Abstract
Soil invertebrate survival in freezing temperatures has generally been considered in the light of the physiological adaptations seen in surface living insects. These adaptations, notably the ability to supercool, have evolved in concert with surface invertebrates' ability to retain body water in a dry environment. However, most soil invertebrates are orders of magnitude less resistant to desiccation than these truly terrestrial insects, opening the possibility that the mechanisms involved in their cold-hardiness are also of a radically different nature. Permeable soil invertebrates dehydrate when exposed in frozen soil. This dehydration occurs because the water vapor pressure of supercooled water is higher than that of ice at the same temperature. The force of this vapor pressure difference is so large that even a few degrees of supercooling will result in substantial water loss, continuing until the vapor pressure of body fluids equals that of the surrounding ice. At this stage, the risk of tissue ice formation has been eliminated, and subzero survival is ensured. Here we show that these soil invertebrates do not base their winter survival on supercooling, as do many other ectotherms, but instead dehydrate and equilibrate their body-fluid melting point to the ambient temperature. They can achieve this equilibration even at the extreme cooling rates seen in polar soils.
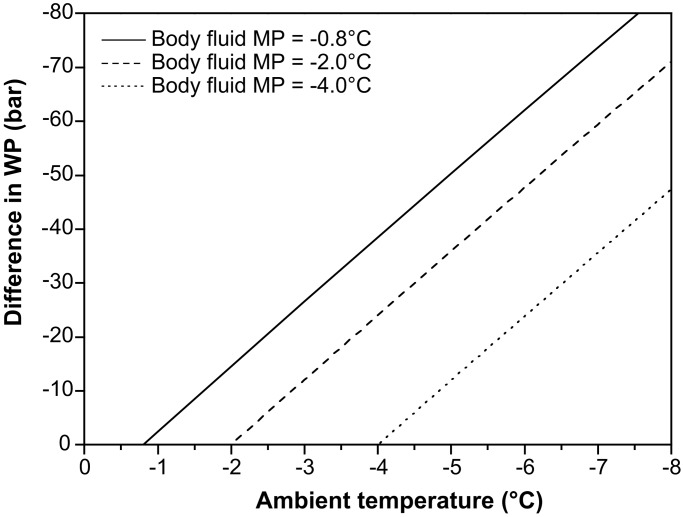
Most studies of cold-tolerance mechanisms in soil organisms have adopted the conceptual framework developed with terrestrial insects. According to this “classic” view, overwintering species either seek to avoid freezing by prolonged and extensive supercooling of their body fluids, i.e., “freeze avoidance,” or are able to tolerate extracellular ice formation in their body fluids, i.e., “freeze tolerance” (1–3). Because most soil invertebrates are unable to survive ice formation within their tissues (4–9), the majority of studies of overwintering physiology in this group have been concerned with measurements of supercooling points, under the assumption that freeze avoidance by supercooling is the relevant adaptation to low-temperature survival. In small soil invertebrates with high integumental water permeability (e.g., enchytraeids, nematodes, and euedaphic Collembola), which completely dominate arctic and subarctic soil ecosystems (10), reported supercooling abilities (typically between −5 and −12°C) (6, 8, 11–14) are inadequate to explain winter survival. Recently, a third and fundamentally different strategy has been proposed for these animals. Thus, it has been shown that soil invertebrate species with low cuticular desiccation resistance will dehydrate when exposed in frozen soil (13–16). This dehydration occurs because the water vapor pressure of supercooled water is higher than that of ice at the same temperature (17). In fact, the force of this vapor pressure difference is so large (Fig. 1) that, in organisms with high integumental permeability for water, even a few degrees of supercooling will result in substantial water loss. This water loss will continue until, at equilibrium, the vapor pressure of the (unfrozen) body fluids equals that of the surrounding ice. At this stage, the animal is no longer supercooled, because the melting point (MP) (and equilibrium freezing point) of the animals' body fluids equals the ambient temperature. At this time, the risk of tissue ice formation or ice inoculation from the surrounding soil has been eliminated, and subzero survival is ensured.
