Abstract
Increasing evidence suggested the multifactorial nature of nocturia, but the true pathogenesis of this condition still remains to be elucidated. Contemporary clinical medications are mostly symptom-based, either aimed at reducing nocturnal urine volume or targeting autonomic receptors within the bladder to facilitate urine storage. The day-night switch of the micturition pattern is controlled by circadian clocks located both in the central nervous system and in peripheral organs. Arousal threshold and secretion of melatonin and vasopressin increase at nighttime to achieve high-quality sleep and minimise nocturnal urine production. In response to the increased vasopressin, the kidney reduces glomerular filtration rate and facilitates reabsorption of water. Synchronously, in the bladder, circadian oscillation of crucial molecules occurs to reduce afferent sensory input and maintain sufficient bladder capacity during the night sleep period. Thus, nocturia might occur as a result of desynchronization in one or more of these circadian regulatory mechanisms. Disrupted rhythmicity of the central nervous system, kidney and bladder (known as the brain-kidney-bladder circadian axis) contribute to the pathogenesis of nocturia. Novel insights into the chronobiologic nature of nocturia will be crucial to promote a revolutionary shift towards effective therapeutics targeting the realignment of the circadian rhythm.
Introduction
Humans have a set of well-orchestrated mechanisms to prevent nocturia, defined as waking up to urinate during the primary sleep period.1-3 However, these intricate mechanisms are vulnerable during urological, nephrotic, neurological, endocrine, psychological and circulatory disturbances, causing nocturia and its related sequelae, including poor sleep, fatigue, reduced quality of life, depression or anxiety, bone fractures from falls, and increased risk of cardiovascular events and mortality.4-9 Results from a 2013 review of 43 studies reporting nocturia prevalence estimated that up to 20% of individuals aged 20–40 years old, and 60% of individuals > 70 years old need to void at least twice during the main sleep period.10 Results from several subsequent surveys of community-based or hospital-based populations reported that among middle-aged and older people (> 40 years old), 65–91% of men and 44–66% of women reported ≥2 voids per night despite some differences between the studies in definitions, outcome criteria, and ethnic representation of participants.11-15
In the past, nocturia has often been considered to be caused by an enlarged prostate or an overactive bladder (OAB) .16-18 This concept has been questioned based on evidence that removal of the prostate (by either transurethral resection or radical prostatectomy) or conventional therapies for OAB do not eradicate nocturia in a considerable proportion of patients, suggesting the involvement of other pathophysiological factors.19-21
Potential causes of nocturia
In general, nocturia might arise from one or more aetiologies including external triggers, changes in the rate of urine production during the sleep phase, or storage capacity of the urinary bladder.
Sleep disorders
A reciprocal relationship exists between sleep disorders and nocturia, as each of these conditions can be a trigger for the other.22,23 Frequent awakening arising from nocturia disrupts the normal sleep cycle, particularly the restorative slow-wave sleep phase, which worsens the quality-of-life. This effect particularly affects elderly patients, who show increasing fragmentation of the sleep cycle, which is a risk factor for some systemic disorders, such as cardiovascular and metabolic disorders .24-27 Nocturia is affected by external factors that disrupt the sleep cycle, such as obstructive sleep apnoea (OSA), insomnia, restless legs syndrome (also known as periodic limb movements), non-rapid eye movement parasomnias, or rapid eye movement sleep behaviour disorder.28 These factors can reduce the brain arousal threshold or the depth of sleep, disrupting nocturnal urine production.28,29 Furthermore, circadian dysregulation of these external factors can further fragment the sleep cycle, increasing the incidence of nocturia.
Much of the focus on primary sleep disorders and nocturia has been on the pathophysiology of OSA and nocturia. Increase in venous return induced by periodic raised inspiratory efforts associated with OSA is one mechanism that might act by inducing more negative intrapleural pressures, with a consequent increased release of atrial natriuretic peptide (ANP) and induction of a natriuresis.30,31 This hypothesis is corroborated by the observation that continuous positive airway pressure ventilation to mitigate against OSA reduces the incidence of nocturia.32
Nocturnal polyuria
Nocturnal polyuria is defined as an increase of nocturnal urine output by 20% in young adults (< 25 years old), by 20–33% in middle-age individuals (25–65 years old), and by 33% in those > 65 years old.2,33-35 Nocturnal polyuria is considered to be the major contributing factor to nocturia, accounting for 57– 64% of nocturia instances.36 Nocturnal polyuria is a heterogenous condition that can arise as a result of high free water and high sodium clearance rate, either alone or in combination.37 Nocturnal polyuria is associated with an array of systemic external factors including cardiovascular diseases, such as congestive heart failure and hypertension induced by salt retention; third-space fluid sequestration and dysregulated circadian secretion of arginine vasopressin (AVP, also known as antidiuretic hormone (ADH)); or renal tubular dysfunction.7,33,34,38 Nocturnal polyuria occurring in the absence of identifiable factors is known as nocturnal polyuria syndrome .37,39-42
Global polyuria
Global polyuria is defined as a 24-hour urine output ≥40 ml/kg.1 This condition might be caused by primary polydipsia, diabetes insipidus, pituitary tumours, hypopituitarism or some pharmaceuticals, such as diuretics, selective serotonin reuptake inhibitors, Ca2+ channel blockers, tetracycline, or lithium.41,43,44
Reduced nocturnal bladder capacity
The reservoir ability of the bladder can either be affected by a large residual volume, structural abnormalities (such as bladder outlet obstruction, bladder contracture, and fibrosis), or functional deficiencies (such as OAB, underactive bladder (UAB), cystitis, bladder pain syndrome (BPS), and neurogenic bladder). This condition is usually characterized by increased nocturnal voiding episodes with a reduced mean void volume.41,43,44
Current treatment of nocturia
The treatment of nocturia is usually tailored around existing pathophysiological changes, which are determined through clinical assessments including medical history, physical examination, bladder diary, laboratory tests, and imaging studies. Lifestyle advice and behavioural modifications are recommended to all patients as a first-line treatment.2,33,34,44-46 These modifications include restricting fluid consumption before sleep, which might help to minimize urine production;46,47 avoiding caffeine and alcohol intake, which could be beneficial in reducing bladder sensitivity;47-49 aerobic exercise to improve nighttime sleep quality;46,47 bladder retraining, which can restore functional bladder capacity;50 reducing peripheral oedema with compression stockings and leg elevation;51 and maintaining continuous positive airway pressure for patients with OSA .52
If no symptom remission is observed with these behavioural strategies, medications are needed to reduce nocturnal urine production. Diuretics might be used to facilitate the expulsion of accumulated fluid during daytime, whereas desmopressin (which is a synthetic replacement for AVP) might be effectively used to promote the reabsorption of water from the distal tubule and collecting ducts of the nephron during the night .2,41,43,44,46,53 Medications for OAB including antimuscarinics and β3-adrenergic agonists can also be prescribed to reduce bladder sensation and detrusor overactivity.53 Diuretics taken at bedtime increase nocturia, but in some patients, especially those with hypertension and lower limb oedema, taking diuretics in the afternoon effectively reduces nocturnal voids and urine volume, owing to the discharge of salt and water before sleeping.53,54 In male patients with an enlarged prostate and voiding dysfunction, α1-adrenergic antagonists, 5α-reductase inhibitors and phosphodiesterase-5 inhibitors can be useful to facilitate bladder emptying.2,41,43,44,46 These pharmaceuticals can be used alone or in combination, depending on each patient’s co-existing conditions.
Regulatory mechanisms of the circadian rhythm
Multiple therapeutic options are available for nocturia working either by reducing voiding episodes or nocturnal urine volume, but many patients remain refractory to treatment, mainly owing to the heterogeneous nature of nocturia pathogenesis. The aetiology of nocturia is multifactorial, and can be attributed to disruptions in the rhythms of sleep-wake cycles, urine production, and bladder capacity. Thus, nocturia can be understood by having a comprehensive perspective of rhythm disturbances. Results from studies on the circadian rhythmic nature of daily urine excretion in healthy children and young adults showed that up to 75% of total urine output occurs during the daytime, with a major peak between 6PM and 10PM.55,56 This rhythm is strikingly different in elderly women and men with nocturia, with a peak rate of diuresis shifting towards late hours, during the sleep phase of the cycle.57 Nocturia has been shown to have a chronobiological nature involving a set of autonomous but integrated circadian rhythmic mechanisms in the brain, kidney and bladder, known as the brain-kidney-bladder circadian axis . Sleep disorders, irregular lifestyle, neurodegenerative diseases and metabolic diseases are all associated with circadian disruption as well as nocturnal polyuria and increased urinary frequency . Thus, understanding the role of circadian axis disruptions as a potential cause of nocturia is imperative.2,58,59
In this Review, we introduce the molecular mechanisms of the circadian clock regulatory cycle and discuss how desynchronized circadian rhythms of the brain, kidney and bladder might contribute to nocturia.
Regulation of the circadian rhythm—transcription-translation feedback loop
The daily rhythm of human behaviour and physiology is regulated by the transcription-translation feedback loop (TTFL), which exists both in the brain and peripheral metabolic tissues (Figure 1).60-62 The TTFL mainly consists of a primary and a secondary cycle in which the transcription of promotive and repressive clock genes oscillate in an autoregulatory fashion with a repeated cycle of 24 hours in synchrony with the solar day.60,63-65 A master circadian pacemaker located in the hypothalamic suprachiasmatic nucleus (SCN) sychronizes the rhythm of other central nucleus components, whereas peripheral tissues set their own autonomous circadian clocks, which are integrated to synchronize their pace with the core clock (Figure 1).60,62,66
Figure 1. Transcription-translation feedback loop and the brain-kidney-bladder circadian axis.
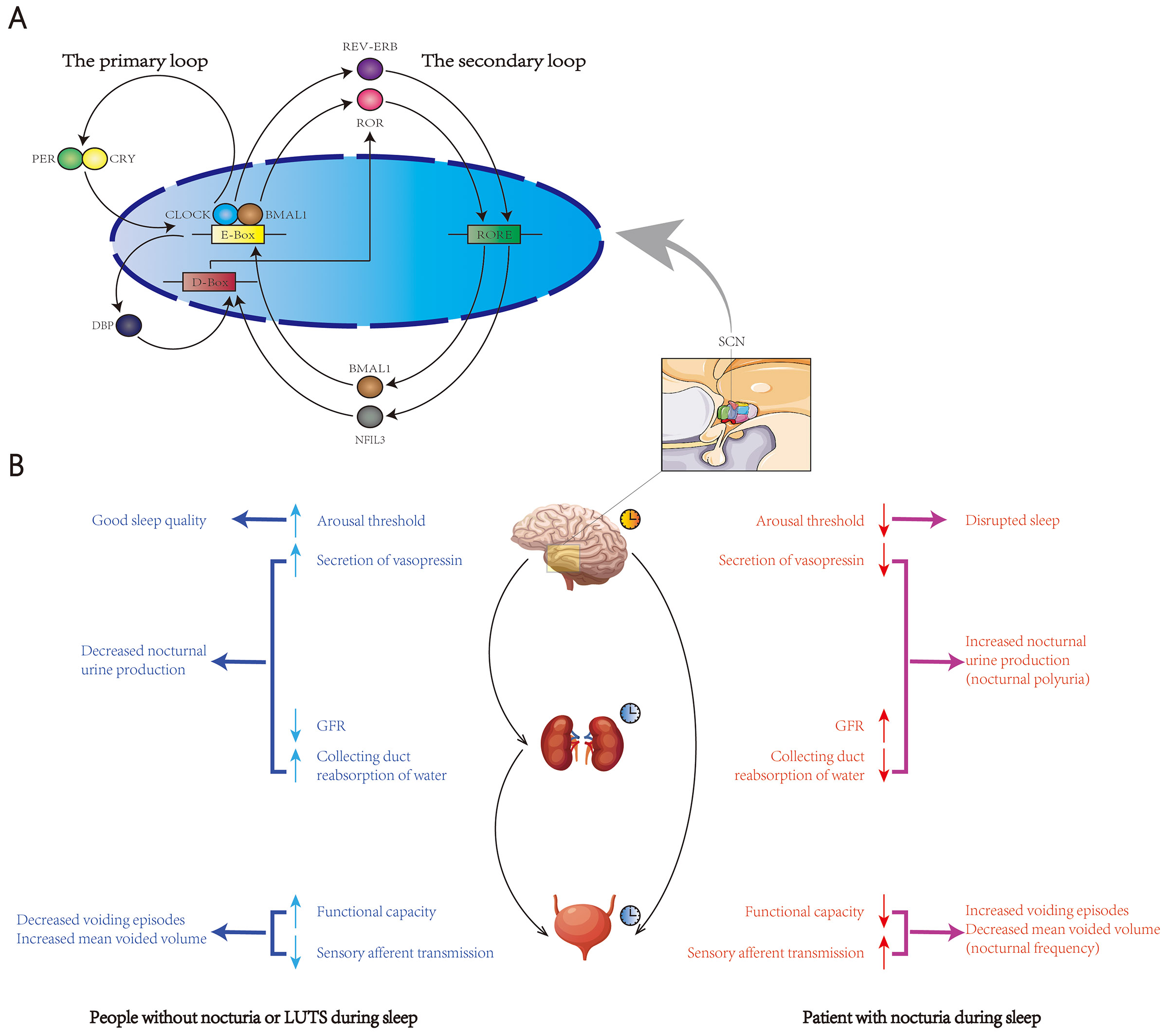
The central circadian pacemaker, located in the suprachiasmatic nucleus (SCN), is regulated by the transcription-translation feedback loop (TTFL), which mainly consists of a primary and a secondary loop (A). In the primary loop, circadian locomotor output cycles kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1) heterodimers bind to the E box of the promoters of the target genes period (PER) and cryptochrome (CRY) and induce their transcription. In a negative feedback fashion, the excessive production of PER and CRY proteins inhibits the transcriptional activity of CLOCK–BMAL1 and leads to the self-degradation of PER and CRY . Once the level of PER and CRY drop to sufficient levels, CLOCK— BMAL1-mediated transcription can be restarted. In the secondary loop, the CLOCK— BMAL1 complex also induces the expression of the nuclear receptors REV-ERB, retinoid-related orphan receptor (ROR), and D-box binding protein (DBP). REV-ERB and ROR repress and activate the transcription of BMAL1, respectively, through binding to the ROR/REV-ERB-response element (RORE). DBP activates D-box-dependent transcription, leading to the expression of ROR. This process can be inhibited by NFIL3, a transcriptional repressor that binds to D-box genes. NFIL3 transcription can be activated or repressed by the binding of REV-ERB and ROR to RORE, respectively. The TTFL also exists in multiple peripheral organs besides SCN. Nocturia has been shown to have a chronobiological nature involving a set of autonomous but integrated circadian rhythmic mechanisms in the brain, kidney and bladder, known as the brain-kidney-bladder circadian axis (B). In people without nocturia or LUTS during sleep (Ba) , the brain sets up a high arousal threshold during the main sleep period to avoid external and internal disturbance. Vasopressin is periodically secreted from the posterior pituitary gland to promote the reabsorption of water in the collecting duct of the nephron. To further reduce urine production, the kidney has its own circadian pattern with reduced glomerular filtration rate (GFR) during sleep. Bladder function also changes in a circadian fashion, with increased storage capacity and decreased sensory afferent transmission during main sleeping hours to minimize the voiding episodes. Nocturia (Bb) might arise from disruption in one or more of these circadian regulation patterns in the brain-kidney-bladder axis, which leads to sleep disorders, increased nocturnal urine production and reduced nocturnal bladder capacity.
