Abstract
Mutations in the adenomatous polyposis coli (APC) gene are responsible for familial adenomatous polyposis coli and also sporadic colorectal cancer development. By using antibodies raised against the N-terminal region of APC protein, we have detected the variable masses of endogenous APC proteins in individual cell lines established from human colorectal carcinomas caused by nonsense mutations of the gene. Phosphorylation of immunoprecipitates of full-length and truncated APC were observed in in vitro kinase reaction, indicating association of APC with protein kinase activity. The kinase activity complexed with APC was sensitive to heparin and used GTP as phosphoryl donor, suggesting an involvement of casein kinase 2 (CK2). Both CK2α- and β-subunits were found to associate with APC in immunoprecipitates as well as in pull-down assays, with preferential interaction of APC with tetrameric CK2 holoenzyme. In synchronized cell populations, the association of APC with CK2 was cell cycle dependent, with the highest association in G2/M. Unexpectedly, APC immunoprecipitates containing full-length APC protein inhibited CK2 in vitro, whereas immunoprecipitates of truncated APC had little effect. This was confirmed by using recombinant APC, and the inhibitory region was localized to the C terminus of APC between residues 2086 and 2394. Overexpression of this fragment in SW480 cells suppressed cell proliferation rates as well as tumorigenesis. These results demonstrate a previously uncharacterized functional interaction between the tumor suppressor protein APC and CK2 and suggest that growth-inhibitory effects of APC may be regulated by inhibition of CK2.
The tumor suppressor adenomatous polyposis coli (APC) encoded by the APC gene is linked to familial adenomatous polyposis (1, 2). APC is inactivated by mutations, frequently involving C-terminal truncations of the protein, which are an early event in the development of most colon adenomas and carcinomas. Full-length APC functions as a negative regulator of Wnt signaling, which regulates embryonic pattern formation. Current models suggest that the tumor suppressor effect of APC is from APC-dependent degradation of β-catenin and subsequent suppression of growth-promoting signals. Wild-type APC, in concert with other cellular proteins such as Axin, Dishevelled, GSK3β, β-TrCP, CK1β, and protein phosphatase 2A, decreases the intracellular level of β-catenin (3–6). On the other hand, mutant inactivated APC observed in colon cancers activates Wnt signaling by allowing β-catenin to accumulate and translocate into the nucleus and enhancing β-catenin-TCF-mediated transcription of proliferative genes such as myc, cyclin D1, PPARγ, TCF-1, siamois, and engrailed, resulting in a production of continuous proliferation signals (3).
In addition to β-catenin, other targets are likely to regulate mechanisms by which APC suppresses cell proliferation. Involvement of phosphoinositide metabolism and phospholipase C enzymes (7, 8), phospholipase A2 (9, 10), and phosphatidylinositol 3-kinase (11) in colorectal tumorigenesis caused by APC dysfunction after mutagenesis has been reported. Molecular events such as protein phosphorylation also play important roles in APC-dependent cell proliferation. APC protein has been reported to be phosphorylated by protein kinases including GSK3β, protein kinase A, p34cdc2, and CK1β (5, 6, 12). With GSK3β, the phosphorylated APC becomes associated in a stable complex formed by axin, GSK3β, and β-catenin, resulting in destabilization of β-catenin. APC phosphorylation by tyrosine kinase (13) and hyperphosphorylation of APC after cell-cycle arrest with nocodazole (14) also have been described, although the kinases involved have not been identified yet. Thus, a mechanistic link between APC and phosphorylation in suppression of cell proliferation is still unclear.
To characterize the biochemical significance of APC on cell proliferation and progression of colorectal cancer, we analyzed protein kinases that associate with normal or truncated APC. Here, we show that APC directly interacts with casein kinase 2 (CK2) in a cell cycle-dependent manner. We demonstrate further that the C-terminal region of APC strongly suppresses the kinase activity of CK2, although APC-CK2 interactions involve the N-terminal region of APC. Thus, in colorectal carcinomas, truncated APC mutants lacking the C-terminal domain bind to CK2 but fail to effectively suppress CK2 activity. The results indicate that heterocomplex formation between CK2 and full-length APC regulates CK2 activity in vivo and has regulatory effects on cell-cycle progression. These findings provide insight into understanding the molecular link between APC and the regulation of phosphorylation-dependent signal transduction.
