Abstract
Farming communities are very concerned about salt stress because of its negative impact on crop productivity. This study evaluated the ability of the Methylobacterium oryzae CBMB20 and exogenous trehalose treatments on Arabidopsis thaliana growth under salt stress conditions. A. thaliana growth was enhanced using M. oryzae CBMB20 as a bioinoculant in both saline and non-saline environments. In addition to better photosynthetic efficiency and endogenous trehalose content, the inoculation of M. oryzae CBMB20 produced improved growth parameters, such as increased rosette fresh weight and shoot length. Reduced levels of proline and malondialdehyde (MDA) under salt stress (150 mM NaCl) further indicated that the inoculated plants had enhanced tolerance to salinity. GFP-tagged M. oryzae CBMB20 was also used in spatial distribution experiments, which showed that the bacteria colonized A. thaliana's root, shoot, and hypocotyl. By increasing shoot length and total fresh weight, the exogenous application of trehalose also markedly enhanced plant growth. Proline and MDA contents were decreased by exogenous trehalose during salt stress, while the endogenous trehalose concentration in A. thaliana remained unaffected. The use of trehalose and M. oryzae CBMB20 can both have a good impact on plant development and stress tolerance in saline environments.
Keywords: Arabidopsis thaliana, Methylobacterium oryzae, PGPR, salt stress, trehalose, osmolytes
Introduction
Plants are routinely exposed to a range of abiotic stimuli during their life cycle, such as heat, cold, salt, drought, and heavy metals. These stresses have a negative impact on plants' development, growth, and yield [1]. One significant abiotic stressor that has a global impact on agricultural productivity and food security is soil salinity. Twenty percent of irrigated lands, or over 62 million hectares, are currently affected by excessive salinity, and by 2050, it is predicted to affect more than 50% of arable areas [2]. Many plants become susceptible to soil salinization, which lowers turgor, reduces harvest quantity and quality, and slows down growth and development rates. Every important aspect of plant life is impacted by salt stress, including germination, growth, pigments involved in photosynthetic processes, water relations, nutritional balance, oxidative stress, and yield. Therefore, methods such as soil flushing, liming, plant breeding and genetic engineering of salt tolerant crops are employed to alleviate soil salinity. However, these strategies can lead to the genetic erosion of native crop species, are time consuming, expensive, and have limited success [3]. Plants also employ various strategies to alleviate salt stress with the help of endosphere, phyllosphere, and rhizosphere microbiomes [4].
Harnessing the plant microbiome to improve productivity and overcome multiple stresses is a key to sustainable crop production [5]. Plant growth-promoting rhizobacteria (PGPR) are recognized for alleviating both abiotic and biotic stresses encountered by plants [6, 7]. Plant growth is significantly improved by PGPR through direct and indirect mechanisms [8]. Directly, they enhance plant growth by regulating phytohormone levels, increasing siderophore production, and improving nutrient availability through nitrogen fixation and phosphorus/ion solubilization [9]. Indirectly, PGPR promote plant growth by enhancing plant tolerance to both biotic and abiotic stresses [10]. The use of PGPR in agriculture has been recommended to achieve sustainable and eco-friendly crop production, particularly for stress alleviation [11]. PGPR like Azospirillum, Arthrobacter, Azotobacter, Bacillus, Burkholderia, Enterobacter, Pseudomonas, and Methylobacterium oryzae have been demonstrated to enhance salt tolerance in various crops [12-14]. These bacteria can reduce the toxic effects of high salinity by increasing biomass, chlorophyll content, and the production of osmoprotectants such as proline and malondialdehyde (MDA) [15].
Several ACC deaminase-producing halotolerant bacteria isolated from coastal saline soils are reported to have plant growth-promoting activities under salinity [16]. The occurrence of the genus Methylobacterium has been reported in more than 70 plant species either as free living, as endophytic, or as epiphytic symbiotic bacteria. Methylobacterium oryzae CBMB20 is a pink-pigmented bacterium that produces 1-aminocyclopropane-1-carboxylate deaminase (ACCD) [17, 18], along with indole-3-acetic acid (IAA), extracellular polysaccharides (EPS), polyhydroxybutyrate and biofilm [19]. According to Wang [20], inoculating ACC deaminase producing bacteria enhances the photosynthetic characteristics of salt-stressed pea plants. M. oryzae CBMB20 have been reported to enhance plant growth and productivity through enhanced nutrient accumulation [21, 22] and production of phytohormone [23, 24]. Rice cultivars treated with M. oryzae CBMB20 showed improved plant vacuolar H+ ATPase activity, and photosynthetic characteristics and decreased ACC accumulation, ACO activity and VOC emission there by the effect of salinity was alleviated [25]. Apart from conferring ACC deaminase-mediated salinity tolerance, M. oryzae CBMB20 also induces defense responses in tomatoes [26, 27].
Under salt stress, osmolytes like glycine betaine, proline, and trehalose can help maintain cell turgor, stabilize proteins, scavenge reactive oxygen species (ROS), and protect membranes from oxidative damage. Therefore, it has been proposed that applying osmolytes externally, either individually or in combination, is an effective strategy for mitigating the effects of salt stress in plants [28]. Exogenous application of trehalose has shown promising potential for crop improvement. For instance, low concentrations of trehalose can decrease sodium ion accumulation in plants, while higher concentrations can prevent chlorophyll loss in leaves and root damage caused by high salt levels, thereby alleviating salt-induced damage [29, 30]. Additionally, research has shown that exogenous trehalose significantly affects ion imbalance, ROS proliferation, and programmed cell death induced by salt stress in Arabidopsis thaliana [31], maize [32], Catharanthus roseus [33] and tomato [28]. Furthermore, trehalose significantly decreased MDA accumulation in tomato, suggesting that it can reduce cell membrane damage and enhance salt tolerance in the plant [28]. However, report on ACC deaminase producing bacteria and in combination with trehalose on salt stress alleviation is scanty. This study aimed to assess the effects of ACC deaminase producing M. oryzae CBMB20 and the application of trehalose on Arabidopsis thaliana, specifically focusing on their potential to alleviate salt stress. Key aspects investigated include plant growth, proline content, and levels of chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids, MDA, and trehalose sugar production.
Materials and Methods
Plant Material and Salt Tolerance Assay
The wild type Arabidopsis thaliana Columbia 0 ecotype (Col-0 accession) seeds were surface disinfected for 5 min in 30% NaOCl and rinsed well with sterile water. The surface disinfected seeds were re-suspended in sterile water and stored at 4°C in the dark for 72 h. The seeds were then placed on 0.4% agar plates containing ½ strength MS medium + 0.5% sucrose (pH 5.7). The plates were incubated in growth chamber at 22 ± 2°C with 16 h light/8 h dark photoperiods, at a light intensity (photon flux density) of 99.5 μmole m-2s-1. In the experiment, 10-day-old Col-0 seedlings were transplanted into plastic plots (dimensions: inner diameter = 9 cm; outer diameter = 10 cm; depth = 9 cm) containing 100 g nursery soil (Nongwoo-Bio Co., Ltd., Republic of Korea; composition: 58.8% coco peat, 17% peat moss, 10% perlite, 10% vermiculate, 4% zeolite, 0.004% pyroligneous acid and 0.01% wetting agent) and grown in a growth chamber. Salt stress was induced gradually by applying 25 mM of sodium chloride solution to each pot on alternative days to avoid osmotic shock; and the desired salt concentrations of 50, 100, 150 and 200 mM were achieved after 2, 6, 10, and 14 days, respectively. Preliminary tests revealed that A. thaliana plants can tolerate up to 150 mM NaCl, but at 200 mM NaCl, the stress symptoms become quite severe such as retarded growth, leaf senescence and loss of turgor. After 14 days of NaCl treatment, the morphology of the plants was observed, and the physiological indices were measured to determine the optimal NaCl concentration. Based on the results obtained in this study, 150 mM NaCl was chosen for further experiments.
