Abstract
The 2-cysteine peroxiredoxins (2-Cys Prx) constitute an ancient family of peroxide detoxifying enzymes and have acquired a plant-specific function in the oxygenic environment of the chloroplast. Immunocytochemical analysis and work with isolated intact chloroplasts revealed a reversible binding of the oligomeric form of 2-Cys Prx to the thylakoid membrane. The oligomeric form of the enzyme was enhanced under stress. The 2-Cys Prx has a broad substrate specificity with activity toward hydrogen peroxides and complex alkyl hydroperoxides. During the peroxide reduction reaction, 2-Cys Prx is alternatively oxidized and reduced as it catalyzes an electron flow from an electron donor to peroxide. Escherichia coli thioredoxin, but also spinach thioredoxin f and m were able to reduce oxidized 2-Cys Prx. The midpoint redox potential of −315 mV places 2-Cys Prx reduction after Calvin cycle activation and before switching the malate valve for export of excess reduction equivalents to the cytosol. Thus the 2-Cys Prx has a defined and preferential place in the hierarchy of photosynthetic electron transport. The activity of 2-Cys Prx also is linked to chloroplastic NAD(P)H metabolism as indicated by the presence of the reduced form of the enzyme after feeding dihydroxyacetone phosphate to intact chloroplasts. The function of the 2-Cys Prx is therefore not confined to its role in the water–water cycle pathway for energy dissipation in photosynthesis but also mediates peroxide detoxification in the plastids during the dark phase.
Reactive oxygen species (ROS) take part in the regulation of many cellular processes. Low concentrations of ROS are required as substrates and signals in cell metabolism, growth, and differentiation (1, 2). At elevated concentrations, ROS participate in defense reactions and trigger changes in gene expression and apoptosis, but they also can damage macromolecules and membranes (3). Consequently, each cell has a multilevel and cross talking defense system against oxidative damage consisting of low molecular weight antioxidants and enzymes (4, 5). The peroxiredoxins, and among these the subgroup of 2-cysteine peroxiredoxins (2-Cys Prx), belong to the enzymic antioxidants (6).
The 2-Cys Prx are ubiquitous enzymes that reduce a broad range of peroxides by an intermolecular thiol-disulfide transition (7). First, a more N-terminal Cys-residue is oxidized by the peroxide. The resulting sulfenic acid intermediate (Cys-SOH) then interacts with a second conserved Cys from the other subunit by C-terminal tail swapping (8). Recent crystal structure analysis with rat 2-Cys Prx (HBP23) demonstrated that the active site is largely hydrophobic with the more C-terminally located Cys being exposed in the oxidized 2-Cys Prx form to allow for rereduction by electron donors such as thioredoxin (Trx) (8). In addition to the functional dimer, the occurrence of multimeric complexes was observed in yeast, Crithidia fasciculata, and man (7, 9, 10). The authors hypothesized a regulatory role of oligomerization on 2-Cys Prx activity.
In plants, the 2-Cys Prx is a nuclear encoded chloroplast protein (11), whereas the yeast homolog is localized in the cytosol as well as most mammalian 2-Cys Prx (12, 13). The Arabidopsis genome contains two 2-Cys Prx genes (14). Analysis of transgenic Arabidopsis with reduced 2-Cys Prx amounts demonstrated that the 2-Cys Prx protects chloroplast proteins from oxidative damage. Partial suppression of 2-Cys Prx expression caused impairment of photosynthesis and increased oxidative damage of chloroplast proteins during early plant development (14).
The chloroplast is characterized by highly variable reduction potentials and a very active oxygen metabolism, depending on environmental parameters such as light, temperature, and acceptor availability (2). Thus the 2-Cys Prx is in a physiological environment distinct from any subcellular compartment of yeast, bacteria, or mammals and is likely to serve specific functions in context with photosynthesis and other chloroplast metabolic pathways.
This paper is the first detailed analysis of the catalytic function of the plant 2-Cys Prx. The basic questions addressed concerned the suborganellar localization, the propensity to oligomerize, the reconstitution of a photosynthetically relevant electron transport chain for rereduction, and possible regulatory mechanisms. Among other results, it will be shown that the 2-Cys Prx attaches to the thylakoid membrane, that the redox state of the 2-Cys Prx depends on the NAD(P)H system, and that the midpoint redox potential defines an antioxidant defense role of the 2-Cys Prx downstream of Calvin cycle activation and upstream of activation of the malate valve. From the data, a novel model of functional regulation is deduced for PRX activity in chloroplasts.
Material and Methods
Plant Growth.
Barley (Hordeum vulgare var. Gerbel) was grown in soil culture under controlled conditions with 14-h light phase at a photosynthetic active radiation of 100 μmol quanta m−2 s−1 and 25°C and 10 h of darkness at 20°C. For etiolation, the seedlings were kept in permanent darkness for 8–10 d.
Isolation of Intact Chloroplasts and Incubation Conditions.
Barley leaves (10 g) of 10- to 12-day-old seedlings were homogenized in an ice-cold buffer containing 330 mM sorbitol, 50 mM Mes-KOH (pH 6.5), 2 mM ascorbate, and 5 mM MgCl2. Chloroplasts were purified on a Percoll gradient (11) and resuspended in sorbitol buffer. Chloroplasts were incubated in medium with or without ascorbic acid for 5 or 60 min, sedimented, and lysed by addition of 5 mM K-Pi (potassium phosphate) buffer, pH 7.5. After centrifugation at 14,000 × g, the supernatant was analyzed as soluble phase. The thylakoid sediment was washed with K-Pi buffer twice. Both fractions were loaded on SDS/PAGE. To test for redox coupling of the 2-Cys Prx redox state to the NAD(P)H system, chloroplasts were added to sorbitol medium with 10 mM ascorbic acid, 20 mM dihydroxyacetone phosphate (DHAP), or 20 mM 3-phosphoglycerate (3-PGA). After 15′ incubation, stop solution was added to a final concentration of 50 mM N-ethylmaleimide, 1.25% (wt/vol) SDS, and 5 mM Tris⋅Cl, pH 7.6. The samples were diluted with loading buffer and analyzed by standard SDS/PAGE (12% acrylamide) as referenced in Baier and Dietz (11).
