time.
These experiments provide evidence that the respiratory motor pattern of crystallized song is modulated in real time by somatosensory feedback. This feedback elicits compensatory motor adjustments within the same syllable to perturbations in respiratory pressure. Konishi (1) suggested that during song development a bird might memorize a match between auditory and nonauditory feedback. Somatosensory feedback after song crystallization may continue to play an important role in allowing rapid online adjustments of the song motor program to varying peripheral conditions and may help maintain or delay the deterioration of song when auditory feedback is distorted (5) or eliminated (1, 3, 4). The fact that pressure perturbations affect only the magnitude, but not the temporal aspects, of the respiratory motor pattern is consistent with the hypothesis that this pattern is determined by a central pattern generator (26).
The compensatory relaxation of the expiratory muscles, in response to the increased load caused by a transient slight inflation of the air sac, minimizes the change in subsyringeal pressure and syringeal airflow because of the injection of air into the air sac. We hypothesize that this is an adaptive motor response that functions to maintain the acoustic stereotypy of the syllable by protecting the syringeal sound generators from unpredictable fluctuations in respiratory pressure that may accompany changes in posture, physical activity, or the level of arousal. The lack of a clear, similar response when the bird is not singing and the fact that flow rates during minibreaths appear to vary with changing pressure are consistent with this interpretation.
The extent of somatosensory feedback to the respiratory motor system during silent respiration is uncertain for reasons described above, but our data strongly suggest that its influence is substantially increased during song when it presumably facilitates close coordination between the vocal and respiratory subsystems. Manogue and Paton (27) described a similar respiratory gating, during quiet respiration, of the access of song control nuclei to the hypoglossal motor neurons innervating the syrinx. Electrical stimulation of HVc or robustus archistriatalis during expiration elicited stimulus locked potentials in both the ipsilateral and contralateral tracheosyringeal nerves. Similar stimuli delivered during inspiration had a reduced effect on motor nerve activity. Manogue and Patton (27) suggested that the relative disengagement of the vocal control telencephalic nuclei from nXII during inspiration permits the same motor neurons to function effectively both during passive breathing, when they may serve to maintain an appropriate airway aperture and are under medullary motor control, and during singing, when they and the respiratory network are under learned, voluntary control of telencephalic nuclei.
Our data indicate that, even within the expiratory phase of respiration, the motor program for most syllables is more accessible to somatosensory feedback early in the syllable than late in its production. Sensory feedback may be most valuable in the motor planning or early execution phase of the vocal gesture producing the syllable so that its motor program can be adjusted for variation in respiratory pressure.
Possible Receptors and Pathways.
The somatosensory receptors mediating this expiratory muscle response to changes in respiratory pressure have not been identified in birds. Bird respiration does not rely on a muscular diaphragm and receptors sensing changes in respiratory pressure or volume could in theory be located almost anywhere in the thoracoabdominal cavity or the respiratory musculature. Alternatively, changes in the rate of airflow might be detected by receptors in the airways. There are no data on the presence or distribution of muscle spindle organs in the respiratory muscles of songbirds although they are sparsely present in the transverse abdominal muscles of the domestic fowl (28). The air sacs themselves are said to be richly innervated (29) and Ballam et al. (30) have suggested on physiological grounds that at least some of this innervation might be supplied by slowly adapting vagal mechanoreceptors such as those that Gleeson and Maloney (31) identified in the vagus nerve as being sensitive to air sac expansion. There is also a richly innervated saccopleural membrane between the wall of the thoracic air sac and the parietal pleura (29), which could also contain mechanoreceptors.