Microbial Culture and Growth Conditions
Methylobacterium oryzae CBMB20 (obtained from Department of Agricultural Chemistry, Chungbuk National University, Republic of Korea) used in this study was maintained in ammonium mineral salts (AMS) medium (K2HPO4 0.7 g/l; KH2PO4 0.54 g/l; MgSO4·7H2O 1.0 g/l; CaCl2·2H2O 0.2 g/l; FeSO4·7H2O 4.0 mg/l; ZnSO4·7H2O 100 μg/l; MnCl2·4H2O 30 μg/l; H3BO3 300 μg/l; CoCl2·6H2O 200 μg/l; CuCl2·2H2O 10 μg/l; NiCl2·6H2O 20 μg/l; Na2MoO4·2H2O 60 μg/l; NH4Cl 0.5 g/l) supplemented with 0.5% sodium succinate as the sole carbon source at 30°C. Single colonies were inoculated in Ammonium Mineral Salts (AMS) medium broth and grown aerobically on a rotary shaker (150 rpm) at 30°C for up to 72 h. Prior to inoculation, bacterial cells were harvested by centrifugation (9,000 rpm, 10 min, 4°C), washed 3 times with sterile 30 mM MgSO4 to remove the growth medium and re-suspended in 30 mM MgSO4 to a final population density of 108 CFU ml-1 and used for inoculation experiments.
Microbial Treatment and Imposing Salt Stress
The effectiveness of bacterial treatment in improving plant resistance to salt stress was assessed using a modified version of the methods [34]. Briefly, uniformly developed, 10-days-old healthy A. thaliana seedlings were transferred aseptically from ½ strength Murashige and Skoog (MS) agar plates to pots filled with nursery soil as described previously, one seedling per pot was maintained. Each treatment included three replicates, with a total of ten plants per treatment using a completely randomized design. After three days of acclimation, the plants were root-treated with 10 ml of a bacterial suspension (~ 108 CFU ml-1). Control A. thaliana plants were inoculated with sterile 30 mM MgSO4. Five days after bacterization, salt stress was induced gradually by applying 25 mM of sodium chloride solution to each pot on alternative days to avoid osmotic shock; and the desired salt concentrations of 150 mM was achieved after 10 days. Parallel controls were maintained by irrigating with tap water. The leaching of water from the pots was prevented by retaining the soil water to a level below water holding capacity. The soil electrical conductivity of 0, and 150 mM treatments were 1.31 and 16.23 dS/m, respectively, at the time of harvest. Fourteen days after exposing to salt stress, the plants were uprooted for growth parameter measurements.
Exogenous Trehalose Application and Assessing Salinity Tolerance
In this experiment, the 10-days-old wild-type Col-0 seedlings were transplanted into plastic plots (dimensions: inner diameter = 9 cm; outer diameter = 10 cm; depth = 9 cm) containing 100 g nursery soil and grown in a growth chamber. To study the role of trehalose against salinity, trehalose at the concentration of 0, 5, 10 and 15 mM was applied exogenously in the roots at the rate of ten ml per plant [35]. After 14 days of treatment, the seedlings were harvested for the assay of phenotypic changes. Based on the above experiment, four treatments were set and 10 mM trehalose was chosen for the further experiment based on physical parameters of plants.
A pot culture experiment was carried out, as detailed in the preceding section, to assess the impact of trehalose on reducing salt stress. Briefly, the 10-days-old wild-type Col-0 seedlings were transplanted into plastic plots (dimensions: inner diameter = 9 cm; outer diameter = 10 cm; depth = 9 cm) containing 100 g nursery soil and grown in a growth chamber. After 5 days of exogenous trehalose application, salt stress was induced gradually by applying 25 mM of sodium chloride solution to each pot on alternative days to avoid osmotic shock; and the desired salt concentrations of 150 mM was achieved after 10 days. Control plants were watered with tap water throughout the experiment, and to prevent water leaching, soil moisture was maintained below its water-holding capacity. The plants were harvested at 14 days after NaCl treatment the plants were uprooted for growth parameter measurements.
Phenotypic Assay and Photosynthetic Pigment Analysis
The parameters assessed included the whole fresh weight, shoot length, root length, and rosette fresh weight of Arabidopsis. The shoots of these seedlings were detached, rinsed with deionized water, dried, and weighed. Photosynthetic pigments (Chl a, Chl b, and carotenoids) were measured following the procedures described by Sumanta [36]. Briefly, the leaf samples (0.3 g) were homogenized in a tissue homogenizer using 80% (w/v) acetone. The homogenate was centrifuged at 6,000 × g, and the supernatant was used for pigment analysis. Absorbance of the extracted pigments was measured in UV-Visible Spectrophotometer (NEO-D3117, NEOGEN, Republic of Korea) at 480 nm, 510 nm, 645 nm and 663 nm. The photosynthetic pigment content was calculated using the following equations:
Chl a = [12.7 (OD663) -2.69 (OD645) × [(final volume of filtrate/1000) × 0.3)]
Chl b = [22.9 (OD645) -4.68 (OD663) × [(final volume of filtrate/1000) × 0.3)]
Carotenoid = [7.6 (OD480) -1.49 (OD510) × [(final volume of filtrate/1000) × 0.3)]
Determination of MDA Concentration
Malondialdehyde content was measured using the thiobarbituric acid (TBA) test, in which two molecules of TBA react with MDA to form a pinkish-red chromogen [37]. 0.5 g of ground frozen tissue was combined with 5 ml of 0.1% (w/v) trichloroacetic acid (TCA) in a 2 ml microcentrifuge tube. The tubes were centrifuged at 6,000 × g for 10 min, and 200 μl of the supernatant was mixed with 800 μl of 0.5% (w/v) TBA in 20% (w/v) TCA. The tubes were then incubated on a dry bath for 30 min at 100°C. After incubation, the tubes were cooled on ice to halt the reaction and centrifuged for 15 min at 6,000 × g. The supernatant absorbance was measured at 532 and 600 nm using a UV-Vis spectrophotometer, with 0.5% (w/v) TBA in 20% (w/v) TCA used as the blank. The MDA content was calculated by subtracting A532 from A600 and multiplied by the extinction coefficient 155 mm-1 cm-1.
Determination of Proline Concentration
Leaf samples (0.3 g) were extracted in 3% sulfosalicylic acid and centrifuged for 10 min at 6,000 × g. Two ml of the supernatant were incubated with an equal volume of acid ninhydrin and glacial acetic acid at 100°C for 1 h. The reaction was stopped by placing the samples in an ice bath, and proline was separated using toluene. Absorbance was then recorded at 520 nm using a UV-Vis spectrophotometer [38]. The proline content was determined using a standard curve plotted with the known concentration of L-proline and expressed as unit μmoles of proline/g fresh weight.