Heterologous Expression and Site-Directed Mutagenesis of 2-Cys Prx.
Wild-type and mutant forms of 2-Cys Prx were expressed as His-tagged protein by using the pQE-30 vector and M15[pREP4] cells. The wild-type cDNA of Hv-2-Cys Prx was integrated into the BamHI-site as described before (11). Site-directed mutations were introduced with two subsequent PCRs by using Pfu (Pyrococcus furiosus) polymerase (Stratagene) and verified by sequencing. The primers used were: pQE-30–5′: ggcgtatcacgaggccctttcg, pQE-30–3′: cattactggatctatcaacagg, CysPrx-5′: atataggatccgattcgaggacggc, 2-CysPrx-3′: atataggtaccctagatagcagcgaa, Cys-64–Ser-5′: cttcgtctccccaactg, Cys-64–Ser-3′: cagttggggagacgaag, Cys-185–Ser-5′: cgaggtctccccggcag, and Cys-185–Ser-3′: ctgccggggagacctcg.
Protein Purification.
Heterologously expressed protein [2-Cys Prx, Escherichia coli Trx, and Trx reductase (TR)] was purified from frozen cell pellets obtained from 1-liter cultures induced with 0.4 mM isopropyl β-d-thiogalactoside for 4 h. The cells were suspended in lysis buffer containing 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 10 mM ascorbate, and 0.5 mg/ml lysozyme, pH 8.0 (NaOH). The solution was shaken at 4°C for 60 min and spun at 15,000 rpm at 4°C for 30 min. The supernatant was loaded onto the Ni-NTA column, previously equilibrated with a solution of 50 mM NaH2PO4, 300 mM NaCl, and 20 mM imidazole, pH 8.0 (NaOH) (buffer A). The loaded resin was washed with 20-column vol of buffer A, followed by 20 vol of buffer A supplemented with 20% glycerol. The protein was eluted with 250 mM imidazole in buffer A, dialyzed, and stored at −20°C. Recombinant spinach Trx f and m, Synechocystis Fd-dependent TR (15–16), spinach Fd, and Fd:NADP+ reductase were purified to homogeneity. E. coli Trx and TR were overexpressed and purified as described (17).
Gel Permeation Chromatography.
The aggregation state of 2-Cys Prx was determined by gel filtration on a Superdex 75 HR 10/30 column (Amersham Pharmacia), equilibrated in K-Pi buffer (40, 200, 500 mM) at a flow rate of 0.5 ml/min. Protein samples (100 μl) were injected in the system. The elution profile was monitored at 280 nm (recombinant protein) or tested for 2-Cys Prx protein by Western blot analysis (crude extract).
Trx-Dependent Activity Assay.
Rates of peroxide reduction were determined in a coupled assay with E. coli Trx and TR by monitoring NADPH oxidation. The assay typically contained 50 μM peroxide in 100 mM K-Pi buffer (pH 7.0), 1 mM EDTA, 0.1 mM NADPH, 3.2 μM 2-Cys Prx, 8.3 μM Trx, and 3.2 μM TR at 20°C. The initial rate of NADPH oxidation was calculated from the slope between 12 and 42 s after addition of the peroxide substrate and corrected for the background oxidation of NADPH in the absence of 2-Cys Prx. The assay with plant Trx contained 50 mM Tris⋅Cl (pH 8.0), 10 mM NADPH, 2.2 μM ferredoxin:NADP reductase (FNR), 10 μM ferredoxin (Fd), 1.75 μM Fd:Trx reductase (FTR), 10 μM Trx m or Trx f, and 8 μM 2-Cys Prx. After 30 min at room temperature, the redox state was arrested with the same volume of Tris buffer supplemented with 100 mM N-ethylmaleimide. Aliquots corresponding to 0.4 μg of 2-Cys PRX were separated by SDS/PAGE, and the redox state of 2-Cys Prx was analyzed by Western blotting.
Oxidation-Reduction Midpoint Potential.
The recombinant wild-type Hv-2-Cys Prx was titrated for redox-state at pH 7.0. The 2-Cys Prx (3.6 μM) was incubated in Mops buffer (100 mM) containing 2 mM total DTT. After 3 h at ambient temperature, monobromobimane was added at a final concentration of 10 mM. The samples were prepared for fluorescence analysis as described by Hirasawa et al. (18).
Immunocytochemistry.
Sections of 1 mm2 were cut from the acropetal part of the leaves of light and dark grown, 8-day-old barley seedlings and fixed in 2.5% (wt/vol) glutaraldehyde (Serva) in buffer (50 mM KH2PO4/Na2HPO4, pH 7.0) for 45 min. The tissue was dehydrated in a graded acetone series and embedded in Transmit resin (TAAB Laboratories Equipment, Berkshire). Ultrathin sections of 60 nm were made with a diamond knife (DuPont) on an Ultracut microtome (Reichert Ultracut E) and placed onto 400 mesh gold grids.
For immunocytochemistry (19), samples were immunolabeled with rabbit antiserum (1:100) that was raised against a peptide of the barley 2-Cys Prx (20) and diluted in 10 mM BSA/TBS containing 0.05% (wt/vol) NaN3 for 1 h. Grids were rinsed five times with TBS and incubated for 1 h with gold-conjugated (15 nm) anti-rabbit IgG (Sigma) at a 1:30 dilution in BSA/TBS. Samples were counterstained with 0.1% (wt/vol) uranyl acetate (5 sec) followed by 2% (wt/vol) lead citrate (5 sec). Preparations were examined with a Hitachi H500 electron microscope at 75 kV.
Results
Chloroplast 2-Cys Prx Catalyzes Trx-Dependent Peroxide Reduction.