Figure 1.
Examples of differences in water potential (WP) between the body fluids of a supercooled animal and the surrounding ice at varying ambient temperature. The more intense supercooling becomes, the larger becomes the WP difference. A negative value of WP difference means that the organism loses water to the surrounding ice. The water potential (ψ) of body fluids at a given temperature has been calculated by using Van't Hoff's equation: ψ = Osm⋅R⋅T, where Osm is the osmolality of body fluids, R is the gas constant, and T is absolute temperature (°K). The MP of body fluids is calculated by application of the osmolal MP depression constant (−1.86°C osmol−1 kg). The MP of a solution is defined by the vapor pressure of ice when there is equilibrium between ice and solution in the system. Thus, the “osmotic pressure of ice,” or water potential of ice, can be calculated by transforming the temperature of ice (ambient temperature) to osmolality and then using Van't Hoff's equation.
Animals that possess this strategy need to be tolerant to desiccation, and indeed they are (14, 18). The few studies that have addressed this “protective dehydration mechanism” suggest that earthworm cocoons, enchytraeids, and certain soil-dwelling insects belonging to the Collembola will dehydrate and equilibrate with the surrounding water vapor pressure in a frozen soil. However, in these studies, temporal changes in body-fluid MPs were measured after a sudden transfer to constant subzero temperatures. Under such conditions, freeze-avoiding organisms are bound to rely on supercooling until equilibrium has been reached. In the field, overwintering soil animals will be subjected to the combined hazards of fluctuating freezing temperatures, the danger of body-water freeze inoculation via contact with ice crystals in the environment, and, in arctic and subarctic regions, exposure to low subzero temperatures for the entire winter. For these animals, therefore, supercooling is, in our opinion, unlikely to be a sustainable winter survival strategy. On the other hand, for the dehydration mechanism to be effective, the important question is: can these organisms lower their body-fluid MP quickly enough and hence avoid the need for supercooling to meet the cooling rates that they will encounter in their habitat?
To address this question, we have conducted experiments in which individuals of the collembolan Onychiurus arcticus (Tullberg) and cocoons of the earthworm Dendrobaena octaedra (Savigny) were exposed to declining subzero temperatures in vials containing crushed ice to simulate exposure in frozen soil. These two organisms represent cold-hardy soil invertebrates with very limited desiccation resistance, causing them to become severely dehydrated when exposed to ice in their environment (14–16). We measured the development in body-fluid MPs in two experiments mimicking “worst-case” but realistic natural temperature declines in early winter arctic soils. In addition, we reevaluated data from a previous study by Worland et al. (16) on dehydration and trehalose accumulation in O. arcticus at subzero temperatures. Using data from this independent but similar experiment, we have calculated MP depression for O. arcticus to corroborate our own findings.
Materials and Methods
Animal Material.
D. octaedra cocoons were obtained from a laboratory culture with a Danish population and allowed to develop at 20°C on moist filter paper in Petri dishes for approximately 14 days until early-stage embryos were visible (19). Adult O. arcticus were collected at Stuphallet, Ny Aalesund, Spitzbergen, and maintained in a laboratory culture at 10°C using moist peat as a substrate. Dried baker's yeast and chicken manure were added as food. Before the experiment, both cocoons and collembolans were acclimated for 2–4 weeks at 2°C under optimal moisture conditions. The collembolans were not fed during this period. The cocoons used in the experiments had a fresh weight (FW) of 2.5–5 mg and a water content (WC) of ≈3 g⋅g−1 dry weight (DW). The collembolans had a FW of 0.5–1.0 mg and a WC of ≈3 g⋅g−1 DW).
Frost-Exposure Experiment 1.
