Abstract
Linear hierarchies, the classical pecking-order structures, are formed readily in both nature and the laboratory in a great range of species including humans. However, the probability of getting linear structures by chance alone is quite low. In this paper we investigate the two hypotheses that are proposed most often to explain linear hierarchies: they are predetermined by differences in the attributes of animals, or they are produced by the dynamics of social interaction, i.e., they are self-organizing. We evaluate these hypotheses using cichlid fish as model animals, and although differences in attributes play a significant part, we find that social interaction is necessary for high proportions of groups with linear hierarchies. Our results suggest that dominance hierarchy formation is a much richer and more complex phenomenon than previously thought, and we explore the implications of these results for evolutionary biology, the social sciences, and the use of animal models in understanding human social organization.
Linear hierarchies, classic pecking-order structures, are formed readily in nature and the laboratory by many species: some insects and crustaceans and various fish, birds, and mammals including human children and adolescents (1–10). However, the probability of generating linear hierarchies by chance alone is low. We do not know how these social structures develop their linear form, and even the types of mechanisms that might produce linearity are controversial. In this paper we evaluate hypotheses concerning the two most commonly proposed factors for explaining the formation of linear hierarchies through a series of experimental studies using cichlid fish.
Two individuals have a dominance relationship if one chases, threatens, or bites, but receives little or no aggression, from the other. Dominance hierarchies, known in the mathematical literature as tournaments, are social structures consisting of dominance relationships between all pairs of individuals in a group. In a linear hierarchy one individual dominates all the other individuals in a group, a second dominates all but the first, and so on down to the last individual who is dominated by all the others. Dominance relationships in a linear hierarchy are always transitive. For any three individuals (triad) in the group, if A dominates B and B dominates C, then A also dominates C. If a hierarchy is not linear, it contains at least one intransitive triad (A dominates B, B dominates C, but C dominates A), and the more intransitive triads there are, the further the hierarchy is from linearity (by many measures of linearity). Perfectly linear hierarchies are most common in groups under 10 members, and as groups grow larger, irregularities may appear (11). Rank in hierarchies influences such important things as behavior, physiology, health, and ability to produce offspring (12–16).
The first and most often suggested hypothesis concerning the mechanisms accounting for linearity is that individuals' positions in hierarchies are predetermined by differences in dominance ability. We term this the “prior attributes” hypothesis. It proposes that the ladder-like structure of linear hierarchies can be explained by another, preexisting ladder-like structure, one on which individuals about to form a hierarchy are ranked by attributes indicative of their dominance ability. According to this hypothesis, the animal highest in dominance attributes takes the top position in the hierarchy, the animal second-highest takes the next position, and so on.
General support for this hypothesis comes from the many studies that demonstrate the association, sometimes quite high, between various attributes of individuals and their positions in hierarchies (2, 17, 18). The attributes that are correlated with rank are varied sorts, depending on study and species, but age, sex, physical size and strength, physiology, and level of aggressiveness are among the most common (12–16, 18). More specifically, some researchers have shown that in groups of three animals with great discrepancies in prior attributes (e.g., A, a recent winner and 30–40% larger than the others; B and C of similar size, but B a recent winner; and C a recent loser), individuals more often form hierarchies according to their rank in attributes than expected by chance alone (19, 20). Other researchers have argued that attribute differences ultimately determine the rank order of individuals in hierarchies by dictating the behavioral strategies used during hierarchy formation (21, 22).
The second hypothesis is that processes of social interaction among group members are the mechanisms that generate linear hierarchies, and these processes are not predetermined by differences in individuals' attributes (23, 24). We term this the “social dynamics” hypothesis. Although researchers have not yet demonstrated experimentally which specific dynamics actually generate linear hierarchies, possibilities include (i) winner effects, with individuals winning earlier contests increasing their probability of winning later ones (25, 26), (ii) loser effects, with individuals losing earlier contests, increasing their probability of losing later ones (25, 26), and (iii) bystander effects, with individuals observing others' encounters and adjusting their behavior accordingly (27–29). In this hypothesis, if social interaction in a group context were prohibited, hierarchies should not develop their usual linear structures. Thus the behavior that occurs when groups are assembled would be central to explaining the structure of hierarchies rather than being derivative. In this case, dominance hierarchies would be “self-organizing” or “self-structuring” systems, the overall structures of which are determined by interaction among the elements comprising the systems (30–32).
Landau (33) and Chase (34) provided some initial support for this hypothesis by demonstrating that stringent mathematical conditions were required to generate highly linear hierarchies on the basis of prior differences among individuals: extremely high correlations between ranking on prior attributes and rank in dominance hierarchies and highly skewed distributions for the probabilities of winning encounters among the members of groups. Examination of the relevant data indicated that such conditions were rarely fulfilled.
