Abstract
Background
In randomized controlled trials, add-on cannabidiol (CBD) has been shown to reduce seizure frequency in patients with Lennox-Gastaut syndrome, Dravet syndrome and Tuberous sclerosis complex. Real-world studies provide insights into the drug’s profile in other off-label indications. This study evaluated factors predicting efficacy, retention, and tolerability of add-on CBD used for off-label treatment in clinical practice for patients with refractory focal-onset, genetic generalised, and other unclassified epilepsies.
Methods
A retrospective cohort study recruiting all patients who had started CBD between 2019 and 2023 for off-label treatment at six German epilepsy centres. Data on baseline and follow-up were obtained from patients’ medical records.
Results
A total of 108 patients (mean age 27.3; median 36; range 1.4–68 years, 56 male) were treated with CBD. At three months, 42 (38.9% considering all 108 patients that started CBD) reported at least a 50% reduction in seizures, including 28 patients (25.9%) with a 50–74% reduction, and 14 (13%) with a reduction of 75–99%. Among those 48 patients experiencing tonic-clonic seizures (TCS), at least 50% response was reported by 45.8%, and eight (16.7%) patients were free of TCS. Sex, age, epilepsy syndrome, concomitant clobazam (CLB) use, and the number of concomitant or previous ASMs were not predictive of response. Mean seizure days per month significantly decreased from a mean of 16.8 (median: 13.5) to 14.5 (median 10, p = 0.002). The probability of patients remaining on CBD treatment was 85.2% (n = 92/108, 16 discontinuations) at three months, 73.5% at six months and 61.1% at twelve months; retention was better in children or adolescents compared to adults (log-rank p = 0.014). Using the CGI-C for overall impression, 69 (63.0%) patients were rated as very much, much, or minimally improved; for behaviour, 60 (55.6%) reported within this range of improvement. TEAEs were reported in 41 (38%) patients. The most frequent were diarrhoea (n = 15), sedation (n = 13), and nausea and vomiting (n = 7).
Conclusions
Our results suggest CBD to be an effective ASM, with 50% responder rates similar to those observed in regulatory trials for other ASMs licensed in focal epilepsies. Its off-label use in refractory focal-onset, genetic generalised, and other unclassified epilepsies seems to be safe and well-tolerated.
Highlights
Off-label use of cannabidiol in 108 patients with refractory focal, genetic generalised and other epilepsies.
Retention rate was 85.2% at three months, 73.5% at six months and 61.1% at 12 months follow-up.
50% responder rate was 39% at three months and 42% at one year.
Using CGI-C, 63.0% were rated as improved regarding overall impression and 55.6% regarding behaviour.
Adverse events were observed in 38% of patients, predominantly gastrointestinal symptoms (diarrhoea, nausea and vomiting) and sedation.
Introduction
Anti-seizure medications (ASMs) play a central and crucial role in the treatment of people with epilepsy, the majority of whom require ASM treatment for an extended period of time. Since up to 30% of patients are refractory to ASM [1, 2], the development of new therapeutic options is strongly warranted. Due to ongoing seizures, patients with drug-refractory epilepsies are affected by increased risk of injury, morbidity and mortality, social stigma, reduced employment opportunities and impaired quality of life for both themselves and their caregivers [3–8]. Any newly introduced ASM would provide an opportunity to achieve better seizure control for some patients [9–11].
Cannabidiol (CBD, Epidyolex®) is an ASM recently approved by the European Medicines Agency (EMA) as an adjunctive therapy in patients aged ≥ 2 years of age for seizures associated with Lennox-Gastaut-Syndrome (LGS) or Dravet Syndrome (DS) in conjunction with clobazam (CLB), or as adjunctive therapy for seizures associated with Tuberous Sclerosis Complex (TSC). In the US, it is approved by the Food and Drug Administration (FDA) for the treatment of seizures associated with LGS, DS, or TSC in patients ≥ 1 year of age. These approvals were based on several double-blind, randomised controlled trials (RCTs) that showed the efficacy of CBD as an adjunctive therapy to standard ASMs in reducing drop seizures in LGS [12, 13], convulsive seizures in DS [14, 15], and TSC-associated seizures [16, 17] compared with placebo.
However, results from regulatory, clinical trials are difficult to extrapolate to clinical practice, as these studies are limited by their short duration, rigid inclusion and exclusion criteria, which exclude the majority of epilepsy patients, and lack of dosing flexibility [18, 19]. Upon the introduction of a new ASM, there is very limited information about the potential efficacy and tolerability in a naturalistic clinical setting, especially if, like CBD, they were tested in an orphan drug designation. In the absence of RCTs for off-label uses of CBD, real-world evidence offers essential clinical insights into its efficacy, tolerability and retention—a robust factor which combines aspects of both the former—across broader patient populations with epilepsy [20–23].
Our multicentre study aimed to give insights into retention, efficacy and tolerability in a large cohort of patients with different epilepsy syndromes during the first year of treatment with CBD. Furthermore, we aimed to identify predictors of efficacy and tolerability, and examined outcomes and treatment-emergent adverse events (TEAEs).
Methods
Study settings and design
This study was performed at six German epilepsy centres (Frankfurt am Main, Freiburg i. Br., Greifswald, Lingen [Münster], Marburg, Radeberg). All patients with refractory focal-onset, genetic generalised, and other unclassified epilepsies treated with CBD in one of the enrolling epilepsy centres between 2019 and 2023 were included. The retrospective analysis was approved by the ethics committee of the University of Frankfurt. Informed consent was waived due to the retrospective nature of the study design, and STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were followed [24]. The study was not sponsored or funded by any third party.