Determination of Cellular Trehalose Concentration
Trehalose content in leaves was measured using the Li method [39]. One gram of leaf was homogenized in 5 ml of 80% (v/v) hot ethanol and centrifuged at 9000 rpm for 20 min. The supernatant was evaporated at 80°C and suspended in 5 ml of distilled water. A 100 μl aliquot of this solution was mixed with 150 μl of 0.2N H2SO4 and boiled at 100°C for 10 min then cooled on ice. Further, 150 μl of 0.6 N NaOH was added, and the mixture was boiled for another 10 min to destroy reducing sugars before being cooled again. Finally, 2.0 ml of anthrone reagent (0.2 g anthrone per 100 ml of 95% H2SO4) was added to the mixture, which was then boiled for 10 min to develop colour and subsequently cooled. Absorbance was measured at 630 nm, and trehalose concentration was determined using a standard curve.
Microscopic Analysis of Bacterial Colonization by Green Fluorescence Protein (GFP)-Tagged M. oryzae CBMB20
Single colonies of GFP-tagged M. oryzae were grown in AMS medium amended with kanamycin (20 μg/ml) to mid-exponential phase at 30°C. The bacterial cell was pelleted by centrifugation (10,000 rpm, 10 min, 4°C) and washed twice in sterile 30 mM MgSO4. The bacterial suspension was adjusted to a population density of 108 CFU ml-1 in sterile 30 mM MgSO4. For seed treatment, surface sterilized A. thaliana seeds were incubated in shaker at 60 rpm for 1 h in 2 ml of bacterial suspension in 30 mM MgSO4 and with sterile 30 mM MgSO4 as control. The seeds were grown on plates containing ½ MS medium for 4 days. Then seedlings with uniform size were transferred to square of a plate containing ½ MS agar with 150 mM NaCl and without NaCl. After 10 days, the plantlets were washed with phosphate buffer solution and examined under the confocal microscope.
Statistical Analysis
The data were analyzed by an analysis of variance (ANOVA) using the general linear model version 9.1; SAS institute Inc, Cary, NC, USA. Means were compared by Tukey’s post hoc test.
Results
Growth Promotion of Arabidopsis thaliana by M. oryzae CBMB20
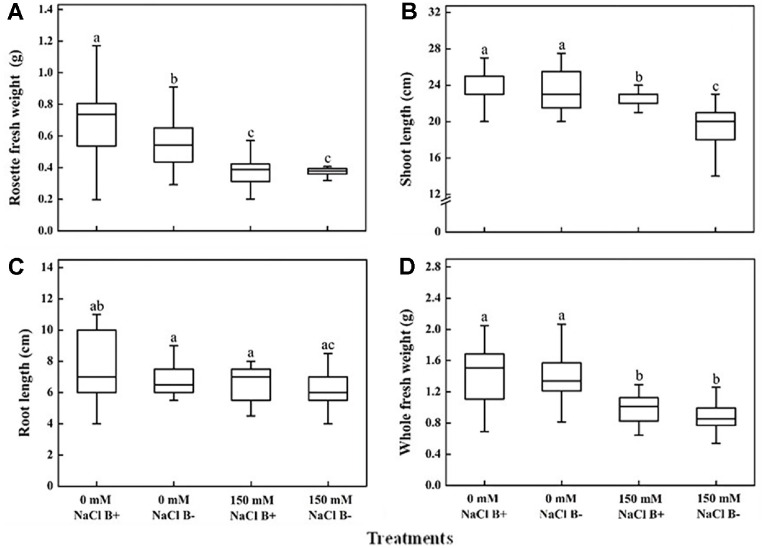
In this study, the interaction between M. oryzae CBMB20 and Arabidopsis was explored under salt stress conditions and demonstrated that salt stress impaired shoot and root development. Under different levels (0–200 mM) of salt stress conditions, all plants survived. As shown in Fig. 1, bacterial inoculation reduced the symptoms of leaf chlorosis induced by salt stress. Imposing of salt stress significantly reduced the Rosette fresh weight, shoot and root lengths. However, inoculation of M. oryzae CBMB20 significantly improved shoot (p < 0.01) and root lengths under salt stress condition (Fig. 2). Even though higher fresh weight was observed in the plants treated with M. oryzae CBMB20 the results are not significant under salt stress (150 mM).
Fig. 1. The growth of Arabidopsis thaliana plants after inoculation with M. oryzae CBMB20 under salt stress and non-stress conditions.
Bacteria+ (treated with M. oryzae CBMB20); Bacteria- (not treated with M. oryzae CBMB20).
Fig. 2. Effects of M. oryzae CBMB20 on plant growth under salt stress and non-stress conditions.
Arabidopsis development phenotypes were analyzed in response to salt stress, inoculated with (B+) and without (B-) M. oryzae CBMB20 under 0 mM NaCl and 150 mM NaCl. Figures show (A) Rosette Fresh Weight, (B) Shoot Length, (C) Root Length, and (D) Whole Fresh Weight. Different letters indicate the significant difference among treatments (p < 0.05); according to one way ANOVA test; Data are the mean ± SD, n = 21.
Photosynthetic Pigment Changes by M. oryzae CBMB20
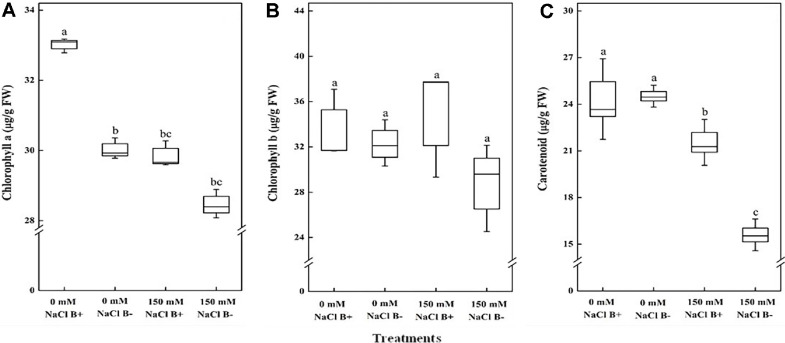
In order to investigate the effects of M. oryzae CBMB20 on the photosynthetic efficiency of plants under salt stress, the leaf chlorophyll and carotenoid contents were measured. Leaf chlorophyll and carotenoid content of Arabidopsis plant is significantly reduced because of salt stress. The leaf chlorophyll a content in plants exposed to M. oryzae CBMB20 were 10% higher than un-inoculated plants under non-stress conditions (Fig. 3A). However, there was no significant increase in chlorophyll b content under both conditions (Fig. 3B). Higher carotenoid contents in plants treated with M. oryzae CBMB20 under salt stress conditions showed 40% increase compared to control (Fig. 3C).
Fig. 3. Effects of M. oryzae CBMB20 on photosynthetic pigments of Arabidopsis plants grown under salt stress and non-stress conditions.
Figures show (A) Chlorophyll a, (B) Chlorophyll b, and (C) Carotenoid. The data represents the mean ± SD of three replicates and the different letters indicate the significant difference among treatments (p < 0.05); according to one way ANOVA test. Treatments details are the same as in Fig. 2.
Proline, Lipid Peroxidation, and Cellular Trehalose by M. oryzae CBMB20
The concentration of proline and MDA content were significantly increased in the plants when exposed to salt stress (Fig. 4). Arabidopsis plants under salt stress conditions showed higher proline content, the inoculation of plants had significantly (p < 0.001) lowered proline concentration compared to the non-inoculated plants under salt stress and non-stress conditions (Fig. 4A).
Fig. 4. Effects of M. oryzae CBMB20 on (A) Proline content, (B) MDA content and (C) Cellular trehalose concentration of leaves in Arabidopsis plants grown under salt stress and non-stress conditions.
The data represents the mean ± SD of three replicates and the different letters indicate the significant difference among treatments (p < 0.05); according to one way ANOVA test. Treatments details are the same as in Fig. 2.