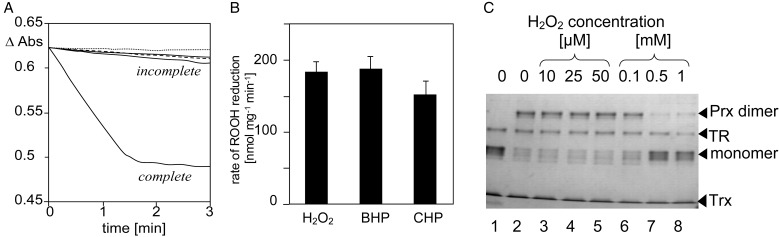
The biochemical features of the chloroplast 2-Cys Prx were investigated by reconstituting the peroxide-reducing activity with E. coli Trx, E. coli TR, and NADPH. Fig. 1A reveals low rates of H2O2 reduction, as measured by monitoring NADPH oxidation, when any single component of the electron transport chain was omitted from the system. Only the complete system reduced H2O2 at the Vmax of 180 nmol (mg protein⋅min)−1. The stoichiometry between H2O2 reduction and NADPH oxidation was unity in these experiments. Interestingly, reduction of butylhydroperoxide and cumenehydroperoxide proceeded at almost the same rates as H2O2 reduction, showing the broad substrate specificity of 2-Cys Prx (Fig. 1B). In the Trx-TR system, the rate of reduction of H2O2 was proportional to the Trx amount at concentrations <6.2 μM and increased linearly with the 2-Cys Prx amount in the test assay. Mutation of the catalytic cysteine residues inactivated 2-Cys Prx and decreased the rate of absorption change to the basal level. The result confirmed the specificity of the assay. The pH dependency showed a pronounced maximum at pH 7 (not shown). The H2O2-reducing activity also was studied as a function of H2O2 concentration. For technical reasons, the spectrophotometric assay could only be used down to an initial concentrations of 10 μM H2O2. The rate was not stimulated when the concentration was increased to 25 and 50 μM H2O2. From that and the data shown in Fig. 1A, a Km(H2O2) of 2 μM can be deduced. At elevated H2O2 concentrations, an inhibition of 2-Cys Prx activity was observed. Concomitantly, the 2-Cys Prx started to migrate with the apparent molecular mass of the monomer in polyacrylamide separations under nonreducing denaturing conditions (Fig. 1C). Monomerization after SDS treatment is typical for the fully reduced form (21). Under highly oxidizing conditions, the presence of the monomeric form is explained by over-oxidation of the thiol groups of the catalytic Cys residues to the sulfinic acid forms. Crystal structure analysis of erythrocyte 2-Cys Prx (10) demonstrates that the over-oxidized Cys-residue and the second conserved cysteine residue cannot form disulfide bridges any more. Consequently, dimers with both catalytic centers over-oxidized do not form disulfide bridges and run as monomers under denaturing conditions.
Figure 1.
Peroxide reduction by recombinant Hv-2-Cys Prx. (A) Kinetics of reduction as measured by time-dependent absorption changes in an assay containing 2-Cys Prx. E. coli-Trx, E. coli-TR, NADPH, and H2O2 (complete system) or in the absence of each single component (incomplete system, traces from the top: −H2O2, −TR, −Trx, and −2-Cys Prx). (B) Comparison of initial rates of reduction of H2O2, butylhydroperoxide (BHP), and cumenehydroperoxide (CHP) at a concentration of 25 μM. The stoichiometry between peroxide reduction and NADPH oxidation was shown to be unity. (C) Inactivation of recombinant 2-Cys Prx at high H2O2 concentrations as investigated by nonreducing SDS/PAGE. Lane 1 shows the reduced 2-Cys PRX in the complete reconstitution system. For lanes 2–8, the reconstitution system was supplemented with increasing H2O2 concentrations and dialyzed for 4 h to 40 mM K-Pi for a mild oxidizing of 2-Cys Prx and dimer formation.
Immunocytochemistry Shows (Partial) Association of 2-Cys Prx with the Thylakoid Membrane.
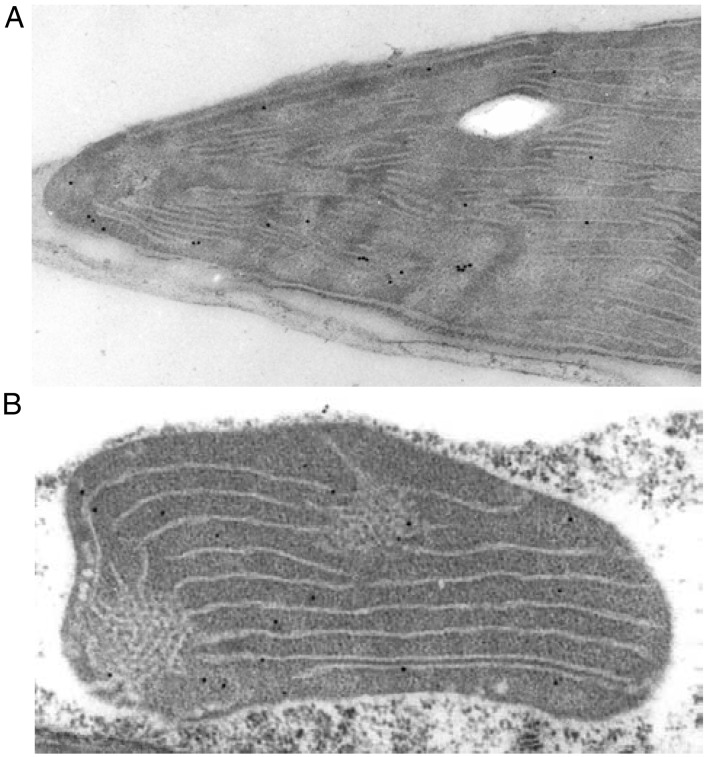
Previously, the 2-Cys Prx preprotein has been shown to be posttranslationally targeted to the chloroplast and the mature 2-Cys Prx protein was localized in the chloroplast (11). However the suborganellar compartmentation has been unknown. The subcellular and organellar localization of 2-Cys Prx was analyzed immunocytochemically in cross sections of mature fully greened or etiolated leaves. The ImmunoGold label was detected within the plastids. A thorough analysis of the distance of gold particles to thylakoid membranes demonstrated that 2-Cys Prx preferentially is attached to the stromal side of the stromal thylakoids (Fig. 2A). In the set of chloroplast cross sections analyzed, 72% of 159 ImmunoGold particles were next to thylakoids. In contrast, in etioplasts the 2-Cys Prx signals appeared distributed more evenly across the plastid sections and only 20–30% of the ImmunoGold signals in the vicinity of the thylakoid lamella (Fig. 2B). Control studies with preimmune serum showed no binding.