The epilepsy diagnosis was based on the definitions proposed by the ILAE and the International Bureau for Epilepsy [25, 26]. No patients treated on-label for LGS [27], DS [28] or TSC [29] were included in the present analysis; these analyses are reported separately [30]. The treating physician at each study site provided information on epilepsy syndrome, aetiology, semiology, demographics, concomitant and previous ASMs, and seizure frequency in the three months prior to CBD treatment (defined as baseline). Patients were interviewed about the occurrence of TEAEs at each visit, and TEAEs were documented according to WHO criteria. Patients were usually seen every three to six months. Follow-up data, collected through patients’ medical records, included target and maximal doses of CBD, seizure frequency, TEAEs, retention of CBD, and discontinuation of CBD categorised according to the following reasons: TEAEs, lack of effectiveness, both TEAEs and lack of effectiveness, or not reported.
Seizure reduction was analysed at three-, six- and 12-month follow-ups regarding the preceding 3, 6, 12 months period since start of CBD for total seizures and tonic-clonic seizures (TCS, this term includes focal to bilateral tonic-clonic, bilateral tonic-clonic and generalised tonic-clonic seizures according to the updated ILAE criteria [31]), where data was available. A 25% responder rate was defined as a 25% or greater seizure reduction compared to the defined baseline, a 50% responder rate meant a 50% or greater seizure reduction, and a 75% responder rate meant a 75% or greater seizure reduction compared to baseline. No response was defined as a change (decrease or increase) in seizure frequency by less than 25% compared to baseline. Seizure increase was defined as a 25% or greater increase in seizure frequency compared to baseline. In addition, change in seizure occurrence was recorded as the average number of seizure days per month, regardless of seizure type at baseline and final follow-up. Physicians rated the overall and behavioural clinical change (CGI-C) during CBD treatment on a 7-point rating scale, categorised from very much improved to very much worse. Retention was defined as patients continuing CBD treatment after three, six and 12 months, and the rate was estimated using Kaplan-Meier survival curves.
Data entry and statistical analysis
The statistical analyses described above, including descriptive analyses, were performed using IBM SPSS Statistics, version 28 (IBM Corp., Armonk, NY, USA). Retention time was displayed using Kaplan-Meier survival curves. Mann-Whitney-U, chi-squared and log-rank tests were used for statistical analysis, and p-values < 0.05 were regarded as statistically significant.
Results
Patient characteristics at baseline
A total of 108 patients with a mean age of 27.3 ± 14.7 years (median 26; range 1.4–68 years) were treated with CBD, of whom 56 patients were male (51.9%). Among the cohort were 32 children and adolescents (29.6%), with 76 (70.4%) adults 18 years or older. The patients had a mean epilepsy duration of 17.8 ± 11.7 years (median 16; range 1–57 years) with a mean age of onset of 8.8 ± 9.6 years (median 5.0; range 0.1–44.0 years). All patients had drug-refractory epilepsy; 74 (68.5%) had focal-onset epilepsy, 14 (13.0%) had genetic generalised epilepsy, and 20 (18.5%) had other or unclassified epilepsy. They were taking a mean of 2.7 ± 1.0 ASMs (median: 3, range: 1–6 ASMs) before starting CBD. Concomitant use of CLB was reported in 44 (40.7%) patients, with a mean dose of 10.7 mg (median 10, range 2.5–30 mg) corresponding to a mean of 0.16 mg per kg bodyweight (median 0.13; range 0.03–0.42). Other mainly used ASMs were lamotrigine (n = 37, 34.3%, mean dose 363 mg), valproate (n = 36, 33.3%, mean dose 1318 mg), brivaracetam (n = 32, 29,6%, mean dose 246 mg), perampanel (n = 28, 25.9%, mean dose 8 mg), lacosamide (n = 27, 25%, mean dose 400 mg), and topiramate (n = 19, 17.6%, mean dose 199 mg). In the past, the patients had experienced failed treatment with a mean of 6.8 ± 3.6 ASMs (median: 7, range: 1–18 failed ASMs; current ASM not included).
Treatment with Cannabidiol
The starting dose of CBD in patients varied between 12.5 mg and 1200 mg per day, with a mean of 170.1 ± 155.6 mg and a median of 100 mg (median 2.2 mg per kg bodyweight per day, 2.8 in children vs. 1.8 in adults, p = 0.017). The target dose ranged between 100 mg and 1600 mg, with a mean of 628.8 ± 333.3 mg and a median of 600 mg (median 10 mg per kg bodyweight per day, 16.7 in children vs. 9.1 in adults, p < 0.001); target dose was achieved within a median time of 3 weeks. The maximum dose ranged between 100 mg and 2400 mg with a mean of 791.5 ± 468.8 mg and a median of 700 mg (median 12.8 mg per kg bodyweight, 22.7 in children vs. 10.9 in adults, p < 0.001).
Outcome and predictors of response at three months
At three months of follow-up, 66 (61.1%, 66/108 considering all patients that started CBD) reported a 25% or greater reduction in seizures, there was no difference between those on (n = 27/44; 61.4%) and without CLB (n = 39/64; 60.9%; p = 0.964). This included 24 patients (22.2%) with a 25–49% reduction in seizures, 28 patients (25.9%) with a 50–74% reduction, and 14 (13%) with a reduction of 75–99%. Responder rates are presented in Fig. 1A. In 31 patients (28.7%), the seizure frequency was unchanged, while six patients (5.6%) reported an increase. For five patients, no seizure outcome data was available.
Fig. 1.
Responder rates at three months for (A) total seizures and (B) tonic-clonic seizures (TCS), and at 12 months for (C) total seizures and (D) tonic-clonic seizures (TCS)
Of the 48 patients experiencing TCS, five (10.4%) reported a 25–49% reduction of TCS, seven (14.6%) reported a 50–75% reduction, and four (8.3%) reported a 75–99% reduction. Eight (16.7%) patients reported being free of TCS. There was no difference in TCS response between those on and without CLB (p = 0.768). Two (4.2%) patients had an increase in TCS, while in six (12.5%), TCS frequency was unchanged; for details, please refer to Fig. 1C. Data was unavailable for 16 patients. Table 1 shows details for responders and non-responders in terms of sex, age, epilepsy syndrome, concomitant CLB use, concomitant and previous number of ASMs. No significant differences were found in the chi-squared analysis (p > 0.05 across all parameters).