In this study, M. oryzae CBMB20 inoculation significantly lowered the malondialdehyde under salt stress conditions and MDA content increased significantly (p < 0.001) under high salinity in non-inoculated plants (Fig. 4B), indicating that M. oryzae CBMB20 plays a role in alleviating oxidative stress caused by high salinity. In both non-stress and salt stress conditions, plants exposed to M. oryzae CBMB20 accumulated higher trehalose (Fig. 4C). Accumulation of trehalose was significantly increased by M. oryzae CBMB20 application compared to the plants not treated with bacteria. However, there was no significant difference in trehalose production between inoculated and non-inoculated plants under non-stress conditions.
Spatial Distribution of M. oryzae CBMB20 Strain in Arabidopsis
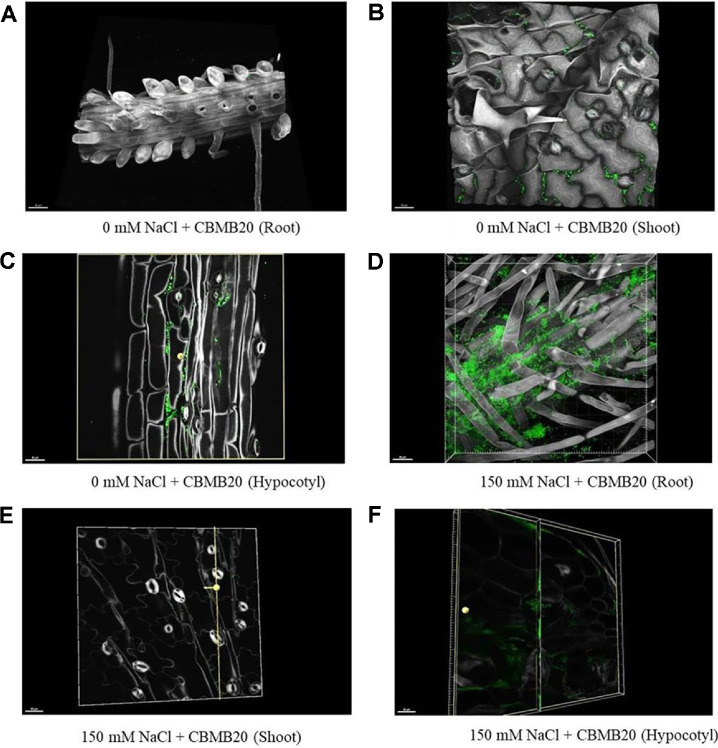
The extent of M. oryzae CBMB20 colonization in A. thaliana plantlets in roots and shoots was investigated using GFP-tagged M. oryzae CBMB20. Two weeks after seed treatment with bacterial suspension of GFP-expressing strain, seedlings exhibited GFP signals in various internal tissues of the plants such as roots, shoots and hypocotyl of Arabidopsis (Fig. 5).
Fig. 5. Confocal microscopic images of the persistence of GFP-tagged M. oryzae CBMB20 in roots, shoot and hypocotyl of Arabidopsis thaliana.
(A) In root under non-stress condition, (B) in shoot under non-stressed condition, (C) in hypocotyl under non-stress condition, (D) in root under salt stress condition, (E) in shoot under salt stress condition, and (F) in hypocotyl under salt stress condition.
Exogenous Trehalose Application on Plant Growth
Under both salt stress and non-stress conditions, exogenous application of trehalose positively impacted plant growth and development. In this study, exogenous application of trehalose significantly (p ≤ 0.05) alleviated the negative effects of salt stress by increasing shoot length in both stress and non-stress plants (Fig. 6). As shown in the figure, trehalose alleviated the symptoms of leaf chlorosis caused by salt stress. Application of trehalose increased fresh weight, shoot and root lengths in non-stress condition, but it’s not significant (Fig. 7). However, whole fresh weight and shoot length were significantly increased under stress conditions (Fig. 7A and 7B). The application of trehalose did not increase the rosette fresh weight and root length under salt stress condition (Fig. 7C and 7D).
Fig. 6. The growth of Arabidopsis thaliana plants after exogenous trehalose application under salt stress and non- stress conditions.
Fig. 7. Effect of exogenous applied trehalose on plant growth under salt stress and non-stress conditions.
Arabidopsis development phenotypes were analyzed in response to salt stress, application (Tre+) or without (Tre-) of 10 mM Trehalose under 0 mM NaCl and 150 mM NaCl concentration. (A) Whole Fresh Weight, (B) Shoot Length, (C) Rosette Fresh Weight, and (D) Root Length. Different letters indicate the significant difference among treatments (p < 0.05); according to one way ANOVA test; Data are the mean ± SD, n = 21.
Exogenous Trehalose Application on Proline, Lipid Peroxidation, and Cellular Trehalose
Under non stress condition application of trehalose reduced proline and MDA content in Arabidopsis leaves (Fig. 8A and 8B). However, exogenous application of trehalose increased cellular trehalose content in plants (Fig. 8C), but the difference is not significant. Under salt stress condition plant proline and MDA was significantly (p < 0.05) reduced compared to untreated plants. Similar to non-stress condition exogenous application of trehalose increased cellular trehalose content in plants but the values are not significant.
Fig. 8. Effects of exogenous trehalose application on (A) Proline content, (B) MDA content, and (C) Cellular trehalose concentration of leaves in Arabidopsis plants grown under salt stress and non-stress conditions.
The data represents the mean ± SD of three replicates and the different letters indicate the significant difference among treatments (p < 0.05); according to one way ANOVA test. Treatments details are the same as in Fig. 7.
Discussion
Microbes associated to plants are generally prevalent and exhibit beneficial activity as PGPR by promoting plant growth, decreasing pathogensis, and mitigating stresses through a variety of underlying mechanisms. However, the exact process by which microorganisms mitigate stress differs depending on the kind and species of plant, and the degree of stress is still unknown. In this study, A. thaliana is used as a test plant in order to understand the mechanisms of salinity stress mitigation by M. oryzae CBMB20 and exogenous application of trehalose.
It was suggested that the PGP activity of certain bacteria depends on stress because some PGPR were shown to stimulate plant development under both normal and stressed environments, while others were only effective under stressful conditions and had no growth promotion effect under ideal conditions [40]. Results of this study showed the plant growth promoting ability of M. oryzae CBMB20 in non-stress and saline stress conditions. Plant growth promoting nature of the M. oryzae CBMB20 may rely on the production of phytohormones [41], ACC deaminase production [42] and enhanced photosynthetic traits [25]. Similar to this, Methylobacterium sp. 2A-inoculated plants outperformed non-inoculated plants in terms of biomass, root and shoot growth, and chlorophyll content in saline environments [43]. In line with this, bacterial inoculation dramatically decreased the negative impacts of stress, as seen by the significantly longer shoots of soybean plants [44]. Indeed, there have been many reports showing the stress mitigation by bacterial inoculation [45, 46].
As evidenced by the results, exogenous application of trehalose mitigated the negative effects of salt stress on growth parameters in Arabidopsis. These findings are consistent with previous studies, such as those conducted on rice [47], Arabidopsis [48], wheat [49] and maize [50]. Higher concentrations of trehalose were found to be supra-optimal and negatively impacted plant growth. Interestingly, the exogenous application of trehalose did not affect chlorophyll content in either non-stress or salt stress plants. In this study, the administration of trehalose enhanced plant growth, suggesting possible stress tolerance. Tomato growth was enhanced by exogenous trehalose, which also increased the plant's height, fresh mass, and relative water content of its leaves [28].