In his “jigsaw puzzle” model, Chase (23) classified various sequences by which dominance relationships could form in triads of animals. Some of these sequences ensured the development of transitive dominance relationships and thus the efficient production of linear hierarchies, whereas others led to either transitive or intransitive relationships and thus, possibly, nonlinear hierarchies. Researchers applying the model have found high proportions of the sequences ensuring transitivity in a range of species: chickens, rhesus monkeys, Japanese macaques, cichlid fish, and crayfish (4, 5, 23, 35–38). Winner, loser, and bystander effects may account for the high proportion of sequences ensuring transitive relationships, and researchers using mathematical models and computer simulation have demonstrated that if these effects occur during hierarchy formation, they can enhance the production of linear structures even when all individuals are identical initially in prior attributes (39–42).
Methods
Study Species.
We used female Metriaclima zebra (formerly Pseudotropheus zebra), OB morph, as the species for our experiments. This cichlid fish is native to east Africa, aggressive in nature, and readily forms dominance hierarchies in the laboratory. We obtained the fish as surplus stock from the New York Aquarium for Wildlife Conservation and from a commercial breeder in Florida.
Experimental Design.
In designing our experiments, we faced a vexing problem. Previous research suggested that differences in some relatively obvious attributes, chiefly weight, might be important for predicting hierarchy ranks in fish (43). But what about differences in undiscovered attributes, or if discovered, attributes that could not be measured easily or could be measured only in invasive and upsetting ways that might change the fish's scores on the attributes measured? Thus, in our experiments we decided to control for weight and to use an experimental design that would allow us to assess the effects of all these other potentially important attributes simultaneously: the unknown and hard-to-measure ones. In the second experiment's Discussion, we consider the potential importance of weight differences in the production of linear hierarchies.
To evaluate these undiscovered and difficult-to-measure attributes, we designed two experiments. The first experiment directly tested the prior attributes hypothesis. In it we assembled groups of fish to form hierarchies, separated them long enough to forget one another (44, 45), and then brought them back together to form a second hierarchy. In this experiment, we wanted to “rewind the tape” of each fish in a group to the greatest extent possible and let the individuals form a second hierarchy starting from scratch again. If rank on prior attributes determined hierarchy rank in the first hierarchy, then rank on the attributes should do so in the second hierarchy, because we would avoid all possible things that might alter the fish's rankings on attributes. In other words, if rank on prior attributes accounted for hierarchy ranks, we would expect identical rankings for individuals in both hierarchies. If, however, rank on the prior attributes did not determine hierarchical rank, the first and second hierarchies should not be identical, and a considerable proportion of fish should change ranks between them.
The second experiment directly contrasted whether the prior attributes or social dynamics hypotheses could account better for the high proportion of linear hierarchies commonly observed. In this experiment we formed hierarchies by two methods: round-robin competition and group assembly. In round-robin competition all the fish in a group met one another, but they did so only in separate, pairwise encounters, out of sight of other fish, and with 1 or 2 days between successive encounters. As a result, interactional processes that might occur normally in a group context were prohibited (e.g., observing others' contests, attacking others after a win, or being attacked oneself after a loss), but the ability of individual qualities (“biological,” “psychological,” or “sociological”) to control the outcomes of these contests was not inhibited. In group assembly, we placed all the fish in a group in a tank simultaneously to establish their relationships in whichever way they chose without interfering with their interactional processes.
Experiment One
We took fish from their stock tanks, weighed them, and placed them in separate isolation chambers, 21 liters in volume, for 2 weeks before they took part in a trial to remove any possible effects of relationships that were established in their stock tanks (44, 45). We made up groups of four fish allowing a 7% maximum difference between the heaviest and lightest fish using their weights at isolation. We fed all fish in a group identical rations over the course of the experiment (≈1.5% of body weight per day). When the 2-week isolation period was complete, we simultaneously transferred the fish in a group to a 76-liter observation tank in which the individuals were separated by partitions. We then removed the partitions and returned 24 h later to observe from behind one-way mirrors. We recorded all instances of nips, chases, and mouth fighting (46) and considered that two fish had a stable relationship if one of the fish (the dominant) delivered six aggressive acts, in any combination of nips and chases, to the other without retaliation. Mouth fighting was considered as a mutually aggressive act, and we began recounting consecutive aggressive acts by either fish after an incidence of this behavior. If after ≈30 min of observation a group had a stable hierarchy, i.e., all of the pairs had stable relationships, we terminated observations. If all relationships were not stable, we performed two or three more observations that day and, if needed, the next day until relationships were stable. After a stable hierarchy was achieved, we transferred the fish back to their original, separate chambers for 2 more weeks of isolation so that they would forget one another (44, 45). Finally, we reassembled them to form a second hierarchy with the same conditions and procedures as used for the first hierarchy.