Table 1.
Clinical characteristics and outcome on follow-up of 3 months
| all patients n = 108 |
non responders n = 42 |
responders n = 66 |
p-value | |
|---|---|---|---|---|
| n | % (n) | |||
| total | 108 | 38.9 (42) | 61.1 (66) | |
| sex | 0.758 | |||
| male | 56 | 37.5 (21) | 62.5 (35) | |
| female | 52 | 40.4 (21) | 59.6 (31) | |
| age range | 0.055 | |||
| < 18 years | 32 | 25 (8) | 75 (24) | |
| ≥ 18 years | 76 | 44.7 (34) | 55.3 (42) | |
| Epilepsy syndrome | 0.925# | |||
| Focal-onset epilepsy | 74 | 39.2 (29) | 60.8 (45) | |
| Genetic generalised epilepsy | 14 | 50 (7) | 50 (7) | |
| other and unclassified | 20 | 30 (6) | 70 (14) | |
| Concomitant use of clobazam | 0.964 | |||
| Use of clobazam | 44 | 38.6 (17) | 61.4 (27) | |
| No use of clobazam | 64 | 39.1 (25) | 60.9 (39) | |
| number of concomitant ASM at start of CBD | 0.203 | |||
| 1–2 ASM | 52 | 32.7 (17) | 67.3 (35) | |
| 3 or more ASM | 56 | 44.6 (25) | 55.5 (31) | |
| previously failed ASMs (without current) | 0.820 | |||
| 6 ASM and below | 27 | 37.0 (10) | 63.0 (17) | |
| ≥ 7 ASM | 81 | 39.5 (32) | 60.5 (49) | |
ASM, anti-seizure medication; CBD, cannabidiol; #focal vs. non-focal
Seizure days
At baseline, patients reported a high seizure burden, with 16.8 ± 11.3 seizure days per month (median: 13.5, range: 0–30). The burden was significantly higher in children and adolescents, with 22.1 ± 11 seizure days per month (median: 30, range: 1–30), than in adults, who had 14.7 ± 10.8 seizure days per month (median: 12, range: 0–30, p = 0.011) during the three-month baseline phase. One adult had been seizure-free during the three months of baseline prior to treatment.
At the final follow-up point, the mean seizure days per month significantly decreased to 14.5 (median 10, range 0–30) days per month in the previous three months of CBD treatment (p = 0.002). Among children and adolescents, seizure days reduced to a mean of 20.2 (median 27.5, p = 0.064), and among adults to 12.2 (median 7.5, p = 0.011) seizure days per month. Figure 2A shows the number of seizure days per month at baseline and final follow-up.
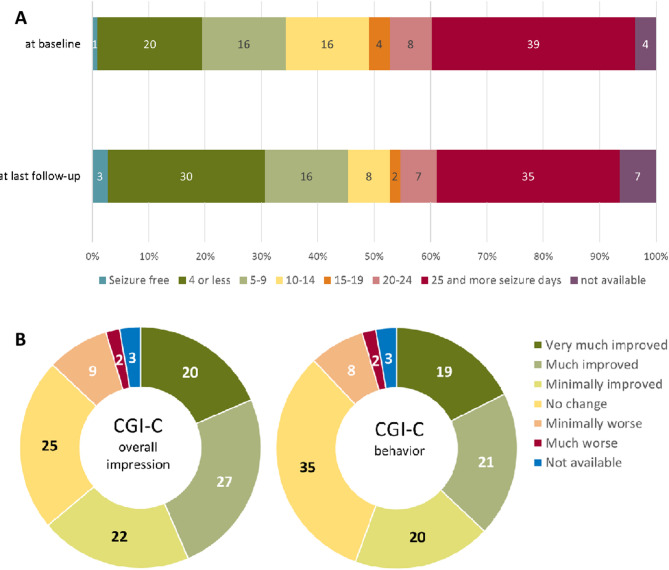
Fig. 2.
(A) Percentage of patients according to seizure days per month across seven incremental categories at baseline and final follow-up, after initiation of cannabidiol (B) Physician-assessed Clinical Global Impression of Change (CGI-C) for the overall impression and (C) behavioural change
Retention analysis and long-term response
The probability of patients remaining on CBD treatment was 85.2% (n = 92/108, 16 discontinued) at three months, 73.5% (n = 75/102, 27 discontinued, 6 with no-follow-up) at six months and 61.1% (n = 58/95, 37 discontinued, 13 with no-follow-up) at twelve months. Kaplan-Meier survival curves show the retention over time (Fig. 3A). The retention was better in children and adolescents compared to adults (log-rank p = 0.014, Fig. 3B). There was no difference observed regarding comedication with CLB (log-rank p = 0.062, Fig. 3C) or the number of concomitant ASMs (log-rank p = 0.699, Fig. 3D).
Fig. 3.
Retention rate of cannabidiol (CBD) in the complete cohort (A), and stratified by age (B; long-rank p-value: 0.014); concomitant clobazam (C; long-rank p-value: 0.062), for number of concomitant ASMs (D; long-rank p-value: 0.699), ASM = anti-seizure medication
The reasons for discontinuation of CBD at one year were TEAEs (n = 16, 14.8%), insufficient efficacy (n = 16, 14.8%), or both (n = 5, 4.6%). TEAEs associated with discontinuation were mainly diarrhoea (n = 7; 6.5%), nausea and vomiting (n = 6, 5.6%), and sedation (n = 5; 4.6%). For details, please refer to Table 2.
