Abstract
The tsetse fly-transmitted protozoan parasite Trypanosoma brucei is the causative agent of human African sleeping sickness and the cattle disease Nagana. The bloodstream form of the parasite uses a dense cell-surface coat of variant surface glycoprotein to escape the innate and adaptive immune responses of the mammalian host and a highly glycosylated transferrin receptor to take up host transferrin, an essential growth factor. These glycoproteins, as well as other flagellar pocket, endosomal, and lysosomal glycoproteins, are known to contain galactose. The parasite is unable to take up galactose, suggesting that it may depend on the action of UDP-glucose 4′-epimerase for the conversion of UDP-Glc to UDP-Gal and subsequent incorporation of galactose into glycoconjugates via UDP-Gal-dependent galactosyltransferases. In this paper, we describe the cloning of T. brucei galE, encoding T. brucei UDP-Glc-4′-epimerase, and functional characterization by complementation of a galE-deficient Escherichia coli mutant and enzymatic assay of recombinant protein. A tetracycline-inducible conditional galE null mutant of T. brucei was created using a transgenic parasite expressing the TETR tetracycline repressor protein gene. Withdrawal of tetracycline led to a cessation of cell division and substantial cell death, demonstrating that galactose metabolism in T. brucei proceeds via UDP-Glc-4′-epimerase and is essential for parasite growth. After several days without tetracycline, cultures spontaneously recovered. These cells were shown to have undergone a genetic rearrangement that deleted the TETR gene. The results show that enzymes and transporters involved in galactose metabolism may be considered as potential therapeutic targets against African trypanosomiasis.
Keywords: UDP-Gal‖galE‖epimerase
The tsetse fly-transmitted protozoan parasite Trypanosoma brucei causes human African sleeping sickness and the related cattle disease Nagana. There are 300,000–500,000 cases of the human disease per year in sub-Saharan Africa (1). The bloodstream form of the parasite divides by binary fission in the blood, lymph, and interstitial fluids of the mammalian host, and causes cachexia and anaemia in cattle and neurological disturbances in man. These conditions are fatal if not treated, and existing chemotherapies are toxic and difficult to administer. The need for new, less-toxic therapeutics is widely acknowledged (1).
The bloodstream form of T. brucei is rich in galactose-containing glycoproteins, most notably the variant surface glycoprotein (VSG) that, at 5 × 106 homodimers per cell, forms a dense cell-surface coat. The VSG coat is a macromolecular diffusion barrier that protects the parasite from the innate immune system and also enables the parasite to undergo antigenic variation. Thus, although an individual parasite only expresses one VSG gene at a time, the parasite population can stay ahead of the host's specific immune response to expressed VSGs when a few parasites switch expression to one of several hundred genes encoding immunologically distinct VSGs (2).
Bloodstream-form T. brucei parasites use facilitated diffusion through a hexose transporter to obtain glucose from the host (3). However, galactose does not compete for this transporter and the parasite cannot take up [14C]Gal (4). This lack of Gal transport was corroborated by unsuccessful attempts to metabolically label VSG with [3H]Gal (G. Wall and M.A.J.F., unpublished data) under conditions that were successful for [3H]Man labeling (5). Based on these observations, we considered it likely that the principal source of Gal in T. brucei might be via the epimerization of UDP-Glc to UDP-Gal by UDP-Glc-4′-epimerase (EC 5.1.3.2). This NADH-dependent oxidoreductase is found in most prokaryotes and eukaryotes, and interspecies sequence similarity is typically 40% or better. The precise roles of conserved residues in substrate binding and catalysis can be inferred from x-ray crystallographic studies (6).
In this study, we describe the cloning of the galE gene encoding the T. brucei UDP-Glc-4′-epimerase, the characterization of the recombinant enzyme, and the creation of a T. brucei conditional galE null mutant that shows that the enzyme, and galactose metabolism, are essential to the parasite.
Materials and Methods
Cloning of T. brucei galE.
Procyclic trypanosomes (strain 427) were grown in SDM-79 medium (7) and genomic DNA was prepared by standard methods. A 1,764-bp genomic clone was obtained by PCR amplification with a forward primer to the 5′-untranslated region (UTR) (5′-gcatacgcacatgtacatgtc-3′) and a reverse primer to the 3′-UTR (5′-caagtgcttaaatagcagtcc-3′), designed from the end sequences of the 10P15 clone identified by tblastn search of the T. brucei database with the human galE sequence. Primers (forward, 5′-gcgatgttgttacacaagtgcg-3′; reverse, 5′-ccacttctataacctcacgcac-3′) were used to generate a 574-bp T. brucei galE probe that was labeled with dUTP fluorescein by random priming (Gene Images kit, Amersham Pharmacia) for use in Southern Blotting. PCR amplification of the T. brucei galE ORF from T. brucei bloodstream-form and procyclic-form cDNA was performed with a forward primer against the miniexon (5′-ggcccgctattattagaacagtttctgta-3′) and a specific reverse primer to the 3′ end of the gene (5′-cgatgcgtacccattagggtgagtg-3′).