Figure 2.
Immunocytochemical detection of 2-Cys Prx protein in a leaf cross section of light-grown or etiolated 8-day-old barley seedling. ImmunoGold labeling in a partial section of a mesophyll cell from light-grown leaves (A) and from etiolated leaves (B).
2-Cys Prx Oligomerizes and Oligomerization Mediates Thylakoid Membrane Attachment.
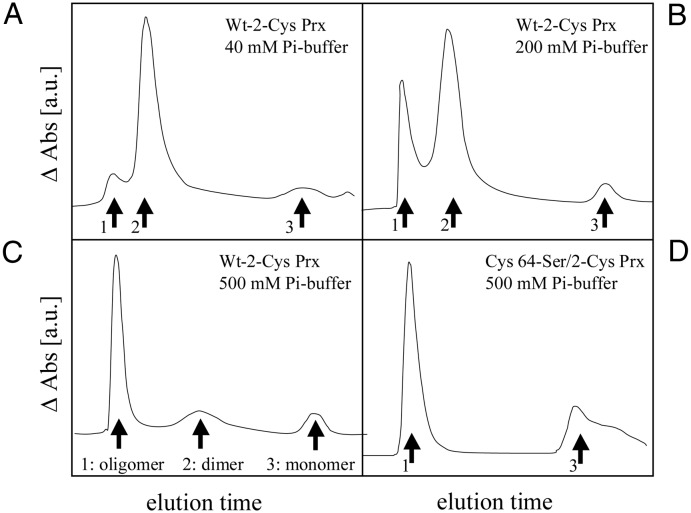
Size exclusion chromatography allows to distinguish monomeric, dimeric, and multimeric Prx (Fig. 3). Recombinant wild-type and Cys-64→Ser mutant form of 2-Cys Prx were separated on Superdex 75 HR material. At low ion concentration, the predominant form of 2-Cys Prx was the dimer (Fig. 3A). With increasing ion concentration, the proportion of the oligomeric form of 2-Cys Prx increased and was the predominant form at 500 mM K-phosphate (Fig. 3 B and C). Oligomerization at high salt concentrations was also seen with the Cys-64→Ser mutant form of 2-Cys Prx showing that the disulfide bridge formation is not required for oligomerization (Fig. 3D). The same aggregation behavior of 2-Cys Prx was observed in crude extracts from barley by size exclusion chromatography followed by immunodetection, i.e., separation as dimer at 40 mM, roughly equal abundance of multimer and dimer at 200 mM and main presence as oligomer at 500 mM K-Pi buffer (data not shown).
Figure 3.
Salt concentration dependence of 2-Cys Prx oligomerization state. Heterologously expressed wild-type and Cys-64→Ser mutant form, respectively, was separated by size exclusion chromatography. The peak labeled as oligomeric form run in the exclusion volume of the Superdex 75 column. On Superose 12 material, the oligomeric form separated as a complex of ≈500 kDa (result not shown). Wild-type 2-Cys Prx in Pi buffer at 40 mM concentration (A), wild-type 2-Cys Prx in Pi buffer (200 mM) (B), wild-type 2-Cys Prx in Pi buffer (500 mM) (C), and Cys-64→Ser mutant form of 2-Cys Prx in Pi buffer of 500 mM concentration (D).
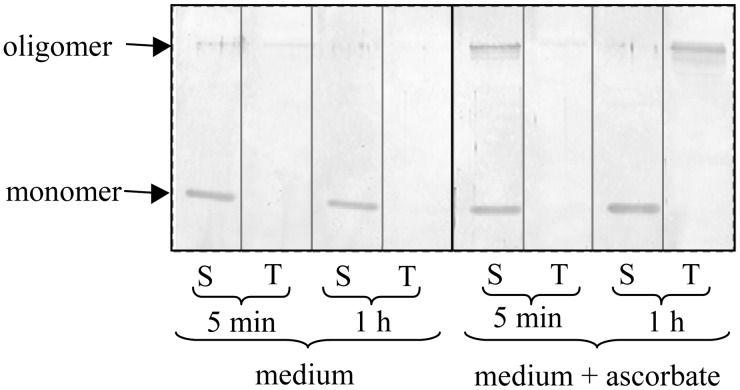
In addition to high salt, reducing conditions favored aggregation of 2-Cys Prx dimers to oligomers and attachment to thylakoid membranes in organello. Intact chloroplasts were incubated in the absence or presence of reducing compounds for 5 or 60 min, followed by hypoosmotic lysis. Proteins of the thylakoid and soluble fractions were separated by reducing SDS/PAGE and analyzed by Western blotting with anti-2-Cys Prx (Fig. 4). The 2-Cys Prx was mainly present in the soluble form under nonreducing conditions (lanes 1–4). Supplementation of the medium with 10 mM ascorbate caused aggregation of 2-Cys Prx, which still was in the stroma after 5 min (lanes 5 and 6) and partly attached to the thylakoid membranes after 60 min of incubation (lanes 7 and 8). Ascorbic acid is likely to create microoxic conditions in the chloroplast, which allow endogenous reducing processes to mediate 2-Cys Prx aggregation. Oligomerized 2-Cys Prx also was detected after addition of DTT to heterologously expressed protein followed by gel filtration. It should be noted that the high salt and reducing conditions did not induce association to a hyperaggregate which could be spun down at 10,000 × g (result not shown).
Figure 4.