Materials and Methods
Cell Culture.
KMS-4 and KMS-8 cells are colorectal carcinoma cells derived from familial adenomatous polyposis patients (15), and SW480 and DLD-1 cells are colorectal carcinoma cells from nonfamilial colorectal carcinoma patients. All four lines were grown in RPMI 1640 medium containing 10% (vol/vol) FBS. TIG-3 and TIG-7 cells are normal human fetal lung fibroblasts grown in DMEM supplemented with 10% (vol/vol) FBS. Normal cells used in this study were at less than 30 population doublings. All cells were maintained at 37°C in a humidified, 5% CO2 incubator. For cell synchronization, logarithmically growing TIG-3 and SW480 cells were starved in 0.2% FBS for 48 h. Cells were collected in G0 phase or released into fresh medium containing 10% (vol/vol) FBS and cultured for an additional 14–16 h to obtain cell populations synchronized at S phase. Cells were arrested at pro-metaphase by adding 50 ng/ml nocodazole to the growth medium 30 min after release, allowed to incubate for an additional 24 h, and collected by mechanical shake-off or by gentle pipetting. Cell growth was monitored by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay at the indicated time point. Colony formation assay was performed in 0.3% soft agar; the number of colonies was counted after 3 weeks in triplicate plates.
Immunoprecipitations and Immunoblotting.
Logarithmically growing cells were washed with PBS and harvested in a lysis buffer consisting of 50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 10 mM β-glycerophosphate, 1 mM sodium orthovanadate, 0.5% Nonidet P-40, 1% deoxycholic acid, 0.1% SDS, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml pepstatin, and 10 μg/ml leupeptin. Lysates were clarified by centrifugation at 100,000 × g for 30 min at 4°C to obtain the supernatant fraction whose protein concentrations were measured and normalized to 2–5 mg/ml protein. Total protein extracts then were precleared by incubation with protein A-Sepharose for 30 min and then incubated at 4°C for 2 h with polyclonal anti-APC antibodies. The latter were raised in rabbits immunized with a synthetic peptide corresponding to residues 114–127 of human APC, SSRSGECSPVPMGSFPRRGFVN. Immune complexes were recovered with protein A-Sepharose, washed three times with 50 mM Tris⋅HCl, pH 7.8/0.1 M NaCl/0.5% Nonidet P-40, and solubilized with Laemmli's sample buffer (16). The proteins were separated by SDS/PAGE and then were transferred to Immobilon-P membrane (Millipore). The membranes were probed with mouse monoclonal anti-APC antibody (OP-44; Calbiochem) and horseradish peroxidase-conjugated protein A (Amersham Pharmacia) and developed by enhanced chemiluminescence (DuPont).
In Vitro Phosphorylation Assays.
Immunoprecipitates obtained by using anti-APC polyclonal antibodies were incubated at 30°C for 15 min with 20 mM Hepes, pH 7.4/10 mM β-glycerophosphate/5 mM MgCl2/10 μg/ml aprotinin/5 μg/ml leupeptin/1 mM PMSF/0.2 mM ATP/1 μCi [γ-32P]ATP or [γ-32P]GTP in the presence or absence of 10 ng/ml heparin. In some experiments, recombinant GST-CK2α and GST-CK2β were added after purification. Phosphorylation reactions were stopped by addition of 4× Laemmli sample buffer and boiled for 5 min. Samples were separated by SDS/5% PAGE and detected by autoradiography. The catalytic activity of CK2 also was determined by P81 phosphocellulose binding by using a specific CK2 substrate peptide, RRREEETEEE, as described (17).
Production of Recombinant Proteins and Transfections.