Functional Complementation.
T. brucei galE ORF was amplified from the genomic clone and subcloned into the BamHI and HindIII sites of pUC18 (RFO) or with an additional one (RF + 1) or two (RF + 2) adenosine bases immediately 5′ to the initiating ATG. The forward and reverse primers were 5′-cgcGGATCC(aa)atgcgcgttttggtttg-3 and 5′-cccAAGCTTttacaacttagtggtcc-3′, respectively, where the capital letters denote restriction sites and the bases in parentheses indicate the additional adenosine base(s). DC41 galE− Escherichia coli was transformed with the pUC18-galE plasmids by electroporation. The transformed cell lines were grown in LB medium with ampicillin and isolated clones were analyzed to ensure that they carried the correct plasmid. These clones were expanded and streaked onto McConkey agar plates (containing ampicillin and Gal as the only carbon source) pretreated with 100 μl of 0.1 M isopropyl β-d-thiogalactoside (IPTG).
Expression and Purification of Recombinant UDP-Glc-4′-epimerase.
The T. brucei galE ORF was amplified from the genomic clone, silencing an NdeI site, and subcloned into the NdeI/BamHI sites of pET15b (Novagen) for expression in BL21(DE3)pLysS E. coli cells (Novagen). The gene was amplified in two segments by using Pfu (Promega). The 5′ end of the ORF was amplified by using 5′-ggaattcCATATGcgcgttttggtttgtgg-3′ and 5′-gttttgactcgccataaggactttctgggctc-3′ and the 3′ end was amplified using 5′-gagcccagaaagtccttatggcgagtcaaaac-3′ and 5′-cgcGGATCCttacaacttagtggtcc-3′ as forward and reverse primers, respectively. The two PCR products were used together in a further PCR reaction to yield a product containing the silent mutation of the NdeI site (italics) and 5′-NdeI and 3′-BamHI restriction sites (capital letters).
Expression was induced overnight at room temperature by adding 50 μM IPTG to cells at a density of 0.5 OD600 units. Cells were harvested, washed in PBS, and lysed by two freeze–thaw cycles and high-pressure lysis in a French press. The lysate was cleared by centrifugation (31,000 × g, 30 min, 4°C) and the supernatant was passed through a 0.45-μm filter and loaded onto a 5-ml HiTrap Chelating Sepharose HP column (Amersham Pharmacia Biotech AB) charged with Ni2+. The column was washed with 50 mM Tris (pH 7.6)/250 mM NaCl until the A280 trace was stable and then eluted with a gradient to 0.4 M imidazole in the same buffer over 100 ml. The flow rate was 5 ml/min and 4-ml fractions were collected. A280 peak fractions were analyzed by 10% SDS/PAGE and those containing the 46-kDa epimerase were pooled and dialyzed against 50 mM Tris (pH 7.6). The pooled fractions were concentrated (Vivapore 7,500 MWCO concentrator, Sartorius Vivascience) and checked by SDS/PAGE and matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometry. The stock solution was adjusted to 20% (wt/vol) glycerol (final concentration, 2.5 mg protein/ml) and stored in aliquots at −80°C.
Enzyme Assay.
Assays were performed using a coupled assay according to ref. 8. Briefly, 1-cm path-length cuvettes were charged with 910 μl of 100 mM glycine-NaOH (pH 8.7)/1 mM β-NAD+ (Sigma)/40 μl of 20 mM UDP-Gal (Sigma)/40 μl of 1 unit/ml UDP-Glc dehydrogenase (Calbiochem). The reaction was started by adding 10 μl of epimerase diluted in 50 mM Tris⋅HCl (pH 7.6)/1% BSA/1 mM DTT/1 mM EDTA/1 mM β-NAD+. Net NADH production was measured by following changes in A340 at 27°C in a Shimadzu UV-1601 spectrophotometer. A linear relationship between Vo and enzyme concentration was established over the range 0–1 μg/ml, and 0.5 μg/ml was used for determining the specific activity and Km for UDP-Gal.
Generation of a T. brucei Bloodstream Form of galE Conditional Null Mutant.
The 309-bp 5′- and 266-bp 3′-UTR sequences immediately adjacent to the start and stop codons of the T. brucei galE ORF were PCR amplified from the genomic clone by using Pfu with 5′-ataagaatGCGGCCGCgcatacgcacatgtac-3′ and 5′-gtttaaacttacggaccgtcaagcttggtaaagaggttctttcc-3′ and 5′-gacggtccgtaagtttaaacggatccctttaactggcagtac-3′ and 5′-ataagtaaGCGGCCGCcaagtgcttaaatagc-3′ as forward and reverse primers, respectively. The two PCR products were used together in a further PCR reaction to yield a product containing the 5′-UTR linked to the 3′-UTR by a short HindIII, PmeI, and BamHI cloning site (italics) and NotI restriction sites at each end (capital letters). The PCR product was cloned into the NotI site of the pGEM-5Zf(+) vector (Promega) and the HYG and PAC drug resistance genes were introduced into the targeting vector via the HindIII and BamHI cloning sites.