The 35- to 70-ms latency that we observed in alert, singing birds between air injection and the change in the abdominal expiratory muscle EMG represents an upper limit because it includes the time required for air sac pressure to increase. An increase in air sac pressure typically is evident within 1–2 ms after the onset of the air pulse and rises rapidly, peaking at ≈22 ± 6 ms (mean ± SD, n = 31; three syllables, bird 141) before declining to normal. The rate of increase in air sac pressure depends on the injection pressure and is independent of pulse duration. The minimum change in air sac pressure required to elicit an EMG response has not been determined but the fact that EMG amplitude is sometimes reduced after air injections too small to produce a change in air sac pressure measurable with our methods, indicates a high sensitivity to changes in respiratory pressure. The time required for pressure to reach the response threshold probably represents a very small portion of the expiratory EMG latency as we have measured it. It is possible that the response of cardinal expiratory muscles to air injection may include the medullary nucleus retroambigualis (RAm), which contains premotor expiratory neurons (32, 33). RAm also receives descending inputs from the song control nuclei, robustus archistriatalis in the telencephalon, and the dorsomedial intercollicular nucleus in the midbrain (34, 35). These connections between RAm and the song control system could provide close coordination of vocal and respiratory motor patterns. The time required for a sensory stimulus signaling a change in respiratory pressure to reach RAm and elicit an effect on the expiratory muscle EMG has not been measured. RAm produces a burst of action potentials during each expiration, the first of which precedes the onset of the abdominal expiratory muscle EMG by ≈20–50 ms in an anesthetized songbird (36).
Comparison with Mammals and Relationship to Human Speech.
Segmental stretch reflexes are well documented in mammalian respiration. In mammals, the excitability of abdominal muscles is modulated during silent respiration by feedback from stretch receptors in the abdominal wall and from a variety of other receptors in the respiratory tree (37, 38). In cats, vagal feedback elicited by an increase in tidal volume augments abdominal muscle expiratory activity (39). Unloading these muscles by bypassing the resistance of the upper airway is followed by decreased muscle activity after a latency of 15–20 ms, presumably because of the unloading of the abdominal spindle afferents (40).
During human speech, abdominal muscles assist in maintaining a constant subglottic pressure by contracting as lung volume drops into the expiratory reserve (41). By preventing caudal displacement and shortening of the diaphragm as the rib cage volume is reduced during expiration, these muscles may also facilitate the ability of the diaphragm to generate periodic, brief inspirations during conversational speech (42, 43).
An understanding of the role of somatosensory feedback in birdsong production may contribute to a better appreciation of its role in human speech. Both speech and birdsong share a number of features including the important role of motor learning and the necessity for precise coordination between vocal and respiratory motor patterns. The role of afferent feedback in speech production is unclear (44, 45). Various investigators have examined expiratory muscle responses to changes in load during repetitive monosyllabic utterances (e.g., refs. 46–48). In none of these studies, however, is there a clear relevance of a respiratory response to normal speech. Newsome Davis and Sears (49) reported that both inspiratory and expiratory intercostal muscles in humans respond to sudden, brief changes in load with an initial reduction in electrical activity at a latency of ≈22 ms. This is followed by an increase in activity at a latency of 50–60 ms, attributed to the stretch reflex. Increasing the load on the expiratory intercostals when the subject was singing a note at a constant pitch produced a larger excitatory response in these muscles than at a similar flow rate without phonation. The authors hypothesized that the initial reduction in EMG is initiated by tendon organs but is normally suppressed by descending central control unless a learned movement encounters an unexpected load. Inhibiting the EMG may prevent a premature inappropriate response to an unexpected load and permit the servo loop for control during predictable loads to have a higher gain (49). Newsome Davis and Sears (49) increased load by impeding expiratory airflow at the mouth, whereas we increased the respiratory pressure driving expiration. The expiratory muscle response necessary to compensate for the load perturbation is thus increased contraction (stretch reflex) in the human paradigm but relaxation in our avian paradigm.
Acknowledgments
We are grateful to D. Parson for computer programming and S. Ronan for technical assistance. We thank Dr. R. Mooney and two anonymous reviewers for helpful comments on a draft of this manuscript. This work was supported by National Institutes of Health Grant NS29467 (to R.A.S.).
