Abstract
Drug-coated balloons (DCBs) provide a stent-free alternative, reducing risks like stent thrombosis and in-stent restenosis and the need for prolonged dual antiplatelet therapy. Recent studies show that DCBs can be effective and safe across various coronary artery diseases (CADs) when lesions are adequately prepared. Specifically, all coronary lesions are treated using the provisional approach, where active lesion preparation is followed by DCB or drug-eluting stent treatment, depending on the results. This approach means DCB is considered the default device before initiating intervention, with efforts focused on obtaining adequate lesion preparation. Depending on the result, DCB or drug-eluting stent is selected, which is termed DCB-based percutaneous coronary intervention. Therefore, this second report of the Asia-Pacific Consensus Group provides practical guidelines (DCB-based percutaneous coronary intervention) based on the latest evidence for DCB treatment in CAD and aims to expand its application across various CADs, facilitating its effective use in real-world clinical practice.
Key Words: balloon angioplasty, coronary artery disease, drug-eluting stent, percutaneous coronary intervention
Central Illustration
Highlights
-
•
DCB treatment is recommended for ISR lesions and de novo small vessel disease in CAD.
-
•
DCB provides an appealing option for managing various de novo coronary lesions.
-
•
DCB-based PCI can improve outcomes in cases with small stent burden compared with DES-only PCI.
-
•
Physiology- or imaging-guided PCI plays a crucial role in both DES and DCB treatment.
Recent advancements in percutaneous coronary intervention (PCI), specifically with drug-eluting stent (DES) development, have led to remarkable improvements in safety and efficacy. However, the persistent occurrence of recurrent events, including late stent thrombosis and restenosis, with an annual risk of nearly 2% without a plateau post–stent implantation, emphasize the need for alternative treatment modalities.1,2 Despite improvements in technology, complex lesions, including multiple or diffuse lesions, particularly in small vessels, tend to yield suboptimal clinical outcomes.3 This is linked to multiple and longer stent use, which increases the risks of stent-related issues.4
Drug-coated balloon (DCB) introduction has heralded a new era in the treatment landscape of coronary artery disease (CAD), providing patients with an alternative or complementary option to conventional stent use.5, 6, 7 Managing CAD via DCB treatment involves the rapid and uniform transfer of antiproliferative drugs into the vessel wall during single balloon inflation, using a lipophilic matrix without requiring permanent implants.8 Applying a DCB ensures that no foreign bodies remain within the vessel lumen, allowing the blood vessel to naturally remodel to its original configuration.5 Additionally, DCB eliminates the risk of stent-associated maladaptive biological responses leading to restenosis and thrombosis and actively promotes favorable natural vascular healing and positive remodeling.6,9, 10, 11, 12, 13 DCB treatment is currently recommended for managing in-stent restenosis (ISR) lesions originating from bare-metal stents (BMS) and DES and treating de novo small vessel disease.14,15 The first Asia-Pacific Consensus Group recommendation and recent research have highlighted DCB treatment as a favorable option to conventional stent-based strategies across diverse CAD contexts.6,16
In the Asia-Pacific region, patient demographics and disease characteristics vary from those observed in Europe.17 Coronary angiography shows that Asia-Pacific patients usually present with smaller coronary arteries but longer lesion lengths than Western patients.18 This coronary manifestation, characterized by “small and diffuse CAD,” may indicate the higher prevalence of diabetes mellitus among Asia-Pacific patients.19 Small-vessel CAD is typically associated with a poorer prognosis for higher restenosis, late lumen loss, and revascularization rates than large vessel CAD, mainly because of its limited capacity to accommodate neointimal growth post–stent placement.20,21 Furthermore, accumulating evidence suggests that Asia-Pacific patients could manifest a unique risk-benefit scenario regarding antiplatelet therapy, typically characterized by diminished risk of thrombosis and elevated risk of bleeding, respectively.22 Therefore, Asia-Pacific patients with these characteristics may derive relatively greater benefits from DCB treatment, preventing the use of foreign materials and necessitating a shorter DAPT duration in CAD management than other ethnicities.
The objective of these recommendations from the consensus group is to provide Asia-Pacific patients with CAD with practical guidelines founded on the latest evidence. Furthermore, the intent is to expand the DCB treatment to encompass a broader spectrum of CAD and facilitate their efficient and appropriate application in real-world clinical settings by DES supporting DCB or through a hybrid approach.
Technical Aspect of DCB Application
Optimal lesion preparation
The success of DCB treatment requires not only resolving epicardial coronary artery stenosis but also optimizing coronary blood flow, typically accomplished through meticulous lesion preparation, commonly with balloon angioplasty (BA), to ensure sufficient myocardial perfusion. A previous study suggested that some factors, including percent diameter stenosis, intimal tear or dissection, and a pressure gradient of ≥20 mmHg, are linked to an increased risk of acute closure post-BA.23 However, other studies have suggested that coronary dissection, when accompanied by a TIMI flow grade of 3 or uncomplicated, non–flow-limiting dissections, is associated with favorable outcomes and predicts a reduced restenosis rate.24
Compared with conventional BA, which primarily resolves stenosis or creates a passage for DES, DCB is not intended for BA but for delivering a coated antiproliferative drug to the vessel wall. Therefore, optimal lesion preparation is crucial before DCB application to ensure successful local drug delivery. Bailout stenting can be prevented by selecting the final device after meticulously preparing the lesion. This preparation’s primary objectives include enhancing blood flow through induced dissection and facilitating uniform drug delivery.
The primary method for preparing lesions involves conducting conventional angioplasty using a semi-compliant or noncompliant balloon with a balloon-to-vessel ratio of 1.0 and applying inflation pressure beyond nominal levels.6,7 In cases where balloon delivery difficulties, vessel underfilling, or potential undersizing are expected, starting the procedure with a smaller balloon followed by reevaluating the vessel size after administering vasodilators might be advisable.7 Subsequently, with improved flow, selecting the next balloon that matches the vessel size is necessary. In situations involving complex lesions, different strategies may be required, including using noncompliant high-pressure balloons, scoring or cutting balloons, or considering advanced procedures (including rotablation, orbital or directional atherectomy, or intravascular lithotripsy). These devices are associated with a lower risk of severe dissection and a higher incidence of optimal angiographic outcomes than conventional semicompliant balloons.25, 26, 27 Intravascular imaging techniques (including intravascular ultrasound [IVUS] or optical coherence tomography [OCT]) or functional assessments (fractional flow reserve [FFR] or instantaneous wave-free ratio) can provide valuable insights into treatment optimization.25,28,29 Recently, the ULTIMATE-III (Intravascular Ultrasound Versus Angiography Guided Drug-coated Balloon) trial comparing IVUS- and angiography-guided DCB treatments for coronary de novo lesions in high-bleeding risk patients demonstrated that IVUS-guided DCB treatment is associated with a lower late lumen loss at 7 months follow-up than angiography guidance.30
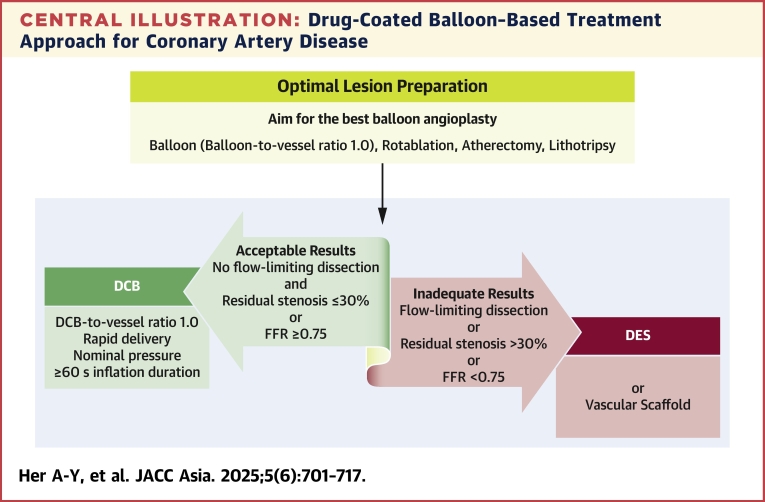
Although all coronary lesions could potentially be candidates for DCB treatment, lesions that fail to achieve optimal lesion preparation may represent a contraindication for DCB use. The following 2 criteria must be met after lesion preparation before applying a DCB: achieving TIMI flow grade 3 without flow-limiting dissection and ensuring that residual stenosis is ≤30% (as shown in the Central Illustration).6,7 When conducting angiography, confirming the complete absence of delayed contrast clearance, not only from the vessel lumen and its walls but also from any potential dissection planes, is essential. Recent data have demonstrated that most non–flow-limiting dissections, classified as less than type C, underwent a benign healing process post-DCB treatment, usually resulting in positive remodeling during follow-up.9,10,31 Although the long-term effects of antiproliferative drugs on the dissection degree remain unknown, current evidence suggests that long-term vascular remodeling varies depending on the dissection extent.25,32 Notably, the presence of dissections extending into the tunica media tends to lead to greater lumen expansion, particularly when the flow is sustained.33 Post-DCB use, a final assessment was conducted at least 5 minutes after administering an intracoronary vasodilator bolus to prevent any residual acute vessel closure.
Central Illustration.
Drug-Coated Balloon–Based Treatment Approach for Coronary Artery Disease
Drug-coated balloon (DCB)–based percutaneous coronary intervention prioritizes optimal lesion preparation, using DCB as the default device and deciding between DCB or drug-eluting stent (DES) based on outcomes. This approach minimized stent use, ensuring effective drug delivery and reducing the need for bailout stenting. FFR = fractional flow reserve.
FFR-guided DCB treatment
FFR application post-BA in larger vessels helps determine whether a DCB or DES is the preferred treatment for the lesion. In de novo large vessel disease cases, FFR-guided DCB treatment is a crucial tool for preventing acute ischemic events immediately after the procedure, including acute recoil and severe dissection. When the post-BA FFR value suggests DCB suitability (cutoff FFR ≥0.75), it ensures a favorable outcome with adequate anatomical and physiological patency during follow-up examinations (Supplemental Figure 1).28 Furthermore, FFR-guided DCB treatment demonstrated sustained anatomical and physiological patency, along with plaque redistribution and vessel remodeling, without acute vessel closure, chronic elastic recoil, or restenosis throughout the follow-up.11,12 Although no validated data exist to define the optimal FFR threshold post-BA, current consensus suggests a threshold of 0.75 as a reasonable compromise based on clinical efficacy.6 An alternative for functional assessment after lesion preparation can be angiography-guided physiology, such as quantitative flow ratio. However, although postprocedural quantitative flow ratio gradient was an independent predictor of target lesion revascularization (TLR) in the DCB treatment,34 further evidence and studies are required to clarify its role in this context.