In this initial experiment, we investigated the MP lowering in O. arcticus exposed to gradually decreasing temperatures (1°C day−1) in the range 0 to −6.5°C. Exposure over ice was achieved by placing groups of three to four adult O. arcticus in an open polyethylene vial (inner diameter, 6 mm; height, 10 mm), which was then placed on top of moist peat (5.3 g of water g−1 of dry peat) in a 14-ml glass vial with a tightly fitting lid. The vials were subjected to freezing temperatures in a programmable Binder MK heating/cooling cabinet (Binder, Tuttlingen, Germany) in which the temperature could be controlled with a precision of ±0.2°C. The temperature was programmed to decrease by 1°C day−1 from +1 to −6.5°C and then remain at this temperature until the experiment was terminated after 14 days. Control animals were exposed at +1°C over moist peat. At ≈1-day intervals, body-fluid MP was measured by using a Wescor HR 33T Dew Point Microvoltmeter connected with C-52 sample chambers operated in the dewpoint mode (Wescor, Logan, UT). Groups of three to four individuals were placed in the sample holder (diameter, 7 mm; depth, 2.5 mm), rapidly opened with two needles to expose body fluids, and then placed in the C-52 sample chamber (14). Using this apparatus, MP could be estimated with a precision of ±0.1°C in the range −0.2 to −7.5°C.
Frost-Exposure Experiment 2.
In this subsequent experiment, we simulated the onset of winter temperatures from the habitat where we collected O. arcticus at Stuphallet, Spitzbergen, by using data from October to November 1993 (20). Individual D. octaedra cocoons were lightly freed of surface water by blotting with filter paper, then placed in a small plastic vial (height, 5 mm; diameter, 9 mm), which was in turn placed on top of ≈2 ml of crushed ice in a 10-ml glass vial. This vial was closed with a tightly fitting lid. Single collembolans were placed in similar plastic cups and confined by means of a 200-μm nylon mesh at the open end of the vial. At time intervals 2–7 days apart, covering a total period of 40 days, individual body-fluid MPs were determined by using differential scanning calorimetry (DSC). The calorimeter used was a Perkin–Elmer DSC-7, equipped with an Intercooler II. In the calorimeter, samples of single individuals were placed in sealed aluminium pans with an empty pan as reference. Samples were cooled to −60°C at a rate of 5°C min−1, held at −60°C for 5 min, and then warmed to a target temperature of 5°C with a rate of 1°C min−1. The calorimeter was calibrated with gallium (Tm = 30.0°C) and the eutectic point of aqueous NaCl solution (Tm = −21.1°C). The body-fluid MP was estimated as the temperature at the apex of the melt endotherm (6). The detection limit of this setup was equivalent to the melting endotherm of 10 μg of ice.
Reevaluation of Data from Worland et al. (16).
Worland et al. (16) reported water loss and trehalose accumulation in O. arcticus held in closed containers with moist plaster of Paris at subzero temperatures. The collembolans were therefore exposed to the vapor pressure of ice, as was the case in the experiments described in the previous sections. We calculated the content of osmotically active water (OAW) of O. arcticus by subtracting the osmotically inactive water (OIW) from the total WC given in Worland et al.'s figure 2a (16). OIW was calculated by using the formula given in Worland et al.'s figure 1 (16). Under the assumption that O. arcticus does not osmoregulate during dehydration, it is possible to calculate the contribution to decreased MP originating from original solutes during dehydration. Studies of related and functionally similar collembolan species suggest that the ability to osmoregulate is extremely limited in these organisms (21, 22). Assuming that the concentration of solutes is proportional to MP, the contribution of original solutes to MP is then the initial MP (set at −0.8°C) multiplied by the ratio OAWinit/OAWdehy (the ratio between initial OAW and OAW of dehydrated animals at time t). Trehalose, which is synthesized during dehydration, also makes a significant contribution to the reduction in body-fluid MP. First, the body-fluid concentration was calculated by reading the amount of trehalose produced from Worland et al.'s figure 4b (16) (μg⋅mg−1 fresh weight) and calculating the molal concentration on the basis of OAW. The contribution of trehalose to the body-fluid MP was then calculated by applying the osmolal MP depression constant of −1.86°C osmol−1 kg. The overall body-fluid MP was finally estimated by adding the contributions from trehalose and original solutes.