Results.
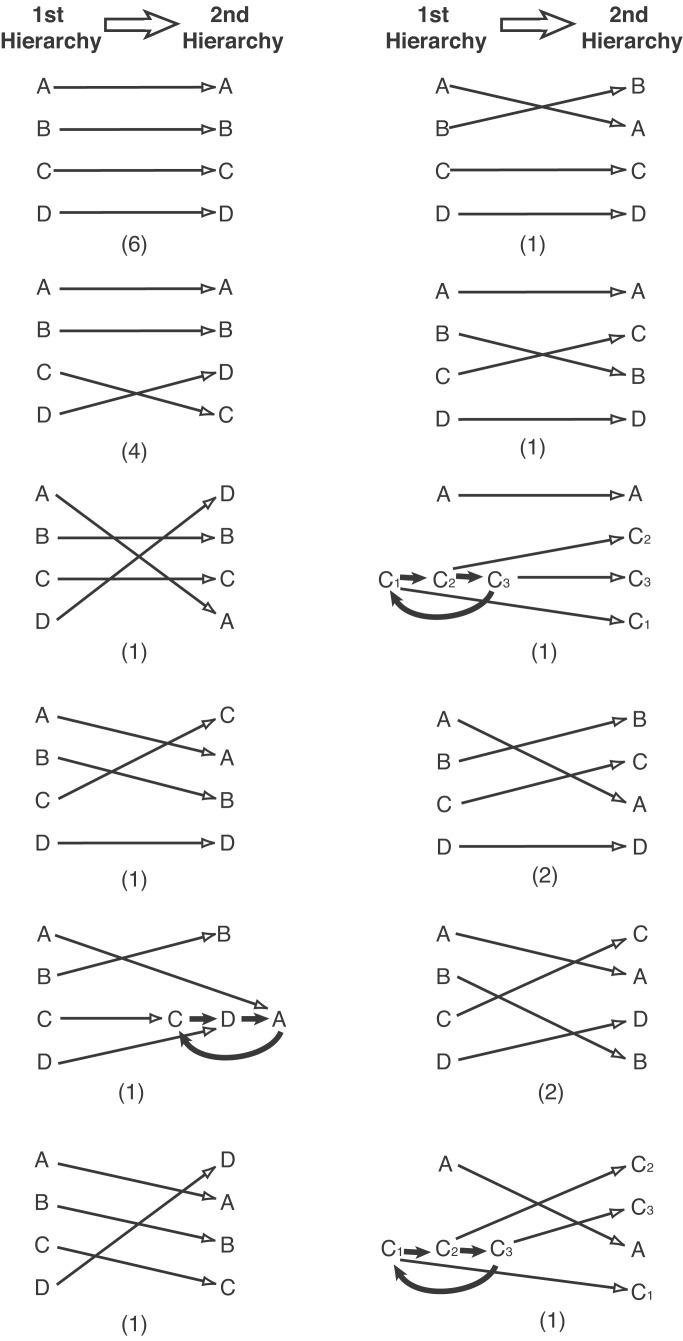
Fig. 1 shows the first and second hierarchies for the 22 groups of fish we observed. Nearly all the groups formed linear hierarchies both times they met (90.9 and 95.5%, respectively). Fig. 1 also shows the transition patterns between the ranks of individual fish in the first and second hierarchies, with all fish keeping the same ranks in both hierarchies, the top two fish in the first hierarchy swapping ranks in the second hierarchy, the bottom two fish in the first hierarchy swapping ranks in the second hierarchy, etc. Inspection indicates the great diversity in the relationships between the two hierarchies. Only four transition patterns occurred in more than one group of fish, and 12 different patterns occurred, or 54.5%, out of the maximum of 22 possible if each group of fish had formed a different transition pattern. This figure shows that it is difficult to achieve the same hierarchy twice.
Figure 1.
Transition patterns between ranks of fish in the first and second hierarchies. Frequencies of experimental groups showing each pattern are indicated in parentheses. Open-headed arrows indicate transitions of rank. Solid-headed arrows show dominance relationships in intransitive triads; all the fish in an intransitive triad share the same rank.
Table 1 shows the proportion of groups in which the two hierarchies were identical, i.e., in which all fish had the same ranks in both, as well as the proportions of groups in which two (half) or more fish changed ranks. The percentage of identical hierarchies (27.3) is significantly higher than would be expected if second hierarchies were random linear orders, that is, if the hierarchies were linear and the fish could take any rank (one-sided binomial test: n = 22, P < 0.001). This is strong evidence that prior attributes do affect hierarchy formation.