Abbreviations
- EMG
electromyogram
- RAm
nucleus retroambigualis
References
- 1.Konishi M. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 2.Pytte C L, Suthers R A. J Neurobiol. 2000;42:172–189. doi: 10.1002/(sici)1097-4695(20000205)42:2<172::aid-neu2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Nordeen K W, Nordeen E J. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 4.Okanoya K, Yamaguchi A. J Neurobiol. 1997;33:343–356. [PubMed] [Google Scholar]
- 5.Leonardo A, Konishi M. Nature (London) 1999;399:466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 6.Waldstein R. J Acoust Soc Am. 1990;88:2099–2114. doi: 10.1121/1.400107. [DOI] [PubMed] [Google Scholar]
- 7.Bottjer S W, Arnold A P. J Comp Neurol. 1982;210:190–197. doi: 10.1002/cne.902100209. [DOI] [PubMed] [Google Scholar]
- 8.Bottjer S W, Arnold A P. J Neurosci. 1984;4:2387–2396. doi: 10.1523/JNEUROSCI.04-09-02387.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi M. In: Annual Reviews in Neuroscience. Cowan W M, editor. Vol. 8. Palo Alto, CA: Annual Reviews; 1985. pp. 125–170. [Google Scholar]
- 10.Vu E T, Mazurek M E, Kuo Y-C. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu E T, Schmidt M F, Mazurek M E. J Neurosci. 1998;18:9088–9098. doi: 10.1523/JNEUROSCI.18-21-09088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartley R S. Respir Physiol. 1990;81:177–187. doi: 10.1016/0034-5687(90)90044-y. [DOI] [PubMed] [Google Scholar]
- 13.Suthers R A, Goller F. In: Current Ornithology. Nolan V Jr, Ketterson E, Thompson C F, editors. Vol. 14. New York: Plenum; 1997. pp. 235–288. [Google Scholar]
- 14.Suthers R A. J Neurobiol. 1997;33:632–652. [PubMed] [Google Scholar]
- 15.Goller F, Suthers R A. J Neurobiol. 1999;41:513–523. doi: 10.1002/(sici)1097-4695(199912)41:4<513::aid-neu7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Suthers R A, Goller F, Pytte C. Philos Trans R Soc London B. 1999;354:927–939. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicario D S. J Neurobiol. 1991;22:63–73. doi: 10.1002/neu.480220107. [DOI] [PubMed] [Google Scholar]
- 18.Vicario D S. Curr Opin Neurobiol. 1991;1:595–600. doi: 10.1016/s0959-4388(05)80034-0. [DOI] [PubMed] [Google Scholar]
- 19.Dittus W P J, Lemon R E. Anim Behav. 1969;17:523–533. [Google Scholar]
- 20.Lemon R E, Scott D M. Can J Zool. 1966;44:191–197. [Google Scholar]
- 21.Yamaguchi A. Dissertation. Davis: Univ. of California; 1996. [Google Scholar]
- 22.Halkin S L, Linville S U. In: The Birds of North America. Poole A, Gill F, editors. Philadelphia: The Birds of North America; 1999. , No. 440, pp. 1–32. [Google Scholar]
- 23.Wild J M, Goller F, Suthers R A. J Neurobiol. 1998;36:441–453. doi: 10.1002/(sici)1097-4695(19980905)36:3<441::aid-neu11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Suthers R A. Nature (London) 1990;347:473–477. [Google Scholar]
- 25.Konishi M. Z Tierpsychol. 1963;20:770–783. [PubMed] [Google Scholar]
- 26.Gracco V L, Abbs J H. Exp Brain Res. 1988;71:515–526. doi: 10.1007/BF00248744. [DOI] [PubMed] [Google Scholar]
- 27.Manogue K R, Paton J A. Brain Res. 1982;247:383–387. doi: 10.1016/0006-8993(82)91265-3. [DOI] [PubMed] [Google Scholar]