Attachment of oligomerized 2-Cys Prx to the thylakoid membrane in intact chloroplasts. Chloroplasts were isolated from Hordeum leaves and incubated in the absence (lanes 1–4) or presence (lanes 5–8) of 10 mM ascorbic acid. After 5 or 60 min, the chloroplasts were lysed by hypoosmotic shock and the thylakoids (T) separated from the soluble stroma (S) by centrifugation. Proteins from both fractions were separated by SDS/PAGE and analyzed in a Western blot analysis with anti-2-Cys Prx.
Plant Trx m and f Reduce Oxidized 2-Cys Prx.
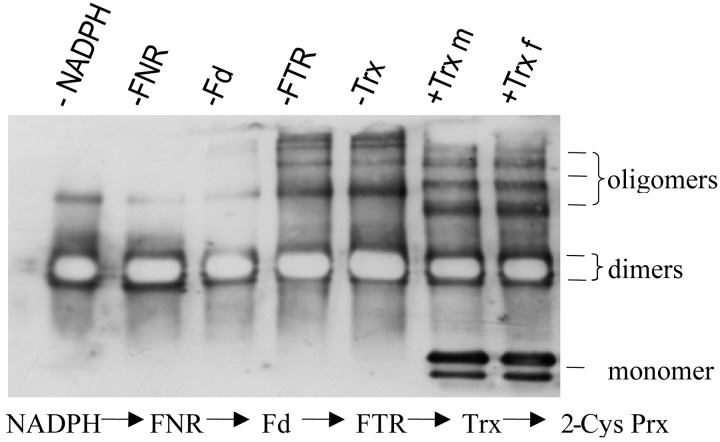
The previous activity tests of 2-Cys Prx were performed with E. coli Trx. Thus it was important to show that higher plant Trxs also can donate electrons to oxidized 2-Cys Prx. An enzyme assay was set up containing NADPH, ferredoxin:NADP reductase (FNR), and ferredoxin (Fd) from spinach, ferredoxin:TR (FTR) from Synechocystis, and Trx f or Trx m, all from spinach (Fig. 5). The samples were analyzed by Western blotting using anti-2-Cys Prx Ab. The reduced monomeric form of the 2-Cys Prx was detected at 24 kDa only when the complete electron transfer system was present in the assay. Both, Trx f and Trx m reduced oxidized 2-Cys Prx and also stimulated oligomer formation. In a similar test system as described above (Fig. 1), Trx m also supported H2O2 reduction by barley 2-Cys Prx, however, at lower rates than E. coli Trx, which could indicate a limitation in regeneration (data not shown).
Figure 5.
Reduction of heterologously expressed 2-Cys Prx in an enzymic system containing spinach Trx f or m. The complete electron transport chain is also depicted. After incubation for 30 min, the samples were separated by denaturing nonreducing SDS/PAGE and analyzed in a Western blot analysis with anti-2-Cys Prx. Lanes 1–5 represent samples in which each one component of the complete system had been omitted as indicated on top of each lane. Lane 6 depicts the result with Trx m and lane 7 with Trx f. The position of monomeric reduced 2-Cys Prx is shown. The protein band beneath the monomer indicates degradation. It was a repeated observation that the reduced 2-Cys Prx exhibited a higher propensity for degradation.
The Midpoint Redox Potential of 2-Cys Prx Is −315 mV.
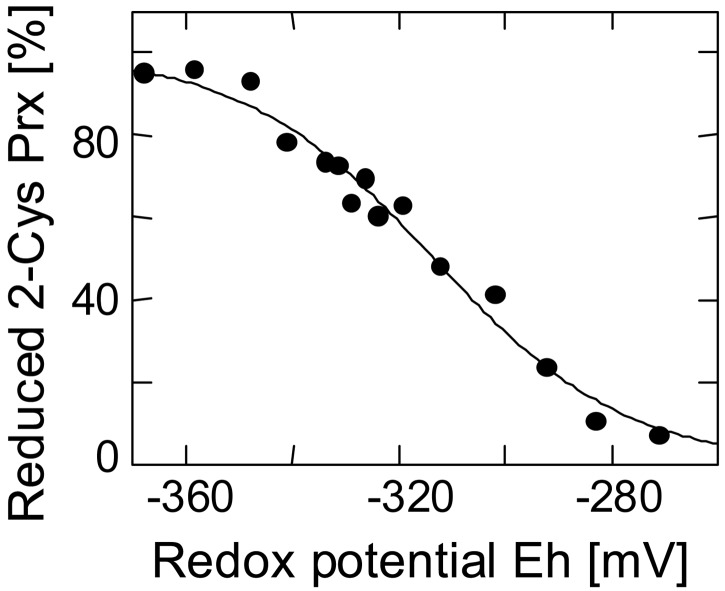
The chloroplast is an organelle with drastically changing redox state depending on developmental conditions and photosynthetic parameters such as light and electron acceptor availability. Because the physiological function of the 2-Cys Prx depends on reduced cysteine residues in the catalytic center, the midpoint redox potential characterizes the conditions under which the 2-Cys Prx may function in peroxide detoxification. The redox state of 2-Cys Prx was titrated in a fluorometric assay by using monobromobimane in the presence of varying ratios of DTTreduced/DTToxidized (Fig. 6). The midpoint redox potential was determined to be −315 mV.
Figure 6.
Titration of the redox midpoint potential of heterologously expressed 2-Cys Prx. The redox potential of the samples was adjusted by varying the ratio of DTToxidized/DTTreduced. After reacting reduced thiol groups with monobromobimane, the samples were analyzed for bound fluorophore.
Modulation of the Chloroplastic NADPH System Affects the Redox State of 2-Cys Prx.