Table 1.
Percentage of groups with different numbers of fish changing ranks between first and second hierarchies (n = 22)
| No. of fish changing ranks | Percentage of groups |
|---|---|
| 0 | 27.3 |
| 2 | 36.4 |
| 3 | 18.2 |
| 4 | 18.2 |
On the other hand, in order for the prior attributes hypothesis in and of itself to be a robust explanation of linear hierarchies, a high proportion of identical first and second hierarchies would be required. If we set the standard for prior attributes to account for linear structures, for example, at a moderate level of 75% identical hierarchies or even at a low level of 50%, the 27% result would be significantly smaller than either of these standards (one-sided binomial test: n = 22, P < 0.001 and P < 0.03, respectively). In this light, 27% of the groups with identical hierarchies is very small.
Discussion.
When we rewound the tape of the fish to form new hierarchies, we usually did not get the same hierarchy twice. The linearity of the structures persisted and the individuals stayed the same, but their ranks did not. Thus our results differ considerably from those predicted by the prior attributes hypothesis. The fact that more identical hierarchies occurred than expected by chance alone supports the hypothesis that rank on prior attributes influences rank within hierarchies but not the hypothesis that rank on prior attributes of itself creates the linear structure of the hierarchies. Although 50% of the fish changed ranks from one hierarchy to the other, almost all the hierarchies were linear in structure. Some factor other than differences in attributes seems to have ensured high rates of linearity. In the next experiment, we tested to determine whether that factor might be social dynamics.
It might seem possible that “noise,” random fluctuations in individuals' attributes or behaviors, could account for the observed differences between the first and second hierarchies. However, a careful consideration of the ways in which fluctuations might occur shows that this explanation is unlikely. For example, what if the differences were assumed to have occurred because some of the fish changed their ranks on attributes from the first to the second hierarchies? To account for our results, this assumption would require a mixture of stability and instability in attribute ranks at just the right times and in just the right proportion of groups. The rankings would have had to have been stable for all the fish in all the groups for the day or two it took them to form their first hierarchies (or we would not have seen stable dominance relationships by our criterion). Then, in three-quarters of the groups (but not in the remaining one-quarter) various numbers of fish would have had to have swapped ranks on attributes in the 2-week period of separation so as to have produced different second hierarchies. And finally, the rankings on attributes for all the fish in all the groups would have had to have become stable once more for the day or two it took them to form their second hierarchies.
Alternatively, instead of attribute rank determining dominance rank as in the prior attribute model, dominance in pairs of fish might be considered to have been probabilistic, such that at one meeting one might dominate, but at a second meeting there was some chance that the other might dominate. The problem with this model is that earlier mathematical analysis demonstrates that in situations in which one of each pair in a group has even a small chance of dominating the other, the probability of getting linear hierarchies is quite low (34). And even in a more restrictive model in which only pairs of fish that are close in rank in the first hierarchies have modest probabilities of reversing their relationships, such as the level (0.25) we observed in this experiment, the probability of getting as many linear hierarchies as we observed is still very low (details are available from the authors).
We know of only one other study (47) in which researchers assembled groups to form initial hierarchies, separated the individuals for a period, and then reassembled them to form a second hierarchy (but see Guhl, ref. 48, for results in which groups had pairwise encounters between assembly and reassembly). Unfortunately, their techniques of analysis make it impossible to compare results, because they examined correlations between the frequency of aggressive acts directed by individuals in pairs toward one another in the two hierarchies rather than comparing the ranks of individuals. With these techniques it is possible to get a positive correlation and thus a “replication” of an original hierarchy in situations in which several animals actually change ranks from the first to the second hierarchies.
Experiment Two
We took fish from their stock tanks, weighed them, and made up groups of four and five fish using weights at isolation; in a set of four the largest was no more than 7% heavier than the smallest, and in a set of five, no more than 9% heavier. After 2 weeks of isolation, as in Experiment One, we formed hierarchies by round-robin competition and group assembly.
In round-robin competition we randomly selected two pairs of fish from a set for the first round of encounters. Each pair was transferred to a 21-liter observation tank separated by a partition and given 2 h to acclimate to the tank. We then removed the partition and observed them through a one-way mirror. Again, all instances of nips, chases, and mouth fighting were recorded until one fish reached dominance over the other (a total of 15 consecutive, aggressive acts against the other without retaliation). Of these 15 we scored only nips for the first seven acts and any combination of nips and chases for the remaining eight. As before, we considered mouth fighting as a mutually aggressive act and began recounting consecutive acts by either fish after such an incidence. When one fish reached the dominance criterion, we separated them and returned them to their isolation tanks.