Although coronary physiology aids in step-by-step flow assessment during PCI, integrating intravascular imaging and physiology can enhance procedural outcomes. IVUS or OCT is essential for assessing plaque composition, ensuring adequate lesion preparation, and optimizing vessel sizing to minimize complications such as dissections.35 Specifically, residual dissections have been linked to positive vessel remodeling at follow-up and intravascular imaging use and over-reaction should not lead to an overestimation of bail-out stenting to seal non–flow-limiting dissections (Supplemental Figure 1).36,37 Therefore, emerging technologies like optical and ultrasonic flow ratios show promise for advancing DCB treatment guidance and optimization.
Local drug delivery optimization
Considering a DCB application is warranted when the angiographic results meet the criteria. Geographic miss refers to the situation where the drug delivery does not adequately cover the entire length of the targeted lesion or the adjacent reference segments.6 This can result from improper positioning of the balloon during inflation, leading to incomplete treatment of diseased areas or exposing healthy vessel segments to unnecessary drug delivery. Like with DES, geographic miss has been identified as an independent predictor of restenosis in DCB treatment.38,39 To avoid geographic mismatch, the angle that best shows the lesion is displayed on the screen as a roadmap, and the procedure is continued from that view. Furthermore, the DCB must extend at least 2 to 3 mm beyond the predilated segment on both sides. Ensuring accurate sizing is essential, and OCT can help overcome the underestimation associated with angiography and the overestimation observed with IVUS, especially in small vessels.40 The DCB diameter should match the target vessel diameter, adhering to the recommended balloon-to-vessel ratio of 1.0. Additionally, maintaining the DCB inflation for at least 60 seconds at nominal pressure ensures sufficient drug delivery to the vascular wall and reduces the risk of additional dissection or the need for bailout stenting.41, 42, 43 However, if a patient cannot tolerate an extended inflation duration, a shorter inflation time (eg, 30 seconds) may be acceptable. Understanding that DCB is primarily aimed at drug delivery rather than solely addressing vessel stenosis as in traditional BA is essential. According to the manufacturer’s guidelines, the DCB should reach the lesion within 2 minutes of vascular access to ensure expedited delivery whenever feasible. However, prolonged drug exposure to the aqueous environment (with an inflation delay >25 seconds) or insufficient contact time with the target lesion (total inflation time <60 seconds) may increase target lesion failure (TLF) risk by approximately 2.0-fold each.41 Therefore, proper procedural optimization achievement should be continuously assessed from the initial decision to the DCB procedure completion.
DCB-based PCI
DCB-based PCI is a procedure where DCB is employed as the first-choice default device for all coronary lesions, aimed at achieving optimal lesion preparation (Central Illustration). The decision to use a DCB or DES is based on the lesion preparation outcomes. This strategy underlines the critical role of optimal lesion preparation before deciding on the final treatment device, either a DCB or DES. Compared with conventional stent implantation, which uses predilation to facilitate stent placement, DCB treatment mandates adequate lesion preparation to maximize luminal gain, ensure sufficient coronary blood flow, and effectively deliver antiproliferative drugs to the vessel wall. This DCB-based approach reduces the overall use of stents and helps prevent the need for bailout stenting post-DCB treatment.
Recent studies suggest that a strategy to reduce stent use can enhance clinical outcomes compared with the traditional DES-alone approach, particularly in patients with multivessel CAD.44,45 When lesion preparation is inadequate or flow-limiting dissection occurs, switching to DES implantation is viewed as a provisional adjustment to manage significant residual stenosis or flow-limiting dissections rather than as a bailout. This approach, highly feasible in real practice, can be applied regardless of various lesion characteristics and clinical presentations based on the lesion preparation results.
DCB Application According to Lesion Characteristics
In-stent restenosis
ISR treatment remains a significant clinical challenge despite advancements in newer-generation DES with improved performance. Historically, whether conventional BA or the stent-in-stent approach is more effective for managing ISR has been considerably debated. Previous trials have demonstrated that treating BMS- and DES-ISR with BA or first-generation DES leads to high revascularization rates and increased long-term risk of stent thrombosis compared with DCB treatment.46,47 Subsequent research, including the RIBS (Restenosis Intra-Stent of Bare Metal Stents: Paclitaxel-coated Balloon vs Everolimus-eluting Stent) V trial, has compared the efficacy of DCB and second-generation DES in patients with BMS-ISR.48 Although late angiographic findings favored the DES group over the DCB group, similar rates of restenosis and clinical outcomes were observed in both groups. Although the RIBS IV trial favored second-generation DES over DCB in treating DES-ISR,49 the more recent DARE (Drug-Eluting Balloon for In-Stent Restenosis) trial demonstrated that DCB is comparable to second-generation DES in terms of 6-month minimal lumen diameter and target vessel revascularization (TVR) up to 1 year in patients with any ISR type.50
Evidence from previous trials shows that DCB is superior to BA alone and is comparable to first-generation DES in clinical outcomes for patients with both BMS- and DES-ISR. The recent ESC guidelines on chronic coronary syndrome recommend both DCB and DES as potential treatments for DES ISR but identify DES as the preferred strategy over DCB.51 DCB treatment outcomes for BMS- and DES-ISR may vary caused by differences in restenotic tissue and original stent characteristics.52,53 However, DCB is preferred for ISR lesions when adequate lesion preparation is achieved post-BA, as it delivers antirestenotic drugs without adding extra stent layers, which is particularly beneficial in DES-ISR. Although new-generation DES stents may slightly reduce the need for TLR in the ISR lesions,54 the risk of additional stent layers makes DCB the preferred choice for BMS- and DES-ISR when angiographic outcomes post-BA are favorable.
Additionally, the use of intravascular imaging modalities, including IVUS and OCT, is recommended to identify the morphological causes of ISR, which helps in achieving optimal lesion preparation, satisfactory angiographic outcomes, and successful drug delivery.
De novo lesion
Small vessel disease
Coronary small vessel disease, characterized by CAD affecting vessels with a diameter <3 mm poses a significant challenge in coronary revascularization. This is caused by increased risks of restenosis and stent thrombosis post-DES implantation.55,56 However, DCB treatment shows notable potential in small vessel disease, as the percentage of lumen loss post-stent placement constitutes a larger portion of the total lumen diameter in small vessels than in larger ones. In the randomized BASKET-SMALL (Basel Kosten Effektivitäts Studie-Drug-Coated Balloons in Small Coronary Artery Lesions) 2 trial, DCB treatment proved as effective as second-generation DES for treating de novo small vessel disease. Over a 3-year follow-up, both groups showed comparable rates of major adverse cardiac events (MACE).15 Additionally, results from the PICCOLETO (Drug Eluting Balloon Efficacy for Small Coronary Vessel Disease Treatment) II trial, which targeted patients with small vessel disease, demonstrated that DCB treatment exhibited superiority over DES (everolimus-eluting stent). Specifically, DCB showed significantly lower late lumen loss than DES.57 A recent comprehensive meta-analysis of 29 randomized controlled trials (RCTs) involving 8,074 patients with small vessel CAD concluded that the clinical outcomes between DCB and newer-generation DES did not significantly differ.58 Therefore, DCB treatment shows comparable efficacy to second-generation DES in managing de novo small vessel disease, with potential superiority in reducing late lumen loss compared to DES (Table 1).
Table 1.
Summary of Clinical Trials of DCB on Treatment of De Novo Coronary Lesions
| Trial Name or First Author Design | Treatment | n | Angiographic Follow-up | Clinical Follow-up |
|---|---|---|---|---|
| Small vessel disease | ||||
| BASKET-SMALL 215/RCT | DCB (Sequent Please) vs DES (Xience or Taxus Element) | 382 vs 376 | - | MACE: 15% (DCB) vs 15% (DES), HR: 0.99; P = 0.95 at 3 y |
| PICCOLETO II57/RCT | DCB (Elutax SV) vs DES-E | 118 vs 114 | LLL: 0.04 mm (DCB) vs 0.17 mm (DES); P = 0.001 for noninferiority; P = 0.03 for superiority at 6 mo | MACE: 6% (DCB) vs 8% (DES); P = 0.55 at 12 mo |
| Large vessel disease | ||||
| Shin et al28/registry-observational | DCB (Sequent Please) vs BMS (Vision) or DES (Resolute Integrity or Xience Prime) | 45 vs 22 | LLL: 0.05 ± 0.27 mm (DCB) vs 0.40 ± 0.54 mm (Stent); P = 0.022; FFR: 0.85 ± 0.08 mm (DCB) vs 0.85 ± 0.05 mm (Stent); P = 0.973 at 9 mo | - |
| Her et al9/registry-observational | DCB (nonsmall) (Sequent Please) vs DCB (small) (Sequent Please) | 100 vs 127 | LLL: 0.03 ± 0.22 mm (nonsmall) vs 0.06 ± 0.25 mm (small); P = 0.384 at 6 mo | TVF: 7% (nonsmall) vs 8% (small); P = 0.596 at 6 mo |
| Gitto et al64/registry-PS matched | DCB-based (Magic Touch or Selution or IN.PACT Falcon or RESTORE) vs DES (contemporary) | 147 vs 701 | TLF: 4.1% (DCB-based) vs 9.8% (DES); P = 0.148 at 2 y | |
| Lin et al65/meta-analysis | DCB vs DES | 152 vs 169 | LLL: SMD: −0.07; P = 0.548 at 6-9 mo | TLR: RR: 1.17; P = 0.746 at 6-9 mo |
| REC-CAGEFREE I66/RCT | DCB (Swide) vs DES-S | 1,133 vs 1,139 | DoCE: 6.4% (DCB) vs 3.4% (DES); P = 0.0008 at 2 y | |
| Bifurcation disease | ||||