Results
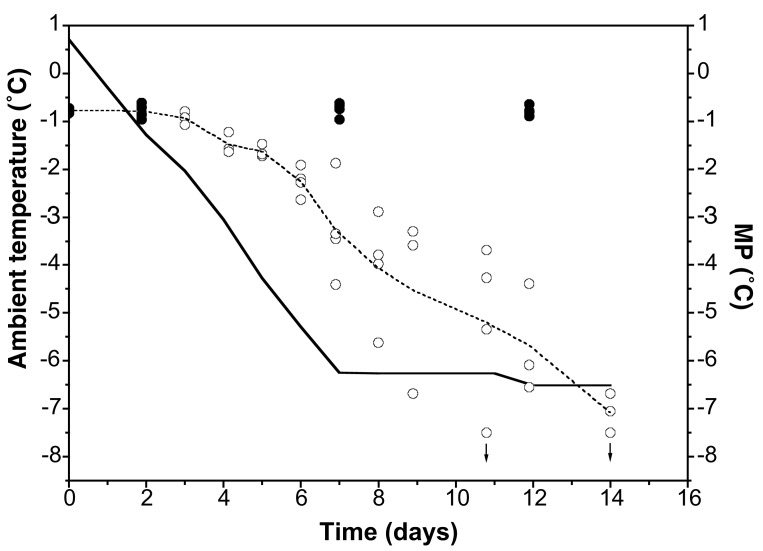
In our first experiment, O. arcticus was subjected to a constant cooling rate. After an initial lag phase, average body-fluid MP appeared to decrease at a slightly lower rate than the ambient temperature, approximately −0.7°C day−1 (Fig. 2). Control animal MP remained at −0.8°C. Ambient temperature reached the target temperature of −6.5°C after 7 days, whereas average MP of O. arcticus reached this temperature after 13 days. On average, groups of O. arcticus supercooled 1–2°C during this period. In the first 6 days of the experiment (where ambient temperature dropped from +1°C to −6°C), there was little variation in MP. At these sampling times, individuals of O. arcticus were active and moving when removed from the freezer. At subsequent sampling times the animals were increasingly dehydrated and entered an immobile state. At these sampling times, it was therefore impossible to distinguish individuals inactive because of dehydration from individuals that had died because of freezing. The large variation in MP between samples seen at this time could therefore be due to occasional frozen individuals, which would have ceased to dehydrate at death, when their body fluids froze. These individuals would therefore increase the overall WC of the sample, leading to measurement of a higher average MP than if only unfrozen individuals had been included in the sample. Even though average MP reached the ambient temperature only after 13 days, it should be noted that the samples with the lowest MPs reached equilibrium with ambient already after 8–9 days.
Figure 2.
Body-fluid MPs of groups of three to four specimens of O. arcticus at decreasing temperatures when held in an atmosphere saturated with the vapor pressure of ice. Solid line, ambient temperature in the freezing cabinet; open circles, MP of individual groups of O. arcticus exposed over ice; closed circles, MP of individual groups of O. arcticus exposed over water at +1°C (control); dashed line, mean MP. Arrows at points indicate that the MP was lower than the range of the psychrometer, −7.5°C. In the time period of 4–12 days, the average MP decreased by 0.7°C day−1 (linear regression, R2 = −0.87; P < 0.0001).