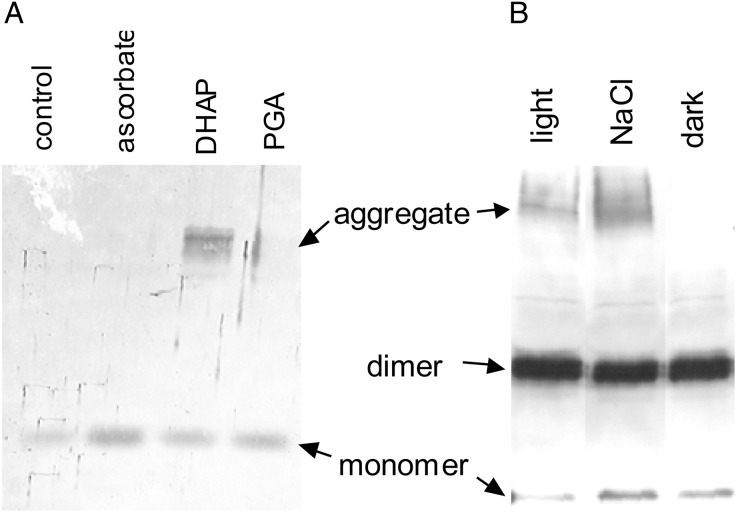
The next set of experiments investigated the coupling between the NAD(P)H system and the redox state of the 2-Cys Prx. Intact isolated chloroplasts were suspended in isoosmotic media supplemented with DHAP or 3-PGA. By enzymic coupling through stromal PGA kinase, glyceraldehyde-3-phosphate dehydrogenase and triosephosphate isomerase, 3-PGA, DHAP, NAD(P)H + H+, and NAD(P)+ are in mass action equilibrium (22). DHAP and 3-PGA externally supplied to intact chloroplasts are exchanged against stromal Pi. Consequently, the NAD(P)H/NA(D)P+-ratio will increase upon feeding DHAP and decrease upon feeding 3-PGA. Under control conditions and in the presence of ascorbic acid and 3-PGA, the 2-Cys Prx was present as DTT-reducible dimer. In a converse manner, exogenous supply of DHAP caused appearance of the band with low mobility suggesting reduction-dependent oligomerization (Fig. 7A).
Figure 7.
Aggregate formation of 2-Cys Prx in intact chloroplast by exogenous DHAP and oligomerization under stress. (A) Isolated barley chloroplasts were incubated either without any further addition (lane 1) or with 10 mM ascorbic acid (lane 2), 20 mM dihydroxyacetone phosphate (lane 3) or 20 mM 3-PGA (lane 4) for 15 min. The samples were analyzed by denaturing reducing SDS/PAGE and Western blotting with anti-2-Cys Prx Ab. (B) Barley plants were subjected to salt stress (250 mM for 2 d) and extracted with N-ethylmaleimide. The control and NaCl-stressed leaves were harvested under illumination. Additionally, nonstressed leaves were analyzed after incubation for 4 h in the dark.
Oligomerization of 2-Cys Prx Also Occurs Under Stress.
In photosynthetic tissues, stressful growth conditions cause overreduction of the photosynthetic apparatus and production of ROS. Barley seedlings were subjected to salt stress. Proteins of primary leaves were analyzed for the aggregation state of the 2-Cys Prx. Salt stress caused the appearance of oligomers (Fig. 7B). This suggests that salinity stress-induced oligomerization triggers membrane attachment and allows for detoxification of peroxides at the site of production in immediate vicinity of the thylakoid membrane.
Discussion
This study gives the first detailed characterization of the plant 2-Cys Prx in the context of photosynthesis. Three sets of biochemical properties appear to be crucial for the understanding of the plant-specific function of the 2-Cys Prx in the chloroplast and will be discussed in the following, i.e., (i) the aggregation/oligomerization and reversible thylakoid association, (ii) the broad substrate specificity and the regeneration by Trx and from the NAD(P)H system, and (iii) the integration of the 2-Cys Prx function into the chloroplast metabolism in the light of the rather negative midpoint potential of −315 mV.
The Reversible Attachment of 2-Cys Prx to the Thylakoids as Regulatory Mechanism.
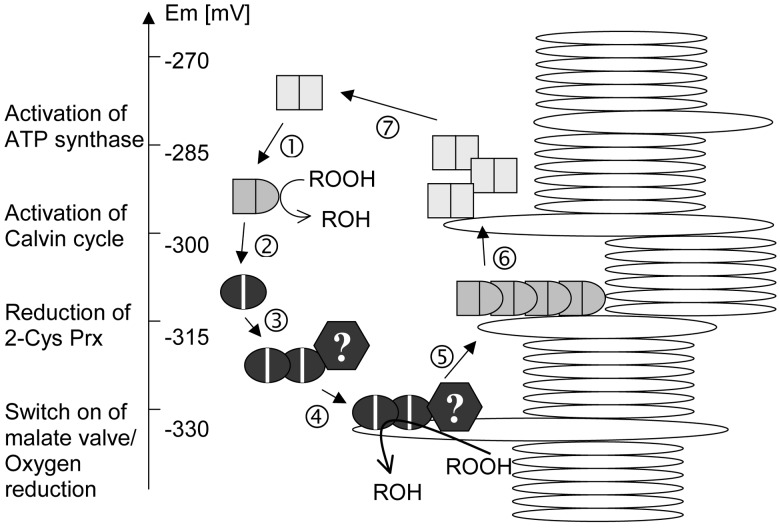
The chloroplast 2-Cys Prx associated with the thylakoid membrane under reducing conditions. Association with the thylakoids was accompanied by aggregate formation. It is unlikely that the extraction procedure led to a complete recovery of the oligomeric form present in vivo. Therefore, the portion of oligomerized 2-Cys Prx may be underestimated from the experiments. Oligomerization has been described as an important regulatory mechanism for 2-Cys Prx in erythrocytes (10). Oligomerization and membrane attachment may be seen as part of a mechanism to regulate 2-Cys Prx via the chloroplast redox potential (Fig. 8): At redox potentials above −300 mV, the 2-Cys Prx is present as oxidized dimers, which are unbound and free to diffuse in the stroma. At redox potentials below −310 mV, 2-Cys Prx is increasingly reduced, aggregated by lipophilic interactions, and recruited to the thylakoid membrane (compare also Fig. 4). When the reduction potential of the chloroplast turns more negative, ROS are synthesized particularly at photosystem I (23). Peroxides are immediately detoxified by the thylakoid-attached 2-Cys Prx complexes at the site of their photoproduction. The regulatory circuit (Fig. 8) is completed when oxidized complexes of 2-Cys Prx dissociate from the thylakoid after a drop of the reduction potential to values above −300 mV. Although evidence for steps 1–6 is available, step 7 still is based on a (necessary) assumption. Additional factors (box with question mark), e.g., cyclophilins or Trxs, may participate in membrane attachment (24, 25). The additional protein bands seen in the fully reconstituted system depicted in Fig. 5 also indicate heteromeric protein–protein interaction.