| PEPCAD-BIF68/RCT | DCB (Sequent Please) vs POBA in SB | 32 vs 32 | Restenosis: 6% (DCB) vs 26% (POBA); P = 0.045; LLL: 0.13 mm (DCB) vs 0.51 mm (POBA); P = 0.013 at 9 mo | TLR: n = 1 (DCB) vs n = 3 (POBA) |
| DCB-BIF70/RCT | DCB (Paclitaxel-coated balloon) vs POBA (NCB) in SB | 391 vs 393 | MACE: 7.2% (DCB) vs 12.5% (POBA); P = 0.013 at 1 y | |
| Her et al/registry-observational71 | DCB (only MV) (Sequent Please) | 16 MBs/32 SBs | SB os lumen area: 0.92 ± 0.68 mm2 (pre) vs 1.03 ± 0.77 mm2 (post) vs 1.42 ± 1.18 mm2 (at 9 mo) | - |
| Pan et al72/registry-PS matched | DCB (Sequent Please) + DES vs DES-only (new-generation) | 199 vs 398 | LLL: 0.13 ± 0.42 mm (DCB) vs 0.42 ± 0.62 mm (DES); P < 0.001 at 1 y | TLF: 7.56% (DCB) vs 14.36% (DES), log-rank P = 0.024 at 2 y; TLR: 2.91% (DCB) vs 9.42% (DES); P = 0.007 |
| Chronic total occlusion lesion | ||||
| PEPCAD-CTO77/registry-variable matching | DCB (Sequent Please) + BMS (Coroflex Blue) vs DES-P | 48 vs 48 | LLL: 0.64 ± 0.69 mm (DCB) vs 0.43 ± 0.64 mm (DES); P = 0.14; Restenosis: 28% (DCB) vs 21% (DES); P = 0.44 at 6 mo | MACE: 15% (DCB) vs 19% (DES); P = 0.58 at 12 mo |
| Shin et al79/registry-PS matched | DCB-based (Sequent Please) vs DES-only (2nd generation) | 200 vs 661 | MACE: 3.1% (DCB) vs 13.2% (DES); P = 0.001 at 2 y | |
| Diffuse long and multivessel disease | ||||
| Costopoulos et al80/registry-variable matching | DCB (IN.PACT Falcon + Pantera Lux) ± DES vs DES (2nd generation) | 93 vs 93 | - | MACE: 21% (DCB) vs 23% (DES); P = 0.74; TVR: 15% (DCB) vs 12% (DES); P = 0.44; TLR: 10% (DCB) vs 9% (DES); P = 0.84 at 2 y |
| Yang et al81/registry-observational | DCB (Sequent Please) ± DES (new-generation) vs DES (new-generation) | 360 vs 831 | LLL: 0.06 ± 0.61 mm (DCB) vs 0.41 ± 0.64 mm (DES); P < 0.001; MLD: 1.74 ± 0.59 mm (DCB) vs 1.94 ± 0.65 mm (DES); P = 0.002 at 9-12 mo | TLR: 7% (DCB) vs 8% (DES), log-rank P = 0.636; MACE: 11% (DCB) vs 14% (DES), log-rank P = 0.324; Thrombosis: 0 (DCB) vs 1% (DCB), log-rank P = 0.193 at 3 y |
| Shin et al44/registry-PS matched | DCB-based (Sequent Please) vs DES (2nd generation) | 254 vs 254 | - | MACE: 4% (DCB) vs 11% (DES); P = 0.002 at 2 y |
BMS = bare-metal stent; DCB = drug-coated balloon; DES = drug-eluting stent; DES-E = everolimus drug-eluting stent; DES-P = paclitaxel drug-eluting stents; DES-S = sirolimus drug-eluting stent; DoCE = device-oriented composite endpoint; FFR = fractional flow reserve; LLL = late lumen loss; MACE = major adverse cardiac events; MLD = minimal lumen diameter; MV = main vessel; NCB = noncompliant balloon; POBA = plain old balloon angioplasty; PS = propensity score; RCT = randomized controlled trial; SB = side-branch; SMD = standard mean deviation; TLF = target lesion failure; TLR = target lesion revascularization; TVF = target vessel failure; TVR = target vessel revascularization.
Large vessel disease
Given the favorable outcomes of DCB treatment in small vessel disease, increasing evidence supports its efficacy and safety in treating de novo lesions in large coronary vessels (≥3.0 mm) (Table 1).59 When residual dissections and limited acute luminal gain persist post-predilation, FFR application can provide a more precise assessment of functional results. Recent studies conducted in Korea have shown that FFR-guided DCB treatment is safe and effective for managing de novo lesions in large coronary vessels, including in individuals with acute coronary syndrome.28,60 Favorable anatomical and physiological outcomes were observed during the 9-month follow-up. Notably, DCB treatment led to significantly lower late lumen loss than DES treatment, with no difference in FFR values between the 2 groups. The authors suggest that DCB treatment enhances coronary blood flow restoration by modifying plaque, resulting in a gradual enlargement of the minimal lumen area, as demonstrated by intravascular imaging techniques, including IVUS or OCT.11,12,61,62 Additional evidence from Europe has demonstrated that using FFR-guided DCB-alone strategy is feasible and well-tolerated for treating stable CAD. This approach results in positive remodeling without compromising lumen integrity, as confirmed by OCT findings during the 6-month follow-up.63
Recent studies have indicated that DCB treatment is equally effective and safe for managing de novo lesions in nonsmall and small coronary vessels.9 Her et al9 demonstrated that the target vessel failure rate did not significantly differ between the 2 groups over a 3.4-year follow-up. In another recent study focusing on lesions of the left anterior descending artery, Gitto et al64 found that DCB-based treatment for left anterior descending lesions was safe, with acceptable rates of adverse events at the 2-year follow-up compared with contemporary DES-based treatment. DCB-based treatment was found to be associated with a lower risk of TLF than DES-alone–based PCI, mainly attributable to a reduction in TLR rates. Furthermore, a recent meta-analysis indicated that DCB treatment showed comparable efficacy to DES implantation in treating de novo large coronary lesions, with similar rates of TLR observed at the 6- to 9-month follow-up.65 This suggests that DCB treatment for large coronary vessel disease leads to sustained favorable anatomical patency, vascular healing, and promising long-term clinical outcomes.
In the most recent randomized trial comparing DCB and DES for large vessel disease, the REC-CAGEFREE I (Drug-Coated Balloon Angioplasty With Rescue Stenting Versus Intended Stenting for the Treatment Oo Patients With De Novo Coronary Artery Lesions) trial, it was found that in patients with de novo, noncomplex coronary artery disease, regardless of vessel diameter, a DCB treatment with rescue stenting failed to demonstrate noninferiority to DES implantation in terms of device-oriented composite endpoint (DoCE) at 2 years.66 While this study demonstrated a significant interaction between reference vessel diameter and DoCE occurrence, the final conclusions should ideally await the results of ongoing studies, such as the REVERSE (Randomized Trial of Drug-Coated Balloon Versus Drug-Eluting Stent for Clinical Outcomes in Patients With Large Coronary Artery Disease; NCT05846893), and future researches in this area. The REVERSE trial is currently evaluating the safety and effectiveness of DCB compared with DES for large vessel disease (≥3.0 mm), with results expected to provide valuable insights into clinical outcomes in de novo large vessel CAD.
Bifurcation lesion
Long-term clinical outcomes for patients with coronary bifurcation lesions usually show unfavorable outcomes, marked by an increased risk of cardiac death, myocardial infarction (MI), and the need for revascularization in the main vessel and side-branch.6,7,67 According to findings from the PEPCAD-BIF (Paclitaxel-Eluting Percutaneous Coronary Angioplasty-Balloon Catheter versus Conventional Balloon Angioplasty For Coronary Bifurcations) trial and a meta-analysis, the DCB group exhibited a significantly lower restenosis rate than the BA group, particularly when the side-branch was treated with DCB (Table 1).68,69 The recent DCB-BIF (Comparison of Noncompliant Balloon With Drug-Coated Balloon Angioplasties for Side Branch After Provisional Stenting for Patients With True Coronary Bifurcation Lesions) trial showed that in patients with true coronary bifurcation lesions undergoing provisional stenting, main vessel stenting combined with a DCB for the compromised side-branch resulted in a lower 1-year composite outcome rate compared to a noncompliant balloon.70 The Korean OCT study, focusing on cases where only the main vessel underwent a DCB-alone treatment approach, supported DCB treatment safety for bifurcation lesions.71 Although the side-branch was not directly treated, the study observed an increase in the lumen area at the side-branch ostium after 9-month follow-up. Regarding left main bifurcation lesions, the latest study demonstrated that applying a hybrid strategy combining DCB and DES yielded favorable outcomes, particularly in terms of late lumen loss and TLF, compared to a DES-alone approach (provisional stenting or two-stent strategies).72 DES-alone–treated patients had a significantly higher late lumen loss at 1 year than patients in the DCB group. Furthermore, the DCB group exhibited a lower TLF incidence within 2 years than the DES group. Recent studies suggest that DCB treatment post–coronary atherectomy for bifurcation lesions, including those in the left main coronary artery, could reduce the need for stents, eliminate complex stent placement techniques, and lead to favorable clinical outcomes.27,73 Particularly in bifurcation lesions, DCB treatment shows promising outcomes, including low restenosis rates, improved side-branch lumen area, and favorable clinical results compared with conventional approaches, such as DES-alone strategies.72,74 Although the optimal strategy and specific role of DCB treatment in bifurcation diseases remain unclear, DCB is gradually being recognized as a possible alternative option for managing bifurcation lesions.
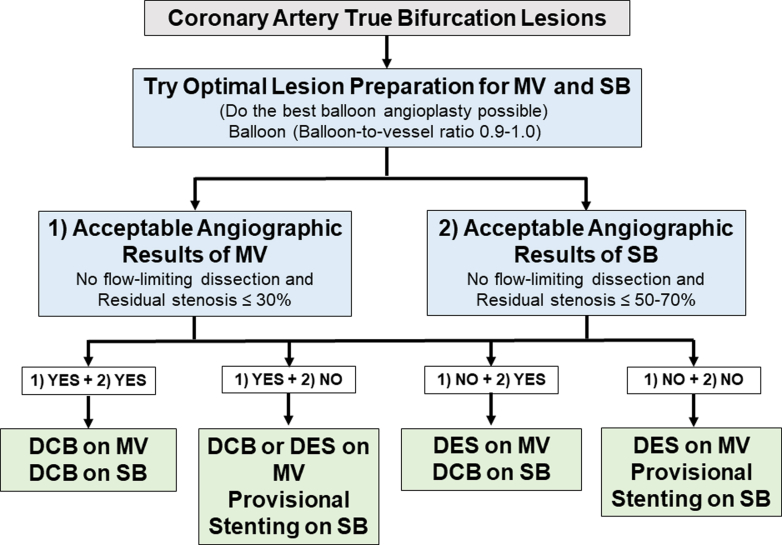
The initial step involves predilating the main vessel and/or side-branch using conventional balloons with a balloon-to-vessel ratio of 0.9 to 1.0 and an inflation pressure higher than nominal (Figure 1). DCB application to the side-branch is feasible if no flow-limiting dissection occurs and residual stenosis is ≤30% in the main vessel and ≤70% in the side-branch with TIMI flow grade 3. The DCB should extend 4 to 5 mm into the main vessel and 2 to 3 mm distally beyond the predilated area, using a DCB-to-vessel ratio of 0.9 to 1.0 for at least 60 seconds. Subsequently, DCB can be similarly applied to the main vessel, extending the balloon-covered length 2 to 3 mm beyond both sides of the predilated area. However, in cases of suboptimal lesion preparation, if the result remains unsatisfactory despite additional lesion preparation, deploying a DES in the main vessel and performing provisional stenting in the side-branch may be considered. Alternatively, employing a DES in the main vessel while adopting a DCB-alone approach in the side-branch may be considered reasonable and has shown efficacy based on prior evidence, although further scientific evaluations are needed.