Leaf chlorophyll concentration serves as an indicator of salt tolerance. Chlorophyll degradation, caused by ions (such as NaCl or Cl) or ROS, leads to the deterioration of cell organelles, particularly in leaf tissue. It is clear that in saline environments, microbial inoculation can increase stomatal conductance and plants' availability of CO2. Specifically, one typical characteristic of microbe-primed plant salt tolerance is an increase in the amounts of chlorophyll and carotenoid in salt-stressed plants [51]. Inoculation with PGPR has been reported to enhance chlorophyll content in various plants compared to controls [52]. PGPR enhances photosynthesis through a number of ways, such as improved chlorophyll content accumulation [53] and increased plant water use efficiency (WUE) [54]. The pink-pigmented bacterium M. oryzae CBMB20 also produces carotenoids [55], which have strong redox characteristics and function as potent antioxidants [56].
It has been demonstrated that inoculation with ACC deaminase-containing bacteria significantly suppresses ethylene synthesis, thereby reducing chlorophyll degradation and preventing leaf senescence associated with elevated ethylene levels [57]. Inoculation of M. oryzae CBMB20 increased the chlorophyll content in Arabidopsis plants under salt stress condition than that of control plants. In line with this, use of Methylobacterium sp. 2A was found to improve the chlorophyll of potato plants grown under salt stress condition [43]. The quantity of chlorophyll in leaves dramatically dropped following treatment with salt stress. But after exogenous trehalose treatment, the content of chlorophyll improved.
Plant growth is inhibited by salinity stress, which also causes oxidative and osmotic stress, particularly ion (Na+ and Cl− ion) toxicity. However, plants mitigate the consequences of salt stress in a number of ways, including the production of suitable solutes (osmolytes), the activation of an antioxidant defense system, and the adaptive regulation of hormones connected with stress. Osmotolerance is achieved through the accumulation of compatible solutes, which protect cells from salt-induced damage [58].
Proline is well known as a compatible solute and is a key compound in assessing salt tolerance [59]. Proline builds up as a solute in plants and is essential for a number of physiological functions, such as protein solvation and structural stability, membrane integrity preservation, lipid membrane reduction and oxidation, reactive oxygen species scavenging, and buffering of cellular redox potential under stress. In the present study M. oryzae CBMB20 inoculation reduced the proline content. Proline accumulation in plants is the first response to stress in order to protect cells from injury. It has been reported that proline enhances plant tolerance to salt stress by not only regulating osmotic pressure but also stabilizing various proteins, enzymes, and cell membranes [60]. Osmolytes help prevent cell membrane disruption and enhance membrane stability under salt stress conditions.
In contrast, proline levels were significantly lower in plants exposed to bacteria compared to those that were not. Other studies have also reported that PGPR reduce proline content. The physiological importance of proline accumulation in salt-stressed plants is controversial and the effects of microbial inoculations on plant proline accumulation during saline stress also differ [51]. For example, the proline levels in Arachis hypogaea shoots inoculated with six PGPR strains were markedly reduced under salt stress conditions [61]. As salt concentration increased, the proline content in rice also increased. However, rice inoculated with PGPR exhibited lower proline levels compared to non-inoculated plants [62]. Additionally, Bacillus amyloliquefaciens SQR9 inoculation decreased proline levels in maize under salt stress conditions [63]. It is possible that plants treated with PGPR experienced less severe salt stress, resulting in lower proline accumulation compared to untreated plants [61].
Exogenous application of trehalose reduced the salinity-induced damage in the plants by regulating the levels of osmotic substances. Proline has been reported to assist with osmotic adjustment under stress conditions [64]. Since both proline and cellular trehalose act as osmoprotectants, the decrease in proline accumulation observed with exogenous trehalose treatment under salt stress suggests either a compensatory role for trehalose or a reduced need for proline. Consistent with previous reports, exogenous trehalose was found to lower proline levels in plants under salt stress [65]. Another recent study found that, exogenous trehalose application decreased proline content in wheat plants under salt stress condition [66]. Plants use defensive adaptation mechanisms, such as osmotic regulation or osmoprotection, to survive in challenging environments.
MDA concentration is used as a gauge to ascertain the plant's redox state and directly correlated with oxidative damage to the cell membrane in plants [67]. In the present study, Arabidopsis plants subjected to salt stress exhibited reduced MDA levels when inoculated with M. oryzae CBMB20. In accord with, both liquid and chitosan-immobilized inoculation M. oryzae CBMB20 cells showed promise in reducing tomato plant salt stress by registering lower MDA level [19]. Lower MDA concentrations are indicative of reduced membrane damage or enhanced salt tolerance in plants [7, 68]. When compared to other treatments, Pseudomonas spp. inoculation dramatically reduced the MDA concentration in red pepper under salt stress conditions [69].
MDA levels indicate the extent of lipid peroxidation in cell membranes, which is closely associated to the plant's effective enzymatic clearance systems [70]. Previous studies have demonstrated that exogenous trehalose positively reduced MDA levels under stress conditions [71-73]. These studies showed that high MDA accumulation indicated membrane lipid peroxidation, which is typically lower in stress-resistant plants. As observed in this study, MDA concentration dramatically increased following salt treatment, suggesting that salt stress severely damaged plant cell membranes and caused a high production of reactive oxygen species and free radicals that damaged the membrane. Following exogenous trehalose treatment, the MDA content decreased. A recent report showed that treatment of wheat seeds with trehalose significantly reduced the MDA content under salt stress [66]. In the present study, trehalose reduced MDA levels in plants, highlighting its role as a protective agent for membranes under abiotic stresses [74, 75].
Trehalose is a disaccharide that works in concert with other antioxidant apparatuses in plant cells to shield cells from ROS during times of stress [76]. Salinity stress resulted in a significant increase in cellular trehalose content in Arabidopsis plants compared to controls with bacterial inoculation. Cellular trehalose production can serve as a stress-reducing strategy, as it supports plant growth under both drought and high salt conditions [4]. Higher concentrations of cellular trehalose have been found in the root nodules of Phaseolus vulgaris and Medicago truncatula in response to drought and salt stress. This indicates the crucial role of cellular trehalose in signaling during plant-bacteria interactions, enhancing plant yield, growth, and adaptation to harsh conditions [77]. Furthermore, the enhancement of tomato root development and growth under saline conditions was totally hindered by the mutation of cellular trehalose synthesis in Pseudomonas putida UW4, indicating a crucial function for osmolytes produced by microbes in plant salt tolerance [78].
Exogenous application of trehalose did not have any impact on the endogenous concentration of trehalose in the present study. This is in concordance with previous results, which also reported no significant change in cell trehalose levels following exogenous application [79]. According to Redillas [80], exogenously administered trehalose boosts the activity of defense-response proteins in crops and enhances rice plants' resilience to salty environments [65].
Root colonization by inoculated bacteria is essential for establishing beneficial interactions between bacteria and their host plants [81, 82]. Linking reporter genes, such as GFP, to plasmids used for transforming specific bacteria has greatly improved our understanding of rhizobacteria localization within plants. It is important to note that GFP-labeled cells were observed inside the roots of A. thaliana treated with the GFP-labeled strain, indicating that the strain colonizes the roots effectively. An endophyte successfully colonized the roots of various plant species, such as Arabidopsis [6], corn [83] and switch grass [84]. Overall, M. oryzae CBMB20 not only promoted plant growth and effectively colonized root tissues but also mitigated the adverse effects of salt stress. This strain enhances salt stress tolerance in A. thaliana, enabling the plants to thrive under stressful conditions. The study demonstrated that the GFP technique effectively assesses the colonization ability of various rhizobacterial species, though it may not apply to all species.