Table 2.
Characteristics of reported treatment-emergent adverse events on cannabidiol, their frequency and related discontinuation at 12 months (total n = 108)
| Adverse events | Reported n (%) |
Leading to withdrawal n (%) |
|---|---|---|
| Overall | 41 (38) | 21 (19.5) |
| GI symptoms | 21 (19.5) | 11 (10.2) |
| Diarrhoea | 15 (13.9) | 7 (6.5) |
| Nausea / vomiting | 7 (6.5) | 6 (5.6) |
| Loss of appetite / weight loss | 3 (2.8) | 1 (< 1) |
| Sedation | 13 (12.0) | 5 (4.6) |
| CNS/Ataxia | 3 (2.8) | 2 (1.9) |
| Dizziness | 2 (1.9) | 1 (< 1) |
| Ataxia | 1 (< 1) | 1 (< 1) |
| Psychobehavioural | 10 (9.3) | 7 (6.5) |
| Aggression / Irritability | 3 (2.8) | 2 (1.9) |
| Behavioural | 3 (2.8) | 2 (1.9) |
| Anxiety | 1 (< 1) | 1 (< 1) |
| other | 3 (2.8) | 2 (1.9) |
| Skin | 3 (2.8) | 1 (< 1) |
| Elevated liver enzymes | 1 (< 1) | 1 (< 1) |
| Other | 3 (2.8) | 1 (< 1) |
Among those on treatment at 12 months, 41 reported a 25% or greater reduction in seizures (57.7%, 41/71). This included 11 patients (15.5%) with a 25–49% reduction, 16 patients (22.6%) with a 50–74% reduction and 14 patients (19.7%) with a 75–99% reduction in seizure frequency; details are presented in Fig. 1B. In patients experiencing TCS (n = 26) at 12 months, 13 (50%) reported a 50% or greater reduction. Among these, four (15.4%) patients were free of TCS, six (23.1%) had a 50–75% reduction, and three (11.5%) had a 75–99% reduction. In three patients, TCS frequency was unchanged, and for ten patients, no data was available. Details are presented in Fig. 1D.
Overall change, behavioural change and treatment-emergent adverse effects
Using the CGI-C for overall impression, 20 (18.5%) patients were rated as very much improved at the last follow-up, 27 (25%) patients were much improved, and 22 (20.4%) patients were minimally improved. Twenty-five (23.1%) patients showed no change. Nine (8.3%) patients were rated minimally worse and two (1.9%) were rated much worse. No patients were rated as very much worse (Fig. 2B). The CGI-C for behaviour showed 19 (17.6%) patients rated as very much improved, 21 (19.5%) as much improved, and 20 (18.5%) as minimally improved. For further details, please refer to Fig. 2C.
Treatment-emergent adverse effects (TEAEs) were reported in 41 (38%) patients. The most frequent were diarrhoea (15 patients), sedation (13 patients), and nausea and vomiting (7 patients). For details, please refer to Table 2.
Discussion
This study reflects experiences with off-label use of CBD during the first years of market access in a cohort of 108 patients with drug-refractory focal-onset, genetic generalised and other epilepsy syndromes, excluding LGS, DS or TSC.
The observed efficacy of CBD was well in line with that observed during RCTs and open-label extension studies for LGS [12, 13], DS [14, 15], and TSC [16], with 50% responder rates of 39% at three months and 42% at one year. The retention rates at three (85%), six (73%), and 12 months (61%) are encouraging, and align well with findings from other post-marketing studies on CBD [32–34]. In addition, the retention is similar to or above that of other ASMs reported in post-marketing studies, including brivaracetam, cenobamate, eslicarbazepine, lacosamide, lamotrigine, levetiracetam, perampanel, topiramate, valproate, and zonisamide in focal or generalised epilepsies [35–47], however, any direct comparisons should be interpreted with caution. In particular, retention data may appear favorable; but cross-study comparisons are inherently limited due to differences in study design and the lack of head-to-head trials [48].
So far, data on the use of CBD in genetic generalised epilepsies has been heterogeneous [49, 50]; however, in our study, patients with this epilepsy type exhibited a 50% responder rate of 52.6% (10/19), suggesting a role for CBD in managing some of these patients. Our results suggest that CBD may offer clinical benefits in certain cases of treatment-resistant epilepsies beyond currently approved indications [51]; however, such use should be considered exploratory, and further prospective studies are needed to establish its efficacy and safety across different epilepsy syndromes.
Mechanism-of-action data support this observation, suggesting that CBD may achieve anti-seizure effects through various pathways, including the modulation of intracellular calcium via GPR55 and TRPV1 receptors and the regulation of adenosine signalling [52]. Although the exact mechanisms remain under investigation, these targets appear to act broadly rather than being limited to specific epilepsy aetiologies or syndromes [52].
Findings on differences in retention rates of CBD between children and adults differ across studies. We observed better retention and higher median daily dosage per kg of body weight for CBD in children, with 22.7 mg per kg compared to 10.9 mg per kg body weight in adults. Higher weight-adjusted dosing in children was also reported in earlier studies on the use of CBD [32, 53]. A retrospective, single-centre study from the University of Wisconsin–Madison [54] reported no differences in retention rates, with 77.2% of paediatric patients (n = 57) and 76.5% of adult patients (n = 51) remaining on CBD over an average of 20 months. In contrast, results from the Italian Expanded Access Program showed higher retention rates in adults than children [55]. Expanded Access Program data from the US revealed no significant differences in seizure frequency reduction between children up to 2 years and adults [56].