In the primary loop, the active components of the TTFL are heterodimers of the transcription factors BMAL1 and CLOCK .60 The CLOCK—BMAL1 heterodimer binds to the E box of the cis-promoter and enhancer regions of the Period 1 (PER1), Period 2 (PER2), Cryptochrome 1 (CRY1) and Cryptochrome 2 (CRY2) genes, inducing transcription of these genes (Figure 1A).67 The accumulation of PER and CRY proteins to a certain concentration will lead to dimerization and translocation into the nucleus, where these proteins bind to CLOCK—BMAL1 complexes and repress CLOCK—BMAL1-mediated transcription .67 PER and CRY proteins also undergo a number of post-translational modifications, resulting in a proteasome-induced 24-hour rhythmic degradation , which reboots CLOCK—BMAL1-mediated transcription, keeping the cycle running.60
In the secondary loop, the CLOCK—BMAL1 complex induces transcription of the nuclear receptors retinoid-related orphan receptors (ROR, including RORα, RORβ and RORγ) and REV-ERB (REV-ERBα and REV-ERBβ; Figure 1A).60 REV-ERB and ROR subtypes compete for binding to REV-ERB—ROR response elements in the promoter and enhancer regions of target genes, with REV-ERB repressing and ROR activating transcription of BMAL1, respectively .67
Circadian rhythm-mediated regulation of physiological functions
The hierarchical organization of the circadian system, from the master pacemaker to extra-SCN clocks, regulates multiple physiological functions. For example, blood pressure follows a circadian pattern characterized by increased blood pressure during wakefulness and decreased blood pressure during sleep.68 In healthy individuals, plasma glucose tolerance also undergoes time-dependent variations, with an increase in the morning and a decrease in the evening, and is positively correlated with rhythmic pancreatic insulin secretion.69 Adipocytes have their own peripheral clock coordinated with the central pacemaker to regulate lipid metabolism by regulating the expression of several crucial enzymes for lipolysis, such as adipose triglyceride lipase, lipoprotein lipase and hormone-sensitive lipase.70
Some circadian regulation mechanisms are also involved in regulating the timing of urine production and voiding behaviour. For example, strengthening of synaptic connections following synaptic high-frequency stimulation, which leads to memory formation and long-term potentiation, is closely regulated through the circadian oscillation of proteins of the cyclic AMP—mitogen-activated protein kinase—cAMP responsive element-binding proteins (cAMP—MAPK—CREBP) pathway, as well as through circadian variation of N-methyl-D-aspartate receptor-evoked calcium current in central neurons.71-73 Both MAPK and glutamatergic signalling were shown to participate in bladder sensory and nociception input, suggesting potential circadian features of these molecules in regulating rhythmic bladder function.74-77
A bidirectional connection exists also between circadian genes and metabolic pathways, both in central and peripheral tissues. Glycogen and fatty acid synthesis peaks during the feeding period, whereas the plasma level of total amino acids and oxidative metabolism peak during fasting, and this regulation is primarily accomplished through circadian rhythm-dependent expression of crucial enzymes.63,65,78-80 For example, in glucose metabolism, peak levels of glucokinase and the hepatic glucose transporter protein GLUT2, which promote glucose storage by facilitating glycogenesis, coincide with feeding; whereas protein kinase A, which promotes glycogenolysis and gluconeogenesis to supply glucose, reaches expression peaks during fasting.63 The oscillatory homeostasis of glucose might in turn influence transcription of clock genes within SCN pacemaker neurons and communication pathways between SCN and extra-SCN neurons, as neuronal projections originating in the SCN directly synapse in the lateral hypothalamic area, which consists of neurons important in peripheral glucose metabolism.81 The adenosine monophosphate-dependent protein kinase sirtuin 1 and the mammalian target of rapamycin (mTOR) also participate in both glucose metabolism and circadian oscillations of SCI and extra-SCN neurons.81 Metabolic balance is an essential element in maintaining normal lower urinary tract function. Thus, circadian integration of metabolism can be hypothesized to be connected with nocturnal voiding behaviour.
Dysregulation of circadian rhythm in pathophysiological conditions
Off-cycle exposure to environmental cues can disrupt circadian homeostasis and have detrimental effects on human behaviour, including sleep-wake cycles and rest-activity rhythms, and health conditions. For instance, shift workers or travellers who experience rapid changes in time zones are prone to suffer from rhythmic desynchrony, which can produce a vicious cycle contributing to a series of metabolic, endocrine and cardiovascular disorders such as obesity, insulin resistance, disrupted cortisol rhythms and increased blood pressure.81-84 Similarly, sleep deprivation, poor sleep quality and narcolepsy substantially dampen the rhythmic expression of clock genes, and are associated with diabetes, metabolic syndrome and obesity.81,85,86 Exercise protocols with a specific timing can improve sleep quality and facilitate the adaptation to work shifts , in turn ameliorating negative metabolic consequences of this lifestyle .83 Inverted sleep-wake cycles and insomnia are both important risk factors for nocturia .28 Conversely, frequent nocturnal urination might contribute to dysregulated circadian sequalae, such as circadian blood pressure variation and depressive symptoms.5,87,88 Lengthening the first uninterrupted sleep period through oral intake of low-dose desmopressin might be beneficial to reduce blood glucose levels in patients with nocturia.89 Together, this evidence highlighted the role of dysregulated circadian rhythms as a contributing factor for nocturia and also as a potential target for treatment.28,90
Circadian rhythm disturbance is a manifestation of neurodegenerative diseases, and could also be a risk factor for developing Alzheimer’s disease and related dementia, as well as Parkinson’s disease.91 For example, patients with moderate-to-severe Alzheimer’s disease are prone to have inverted rest-activity patterns, increased levels of sleep fragmentation, and depleted secretion of melatonin, vasoactive intestinal polypeptide, arginine vasopressin and neurotensin, possibly owing to loss of crucial neuronal populations in the SCN.91,92 Conversely, results from several longitudinal studies with long-term follow-up monitoring suggested that elderly individuals (aged 65 years and older) with reduced amplitude of circadian rhythms and circadian phase shifts, such as less consolidated night-time sleep patterns, experienced increased daytime napping, and cognitive decline, preceding the risk of developing neurodegenerative diseases.93-96 Additionally, aberrant DNA methylation of BMAL1 and subsequent disruption of the rhythmic transcription of this gene were identified in post-mortem frontal cortex samples from patients with Alzheimer’s disease, indicating that epigenetic deregulation of circadian rhythms might contribute to cognitive impairment and neurodegeneration.97 Coherent with these results, Bmal1-deficient mice showed neurological phenotypes including severe spontaneous astrocyte proliferation, increased neuroinflammation, oxidative damage and synaptic degeneration, together with impaired functional brain connectivity, learning and memory.98 In addition to the reciprocal relationship between circadian disruption and neurodegenerative diseases, mice harbouring modifications in various types of clock genes showed changes in the circadian rhythm of urination, including disruptions in diurnal rhythms of neuroendocrine function, urine production, and bladder capacity (Table 1).99-103 This evidence suggests that disrupting clock gene expression might be associated with both neurodegenerative diseases and nocturia.
Table 1.
Clock gene abnormalities along the brain-kidney-bladder circadian axis in relation to nocturia
| Location | Clock genes | Proposed influence on nocturia |
|---|---|---|
| Brain | Bmal1 | Overexpression of Bmal1 obliterated the circadian rhythm of melatonin secretion.114 Paraventricular nuclei-specific Bmal1 ablation suppressed the expression of AVP during the daytime, resulting in a reduction in plasma AVP concentration.130 |
| Brain | Clock | The interactions between Clock and HIF-α induce the expression of AVP expression.144 |
| Brain | Per1 and Per2 | PER1 is rhythmically expressed in the pineal body as one of the regulators of melatonin synthesis.267,268 |
| Brain | Cry1 and Cry2 | Suppression of AVP expression and arrythmia were observed in Cry1 and Cry2 double-knockout mice.269 |
| Kidney | Bmal1 | Ablation of Bmal1 expression in renin-secreting granular cells disrupts the circadian pattern of renin protein expression in kidney tissue and results in a moderate reduction of plasma aldosterone levels.164 Deletion of Bmal1 in podocytes results in loss of circadian rhythmicity of glomerular filtration rate, along with disruption in the circadian patterns of urinary creatinine, Na+, K+, and water excretion, and diurnal pattern of plasma aldosterone levels.165 |
| Kidney | Clock | Clock-null mice showed loss of circadian rhythm in plasma aldosterone levels, as well as in urinary Na+, K+ and water excretion .159,162 |
| Kidney | Per1 and Per2 | ✓Per1 regulates several genes encoding crucial proteins involved in solute reabsorption along the nephron, including ENaC, Na+-Cl− co-transporter, the Na+-glucose co-transporter, and Na+-K+-ATPase positive regulator.168,170-172 Per1 and Per2 double knockout mice showed disturbed circadian rhythm in urine production.103 Following global or kidney-specific Per1 deletion, renal ET-1 gene expression and inner medullary ET-1 peptide levels increased, suggesting that Per1 negatively regulates the endothelin axis in the kidney.172,175 |
| Kidney | Cry1 and Cry2 | Global Cry1 and Cry2 deletion result in increased plasma aldosterone level and reduced circadian aldosterone oscillations.161 |
| Bladder | Bmal1 | The circadian rhythmic expression of Bmal1 is regulated by the glucocorticoid receptor signalling pathway in a concentration-dependent and time-dependent manner in the human urothelium.222 |
| Bladder | Clock | Clock-mutant mice lose circadian variations in urine volume voided per micturition.102 |
| Bladder | Per1 and Per2 | The circadian expression of intrinsic Per2 positively correlated with detrusor contractile response to carbachol, suggesting the participation of clock genes in rhythmic bladder function.203 Per2 expression and response to mechanical stimulation are substantially reduced with ageing, which might contribute to age-related bladder dysfunctions.203 |
| Bladder | Cry1 and Cry2 | Cry-null mice show no circadian rhythms in urine volume voided per micturition.101 |
| Bladder | Rev-Erbα | Rev-Erbα regulates the transcription of connexin 43, a gap junction protein responsible for diurnal variation in bladder sensitivity and capacity.101 |
AVP, arginine vasopressin; BMAL1, brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1; CLOCK, circadian locomotor output cycles kaput; CRY, cryptochrome; ENaC, epithelial sodium channel; ET-1, endothelin-1; PER, period.
Dysregulation of circadian rhythm of the central clock as a potential cause of nocturia
A central clock in the SCN controls the body's circadian rhythms and synchronizes peripheral clocks in most organs, tissues and cells through the autonomic nervous system, hormonal signals, and behavioural control.62 Disruption of the central clock in the central nervous system and neuroendocrine system can affect lower urinary tract function.
Many pathologies including cardiovascular disorders, pulmonary dysfunction and hepatic failure, as well as aging, are associated with increased nocturia,42,104-107 suggesting a common mechanism. Furthermore, in healthy elderly individuals, loss of circadian rhythmicity of AVP secretion, as well as increased plasma levels of natriuretic peptides increase nocturia.108-110 Desynchronization of circadian rhythmicity in the central nervous system has also been suggested as a potential contributor to nocturia in individuals with metabolic disorders or experiencing disruption of sleep.81,111
Circadian regulation of suprachiasmatic nucleus hormones in nocturia— melatonin and arginine vasopressin
The SCN acts as an intrinsic central clock with a period of ~24 hours, communicating with other brain centres and with peripheral tissues through the neuroendocrine system or through projections to both branches of the autonomic nervous system.62,66 Circadian rhythm controls specific functions of different tissues, but also general tissue metabolism and sleep, which in turn regulate SCN activity through a feedback loop.81,111 The extent of communication between the peripheral and the central clock depends on integration of input signals and environmental cues, which have the ability to reset the magnitude and phase of the central clock and, in turn, modulate output signals. SCN can be regulated by photic input from the retina and non-photic inputs.66
SCN receiving photic input from the retina enters a light-dark cycle , in which a synchronized SCN output pathway through the sympathetic superior cervical ganglion projects to the pineal gland to release the tryptophan derivative melatonin.62 Regulation by the central clock increases melatonin secretion in the dark phase. Among melatonin’s sites of action is also SCN, where melatonin binds to G-coupled MT1 and MT2 receptors, with the effect of promoting sleep and fatigue-like states and, ultimately, reducing urine output.112-114 Slow-release melatonin preparations, such as circadin or MT1 and MT2 receptor agonists, have been used for nocturia treatment in several clinical trials.115 As observed in a study in rats, the possible mechanism of action of melatonin in this context could be the activation of the inhibitory γ-aminobutyric acid receptor, leading to increased bladder capacity and decreased urine volume.116,117 Results from a cross-sectional observational study showed higher urinary 6-sulfatoxymelatonin concentration in the elderly population (≥60 years old) with nocturia than in age-matched individuals without nocturia, suggesting an inverse correlation between melatonin secretion and nocturia.118 Thus, melatonin supplement could be a potential therapeutic option, as shown in a randomized clinical trial in which 2 weeks of oral intake of melatonin (2mg/day) significantly alleviated nocturia (−1.0 (−3.0– 0.0) versus 0.0 (−2.3– 1.3) episodes per night; p < 0.001) and increased median duration of the first uninterrupted sleep ( 1.0 (−0.3– 4.5) versus 0.0 (−3.0– 2.3) hours; p < 0.001) compared with patients receiving placebo .119 Similarly, in patients with neurological conditions such as Parkinson’s disease, treatment with melatonin significantly reduced nocturia episode (P=0.013) and symptom scores (P=0.01).120. However, in another trial including patients with multiple sclerosis, a similar dosage of circadin (2mg/day), did not significantly alter nocturia episodes (1.4/night after melatonin treatment compared with 1.6/night in the placebo group; P=0.85), suggesting that further validation is needed to determine the clinical value of this therapy.121
AVP has a dual role in the context of controlling urine output. AVP is synthesized in magnocellular neurons of the hypothalamic supraoptic and paraventricular nuclei and is transported along neurons in the hypothalamic-hypophyseal tract to the posterior pituitary gland, from where AVP is secreted in response to changes of plasma osmolality.122 Secreted AVP regulates water reabsorption at the collecting duct through increasing cell surface expression of aquaporin-2 channels on the cell membrane of collecting duct cells.123 AVP release follows a pronounced circadian rhythmicity, especially in young individuals (with a mean age of 25 years old), with a nadir in the late afternoon and a peak at night110; thus, the anti-diuretic effect of AVP helps to regulate nocturnal urine volume.124 Additionally, ~20% of SCN neurons contain AVP-positive neurons, which show circadian rhythmic AVP mRNA expression with a peak during daytime, and also contain the E-box element in the AVP promoter region (the target of CLOCK and BMAL1 transcription factors).125,126 The SCN then outputs to the magnocellular neurosecretory cells in the paraventricular nucleus through AVP-immunoreactive synapses, which synchronize the central clock and circadian AVP humoral release.126,127 Thus, disruption of clock-guarding genes and circadian activity would affect circadian pattern of plasma AVP levels, causing dysregulated water reabsorption and increased urine output during the sleep period. Proper AVP secretion and regulation is, therefore, important for normal circadian urinary function, and disruption of these processes can cause urinary dysregulation leading to nocturia.128-130
Circadian misalignment during sleep disorders and central nervous system hypoxia in nocturia
Results from several studies have shown that reduced sleep quality, assessed objectively or subjectively, is associated with increased incidence of nocturia.131,132 Furthermore, shift workers experience an increased incidence of nocturia.133,134 Sleep deprivation generates increased salt and water excretion at night, mainly related to dysregulation of the renin-angiotensin-aldosterone system (RAAS).135 Disrupted sleep quality, even increasing overall sleep duration, is also associated with nocturia.136 However, responses to stress through dysregulation of circadian rhythms can be variable, making it difficult to identify causative mechanisms. The idea that some individuals experience high sleep reactivity (which refers to the degree of sleeping difficulty under stress) has been proposed as an explanation for the variability of disordered sleep patterns in relation to stress exposure, but how sleep reactivity influences the variability in the incidence of nocturia remains to be explored.137
The relationship between OSA and nocturia has been extensively studied. OSA is a common condition induced by intermittent collapse of the upper respiratory tract during sleep, accompanied by periods of hypoxemia, and is considered as a risk factor for nocturia secondary to nocturnal polyuria.30,138 Furthermore, OSA and nocturia share dominant demographic risk factors, such as advancing age and high BMI.139 Thus, not surprisingly, treatment of OSA with continuous positive airway pressure ventilation substantially reduces nocturia episodes.139 However, the relationship between OSA and nocturia is not exclusive, as nocturnal polyuria is independently associated with changes in circadian variation of extracellular fluid volume.140 Additionally, chronic obstructive pulmonary disease with concomitant hypoxemia is also associated with a prevalence of nocturia as high as that reported with OSA. Overall, ~15% of patients with chronic obstructive disease have OSA as a co-morbidity, and this condition is known as overlap syndrome.107 The incidence of nocturia is significantly higher in patients with the overlap syndrome (63.5%) than in patients with OSA alone (63.5% versus 58.0%, P<0.01) , but patients with the overlap syndrome are also substantially older (mean age of 63.5 and 56.9 years old in the overlap syndrome and the OSA groups, respectively).107 Thus, to date, whether the presence of any of these two pulmonary disorders is an additional risk factor for nocturia for patients with either disorder alone is unclear.