We continued the rounds of encounters until all fish in a set had met one another. Each fish in sets of four had 2 days between encounters, and in sets of five each had 1 day except for the odd fish out, which had 2 days. Where possible, we matched winners to winners and losers to losers.
In group assembly we simultaneously transferred all fish in a set from their isolation tanks to a 76-liter aquarium; observations began 24 h later. We observed the fish and determined stable dominance relationships and hierarchies using the same procedures as described for experiment one.
We used six rather than 15 consecutive aggressive acts in group assembly to determine stable dominance relationships, because upon first meeting, as in round-robin competition, fish often exchange aggressive acts before one clearly establishes dominance and initiates all aggressive activity; such contests require a fairly large number of consecutive acts to ensure a stable relationship. In contrast, after some time together, relationships are often in place, fish do not trade acts back and forth, thus fewer acts suffice to determine which fish in a pair is dominant.
Results.
As indicated earlier in the discussion of our experimental design, both hypotheses predicted that the hierarchies formed through group assembly should be linear, but they disagreed about the extent of linear hierarchies formed via round-robin competition. Although the prior attributes hypothesis anticipated linear structures, the social dynamics hypothesis forecasted nonlinear ones, because round-robin competition did not allow interaction in a group context.
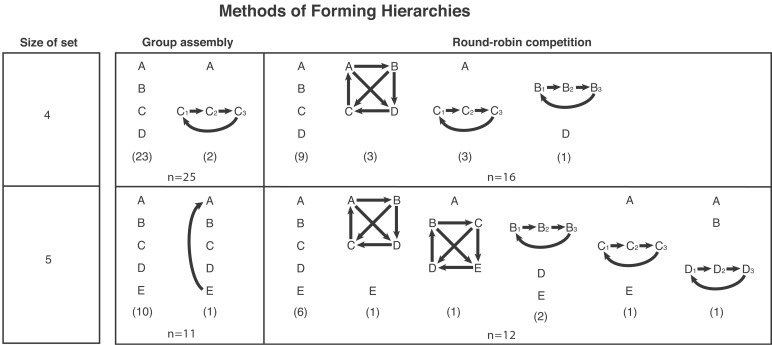
Fig. 2 shows the various hierarchy structures and frequencies of sets of fish forming them in round-robin competition and group assembly. Most of the hierarchies formed under group assembly were linear, and the few others tended to show relatively simple structural deviations from linearity. In contrast, many hierarchies formed with round-robin competition were not linear, including several with quite complicated structures.
Figure 2.
The structure of hierarchies forming in group assembly and round-robin competition for sets of four and five fish. An animal dominates all those listed below it except as indicated by heavy arrows; three fish in an intransitive triad sharing the same rank are placed on the same level in a hierarchy. Frequencies of experimental groups showing each structure are indicated in parentheses.
In Table 2, the probabilities of linear and nonlinear hierarchies in sets of four and five fish expected by chance alone (if each fish had an independent 0.5 probability of dominating each other) are compared with the proportions observed in round-robin competition and group assembly. Although round-robin competition only produced significantly higher proportions of linear hierarchies than expected by chance in sets of five fish, group assembly did so in sets of both sizes (one-sided binomial tests: round robin competition, n = 16, P = 0.10 in sets of four fish, and n = 12, P < 0.005 in sets of five; group assembly, n = 25, P < 0.001 in sets of four and n = 11, P < 0.001 in sets of five).
Table 2.
Percentage of linear structures expected in random hierarchies and observed in round-robin competition and group assembly in sets of four and five fish
| Size of set | Method of forming hierarchy
|
||
|---|---|---|---|
| Random, % | Round robin, % | Group assembly, % | |
| 4 | 37.5 | 56.2 (n = 16) | 92.0 (n = 25) |
| 5 | 11.7 | 50.0 (n = 12) | 90.9 (n = 11) |
Concerning support for the hypotheses, further inspection of Table 2 indicates that the proportions of linear hierarchies formed with group assembly were much higher than those established with round-robin competition. With group assembly over 90% of the hierarchies in both sizes were linear, but with round-robin competition, only about half, 54 and 50%, were linear in sets of four and five, respectively. Statistical tests indicate that these differences are highly significant (Fisher's exact test: P < 0.002 for sets of four, and P < 0.05 for sets of five).
Discussion.