- 28.DeWet P D, Fedde M R, Kitchell R L. J Morphol. 1967;123:17–34. doi: 10.1002/jmor.1051230103. [DOI] [PubMed] [Google Scholar]
- 29.McLelland J. In: Form and Function in Birds. King A S, McLelland J, editors. Vol. 4. New York: Academic; 1989. pp. 221–279. [Google Scholar]
- 30.Ballam G O, Clinton T L, Kaminski R P, Kunz A L. J Appl Physiol. 1985;59:991–1000. doi: 10.1152/jappl.1985.59.3.991. [DOI] [PubMed] [Google Scholar]
- 31.Gleeson M, Moloney V. In: Form and Function in Birds. King A S, McLelland J, editors. Vol. 4. London: Academic; 1989. pp. 439–484. [Google Scholar]
- 32.Wild J M. J Neurobiol. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Wild J M. Brain Res. 1993;606:119–124. doi: 10.1016/0006-8993(93)91001-9. [DOI] [PubMed] [Google Scholar]
- 34.Wild J M. J Comp Neurol. 1993;338:225–241. doi: 10.1002/cne.903380207. [DOI] [PubMed] [Google Scholar]
- 35.Wild J M, Li D, Eagleton C. J Comp Neurol. 1997;377:392–413. doi: 10.1002/(sici)1096-9861(19970120)377:3<392::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Wild J M. Brain Behav Evol. 1994;44:192–209. doi: 10.1159/000113577. [DOI] [PubMed] [Google Scholar]
- 37.Frazier D T, Xu F, Lee L-Y. In: Neural Control of the Respiratory Muscles. Miller A D, Bianchi A L, Bishop B P, editors. Boca Raton, FL: CRC; 1997. pp. 131–141. [Google Scholar]
- 38.Bishop B. In: Neural Control of the Respiratory Muscles. Miller A D, Bianchi A L, Bishop B P, editors. Boca Raton, FL: CRC; 1997. pp. 35–46. [Google Scholar]
- 39.Fergosi R F. J Appl Physiol. 1994;76:602–609. doi: 10.1152/jappl.1994.76.2.602. [DOI] [PubMed] [Google Scholar]
- 40.Remmers J E, Bartlett D., Jr J Appl Physiol. 1977;42:80–87. doi: 10.1152/jappl.1977.42.1.80. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto T, Nonaka S, Katada A. In: Neural Control of the Respiratory Muscles. Miller A D, Bianchi A L, Bishop B P, editors. Boca Raton, FL: CRC; 1997. pp. 249–258. [Google Scholar]
- 42.Estenne M, Zocchi L, Ward M, Macklem P T. J Appl Physiol. 1990;68:2075–2082. doi: 10.1152/jappl.1990.68.5.2075. [DOI] [PubMed] [Google Scholar]
- 43.Hoit J D, Plassman B L, Lansing R W, Hixon T J. J Appl Physiol. 1988;65:2656–2664. doi: 10.1152/jappl.1988.65.6.2656. [DOI] [PubMed] [Google Scholar]
- 44.Kent R D. In: Speech Production: Motor Control, Brain Research, and Fluency Disorders. Hulstijn W, Peters H F M, Lieshout P H H M V, editors. Amsterdam: Elsevier; 1997. pp. 13–36. [Google Scholar]
- 45.Smith A. Crit Rev Oral Biol Med. 1992;3:233–267. doi: 10.1177/10454411920030030401. [DOI] [PubMed] [Google Scholar]
- 46.Macefield V G, Gandevia S C, McKenzie D K, Butler J E. In: Vocal Fold Physiology: Controlling Complexity and Chaos. Davis P J, Fletcher N H, editors. San Diego: Singular; 1996. pp. 219–234. [Google Scholar]
- 47.Mead J, Reid M B. J Appl Physiol. 1988;64:2314–2317. doi: 10.1152/jappl.1988.64.6.2314. [DOI] [PubMed] [Google Scholar]
- 48.Baken R J, Orlikoff R F. In: Laryngeal Function in Phonation and Respiration. Baer T, Sasake C T, Harris K S, editors. Boston: College-Hill Press; 1987. pp. 273–290. [Google Scholar]
- 49.Newsome Davis J, Sears T A. J Physiol (London) 1970;209:711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]