A hallmark of tissue hypoxia is the activation of hypoxia-inducible factors (HIFs).141 HIFs are a family of transcription factors that form dimers consisting of an oxygen-regulated subunit (HIF-α) and a constitutively expressed subunit (HIF-β) , and encode genes that lead to restoration of tissue normoxia.141 HIF-α and clock proteins are members of the same transcription factor superfamily and together can synergistically enhance the expression of target genes, and can also generate circadian rhythm misalignment as a result of hypoxia variations at different times of the day.142,143 Specifically, in the SCN, HIF-α and CLOCK cooperate to promote transcription of AVP .144 Hypoxia was hypothesized to desynchronize the circadian rhythm of AVP secretion, which is increased in the sleep phase normally, but results from human studies were conflicting. In a small study including 4 patients with post-stroke nocturia and nocturnal polyuria, the normal circadian rhythm of plasma AVP concentration was absent, as measured every 4 hours from 8AM to 8AM the next morning.106 However, in another study including patients with and without OSA, nocturnal secreted AVP, assessed through a single early morning urinary AVP measurement (normalized to the creatinine value) taken at 6AM, was similar in both groups (6.7pg/ml/Cre in OAS group versus 6.8 pg/ml/Cre in non-OSA group, P=0.36).109 Large trials are needed to determine the link between diurnal AVP secretion, hypoxemia and nocturnal polyuria .
Dysregulation of circadian rhythm of renal function as a potential cause of nocturia
Most physiological renal processes follow a circadian pattern of activity, such as urine secretion and water reabsorption, in response to the rhythmic fluctuation of blood pressure, electrolyte and hormones.145,146 Disturbance of the renal circadian rhythms is increasingly recognized as a risk factor for nocturia, suggestive of nocturnal polyuria.
Physiology of rhythmic renal function
In healthy humans, glomerular filtration rate (GFR) oscillates between 120ml/min and 90 ml/min, reaching a peak in the waking phase at ~2–3PM, and a minimum during the middle of the sleep phase.147 Na+, K+ and Cl− excretions peak between 9AM and 12PM, and then progressively decrease during the night time.148 Urine production follows a similar pattern, with increased volumes during the day and lowest during the night .146 Regulators of salt and water excretion, such as the hormones AVP and aldosterone, show a circadian pattern of activity.146 AVP plasma levels are higher at night than during the day, which helps to regulate nocturnal urine volume.124 Aldosterone is secreted in response to variations in blood pressure and volume, as well as in plasma Na+ levels, and primarily regulates Na+ reabsorption through the epithelial sodium channel (ENAC) in the distal nephron.149 In humans, plasma aldosterone levels show a diurnal pattern that parallels that of GFR, peaking in the first half of the day and reducing to a nadir in the middle of the night.150
This rhythmicity of renal function was initially thought to be a reaction of the kidneys to control water and solute balance in the body and maintain homeostasis in response to changes in circadian activity or changes in food and water intake.151 This view was challenged by evidence that renal excretory rhythms can still persist for several days after periodic behavioural activity was switched , or fluid and food intake were controlled, or in conditions in which diurnal activity and feeding cycles were inverted.152-156 This evidence generated the hypothesis that the rhythmicity of renal function is a self-sustained mechanism that might enable the kidney to anticipate the predictable circadian challenges to homeostasis.151 The molecular basis of this mechanism remained elusive until the discovery of the mammalian circadian clock system.
Potential molecular mechanisms underlying rhythmic renal function
Hundreds of putative clock-controlled genes have been identified throughout the nephron.157 Rhythmic transcription has been identified for several crucial genes involved in renal salt and water transport, including genes encoding ion channels and receptors, such as the Na+/H+ exchanger-3 (NH3, encoded by SLC9A3), the α-subunit of epithelial Na+ channels (α-ENaC, encoded by SCNN1A), aquaporins (AQP1, AQP2, and AQP3) and renal AVP receptors (V1AR and V2R).146,158
Disruption of the circadian clock through genetic ablation of different clock genes results in profound changes to the kidney transcriptome and to the rhythmicity and regulation of renal function.151,157-160 For example, in mice with global Cry1 and Cry2 deletion, plasma aldosterone levels were significantly increased at both circadian time 0 and 12 (P<0.01), and circadian aldosterone oscillations were enhanced compared with wild-type mice 161 Clock-null mice also showed disruption in the circadian rhythm of plasma aldosterone levels, reduction of circadian rhythmicity of urinary Na+, K+ and water excretion, and a substantial reduction in blood pressure.159,162 Results from other studies showed that Clock-null mice also had altered circadian rhythm of 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis in the kidney, leading to both a delayed shift in the acrophase (peak point of the cell cycle) and reduced average 24-hour levels of 20-HETE .162 Endogenous 20-HETE, released into the renal microcirculation , is a powerful regulator of renal tonus, Na+ reabsorption and K+ secretion, and modulates both the myogenic and tubuloglomerular feedback-mediated regulation of glomerular filtration.163 Results from studies in mice in which clock genes were selectively deleted in the kidney further highlighted the influence of the circadian clock on the modulation of the expression of RAAS components and other regulatory mechanisms.164 Specifically, ablation of Bmal1 in renin-secreting granular cells in mice disrupted the circadian pattern of renin protein expression in kidney tissue and resulted in a moderate reduction of plasma aldosterone levels.164 Further analysis of these mice showed increased GFR and urine volume and changes in the circadian rhythm of urinary Na+ excretion, suggesting that local renal circadian clocks control body fluid homeostasis.164
Ablation of Bmal1 in podocytes (which are highly specialized glomerular cells essential for protein retention during glomerular filtration) in mice resulted in loss of circadian rhythmicity of GFR.165 In these mice, disruption of GFR circadian rhythmicity was accompanied by substantial changes to the circadian patterns of urinary creatinine, Na+, K+, and water excretion, and by a dysregulation of the diurnal pattern of plasma aldosterone levels165. These findings highlighted the importance of peripheral kidney circadian clocks in regulating renal function oscillations. Conversely, in another study, the ablation of Bmal1 in renal tubular cells in mice did not result in obvious abnormalities in renal Na+, K+ or water handling.166 The fact that renal excretory rhythms were not affected by the inactivation of the molecular clock in renal tubular cells indicates that these rhythms are mainly regulated by external circadian time cues. One of these cues is probably diurnal oscillations in aldosterone levels. Aldosterone-mediated regulation of renal tubular Na+ reabsorption involves multiples mechanisms that include regulation of ENAC expressed on the apical membrane of principal cells in the collecting duct.167 SCNN1, the gene encoding α-ENaC, is regulated by PER1.168 Results from an in vivo study in Per1 knockout mice have shown downregulation in ENaC expression, along with increased concentration of sodium in the urine and reduced blood pressure compared to wild-type mice. 168 . PER1 expression is positively regulated by aldosterone.168 Upregulated PER1 expression was observed in an aldosterone-stimulated renal collecting duct cell line and in the kidney of aldosterone-treated rats through mineralocorticoid and glucocorticoid receptor-mediated signalling.169 Together, evidence that PER1 is regulated by aldosterone and regulates ENaC suggest a role for PER1 in mediating the downstream effects of aldosterone on ENaC in the renal collecting duct cells (Figure 2).168 PER1 also regulates the expression of several other genes encoding crucial proteins involved in solute reabsorption along the nephron (Figure 2), including the Na+-Cl− co-transporter (NCC, encoded by SLC12a3) and its regulatory kinases with-no-lysine kinases 1 and 4 (WNK1 and WNK4), the NHE3 and the Na+-glucose co-transporter (SGLT) , the Na+-K+-ATPase positive regulator FXYD domain containing ion transport regulator 5 (FXYD5), and the negative regulators of ENaC caveolin 1 and ubiquitin-conjugating enzyme E2 E3 (UBE2E3).168,170-172
Figure 2. PER1-mediated circadian control of the nephron.
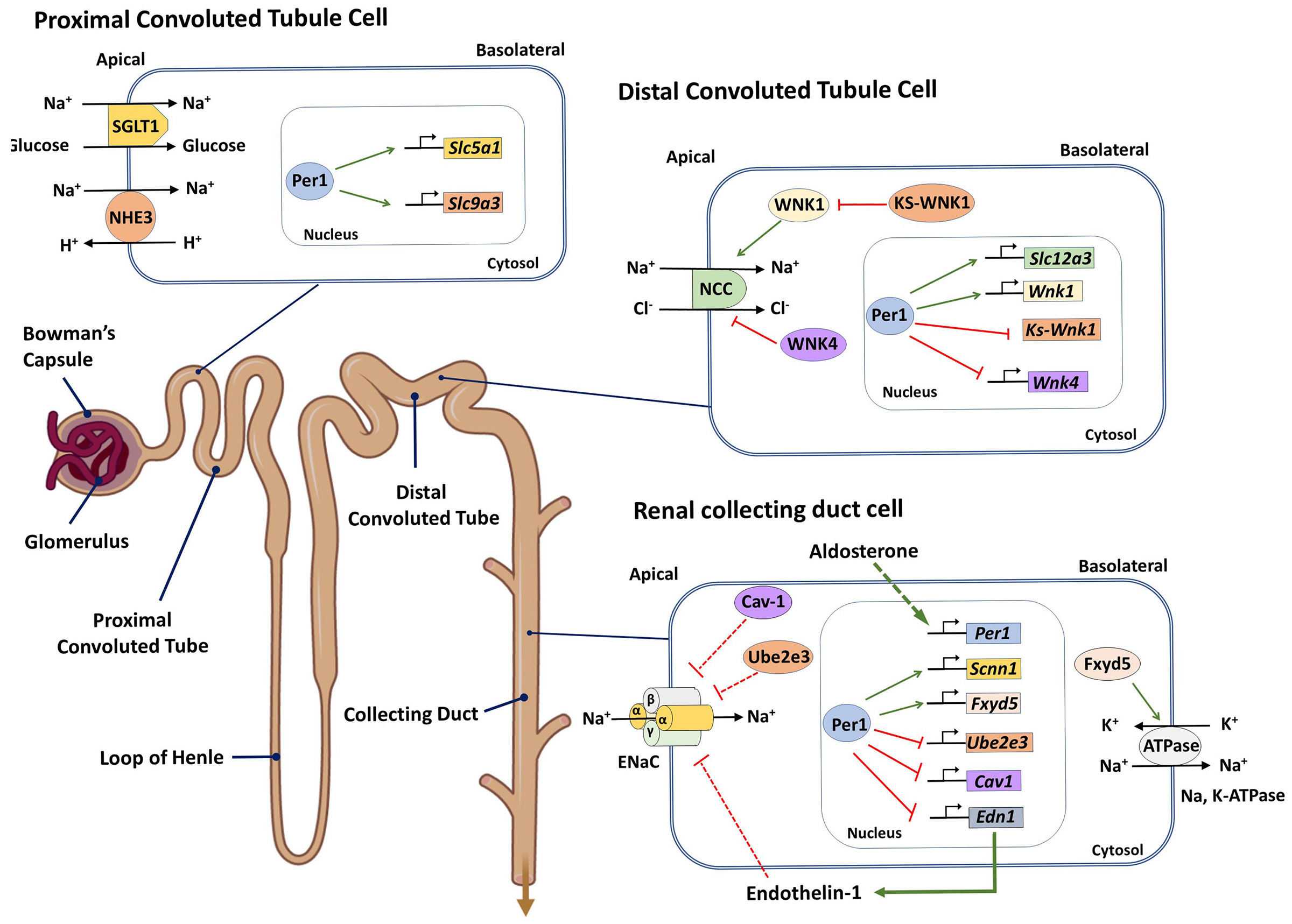
Period 1 (PER1) was shown to be involved in the transcriptional regulation of genes encoding crucial proteins modulating solute reabsorption along the nephron. In proximal tubule cells, PER1 positively regulates the expression of SLC5a1 and SLC9a3 (encoding the sodium-glucose co-transporter 1 (SGLT1) and Na+/H+ exchanger 3 (NHE3), respectively)171. In distal tubule cells, PER1 regulates the expression levels of SLC12a3 (encoding the Na-Cl co-transporter (NCC)), and of genes encoding components of the with-no-lysine kinase (WNK) pathway, such as WNK1, WNK4 and KS-WNK1 (a kidney-specific WNK1 isoform), which regulate NCC activity133. In collecting duct cells, PER1 regulates the expression of the Na, K-ATPase positive regulator FXDY5, in turn modulating this Na, K-ATPase activity. Additionally, PER1 regulates the activity of the epithelial sodium channel (ENaC), both by regulating the expression of SCNN1, which encodes the α-ENaC subunit, and of UBE2e3 and caveolin-1 (CAV1), which are negatively regulators of ENaC. PER1 also negatively regulates the expression of END1, encoding Endothelin-1, which is a negative regulator of ENaC. In the collecting duct cells, PER1 is also positively regulated by aldosterone and mediates the downstream effects of aldosterone on ENaC .
The endothelin-1 (ET-1) axis in the kidney is also influenced by PER1 (Figure 2).172,173 Endothelin regulates Na+ handling in the collecting duct mainly through the activation of ETB receptors.174 These receptors inhibit renal tubular Na+ reabsorption and promote Na+ excretion in response to an increased NaCl intake, through MAPK-mediated ENaC phosphorylation, which reduces both ENaC activity and number .175 In mice with global or kidney-specific Per1 deletion in the distal nephron and collecting duct, renal Et-1 expression and inner medullary Et-1 peptide levels increased, suggesting that Per1 negatively regulates the endothelin axis in the kidney.146,158,172,175 Coordination of these opposite effects of aldosterone and ET-1 on Na+ handling in the kidney is another example of how circadian clock regulates and drives the rhythmicity of renal function.
Potential role of desynchronized renal rhythmicity in nocturnal polyuria
In healthy individuals, nocturnal polyuria commonly develops with ageing and is the major factor contributing to nocturia.176 With advancing age, circadian rhythms of diuresis-regulating hormones are dysregulated, and the concentrating capacity and the ability of the kidney to retain Na+ are reduced. 108,110,177-179 Similar to hormones, several genes involved in renal salt and water transport also show a circadian pattern of expression and activity that influences renal rhythmicity.146,158 Changes in the expression of clock genes have also been reported in the ageing mouse kidney. 177,180 Dysregulation of renal rhythmicity involving changes in the circadian patterns of diuresis-regulating hormones or downstream molecular mediators of renal salt and water transport warrant consideration as potential factors in the aetiology of nocturnal polyuria in healthy ageing adults.
Results from studies including healthy young and old adults have clearly shown that ageing alters the secretory rhythm of hormones that regulate salt-water balance.181,182 For example, results from an observational study including old adults (>60 years old) with and without nocturia showed that the circadian rhythm of AVP secretion was attenuated or lost in individuals with nocturia whose diurnal variation in urine output was disrupted.181 Gender differences in AVP levels during ageing have also been reported in a clinical study, with higher AVP plasma concentration observed in males than in females, suggesting a greater response in AVP and renin-angiotensin system sensitivity in men. .183 The plasma levels of ANP, which could also be implicated in nocturnal polyuria through increasing renal Na+ excretion, in turn suppressing AVP release, and decreasing RAAS activity ,184 have been shown to be higher in elderly (65–75 years old) than in young (20–25 years old) adults.178 In the same study, a clear circadian pattern of plasma ANP with an acrophase early in the afternoon was also shown in young but not in elderly individuals, indicating that the inhibitory action of ANP on the circadian phasic increase of renin, aldosterone, and cortisol is lost with ageing .178 However, in a study including age-matched old men (mean age: 62.8–63.3 years old) with nocturnal polyuria, with nocturia not associated with nocturnal polyuria, and without nocturia, no differences in plasma renin and aldosterone levels were shown among the three groups.185 Results from a study including men ≥60 years old with or without nocturnal polyuria also showed no differences in AVP levels between the two groups.186 Moreover, the use of desmopressin to treat nocturnal polyuria in elderly patients showed limited effect and does not always alleviate the symptoms.187 These findings indicate that loss or attenuation of circadian rhythms for diuresis-regulating hormones cannot fully explain the nocturnal shift in urine production in healthy old adults, and highlight the contribution of other factors in altering the rhythmicity of water and solute excretion.