Figure 1.
DCB Treatment Approach for True Bifurcation Lesion
The procedure involves predilating vessels with a balloon-to-vessel ratio of 0.9 to 1.0 and applying drug-coated balloon (DCB) if flow-limiting dissection is absent and residual stenosis meets criteria in main vessel and/or side-branch. DCB should cover the predilated area with appropriate overlap, but if results are unsatisfactory, drug-eluting stent (DES) implantation in the main vessel and/or side-branch may be considered. MV = main vessel; SB = side branch.
Chronic total occlusion lesion
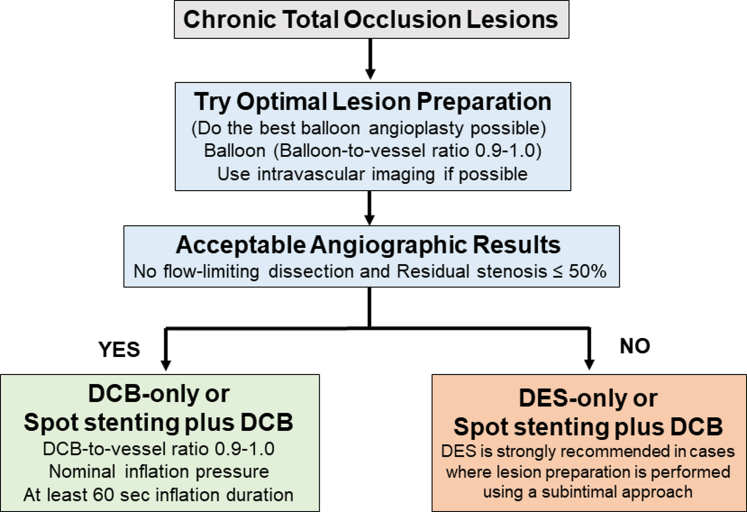
Chronic total occlusion (CTO) presents substantial technical challenges during PCI, particularly in accurately sizing stents post-BA. This challenge usually leads to underestimated stent dimensions and the risk of late stent malapposition. Furthermore, adopting a “full-metal jacket” PCI approach involving overlapping DES has been associated with an increased incidence of adverse events.75 Previous research on “full-metal jacket” PCI identified the number of DES in the target vessel and the presence of persistent distal luminal narrowing as 2 significant predictors of TLF.76 The PEPCAD CTO (Percutaneous Transluminal Coronary Angioplasty-Balloon Catheter in Coronary Artery Disease to Treat Chronic Total Occlusions) trial reported no significant differences in angiographic findings and clinical outcomes between the BMS+DCB- and DES-treated groups (Table 1).77 A recent study also showed that employing a DCB-alone approach without stenting was feasible and well-tolerated for treating CTO lesions, particularly when satisfactory outcomes were achieved with predilation.78 Using a DCB-based treatment strategy (either alone or combined with DES) presents promising opportunities for effectively managing such lesions.79 Specifically, the DCB-based treatment strategy was used to treat a CTO lesion, showing that with adequate lesion preparation, DCB treatment can be safely applied, provided TIMI flow grade 3 and residual stenosis ≤50% are achieved (Figure 2). However, in cases where lesion preparation is performed using a subintimal approach, the safety and clinical evidence supporting the use of DCB remain uncertain. Therefore, in such cases, DES implantation should be recommended to ensure optimal flow and durable long-term outcomes.
Figure 2.
DCB Treatment Approach for Chronic Total Occlusion Lesion
The DCB-based strategy effectively treats CTO lesions with adequate preparation and TIMI flow grade 3 and the residual diameter stenosis ≤50%, but DES is preferred if a subintimal approach is used, even if criteria are met. Abbreviations as in Figure 1.
Diffuse long and multivessel diseases
In situations involving diffuse long and multivessel lesions and those with a high burden of stents, the deployment of multiple lengthy metallic devices within coronary arteries may impair the restoration of vasomotion in the treated segment.6 This compromise can cause stent-related complications, including ISR, stent thrombosis, and neoatherosclerosis, potentially affecting the feasibility of subsequent CABG surgery. A retrospective analysis comparing the management of diffuse lesions (>25 mm) included patients treated with a DCB alone or combined with DES and those treated with DES alone.80 At the 2-year follow-up, no significant differences in clinical outcomes were found between the DCB strategy or DES-alone approach. Another observational study focusing on lesions longer than 25 mm showed that the long-term efficacy and safety outcomes associated with the DCB strategy, either alone or combined with DES, were comparable to the DES-alone approach in diffuse coronary lesions. This conclusion was supported by similar rates of TLR and MACE observed during the 3-year follow-up.81 Shin et al44 investigated multivessel CAD and reported that adopting a DCB-based strategy, either alone or combined with DES, substantially reduced the stent burden (Table 1). Furthermore, this approach resulted in a significantly lower MACE incidence than DES-alone treatment during the 2-year follow-up.44 These findings suggest that using a DCB-based treatment strategy for diffuse long and multivessel diseases effectively reduces the stent burden, particularly in small vessel lesions, and shows potential for improving long-term outcomes by decreasing stent-related complications.
For diffuse long and multivessel diseases, the PCI target lesions are first determined, followed by BA to assess suitability for DCB treatment. Specifically, predilation with a semicompliant or noncompliant balloon at a balloon-to-vessel ratio of 1.0 is mandatory. Post-BA, stenting is deferred for all types of dissections (A to E), provided TIMI flow grade 3 is achieved. PCI with stent implantation without DCB use is recommended in cases of flow-limiting dissection post-predilation (TIMI flow grade <3) and residual diameter stenosis >30%. However, in exceptional cases with normal flow (TIMI flow grade 3) and residual diameter stenosis ≤30%, the operator may opt to use DES or DCB if the patient complains of new-onset chest pain post-BA or if ST-segment changes or progression of dissection is evident.44 The DCB was inflated to its nominal pressure for at least 60 seconds, ensuring it covered the full length of the lesions dilated with predilatation balloons.
Drug-Coated Balloon Application According to Patients’ Characteristics
High-bleeding risk patients
Post-PCI bleeding is associated with higher mortality rates and can lead to additional complications, including MI and prolonged hospital stays, underscoring the importance of preventive strategies.7,82 Despite the shorter DAPT duration required post–new-generation DES implantation than post–old-generation stent, the possibility of discontinuing antiplatelet agents may arise sooner post-DCB treatment, particularly in cases of severe, life-threatening bleeding than DES implantation. The DEBUT (Drug-Coated Balloon for Treatment of De-Novo Coronary Artery Lesions in Patients With High Bleeding Risk) trial demonstrated that DCB treatment was superior to BMS in high-bleeding risk patients, showing significantly lower rates of MACE during the 9-month follow-up (Table 2).83 High-bleeding risk patients have shown superior efficacy in angiographic and physiologic patencies during the 9-month follow-up with FFR-guided DCB treatment, requiring only 1 month of DAPT, compared with BMS.84 Recent studies show no significant difference in thrombotic event rates between single antiplatelet agent after DCB and standard antiplatelet regimens, with lower major bleeding rates.85,86 These results suggest it could be a preferred option for this patient subgroup. Therefore, for high-bleeding risk patients, DCB treatment presents advantages over stent implantation, indicating a need for future comparisons with newer-generation DES.
Table 2.
Summary of Clinical Trials of DCB on Treatment According to Clinical Characteristics
| Trial Name or First Author Design | Treatment | N | Angiographic Follow-up | Clinical Follow-up |
|---|---|---|---|---|
| High bleeding risk | ||||
| DEBUT83/RCT | DCB (Sequent Please) vs BMS (Integrity) | 102 vs 106 | - | MACE: 1% vs 14%; RR: 0.07; P < 0.00001 for noninferiority; P = 0.00034 for superiority at 9 mo |
| Shin et al84/RCT | DCB (Sequent Please) vs BMS (Vision) | 20 vs 20 | LLL: 0.2 ± 0.3 mm (DCB) vs 1.2 ± 0.8 mm (DES); P < 0.001; Restenosis: 0 (DCB) vs 25% (DES); P = 0.049; FFR: 0.87 ± 0.06 (DCB) vs 0.89 ± 0.06 (DES); P = 0.254 at 9 mo | TVR: 0 (DCB) vs 15% (BMS) at 12 mo |
| Diabetes mellitus | ||||
| DEAR89/Retrospective head-to-head comparison | DCB (DIOR) ± BMS vs BMS vs DES (Cypher, Taxus, Endeavor, Biodegradable Paclitaxel Eluting stent) | 92 vs 96 vs 129 | - | MACE: 13% (DCB) vs 32% (BMS); P = 0.003; 19% (DES); P = 0.29; TVF: 11% (DCB) vs 30% (BMS); P = 0.003; 19% (DES); P = 0.13 at 12 mo |
| BASKET-SMALL 290/RCT | DCB (Sequent Please) vs DES (Xience or Taxus Element) | 122 vs 130 | - | MACE: 19% (DCB) vs 22% (DES); HR: 0.82; P = 0.51; TVR: 9% (DCB) vs 15% (DES); HR: 0.40; P = 0.036 at 3 y |
| Her et al45/Registry-PS matched | DCB-based (Sequent Please) vs DES-only (2nd generation) | 104 vs 115 | MACE: 2.9% (DCB) vs 13.9% (DES), log-rank P = 0.003; Cardiac death: 0% (DCB) vs 3.5% (DES), log-rank P = 0.044 at 2 y | |
| Acute myocardial infarction | ||||
| REVELATION93/RCT | DCB (Pantera Lux) vs DES (Orsiro) | 60 vs 60 | FFR: 0.92 ± 0.05 (DCB) vs 0.91 ± 0.06 (DES); P = 0.27; LLL: 0.05 mm (DCB) vs 0.00 mm (DES); P = 0.51 at 9 mo | MACE: 3% (DCB) vs 2% (DES); P = 1.00 at 9 mo |
| PEPCAD NSTEMI94/RCT | DCB (Sequent Please and Sequent Please Neo) vs BMS or DES (new-generation limus-eluting) | 104 vs 106 | TLF: 4% (DCB) vs 7% (Stent); P = 0.53 at 9 mo | MACE: 7% (DCB) vs 14% (DES); P = 0.11 at 9 mo |
Abbreviations as in Table 1.