The T. brucei galE ORF was amplified from the genomic clone by using Pfu and 5′-cccAAGCTTatgcgcgttttggtttg-3′ and 5′-cgcGGATCCttacaacttagtggtcc-3′ as forward and reverse primers, respectively, where the capital letters indicate HindIII and BamHI restriction sites. The product was ligated into the HindIII/BamHI cloning site of the pLew100 tetracycline-inducible expression vector (9).
The above plasmids were prepared using Qiagen Maxi-Prep kits, digested with NotI, precipitated and washed twice with 70% ethanol, redissolved in sterile water, and used for electroporation of the parasites. All genetic modifications were performed using strain 427 (variant 221) bloodstream forms stably transfected to express T7 RNA polymerase and tetracycline repressor (TETR) protein under continuous G418 selection (26). For convenience, these are referred to here as “wild-type” cells. The cultivation and transfection of these cells is described in ref. 9 with minor modifications described in ref. 10. Once stable conditional galE null mutant clones were obtained, all selection drugs (except G418) were removed from the medium. Tet-system-approved FCS (CLONTECH) was used in experiments on the effects of tetracycline removal.
Northern Blots.
Total RNA for Northern blotting was prepared by standard guanidinium thiocyanate methods from 1 × 108 cells. Samples of RNA (5 μg) were run on formaldehyde agarose gels and transferred to Hybond N nylon membrane (Amersham Pharmacia) for hybridization with [α-32P]dCTP-labeled T. brucei galE probe (Stratagene, Prime-It RmT Random Primer labeling kit). The control β-tubulin probe was used after the T. brucei galE probe had decayed.
Analysis of Tetracycline Response Elements.
The T. brucei galE conditional mutants contain the TETR gene under G418 selection (i.e., adjacent to a NEO gene) and the ectopic galE gene downstream of two tetracycline operator (TetOp) sequences. The region of DNA containing the TETR gene was PCR amplified from genomic DNA by using flanking primers (5′-gaagtgcttgacattggg-3′ and 5′-gaacgcagacgatttcgc-3′). The region of DNA containing the TetOp sequences was PCR amplified from genomic DNA by using flanking primers (5′-cgtggacacgacctc-3′ and 5′-cacgtctcccacctc-3′).
For Southern blot analysis, probes to the TETR and NEO genes were prepared by PCR amplification from the pLew114hyg5′ plasmid (26) by using the primers 5′-cagcgcattagagctgc-3′ and 5′-ccgaataagaaggctgg-3′ and 5′-ccaagcttggatggattg-3′ and 5′-caagaaggcgatagaagg-3′, respectively. The probes were labeled with dUTP-fluorescein and used to probe NotI/SalI digests.
Results
Cloning of the T. brucei galE Gene.
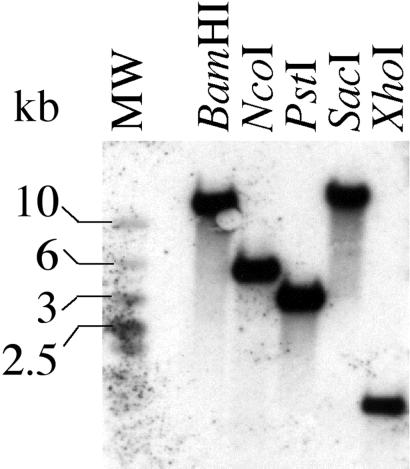
A tblastn search of The Institute of Genomic Research (TIGR) database with the human UDP-Glc-4′-epimerase protein sequence identified a sheared DNA library clone (10P15) with significant homology to the C-terminal 98-aa residues followed by 298 bp of putative 3′-UTR. The other end sequence of the clone was presumed to represent 515 bp of 5′-UTR. A genomic clone was obtained using genomic DNA template and primers designed to the putative 5′- and 3′-UTRs. This 1,764-bp clone (GenBank accession no. AJ430490) contained a 1,188-bp ORF, encoding a protein with significant homology to known UDP-Glc-4′-epimerases (Fig. 1), flanked by 309 and 267 bp of 5′- and 3′-UTR, respectively. PCR amplification of a 1.2-kb fragment generated from both bloodstream and procyclic form T. brucei cDNA, using primers based on the predicted C terminus of the T. brucei epimerase and the miniexon (a 5′-spliced leader common to all T. brucei mRNAs), confirmed that the gene is transcribed in both life cycle stages. Southern blotting showed that the gene is single-copy per haploid genome (Fig. 2).
Figure 1.
clustal v alignment of T. brucei (Tb), E. coli (Ec), Saccharomyces cerevisiae (Sc), Caenorhabditis elegans (Ce), Drosophila melanogaster (Dm), and human (Hu) UDP-Glc-4-epimerase predicted amino acid sequences. The reverse-text indicates residues that match the consensus sequence.