Age-related changes in the circadian clock regulation of the kidney transcriptome also contributes to nocturnal polyuria and nocturia. In a study including young (6 months), old (18 months), and aged (27 months) male mice, an age-associated decline in the number of rhythmically expressed genes (REGs) was shown across various tissues, with the largest change in REGs observed in the kidney (75% decline from young to old mice).180 Notably, ageing was shown to blunt the oscillatory expression patterns of the Scnn1a and Atp1a1 genes, which encode the alpha subunits of ENaC and Na+/K+-ATPases, respectively.180 Loss of diurnal pattern of expression of these genes affects the renal circadian rhythmicity of Na+ and K+ handling, leading to increased nocturnal Na+ excretion and contributing to nocturnal polyuria .37 A decline in clock genes Per1 and Per2 rhythmicity was also observed in the aging mouse kidney.177,180 Thus, age-related decline in renal PER1 rhythmicity might have a role in driving changes in the diurnal pattern of Na+ secretion that contributes to nocturnal polyuria, even when aldosterone levels remain unaltered.
Desynchronization of renal rhythmicity might also have a role in nocturnal polyuria in other contexts. For example, in old men (mean age :61.1 years old) with nocturia, decreased day-to-night ratios of diuresis are associated with increased nighttime mean arterial pressure, which could be responsible for nocturnal polyuria, as essential hypertension has also been implicated in the pathophysiology of nocturnal polyuria.188-190 Dysregulation of water and electrolyte excretion rhythms was shown to be closely associated with abnormal blood pressure and a non-dipping pattern (which refers to a loss of nocturnal decrease in blood pressure during sleep) during nighttime.191,192 In one of these studies, the enhanced nocturnal natriuresis and reduced fall in blood pressure in non-dippers were normalized by dietary Na restriction, suggesting the importance of circadian urinary sodium exertion in maintaining variation of blood pressure.191 In other studies, dietary Na restriction was also shown to reduce nocturnal polyuria in patients with excessive salt intake, as well as in renal allograft recipients, indicating that restoring circadian natriuresis through lifestyle modification could be effective as a behavioural therapy for nocturnal polyuria .193,194 These findings, combined with the evidence that Cry-null mice develop salt-sensitive non-dipper hypertension,161 suggest the involvement of the peripheral circadian clock and a potential role for desynchronization of renal rhythmicity in the pathogenesis of nocturnal polyuria.
Dysregulation of circadian rhythm of the bladder as a potential cause of nocturia
In coordination with the central clock, the bladder expresses its own peripheral clock genes in a circadian fashion, which leads to time-dependent variation in bladder sensation and excitability.
Physiology of diurnal and nocturnal bladder function
Humans have a diurnal variation in the amount of urine volume voided per micturition, which increases at night and often reaches a maximum value during the first urine in the morning.195,196 Mice and rats also show a diurnal variation in voided volumes, indicating the existence of common underlying mechanisms of this process in mammals.101,102,197-202 A diurnal rhythm in detrusor smooth muscle responses to stimulation by carbachol was shown in isolated mice bladder samples , with peak contractile activity at 12 hours, which correlates with the intrinsic peak level of Per2, suggesting that muscarinic-mediated bladder contractility might undergo circadian control .203 In guinea-pigs, diurnal differences were also observed in the effects of stroking and stretch on the activity of bladder low-treshold and high-threshold muscular mucosa and afferent nerves, with higher response observed during the day than at night .204 In humans, maximum urine flow rates were shown to be reduced overnight and early in the morning .196,205-207 Together, these findings suggest that the bladder has a diurnal rhythm that shifts toward storing urine during the inactive (sleep) period and expelling urine during the active (awake) period. Cystometric and flowmetric data including video sequences from implantable telemetric monitors in miniature pigs showed day-to-night differences in urinary frequency, possibly as a result of the circadian rhythmic detrusor contractility and mechanosensory input.208 However, in organ bath studies using mouse bladder strips, the contractile response to an electrical field stimulation set to directly stimulate the detrusor smooth muscle did not show a circadian pattern in muscle contraction.203,209 Thus, the diurnal variation in urine flow rates might require intricated coordination between central and peripheral circadian rhythmic mechanisms, rather than depend on the contractile properties of an isolated organ.
Potential molecular mechanisms underlying rhythmic bladder function
Increasing evidence suggests that the peripheral bladder clock is involved in the generation of diurnal rhythmicity of bladder function. Results from early studies showed the circadian rhythmic expression of clock genes in the bladder and led to identification of a group of genes whose expression vary diurnally, introducing the idea of clock-mediated regulation of genes involved in bladder storage and voiding function.101,210 Connexin 43 (CX43), a gap junction protein with major roles in intercellular signalling and modulation of bladder hypersensitivity, contributes to the diurnal variation of bladder capacity, and is also transcriptionally regulated by the clock gene REV-ERBα.101
Results from subsequent studies showed that urothelial regulation of bladder function is also influenced by the molecular clock (Figure 3).100,197,211-214 In addition to a barrier function, the urothelium has a central role in mechanisms of bladder sensation and perception of the extent of bladder fullness.215,216 In mouse bladder mucosa, some molecular mediators of the urothelial response to mechanical stimulation, including the mechano-sensor Piezo1; the mechano-sensitive transient receptor potential cation channel subfamily V member-4 (TRPV4) receptor; the connexin 26 (CX26) channel; and the vesicular nucleotide transporter (VNUT); were shown to undergo diurnal fluctuation.213,214,217 In primary cultures of mouse urothelial cells, TRPV4 and Piezo1 were also shown to be involved in the circadian rhythm of stretch-induced changes in intracellular Ca2+ concentration .212
Figure 3. Putative role of the urothelium in the physiological diurnal rhythm of bladder capacity (modified from ref 196).
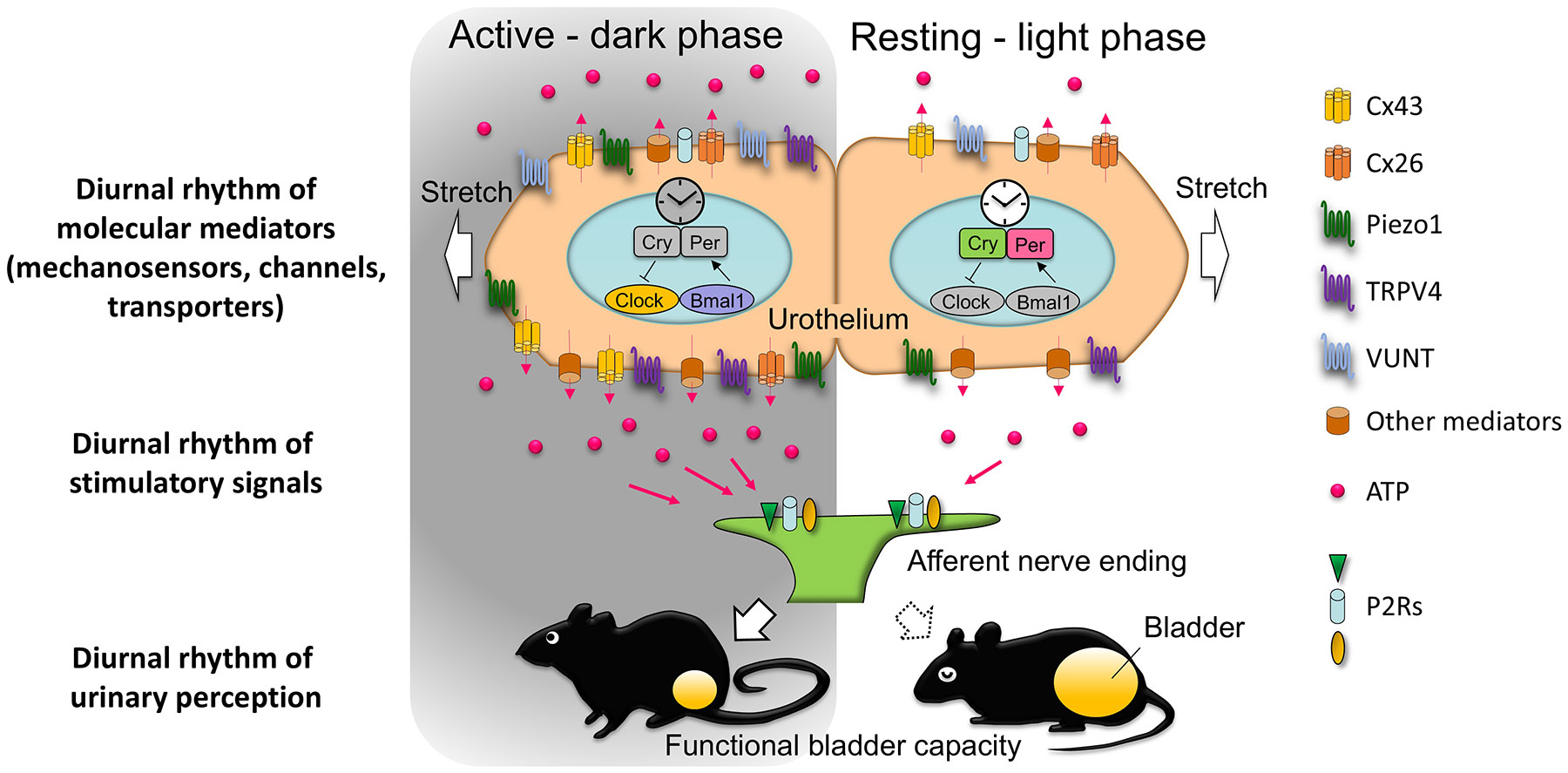
The circadian modulation of bladder function by the peripheral bladder clock is shown. The diurnal rhythm of molecular mediators, including CX43, CX26, VNUT, and mechanosensors such as Piezo1 and TRPV4, regulates ATP release in response to bladder distension, influencing urinary sensation and bladder capacity. During the active (dark) phase, the expression of CX43, CX26 and VNUT peaks, facilitating increased ATP release through the haemichannels formed by CX43 and CX26. This process leads to increased bladder sensitivity and modulation of bladder capacity. The mechanosensors Piezo1 and TRPV4 also show diurnal fluctuations, contributing to the circadian control of ATP release. Released ATP activates P2 receptors on afferent nerves, mediating the bladder’s sensory response to distension.
In the resting (light) phase, the expression of these mediators decreases, resulting in reduced ATP release and reduced bladder excitability, supporting time-dependent regulation of bladder function in alignment with the circadian cycle.
Abbreviations: Cx:26, connexin 26; Cx43, connexin 43; TRPV4, transient receptor potential cation channel subfamily V member 4; VNUT, vesicular nucleotide transporter; P2Rs, purinergic type 2 receptors.
The urothelium responds to bladder distension by releasing important urothelial mechanotransmitters, including ATP.215,216 Growing evidence suggests a circadian rhythmic pattern of purinergic signalling, which might contribute to periodically changes in bladder excitability..218 Within the bladder, ATP release into the lumen has a diurnal pattern, and urothelial CX43 has been shown to have a role in this pattern, possibly through forming hemichannels to enable cellular ATP efflux.100 Systemic Cx43 heterozygous mice showed larger functional bladder capacity than wild-type mice both in active and inactive phases as measured through the automated voided stain-on-paper technique.101 Mice with a urothelium-specific Cx43 deletion showed a larger bladder capacity than wild-type mice, with less luminal ATP release in response to bladder distention only during the active phase.197 The expression of CX43 in the urothelium follows a circadian rhythm and peaks during the active phase 100, 197 Thus, these findings suggest that CX43 in the urothelium has a role in perception and regulation of responses to bladder distension in the active phase, possibly through altering the number of CX43 hemichannels that mediate ATP release.100
Understanding how the peripheral clock of the bladder is tuned independently from CNS regulation is an important issue.219 The major entrainers of the peripheral clock vary in different organs and tissues; for example, in the liver, major entrainers of functional rhythm are dietary factors, as evidenced by data showing that restricted feeding can rapidly shift the liver’s rhythm by 10 hours, with the presence of an intact central clock .220 Mechanisms underlying synchronization of the peripheral clock involve hormones such as glucagon and growth factors such as IGF-1, which are responsive to dietary intake and help synchronize the liver's clock to metabolic signals, overriding cues from the central clock.221 In the bladder, local activation of muscarinic and purinergic receptors was shown to influence the peripheral bladder clock by shifting peak expression of PER2 to an earlier time, indicating that local receptors could regulate bladder rhythms.203 Glucocorticoids have been identified as synchronizing agents of the bladder clock.222 In immortalized human urothelial cells, glucocorticoids were shown to modulate the circadian expression of BMAL1 in a concentration-dependent and time-dependent manner .222 In adrenalectomized mice, treatment with corticosterone in in the early sleep period, which is the opposite phase of corticosterone physiological peak, resulted in an 8–12 hour shift in the expression of the major clock genes and loss of diurnal rhythm of volume voided per micturition.222 In a study in mice, peripheral clocks were also shown to function in the urethral sphincter and in the L4/L5 spinal cord, whereas no rhythm of clock gene expression in the periaqueductal grey matter and pontine micturition centre was reported.223 Results from these studies suggest that excitatory receptors, neurotransmitters and hormones could regulate the rhythmic pattern of peripheral clock genes. The diurnal variation in the micturition pathway might also be related to diurnal differential regulation of bladder function. Studying this regulatory mechanism is a topic for future research.
The role of disrupted rhythmic bladder function in nocturia
In mice with disrupted biological clocks, such as Cry-null and Clock mutant mice, no circadian rhythms were observed in urine volume voided per micturition, indicating that bladder function is influenced by the circadian clock .101,224 Under normal physiological conditions, diurnal rhythms generated by the circadian clock system gradually decline in function with ageing.225 In mice, a decrease of Per2 expression in the bladder and loss of normal Per2 response to local activation of muscarinic and purinergic receptors was observed with ageing.203 In a study including patients with or without OAB symptoms , higher urinary ATP concentration was observed in in patients with OAB than in patients without OAB and the ratio of ATP-to-creatine positively correlated with age.226 These results could explain the evidence that both prevalence of OAB and severity of nocturnal frequency increase with age,227-229 suggesting that these phenomena could be possibly ascribable to the loss of clock gene expression rhythmicity and the disruption of diurnal variation of mechanosensory receptors.
A relationship between disruption of the bladder clock and diurnal rhythmicity of bladder function has also been reported in pathological mouse and rat models .200-202,230 Restraint-stressed mice and spontaneously hypertensive rats showed altered rhythms of TRPV4, Piezo1, and CX26 expression in the bladder, which were accompanied by a decrease or loss of diurnal variations of bladder capacity, indicating that systematic disorders might affect voiding behaviour by influencing gene expression rhythms within the bladder .200-202 In Dahl salt-sensitive rats, salt loading resulted in altered expressions of clock genes in the bladder and increased bladder capacity, which were partially restored when salt intake was reduced, indicating a close relationship between lifestyle, peripheral clock, and circadian voiding pattern.230 Thus, increasing evidence suggest a role for the bladder clock in generation of a diurnal rhythm of bladder capacity, and a potential relationship between disruption of clock-regulated bladder capacity and nocturia. As knowledge in this area increases, major advances are expected in the development of novel chronotherapies for nocturia.
Future research on chronotherapies
The disruption of one or more components within the brain-kidney-bladder circadian axis has an essential role in the onset of nocturia. Thus, understanding the interrelated molecular basis of circadian regulation and dysregulation is imperative to develop strategies for prevention, management and treatment of nocturia based on chronobiology.
Chronotherapies based on environmental and behavioural changes.