Diabetes mellitus
Patients with diabetes are well-known to be at an increased risk of cardiovascular-related events, usually presenting with extensive diffuse disease necessitating longer and smaller stents compared to those without diabetes.87 Furthermore, they typically experience poorer outcomes post-PCI, including higher incidences of ISR, stent thrombosis, MI, and mortality, than patients without diabetes.88 The previous trial showed that patients with diabetes treated with DCB followed by BMS had significantly better outcomes than those treated with BMS alone, and their results were comparable to those of DES-treated patients.89 Moreover, a recent subgroup analysis of the BASKET-SMALL 2 trial demonstrated that DCB treatment was noninferior to DES and showed a significantly lower TVR rate than DES in patients with diabetes.90 In a recent Korean study, Her et al45 demonstrated that among individuals with multivessel CAD, the clinical benefits of using a DCB-based revascularization strategy (either alone or combined with DES) appear to be more significant in patients with diabetes than in those without diabetes during the 2-year follow-up (Table 2). Furthermore, the DCB-based treatment group exhibited a significantly lower MACE risk in patients with diabetes than the DES-only group, whereas this risk reduction was not observed in nondiabetic patients. These findings suggest that adopting a DCB-based PCI strategy, particularly in patients with diabetes, substantially reduces the MACE risk compared with conventional treatments involving DES alone. Although additional data and further analyses are necessary, DCB treatment might represent a favorable option compared with DES implantation for patients with diabetes.
Acute MI
Acute myocardial infarction (AMI), being the typical example of atherothrombotic lesions, has made significant strides in reducing repeat revascularization needs with stent placement. However, patients with AMI still encounter ongoing risks of stent thrombosis and ISR.91 Therefore, DCB treatment could be considered an alternative therapeutic approach for AMI, particularly when the coronary flow is restored and residual stenosis is minimal post-thrombus aspiration and balloon dilation.6,92 In the randomized REVELATION (Revascularization with Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction) trial, DCB treatment demonstrated noninferiority to DES in terms of FFR during the 9-month follow-up (Table 2).93 These findings suggest that DCB treatment could be a safe and effective approach for treating ST-segment elevation myocardial infarction (STEMI), provided that the culprit lesion is sufficiently prepared. In patients with non-STEMI, Scheller et al94 demonstrated that DCB treatment was noninferior to stenting with BMS or DES. A recent meta-analysis also indicated the feasibility of DCB treatment compared to DES implantation.95 The clinical safety and angiographic efficacy of DCB treatment were validated across a spectrum of adverse cardiovascular-related events, including MACE, cardiac mortality, all-cause mortality, MI, bleeding events, TVR, TLR, and late lumen loss. Finally, DCB treatment has shown noninferiority to DES in both STEMI and non-STEMI, suggesting it to be a safe and effective alternative supported by evidence across various acute coronary syndrome cases, affirming its clinical safety and angiographic efficacy in managing adverse cardiovascular-related outcomes. These results highlight the necessity for further research on DCB treatment, ideally through larger randomized trials using DES as control groups.
For patients with AMI, after identifying the infarct-related lesion, thrombus aspiration is conducted if the thrombus is observed, as determined by the operator (Figure 3). Although intravascular imaging can assist in the detection and confirmation of thrombus presence, as well as in assessing its successful removal, its role in thrombus burden quantification and evaluation during index procedures in AMI patients is relatively limited. Predilation with a plain balloon was mandatory, using a balloon-to-vessel ratio of 1.0. Stenting is deferred in all types of dissections (A to E) if TIMI flow grade 3 is achieved after predilatation.96 Visual residual stenosis ≤30% without flow-limiting dissection is considered an adequate lesion preparation and followed by DCB treatment. If lesion preparation is inadequate or a flow-limiting dissection occurs before DCB application, switching to DES implantation without applying DCB is considered a provisional strategy rather than a bailout. The DCB is inflated to nominal pressure for at least 60 seconds, extending the DCB at least 2 mm beyond the initial pre-dilatation. Following the application of DCB, the final assessment is conducted at least 5 minutes after intracoronary vasodilator administration. In cases of substantial thrombus burden, a rescue strategy with glycoprotein IIb/IIIa receptor inhibitors is utilized.
Figure 3.
DCB Treatment Approach for Acute Myocardial Infarction
In acute myocardial infarction patients, the use of intravascular imaging can assist in the detection and confirmation of thrombus presence. Predilation with a 1.0 balloon-to-vessel ratio is required, followed by DCB treatment if the residual stenosis is ≤30% without flow-limiting dissection and the thrombus burden is low. If lesion preparation is inadequate or a high thrombus burden persists, DES implantation is employed as a provisional strategy. Abbreviations as in Figure 1.
Sirolimus-Coated Balloons Treatment
Paclitaxel continues to be widely used for balloon coating because of its high lipophilicity and potent antiproliferative effects.97 However, recent studies have increasingly focused on alternative coatings and drug delivery technologies.5,98,99 All currently available DES release sirolimus or its analogs, demonstrating superior outcomes compared with paclitaxel-eluting stents across various indications. Moreover, paclitaxel-eluting stents exhibit a higher stent thrombosis incidence than BMS and sirolimus-eluting stents.100 Despite the superior safety and effectiveness of limus-eluting stents in coronary lesions compared with paclitaxel, sirolimus may encounter challenges when used in DCBs. When considering the mechanism of DCB treatment, these devices require rapid drug release and short transit times. However, sirolimus’s low lipophilicity and limited ability to penetrate and persist within the target vessel wall, particularly in the adventitia, hinder these requirements. In the first RCT comparing a novel sirolimus-coated balloon (SCB) to a widely used paclitaxel-coated balloon (PCB) for de novo coronary lesions, the SCB with a crystalline coating showed comparable angiographic outcomes.13 During the 6-month follow-up, in-segment late lumen loss measured 0.10 ± 0.32 mm and 0.01 ± 0.33 mm in the SCB and PCB groups, respectively. However, a higher late lumen enlargement incidence was noted among PCB-treated patients. In the TRANSFORM (Treatment of Small Coronary Vessels: MagicTouch Sirolimus Coated Balloon) I trial, patients with de novo single-vessel disease (≤2.75 mm) were randomly assigned to receive the MagicTouch SCB or the SeQuent Please Neo PCB.37 This was primarily aimed at evaluating in-segment net lumen gain assessed by angiography during the 6-month follow-up. The SCB did not achieve noninferiority in terms of angiographic net gain at this time point compared with the PCB. This difference was primarily driven by a smaller late lumen loss and a higher incidence of late lumen enlargement with PCB. These findings indicate that paclitaxel demonstrates superior angiographic outcomes compared with sirolimus in DCB treatment, contrasting with its performance in stent-based drug delivery. However, larger-scaled studies with adequate sample sizes and sufficient statistical power are necessary to assess clinical outcomes and establish the efficacy of SCB compared to PCB in coronary lesions.
Future Direction of DCB Treatment
Ongoing randomized controlled trials
Recent data suggest that DCB treatment provides improved safety and efficacy across various clinical and angiographic scenarios (Tables 1 and 2). However, ongoing trials are being conducted to provide clear evidence regarding the DCB treatment of de novo large coronary vessels, bifurcation lesions, high-bleeding risk patients, and the use of sirolimus-coated balloons (Table 3).
Table 3.
List of Current Ongoing Randomized Controlled Trials of Drug-Coated Balloon Treatment in Coronary Artery Disease
| NCT# Trial Name (Year) | Device (n) | Study Population | Primary Endpoint | Follow-up (mo) |
|---|---|---|---|---|
| NCT05846893 REVERSE (2023) | SeQuent Please NEO DCB (718) vs current-generation DES (718) | De novo large CAD (≥3.0 mm by visual estimation) | NACE: composite of all-cause death, nonfatal MI, TVR, or major bleeding | 12 |
|
NCT06084000 STENTLESS (2023) |
Bingo DCB (1,350) vs second-generation DES (1,350) | De novo large CAD (≥2.75 mm diameter) | MACE: composite of cardiac death, target-vessel MI, and TVR | 12 |
| NCT04664283 (2021) | DCB (127) by OCT-guided vs DES (127) by OCT-guided | De novo large CAD (vessel diameter 3.0-4.5 mm) | Late lumen loss by QCA | 12 |
|
NCT04242134 DCB-BIF (2020) |
PS-DCB (392) vs PS-NCB (392) | De novo true bifurcation lesions | MACE: composite of cardiac death, MI, and TLR | 12 |
|
NCT05221931 DCB-HBR (2022) |
DES (Ultimaster) (675) vs DCB (PCB) (675) | De novo coronary lesions in patients with high bleeding risk | TVF: composite of cardiovascular death, target-vessel MI, and TVR | 24 |
|
NCT04814212 DEBATE (2022) |
SeQuent® Please NEO DCB (273) vs DES (273) | De novo stable CAD or ACS patients at high risk of bleeding | MACE and major bleeding (MACE: composite of cardiac death, nonfatal MI, and TLR) | 12 |
|
NCT04893291 TRANSFORM II (2021) |
SCB (Magic Touch) DCB (910) vs EES DES (910) | De novo small and medium-sized coronary lesions (2-3 mm) | TLF and NACE (TLF: composite of cardiac death, all-cause death, MI, TLR, TVR, and bleeding; NACE: composite of all-cause death, MI, ischemic stroke, and major bleeding) | 12 |
|
NCT04859985 SELUTION DeNovo (2021) |
SCB (SELUTION SLR) DCB (1,663) vs DES (1,663) | De novo chronic coronary syndrome, unstable angina or NSTEMI | TVF (TVF: composite of cardiac death, target-vessel–related MI, and TVR) | 12 |
ACS = acute coronary syndrome; CAD = coronary artery disease; EES = everolimus eluting stent; MI = myocardial infarction; NACE = net adverse clinical event; OCT = optical coherence tomography; PCB = paclitaxel coated balloon; QCA = quantitative coronary analysis; SCB = sirolimus coated balloon; other abbreviations as in Table 1.