Group assembly experiments allowed fish to interact in a group context, and nearly all the hierarchies were linear. But when we prohibited this form of interaction in round-robin competition, only about half the hierarchies were linear. Social interaction greatly enhanced the formation of linear hierarchies. Consequently, there is strong support for the social dynamics hypothesis. The prior attributes hypothesis also finds support in that sets of five fish meeting in round-robin competition formed linear hierarchies at a rate significantly higher than chance.
It might be argued that differences in a certain class of prior attributes (social attributes) could only be expressed in group contexts but not in round-robins, e.g., the ability to react strategically to others' contests. However, if such attributes existed and were vital to forming linear hierarchies, they should have manifested in the first experiment in which fish met in groups. We should have seen a large proportion of identical first and second hierarchies, but we did not.
Our results do not demonstrate that social dynamics by themselves can produce linear hierarchies. In the group assembly experiments, in which group interaction proved so effective in producing linear hierarchies, the individuals still varied in prior attributes. For this reason we say that our results demonstrate that social interaction greatly enhances the production of linear hierarchies rather than that social dynamics by themselves generate linear hierarchies. To determine whether social dynamics on their own can generate high proportions of linear hierarchies, we propose investigation of hierarchy formation in genetically identical fish or other animals with equal physical characteristics. We conjecture that even without attribute differences such animals would form linear hierarchies as readily as those with variation in attributes.
Would some variation in weight, the one prior attribute we controlled, be sufficient to produce high rates of linear hierarchies even without interaction in a group context? We suggest that the differences required would be high, probably greater than that found in many groups forming linear hierarchies either in nature or the laboratory. For example, based on our experiments with pairs of fish, we estimate that even when each individual was ≈25% heavier than the next (C 25% heavier than D, B 25% heavier than C, and A 25% heavier than B), only ≈34% of the hierarchies formed would be linear and identical to the prior ranking on weight. And at this variation, A would be about twice the weight of D! (also see Francis, ref. 43, for an insightful discussion of the social mediation of weight differences in established hierarchies).
General Discussion
Our results support both the prior attributes and social dynamics hypotheses. More identical hierarchies appeared in the first experiment and more linear hierarchies in the second experiment (in sets of five fish) than expected by chance alone. But the rates of identical hierarchies still were relatively low, and the rates of linear hierarchies from round-robin competition were significantly less than those from group assembly. To ensure that most hierarchies were linear, as they are in many nature- and laboratory-based studies, the fish had to interact in a group context. Linear hierarchies did not consistently preexist on some attribute ranking, ready to be revealed when individuals met. Instead, social dynamics were crucial to the production of these social structures. Although variation in attributes significantly affected the respective placement of individuals on hierarchy ladders, social dynamics were necessary for the dependable manufacture of the ladders themselves.
These findings require a reconceptualization of the phenomenon of dominance hierarchies. Linear structures should not be assumed to result simply from variation among individuals or from cumulative conflicts among pairs of individuals. Instead, to account for the common occurrence of linear hierarchies and provide more accurate accounts of how individuals acquire their ranks, we must look at patterns of interaction across whole groups and understand how these patterns produce hierarchy ladders. Even in dominance hierarchies among simple creatures, in which individual differences in raw physical power, aggression, and strategy might seem crucial, interaction processes are vital in providing the typical forms of social organization that we observe. Simply put, the formation of dominance hierarchies is a richer and more complex phenomenon than has been thought previously.
The importance of interaction among individuals for producing the patterns of organization in dominance hierarchies reveals these structures as self-organizing or self-structuring systems. These experiments are an empirical demonstration that dominance hierarchies are indeed self-organizing, and they confirm previous theoretical work (40–42).
But what particular social processes actually promote the formation of linear hierarchies, and how do they do so? Our results here show that these processes are at the core of the as-yet-unanswered riddle of hierarchy formation. Empirical and theoretical work on winner, loser, and bystander effects (25–29, 39–42) and applications of Chase's jigsaw puzzle model (5, 23, 35–38) seem to be very promising starting points. Both of these lines of research suggest that linear structure is promoted by positive feedback to initial wins and losses during hierarchy formation, i.e., when an animal dominating in one contest goes on to dominate in others and when an animal becoming subordinate in one contest goes on to be subordinate in others. We suggest that further investigation of these and other dynamical patterns hold the key to understanding how hierarchies come to develop linear structures so often.