Some environmental and lifestyle modifications are known to alter circadian timing, contributing to a variety of health conditions.231,232 For example, the master clock is mostly regulated by ambient light perceived by the retina;233 thus, non-invasive light therapy might be effective in maintaining and resynchronizing circadian rhythmicity.233,234 This hypothesis is supported by clinical evidence showing that timed light exposure for critically ill patients in intensive care units normalised the circadian phase of physiological behaviour (as determined by the urinary 6-sulfatoxymelatonin rhythm), as well as prevented and shortened postoperative delirium episodes.235-238 The feeding-fasting cycle in humans also follows a natural circadian pattern, and irregular feeding behaviour can reset many circadian clocks in peripheral endocrine glands and metabolic organs, exerting deleterious effects on metabolic health and energy homeostasis.239 Consequently, in men with prediabetes (with both elevated HbA1c levels and impaired glucose tolerance), time-restricted feeding (consisting of a limited food access period during the active phase) was shown to be effective in treating type II diabetes through improving insulin sensitivity and β cell responsiveness,240 and also in preventing other metabolic disorders, such as obesity, cardiovascular events, hepatic steatosis, and hypercholesterolemia .241 Moreover, in elder adults with chronic insomnia, scheduled behavioural activity (such as aerobic physical exercise )was shown to regulate rhythms and cause phase shifts, in turn improving sleep quality, mood, and quality of life.242-245
Lifestyle interventions are considered to be the first-line approach in the management of nocturia. These modifications include limiting drinking before sleep, encouraging physical exercise and restricting salt intake. Further evidence is needed to confirm the relevance of timed fluid consumption, timed physical activity, and timed sleep-waking patterns in relieving nocturia and modifying circadian control mechanisms.
Chronotherapies to optimise drug efficacy
A high number of drug targets and metabolizing enzymes are expressed in a circadian fashion; thus, chronotherapy approaches can be used to optimize drug efficacy .59,231,232,246,247 The absorption, distribution, metabolism and elimination of drugs are largely affected by rhythmic changes in liver metabolism and renal glomerular filtration that are regulated by clock genes .247 Thus, circadian-controlled delivery of therapeutics might be developed according to the biological rhythms of different conditions to achieve maximum efficacy and minimum adverse effects. For example, timed melatonin therapy has been proposed to rectify the circadian misalignment owing to jet lag and shiftwork, as melatonin given in the late biological afternoon or early biological morning (over a 24-hour cycle) resulted in advanced and delayed circadian rhythms, respectively.248 The hepatotoxicity of cyclophosphamide, a medication used as chemotherapy for cancer and autoimmune diseases, is also time-dependent, and is attributed to diurnal oscillations of the expression and metabolism of cyclophosphamide-metabolizing enzymes (such as CYP2B10), which have been shown to be controlled by CLOCK.249 Myeloid cells were shown to adhere to atherosclerotic lesions in a circadian fashion, and timed pharmacological neutralization of the CCL2—CCR2 axis (a signalling pathway that triggers chemokine adhesion and immobilization on arterial vessels) during the peak of arterial myeloid cell recruitment inhibits atherosclerosis without disturbing microvascular recruitment.250 Similarly, chronotherapeutic schedules of several anticancer agents has shown benefit in improving tolerability and reducing cytotoxicity towards healthy cells without jeopardizing tumour control.251
Considering the role of central and peripheral circadian rhythm disruption in the pathogenesis of nocturia, chronotherapeutic approaches can be established in the future. Future research should focus on developing novel medications and modulate their pharmacokinetics to reach peak plasma concentration during the main sleep period to reduce urine production and increase bladder storage functional capacity during sleep.
Chronotherapies targeting manipulation of the central oscillators
Direct manipulation of the TTFL using small-molecule modulators is an intriguing approach to regulate central oscillators. For example, stabilizing CRY expression could lengthen the period and reduce the amplitude of circadian oscillators. Treatment with central circadian clock modulators, such as KL001, SHP656, KL101, TH301 and KS15, was shown to improve glucose tolerance in patients with diabetes ,252 as well as enhance brown adipocyte differentiation in vitro253 and inhibit cell proliferation of glioblastoma stem cells and breast cancer cells, indicating potential of these therapies in future clinical applications .254,255 Moreover, chemicals that modulate the secondary feedback loop in the clock cycle have also been shown to potentiate circadian wheel running activity and clock gene oscillation and, therefore, might restore metabolic homeostasis in several behavioural and metabolic diseases .232 For example, selective agonists of REV-ERB (SR0990 and SR9011) have been shown to restore glucose metabolism, reduce diet-induced obesity, and ameliorate anxiety through altering circadian behaviour and targeting core clock gene (BMAL1, PER1 and PER2) expression in the hypothalamus of mice, suggesting a promising role for REV-ERB agonists in resynchronizing rhythms of behaviour and metabolic processes .256,257 Additionally, nobiletin, a direct ROR agonist, improves metabolic homeostasis and protects against inflammation and atherosclerosis through enhancing the amplitude and lengthening the period of circadian rhythms, as evidenced by the remodelling of clock gene expression observed in both PER2::Luc reporter cells and transgenic mice receiving nobiletin treatment.258-260
In summary, small-molecule modulators targeting central and peripheral clock genes might have great potential in reversing metabolic, behavioural and attentional disorders, although safety and effectiveness of these agents still remain to be explored in clinical trials.
Future perspectives of chronotherapies in nocturia
To date, none of the available regulators of clock oscillators, behavioural chronotherapies, or circadian-controlled dosing modalities have been shown to have a clear effect in the treatment of nocturia. In a restraint-stress mouse model, the disrupted circadian voiding pattern accompanied by clock gene abnormalities could be reversed through oral administration of PF670462, an inhibitor of the enzyme responsible for PER phosphorylation, indicating that the modulation of clock genes could be a potential therapeutic approach for nocturia .201 In a subsequent study from the same group, the inhibitory effects of GsMTx4, a Piezo1 inhibitor, were shown to change in a circadian rhythmic fashion.261 In mice, both a low dose (0.75mg/kg) of GsMTx4 given during the nadir expression of Piezo1 and a high dose (1.5mg/kg) of GsMTx4 given during the zenith expression of Piezo1 significantly (P<0.01) reduced voiding frequency , suggesting that the time of medication might affect the therapeutic effect .261 In a study in which metabolomic analysis was carried out on urine samples from elderly men aged 65–80 years, increased urinary serotonin, and decreased levels of 3-hydroxypropionic acid (a metabolite of beta-alanine metabolism involved in psychological disorders) and 3-indoleacetonitrile (a metabolite of tryptophan metabolism involved in metabolic syndrome pathogenesis) were shown to be highly associated with nocturia, suggesting redressing the circadian regulation of these metabolic pathways as a potential treatment for nocturia .262 Additional evidence from studies in mice showed that serum fatty acid metabolites fluctuate with circadian rhythm in wild-type mice, but the fluctuations are absent in mice with a Clock gene mutation.263 Specifically, the loss of regular circadian rhythm regulation of fatty acid metabolism led to increased serum levels of palmitoylethanolamide, which activates the pontine micturition centre and induces nocturia through activating G protein-coupled receptor 55 in the urothelium.264 Together, these results suggest that regulators of fatty acid metabolic rhythms during the sleep phase might be a potential chronotherapy for nocturia. From a clinical perspective, initial results from a 2023 clinical trial including patients with nocturia ≥ 50 years old showed that treatment with a mixture of nobiletin and tangeretin extracted from Citrus depressa peels significantly reduced nighttime voiding frequency (0.5 times, p=0.04) compared with placebo.265 However, no significant improvement was shown in nocturnal bladder capacity and nocturnal polyuria index (P=0.61 and P=0.3, respectively). In another pilot study to investigate the effect of hormonal therapy on voiding patterns and renal circadian rhythms in postmenopausal women, oestrogen treatment for 3 months did not significantly change nocturnal parameters calculated from a frequency-volume chart (P=0.056 for number of nocturnal voids; P=0.6 for mean nocturnal voided volume; P=0.2 for nocturnal urine volume), although the same hormonal treatment restored circadian rhythm of free water clearance (increased during daytime and decreased during nighttime) in post-menopausal women with nocturnal polyuria .266 Considering the ambiguous results from existing studies, future studies are needed to define the most appropriate circadian medications, treatment dosage, and delivery modality to treat nocturia.
Conclusions
Coordination of the brain-kidney-bladder circadian axis is essential for normal lower urinary tract function, and disruptions of this axis can result in nocturia. Increasing evidence shows the existence of common mechanisms among clock gene regulation, sleep behaviour, urine production and voiding patterns, highlighting the potential benefits of chronotherapies for nocturia. However, many questions remain unsolved. For example, the feasibility of using circadian behaviour therapy— including timed feeding, sleeping and light exposure— to reduce nocturia should be investigated. Additionally, whether modulators of clock genes could be safely used to manipulate bladder sensitivity and capacity individually for each patient remains to be assessed. Lastly, the interrelated connections among bladder clock genes, rhythmic expression of neurotransmitters and bladder function need to be identified. Understanding the crosstalk among components within the brain-kidney-bladder circadian axis will be valuable to discover safe, effective and cost-efficient therapeutics to resynchronize the clock for patients with nocturia.
Key points.
Growing evidence has shown that disrupted rhythmicity of the central nervous system, kidney and bladder (the brain-kidney-bladder circadian axis) contributes to the pathogenesis of nocturia.
The daily rhythm of human behaviour and physiology is regulated by the transcription-translation feedback loop, which exists both in the brain and in peripheral metabolic tissues, consisting of opposite transcriptional activators (CLOCK and BMAL1) and repressors (PER and CRY).
Disruption of the central clock in the suprachiasmatic nucleus and neuroendocrine system leads to nocturia through impaired sleep quality and misaligned release of hormones such as melatonin and arginine vasopressin.
Most physiological renal processes, such as urine secretion and water reabsorption, follow a circadian pattern of activity; disruptions of this pattern can cause nocturia.
The circadian expression of peripheral clock genes in the bladder leads to time-dependent variations of bladder sensation and excitability, which can be disorganized under pathophysiological conditions contributing to nocturia onset.
Expanding knowledge of the molecular basis of circadian regulation and dysregulation within the brain-kidney-bladder circadian axis will help developing strategies for prevention, management and treatment of nocturia based on chronobiology.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Hashim H. et al. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol. Urodyn 38, 499–508, doi: 10.1002/nau.23917 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Dani H, Esdaille A & Weiss JP Nocturia: aetiology and treatment in adults. Nat Rev Urol 13, 573–583, doi: 10.1038/nrurol.2016.134 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Hashim H & Drake MJ Basic concepts in nocturia, based on international continence society standards in nocturnal lower urinary tract function. Neurourol. Urodyn 37, S20–S24, doi: 10.1002/nau.23781 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa H. et al. Impact of Nocturia on Bone Fracture and Mortality in Older Individuals: A Japanese Longitudinal Cohort Study. J Urol 184, 1413–1418, doi: 10.1016/j.juro.2010.05.093 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Obayashi K, Saeki K, Negoro H & Kurumatani N Nocturia increases the incidence of depressive symptoms: a longitudinal study of the HEIJO-KYO cohort. BJU Int 120, 280–285, doi: 10.1111/bju.13791 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Funada S. et al. Impact of Nocturia on Mortality: The Nagahama Study. J Urol 204, 996–1002, doi: 10.1097/JU.0000000000001138 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Bliwise DL, Wagg A & Sand PK Nocturia: A Highly Prevalent Disorder With Multifaceted Consequences. Urology 133S, 3–13, doi: 10.1016/j.urology.2019.07.005 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Azuero J. et al. Potential associations of adult nocturia. Results from a national prevalence study. Neurourol Urodyn 40, 819–828, doi: 10.1002/nau.24624 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Lazar JM et al. Nocturia is Associated with High Atherosclerotic Cardiovascular Disease Risk in Women: Results from the National Health and Nutrition Examination Survey. J Community Health 46, 854–860, doi: 10.1007/s10900-021-00962-9 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Bosch JLHR & Weiss JP The Prevalence and Causes of Nocturia. Journal of Urology 189, S86–S92, doi: 10.1016/j.juro.2012.11.033 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Malde S. et al. Incidence of Nocturia in Men with Lower Urinary Tract Symptoms Associated with Benign Prostatic Enlargement and Outcomes After Medical Treatment: Results from the Evolution European Association of Urology Research Foundation Prospective Multinational Registry. Eur Urol Focus 7, 178–185, doi: 10.1016/j.euf.2019.07.003 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Song QX et al. The characteristics and risk factors of healthcare-seeking men with lower urinary tract symptoms in China: Initial report from the POInT group. Neurourol Urodyn 40, 1740–1753, doi: 10.1002/nau.24737 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Daugherty M, Ginzburg N & Byler T Prevalence of Nocturia in United States Women: Results From National Health and Nutrition Examination Survey. Female Pelvic Med Reconstr Surg 27, e52–e58, doi: 10.1097/SPV.0000000000000792 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Dutoglu E. et al. Nocturia and its clinical implications in older women. Arch Gerontol Geriatr 85, 103917, doi: 10.1016/j.archger.2019.103917 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Bower WF et al. The association between nocturia, hormonal symptoms and bladder parameters in women: an observational study. BJOG 129, 812–819, doi: 10.1111/1471-0528.16752 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Song Q, Abrams P & Sun Y Beyond prostate, beyond surgery and beyond urology: The "3Bs" of managing non-neurogenic male lower urinary tract symptoms. Asian J Urol 6, 169–173, doi: 10.1016/j.ajur.2017.11.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapple CR et al. Lower Urinary Tract Symptoms Revisited: A Broader Clinical Perspective. Eur. Urol 54, 563–569, doi: 10.1016/j.eururo.2008.03.109 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Abrams P. New words for old: lower urinary tract symptoms for "prostatism". BMJ 308, 929–930, doi: 10.1136/bmj.308.6934.929 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haga N. et al. Postoperative urinary incontinence exacerbates nocturia-specific quality of life after robot-assisted radical prostatectomy. Int J Urol 23, 873–878, doi: 10.1111/iju.13163 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Seki N, Yuki K, Takei M, Yamaguchi A & Naito S Analysis of the prognostic factors for overactive bladder symptoms following surgical treatment in patients with benign prostatic obstruction. Neurourol Urodyn 28, 197–201, doi: 10.1002/nau.20619 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Smith AL & Wein AJ Outcomes of pharmacological management of nocturia with non-antidiuretic agents: does statistically significant equal clinically significant? BJU International 107, 1550–1554, doi: 10.1111/j.1464-410X.2010.09972.x (2011). [DOI] [PubMed] [Google Scholar]