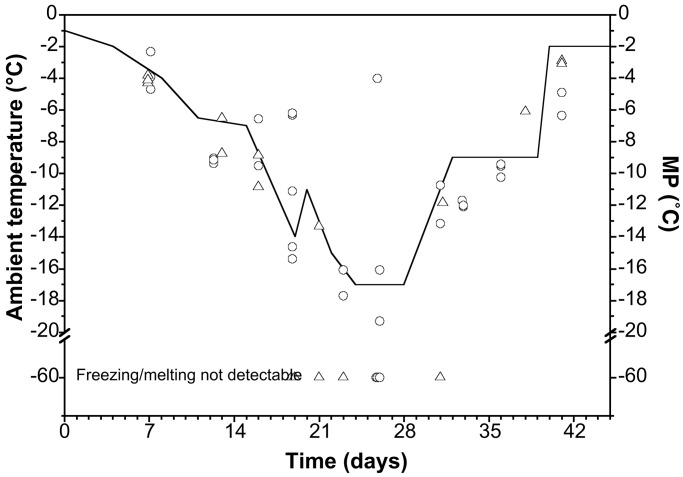
In the second experiment, we used DSC for MP determination, enabling us to determine the MP of single individuals over a much wider range of MPs than was possible using the dewpoint microvoltmeter. During the first 23 days, the temperature dropped from −1 to −17°C, with a maximum rate of about 1°C day−1. In the freezing cabinet, the MPs of both the collembolans and the earthworm cocoons were, on average, at the same level as the cabinet temperature, suggesting that the organisms' body fluids were not supercooled to any significant extent during this period (Fig. 3). In fact, many of the individuals had a MP lower than the ambient temperature, especially at the lowest temperatures. During the experiment, only 3 of 33 O. arcticus had a MP much higher than the ambient temperature (e.g., at day 26). The most likely explanation of these observations is that these individuals had frozen in the freezing cabinet, at which time they had ceased to dehydrate. At cabinet temperatures below −14°C, D. octaedra cocoons showed no melting endotherms, even though these samples had been held at −60°C in the DSC, suggesting that these individuals did not contain any detectable freezeable water. A number of O. arcticus individuals appeared also to be devoid of freezeable water at cabinet temperatures of −17°C. Interestingly, a subsequent temperature increase from the lowest-used temperature at −17°C resulted in an almost simultaneous increase in body-fluid MP of both species (Fig. 3).
Figure 3.
Body-fluid MP depression in collembolans and earthworm cocoons during a natural temperature fall. Circles, MP of individual O. arcticus; triangles, MP of individual D. octaedra cocoons. The solid line indicates the temperature of the freezing cabinet. Points with MP values at −60°C indicate that freezing or melting was not observed even after exposure to −60°C.
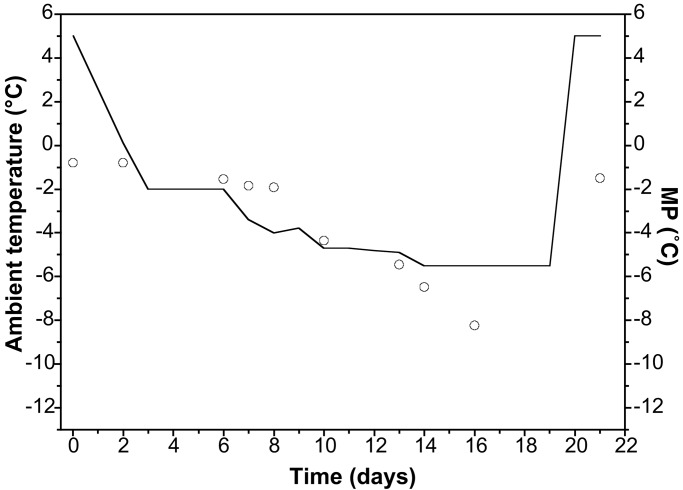
In the experiment by Worland et al. (16), O. arcticus were cooled in steps of 1–2°C day−1, as depicted in Fig. 4. This treatment resulted in an extensive dehydration and a concomitant increase in the trehalose content of O. arcticus. According to our calculations, the body-fluid MP was slightly higher (1–2°C) than the ambient temperature during the first 6 days at subzero temperatures (days 2–8) in which the temperature dropped from −1 to about −4°C. At day 10 (ambient temperature at −4.7°C), body-fluid MPs had approximately equilibrated to ambient and thereafter had a lower MP than the environmental temperature. At day 16, the MP was approximately −8°C, which should be compared with an ambient temperature of −5.5°C. From days 19 to 20, the environmental temperature was increased abruptly from −5.5 to +5°C, causing O. arcticus to absorb water from the moist substrate. This dehydration led to a reduction in trehalose concentration simultaneously with a rapid conversion of trehalose back to glycogen (16). Thus, after 1 day at +5°C, the MP was −1.5°C.
Figure 4.