Conclusion
This study indicates that trehalose may function as an elicitor of biological processes, and that plant growth-promoting bacteria could be a valuable tool for agriculture in saline regions, potentially contributing to global food security and agricultural sustainability. These results demonstrated the potential applications of trehalose and M. oryzae CBMB20 in plant stress management; however, more investigation is required to assess CBMB20's suitability for agronomic management in open-field settings.
Acknowledgments
This work is partly supported by the Chey Institute for Advanced Studies, International Scholar Exchange Fellowship for the academic year of 2023-2024 for Dr. Kyaw’s fellowship in the Republic of Korea. We would also thank Prof. Tongmin Sa of Chungbuk National University for allowing us to use his laboratory to conduct all the experiments in this study and for the Methylobacterium oryzae CBMB20 strain. Also we acknowledge Rangasamy Anandham, Tamil Nadu Agricultural University, India for critical reading of this manuscript.
Footnotes
Author Contributions
Conceptualization, K.K., E.P.K.,; methodology, E.P.K., K.K., R.R., K.R.,; writing—original draft preparation, E.P.K., D.I.W., R.R.,; writing—review and editing, K.R.,; supervision, D.I.W., All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamil A, Riaz S, Ashraf M, Foolad MR. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011;30:435–458. doi: 10.1080/07352689.2011.605739. [DOI] [Google Scholar]
- 3.Mustafa G, Akhtar MS. 2019. Crops and methods to control soil salinity. pp. 237-251. Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches. 10.1007/978-981-13-8805-7_11 [DOI]
- 4.Orozco-Mosqueda MDC, Duan J, DiBernardo M, Zetter E, Campos-García J, Glick BR, et al. The production of ACC deaminase and trehalose by the plant growth-promoting bacterium Pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019;10:1392. doi: 10.3389/fmicb.2019.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compant S, Cassan F, Kostić T, Johnson L, Brader G, Trognitz F, et al. Harnessing the plant microbiome for sustainable crop production. Nat. Rev. Microbiol. 2025;23:9–23. doi: 10.1038/s41579-024-01079-1. [DOI] [PubMed] [Google Scholar]
- 6.Pinedo I, Ledger T, Greve M, Poupin MJ. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015;6:466. doi: 10.3389/fpls.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey S, Gupta S. Diversity analysis of ACC deaminase producing bacteria associated with rhizosphere of coconut tree (Cocos nucifera L.) grown in Lakshadweep islands of India and their ability to promote plant growth under saline conditions. J. Biotechnol. 2020;324:183–197. doi: 10.1016/j.jbiotec.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Baek C, Shin SK, Yi H. Flavobacterium magnum sp. nov., Flavobacterium pallidum sp. nov., Flavobacterium crocinum sp. nov. and Flavobacterium album sp. nov. Int. J. Syst. Evol. Microbiol. 2018;68:3837–3843. doi: 10.1099/ijsem.0.003067. [DOI] [PubMed] [Google Scholar]
- 9.Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:963401. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A. Role of plant growth promoting rhizobacteria in agricultural sustainability - a review. Molecules. 2016;21:573. doi: 10.3390/molecules21050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha Y, Subramanian RB, Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011;33:797–802. doi: 10.1007/s11738-010-0604-9. [DOI] [Google Scholar]
- 12.Forni C, Duca D, Glick BR. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410:335–356. doi: 10.1007/s11104-016-3007-x. [DOI] [Google Scholar]
- 13.Sunithakumari VS, Menon RR, Suresh GG, Krishnan R, Rameshkumar N. 2024. Characterization of a novel root-associated diazotrophic rare PGPR taxa, Aquabacter pokkalii sp. nov., isolated from pokkali rice: new insights into the plant-associated lifestyle and brackish adaptation. BMC Genomics 25: 424. 10.1186/s12864-024-10332-z [DOI] [PMC free article] [PubMed]
- 14.Madhaiyan M, Kim BY, Poonguzhali S, Kwon SW, Song MH, Ryu JH, et al. Methylobacterium oryzae sp. nov., an aerobic, pinkpigmented, facultatively methylotrophic, 1-aminocyclopropane-1-carboxylate deaminase-producing bacterium isolated from rice. Int. J. Syst. Evol. Microbiol. 2007;57:326–331. doi: 10.1099/ijs.0.64603-0. [DOI] [PubMed] [Google Scholar]
- 15.Ali S, Charles TC, Glick BR. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014;80:160–167. doi: 10.1016/j.plaphy.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa TM. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J. Microbiol. Biotechnol. 2010;20:1577–1584. doi: 10.4014/jmb.1007.07011. [DOI] [PubMed] [Google Scholar]
- 17.Roy Choudhury A, Roy SK, Trivedi P, Choi J, Cho K, Yun SH, et al. Label‐free proteomics approach reveals candidate proteins in rice (Oryza sativa L.) important for ACC deaminase producing bacteria‐mediated tolerance against salt stress. Environ. Microbiol. 2022;24:3612–3624. doi: 10.1111/1462-2920.15937. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury AR, Trivedi P, Madhaiyan M, Choi J, Choi W, Park JH, et al. ACC deaminase producing endophytic bacteria enhances cell viability of rice (Oryza sativa L.) under salt stress by regulating ethylene emission pathway. Environ. Exp. Bot. 2023a;213:105411. doi: 10.1016/j.envexpbot.2023.105411. [DOI] [Google Scholar]
- 19.Chanratana M, Han GH, Roy Choudhury A, Sundaram S, Halim MA, Krishnamoorthy R, et al. Assessment of Methylobacterium oryzae CBMB20 aggregates for salt tolerance and plant growth-promoting characteristics for bio-inoculant development. AMB Express. 2017;7:208. doi: 10.1186/s13568-017-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Dodd IC, Belimov AA, Jiang F. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct. Plant Biol. 2016;43:161–172. doi: 10.1071/FP15200. [DOI] [PubMed] [Google Scholar]
- 21.Madhaiyan M, Poonguzhali S, Kwon SW, Sa TM. Methylobacterium phyllosphaerae sp. nov., a pink-pigmented, facultative methylotroph from the phyllosphere of rice. Int. J. Syst. Evol. Microbiol. 2009;59:22–27. doi: 10.1099/ijs.0.001693-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee MK, Chauhan PS, Yim WJ, Lee GJ, Kim YS, Park KW, et al. Foliar colonization and growth promotion of red pepper (Capsicum annuum L.) by Methylobacterium oryzae CBMB20. J. Appl. Biol. Chem. 2011;54:120–125. doi: 10.3839/jabc.2011.021. [DOI] [Google Scholar]
- 23.Madhaiyan M, Poonguzhali S, Ryu J, Sa T. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta. 2006;224:268–278. doi: 10.1007/s00425-005-0211-y. [DOI] [PubMed] [Google Scholar]