The lower-than-recommended median starting dose of 2.2 mg per kg bodyweight per day reflects cautious titration practices in clinical routine, particularly in patients with complex therapy with multiple ASMs or concern for drug interactions. This conservative approach is commonly adopted to improve tolerability, especially in populations outside of the licensed indications. Overall, the TEAE profile observed in our study was in line with the established adverse events profile of CBD. Overall, TEAEs were reported in 37.9% of patients, with discontinuation due to TEAEs occurring in 19.5% of the cohort. The most common TEAEs were gastrointestinal symptoms and sedation. We did not observe any serious TEAEs, and, similar to other post-marketing studies, the overall proportion of patients reporting TEAEs was lower than in RCTs where TEAEs are more rigorously documented. Systematic literature reviews of clinical trials have indicated that CBD is associated with somnolence (particularly when used with CLB), as well as decreased appetite, diarrhoea, and elevated liver enzymes, especially in conjunction with valproate [57–59]. We observed elevated liver enzymes in only one patient; however, data on laboratory tests were not routinely collected. Patients with epilepsy are prone to behavioural and psychiatric TEAEs as well as sleep disorders, with some ASMs reported to worsen these symptoms [60]. In the current study, consistent with findings from other CBD studies, psychobehavioural TEAEs were infrequently observed. In addition, physicians rated 55.6% of patients as showing improvement or no change (32.4%) in behaviour. Overall, CBD appears to have a favourable psychobehavioural profile.
This study is subject to several limitations typical of retrospective chart reviews, including an uncontrolled design, potential gaps or incompleteness in the data, and variability in data collection processes. For example, the absence of detailed records on total seizure counts and TCS may have influenced the accuracy of responder rate calculations. Moreover, seizure frequency data were based on caregiver-reported seizure diaries, which depend on their reliability and accuracy, and may be subject to over- and underreporting [61]. Therapeutic drug monitoring (TDM) was not routinely performed or collected for CBD and other concomitant ASM. Implementing routine TDM for CBD and co-administered ASMs could help minimize TEAEs and support informed dosing adjustments. Recent progress was reported in TDM techniques for precise CBD measurement, that offers significant utility in assessing drug-drug interactions, and might contribute to optimized patient care in complex therapeutic scenarios [62]. The study also lacked a predefined design to statistically evaluate differences between age subgroups, epilepsy types, or the impact of concomitant CLB use. These limitations highlight the need for prospective, controlled studies to provide a more comprehensive understanding of the findings.
Conclusions
In conclusion, our analysis demonstrates that CBD can achieve favourable responder rates in clinical practice, along with good tolerability, among patients with drug-refractory focal-onset epilepsy, genetic generalised epilepsy, and other epilepsy syndromes.
Author contributions
MH, SSB and AS developed the idea for this study. MH, TM, KAK, SK, FvP, GK, II, FR, SSB, and AS participated in the recruitment of patients and data collection. SSB and AS supervised the study. MH and AS conceived the paper and performed the statistical analysis. MH and AS created the charts and figures. MH, TM, KAK, SK, FvP, GK, II, FR, SSB, and AS wrote the paper, discussed the results, contributed to the final manuscript. All authors have read and approve the final submitted manuscript, and agree to be accountable for the work.
Funding
Open Access funding enabled and organized by Projekt DEAL. There is no funding to report.
Data availability
The data of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Declarations
Conflict of interest
MH reports no conflicts of interest.
TM receives speaker’s honoraria from Jazz Pharma, UCB, and Angelini Pharma.
KAK received speaker’s honoraria from Biocodex, Desitin, Eisai, Jazz Pharmaceuticals, and UCB Pharma.
SK received speaker’s honoraria from Bial, Destin Arzneimittel, Eisai, Jazz Pharma, Merck Serono, and UCB.
FvP received personal fees and grants from Angelini Pharma, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, UCB Pharma, Nutricia Milupa GmbH, Neuraxpharm, and Bial.
GK received speaker’s honoraria from Angelini Pharma, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Takeda, UCB Pharma, Neuraxpharm, Stada Arzneimittel, Precisis GmbH, and Alexion Pharmaceuticals.
II received personal fees from Angelini Pharma, Jazz Pharmaceuticals, and Precisis.
FR received personal fees and grants from Angelini Pharma, Desitin Arzneimittel, Dr. Schär Deutschland GmbH, Eisai Pharma, Jazz Pharma, Nutricia Milupa GmbH, Stoke Therapeutics, Takeda, UCB Pharma and Vitaflo Deutschland GmbH. Research support from the European Union (EU-FP7), German Research Foundation (DFG), Federal State of Hesse, Germany, Detlev Wrobel Fonds for Epilepsy Research, Reiss-Stiftung, Dr. Senckenbergische-Stiftung, Kassel-Stiftung, Ernst Max von Grunelius-Stiftung, Chaja-Stiftung.
SSB received personal fees and grants from Angelini Pharma, Biocodex, Biomarin, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Takeda, and UCB Pharma.
AS received personal fees and grants from Angelini Pharma, Biocodex, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Longboard, Neuraxpharm, Stoke Therapeutics, Takeda, UCB Pharma, and UNEEG Medical.
Ethics approval
Ethics committee approval was obtained.
Consent to participate
Waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Susanne Schubert-Bast and Adam Strzelczyk contributed equally as senior authors.