- 22.Araujo AB et al. Sleep related problems and urological symptoms: testing the hypothesis of bidirectionality in a longitudinal, population based study. J Urol 191, 100–106, doi: 10.1016/j.juro.2013.07.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negoro H. et al. Risk analyses of nocturia on incident poor sleep and vice versa: the Nagahama study. Sci Rep 13, 9495, doi: 10.1038/s41598-023-36707-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djavan B, Milani S, Davies J & Bolodeoku J The Impact of Tamsulosin Oral Controlled Absorption System (OCAS) on Nocturia and the Quality of Sleep: Preliminary Results of a Pilot Study. European Urology Supplements 4, 61–68, doi: 10.1016/j.eursup.2004.12.001 (2005). [DOI] [Google Scholar]
- 25.Schneider T & Stanley N Impact of Nocturia on Sleep and Energy. European Urology Supplements 6, 585–593, doi: 10.1016/j.eursup.2007.01.004 (2007). [DOI] [Google Scholar]
- 26.Hirshkowitz M Normal human sleep: an overview. Medical Clinics of North America 88, 551–565, doi: 10.1016/j.mcna.2004.01.001 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Stanley N. The physiology of sleep and the impact of ageing. European Urology Supplements 3, 17–23, doi: 10.1016/S1569-9056(05)80003-X (2005). [DOI] [Google Scholar]
- 28.Papworth E. et al. Association of Sleep Disorders with Nocturia: A Systematic Review and Nominal Group Technique Consensus on Primary Care Assessment and Treatment. Eur Urol Focus 8, 42–51, doi: 10.1016/j.euf.2021.12.011 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Umlauf MG & Chasens ER Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev 7, 403–411, doi: 10.1053/smrv.2002.0273 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Umlauf MG et al. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep 27, 139–144, doi: 10.1093/sleep/27.1.139 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Doyle-McClam M, Shahid MH, Sethi JM & Koo P Nocturia in Women With Obstructive Sleep Apnea. Am J Lifestyle Med 15, 260–268, doi: 10.1177/1559827618782657 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McInnis RP, Dodds EB, Johnsen J, Auerbach S & Pyatkevich Y CPAP Treats Enuresis in Adults With Obstructive Sleep Apnea. J Clin Sleep Med 13, 1209–1212, doi: 10.5664/jcsm.6776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss JP & Everaert K Management of Nocturia and Nocturnal Polyuria. Urology 133S, 24–33, doi: 10.1016/j.urology.2019.09.022 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Tyagi S & Chancellor MB Nocturnal polyuria and nocturia. Int Urol Nephrol 55, 1395–1401, doi: 10.1007/s11255-023-03582-5 (2023). [DOI] [PubMed] [Google Scholar]
- 35.van Kerrebroeck P. et al. The standardisation of terminology in nocturia: Report from the standardisation sub-committee of the International Continence Society. Neurourol. Urodyn 21, 179–183, doi: 10.1002/nau.10053 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Weiss JP, van Kerrebroeck PE, Klein BM & Norgaard JP Excessive nocturnal urine production is a major contributing factor to the etiology of nocturia. J Urol 186, 1358–1363, doi: 10.1016/j.juro.2011.05.083 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Goessaert AS, Krott L, Hoebeke P, Vande Walle J & Everaert K Diagnosing the pathophysiologic mechanisms of nocturnal polyuria. Eur Urol 67, 283–288, doi: 10.1016/j.eururo.2014.09.003 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Hervé F. et al. Is our current understanding and management of nocturia allowing improved care? International Consultation on Incontinence-Research Society 2018. Neurourol. Urodyn 38, 9–7, doi: 10.1002/nau.23961 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Emeruwa CJ, Epstein MR, Michelson KP, Monaghan TF & Weiss JP Prevalence of the nocturnal polyuria syndrome in men. Neurourol Urodyn 39, 1732–1736, doi: 10.1002/nau.24403 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Drangsholt S. et al. Diagnosis and management of nocturia in current clinical practice: who are nocturia patients, and how do we treat them? World J Urol 37, 1389–1394, doi: 10.1007/s00345-018-2511-4 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Gulur DM, Mevcha AM & Drake MJ Nocturia as a manifestation of systemic disease. BJU International 107, 702–713, doi: 10.1111/j.1464-410X.2010.09763.x (2011). [DOI] [PubMed] [Google Scholar]
- 42.Ohishi M, Kubozono T, Higuchi K & Akasaki Y Hypertension, cardiovascular disease, and nocturia: a systematic review of the pathophysiological mechanisms. Hypertens Res 44, 733–739, doi: 10.1038/s41440-021-00634-0 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Everaert K. et al. International Continence Society consensus on the diagnosis and treatment of nocturia. Neurourol. Urodyn 38, 478–498, doi: 10.1002/nau.23939 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Fine ND, Weiss JP & Wein AJ Nocturia: consequences, classification, and management. F1000Res 6, 1627–1627, doi: 10.12688/f1000research.11979.1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drangsholt S. et al. Diagnosis and management of nocturia in current clinical practice: who are nocturia patients, and how do we treat them? World Journal of Urology, 1–6, doi: 10.1007/s00345-018-2511-4 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Gordon DJ, Emeruwa CJ & Weiss JP Management Strategies for Nocturia. Curr Urol Rep 20, 75, doi: 10.1007/s11934-019-0940-2 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Yap TL, Brown C, Cromwell DA, van der Meulen J & Emberton M The impact of self-management of lower urinary tract symptoms on frequency-volume chart measures. BJU Int 104, 1104–1108, doi: 10.1111/j.1464-410X.2009.08497.x (2009). [DOI] [PubMed] [Google Scholar]
- 48.Robinson D, Giarenis I & Cardozo L You are what you eat: the impact of diet on overactive bladder and lower urinary tract symptoms. Maturitas 79, 8–13, doi: 10.1016/j.maturitas.2014.06.009 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Robinson D, Hanna-Mitchell A, Rantell A, Thiagamoorthy G & Cardozo L Are we justified in suggesting change to caffeine, alcohol, and carbonated drink intake in lower urinary tract disease? Report from the ICI-RS 2015. Neurourol Urodyn 36, 876–881, doi: 10.1002/nau.23149 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Majumdar A, Hassan I, Saleh S & Toozs-Hobson P Inpatient bladder retraining: is it beneficial on its own? Int Urogynecol J 21, 657–663, doi: 10.1007/s00192-009-1085-5 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Kaga K. et al. The Efficacy of Compression Stockings on Patients With Nocturia: A Single-Arm Pilot Study. Cureus 14, e28603, doi: 10.7759/cureus.28603 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyazato M. et al. Effect of continuous positive airway pressure on nocturnal urine production in patients with obstructive sleep apnea syndrome. Neurourol Urodyn 36, 376–379, doi: 10.1002/nau.22936 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Sakalis VI et al. Medical Treatment of Nocturia in Men with Lower Urinary Tract Symptoms: Systematic Review by the European Association of Urology Guidelines Panel for Male Lower Urinary Tract Symptoms. Eur Urol 72, 757–769, doi: 10.1016/j.eururo.2017.06.010 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Garrison SR et al. Tolerability of bedtime diuretics: a prospective cohort analysis. BMJ Open 13, e068188, doi: 10.1136/bmjopen-2022-068188 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hillier P, Knapp MS & Cove-Smith R Circadian variations in urine excretion in chronic renal failure. Q J Med 49, 461–478 (1980). [PubMed] [Google Scholar]
- 56.Singh RK, Bansal A, Bansal SK & Rai SP Circadian rhythms of common laboratory profiles in serum and urine of healthy Indians. Prog Clin Biol Res 341B, 559–566 (1990). [PubMed] [Google Scholar]
- 57.Asplund R & Aberg HE Micturition habits of older people. Voiding frequency and urine volumes. Scand J Urol Nephrol 26, 345–349, doi: 10.3109/00365599209181224 (1992). [DOI] [PubMed] [Google Scholar]
- 58.Duffy JF, Scheuermaier K & Loughlin KR Age-Related Sleep Disruption and Reduction in the Circadian Rhythm of Urine Output: Contribution to Nocturia? Curr Aging Sci 9, 34–43, doi: 10.2174/1874609809666151130220343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smolensky MH, Hermida RC, Reinberg A, Sackett-Lundeen L & Portaluppi F Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int 33, 1101–1119, doi: 10.1080/07420528.2016.1184678 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Patke A, Young MW & Axelrod S Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21, 67–84, doi: 10.1038/s41580-019-0179-2 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Greco CM & Sassone-Corsi P Circadian blueprint of metabolic pathways in the brain. Nat Rev Neurosci 20, 71–82, doi: 10.1038/s41583-018-0096-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Q & Kim JY Mammalian circadian networks mediated by the suprachiasmatic nucleus. FEBS J 289, 6589–6604, doi: 10.1111/febs.16233 (2022). [DOI] [PubMed] [Google Scholar]
- 63.Panda S. Circadian physiology of metabolism. Science 354, 1008–1015, doi: 10.1126/science.aah4967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bass J. Circadian topology of metabolism. Nature 491, 348–356, doi: 10.1038/nature11704 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Bass J & Takahashi JS Circadian integration of metabolism and energetics. Science 330, 1349–1354, doi: 10.1126/science.1195027 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dibner C, Schibler U & Albrecht U The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72, 517–549, doi: 10.1146/annurev-physiol-021909-135821 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Asher G & Schibler U Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab 13, 125–137, doi: 10.1016/j.cmet.2011.01.006 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Gumz ML et al. Toward Precision Medicine: Circadian Rhythm of Blood Pressure and Chronotherapy for Hypertension - 2021 NHLBI Workshop Report. Hypertension 80, 503–522, doi: 10.1161/HYPERTENSIONAHA.122.19372 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE & Kalsbeek A Circadian clocks and insulin resistance. Nat Rev Endocrinol 15, 75–89, doi: 10.1038/s41574-018-0122-1 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Lekkas D & Paschos GK The circadian clock control of adipose tissue physiology and metabolism. Auton Neurosci 219, 66–70, doi: 10.1016/j.autneu.2019.05.001 (2019). [DOI] [PubMed] [Google Scholar]
- 71.McCauley JP et al. Circadian Modulation of Neurons and Astrocytes Controls Synaptic Plasticity in Hippocampal Area CA1. Cell Rep 33, 108255, doi: 10.1016/j.celrep.2020.108255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerstner JR & Yin JC Circadian rhythms and memory formation. Nat Rev Neurosci 11, 577–588, doi: 10.1038/nrn2881 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.S S & Sriram K Multi-scale modeling of the circadian modulation of learning and memory. PLoS One 14, e0219915, doi: 10.1371/journal.pone.0219915 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crock LW et al. Metabotropic glutamate receptor 5 (mGluR5) regulates bladder nociception. Mol Pain 8, 20, doi: 10.1186/1744-8069-8-20 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ochodnicky P. et al. Bradykinin modulates spontaneous nerve growth factor production and stretch-induced ATP release in human urothelium. Pharmacol Res 70, 147–154, doi: 10.1016/j.phrs.2013.01.010 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Uy J. et al. Glutamatergic Mechanisms Involved in Bladder Overactivity and Pudendal Neuromodulation in Cats. J Pharmacol Exp Ther 362, 53–58, doi: 10.1124/jpet.117.240895 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yokoyama O, Yoshiyama M, Namiki M & de Groat WC Role of the forebrain in bladder overactivity following cerebral infarction in the rat. Exp Neurol 163, 469–476, doi: 10.1006/exnr.2000.7391 (2000). [DOI] [PubMed] [Google Scholar]
- 78.Xie Z. et al. A review of sleep disorders and melatonin. Neurol Res 39, 559–565, doi: 10.1080/01616412.2017.1315864 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Yanar K, Simsek B & Cakatay U Integration of Melatonin Related Redox Homeostasis, Aging, and Circadian Rhythm. Rejuvenation Res 22, 409–419, doi: 10.1089/rej.2018.2159 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Rutter J, Reick M & McKnight SL Metabolism and the control of circadian rhythms. Annu Rev Biochem 71, 307–331, doi: 10.1146/annurev.biochem.71.090501.142857 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Huang W, Ramsey KM, Marcheva B & Bass J Circadian rhythms, sleep, and metabolism. J Clin Invest 121, 2133–2141, doi: 10.1172/JCI46043 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boivin DB & Boudreau P Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris) 62, 292–301, doi: 10.1016/j.patbio.2014.08.001 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Gabriel BM & Zierath JR Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol 15, 197–206, doi: 10.1038/s41574-018-0150-x (2019). [DOI] [PubMed] [Google Scholar]
- 84.Boivin DB, Boudreau P & Kosmadopoulos A Disturbance of the Circadian System in Shift Work and Its Health Impact. J Biol Rhythms 37, 3–28, doi: 10.1177/07487304211064218 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bolsius YG et al. The role of clock genes in sleep, stress and memory. Biochem Pharmacol 191, 114493, doi: 10.1016/j.bcp.2021.114493 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang Y, Wang H, Li Y, Tao X & Sun J Poor Sleep Quality Is Associated with Dawn Phenomenon and Impaired Circadian Clock Gene Expression in Subjects with Type 2 Diabetes Mellitus. Int J Endocrinol 2017, 4578973, doi: 10.1155/2017/4578973 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsumoto T. et al. Nocturia and increase in nocturnal blood pressure: the Nagahama study. J Hypertens 36, 2185–2192, doi: 10.1097/HJH.0000000000001802 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Furukawa S. et al. Nocturia and Prevalence of Depressive Symptoms in Japanese Adult Patients With Type 2 Diabetes Mellitus: The Dogo Study. Can J Diabetes 42, 51–55, doi: 10.1016/j.jcjd.2017.03.002 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Juul KV, Jessen N, Bliwise DL, van der Meulen E & Norgaard JP Delaying time to first nocturnal void may have beneficial effects on reducing blood glucose levels. Endocrine 53, 722–729, doi: 10.1007/s12020-016-0920-y (2016). [DOI] [PubMed] [Google Scholar]
- 90.Kim JW Effect of Shift Work on Nocturia. Urology 87, 153–160, doi: 10.1016/j.urology.2015.07.047 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Leng Y, Musiek ES, Hu K, Cappuccio FP & Yaffe K Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 18, 307–318, doi: 10.1016/S1474-4422(18)30461-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Videnovic A, Lazar AS, Barker RA & Overeem S 'The clocks that time us'--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 10, 683–693, doi: 10.1038/nrneurol.2014.206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leng Y. et al. Excessive daytime sleepiness, objective napping and 11-year risk of Parkinson's disease in older men. Int J Epidemiol 47, 1679–1686, doi: 10.1093/ije/dyy098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walsh CM et al. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep 37, 2009–2016, doi: 10.5665/sleep.4260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rogers-Soeder TS et al. Rest-Activity Rhythms and Cognitive Decline in Older Men: The Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 66, 2136–2143, doi: 10.1111/jgs.15555 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bokenberger K. et al. Association Between Sleep Characteristics and Incident Dementia Accounting for Baseline Cognitive Status: A Prospective Population-Based Study. J Gerontol A Biol Sci Med Sci 72, 134–139, doi: 10.1093/gerona/glw127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cronin P. et al. Circadian alterations during early stages of Alzheimer's disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement 13, 689–700, doi: 10.1016/j.jalz.2016.10.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Musiek ES & Holtzman DM Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008, doi: 10.1126/science.aah4968 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verma AK, Singh S & Rizvi SI Aging, circadian disruption and neurodegeneration: Interesting interplay. Exp Gerontol 172, 112076, doi: 10.1016/j.exger.2022.112076 (2023). [DOI] [PubMed] [Google Scholar]
- 100.Sengiku A. et al. Circadian coordination of ATP release in the urothelium via connexin43 hemichannels. Sci Rep 8, 1996, doi: 10.1038/s41598-018-20379-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Negoro H. et al. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat Commun 3, 809, doi: 10.1038/ncomms1812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ihara T. et al. The Clockmutant mouse is a novel experimental model for nocturia and nocturnal polyuria. Neurourol. Urodyn 36, 1034–1038, doi: 10.1002/nau.23062 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Noh JY. et al. Circadian rhythms in urinary functions: possible roles of circadian clocks? Int Neurourol J 15, 64–73, doi: 10.5213/inj.2011.15.2.64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldman R. Studies in diurnal variation of water and electrolyte excretion; nocturnal diuresis of water and sodium in congestive cardiac failure and cirrhosis of the liver. J Clin Invest 30, 1191–1199, doi: 10.1172/JCI102538 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiss JP & Blaivas JG. Nocturia. J Urol 163, 5–12 (2000). [PubMed] [Google Scholar]