One implication of our results is that current models in sociobiology are either too simple or too concerned with individual differences to account adequately for the evolution of behaviors leading to dominance hierarchies. Many of these models use game theoretic techniques to consider conflicts between pairs. Although these models are helpful, we concur with Oliveira et al. (27) in that the evolution of behavior in dominance hierarchy formation must be seen as contextual to networks of individuals rather than independent dyads. Forming dominance hierarchies and being a social animal in general may require the evolution of considerable cognitive power in individuals to meet the contingencies of interaction in groups. Another line of evolutionary thinking notes that individuals ranking higher in hierarchies often produce more offspring than those ranking lower. Consequently, if there are genetic linkages to any differences in attributes that help influence higher rank, they would be favored by natural selection (but see Francis, ref. 49, for careful consideration of the requirements for demonstrating selection for attributes associated with dominance). But even if these differences in dominance potential are selected for, our results suggest that they are not doing the heavy lifting in the production of linear hierarchies.
In their seminal work on friendship in human groups, Holland and Leinhardt (50) argue that any network of relationships in which higher-level properties can be modeled adequately using only the properties of actors or pairs of actors has no true social structure. In their words, there is nothing inherently social about the structure of such a network of relationships. By this definition, our fish are genuine social creatures and their dominance hierarchies are true social structures. Fish apparently can be active and aware social actors, not just taking places in dominance hierarchies that reflect their intrinsic, biological characteristics. And if fish can form true social structures, why not other animals? Thus our results suggest that there may be no fundamental discontinuities between social structure in humans and animals.
Finally, our results suggest that dominance hierarchies in fish and perhaps other social structures in “simple” animals might serve as models to help us understand the development of hierarchies and other forms of social organization in humans. Ordinarily, the absence of higher-level cognitive ability, behavioral complexity, language skills, and elaborate cultural forms argue against applying lessons learned from studying social organization in simple creatures to the investigation of social systems in humans. However, finding that social interaction is so important in producing organized structures in fish strengthens the argument for investigating the importance of social dynamics in producing dominance hierarchies and other social structures in humans. Just as animals have served as invaluable models for understanding genetics, health and disease processes, and cognition and perception in humans, animal models may enable us to better understand how some of our social systems develop as they do.
Acknowledgments
We thank Ritu Bapat, Michelle Cornog, Peter Murch, Kristine Seitz, and Nam Thai for help in data collection; Ginny and Charlie Eckstein and Tom Keegan for advice on fish care; Paul Loiselle and the New York Aquarium for Wildlife Conservation for providing fish; Peter Bearman, Al Carlson, Stephen Cole, Andrea Tyree, and Everett Waters for comments on earlier drafts; and Eugene Danner, Inc., Penn Plax Corporation, Python Products, Rena Corporation, and Tetra USA for equipment donations. Support was provided by the Harry Frank Guggenheim Foundation, National Science Foundation Grant SES-9424006, the Guy Jordan Endowment Fund of the American Cichlid Association (to I.D.C.), and grants from the Institute Fellows program at Georgia Institute of Technology and the Georgia Tech Foundation (donation by John Grigsby, to C.T.).
References
- 1.Heinze J. Naturwissenschaften. 1990;77:41–43. [Google Scholar]
- 2.Wilson E O. Sociobiology. Cambridge, MA: Harvard Univ. Press; 1975. [Google Scholar]
- 3.Vannini M, Sardini A. Monitore Zool Ital (NS) 1971;5:173–213. [Google Scholar]
- 4.Goessmann C, Hemelrijk C K, Huber R. Behav Ecol Sociobiol. 2000;48:418–428. [Google Scholar]