References
- 1.Kwan, P., & Brodie, M. J. (2000). Early identification of refractory epilepsy. New England Journal of Medicine, 342(5), 314–319. [DOI] [PubMed] [Google Scholar]
- 2.Kwan, P., Schachter, S. C., & Brodie, M. J. (2011). Drug-resistant epilepsy. New England Journal of Medicine, 365(10), 919–926. [DOI] [PubMed] [Google Scholar]
- 3.Strzelczyk, A., Reese, J. P., Dodel, R., et al. (2008). Cost of epilepsy: A systematic review. Pharmacoeconomics, 26(6), 463–476. [DOI] [PubMed] [Google Scholar]
- 4.Willems, L. M., Zöllner, J. P., Hamann, L., et al. (2023). Unemployment and early retirement among patients with epilepsy - A study on predictors, resilience factors and occupational reintegration measures. Epilepsy & Behavior, 144, 109255. 10.1016/j.yebeh.2023.109255 [DOI] [PubMed] [Google Scholar]
- 5.Willems, L. M., Hochbaum, M., Zöllner, J. P., et al. (2022). Trends in resource utilization and cost of illness in patients with active epilepsy in Germany from 2003 to 2020. Epilepsia, 63(6), 1591–1602. 10.1111/epi.17229 [DOI] [PubMed] [Google Scholar]
- 6.Neligan, A., Bell, G. S., Johnson, A. L., et al. (2011). The long-term risk of premature mortality in people with epilepsy. Brain, 134(Pt 2), 388–395. 10.1093/brain/awq378 [DOI] [PubMed] [Google Scholar]
- 7.Frey, K., Zöllner, J. P., Knake, S., et al. (2020). Risk incidence of fractures and injuries: A multicenter video-EEG study of 626 generalized convulsive seizures. Journal of Neurology, 267(12), 3632–3642. 10.1007/s00415-020-10065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siebenbrodt, K., Willems, L. M., von Podewils, F., et al. (2023). Determinants of quality of life in adults with epilepsy: A multicenter, cross-sectional study from Germany. Neurol Res Pract, 5(1), 41. 10.1186/s42466-023-00265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luciano, A. L., & Shorvon, S. D. (2007). Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Annals of Neurology, 62(4), 375–381. [DOI] [PubMed] [Google Scholar]
- 10.Callaghan, B., Schlesinger, M., Rodemer, W., et al. (2011). Remission and relapse in a drug-resistant epilepsy population followed prospectively. Epilepsia, 52(3), 619–626. 10.1111/j.1528-1167.2010.02929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann, L., Rosenow, F., Strzelczyk, A., et al. (2023). The impact of referring patients with drug-resistant focal epilepsy to an epilepsy center for presurgical diagnosis. Neurol Res Pract, 5(1), 65. 10.1186/s42466-023-00288-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devinsky, O., Patel, A. D., Cross, J. H., et al. (2018). Effect of Cannabidiol on drop seizures in the Lennox-Gastaut syndrome. New England Journal of Medicine, 378(20), 1888–1897. 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 13.Thiele, E. A., Marsh, E. D., French, J. A., et al. (2018). Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet, 391(10125), 1085–1096. 10.1016/s0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- 14.Miller, I., Scheffer, I. E., Gunning, B., et al. (2020 Mar). Dose-Ranging effect of adjunctive oral Cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: A randomized clinical trial. JAMA Neurol, 2. 10.1001/jamaneurol.2020.0073 [DOI] [PMC free article] [PubMed]
- 15.Devinsky, O., Cross, J. H., Laux, L., et al. (2017). Trial of Cannabidiol for Drug-Resistant seizures in the Dravet syndrome. New England Journal of Medicine, 376(21), 2011–2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 16.Thiele, E. A., Bebin, E. M., Bhathal, H., et al. (2021). Add-on Cannabidiol treatment for Drug-Resistant seizures in tuberous sclerosis complex: A Placebo-Controlled randomized clinical trial. JAMA Neurol, 78(3), 285–292. 10.1001/jamaneurol.2020.4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lappe, L., Hertzberg, C., Knake, S., et al. (2024). A multicenter, matched case-control analysis comparing burden of illness among patients with tuberous sclerosis complex related epilepsy, generalized idiopathic epilepsy, and focal epilepsy in Germany. Neurol Res Pract, 6(1), 29. 10.1186/s42466-024-00323-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinhoff, B. J., Staack, A. M., & Hillenbrand, B. C. (2017). Randomized controlled antiepileptic drug trials miss almost all patients with ongoing seizures. Epilepsy & Behavior, 66, 45–48. 10.1016/j.yebeh.2016.10.025 [DOI] [PubMed] [Google Scholar]
- 19.Walker, M. C., & Sander, J. W. (1997). Difficulties in extrapolating from clinical trial data to clinical practice: The case of antiepileptic drugs. Neurology, 49(2), 333–337. [DOI] [PubMed] [Google Scholar]
- 20.Matricardi, S., Scorrano, G., Prezioso, G., et al. (2024). The latest advances in the Pharmacological management of focal epilepsies in children: A narrative review. Expert Review of Neurotherapeutics, 24(4), 371–381. 10.1080/14737175.2024.2326606 [DOI] [PubMed] [Google Scholar]
- 21.Tong, J., Ji, T., Liu, T., et al. (2024). Efficacy and safety of six new Antiseizure medications for adjunctive treatment of focal epilepsy and epileptic syndrome: A systematic review and network meta-analysis. Epilepsy & Behavior, 152, 109653. 10.1016/j.yebeh.2024.109653 [DOI] [PubMed] [Google Scholar]