- 24.Madhaiyan M, Suresh Reddy BV, Anandham R, Senthilkumar M, Poonguzhali S, Sundaram SP, et al. Plant growth-promoting Methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot pathogens. Curr. Microbiol. 2006;53:270–276. doi: 10.1007/s00284-005-0452-9. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee P, Kanagendran A, Samaddar S, Pazouki L, Sa TM, Niinemets U. Methylobacterium oryzae CBMB20 influences photosynthetic traits, volatile emission and ethylene metabolism in Oryza sativa genotypes grown in salt stress conditions. Planta. 2019;249:1903–1919. doi: 10.1007/s00425-019-03139-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy Choudhury A, Trivedi P, Choi J, Madhaiyan M, Park JH, Choi W. Inoculation of ACC deaminase‐producing endophytic bacteria down‐regulates ethylene‐induced pathogenesis‐related signaling in red pepper (Capsicum annuum L.) under salt stress. Physiol. Plant. 2023b;175:e13909. doi: 10.1111/ppl.13909. [DOI] [PubMed] [Google Scholar]
- 27.Indiragandhi P, Anandham R, Kim K, Yim W, Madhaiyan M, Sa TM. Induction of defense responses in tomato against Pseudomonas syringae pv. tomato by regulating the stress ethylene level with Methylobacterium oryzae CBMB20 containing 1-aminocyclopropane-1-carboxylate deaminase. World J. Microbiol. Biotechnol. 2008;24:1037–1045. doi: 10.1007/s11274-007-9572-7. [DOI] [Google Scholar]
- 28.Feng Y, Chen X, He Y, Kou X, Xue Z. Effects of exogenous trehalose on the metabolism of sugar and abscisic acid in tomato seedlings under salt stress. Trans. Tianjin Univ. 2019;25:451–471. doi: 10.1007/s12209-019-00214-x. [DOI] [Google Scholar]
- 29.Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M. Trehalose metabolism in plants. Plant J. 2014;79:544–567. doi: 10.1111/tpj.12509. [DOI] [PubMed] [Google Scholar]
- 30.Islam MO, Kato H, Shima S, Tezuka D, Matsui H, Imai R. Functional identification of a rice trehalase gene involved in salt stress tolerance. Gene. 2019;685:42–49. doi: 10.1016/j.gene.2018.10.071. [DOI] [PubMed] [Google Scholar]
- 31.Garapati P, Feil R, Lunn JE, Van Dijck P, Balazadeh S, Mueller-Roeber B. Transcription factor Arabidopsis activating factor1 integrates carbon starvation responses with trehalose metabolism. Plant Physiol. 2015;169:379–390. doi: 10.1104/pp.15.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry C, Bledsoe SW, Griffiths CA, Kollman A, Paul MJ, Sakr S, et al. Differential role for trehalose metabolism in salt-stressed maize. Plant Physiol. 2015;169:1072–1089. doi: 10.1104/pp.15.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang B, Yang L, Cong W, Zu Y, Tang Z. The improved resistance to high salinity induced by trehalose is associated with ionic regulation and osmotic adjustment in Catharanthus roseus. Plant Physiol. Biochem. 2014;77:140–148. doi: 10.1016/j.plaphy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Fan D, Subramanian S, Smith DL. Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci. Rep. 2020;10:12740. doi: 10.1038/s41598-020-69713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Yao Y, Li J, Zhang J, Zhang X, Hu L, et al. Trehalose alleviated salt stress in tomato by regulating ROS metabolism, photosynthesis, osmolyte synthesis, and trehalose metabolic pathways. Front. Plant Sci. 2022;13:772948. doi: 10.3389/fpls.2022.772948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumanta N, Haque CI, Nishika J, Suprakash R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014;2231:606X. [Google Scholar]
- 37.Chanratana M, Joe MM, Roy Choudhury A, Anandham R, Krishnamoorthy R, Kim K, et al. Physiological response of tomato plant to chitosan-immobilized aggregated Methylobacterium oryzae CBMB20 inoculation under salinity stress. 3 Biotech. 2019;9:397. doi: 10.1007/s13205-019-1923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bates LS, Waldren RPA, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 39.Li ZG, Luo LJ, Zhu LP. Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot. Stud. 2014;55:20. doi: 10.1186/1999-3110-55-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018;9:1473. doi: 10.3389/fpls.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madhaiyan M, Poonguzhali S, Kang BG, Lee YJ, Chung JB, Sa TM. Effect of co-inoculation of methylotrophic Methylobacterium oryzae with Azospirillum brasilense and Burkholderia pyrrocinia on the growth and nutrient uptake of tomato, red pepper and rice. Plant Soil. 2010;328:71–82. doi: 10.1007/s11104-009-0083-1. [DOI] [Google Scholar]
- 42.Yim W, Seshadri S, Kim K, Lee G, Sa TM. Ethylene emission and PR protein synthesis in ACC deaminase producing Methylobacterium spp. inoculated tomato plants (Lycopersicon esculentum Mill.) challenged with Ralstonia solanacearum under greenhouse conditions. Plant Physiol Biochem. 2013;67:95–104. doi: 10.1016/j.plaphy.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Grossi CEM, Fantino E, Serral F, Zawoznik MS, Fernandez Do Porto DA, Ulloa RM. Methylobacterium sp. 2A is a plant growthpromoting rhizobacteria that has the potential to improve potato crop yield under adverse conditions. Front. Plant Sci. 2020;11:71. doi: 10.3389/fpls.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asaf S, Khan AL, Khan MA, Imran QM, Yun BW, Lee IJ. Osmoprotective functions conferred to soybean plants via inoculation with Sphingomonas sp. LK11 and exogenous trehalose. Microbiol. Res. 2017;205:135–145. doi: 10.1016/j.micres.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Poupin MJ, Timmermann T, Vega A, Zuñiga A, González B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS One. 2013;8:e69435. doi: 10.1371/journal.pone.0069435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinedo I, Ledger T, Greve M, Poupin MJ. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015;6:466. doi: 10.3389/fpls.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theerakulpisut P, Phongngarm S. Alleviation of adverse effects of salt stress on rice seedlings by exogenous trehalose. Asian J. Crop Sci. 2013;5:405–415. doi: 10.3923/ajcs.2013.405.415. [DOI] [Google Scholar]
- 48.Bae H, Herman E, Bailey B, Bae HJ, Sicher R. Exogenous trehalose alters Arabidopsis transcripts involved in cell wall modification, abiotic stress, nitrogen metabolism, and plant defense. Physiol. Plantarum. 2005;125:114–126. doi: 10.1111/j.1399-3054.2005.00537.x. [DOI] [Google Scholar]
- 49.Mohamed HI, Akladious SA, El-Beltagi HS. Mitigation the harmful effect of salt stress on physiological, biochemical and anatomical traits by foliar spray with trehalose on wheat cultivars. Fresenius Environ. Bull. 2018;27:7054–7065. [Google Scholar]
- 50.Zeid IM. Trehalose as osmoprotectant for maize under salinity-induced stress. Res. J. Agric. Biol. Sci. 2009;5:613–622. [Google Scholar]
- 51.Liu Y, Xun W, Chen L, Xu Z, Zhang N, Feng H, et al. Rhizosphere microbes enhance plant salt tolerance: toward crop production in saline soil. Comput. Struct. Biotechnol. J. 2022;20:6543–6551. doi: 10.1016/j.csbj.2022.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woo OG, Kim H, Kim JS, Keum HL, Lee KC, Sul WJ, et al. Bacillus subtilis strain GOT9 confers enhanced tolerance to drought and salt stresses in Arabidopsis thaliana and Brassica campestris. Plant Physiol. Biochem. 2020;148:359–367. doi: 10.1016/j.plaphy.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 53.Kim K, Yim W, Trivedi P, Madhaiyan M, Deka Boruah HP, Islam MR, et al. Synergistic effects of inoculating arbuscular mycorrhizal fungi and Methylobacterium oryzae strains on growth and nutrient uptake of red pepper (Capsicum annuum L.) Plant Soil. 2010;327:429–440. doi: 10.1007/s11104-009-0072-4. [DOI] [Google Scholar]