Figure 2.
Southern blot of T. brucei procyclic genomic DNA digested with a panel of restriction endonucleases and probed for T. brucei galE. Approximately five micrograms of DNA, digested overnight with BamHI, NcoI, PstI, SacI, and XhoI restriction enzymes, was loaded per lane. Hybridization was at 60°C overnight. The blot was washed with 1× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7)/0.1% SDS for 20 min at 60°C, followed by 0.5× SSC/0.1% SDS for 20 min at 60°C. The single band in each lane shows that the epimerase is present as a single copy per haploid genome.
Functional Complementation of galE-Deficient E. coli.
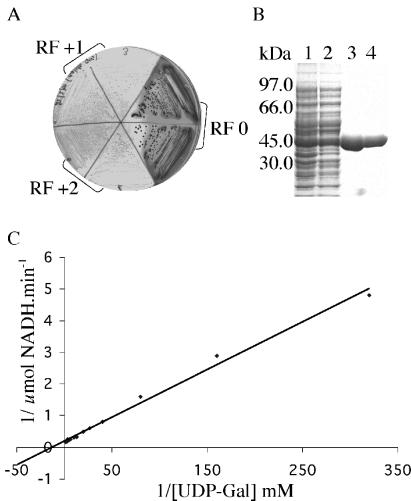
The T. brucei galE gene was subcloned into pUC18 in three frames, placing the gene under the control of the lacZ promoter. The plasmids were electroporated into a galE-deficient E. coli strain to test for functional complementation in the presence of isopropyl β-d-thiogalactoside (IPTG) inducer. In one frame (RF0), the galE− cells were rescued and grew as dark red colonies on McConkey agar (a medium with Gal as the only carbon source). By contrast, transformation with the T. brucei epimerase gene in the RF + 1 and RF + 2 frames failed to rescue the galE− growth defect and these cells grew as small, pale colonies (Fig. 3A).
Figure 3.
Functionality of the T. brucei galE gene and recombinant protein. (A) Functional complementation of a galE-deficient strain of E. coli. Cells expressing T. brucei galE (RF0) from pUC18 formed dark red colonies on McConkey agar. When the epimerase is not expressed and the cells grow poorly. (B) Purification of recombinant T. brucei UDP-Glc-4′-epimerase; 10% SDS/PAGE gel stained with Coomassie blue of total E. coli lysate (lane 1), soluble supernatant (lane 2), Ni2+-Sepharose purified protein (lane 3), and dialyzed purified protein (lane 4). (C) Lineweaver–Burk plot of purified recombinant T. brucei epimerase activity.
Expression and Assay of Recombinant T. brucei UDP-Glc-4′-epimerase.
For biochemical characterization of the T. brucei epimerase, we expressed recombinant protein with an N-terminal His-6-tag in E. coli to yield about 20 mg/l of recombinant protein (Fig. 3B). The purified recombinant protein (Fig. 3B) was assayed using a coupled assay (8) and the specific activity and Km for UDP-Gal were determined (Fig. 3C). The specific activity of the recombinant T. brucei enzyme was 5.9 units/mg, similar to that measured under identical conditions for a commercially available epimerase from Streptococcus thermophilus (16.7 units/mg). The apparent Km of the T. brucei epimerase for UDP-Gal was 77 μM, similar to that measured for the S. thermophilus enzyme (91 μM). These values of specific activity and Km are well within the normal ranges of 0.012–210 units/mg and 8–260 μM, respectively, reported for a UDP-Glc-4′-epimerases from a variety of sources (11).
The T. brucei galE Gene Is Essential to the Bloodstream Form of Trypanosomes.
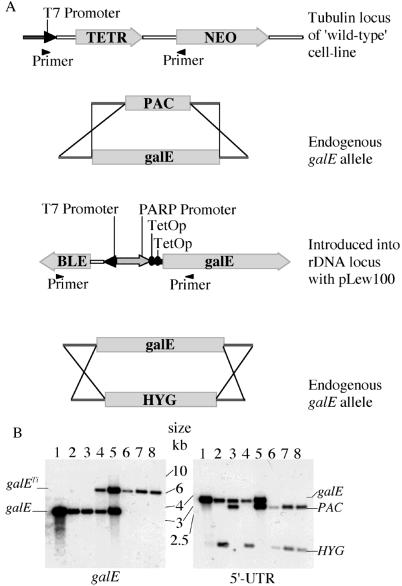
Although it was possible to replace one T. brucei galE allele by homologous recombination with either PAC or HYG, several attempts to replace the second allele with the complementary drug-resistance gene failed, suggesting that galE might be an essential gene. To investigate this, a conditional null mutant was created. A trypanosome cell line that constitutively expresses T7 RNA polymerase and the tetracycline repressor (TETR) protein under G418-neomycin selection was used (9). An ectopic epimerase gene was introduced into the trypanosome rDNA locus (using the pLew100 expression vector), driven by a trypanosome (procyclin) promoter and regulated by two TetOp sequences (Fig. 4A). Thus, the addition of tetracycline relieves binding of the TETR protein to the operators and allows expression of the epimerase. Three conditional null mutant clones were obtained in this way, and Southern blots confirmed the replacement of both chromosomal galE alleles with antibiotic resistance genes (Fig. 4B).