- 22.Liu, S., He, Z., & Li, J. (2023). Long-term efficacy and adverse effects of Cannabidiol in adjuvant treatment of drug-resistant epilepsy: A systematic review and meta-analysis. Therapeutic Advances in Neurological Disorders, 16, 17562864231207755. 10.1177/17562864231207755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lattanzi, S., Trinka, E., Striano, P., et al. (2021). Highly purified Cannabidiol for epilepsy treatment: A systematic review of epileptic conditions beyond Dravet syndrome and Lennox-Gastaut syndrome. Cns Drugs, 35(3), 265–281. 10.1007/s40263-021-00807-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm, E., Altman, D. G., Egger, M., et al. (2007). Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bmj, 335(7624), 806–808. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheffer, I. E., Berkovic, S., Capovilla, G., et al. (2017). ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia, 58(4), 512–521. 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher, R. S., Cross, J. H., French, J. A., et al. (2017). Operational classification of seizure types by the international league against epilepsy: Position paper of the ILAE commission for classification and terminology. Epilepsia, 58(4), 522–530. 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 27.Specchio, N., Wirrell, E. C., Scheffer, I. E., et al. (2022). International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE task force on nosology and definitions. Epilepsia, 63(6), 1398–1442. 10.1111/epi.17241 [DOI] [PubMed] [Google Scholar]
- 28.Zuberi, S. M., Wirrell, E., Yozawitz, E., et al. (2022). ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE task force on nosology and definitions. Epilepsia, 63(6), 1349–1397. 10.1111/epi.17239 [DOI] [PubMed] [Google Scholar]
- 29.Northrup, H., Aronow, M. E., Bebin, E. M., et al. (2021). Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatric Neurology, 123, 50–66. 10.1016/j.pediatrneurol.2021.07.011 [DOI] [PubMed] [Google Scholar]
- 30.Strzelczyk, A., Schubert-Bast, S., von Podewils, F., et al. (2025). Real-world experience of Cannabidiol in conjunction with clobazam for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome: Results from a retrospective multicentre chart review in Germany. Epilepsy & Behavior, 166, 110302. 10.1016/j.yebeh.2025.110302 [DOI] [PubMed] [Google Scholar]
- 31.Beniczky, S., Trinka, E., Wirrell, E., et al. (2025). Updated classification of epileptic seizures: Position paper of the international league against epilepsy. Epilepsia,66(6),1804–1823. 10.1111/epi.18338 [DOI] [PMC free article] [PubMed]
- 32.Kühne, F., Becker, L. L., Bast, T., et al. (2023). Real-world data on Cannabidiol treatment of various epilepsy subtypes: A retrospective, multicenter study. Epilepsia Open, 8(2), 360–370. 10.1002/epi4.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espinosa-Jovel, C., Riveros, S., Bolanos-Almeida, C., et al. (2023). Real-world evidence on the use of Cannabidiol for the treatment of drug resistant epilepsy not related to Lennox-Gastaut syndrome, Dravet syndrome or tuberous sclerosis complex. Seizure, 112, 72–76. 10.1016/j.seizure.2023.09.015 [DOI] [PubMed] [Google Scholar]
- 34.Barnes, J. P., Dial, H., Owens, W., et al. (2024). Adherence and discontinuation of prescription Cannabidiol for the management of seizure disorders at an integrated care center. Epilepsy Research, 200, 107300. 10.1016/j.eplepsyres.2024.107300 [DOI] [PubMed] [Google Scholar]
- 35.Novy, J., Bartolini, E., Bell, G. S., et al. (2013). Long term retention of lacosamide in a large cohort of people with medically refractory epilepsy: A single centre evaluation. Epilepsy Research, 106(1-2), 250–256. [DOI] [PubMed] [Google Scholar]
- 36.Flores, L., Kemp, S., Colbeck, K., et al. (2012). Clinical experience with oral lacosamide as adjunctive therapy in adult patients with uncontrolled epilepsy: A multicentre study in epilepsy clinics in the united Kingdom (UK). Seizure-European Journal of Epilepsy, 21(7), 512–517. 10.1016/j.seizure.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 37.Marson, A. G., Al-Kharusi, A. M., Alwaidh, M., et al. (2007). The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: An unblinded randomised controlled trial. Lancet, 369(9566), 1000–1015. 10.1016/S0140-6736(07)60460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catarino, C. B., Bartolini, E., Bell, G. S., et al. (2011). The long-term retention of Zonisamide in a large cohort of people with epilepsy at a tertiary referral centre. Epilepsy Research, 96(1–2), 39–44. 10.1016/j.eplepsyres.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 39.Betts, T., Yarrow, H., Greenhill, L., et al. (2003). Clinical experience of marketed Levetiracetam in an epilepsy clinic-a one year follow up study. Seizure, 12(3), 136–140. [DOI] [PubMed] [Google Scholar]
- 40.Strzelczyk, A., Zaveta, C., von Podewils, F., et al. (2021). Long-term efficacy, tolerability, and retention of Brivaracetam in epilepsy treatment: A longitudinal multicenter study with up to 5 years of follow-up. Epilepsia, 62(12), 2994–3004. 10.1111/epi.17087 [DOI] [PubMed] [Google Scholar]
- 41.Willems, L. M., Zöllner, J. P., Paule, E., et al. (2018). Eslicarbazepine acetate in epilepsies with focal and secondary generalised seizures: Systematic review of current evidence. Expert Rev Clin Pharmacol, 11(3), 309–324. 10.1080/17512433.2018.1421066 [DOI] [PubMed] [Google Scholar]
- 42.Liguori, C., Santamarina, E., Strzelczyk, A., et al. (2023). Perampanel outcomes at different stages of treatment in people with focal and generalized epilepsy treated in clinical practice: Evidence from the PERMIT study. Frontiers in Neurology, 14, 1120150. 10.3389/fneur.2023.1120150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villanueva, V., Laloyaux, C., D’Souza, W., et al. (2023). Effectiveness and tolerability of 12-Month Brivaracetam in the real world: EXPERIENCE, an international pooled analysis of individual patient records. CNS Drugs, 37(9), 819–835. 10.1007/s40263-023-01033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberti, R., Assenza, G., Bisulli, F., et al. (2024). Adjunctive cenobamate in people with focal onset seizures: Insights from the Italian expanded access program. Epilepsia, 65(10), 2909–2922. 10.1111/epi.18091 [DOI] [PubMed]