- 106.Sakakibara R. et al. Nocturnal polyuria with abnormal circadian rhythm of plasma arginine vasopressin in post-stroke patients. Intern Med 44, 281–284, doi: 10.2169/internalmedicine.44.281 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Adler D. et al. Clinical presentation and comorbidities of obstructive sleep apnea-COPD overlap syndrome. PLoS One 15, e0235331, doi: 10.1371/journal.pone.0235331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Asplund R & Aberg H. Diurnal variation in the levels of antidiuretic hormone in the elderly. J Intern Med 229, 131–134, doi: 10.1111/j.1365-2796.1991.tb00320.x (1991). [DOI] [PubMed] [Google Scholar]
- 109.Hoshiyama F et al. The impact of obstructive sleep apnea syndrome on nocturnal urine production in older men with nocturia. Urology 84, 892–896, doi: 10.1016/j.urology.2014.02.073 (2014). [DOI] [PubMed] [Google Scholar]
- 110.Forsling ML, Montgomery H, Halpin D, Windle RJ & Treacher DF Daily patterns of secretion of neurohypophysial hormones in man: effect of age. Exp Physiol 83, 409–418, doi: 10.1113/expphysiol.1998.sp004124 (1998). [DOI] [PubMed] [Google Scholar]
- 111.Potter GD et al. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr Rev 37, 584–608, doi: 10.1210/er.2016-1083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol 175, 3190–3199, doi: 10.1111/bph.14116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brzezinski A, Rai S, Purohit A & Pandi-Perumal SR Melatonin, Clock Genes, and Mammalian Reproduction: What Is the Link? Int J Mol Sci 22, doi: 10.3390/ijms222413240 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bian J, Wang Z, Dong Y, Cao J & Chen Y Role of BMAL1 and CLOCK in regulating the secretion of melatonin in chick retina under monochromatic green light. Chronobiol Int 37, 1677–1692, doi: 10.1080/07420528.2020.1830790 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Burke CA, Nitti VW & Stothers L Melatonin and melatonin receptor agonists in the treatment of nocturia: A systematic review. Neurourol Urodyn 43, 826–839, doi: 10.1002/nau.25443 (2024). [DOI] [PubMed] [Google Scholar]
- 116.Liu J. et al. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol 56, 361–383, doi: 10.1146/annurev-pharmtox-010814-124742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsuta Y. et al. Melatonin increases bladder capacity via GABAergic system and decreases urine volume in rats. J Urol 184, 386–391, doi: 10.1016/j.juro.2010.03.002 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Obayashi K, Saeki K & Kurumatani N Association between melatonin secretion and nocturia in elderly individuals: a cross-sectional study of the HEIJO-KYO cohort. J Urol 191, 1816–1821, doi: 10.1016/j.juro.2013.12.043 (2014). [DOI] [PubMed] [Google Scholar]
- 119.Leerasiri P, Pariyaeksut P, Hengrasmee P & Asumpinwong C Effectiveness of melatonin for the treatment of nocturia: a randomized controlled trial. Int Urogynecol J, doi: 10.1007/s00192-022-05232-3 (2022). [DOI] [PubMed] [Google Scholar]
- 120.Batla A. et al. Exploratory pilot study of exogenous sustained-release melatonin on nocturia in Parkinson's disease. Eur J Neurol 28, 1884–1892, doi: 10.1111/ene.14774 (2021). [DOI] [PubMed] [Google Scholar]
- 121.Drake MJ et al. Results of a randomized, double blind, placebo controlled, crossover trial of melatonin for treatment of Nocturia in adults with multiple sclerosis (MeNiMS). BMC Neurol 18, 107, doi: 10.1186/s12883-018-1114-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leng G, Brown CH & Russell JA Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol 57, 625–655, doi: 10.1016/s0301-0082(98)00072-0 (1999). [DOI] [PubMed] [Google Scholar]
- 123.Nielsen S. et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82, 205–244, doi: 10.1152/physrev.00024.2001 (2002). [DOI] [PubMed] [Google Scholar]
- 124.George CP et al. Diurnal variation of plasma vasopressin in man. The Journal of clinical endocrinology and metabolism 41, 332–338, doi: 10.1210/jcem-41-2-332 (1975). [DOI] [PubMed] [Google Scholar]
- 125.Ono D, Honma KI & Honma S Roles of Neuropeptides, VIP and AVP, in the Mammalian Central Circadian Clock. Front Neurosci 15, 650154, doi: 10.3389/fnins.2021.650154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ingram CD et al. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res 119, 351–364, doi: 10.1016/s0079-6123(08)61580-0 (1998). [DOI] [PubMed] [Google Scholar]
- 127.Tousson E & Meissl H Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J Neurosci 24, 2983–2988, doi: 10.1523/JNEUROSCI.5044-03.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gizowski C, Trudel E & Bourque CW Central and peripheral roles of vasopressin in the circadian defense of body hydration. Best Pract Res Clin Endocrinol Metab 31, 535–546, doi: 10.1016/j.beem.2017.11.001 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Buijs RM & Kalsbeek A Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2, 521–526, doi: 10.1038/35081582 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Nakata M. et al. Circadian Clock Component BMAL1 in the Paraventricular Nucleus Regulates Glucose Metabolism. Nutrients 13, doi: 10.3390/nu13124487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bing MH et al. Prevalence and bother of nocturia, and causes of sleep interruption in a Danish population of men and women aged 60-80 years. BJU Int 98, 599–604, doi: 10.1111/j.1464-410X.2006.06390.x (2006). [DOI] [PubMed] [Google Scholar]
- 132.Obayashi K, Saeki K & Kurumatani N Quantitative association between nocturnal voiding frequency and objective sleep quality in the general elderly population: the HEIJO-KYO cohort. Sleep Med 16, 577–582, doi: 10.1016/j.sleep.2015.01.021 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Kim SJ et al. Influence of Circadian Disruption Associated With Artificial Light at Night on Micturition Patterns in Shift Workers. Int Neurourol J 23, 258–264, doi: 10.5213/inj.1938236.118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.C DEN et al. Night shift workers refer higher urinary symptoms with an impairment quality of life: a single cohort study. Minerva Urol Nephrol 73, 831–835, doi: 10.23736/S2724-6051.20.03735-2 (2021). [DOI] [PubMed] [Google Scholar]
- 135.Kamperis K, Hagstroem S, Radvanska E, Rittig S & Djurhuus JC Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol 299, F404–411, doi: 10.1152/ajprenal.00126.2010 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Udo Y. et al. Sleep duration is an independent factor in nocturia: analysis of bladder diaries. BJU Int 104, 75–79, doi: 10.1111/j.1464-410X.2009.08379.x (2009). [DOI] [PubMed] [Google Scholar]
- 137.Kalmbach DA, Anderson JR & Drake CL The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res 27, e12710, doi: 10.1111/jsr.12710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Madhu C. et al. Nocturia: risk factors and associated comorbidities; findings from the EpiLUTS study. Int J Clin Pract 69, 1508–1516, doi: 10.1111/ijcp.12727 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Deger M. et al. Risk factors associated with nocturia in patients with obstructive sleep apnea syndrome. Int J Clin Pract 75, e13724, doi: 10.1111/ijcp.13724 (2021). [DOI] [PubMed] [Google Scholar]
- 140.Niimi A. et al. Sleep Apnea and Circadian Extracellular Fluid Change as Independent Factors for Nocturnal Polyuria. J Urol 196, 1183–1189, doi: 10.1016/j.juro.2016.04.060 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Corrado C & Fontana S Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int J Mol Sci 21, doi: 10.3390/ijms21165611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gabryelska A. et al. Disruption of Circadian Rhythm Genes in Obstructive Sleep Apnea Patients-Possible Mechanisms Involved and Clinical Implication. Int J Mol Sci 23, doi: 10.3390/ijms23020709 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manella G. et al. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc Natl Acad Sci U S A 117, 779–786, doi: 10.1073/pnas.1914112117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ghorbel MT, Coulson JM & Murphy D Cross-talk between hypoxic and circadian pathways: cooperative roles for hypoxia-inducible factor 1alpha and CLOCK in transcriptional activation of the vasopressin gene. Mol Cell Neurosci 22, 396–404, doi: 10.1016/s1044-7431(02)00019-2 (2003). [DOI] [PubMed] [Google Scholar]
- 145.Wuerzner G, Firsov D & Bonny O Circadian glomerular function: from physiology to molecular and therapeutical aspects. Nephrol Dial Transplant 29, 1475–1480, doi: 10.1093/ndt/gft525 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Johnston JG & Pollock DM Circadian regulation of renal function. Free Radic Biol Med 119, 93–107, doi: 10.1016/j.freeradbiomed.2018.01.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pollak MR, Quaggin SE, Hoenig MP & Dworkin LD The glomerulus: the sphere of influence. Clin J Am Soc Nephrol 9, 1461–1469, doi: 10.2215/cjn.09400913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mills JN Human circadian rhythms. Physiol Rev 46, 128–171, doi: 10.1152/physrev.1966.46.1.128 (1966). [DOI] [PubMed] [Google Scholar]
- 149.Rossier BC, Baker ME & Studer RA Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95, 297–340, doi: 10.1152/physrev.00011.2014 (2015). [DOI] [PubMed] [Google Scholar]
- 150.Hurwitz S, Cohen RJ & Williams GH Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol (1985) 96, 1406–1414, doi: 10.1152/japplphysiol.00611.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 151.Firsov D & Bonny O Circadian regulation of renal function. Kidney Int 78, 640–645, doi: 10.1038/ki.2010.227 (2010). [DOI] [PubMed] [Google Scholar]
- 152.Mills JN & Stanbury SW Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol 117, 22–37 (1952). [PMC free article] [PubMed] [Google Scholar]
- 153.Mills JN Diurnal rhythm in urine flow. J Physiol 113, 528–536, doi: 10.1113/jphysiol.1951.sp004593 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mills JN & Stanbury SW Intrinsic diurnal rhythm in urinary electrolyte output. J Physiol 115, 18p–19p (1951). [PubMed] [Google Scholar]
- 155.Ede MC, Faulkner MH & Tredre BE An intrinsic rhythm of urinary calcium excretion and the specific effect of bedrest on the excretory pattern. Clin Sci 42, 433–445, doi: 10.1042/cs0420433 (1972). [DOI] [PubMed] [Google Scholar]
- 156.Moore-Ede MC & Herd JA Renal electrolyte circadian rhythms: independence from feeding and activity patterns. The American journal of physiology 232, F128–135, doi: 10.1152/ajprenal.1977.232.2.F128 (1977). [DOI] [PubMed] [Google Scholar]
- 157.Firsov D & Bonny O Circadian rhythms and the kidney. Nat Rev Nephrol 14, 626–635, doi: 10.1038/s41581-018-0048-9 (2018). [DOI] [PubMed] [Google Scholar]
- 158.Stow LR & Gumz ML The circadian clock in the kidney. J Am Soc Nephrol 22, 598–604, doi: 10.1681/ASN.2010080803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zuber AM et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A 106, 16523–16528, doi: 10.1073/pnas.0904890106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Solocinski K & Gumz ML The Circadian Clock in the Regulation of Renal Rhythms. J Biol Rhythms 30, 470–486, doi: 10.1177/0748730415610879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Doi M. et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16, 67–74, doi: 10.1038/nm.2061 (2010). [DOI] [PubMed] [Google Scholar]
- 162.Nikolaeva S. et al. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 23, 1019–1026, doi: 10.1681/ASN.2011080842 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ge Y. et al. Endogenously produced 20-HETE modulates myogenic and TGF response in microperfused afferent arterioles. Prostaglandins Other Lipid Mediat 102-103, 42–48, doi: 10.1016/j.prostaglandins.2013.03.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tokonami N. et al. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol 25, 1430–1439, doi: 10.1681/ASN.2013060641 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ansermet C. et al. The intrinsic circadian clock in podocytes controls glomerular filtration rate. Sci Rep 9, 16089, doi: 10.1038/s41598-019-52682-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nikolaeva S. et al. Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition. Journal of the American Society of Nephrology : JASN 27, 2997–3004, doi: 10.1681/asn.2015091055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Soundararajan R, Pearce D & Ziera T The role of the ENaC-regulatory complex in aldosterone-mediated sodium transport. Mol Cell Endocrinol 350, 242–247, doi: 10.1016/j.mce.2011.11.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gumz ML Molecular basis of circadian rhythmicity in renal physiology and pathophysiology. Exp Physiol 101, 1025–1029, doi: 10.1113/EP085781 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gumz ML et al. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119, 2423–2434, doi: 10.1172/JCI36908 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Richards J. et al. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 289, 11791–11806, doi: 10.1074/jbc.M113.531095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Solocinski K. et al. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol 309, F933–942, doi: 10.1152/ajprenal.00197.2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stow LR et al. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59, 1151–1156, doi: 10.1161/HYPERTENSIONAHA.112.190892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Richards J. et al. Tissue-specific and time-dependent regulation of the endothelin axis by the circadian clock protein Per1. Life Sci 118, 255–262, doi: 10.1016/j.lfs.2014.03.028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kohan DE, Inscho EW, Wesson D & Pollock DM Physiology of endothelin and the kidney. Compr Physiol 1, 883–919, doi: 10.1002/cphy.c100039 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Douma LG et al. Kidney-specific KO of the circadian clock protein PER1 alters renal Na(+) handling, aldosterone levels, and kidney/adrenal gene expression. Am J Physiol Renal Physiol 322, F449–F459, doi: 10.1152/ajprenal.00385.2021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miller M. Nocturnal polyuria in older people: pathophysiology and clinical implications. Journal of the American Geriatrics Society 48, 1321–1329, doi: 10.1111/j.1532-5415.2000.tb02608.x (2000). [DOI] [PubMed] [Google Scholar]
- 177.Schmitt EE, Johnson EC, Yusifova M & Bruns DR The renal molecular clock: broken by aging and restored by exercise. Am J Physiol Renal Physiol 317, F1087–f1093, doi: 10.1152/ajprenal.00301.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cugini P. et al. Effect of aging on circadian rhythm of atrial natriuretic peptide, plasma renin activity, and plasma aldosterone. J Gerontol 47, B214–219, doi: 10.1093/geronj/47.6.b214 (1992). [DOI] [PubMed] [Google Scholar]
- 179.Kirkland JL, Lye M, Levy DW & Banerjee AK Patterns of urine flow and electrolyte excretion in healthy elderly people. Br Med J (Clin Res Ed) 287, 1665–1667, doi: 10.1136/bmj.287.6406.1665 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wolff CA et al. Defining the age-dependent and tissue-specific circadian transcriptome in male mice. Cell Rep 42, 111982, doi: 10.1016/j.celrep.2022.111982 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moon DG et al. Antidiuretic hormone in elderly male patients with severe nocturia: a circadian study. BJU Int 94, 571–575, doi: 10.1111/j.1464-410X.2004.05003.x (2004). [DOI] [PubMed] [Google Scholar]
- 182.Asplund R. Diuresis pattern, plasma vasopressin and blood pressure in healthy elderly persons with nocturia and nocturnal polyuria. Neth J Med 60, 276–280 (2002). [PubMed] [Google Scholar]
- 183.Tamma G, Goswami N, Reichmuth J, De Santo NG & Valenti G Aquaporins, vasopressin, and aging: current perspectives. Endocrinology 156, 777–788, doi: 10.1210/en.2014-1812 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Carter PG, Cannon A, McConnell AA & Abrams P Role of atrial natriuretic peptide in nocturnal polyuria in elderly males. Eur Urol 36, 213–220, doi: 10.1159/000068000 (1999). [DOI] [PubMed] [Google Scholar]
- 185.Matthiesen TB, Rittig S, Norgaard JP, Pedersen EB & Djurhuus JC Nocturnal polyuria and natriuresis in male patients with nocturia and lower urinary tract symptoms. J Urol 156, 1292–1299 (1996). [PubMed] [Google Scholar]
- 186.Johnson TM 2nd, Miller M, Pillion DJ & Ouslander JG Arginine vasopressin and nocturnal polyuria in older adults with frequent nighttime voiding. J Urol 170, 480–484, doi: 10.1097/01.ju.0000071406.18453.5f (2003). [DOI] [PubMed] [Google Scholar]
- 187.Asplund R, Sundberg B & Bengtsson P Oral desmopressin for nocturnal polyuria in elderly subjects: a double-blind, placebo-controlled randomized exploratory study. BJU Int 83, 591–595, doi: 10.1046/j.1464-410x.1999.00012.x (1999). [DOI] [PubMed] [Google Scholar]