Average MP of O. arcticus at decreasing temperatures when held in an atmosphere saturated with the vapor pressure of ice. Solid line, ambient temperature in the freezing cabinet; circles, average MP of O. arcticus body fluids. MPs are calculated by using data reported in Worland et al. (16).
Discussion
The three sets of data presented in this study concern the changes in body-fluid MP of organisms with high cuticular permeability for water at decreasing subzero temperatures. Thus, in two independent experiments, it was shown that the collembolan, O. arcticus, during the initial phase of the temperature decline decreased its MP almost in parallel to the environmental temperature (Figs. 2 and 4). After a short lag phase, the MP equilibrated with the environmental temperature and later fell to several °C below this. This trend was confirmed in the longer-term experiment, where individual MPs were determined by using DSC (Fig. 3). These results show that MP on a wider time scale followed the environmental temperature quite closely, during both decreasing and increasing temperatures. The experiment with cocoons of the earthworm, D. octaedra, essentially shows the same results (Fig. 3). In combination, these results suggest that supercooling of the body fluids of these two organisms during overwintering is limited to a few days, and that supercooling during this period is restricted to a few °C. These studies were conducted in relatively pure systems, where equilibrium was attained with liquid or ice phases. Even though pore water of the upper soil or litter layers (where these organisms are found) is not pure, the concentration of solutes is generally so low that we consider our experimental conditions to be applicable to natural conditions in the majority of cases.
The observed depression in MP of both collembolan and earthworm body fluids was due to dehydration and the resultant increase in the concentration of original solutes, but also a concomitant accumulation of sugars and polyols (SP). Dehydration causes embryos of D. octaedra cocoons to synthesize and accumulate sorbitol (23). Likewise, O. arcticus will accumulate trehalose during dehydration (16). Thus, it is the combination of dehydration and SP accumulation that enables these organisms to match their body-fluid MP to the current ambient temperature. As is to be expected when following this line of reasoning, the programmed temperature increase from the minimum temperature at −17°C resulted in an almost simultaneous increase in body-fluid MP of both species (Fig. 3). Thus, the reversal of the vapor pressure gradient caused by the increase in ambient temperature induced the organisms to absorb water vapor, thereby diluting the solutes of the body fluids and elevating their MPs. This process is a simple reversal of the dehydration occurring with falling temperatures and bears an interesting parallel to the passive water-vapor absorption mechanism found in similar collembolans during summer drought (21, 22).
Although this vapor pressure difference can explain the major trend in all three experiments, the fact that some individuals overshot and ended up with a MP several degrees below ambient is not directly reconcilable with this theory. However, all these cases occurred where the WC of the organism was extremely low. In this end of the dehydration spectrum, even a small change in WC, increasing or decreasing, would have a large effect on the resulting MP (15, 24). Because of inevitable inaccuracies in WC determinations, the calculated MP of O. arcticus may be imprecise at very low WCs (Worland et al.; Fig. 4) (this paper). Likewise, the MP determinations of very dehydrated specimens in the lower end of the range of the Wescor psychrometer may also be imprecise. During the transfer of O. arcticus specimens to the C-52 sample holders and crushing of the sample, a small amount of water is likely to evaporate, which would lead to a measured MP lower than the actual MP (Fig. 2). In the DSC experiment, the same considerations may apply for those samples where melting endotherms (or freezing exoterms) were not detected (Fig. 3; ambient temperatures at −14 to −17°C). At these temperatures, almost all of the remaining water of the animals can be considered as “bound water” (or OIW) strongly constrained by van der Waals forces to, e.g., proteins and membranes, even though some OIW may be “released” to become OAW at very low water potentials (15, 16). It is therefore likely that so little “freezeable water” remained that it was probably below the detection limit of the DSC apparatus (Peter Westh, personal communication). We conclude that both the earthworm cocoons and the collembolans equilibrated their MP with the ambient temperature and consider the apparent tendency to achieve a MP lower than the ambient temperature to be an experimental error.