For de novo large coronary vessels, the REVERSE trial (NCT05846893), aim to establish the noninferiority of DCB treatment compared with current-generation DES in patients with de novo lesions in large CAD (reference vessel diameter ≥3.0 mm by visual estimation). Similarly, the STENTLESS (STrategies of Scheduled Drug-coated Balloons [DCB] Versus Conventional DES for the interveNTional Therapy of de Novo Lesions in Large Coronary vESSels; NCT06084000) trial is investigating the safety and efficacy of scheduled DCB vs conventional DES strategies for treating de novo lesions in large coronary vessels (≥2.75 mm diameter). Another trial (NCT04664283) intends to determine if DCB is noninferior to DES in treating large vessel disease (3.0–4.5 mm vessel diameter), assessed via OCT.
The DCB-BIF (Drug-coating Balloon Angioplasties for True Coronary Bifurcation Lesions; NCT04242134) trial aims to determine if using DCB compared with conventional BA for the side-branch postprovisional stenting in true coronary de novo bifurcation lesions reduces the incidence of the composite endpoint of MACE at 12 months.101
The DCB-HBR (Drug-Coated Balloon Versus Drug-Eluting Stent for Treatment of De Novo Coronary Lesions in Patients With High Bleeding Risk; NCT05221931) aims to assess the clinical outcomes of DCB versus DES for treating de novo coronary lesions, guided by intravascular imaging optimization in high-bleeding risk patients. Additionally, the DEBATE (Drug-Coated Balloon in Anticoagulated and Bleeding Risk Patients Undergoing PCI; NCT04814212) trial is proposed to determine whether a DCB strategy with a shorter DAPT regimen is noninferior to a DES approach with longer DAPT in patients with stable CAD or acute coronary syndrome at high-bleeding risk, including those on anticoagulation therapy.
The TRANSFORM II (Sirolimus-coated Balloon Versus Drug-eluting Stent in Native Coronary Vessels; NCT04893291) trial aims to evaluate the use of DCB in treating small- and medium-sized de novo coronary lesions (2-3 mm) by comparing MagicTouch DCB with everolimus-eluting stents, focusing on TLF at 12 months of follow-up.102 The SELUTION DeNovo trial (NCT04859985) is designed to demonstrate noninferiority at 1 year for TVF of a treatment strategy with SCB and provisional DES vs DES in de novo coronary lesions.103
Metal-free PCI
Although DES technologies have significantly improved the safety and efficacy of PCI in treating obstructive CAD, the permanent presence of a metallic stent in the coronary artery can result in vascular inflammation, hypersensitivity reactions, accelerated neoatherosclerosis, stent thrombosis, and compromised late luminal enlargement and vasomotor function.104 Therefore, to overcome these issues with DES, bioresorbable scaffolds were developed to support vessel walls for a certain period and then gradually disappear as the vessels heal. However, it was withdrawn from the market caused by its inferior performance compared to contemporary DES in treating CAD.105,106 The failure of bioresorbable scaffolds has further promoted DCBs’ introduction and advancement with the “leave nothing behind” concept, highlighting their growing significance. Nevertheless, because the basic concept of bioresorbable scaffolds is sound, their development should continue. Therefore, considering previous failures, we anticipate that the development of new-generation bioresorbable scaffolds, alongside DCB innovations, could pave the way for a new era of metal-free PCI, representing a significant leap in the treatment of CAD. Including this perspective in the context of DCB therapy highlights critical areas for future research and addresses unanswered questions, fostering innovation and expanding the therapeutic landscape.
Conclusions
Although current recommendations for DCB treatment in CAD are limited to ISR lesions and de novo small vessel disease, it remains an appealing therapeutic option and could play a significant role in managing de novo coronary lesions across various disease subsets. DCB provides an additional tool in PCI that can enhance patient outcomes by addressing lesions previously challenging or less responsive to DES implantation. For more complex lesions, including de novo large vessel disease, bifurcation disease, and complex high-risk PCI, further use of intravascular imaging or functional measurements can provide valuable insights. Advancements in DCB technology, coupled with improvements in imaging techniques, physiologic guidance, and adjunctive pharmacotherapy, will also be essential to improving clinical outcomes in CAD management. DCB treatment is expected to play a pivotal role, particularly in the Asia-Pacific region, where patients usually present with small vessel disease or multiple diffuse lesions leading to increased adverse clinical events with DES implantation. Therefore, prioritizing the proper implementation of DCB procedures is essential to enhance the likelihood of successful outcomes. Finally, newer generation bioresorbable scaffold development and adoption in the future may enable metal-free PCI alongside DCB, potentially leading to changes in the field of PCI and improvements in outcomes for patients with CAD.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to thank Editage for English language editing.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure, please see the online version of this paper.
Appendix
References
- 1.Kufner S., Joner M., Thannheimer A., et al. Ten-year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease. Circulation. 2019;139:325–333. doi: 10.1161/CIRCULATIONAHA.118.038065. [DOI] [PubMed] [Google Scholar]
- 2.Kufner S., Ernst M., Cassese S., et al. 10-year outcomes from a randomized trial of polymer-free versus durable polymer drug-eluting coronary stents. J Am Coll Cardiol. 2020;76:146–158. doi: 10.1016/j.jacc.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed M.O., Polad J., Hildick-Smith D., et al. Impact of coronary lesion complexity in percutaneous coronary intervention: one-year outcomes from the large, multicentre e-Ultimaster registry. EuroIntervention. 2020;16:603–612. doi: 10.4244/EIJ-D-20-00361. [DOI] [PubMed] [Google Scholar]
- 4.Kong M.G., Han J.K., Kang J.H., et al. Clinical outcomes of long stenting in the drug-eluting stent era: patient-level pooled analysis from the GRAND-DES registry. EuroIntervention. 2021;16:1318–1325. doi: 10.4244/EIJ-D-19-00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheller B., Speck U., Abramjuk C., Bernhardt U., Böhm M., Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110:810–814. doi: 10.1161/01.CIR.0000138929.71660.E0. [DOI] [PubMed] [Google Scholar]
- 6.Her A.Y., Shin E.S., Bang L.H., et al. Drug-coated balloon treatment in coronary artery disease: recommendations from an Asia-Pacific Consensus Group. Cardiol J. 2021;28:136–149. doi: 10.5603/CJ.a2019.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeger R.V., Eccleshall S., Wan Ahmad W.A., et al. Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391–1402. doi: 10.1016/j.jcin.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 8.Tesfamariam B. Local arterial wall drug delivery using balloon catheter system. J Control Release. 2016;238:149–156. doi: 10.1016/j.jconrel.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Her A.Y., Yuan S.L., Jun E.J., et al. Drug-coated balloon treatment for nonsmall de-novo coronary artery disease: angiographic and clinical outcomes. Coron Artery Dis. 2021;32:534–540. doi: 10.1097/MCA.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 10.Hui L., Shin E.S., Jun E.J., et al. Impact of dissection after drug-coated balloon treatment of de novo coronary lesions: angiographic and clinical outcomes. Yonsei Med J. 2020;61:1004–1012. doi: 10.3349/ymj.2020.61.12.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ann S.H., Balbir Singh G., Lim K.H., Koo B.K., Shin E.S. Anatomical and physiological changes after paclitaxel-coated balloon for atherosclerotic de novo coronary lesions: serial IVUS-VH and FFR study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ann S.H., Her A.Y., Singh G.B., Okamura T., Koo B.K., Shin E.S. Serial morphological and functional assessment of the paclitaxel-coated balloon for de novo lesions. Rev Esp Cardiol (Engl Ed) 2016;69:1026–1032. doi: 10.1016/j.rec.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad W.A.W., Nuruddin A.A., Abdul Kader M., et al. Treatment of coronary de novo lesions by a sirolimus- or paclitaxel-coated balloon. JACC Cardiovasc Interv. 2022;15:770–779. doi: 10.1016/j.jcin.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 15.Jeger R.V., Farah A., Ohlow M.A., et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396:1504–1510. doi: 10.1016/S0140-6736(20)32173-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.Y., Cho Y.K., Kim S.W., et al. Clinical results of drug-coated balloon treatment in a large-scale multicenter Korean registry study. Korean Circ J. 2022;52:444–454. doi: 10.4070/kcj.2021.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson P., Sayer J., Laji K., et al. Comparison of case fatality in south Asian and white patients after acute myocardial infarction: observational study. BMJ. 1996;312:1330–1333. doi: 10.1136/bmj.312.7042.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zindrou D., Taylor K.M., Bagger J.P. Coronary artery size and disease in UK South Asian and Caucasian men. Eur J Cardiothorac Surg. 2006;29:492–495. doi: 10.1016/j.ejcts.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Nanditha A., Ma R.C., Ramachandran A., et al. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care. 2016;39:472–485. doi: 10.2337/dc15-1536. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama T., Moussa I., Reimers B., et al. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol. 1998;32:1610–1618. doi: 10.1016/s0735-1097(98)00444-6. [DOI] [PubMed] [Google Scholar]
- 21.Siontis G.C., Piccolo R., Praz F., et al. Percutaneous coronary interventions for the treatment of stenoses in small coronary arteries: a network meta-analysis. JACC Cardiovasc Interv. 2016;9:1324–1334. doi: 10.1016/j.jcin.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Kang J., Park K.W., Palmerini T., et al. Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. doi: 10.1055/s-0038-1676545. [DOI] [PubMed] [Google Scholar]
- 23.Ellis S.G., Roubin G.S., King S.B., 3rd, et al. Angiographic and clinical predictors of acute closure after native vessel coronary angioplasty. Circulation. 1988;77:372–379. doi: 10.1161/01.cir.77.2.372. [DOI] [PubMed] [Google Scholar]
- 24.Cappelletti A., Margonato A., Rosano G., et al. Short- and long-term evolution of unstented nonocclusive coronary dissection after coronary angioplasty. J Am Coll Cardiol. 1999;34:1484–1488. doi: 10.1016/s0735-1097(99)00395-2. [DOI] [PubMed] [Google Scholar]