Figure 8.
Model for the regulation of the 2-Cys Prx activity in the chloroplast. The basic unit is the dimer which can either exist fully oxidized (square), fully reduced (oval), or partially oxidized (bullet shaped). The reduced dimer can oligomerize and attach to the thylakoid membrane. The regulatory cycle is linked to the redox potential as indicated by the scale at the left. The 2-Cys Prx cycle is integrated into the hierarchy of electron flow as exemplified with some key processes. See text for further details.
The Midpoint Redox Potential Places 2-Cys Prx Reduction After Calvin Cycle Activation and Before Switching the Malate Valve.
The midpoint redox potential Em of 2-Cys Prx was determined to be −315 mV and, thus, more negative than the Em values of Trx f (−290 mV) and Trx m (−300 mV) (26). The low redox potential explains the observation that glutathione (Em = −240 mV) and cysteine (Em = −241 mV) are not able to reduce oxidized 2-Cys Prx (not shown). It also concurs with the observation that the 2-Cys Prx protein was never found in fully reduced form in plant extracts prepared in the presence of N-ethylmaleimide. Even under the situation of a highly reduced Trx system, the 2-Cys Prx will remain partly oxidized. The redox potential also gives important hints on the physiological context of the 2-Cys Prx-dependent peroxide detoxification. The chloroplast is a compartment with varying redox milieu depending on the conditions for photosynthesis (2, 26). High light, low acceptor availability such as CO2 and NO2−, and low temperatures are conditions that will cause a high electron pressure in the photosynthetic electron transport chain and a very negative redox potential. Conversely, low light, high CO2, and temperature lead to more positive redox potentials. Accordingly, redox information is used to tune the photosynthetic machinery transcriptionally and posttranscriptionally for optimum performance (2). This can be illustrated during a dark-light transition. After beginning of illumination, the electron transport chain and the Trx system change from a rather oxidized to an intermediately reduced state. The redox-modulated enzymes of the Calvin cycle with Em of −280 to −305 mV are activated and carbon assimilation is initiated. It has been documented that there exists a hierarchy of electron transfer in the chloroplast. Electrons are preferentially donated to H2O2 reduction, NO2−, and CO2 assimilation (27). When the electron pressure increases and the redox potential takes more negative values, oxaloacetate reduction and O2 reduction are activated. Oxaloacetate reduction (malate valve) is an important mechanism to dissipate reduction equivalents by oxidation of NADPH and export of malate to the cytoplasm. The controlling enzyme is the NADPH-dependent malate dehydrogenase with a redox potential of −330 mV in sorghum (28). The midpoint redox potential of −315 mV places 2-Cys Prx reduction and, thereby, peroxide reduction through the peroxiredoxin pathway, after Calvin cycle activation and before switching the malate valve. Despite the use of a His-tagged 2-Cys Prx the data on activity, aggregation and redox potential are unlikely to differ from those of the native 2-Cys Prx as concluded from the comparative work with Prx of Crithidia by Montemartini et al. (29).
The Function of 2-Cys Prx in Chloroplast Peroxide Detoxification in the Light.
Peroxide reduction by 2-Cys Prx, coupled to the photosynthetic electron transport chain as first shown in this paper, is an alternative pathway for driving the water–water cycle, which mediates the dissipation of excess excitation energy (23). In the water–water cycle, reduction of O2 at photosystem I consumes electrons pumped by the photosystems into the electron transport chain. The water–water cycle relieves overreduction and protects the photosynthetic apparatus from photooxidative damage (30). Superoxide anion radicals produced in this reaction are detoxified by CuZn superoxide dismutase (SOD), ascorbate peroxidase, Fd, and Fd-dependent monodehydroascorbate reductase. The activity of this ascorbate-dependent reaction sequence is reported to exceed the rate of O2 photoreduction >100-fold. 2-Cys Prx-mediated detoxification of H2O2 represents an alternative water–water cycle. In this case, the reaction sequence is completed by feeding electrons from the electron transport via Fd, Fd-dependent TR, and Trx to the oxidized 2-Cys Prx and release of water. The 2-Cys Prx-dependent water–water cycle is likely to be of great importance given the high sensitivity of the chloroplastic ascorbate peroxidase isoforms to oxidative inactivation. For example under water stress, the activity of ascorbate peroxidase was not detectable (31).
2-Cys Prx-Mediated Peroxide Detoxification in the Dark.
Three observations support the conclusion that 2-Cys Prx also catalyzes peroxide detoxification in the dark. (i) The 2-Cys Prx protein was present in etiolated leaf tissue and was detected by ImmunoGold labeling in etioplasts. (ii) Oxidized 2-Cys Prx could be reduced in part by exogenous addition of DHAP to darkened chloroplasts. (iii) 2-Cys Prx was not fully oxidized in the dark. Plastids have an intensive dark metabolism, e.g., starch and fatty acid metabolism and chlororespiration. Peroxides produced in the dark may be detoxified by partially reduced 2-Cys Prx dissolved as dimers in the stromal space. The stromal pH is thought to be close to 7 in the dark. Thus, the pH optimum of 7 indicates an efficient detoxification by 2-Cys PRX in the dark.
Acknowledgments
Expert technical assistance by Ms. Tanja Thias is gratefully acknowledged. This work was supported by the Deutsche Forschungsgemeinschaft (Di 346/6, FOR 387/TP3), the Fonds der Chemischen Industrie, and the Schweizer Nationalfonds (31-56761.99 to P.S.).