- 54.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004;42:565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Kwak MJ, Jeong H, Madhaiyan M, Lee Y, Sa TM, Oh TK, et al. Genome information of Methylobacterium oryzae, a plantprobiotic methylotroph in the phyllosphere. PLoS One. 2014;9:e106704. doi: 10.1371/journal.pone.0106704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gholizadeh A. Molecular evidence for the contribution of methylobacteria to the pink-pigmentation process in pink-colored plants. J. Plant Interact. 2012;7:316–321. doi: 10.1080/17429145.2012.693208. [DOI] [Google Scholar]
- 57.Arshad M, Shaharoona B, Mahmood T. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.) Pedosphere. 2008;18:611–620. doi: 10.1016/S1002-0160(08)60055-7. [DOI] [Google Scholar]
- 58.Krishnamoorthy R, Aritra C, Denver W, Rangasamy A, Senthilkumar M, Sa T. Salt stress tolerance-promoting proteins and metabolites under plant-bacteria-salt stress tripartite interactions. Appl. Sci. 2022;12:3126. doi: 10.3390/app12063126. [DOI] [Google Scholar]
- 59.Banu MNA, Hoque MA, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Shimoishi Y, et al. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009;166:146–156. doi: 10.1016/j.jplph.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Ramadoss D, Lakkineni VK, Bose P, Ali S, Annapurna K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springerplus. 2013;2:6. doi: 10.1186/2193-1801-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shukla PS, Agarwal PK, Jha B. Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plantgrowth-promoting rhizobacteria. J. Plant Growth Regul. 2012;31:195–206. doi: 10.1007/s00344-011-9231-y. [DOI] [Google Scholar]
- 62.Jha Y, Subramanian RB, Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011;33:797–802. doi: 10.1007/s11738-010-0604-9. [DOI] [Google Scholar]
- 63.Chen L, Liu Y, Wu G, Njeri K, Shen Q, Zhang N, et al. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016;158:34–44. doi: 10.1111/ppl.12441. [DOI] [PubMed] [Google Scholar]
- 64.Ayala-Astorga GI, Alcaraz-Meléndez L. Salinity effects on protein content, lipid peroxidation, pigments, and proline in Paulownia imperialis (Siebold & Zuccarini) and Paulownia fortunei (Seemann & Hemsley) grown in vitro. Electron. J. Biotechnol. 2010;13:13–14. doi: 10.2225/vol13-issue5-fulltext-13. [DOI] [Google Scholar]
- 65.Nounjan N, Nghia PT, Theerakulpisut P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012;169:596–604. doi: 10.1016/j.jplph.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Alhudhaibi AM, Ibrahim MA, Abd-Elaziz SM, Farag HR, Elsayed SM, Ibrahim HA, et al. Enhancing salt stress tolerance in wheat (Triticum aestivum) seedlings: insights from trehalose and mannitol. BMC Plant Biol. 2024;24:472. doi: 10.1186/s12870-024-04964-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arbona V, Hossain Z, López‐Climent MF, Pérez‐Clemente RM, Gómez‐Cadenas A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plantarum. 2008;132:452–466. doi: 10.1111/j.1399-3054.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 68.Sukweenadhi J, Balusamy SR, Kim YJ, Lee CH, Kim YJ, Koh SC, et al. A growth-promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018;9:813. doi: 10.3389/fpls.2018.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samaddar S, Chatterjee P, Choudhury AR, Ahmed S, Sa T. Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol. Res. 2019;219:66–73. doi: 10.1016/j.micres.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Farooq M, Wahid A, Kobayashi NSM, Fujita DBSMA, Basra SM. 2009. Plant drought stress: effects, mechanisms and management. Sustain. Agric. pp. 185-212. 10.1051/agro:2008021 [DOI]
- 71.Kosar F, Akram NA, Ashraf M, Ahmad A, Alyemeni MN, Ahmad P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plantarum. 2021;172:317–333. doi: 10.1111/ppl.13155. [DOI] [PubMed] [Google Scholar]
- 72.Sadak MS, El-Bassiouny HMS, Dawood MG. Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull. Natl. Res. Centre. 2019;43:1–11. doi: 10.1186/s42269-018-0039-9. [DOI] [Google Scholar]
- 73.Mostofa MG, Hossain MA, Fujita M. Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma. 2015;252:461–475. doi: 10.1007/s00709-014-0691-3. [DOI] [PubMed] [Google Scholar]
- 74.Herdeiro RS, Pereira MD, Panek AD, Eleutherio ECA. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim. Biophys. Acta (BBA)-Gen Subj. 2006;1760:340–346. doi: 10.1016/j.bbagen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 75.Perveen S, Shahbaz M, Ashraf M. Triacontanol-induced changes in growth, yield, leaf water relations, oxidative defense system, minerals, and some key osmoprotectants in Triticum aestivum under saline conditions. Turk. J. Bot. 2014;38:896–913. doi: 10.3906/bot-1401-19. [DOI] [Google Scholar]
- 76.Smeekens S. Drought resistance: spraying for yield. Nat. Plants. 2017;3:17023. doi: 10.1038/nplants.2017.23. [DOI] [PubMed] [Google Scholar]
- 77.Lopez M, Tejera NA, Iribarne C, Lluch C, Herrera‐Cervera JA. Trehalose and trehalase in root nodules of Medicago truncatula and Phaseolus vulgaris in response to salt stress. Physiol. Plantarum. 2008;134:575–582. doi: 10.1111/j.1399-3054.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- 78.Orozco-Mosqueda MDC, Santoyo G. Plant-microbial endophytes interactions: scrutinizing their beneficial mechanisms from genomic explorations. Curr. Plant Biol. 2021;25:100189. doi: 10.1016/j.cpb.2020.100189. [DOI] [Google Scholar]
- 79.Samadi S, Habibi G, Vaziri A. Exogenous trehalose alleviates the inhibitory effects of salt stress in strawberry plants. Acta Physiol. Plant. 2019;41:1–11. doi: 10.1007/s11738-019-2905-y. [DOI] [Google Scholar]
- 80.Redillas MC, Park SH, Lee JW, Kim YS, Jeong JS, Jung H, et al. Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotechnol. Rep. 2012;6:89–96. doi: 10.1007/s11816-011-0210-3. [DOI] [Google Scholar]
- 81.Weng J, Wang Y, Li J, Shen Q, Zhang R. Enhanced root colonization and biocontrol activity of Bacillus amyloliquefaciens SQR9 by abrB gene disruption. Appl. Microbiol. Biotechnol. 2013;97:8823–8830. doi: 10.1007/s00253-012-4572-4. [DOI] [PubMed] [Google Scholar]
- 82.Shehata HR, Dumigan C, Watts S, Raizada MN. An endophytic microbe from an unusual volcanic swamp corn seeks and inhabits root hair cells to extract rock phosphate. Sci. Rep. 2017;7:13479. doi: 10.1038/s41598-017-14080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014;97:30–39. doi: 10.1016/j.envexpbot.2013.09.014. [DOI] [Google Scholar]
- 84.Kim S, Lowman S, Hou G, Nowak J, Flinn B, Mei C. Growth promotion and colonization of switchgrass (Panicum virgatum) cv. Alamo by bacterial endophyte Burkholderia phytofirmans strain PsJN. Biotechnol. Biofuels. 2012;5:37. doi: 10.1186/1754-6834-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]