- 25.Shin E.S., Ann S.H., Jang M.H., et al. Impact of scoring balloon angioplasty on lesion preparation for DCB treatment of coronary lesions. J Clin Med. 2023;12:6254. doi: 10.3390/jcm12196254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsui K., Lee T., Miyazaki R., et al. Drug-coated balloon strategy following orbital atherectomy for calcified coronary artery compared with drug-eluting stent: one-year outcomes and optical coherence tomography assessment. Catheter Cardiovasc Interv. 2023;102:11–17. doi: 10.1002/ccd.30689. [DOI] [PubMed] [Google Scholar]
- 27.Kitani S., Igarashi Y., Tsuchikane E., et al. Efficacy of drug-coated balloon angioplasty after directional coronary atherectomy for coronary bifurcation lesions (DCA/DCB registry) Catheter Cardiovasc Interv. 2021;97:E614–E623. doi: 10.1002/ccd.29185. [DOI] [PubMed] [Google Scholar]
- 28.Shin E.S., Ann S.H., Balbir Singh G., Lim K.H., Kleber F.X., Koo B.K. Fractional flow reserve-guided paclitaxel-coated balloon treatment for de novo coronary lesions. Catheter Cardiovasc Interv. 2016;88:193–200. doi: 10.1002/ccd.26257. [DOI] [PubMed] [Google Scholar]
- 29.Chung J.H., Shin E.S., Her A.Y., et al. Instantaneous wave-free ratio-guided paclitaxel-coated balloon treatment for de novo coronary lesions. Int J Cardiovasc Imaging. 2020;36:179–185. doi: 10.1007/s10554-019-01707-5. [DOI] [PubMed] [Google Scholar]
- 30.Gao X.F., Ge Z., Kong X.Q., et al. Intravascular ultrasound vs angiography-guided drug-coated balloon angioplasty: the ULTIMATE Ⅲ trial. JACC Cardiovasc Interv. 2024;17:1519–1528. doi: 10.1016/j.jcin.2024.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Cortese B., Silva Orrego P., Agostoni P., et al. Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv. 2015;8:2003–2009. doi: 10.1016/j.jcin.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M., Hara H., Kubota S., Hiroi Y. Predictors of late lumen enlargement after drug-coated balloon angioplasty for de novo coronary lesions. EuroIntervention. 2024;20:602–612. doi: 10.4244/EIJ-D-23-00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin E.S., Jun E.J., Kim B. Vascular remodeling after drug-coated balloon treatment: insight from optical coherence tomography. Korean Circ J. 2023;53:191–193. doi: 10.4070/kcj.2022.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirigaya H., Okada K., Hibi K., et al. Post-procedural quantitative flow ratio gradient and target lesion revascularization after drug-coated balloon or plain-old balloon angioplasty. J Cardiol. 2022;80:511–517. doi: 10.1016/j.jjcc.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Gao X.F., Ge Z., Kong X.Q., et al. Intravascular ultrasound vs angiography-guided drug-coated balloon angioplasty: the ULTIMATE Ⅲ Trial. JACC Cardiovasc Interv. 2024;17:1519–1528. doi: 10.1016/j.jcin.2024.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Serruys P.W., Tobe A., Ninomiya K., et al. Editorial: Is the axiom of balloon angioplasty, "the more you gain the more you lose", still true in the era of DCB with paclitaxel? Cardiovasc Revasc Med. 2024;69:70–78. doi: 10.1016/j.carrev.2024.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Ninomiya K., Serruys P.W., Colombo A., et al. A prospective randomized trial comparing sirolimus-coated balloon with paclitaxel-coated balloon in de novo small vessels. JACC Cardiovasc Interv. 2023;16:2884–2896. doi: 10.1016/j.jcin.2023.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Koiwaya H., Watanabe N., Kuriyama N., et al. Predictors of recurrent in-stent restenosis after paclitaxel-coated balloon angioplasty. Circ J. 2017;81:1286–1292. doi: 10.1253/circj.CJ-17-0095. [DOI] [PubMed] [Google Scholar]
- 39.Gogas B.D., Garcia-Garcia H.M., Onuma Y., et al. Edge vascular response after percutaneous coronary intervention: an intracoronary ultrasound and optical coherence tomography appraisal: from radioactive platforms to first- and second-generation drug-eluting stents and bioresorbable scaffolds. JACC Cardiovasc Interv. 2013;6:211–221. doi: 10.1016/j.jcin.2013.01.132. [DOI] [PubMed] [Google Scholar]
- 40.Ono M., Kawashima H., Hara H., et al. A prospective multicenter randomized trial to assess the effectiveness of the MagicTouch sirolimus-coated balloon in small vessels: rationale and design of the TRANSFORM I Trial. Cardiovasc Revasc Med. 2021;25:29–35. doi: 10.1016/j.carrev.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Lee H.S., Kang J., Park K.W., et al. Procedural optimization of drug-coated balloons in the treatment of coronary artery disease. Catheter Cardiovasc Interv. 2021;98:E43–E52. doi: 10.1002/ccd.29492. [DOI] [PubMed] [Google Scholar]
- 42.Rhee T.M., Lee J.M., Shin E.S., et al. Impact of optimized procedure-related factors in drug-eluting balloon angioplasty for treatment of in-stent restenosis. JACC Cardiovasc Interv. 2018;11:969–978. doi: 10.1016/j.jcin.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Gurgoglione F.L., Gattuso D., Greco A., Donelli D., Niccoli G., Cortese B. Angiographic and clinical impact of balloon inflation time in percutaneous coronary interventions with sirolimus-coated balloon: a subanalysis of the EASTBOURNE study. Cardiovasc Revasc Med. 2024 doi: 10.1016/j.carrev.2024.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Shin E.S., Jun E.J., Kim S., et al. Clinical Impact of Drug-Coated Balloon-Based Percutaneous Coronary Intervention in Patients With Multivessel Coronary Artery Disease. JACC Cardiovasc Interv. 2023;16:292–299. doi: 10.1016/j.jcin.2022.10.049. [DOI] [PubMed] [Google Scholar]
- 45.Her A.Y., Shin E.S., Kim S., et al. Drug-coated balloon-based versus drug-eluting stent-only revascularization in patients with diabetes and multivessel coronary artery disease. Cardiovasc Diabetol. 2023;22:120. doi: 10.1186/s12933-023-01853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrne R.A., Neumann F.J., Mehilli J., et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381:461–467. doi: 10.1016/S0140-6736(12)61964-3. [DOI] [PubMed] [Google Scholar]
- 47.Lee J.M., Park J., Kang J., et al. Comparison among drug-eluting balloon, drug-eluting stent, and plain balloon angioplasty for the treatment of in-stent restenosis: a network meta-analysis of 11 randomized, controlled trials. JACC Cardiovasc Interv. 2015;8:382–394. doi: 10.1016/j.jcin.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Alfonso F., Pérez-Vizcayno M.J., Cárdenas A., et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V clinical trial (restenosis intra-stent of bare metal stents: paclitaxel-eluting balloon vs everolimus-eluting stent) J Am Coll Cardiol. 2014;63:1378–1386. doi: 10.1016/j.jacc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Alfonso F., Perez-Vizcayno M.J., Cardenas A., et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV randomized clinical trial. J Am Coll Cardiol. 2015;66:23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 50.Baan J., Jr., Claessen B.E., Dijk K.B., et al. A randomized comparison of paclitaxel-eluting balloon versus everolimus-eluting stent for the treatment of any in-stent restenosis: the DARE trial. JACC Cardiovasc Interv. 2018;11:275–283. doi: 10.1016/j.jcin.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Vrints C., Andreotti F., Koskinas K.C., et al. 2024 ESC guidelines for the management of chronic coronary syndromes: developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2024;45:3415–3537. [Google Scholar]
- 52.Lee W.C., Wu C.J., Chen Y.L., et al. Associations between target lesion restenosis and drug-eluting balloon use: an observational study. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee W.C., Fang Y.N., Fang C.Y., et al. Comparison of clinical results following the use of drug-eluting balloons for a bare-metal stent and drug-eluting stent instent restenosis. J Interv Cardiol. 2016;29:469–479. doi: 10.1111/joic.12327. [DOI] [PubMed] [Google Scholar]
- 54.Giacoppo D., Alfonso F., Xu B., et al. Paclitaxel-coated balloon angioplasty vs drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study) Eur Heart J. 2020;41:3715–3728. doi: 10.1093/eurheartj/ehz594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Claessen B.E., Smits P.C., Kereiakes D.J., et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv. 2011;4:1209–1215. doi: 10.1016/j.jcin.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 56.van der Heijden L.C., Kok M.M., Danse P.W., et al. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: Insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am Heart J. 2016;176:28–35. doi: 10.1016/j.ahj.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 57.Cortese B., Di Palma G., Guimaraes M.G., et al. Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: PICCOLETO II randomized clinical trial. JACC Cardiovasc Interv. 2020;13:2840–2849. doi: 10.1016/j.jcin.2020.08.035. [DOI] [PubMed] [Google Scholar]
- 58.Kiyohara Y., Aikawa T., Kayanuma K., et al. Comparison of clinical outcomes among various percutaneous coronary intervention strategies for small coronary artery disease. Am J Cardiol. 2024;211:334–342. doi: 10.1016/j.amjcard.2023.11.043. [DOI] [PubMed] [Google Scholar]
- 59.Uskela S., Kärkkäinen J.M., Eränen J., et al. Percutaneous coronary intervention with drug-coated balloon-only strategy in stable coronary artery disease and in acute coronary syndromes: an all-comers registry study. Catheter Cardiovasc Interv. 2019;93:893–900. doi: 10.1002/ccd.27950. [DOI] [PubMed] [Google Scholar]
- 60.Chung J.H., Lee K.E., Her A.Y., et al. Comparison of fractional flow reserve and angiographic characteristics after balloon angioplasty in de novo coronary lesions. Int J Cardiovasc Imaging. 2019;35:1945–1954. doi: 10.1007/s10554-019-01649-y. [DOI] [PubMed] [Google Scholar]
- 61.Her A.Y., Shin E.S., Lee J.M., et al. Paclitaxel-coated balloon treatment for functionally nonsignificant residual coronary lesions after balloon angioplasty. Int J Cardiovasc Imaging. 2018;34:1339–1347. doi: 10.1007/s10554-018-1351-z. [DOI] [PubMed] [Google Scholar]
- 62.Her A.Y., Shin E.S., Chung J.H., et al. Plaque modification and stabilization after paclitaxel-coated balloon treatment for de novo coronary lesions. Heart Vessels. 2019;34:1113–1121. doi: 10.1007/s00380-019-01346-9. [DOI] [PubMed] [Google Scholar]
- 63.Poerner T.C., Duderstadt C., Goebel B., Kretzschmar D., Figulla H.R., Otto S. Fractional flow reserve-guided coronary angioplasty using paclitaxel-coated balloons without stent implantation: feasibility, safety and 6-month results by angiography and optical coherence tomography. Clin Res Cardiol. 2017;106:18–27. doi: 10.1007/s00392-016-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gitto M., Sticchi A., Chiarito M., et al. Drug-coated balloon angioplasty for de novo lesions on the left anterior descending artery. Circ Cardiovasc Interv. 2023;16 doi: 10.1161/CIRCINTERVENTIONS.123.013232. [DOI] [PubMed] [Google Scholar]