Figure 4.
Construction of T. brucei conditional galE null mutants. (A) Schematic representation showing the location of the TETR gene in the “wild-type” cells, the targeted replacement of the one galE allele with PAC, the introduction of an ectopic tetracycline-inducible copy of galE into the rDNA locus, and replacement of the second galE allele with HYG. The primers indicated are those used to amplify regions of interest in (Fig. 5). (B) Southern blot of conditional galE null mutants and intermediate cell lines. Aliquots (≈5 μg) of PstI-digested DNA were blotted with the T. brucei galE probe (Left) or the 5′-UTR probe (Right). The DNA was from wild-type galE+/+ cells (lane 1), ΔgalE∷HYG cells (lane 2), ΔgalE∷PAC cells (lane 3), galETi ΔgalE∷HYG cells (lane 4), galETi ΔgalE∷PAC cells (lane 5), and conditional galETi ΔgalE∷PAC/ΔgalE∷HYG null mutant clones derived from galETi ΔgalE∷HYG cells (lane 6) and from galETi ΔgalE∷PAC cells (lanes 7 and 8). The probe reveals allelic (galE) copies at 4 kb and ectopic tetracycline-inducible (galETi) copies at 8 kb. The 5′-UTR probe shows differences when the allelic galE copies are replaced by the drug resistance genes. Note that the HYG gene contains a PstI site and therefore shows a larger change.
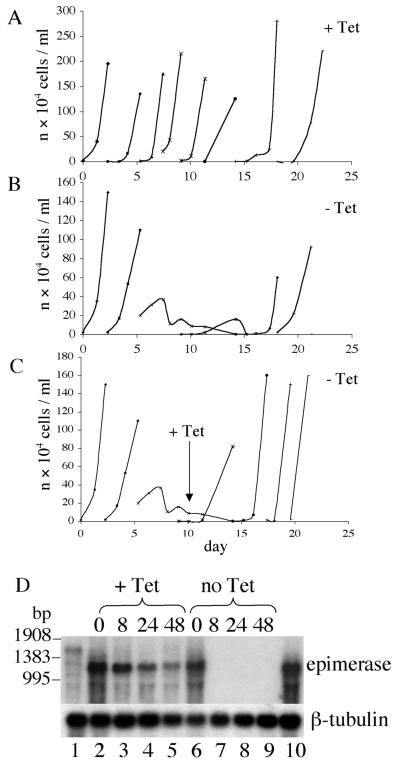
To test whether the galE gene is essential, the conditional null mutant cells were washed three times in medium without tetracycline and cultures (with and without 1 μg/ml of tetracycline) were inoculated at various cell densities. Cells were counted daily and cultures were split when densities approached 3 × 106 cells per ml. In the presence of tetracycline, the cells continued to grow with normal (wild type) kinetics (Fig. 5A), whereas in the absence of tetracycline the cells grew normally for 3–4 days, followed by rapid cell death (Fig. 5B). The few surviving cells failed to divide unless tetracycline was reintroduced at day 10 (Fig. 5C) or until day 16–20, when the cultures spontaneously started to grow once more at normal rates (Fig. 5B). Northern blot analysis showed that galE mRNA was more abundant in the conditional mutant than in wild-type cells, but that it became undetectable within 8 h of tetracycline removal (Fig. 5D). However, the cells continued to divide for 2 days, suggesting that it takes about 48 h for epimerase loss (due to dilution by cell division and protein turnover) to reach unsustainable levels. The Northern blot also showed that the cells that spontaneously recovered after 16 days in the absence of tetracycline were re-expressing galE mRNA.
Figure 5.
Growth and Northern blots of the T. brucei conditional galE null mutants. Representative growth curves for one of the three conditional mutant clones are shown. Cells grown in the presence of tetracycline were washed at day 0 and reintroduced into tetracycline-containing medium (A; +Tet) or tetracycline-free medium (B and C; −Tet). Tetracycline was reintroduced into one of the cultures after 14 days (C). (D) Northern blot of total RNA prepared from “wild-type” T. brucei (lane 1) and conditional galE null mutants grown with (+Tet; lanes 2–5) and without (−Tet; lanes 6–9) tetracycline for 0, 8, 24, and 48 h and from cells that spontaneously re-emerged after 16 days without tetracycline (lane 10). The blot was probed with a T. brucei galE probe (Upper) and a β-tubulin probe (Lower).
The Mechanism of Re-Expression of galE by Conditional Null Mutants.