- 5.Nelissen M H J. Behavior. 1985;94:85–107. [Google Scholar]
- 6.Post W. Anim Behav. 1992;44:917–929. [Google Scholar]
- 7.Barkan C P L, Craig J L, Strahl S D, Stewart A M, Brown J L. Anim Behav. 1986;34:175–187. [Google Scholar]
- 8.Addison W E, Simmel E C. Bull Psychon Soc. 1970;15:303–305. [Google Scholar]
- 9.Hausfater G, Altmann J, Altmann S. Science. 1982;217:752–755. doi: 10.1126/science.217.4561.752. [DOI] [PubMed] [Google Scholar]
- 10.Savin-Williams R C. J Youth Adolesc. 1980;9:75–85. doi: 10.1007/BF02088381. [DOI] [PubMed] [Google Scholar]
- 11.Jameson K A, Appleby M C, Freeman L C. Anim Behav. 1999;57:991–998. doi: 10.1006/anbe.1998.1077. [DOI] [PubMed] [Google Scholar]
- 12.Raleigh M J, McGuire M T, Brammer G L, Pollack D B, Yuwiler A. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky R M, Share L J. Am J Primatol. 1994;32:261–275. doi: 10.1002/ajp.1350320404. [DOI] [PubMed] [Google Scholar]
- 14.Clutton-Brock T H, Albon S D, Guiness F E. Nature (London) 1984;308:358–360. [Google Scholar]
- 15.Beacham J L. Anim Behav. 1988;36:621–623. [Google Scholar]
- 16.Holenkamp K E, Smale L. Anim Behav. 1993;46:451–466. [Google Scholar]
- 17.Drews C. Behavior. 1993;125:283–313. [Google Scholar]
- 18.Jackson W M, Winnegrad R L. Anim Behav. 1988;36:1237–1240. [Google Scholar]
- 19.Cloutier S, Beaugrand J P, Lague P C. Behav Processes. 1996;38:227–239. doi: 10.1016/s0376-6357(96)00034-4. [DOI] [PubMed] [Google Scholar]
- 20.Beaugrand J P, Cotnoir P-A. Behav Processes. 1996;38:287–296. doi: 10.1016/s0376-6357(96)00039-3. [DOI] [PubMed] [Google Scholar]
- 21.Slater P J B. Anim Behav. 1986;34:1264–1265. [Google Scholar]
- 22.Jackson W M. Ethology. 1988;79:71–77. [Google Scholar]
- 23.Chase I D. Behavior. 1982;80:218–240. [Google Scholar]
- 24.Francis R C. Ethology. 1988;78:223–237. [Google Scholar]
- 25.Chase I D, Bartolomeo C, Dugatkin L A. Anim Behav. 1994;48:393–400. [Google Scholar]
- 26.Hsu Y Y, Wolf L L. Anim Behav. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira R F, McGregor P K, Latruffe C. Proc R Soc London Ser B. 1998;265:1045–1049. [Google Scholar]
- 28.Johnsson J I, Akerman A. Anim Behav. 1998;56:771–776. doi: 10.1006/anbe.1998.0824. [DOI] [PubMed] [Google Scholar]
- 29.Silk J B. Anim Behav. 1999;58:45–51. doi: 10.1006/anbe.1999.1129. [DOI] [PubMed] [Google Scholar]
- 30.Bak P. How Nature Works. New York: Springer; 1996. [Google Scholar]
- 31.Resnick M. Turtles, Termites, and Traffic Jams. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- 32.Kauffman S A. The Origins of Order. New York: Oxford Univ. Press; 1993. [Google Scholar]
- 33.Landau H G. Bull Math Biophys. 1951;13:1–19. [Google Scholar]
- 34.Chase I D. Behav Sci. 1974;19:374–382. [Google Scholar]
- 35.Mendoza S D, Barchas P R. J Hum Evol. 1983;12:185–192. [Google Scholar]
- 36.Chase I D. Anim Behav. 1985;33:86–100. [Google Scholar]
- 37.Barchas P R, Mendoza S D. In: Social Hierarchies: Essays Toward a Sociophysiological Perspective. Barchas P R, editor. Westport, CT: Greenwood; 1984. pp. 23–44. [Google Scholar]
- 38.Eaton G G. Int J Primatol. 1984;5:145–160. [Google Scholar]
- 39.Skvoretz J, Faust K, Fararo T J. J Math Soc. 1996;21:57–76. [Google Scholar]
- 40.Theraulaz G, Bonabeau E, Deneubourg J L. J Theor Biol. 1995;174:312–323. doi: 10.1006/jtbi.1998.0789. [DOI] [PubMed] [Google Scholar]
- 41.Hogeweg P. In: Artificail Life I. Langton C, editor. Redwood City, CA: Addison–Wesley; 1989. pp. 297–316. [Google Scholar]
- 42.Hemelrijk C K. Anim Behav. 2000;59:1035–1048. doi: 10.1006/anbe.2000.1400. [DOI] [PubMed] [Google Scholar]
- 43.Francis R C. Anim Behav. 1988;36:1844–1845. [Google Scholar]
- 44.Johnsson J L. Ethology. 1997;103:267–282. [Google Scholar]
- 45.Miklosi A, Haller J, Csanyi V. Behav Processes. 1997;40:97–105. doi: 10.1016/s0376-6357(96)00755-3. [DOI] [PubMed] [Google Scholar]
- 46.Barends G P, Barends-Van Roon J M. Behavior Suppl. 1950;1:1–242. [Google Scholar]
- 47.Dugatkin L A, Alfieri M S, Moore A J. Ethology. 1994;97:94–102. [Google Scholar]
- 48.Guhl A M. In: Social Hierarchy and Dominance. Schein M W, editor. Hutchinson, and Ross, Stroudsburg, PA: Dowden; 1975. pp. 156–201. [Google Scholar]
- 49.Francis R C. Behavior. 1984;90:25–45. [Google Scholar]
- 50.Holland P W, Leinhardt S. In: Perspectives on Social Network Research. Holland P W, Leinhardt S, editors. New York: Academic; 1979. pp. 63–83. [Google Scholar]