We have emphasized that the protective dehydration strategy does not involve extensive or long-term supercooling of body fluids. However, in fact it appears that supercooling is a brief but necessary part of the winter survival process, and that an elegant balance between dehydration and supercooling seems to occur. For example, when considering the special case of an animal body-fluid MP of −2°C surrounded by ice at −3°C ambient temperature (“ambient MP”), we find that a difference in water potential of about 12 bar exists to facilitate dehydration (Fig. 1). Even with a “MP inequality” of only 0.1°C, there is a difference in water potential of about 1.2 bar. This substantial driving force explains why dehydration can occur at such a high rate in animals with high integument conductance. At the same time, it is important to note that the risk of spontaneous or inoculative freezing is probably negligible when the difference in animal MP and ambient temperature is on the order of 0.1°C. There are considerable data from insects showing that the risk of freezing is very low when the ambient temperature is >5°C higher than the standard supercooling point (SCP) (reviewed in ref. 9). The SCP of the animals in the present study was always at least 5°C lower than the ambient temperature used in our experiments (14, 19).
A number of studies have investigated the effects of dehydration on supercooling abilities. Not surprisingly, dehydration will lower the SCP because it increases the concentration of original solutes (9). This lowering of SCP has been shown for collembolans (14, 16, 25), earthworm cocoons (19, 26), and insects (1, 24). However, our rationale here is that, whereas increased supercooling ability because of dehydration may be of importance in desiccation-resistant organisms (e.g., insects), the adaptive value of this phenomenon is probably insignificant in permeable soil invertebrates, because supercooling in these organisms hardly occurs. In invertebrates that really do rely on prolonged supercooling for winter survival, thermal hysteresis molecules, which strongly inhibit the growth of ice crystals, are typically present (27, 28). Interestingly, these molecules have so far not been detected in the small permeable soil invertebrates we are concerned with here, despite a number of attempts to find them (11, 26, 29). Because these animals equilibrate their body-fluid MP to their surroundings, ice crystal formation is impossible and thermal hysteresis molecules an unnecessary adaptation. On the other hand, thermal hysteresis molecules could be advantageous for soil invertebrates by preventing ice formation by inoculative freezing from soil ice during the first winter freeze period with temperatures just below zero. During this period, these animals are still hydrated and the accumulation of sugars and polyols has not begun yet. Previous studies have demonstrated that thermal hysteresis molecules in certain insects can prevent ice penetration through the integument (30, 31).
This “protective dehydration strategy” has so far been studied only in a few species. However, that we here show its effectiveness as an overwintering strategy in two so phylogenetically distant organisms as an earthworm and a collembolan, whose only relevant common feature in this context is their high water permeability, strongly suggests that this frost-induced dehydration is a widespread and effective winter survival strategy in soil invertebrates. Further, the cooling rates we have used in this study are “worst-case scenarios” (20), suggesting that vapor equilibration is possible under the vast majority of cooling rates in natural soils. Many other soil invertebrate species possess the characteristics necessary for the effective use of this strategy, namely small size and high permeability for water. Thus, frost-induced dehydration has been shown in nematodes (32), enchytraeids (13), and chironomid larvae (33, 34). Other soil invertebrates, such as tardigrades and rotifers, could also be candidates. Further study of the cold-hardiness strategies of soil invertebrates seems both to be needed and likely to be rewarding.
Acknowledgments
Steve Coulson is thanked for temperature data from Svalbard. We also thank Peter Westh for constructive criticism and assistance. This study received financial support from the Danish Natural Science Research Council and the Carlsberg Foundation.