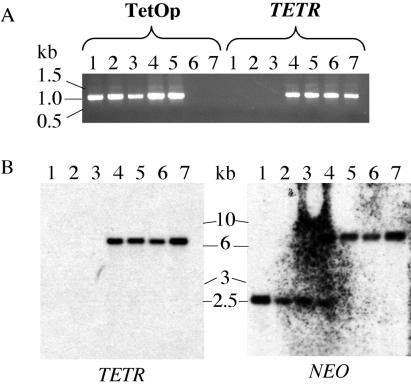
We analyzed the integrity of the genetic components of the tetracycline-inducible system in the parasites from three separate cultures that spontaneously recovered in the absence of tetracycline, as well as those of the conditional mutants grown continuously in the presence of tetracycline or starved of tetracycline for 10 days and then re-induced with the drug. Thus, DNA fragments containing the TetOp sequences upstream of the inducible galE gene and DNA fragments containing the tetracycline repressor protein (TETR) gene were amplified by PCR. The TetOp PCR products from all of the cells that had been transformed with the pLew100-galE construct were identical (Fig. 6A). In contrast, the TETR gene appeared to be absent from the parasites that had spontaneously recovered from cultures without tetracycline, whereas it was present in the other cells (Fig. 6A). The absence of the TETR gene in the recovered cells was confirmed by Southern blot with a TETR probe (Fig. 6B). The deletion of the TETR gene was also evident from a Southern blot with a probe to the NEO gene (the adjacent selectable marker gene that allowed the selection of the TETR-expressing cell lines). The results suggests the deletion of ≈4–5 kb of DNA, including most or all of the TETR gene, in all cases. The cells from the cultures rescued by reintroduction of tetracycline after 10 days appear to be a mixed population with some lacking the TETR gene and others retaining it.
Figure 6.
The mechanism of the recovery of the T. brucei conditional galE null mutants in the absence of tetracycline. (A) Ethidium-bromide-stained agarose gel of PCR products of the TetOp and TETR regions amplified from genomic DNA from conditional mutant cells from three cultures that recovered after 16 days (lanes 1–3), conditional mutant cells induced to grow after the reintroduction of tetracycline after 14 days (lane 4), conditional mutant cells grown continuously in the presence of tetracycline (lane 5), “wild-type” (TETR+) cells grown without tetracycline (lane 6), and “wild-type” (TETR+) cells grown with tetracycline (lane 7). (B) Southern blot of genomic DNA digested with NotI and SalI from the same cells described in A with TETR and NEO probes.
Discussion
Many glycoproteins of the bloodstream form of T. brucei contain galactose. The abundant surface coat VSG homodimers contain d-galactose (β-gal and α-gal) in their glycosylphosphatidylinositol (GPI) anchor side chains and/or N-linked oligosaccharides (12). The T. brucei transferrin receptor (TfR), a VSG-like heterodimer present at about 3,000 copies in the flagellar pocket, the only site of endo- and exocytosis in the cell (13), contains one GPI anchor and seven potential N-glycosylation sites (14, 15). The TfR, like the lysosomal glycoprotein gp150/CB1-gp (16) and the invariant surface glycoprotein ISG100 (17), binds to tomato lectin, suggesting the presence of linear poly-N-acetyl-lactosamine (-3Galβ1–4GlcNAcβ1-) repeats (18). It has been postulated that these β-Gal-containing structures may be involved in retaining the TfR, and other recycling components, in the T. brucei flagellar pocket and the endosomal/lysosomal membrane system (18). In addition to the aforementioned glycoproteins, five other transmembrane glycoproteins, ISG65, ISG75 (19), the ecto-phosphatase AcP115 (20), the Golgi- and lysosome-associated glycoprotein tGLP-1 (21), and the Fla-1 glycoprotein (22), the latter associated with flagellar adhesion to the cell body (23), are known to be glycosylated, but the nature of their carbohydrate chains is unknown. There are also other membrane proteins, such as Tb-29 (24), the cysteine-rich acidic membrane protein CRAM (25), the alanine-rich protein BARP (26), and the products of expression-site-associated genes (ESAGs) 1, 2, 3, 4, 10, and 11 (26, 27), that are predicted to contain carbohydrate in the form of GPI anchors and/or N-linked oligosaccharides. Finally, the flagellar pocket of the parasite contains a filamentous matrix of unknown composition that binds both the β-Gal-specific lectin ricin (28) and tomato lectin (18). Taken together, it is clear that the bloodstream form of T. brucei parasites contains a significant amount and variety of glycoconjugate-associated galactose. However, the importance of galactose metabolism to parasite survival in culture or in vivo was unknown.
It had been noted by others that galactose does not compete with 2-deoxy-glucose for uptake through the parasite hexose transporter (3, 4), and our unsuccessful attempts to metabolically label T. brucei with [3H]Gal were consistent with this view. Thus, we surmised that the most likely source of galactose for glycoconjugate biosynthesis was UDP-Gal derived from UDP-Glc by the action of UDP-Glc-4′-epimerase, and that the importance of galactose metabolism in general to the survival or infectivity of the parasite might be assessed by deletion of the galE gene encoding this enzyme from the parasite.