- 45.Steinhoff, B. J., Georgiou, D., & Intravooth, T. (2024). The cenobamate KORK study-A prospective monocenter observational study investigating cenobamate as an adjunctive therapy in refractory epilepsy, with comparisons to historical cohorts treated with add-on lacosamide, perampanel, and Brivaracetam. Epilepsia Open, 9(4), 1502–1514. 10.1002/epi4.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lattanzi, S., Trinka, E., Zaccara, G., et al. (2022). Third-Generation Antiseizure medications for adjunctive treatment of Focal-Onset seizures in adults: A systematic review and network Meta-analysis. Drugs, 82(2), 199–218. 10.1007/s40265-021-01661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strzelczyk, A., von Podewils, F., Hamer, H. M., et al. (2025). Post-marketing experience with cenobamate in the treatment of focal epilepsies: A multicentre cohort study. CNS Drugs, 39(3), 321–331. 10.1007/s40263-025-01158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thieffry, S., Klein, P., Baulac, M., et al. (2020). Understanding the challenge of comparative effectiveness research in focal epilepsy: A review of network meta-analyses and real-world evidence on antiepileptic drugs. Epilepsia, 61(4), 595–609. 10.1111/epi.16476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azevedo, M., & Benbadis, S. R. (2023). Efficacy of highly purified Cannabidiol (CBD) in typical absence seizures: A pilot study. Epilepsy & Behavior, 149, 109512. 10.1016/j.yebeh.2023.109512 [DOI] [PubMed] [Google Scholar]
- 50.Roebuck, A. J., Greba, Q., Onofrychuk, T. J., et al. (2022). Dissociable changes in Spike and wave discharges following exposure to injected cannabinoids and smoked cannabis in genetic absence epilepsy rats from Strasbourg. European Journal of Neuroscience, 55(4), 1063–1078. 10.1111/ejn.15096 [DOI] [PubMed] [Google Scholar]
- 51.Strzelczyk, A., von Stuckrad-Barre, S., Kurlemann, G., et al. (2025). Off-label-Use von anfallssuppressiver und immunsuppressiver Medikation bei Epilepsien. Clinical Epileptology, 38(1), 54–62.. 10.1007/s10309-024-00735-z
- 52.Reddy, D. S. Therapeutic and clinical foundations of Cannabidiol therapy for difficult-to-treat seizures in children and adults with refractory epilepsies. Experimental Neurology. 2023 2023/01/01/;359:114237. 10.1016/j.expneurol.2022.114237 [DOI] [PubMed]
- 53.Osman, M., Khalil, J., El-Bahri, M., et al. (2024). Decoding epilepsy treatment: A comparative evaluation contrasting Cannabidiol pharmacokinetics in adult and paediatric populations. Chem Biol Interact, 394, 110988. 10.1016/j.cbi.2024.110988 [DOI] [PubMed] [Google Scholar]
- 54.Georgieva, D., Langley, J., Hartkopf, K., et al. (2023). Real-world, long-term evaluation of the tolerability and therapy retention of Epidiolex® (cannabidiol) in patients with refractory epilepsy. Epilepsy & Behavior, 141, 109159. 10.1016/j.yebeh.2023.109159 [DOI] [PubMed] [Google Scholar]
- 55.Iannone, L. F., Arena, G., Battaglia, D., et al. (2021). Results from an Italian expanded access program on Cannabidiol treatment in highly refractory Dravet syndrome and Lennox-Gastaut syndrome. Frontiers in Neurology, 12, 673135. 10.3389/fneur.2021.673135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaston, T. E., Ampah, S. B., Martina Bebin, E., et al. (2021). Long-term safety and efficacy of highly purified Cannabidiol for treatment refractory epilepsy. Epilepsy & Behavior, 117, 107862. 10.1016/j.yebeh.2021.107862 [DOI] [PubMed] [Google Scholar]
- 57.Lattanzi, S., Brigo, F., Trinka, E., et al. (2018). Efficacy and safety of Cannabidiol in epilepsy: A systematic review and Meta-Analysis. Drugs, 78(17), 1791–1804. 10.1007/s40265-018-0992-5 [DOI] [PubMed] [Google Scholar]
- 58.Lattanzi, S., Brigo, F., Trinka, E., et al. (2020). Adjunctive Cannabidiol in patients with Dravet syndrome: A systematic review and Meta-Analysis of efficacy and safety. CNS Drugs, 34(3), 229–241. 10.1007/s40263-020-00708-6 [DOI] [PubMed] [Google Scholar]
- 59.Talwar, A., Estes, E., Aparasu, R., et al. (2023). Clinical efficacy and safety of Cannabidiol for pediatric refractory epilepsy indications: A systematic review and meta-analysis. Experimental Neurology, 359, 114238. 10.1016/j.expneurol.2022.114238 [DOI] [PubMed] [Google Scholar]
- 60.Strzelczyk, A., & Schubert-Bast, S. (2022). Psychobehavioural and cognitive adverse events of Anti-Seizure medications for the treatment of developmental and epileptic encephalopathies. CNS Drugs, 36(10), 1079–1111. 10.1007/s40263-022-00955-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannon, T., Fernandes, K. M., Wong, V., et al. (2024). Over- and underreporting of seizures: How big is the problem? Epilepsia, 65(5), 1406–1414. 10.1111/epi.17930 [DOI] [PubMed] [Google Scholar]
- 62.Pigliasco, F., Cafaro, A., Barco, S., et al. (2024). Innovative LC-MS/MS method for therapeutic drug monitoring of Fenfluramine and Cannabidiol in the plasma of pediatric patients with epilepsy. Journal of Pharmaceutical and Biomedical Analysis, 245, 116174. 10.1016/j.jpba.2024.116174 [DOI] [PubMed] [Google Scholar]
- 63.Holtkamp, M., May, T.W., Berkenfeld, R. et al. (2024) Erster epileptischer Anfall und Epilepsien im Erwachsenenalter. Clin Epileptol,37, 118–139. https://doi.org/10.1007/s10309-024-00663-y 10.1007/s10309-024-00663-y
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available from the corresponding author upon reasonable request.
Not applicable.