Abbreviations
- MP
melting point
- DSC
differential scanning calorimetry
- OAW
osmotically active water
- WC
water content
- OIW
osmotically inactive water
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Zachariassen K E. Physiol Rev. 1985;65:799–831. doi: 10.1152/physrev.1985.65.4.799. [DOI] [PubMed] [Google Scholar]
- 2.Block W. Philos Trans R Soc London B. 1990;326:613–633. [Google Scholar]
- 3.Lee R E. In: Insects at Low Temperature. Lee R E, Denlinger D, editors. New York: Chapman & Hall; 1991. pp. 17–46. [Google Scholar]
- 4.Pickup J. Funct Ecol. 1990;4:257–264. [Google Scholar]
- 5.Wharton D, Block W. Funct Ecol. 1993;7:578–584. [Google Scholar]
- 6.Block W, Bauer R. Cryo-Letters. 2000;21:99–106. [PubMed] [Google Scholar]
- 7.Cannon R, Block W. Biol Rev. 1988;63:23–77. [Google Scholar]
- 8.Holmstrup M, Zachariassen K E. Comp Biochem Physiol. 1996;115A:91–101. [Google Scholar]
- 9.Sømme L. Comp Biochem Physiol. 1982;73A:519–543. [Google Scholar]
- 10.Petersen H, Luxton M. Oikos. 1982;39:287–388. [Google Scholar]
- 11.Aunaas T, Baust J G, Zachariassen K E. Polar Res. 1983;1:235–240. [Google Scholar]
- 12.Block W, Webb N R, Coulson S, Hodkinson I D, Worland M R. J Insect Physiol. 1994;40:715–722. [Google Scholar]
- 13.Sømme L, Birkemoe T. J Comp Physiol B. 1997;167:264–269. [Google Scholar]
- 14.Holmstrup M, Sømme L. J Comp Physiol B. 1998;168:197–203. [Google Scholar]
- 15.Holmstrup M, Westh P. J Comp Physiol B. 1994;164:312–315. [Google Scholar]
- 16.Worland M, Grubor-Lajsic G, Montiel P. J Insect Physiol. 1998;44:211–219. doi: 10.1016/s0022-1910(97)00166-2. [DOI] [PubMed] [Google Scholar]
- 17.Weast R C. Handbook of Chemistry and Physics. Cleveland: CRC; 1989. [Google Scholar]
- 18.Holmstrup M, Westh P. J Comp Physiol B. 1995;165:377–383. [Google Scholar]
- 19.Holmstrup M. Comp Biochem Physiol. 1992;102A:49–54. [Google Scholar]
- 20.Coulson S, Hodkinson I D, Strathdee A, Block W, Webb N, Bale J, Worland M. Arctic Alpine Res. 1995;27:364–370. [Google Scholar]
- 21.Bayley M, Holmstrup M. Science. 1999;285:1909–1911. doi: 10.1126/science.285.5435.1909. [DOI] [PubMed] [Google Scholar]
- 22.Holmstrup M, Sjursen H, Ravn H, Bayley M. Funct Ecol. 2001;15:647–653. [Google Scholar]
- 23.Holmstrup M. Comp Biochem Physiol. 1995;111A:251–255. [Google Scholar]
- 24.Ring R. Comp Biochem Physiol. 1982;73A:605–612. [Google Scholar]
- 25.Worland M R. Eur J Entomol. 1996;93:341–348. [Google Scholar]
- 26.Holmstrup M. J Comp Physiol B. 1994;164:222–228. [Google Scholar]
- 27.Zachariassen K E, Husby J A. Nature (London) 1982;298:865–867. [Google Scholar]
- 28.Duman J G. Annu Rev Physiol. 2001;63:327–357. doi: 10.1146/annurev.physiol.63.1.327. [DOI] [PubMed] [Google Scholar]
- 29.Zettel J. Rev Écol Biol Sol. 1984;21:189–203. [Google Scholar]
- 30.Gehrken U. J Insect Physiol. 1992;38:519–524. [Google Scholar]
- 31.Olsen T M, Sass S J, Li N, Duman J G. J Exp Biol. 1998;201:1585–1594. doi: 10.1242/jeb.201.10.1585. [DOI] [PubMed] [Google Scholar]
- 32.Forge T A, MacGuidwin A E. Can J Zool. 1992;70:1553–1560. [Google Scholar]
- 33.Scholander P F, Flagg W, Hock R J, Irving L. J Cell Comp Physiol. 1953;42:1–56. doi: 10.1159/000054843. [DOI] [PubMed] [Google Scholar]
- 34.Danks H. Can Entomol. 1971;103:1875–1910. [Google Scholar]