- 65.Lin Y., Sun X., Liu H., Pang X., Dong S. Drug-coated balloon versus drug-eluting stent for treating de novo coronary lesions in large vessels: a meta-analysis of clinical trials. Herz. 2021;46:269–276. doi: 10.1007/s00059-020-04938-8. [DOI] [PubMed] [Google Scholar]
- 66.Gao C., He X., Ouyang F., et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I): an open-label, randomised, non-inferiority trial. Lancet. 2024;404:1040–1050. doi: 10.1016/S0140-6736(24)01594-0. [DOI] [PubMed] [Google Scholar]
- 67.Pan M., Lassen J.F., Burzotta F., et al. The 17th expert consensus document of the European Bifurcation Club - techniques to preserve access to the side branch during stepwise provisional stenting. EuroIntervention. 2023;19:26–36. doi: 10.4244/EIJ-D-23-00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kleber F.X., Rittger H., Ludwig J., et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol. 2016;105:613–621. doi: 10.1007/s00392-015-0957-6. [DOI] [PubMed] [Google Scholar]
- 69.Corballis N.H., Paddock S., Gunawardena T., Merinopoulos I., Vassiliou V.S., Eccleshall S.C. Drug coated balloons for coronary artery bifurcation lesions: A systematic review and focused meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X., Tian N., Kan J., et al. Drug-coated balloon angioplasty of the side branch during provisional stenting: the multicenter randomized DCB-BIF Trial. J Am Coll Cardiol. 2025;85(1):1–15. doi: 10.1016/j.jacc.2024.08.067. [DOI] [PubMed] [Google Scholar]
- 71.Her A.Y., Ann S.H., Singh G.B., et al. Serial morphological changes of side-branch ostium after paclitaxel-coated balloon treatment of de novo coronary lesions of main vessels. Yonsei Med J. 2016;57:606–613. doi: 10.3349/ymj.2016.57.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan L., Lu W., Han Z., et al. Drug-coated balloon in the treatment of coronary left main true bifurcation lesion: A patient-level propensity-matched analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1028007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okutsu M., Mitomo S., Ouchi T., et al. Impact of directional coronary atherectomy followed by drug-coated balloon strategy to avoid the complex stenting for bifurcation lesions. Heart Vessels. 2022;37:919–930. doi: 10.1007/s00380-021-02000-z. [DOI] [PubMed] [Google Scholar]
- 74.Her A.Y., Kim B., Kim S., Kim Y.H., Scheller B., Shin E.S. Comparison of angiographic change in side-branch ostium after drug-coated balloon vs drug-eluting stent vs medication for the treatment of de novo coronary bifurcation lesions. Eur J Med Res. 2024;29:280. doi: 10.1186/s40001-024-01877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharp A.S., Latib A., Ielasi A., et al. Long-term follow-up on a large cohort of "full-metal jacket" percutaneous coronary intervention procedures. Circ Cardiovasc Interv. 2009;2:416–422. doi: 10.1161/CIRCINTERVENTIONS.109.886945. [DOI] [PubMed] [Google Scholar]
- 76.Lee P.H., Lee S.W., Yun S.C., et al. Full metal jacket with drug-eluting stents for coronary chronic total occlusion. JACC Cardiovasc Interv. 2017;10:1405–1412. doi: 10.1016/j.jcin.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 77.Wöhrle J., Werner G.S. Paclitaxel-coated balloon with bare-metal stenting in patients with chronic total occlusions in native coronary arteries. Catheter Cardiovasc Interv. 2013;81:793–799. doi: 10.1002/ccd.24409. [DOI] [PubMed] [Google Scholar]
- 78.Jun E.J., Shin E.S., Teoh E.V., et al. Clinical outcomes of drug-coated balloon treatment after successful revascularization of de novo chronic total occlusions. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.821380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shin E.S., Her A.Y., Jang M.H., Kim B., Kim S., Liew H.B. Impact of drug-coated balloon-based revascularization in patients with chronic total occlusions. J Clin Med. 2024;13:3381. doi: 10.3390/jcm13123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costopoulos C., Latib A., Naganuma T., et al. The role of drug-eluting balloons alone or in combination with drug-eluting stents in the treatment of de novo diffuse coronary disease. JACC Cardiovasc Interv. 2013;6:1153–1159. doi: 10.1016/j.jcin.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Yang X., Lu W., Pan L., et al. Long-term outcomes of drug-coated balloons in patients with diffuse coronary lesions. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.935263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palmerini T., Della Riva D., Benedetto U., et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11 473 patients. Eur Heart J. 2017;38:1034–1043. doi: 10.1093/eurheartj/ehw627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rissanen T.T., Uskela S., Eränen J., et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): a single-blind, randomised, non-inferiority trial. Lancet. 2019;394:230–239. doi: 10.1016/S0140-6736(19)31126-2. [DOI] [PubMed] [Google Scholar]
- 84.Shin E.S., Lee J.M., Her A.Y., et al. Prospective randomized trial of paclitaxel-coated balloon versus bare-metal stent in high bleeding risk patients with de novo coronary artery lesions. Coron Artery Dis. 2019;30:425–431. doi: 10.1097/MCA.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 85.Cortese B., Serruys P.W. Single-antiplatelet treatment after coronary angioplasty with drug-coated balloon. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Räsänen A., Kärkkäinen J.M., Eranti A., Eränen J., Rissanen T.T. Percutaneous coronary intervention with drug-coated balloon-only strategy combined with single antiplatelet treatment in patients at high bleeding risk: Single center experience of a novel concept. Catheter Cardiovasc Interv. 2023;101:569–578. doi: 10.1002/ccd.30558. [DOI] [PubMed] [Google Scholar]
- 87.Farkouh M.E., Domanski M., Sleeper L.A., et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 88.Nogic J., Nerlekar N., Soon K., et al. Diabetes mellitus is independently associated with early stent thrombosis in patients undergoing drug eluting stent implantation: analysis from the Victorian Cardiac Outcomes Registry. Catheter Cardiovasc Interv. 2022;99:554–562. doi: 10.1002/ccd.29913. [DOI] [PubMed] [Google Scholar]
- 89.Mieres J., Fernandez-Pereira C., Risau G., et al. One-year outcome of patients with diabetes mellitus after percutaneous coronary intervention with three different revascularization strategies: results from the Diabetic Argentina Registry (DEAR) Cardiovasc Revasc Med. 2012;13:265–271. doi: 10.1016/j.carrev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Wöhrle J., Scheller B., Seeger J., et al. Impact of diabetes on outcome with drug-coated balloons versus drug-eluting stents: the BASKET-SMALL 2 trial. JACC Cardiovasc Interv. 2021;14:1789–1798. doi: 10.1016/j.jcin.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 91.Stone S.G., Serrao G.W., Mehran R., et al. Incidence, predictors, and implications of reinfarction after primary percutaneous coronary intervention in ST-segment-elevation myocardial infarction: the harmonizing outcomes with revascularization and stents in acute myocardial infarction trial. Circ Cardiovasc Interv. 2014;7:543–551. doi: 10.1161/CIRCINTERVENTIONS.114.001360. [DOI] [PubMed] [Google Scholar]
- 92.Fang C.Y., Fang H.Y., Chen C.J., Yang C.H., Wu C.J., Lee W.C. Comparison of clinical outcomes after drug-eluting balloon and drug-eluting stent use for in-stent restenosis related acute myocardial infarction: a retrospective study. PeerJ. 2018;6 doi: 10.7717/peerj.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vos N.S., Fagel N.D., Amoroso G., et al. Paclitaxel-coated balloon angioplasty versus drug-eluting stent in acute myocardial infarction: the REVELATION randomized trial. JACC Cardiovasc Interv. 2019;12:1691–1699. doi: 10.1016/j.jcin.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 94.Scheller B., Ohlow M.A., Ewen S., et al. Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: the randomised PEPCAD NSTEMI trial. EuroIntervention. 2020;15:1527–1533. doi: 10.4244/EIJ-D-19-00723. [DOI] [PubMed] [Google Scholar]
- 95.Kondo Y., Ishikawa T., Shimura M., et al. Cardiovascular outcomes after paclitaxel-coated balloon angioplasty versus drug-eluting stent placement for acute coronary syndrome: a systematic review and meta-analysis. J Clin Med. 2024;13:1481. doi: 10.3390/jcm13051481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin E.S., Bang L.H., Jun E.J., et al. Provisional drug-coated balloon treatment guided by physiology on de novo coronary lesion. Cardiol J. 2021;28:615–622. doi: 10.5603/CJ.a2020.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rowinsky E.K., Donehower R.C. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 98.Verheye S., Vrolix M., Kumsars I., et al. The SABRE trial (sirolimus angioplasty balloon for coronary in-stent restenosis): angiographic results and 1-year clinical outcomes. JACC Cardiovasc Interv. 2017;10:2029–2037. doi: 10.1016/j.jcin.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 99.Scheller B., Mangner N., Abdul Kader M., et al. Combined analysis of two parallel randomized trials of sirolimus-coated and paclitaxel-coated balloons in coronary in-stent restenosis lesions. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.122.012305. [DOI] [PubMed] [Google Scholar]
- 100.Stettler C., Wandel S., Allemann S., et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 101.Gao X.F., Ge Z., Kan J., et al. Rationale and design for comparison of non-compliant balloon with drug-coating balloon angioplasty for side branch after provisional stenting for patients with true coronary bifurcation lesions: a prospective, multicentre and randomised DCB-BIF trial. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-052788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greco A., Sciahbasi A., Abizaid A., et al. Sirolimus-coated balloon versus everolimus-eluting stent in de novo coronary artery disease: rationale and design of the TRANSFORM II randomized clinical trial. Catheter Cardiovasc Interv. 2022;100:544–552. doi: 10.1002/ccd.30358. [DOI] [PubMed] [Google Scholar]
- 103.Spaulding C., Krackhardt F., Bogaerts K., et al. Comparing a strategy of sirolimus-eluting balloon treatment to drug-eluting stent implantation in de novo coronary lesions in all-comers: Design and rationale of the SELUTION DeNovo Trial. Am Heart J. 2023;258:77–84. doi: 10.1016/j.ahj.2023.01.007. [DOI] [PubMed] [Google Scholar]
- 104.Nakazawa G., Otsuka F., Nakano M., et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wykrzykowska J.J., Kraak R.P., Hofma S.H., et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. 2017;376:2319–2328. doi: 10.1056/NEJMoa1614954. [DOI] [PubMed] [Google Scholar]
- 106.Ali Z.A., Gao R., Kimura T., et al. Three-year outcomes with the absorb bioresorbable scaffold: individual-patient-data meta-analysis from the ABSORB randomized trials. Circulation. 2018;137:464–479. doi: 10.1161/CIRCULATIONAHA.117.031843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.