A tblastn search using the human UDP-Glc-4′-epimerase protein sequence revealed a fragment of a putative parasite galE gene that was cloned (Fig. 1) and shown to be functional by complementation of a galE-deficient E. coli mutant (Fig. 3A). Assay of the recombinant protein showed that it had a specific activity and Km for UDP-Gal typical of UDP-Glc-4′-epimerases (ref. 11; Fig. 3C). Attempts to replace both chromosomal copies of the parasite galE gene were unsuccessful, even in the presence of 10 mM galactose in the medium (providing further evidence that the parasite cannot transport this sugar). However, a conditional null mutant could be created by introducing and expressing an ectopic tetracycline-inducible copy of the gene before replacement of the second chromosomal copy (Fig. 4). Withdrawal of tetracycline caused the rapid down-regulation of epimerase mRNA in these cells that was followed by cell death (Fig. 5). A few nondividing parasites survived in these cultures and could be revived by addition of tetracycline. Interestingly, these cultures spontaneously recovered after about 16 days without tetracycline.
The spontaneous recovery of T. brucei tetracycline-inducible conditional null mutants in the absence of tetracycline has been described previously but the mechanisms of reversion have not been described (10, 29). In this case, we analyzed the genotype of the recovered parasites and found that they had undergone a genetic rearrangement to delete the TETR transgene, thus becoming constitutive expressers of the ectopic galE gene. This escape mechanism confirms the essentiality of the galE gene, because parasite survival depended on a significant genetic rearrangement.
Based on current knowledge, one might speculate that a lack of UDP-Gal in the bloodstream form of T. brucei might be lethal because it affects the integrity of the VSG coat and/or the uptake and/or recycling of glycoproteins like the TfR and ISG100, and/or it prevents the synthesis of the flagellar pocket matrix material of the parasite. Of these, effects on the uptake and/or recycling of the TfR may be the most acute because the receptor-mediated uptake of transferrin is known to be essential for cell growth (30). In any case, we may conclude that galactose metabolism is essential to the bloodstream form of T. brucei, and that we might usefully target the trypanosome UDP-Glc-4′-epimerase and/or downstream UDP-Gal transporters and/or UDP-Gal-dependent galactosyltransferases as potential drug targets. The latter group of enzymes may prove to be the most amenable to attack for three reasons. Firstly, the parasite makes several galactosyl structures that do not occur in the mammalian host (12), suggesting the presence of parasite-specific galactosyltransferases. Secondly, as a consequence, inhibitors may be designed from neutral parasite-specific acceptor substrate structures rather than from the common negatively charged UDP-Gal substrate. Thirdly, tblastn searches suggest that even conserved galactosyl structures (e.g., N-acetyl-lactosamine, Galβ1–4GlcNAc) are catalyzed by parasite enzymes that bare insufficient sequence similarity to their mammalian counterparts for identification and which, therefore, may be amenable to selective inhibition. Thus, the present study encourages further work to pinpoint specific galactosyl structures that are essential for parasite survival and to further investigate the therapeutic potential of affecting galactose metabolism in T. brucei.
Acknowledgments
We thank David Parkin for helpful discussions and Alan Fairlamb for access to the spectrophotometer. We are grateful to the T. brucei genome project and The Institute of Genomic Research (TIGR) for the availability of genome survey sequences and EST data, George Cross and colleagues for providing the cell lines and vectors used in making conditional trypanosome mutants, and Iain Donaldson for providing galE-deficient E. coli. This work was supported by Wellcome Trust program Grant 054491. J.R.R. thanks the BBSRC for a Ph.D. studentship.
Abbreviations
- VSG
variant surface glycoprotein
- UTR
untranslated region
- TfR
Trypanosoma brucei transferrin receptor
- TetOp
tetracycline operator
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ430490).