- 188.Mohandas R, Douma LG, Scindia Y & Gumz ML Circadian rhythms and renal pathophysiology. J Clin Invest 132, doi: 10.1172/JCI148277 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Graugaard-Jensen C, Rittig S & Djurhuus JC Nocturia and circadian blood pressure profile in healthy elderly male volunteers. J Urol 176, 1034–1039; discussion 1039, doi: 10.1016/j.juro.2006.04.046 (2006). [DOI] [PubMed] [Google Scholar]
- 190.Weiss JP, Monaghan TF, Epstein MR & Lazar JM Future Considerations in Nocturia and Nocturnal Polyuria. Urology 133S, 34–42, doi: 10.1016/j.urology.2019.06.014 (2019). [DOI] [PubMed] [Google Scholar]
- 191.Fujii T. et al. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis 33, 29–35, doi: 10.1016/s0272-6386(99)70254-4 (1999). [DOI] [PubMed] [Google Scholar]
- 192.Sachdeva A & Weder AB Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension 48, 527–533, doi: 10.1161/01.HYP.0000240268.37379.7c (2006). [DOI] [PubMed] [Google Scholar]
- 193.Matsuo T, Miyata Y & Sakai H Effect of salt intake reduction on nocturia in patients with excessive salt intake. Neurourol Urodyn 38, 927–933, doi: 10.1002/nau.23929 (2019). [DOI] [PubMed] [Google Scholar]
- 194.Okumura Y. et al. Dietary Sodium Restriction Reduces Nocturnal Urine Volume and Nocturnal Polyuria Index in Renal Allograft Recipients With Nocturnal Polyuria. Urology 106, 60–64, doi: 10.1016/j.urology.2017.04.025 (2017). [DOI] [PubMed] [Google Scholar]
- 195.Nakamura S. et al. Circadian changes in urine volume and frequency in elderly men. J Urol 156, 1275–1279 (1996). [PubMed] [Google Scholar]
- 196.Summers SJ et al. Male Voiding Behavior: Insight from 19,824 At-Home Uroflow Profiles. J Urol 205, 1126–1132, doi: 10.1097/ju.0000000000001504 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kono J. et al. Urothelium-Specific Deletion of Connexin43 in the Mouse Urinary Bladder Alters Distension-Induced ATP Release and Voiding Behavior. Int J Mol Sci 22, doi: 10.3390/ijms22041594 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Herrera GM & Meredith AL Diurnal variation in urodynamics of rat. PLoS One 5, e12298, doi: 10.1371/journal.pone.0012298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Negoro H. et al. Development of diurnal micturition pattern in mice after weaning. J Urol 189, 740–746, doi: 10.1016/j.juro.2012.07.140 (2013). [DOI] [PubMed] [Google Scholar]
- 200.Langdale CL, Degoski D, Milliken PH & Grill WM Voiding behavior in awake unrestrained untethered spontaneously hypertensive and Wistar control rats. Am J Physiol Renal Physiol 321, F195–F206, doi: 10.1152/ajprenal.00564.2020 (2021). [DOI] [PubMed] [Google Scholar]
- 201.Ihara T. et al. Intermittent restraint stress induces circadian misalignment in the mouse bladder, leading to nocturia. Sci Rep 9, 10069, doi: 10.1038/s41598-019-46517-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kimura Y. et al. The circadian rhythm of bladder clock genes in the spontaneously hypersensitive rat. PLoS One 14, e0220381, doi: 10.1371/journal.pone.0220381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wu C. et al. Local receptors as novel regulators for peripheral clock expression. FASEB J 28, 4610–4616, doi: 10.1096/fj.13-243295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Christie S & Zagorodnyuk V Time-of-day dependent changes in guinea pig bladder afferent mechano-sensitivity. Sci Rep 11, 19283, doi: 10.1038/s41598-021-98831-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Witjes WP, Wijkstra H, Debruyne FM & de la Rosette JJ Quantitative assessment of uroflow: is there a circadian rhythm? Urology 50, 221–228, doi: 10.1016/s0090-4295(97)00190-8 (1997). [DOI] [PubMed] [Google Scholar]
- 206.Hiramatsu I. et al. Maximum Flow Rate is Lowest in the Early Morning in Hospitalized Men With Nocturia Evaluated Over 24 Hours by Toilet Uroflowmetry. Urology 166, 196–201, doi: 10.1016/j.urology.2022.03.011 (2022). [DOI] [PubMed] [Google Scholar]
- 207.Negoro H. et al. Diurnal differences in urine flow in healthy young men in a light-controlled environment: a randomized crossover design. J Physiol Anthropol 42, 27, doi: 10.1186/s40101-023-00346-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huppertz ND, Kirschner-Hermanns R, Tolba RH & Grosse JO Telemetric monitoring of bladder function in female Göttingen minipigs. BJU Int 116, 823–832, doi: 10.1111/bju.13089 (2015). [DOI] [PubMed] [Google Scholar]
- 209.White RS et al. Evaluation of mouse urinary bladder smooth muscle for diurnal differences in contractile properties. Front Pharmacol 5, 293, doi: 10.3389/fphar.2014.00293 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Negoro H, Kanematsu A, Yoshimura K & Ogawa O Chronobiology of micturition: putative role of the circadian clock. J Urol 190, 843–849, doi: 10.1016/j.juro.2013.02.024 (2013). [DOI] [PubMed] [Google Scholar]
- 211.Ihara T. et al. The Circadian expression of Piezo1, TRPV4, Connexin26, and VNUT, associated with the expression levels of the clock genes in mouse primary cultured urothelial cells. Neurourol Urodyn 37, 942–951, doi: 10.1002/nau.23400 (2018). [DOI] [PubMed] [Google Scholar]
- 212.Ihara T. et al. The oscillation of intracellular Ca(2+) influx associated with the circadian expression of Piezo1 and TRPV4 in the bladder urothelium. Sci Rep 8, 5699, doi: 10.1038/s41598-018-23115-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ihara T. et al. The time-dependent variation of ATP release in mouse primary-cultured urothelial cells is regulated by the clock gene. Neurourol Urodyn 37, 2535–2543, doi: 10.1002/nau.23793 (2018). [DOI] [PubMed] [Google Scholar]
- 214.Ihara T. et al. Clock Genes Regulate the Circadian Expression of Piezo1, TRPV4, Connexin26, and VNUT in an Ex Vivo Mouse Bladder Mucosa. PLoS One 12, e0168234, doi: 10.1371/journal.pone.0168234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Birder LA & Van Kerrebroeck PEV Pathophysiological Mechanisms of Nocturia and Nocturnal Polyuria: The Contribution of Cellular Function, the Urinary Bladder Urothelium, and Circadian Rhythm. Urology 133S, 14–23, doi: 10.1016/j.urology.2019.07.020 (2019). [DOI] [PubMed] [Google Scholar]
- 216.Dalghi MG, Montalbetti N, Carattino MD & Apodaca G The Urothelium: Life in a Liquid Environment. Physiol Rev 100, 1621–1705, doi: 10.1152/physrev.00041.2019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ihara T. et al. The Circadian expression of Piezo1, TRPV4, Connexin26, and VNUT, associated with the expression levels of the clock genes in mouse primary cultured urothelial cells. Neurourol Urodyn, doi: 10.1002/nau.23400 (2017). [DOI] [PubMed] [Google Scholar]
- 218.Ali AAH, Avakian GA & Gall CV The Role of Purinergic Receptors in the Circadian System. Int J Mol Sci 21, doi: 10.3390/ijms21103423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Thoma C. Urinary incontinence: The bladder sets its own clock. Nat Rev Urol 11, 544, doi: 10.1038/nrurol.2014.248 (2014). [DOI] [PubMed] [Google Scholar]
- 220.Stokkan KA, Yamazaki S, Tei H, Sakaki Y & Menaker M Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493, doi: 10.1126/science.291.5503.490 (2001). [DOI] [PubMed] [Google Scholar]
- 221.Ikeda Y. et al. Glucagon and/or IGF-1 Production Regulates Resetting of the Liver Circadian Clock in Response to a Protein or Amino Acid-only Diet. EBioMedicine 28, 210–224, doi: 10.1016/j.ebiom.2018.01.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chihara I. et al. Glucocorticoids coordinate the bladder peripheral clock and diurnal micturition pattern in mice. Commun Biol 6, 81, doi: 10.1038/s42003-023-04464-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Noh JY et al. Presence of multiple peripheral circadian oscillators in the tissues controlling voiding function in mice. Exp Mol Med 46, e81, doi: 10.1038/emm.2013.153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ihara T. et al. The Clock mutant mouse is a novel experimental model for nocturia and nocturnal polyuria. Neurourol Urodyn 36, 1034–1038, doi: 10.1002/nau.23062 (2017). [DOI] [PubMed] [Google Scholar]
- 225.Belancio VP, Blask DE, Deininger P, Hill SM & Jazwinski SM The aging clock and circadian control of metabolism and genome stability. Front Genet 5, 455, doi: 10.3389/fgene.2014.00455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Silva-Ramos M. et al. Urinary ATP May Be a Dynamic Biomarker of Detrusor Overactivity in Women with Overactive Bladder Syndrome. PLOS ONE 8, e64696, doi: 10.1371/journal.pone.0064696 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Homma Y, Yamaguchi O & Hayashi K An epidemiological survey of overactive bladder symptoms in Japan. BJU Int 96, 1314–1318, doi: 10.1111/j.1464-410X.2005.05835.x (2005). [DOI] [PubMed] [Google Scholar]
- 228.Goessaert AS, Krott L, Walle JV & Everaert K Exploring nocturia: gender, age, and causes. Neurourol Urodyn 34, 561–565, doi: 10.1002/nau.22638 (2015). [DOI] [PubMed] [Google Scholar]
- 229.Presicce F. et al. Variations of Nighttime and Daytime Bladder Capacity in Patients with Nocturia: Implication for Diagnosis and Treatment. J Urol 201, 962–966, doi: 10.1097/JU.0000000000000022 (2019). [DOI] [PubMed] [Google Scholar]
- 230.Iwamoto T. et al. Reduced salt intake partially restores the circadian rhythm of bladder clock genes in Dahl salt-sensitive rats. Life Sci 306, 120842, doi: 10.1016/j.lfs.2022.120842 (2022). [DOI] [PubMed] [Google Scholar]
- 231.Griffett K & Burris TP The mammalian clock and chronopharmacology. Bioorg Med Chem Lett 23, 1929–1934, doi: 10.1016/j.bmcl.2013.02.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ruan W, Yuan X & Eltzschig HK. Circadian rhythm as a therapeutic target. Nat Rev Drug Discov 20, 287–307, doi: 10.1038/s41573-020-00109-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.LeGates TA, Fernandez DC & Hattar S Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci 15, 443–454, doi: 10.1038/nrn3743 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gooley JJ Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singap 37, 669–676 (2008). [PubMed] [Google Scholar]
- 235.Gehlbach BK et al. The Effects of Timed Light Exposure in Critically Ill Patients: A Randomized Controlled Pilot Clinical Trial. Am J Respir Crit Care Med 198, 275–278, doi: 10.1164/rccm.201801-0170LE (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ono H, Taguchi T, Kido Y, Fujino Y & Doki Y The usefulness of bright light therapy for patients after oesophagectomy. Intensive Crit Care Nurs 27, 158–166, doi: 10.1016/j.iccn.2011.03.003 (2011). [DOI] [PubMed] [Google Scholar]
- 237.Taguchi T, Yano M & Kido Y Influence of bright light therapy on postoperative patients: a pilot study. Intensive Crit Care Nurs 23, 289–297, doi: 10.1016/j.iccn.2007.04.004 (2007). [DOI] [PubMed] [Google Scholar]
- 238.Yang J. et al. Bright light therapy as an adjunctive treatment with risperidone in patients with delirium: a randomized, open, parallel group study. Gen Hosp Psychiatry 34, 546–551, doi: 10.1016/j.genhosppsych.2012.05.003 (2012). [DOI] [PubMed] [Google Scholar]
- 239.Challet E. The circadian regulation of food intake. Nat Rev Endocrinol 15, 393–405, doi: 10.1038/s41574-019-0210-x (2019). [DOI] [PubMed] [Google Scholar]
- 240.Sutton EF et al. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 27, 1212–1221 e1213, doi: 10.1016/j.cmet.2018.04.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chaix A, Zarrinpar A, Miu P & Panda S Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005, doi: 10.1016/j.cmet.2014.11.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mrosovsky N & Salmon PA A behavioural method for accelerating re-entrainment of rhythms to new light-dark cycles. Nature 330, 372–373, doi: 10.1038/330372a0 (1987). [DOI] [PubMed] [Google Scholar]
- 243.Reid KJ et al. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med 11, 934–940, doi: 10.1016/j.sleep.2010.04.014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Buxton OM, Lee CW, L'Hermite-Baleriaux M, Turek FW & Van Cauter E Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol 284, R714–724, doi: 10.1152/ajpregu.00355.2002 (2003). [DOI] [PubMed] [Google Scholar]
- 245.Meyer N, Harvey AG, Lockley SW & Dijk DJ Circadian rhythms and disorders of the timing of sleep. Lancet 400, 1061–1078, doi: 10.1016/S0140-6736(22)00877-7 (2022). [DOI] [PubMed] [Google Scholar]
- 246.Panda S. The arrival of circadian medicine. Nat Rev Endocrinol 15, 67–69, doi: 10.1038/s41574-018-0142-x (2019). [DOI] [PubMed] [Google Scholar]
- 247.Yu F. et al. Recent advances in circadian-regulated pharmacokinetics and its implications for chronotherapy. Biochem Pharmacol 203, 115185, doi: 10.1016/j.bcp.2022.115185 (2022). [DOI] [PubMed] [Google Scholar]
- 248.Burgess HJ, Revell VL & Eastman CI A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol 586, 639–647, doi: 10.1113/jphysiol.2007.143180 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhao M, Zhao H, Deng J, Guo L & Wu B Role of the CLOCK protein in liver detoxification. Br J Pharmacol 176, 4639–4652, doi: 10.1111/bph.14828 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Winter C. et al. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab 28, 175–182 e175, doi: 10.1016/j.cmet.2018.05.002 (2018). [DOI] [PubMed] [Google Scholar]
- 251.Levi F & Schibler U Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47, 593–628, doi: 10.1146/annurev.pharmtox.47.120505.105208 (2007). [DOI] [PubMed] [Google Scholar]
- 252.Humphries PS et al. Carbazole-containing amides and ureas: Discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg Med Chem Lett 28, 293–297, doi: 10.1016/j.bmcl.2017.12.051 (2018). [DOI] [PubMed] [Google Scholar]
- 253.Miller S. et al. Isoform-selective regulation of mammalian cryptochromes. Nat Chem Biol 16, 676–685, doi: 10.1038/s41589-020-0505-1 (2020). [DOI] [PubMed] [Google Scholar]
- 254.Hirota T. et al. Identification of small molecule activators of cryptochrome. Science 337, 1094–1097, doi: 10.1126/science.1223710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dong Z. et al. Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock. Cancer Discov 9, 1556–1573, doi: 10.1158/2159-8290.CD-19-0215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Solt LA et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68, doi: 10.1038/nature11030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Banerjee S. et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat Commun 5, 5759, doi: 10.1038/ncomms6759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.He B. et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab 23, 610–621, doi: 10.1016/j.cmet.2016.03.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.EE M, AC B & MW H Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annual review of nutrition 36, 275–299, doi: 10.1146/annurev-nutr-071715-050718 (2016). [DOI] [PubMed] [Google Scholar]
- 260.Shinozaki A. et al. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::LUCIFERASE Mouse Embryonic Fibroblasts. PLoS One 12, e0170904, doi: 10.1371/journal.pone.0170904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ihara T. et al. Different effects of GsMTx4 on nocturia associated with the circadian clock and Piezo1 expression in mice. Life Sci 278, 119555, doi: 10.1016/j.lfs.2021.119555 (2021). [DOI] [PubMed] [Google Scholar]
- 262.Kira S. et al. Urinary metabolites identified using metabolomic analysis as potential biomarkers of nocturia in elderly men. World J Urol 38, 2563–2569, doi: 10.1007/s00345-019-03042-9 (2020). [DOI] [PubMed] [Google Scholar]
- 263.Ihara T. et al. Effects of fatty acid metabolites on nocturia. Sci Rep 12, 3050, doi: 10.1038/s41598-022-07096-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ihara T. et al. G protein-coupled receptor 55 activated by palmitoylethanolamide is associated with the development of nocturia associated with circadian rhythm disorders. Life Sci 332, 122072, doi: 10.1016/j.lfs.2023.122072 (2023). [DOI] [PubMed] [Google Scholar]
- 265.Ito H. et al. Effectiveness and Safety of a Mixture of Nobiletin and Tangeretin in Nocturia Patients: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. J Clin Med 12, doi: 10.3390/jcm12082757 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pauwaert K. et al. Does hormonal therapy affect the bladder or the kidney in postmenopausal women with and without nocturnal polyuria: Results of a pilot trial? Maturitas 160, 61–67, doi: 10.1016/j.maturitas.2022.01.009 (2022). [DOI] [PubMed] [Google Scholar]
- 267.Simonneaux V. et al. Daily rhythm and regulation of clock gene expression in the rat pineal gland. Brain Res Mol Brain Res 120, 164–172, doi: 10.1016/j.molbrainres.2003.10.019 (2004). [DOI] [PubMed] [Google Scholar]
- 268.von Gall C. et al. Clock gene protein mPER1 is rhythmically synthesized and under cAMP control in the mouse pineal organ. J Neuroendocrinol 13, 313–316, doi: 10.1046/j.1365-2826.2001.00643.x (2001). [DOI] [PubMed] [Google Scholar]
- 269.Ono D, Honma S & Honma K Differential roles of AVP and VIP signaling in the postnatal changes of neural networks for coherent circadian rhythms in the SCN. Sci Adv 2, e1600960, doi: 10.1126/sciadv.1600960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