Abbreviations
- 2-Cys Prx
2-cysteine peroxiredoxin
- DHAP
dihydroxyacetonephosphate
- K-Pi
potassium phosphate
- 3-PGA
3-phosphoglycerate
- ROS
reactive oxygen species
- Trx
thioredoxin
- TR
Trx reductase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Elstner E F. Der Sauerstoff: Biochemie, Biologie, Medizin. Wienheim, Germany: Verlag Chemie; 1990. [Google Scholar]
- 2.Dietz K J, Link G, Pistorius E K, Scheibe R. Prog Bot. 2002;63:207–245. [Google Scholar]
- 3.Davies K J A. Biochem Soc Symp. 1994;61:1–32. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 4.Baier M, Dietz K J. Prog Bot. 1998;60:283–314. [Google Scholar]
- 5.Noctor G, Foyer C H. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 6.Baier M, Dietz K J. Trends Plant Sci. 1999;4:166–168. doi: 10.1016/s1360-1385(99)01398-9. [DOI] [PubMed] [Google Scholar]
- 7.Chae H Z, Chung S J, Rhee S G. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 8.Hirotsu S, Abe Y, Okada K, Nagahara N, Hori H, Nishino T, Hakoshima T. Proc Natl Acad Sci USA. 1999;96:12333–12338. doi: 10.1073/pnas.96.22.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogoceke E, Gommel D U, Kiess M, Kalisz H M, Flohé L. Biol Chem. 1997;378:827–836. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- 10.Schröder E, Littlechild J A, Lebedev A A, Errington N, Vagin A A, Isupov M N. Structure (London) 2000;8:605–615. doi: 10.1016/s0969-2126(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 11.Baier M, Dietz K J. Plant J. 1997;12:179–190. doi: 10.1046/j.1365-313x.1997.12010179.x. [DOI] [PubMed] [Google Scholar]
- 12.Chae H Z, Kim I H, Kim K, Rhee S G. J Biol Chem. 1993;268:16815–16821. [PubMed] [Google Scholar]
- 13.Immenschuh S, Iwahara S I, Satoh H, Nell C, Katz N, Müller-Eberhard U. Biochemistry. 1995;34:13407–13411. doi: 10.1021/bi00041a018. [DOI] [PubMed] [Google Scholar]
- 14. Dietz, K. J., Horling, F., König, J. & Baier, M. (2002) J. Exp. Bot., in press. [PubMed]
- 15.Schürmann P. In: Protein Sensors of Reactive Oxygen Species: Selenoproteins, Thioredoxin, Thiol Enzymes, and Proteins. Packer L, Sies H, editors. San Diego: Academic; 2002. [Google Scholar]
- 16.Schürmann P. In: Biothiols Part B: Glutathione and Thioredoxin. Packer L, editor. San Diego: Academic; 1995. pp. 274–283. [Google Scholar]
- 17.Yamamoto H, Miyake C, Dietz K J, Tomizawa K I, Murata N, Yokota A. FEBS Lett. 1999;447:269–273. doi: 10.1016/s0014-5793(99)00309-9. [DOI] [PubMed] [Google Scholar]
- 18.Hirasawa M, Schürmann P, Jacquot J P, Manieri W, Jacquot P, Keryer E, Hartman F C, Knaff D B. Biochemistry. 1999;38:5200–5205. doi: 10.1021/bi982783v. [DOI] [PubMed] [Google Scholar]
- 19.Beesley J E. Microscopy Handbooks. Vol. 17. Oxford: Oxford Univ. Press; 1989. pp. 17–29. [Google Scholar]
- 20.Baier M, Dietz K J. Plant Mol Biol. 1996;31:553–564. doi: 10.1007/BF00042228. [DOI] [PubMed] [Google Scholar]
- 21.Baier M, König J, Horling F, Dietz K J. Proc. Int. Cong. Photosynth, 4th. 2001. [Google Scholar]
- 22.Dietz K J, Heber U. Biochim Biophys Acta. 1984;767:432–443. [Google Scholar]
- 23.Asada K. Phil Trans R Soc London B. 2000;355:1419–1431. doi: 10.1098/rstb.2000.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S P, Hwang Y S, Kim Y J, Kwon K S, Kim H J, Kim K, Cha H Z. J Biol Chem. 2001;276:29826–29832. doi: 10.1074/jbc.M101822200. [DOI] [PubMed] [Google Scholar]
- 25.Motohashi K, Kondoh A, Stumpp M T, Hisabori T. Proc Natl Acad Sci USA. 2001;98:11224–11229. doi: 10.1073/pnas.191282098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schürmann P, Jacquot J-P. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:371–400. doi: 10.1146/annurev.arplant.51.1.371. [DOI] [PubMed] [Google Scholar]
- 27.Holtgrefe S, Backhausen J, E, Kitzmann C, Scheibe R. Plant Cell Physiol. 1997;38:1207–1216. [Google Scholar]
- 28.Hirasawa M, Ruelland E, Schepens I, Issakidis-Bourguet E, Miginiac-Maslow M, Knaff D B. Biochemistry. 2000;39:3344–3350. doi: 10.1021/bi9916731. [DOI] [PubMed] [Google Scholar]
- 29.Montemartini M, Nogoceke E, Singh M, Steinert P, Flohé L, Kalisz H M. J Biol Chem. 1998;273:4864–4871. doi: 10.1074/jbc.273.9.4864. [DOI] [PubMed] [Google Scholar]
- 30.Asada K, Endo T, Mano J, Miyake C. In: Stress Responses of Photosynthetic Organisms. Satoh K, Murata N, editors. Amsterdam: Elsevier Science; 1998. pp. 37–52. [Google Scholar]
- 31.Shikanai T, Takeda T, Yamauchi H, Sano S, Tomizawa K I, Yokota A, Shigeoka S. FEBS Lett. 1998;428:47–51. doi: 10.1016/s0014-5793(98)00483-9. [DOI] [PubMed] [Google Scholar]