References
- 1.World Health Organization. African trypanosomiasis. 2001. , Fact sheet number 259 (WHO Publications, Geneva). [Google Scholar]
- 2.Vanhamme L, Pays E, McCulloch R, Barry J D. Trends Parasitol. 2001;17:338–343. doi: 10.1016/s1471-4922(01)01922-5. [DOI] [PubMed] [Google Scholar]
- 3.Tetaud E, Barrett M P, Bringaud F, Baltz F. Biochem J. 1997;325:569–580. doi: 10.1042/bj3250569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenthal R, Game S, Holman G D. Biochem Biophys Acta. 1989;985:81–89. doi: 10.1016/0005-2736(89)90107-7. [DOI] [PubMed] [Google Scholar]
- 5.Güther M L S, Ferguson M A J. EMBO J. 1995;14:3080–3093. doi: 10.1002/j.1460-2075.1995.tb07311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thoden J B, Frey P A, Holden H M. Biochemistry. 1996;35:5137–5144. doi: 10.1021/bi9601114. [DOI] [PubMed] [Google Scholar]
- 7.Brun R, Schoeneberger M. Acta Tropica. 1979;36:289–292. [PubMed] [Google Scholar]
- 8.Vorgias C E, Lemaire H-G, Wilson K S. Protein Expression Purif. 1991;2:330–338. doi: 10.1016/1046-5928(91)90091-v. [DOI] [PubMed] [Google Scholar]
- 9.Wirtz E, Leal S, Ochatt C, Cross G A M. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 10.Milne K G, Güther M L S, Ferguson M A J. Mol Biochem Parasitol. 2001;112:301–304. doi: 10.1016/s0166-6851(00)00369-8. [DOI] [PubMed] [Google Scholar]
- 11.Prosselkov P V, Gross W, Igamberdiev A U, Schnarrenberger C. Physiologia Plantarum. 1996;98:753–758. [Google Scholar]
- 12.Mehlert A, Zitzmann N, Richardson J M, Treumann A, Ferguson M A J. Mol Biochem Parasitol. 1998;91:145–152. doi: 10.1016/s0166-6851(97)00187-4. [DOI] [PubMed] [Google Scholar]
- 13.Overath P, Stierhof Y-D, Wiese M. Trends Cell Biol. 1997;7:27–33. doi: 10.1016/S0962-8924(97)10046-0. [DOI] [PubMed] [Google Scholar]
- 14.Steverding D, Stierhof Y-D, Chaudhri M, Ligtenberg M, Schell D, Beck-Sickinger A G, Overath P. Eur J Cell Biol. 1994;64:78–87. [PubMed] [Google Scholar]
- 15.Salmon D, Hanocq-Quertier J, Paturiaux-Hanocq F, Pays A, Tebabi P, Nolan D P, Michel A, Pays E. EMBO J. 1997;16:7272–7278. doi: 10.1093/emboj/16.24.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley R J, Alexander D L, Cowan C, Balber A E, Bangs J D. Mol Biochem Parasitol. 1999;98:17–28. doi: 10.1016/s0166-6851(98)00155-8. [DOI] [PubMed] [Google Scholar]
- 17.Nolan D P, Jackson D G, Windle H J, Pays A, Geuskens M, Michel A, Voorheis H P, Pays E. J Biol Chem. 1997;272:29212–29221. doi: 10.1074/jbc.272.46.29212. [DOI] [PubMed] [Google Scholar]
- 18.Nolan D P, Geuskens M, Pays E. Curr Biol. 1999;9:1169–1172. doi: 10.1016/S0960-9822(00)80018-4. [DOI] [PubMed] [Google Scholar]
- 19.Ziegelbauer K, Multhaup G, Overath P. J Biol Chem. 1992;267:10797–10803. [PubMed] [Google Scholar]
- 20.Bakalara N, Santarelli X, Davis C, Baltz T. J Biol Chem. 2000;275:8863–8871. doi: 10.1074/jbc.275.12.8863. [DOI] [PubMed] [Google Scholar]
- 21.Lingnau A, Zuffery R, Lingnau M, Russell D G. J Cell Sci. 1999;112:3061–3070. doi: 10.1242/jcs.112.18.3061. [DOI] [PubMed] [Google Scholar]
- 22.Nozaki T, Haynes P A, Cross G A M. Mol Biochem Parasitol. 1996;82:245–255. doi: 10.1016/0166-6851(96)02741-7. [DOI] [PubMed] [Google Scholar]
- 23.Moreira-Leite F, Sherwin T, Kohl L, Gull K. Science. 2001;294:610–612. doi: 10.1126/science.1063775. [DOI] [PubMed] [Google Scholar]
- 24.Gwo-Shu M, Russell D G, D'Alesandro P A, van der Ploeg L H T. J Biol Chem. 1994;269:8408–8415. [PubMed] [Google Scholar]
- 25.Hong Y, Russell D G, Zheng B, Eiki M, Lee M G-S. Mol Cell Biol. 2000;20:5149–5163. doi: 10.1128/mcb.20.14.5149-5163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pays E, Nolan D P. Mol Biochem Parasitol. 1998;91:3–36. doi: 10.1016/s0166-6851(97)00183-7. [DOI] [PubMed] [Google Scholar]
- 27.Redpath M B, Windle H, Nolan D, Pays E, Voorheis H P, Carrington M. Mol Biochem Parasitol. 2000;111:223–228. doi: 10.1016/s0166-6851(00)00305-4. [DOI] [PubMed] [Google Scholar]
- 28.Brickman M J, Balber A E. J Protozool. 1990;37:219–224. doi: 10.1111/j.1550-7408.1990.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 29.Kreiger S, Schwarz W, Ariyanayagam M R, Fairlamb A H, Krauth-Siegel R L, Clayton C. Mol Microbiol. 2000;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- 30.Steverding D. Parasitol Int. 2000;48:191–198. doi: 10.1016/s1383-5769(99)00018-5. [DOI] [PubMed] [Google Scholar]