APC fragments corresponding to codons 2086–2843, 2086–2394, 2226–2560, and 2518–2843 were derived from PCR-amplified cDNA cloned from a human fetal fibroblast library. The following primers were used to generate each fragment: 2086–2394, 5′-TAGGATCCCCAGATTCAGAACATGGTCTAT and 5′-TCGTCGACTCCTTTGGAGGCAGACTCACTT; 2518–2843, 5′-CAGGATCCAATGATGGAAGACCAGCAAAGC and 5′-ACGAATTCAACAGATGTCACAAGGTAAGAC. cDNA encoding fragment 2226–2560 was produced by cleaving the PCR fragment 2086–2843 with XhoI. Full-length cDNA for human CK2 subunits α, α′, and β were amplified by PCR as described (18, 19). All constructs were confirmed by DNA sequencing. APC deletion constructs and CK2 subunits were subcloned into pGEX-4T (Pharmacia) and pGEX-2T, respectively, and transformed into Escherichia coli strain AD3 grown in LB medium. Protein expression was induced with 0.1 mM isopropyl thiogalactopyranoside for the last 2 h. The recombinant glutathione S-transferase (GST)-fusion proteins were purified by affinity adsorption to glutathione-Sepharose, as suggested by the manufacturer (Pharmacia), and eluted from the resin with 10 mM reduced glutathione/20 mM Tris⋅HCl, pH 8.0. The purity and quantity of the fusion proteins were estimated by SDS/PAGE and Bradford analysis. Approximately 10 mg of GST-CK2α and GST-CK2α′, 14 mg of GST-CK2β, and 3 mg of APC fragments were obtained from each 400-ml culture. Purified APC fragments 959-1338 and 1211–2075 (4) were kindly provided by Akira Kikuchi (University of Hiroshima).
For mammalian expression, cDNAs of the above APC fragments were subcloned into pEGFP-C1 (CLONTECH) or pCDNA3-NFLAG (Invitrogen). For transient transfections, cells were transfected by using Lipofectamine-Plus reagent according to manufacturer's instructions (Life Technologies, Gaithersburg, MD). For the generation of stable cell lines, the medium was changed to DMEM containing 10% (vol/vol) FBS the following day, and cells were allowed to recover 1 day before selection with G418 (400 μg/ml). After 3 weeks in G418, clones were isolated and analyzed for APC expression.
Results
Truncated Forms of APC in Colorectal Cancer Cells.
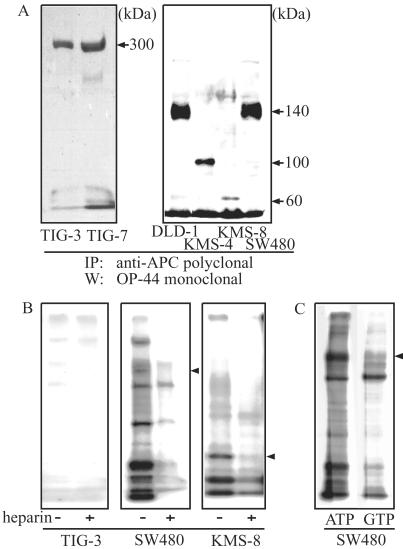
We used rabbit polyclonal antibodies to the N terminus of APC, which were effective in immunoprecipitating endogenous protein. As shown in Fig. 1A, whereas full-length APC protein (≈300 kDa) was observed only in normal TIG-3 and TIG-7 fibroblasts, human colorectal cancer cell lines expressed truncated forms of various sizes. SW480 and DLD-1 cells, derived from human sporadic colorectal carcinomas, expressed a 140-kDa form of APC, which was consistent with previous reports of nonsense mutations present at codon 1338 in SW480 cells and codon 1427 in DLD-1 cells. KMS-4 and KMS-8 cells expressed truncated APC proteins of 100 kDa and 60 kDa, respectively. Thus, immunoprecipitation followed by Western blotting clearly demonstrated the sizes of endogenous APC proteins expressed in different colorectal carcinoma cell lines and validated previous mutational analyses. We also note that the steady-state level of APC in normal cells was less than one-third of those in carcinoma cells. This may reflect differences between normal and carcinoma cells because of DNA alkylation or methylation at the promoter region of the APC gene, as reported (20, 21).
Figure 1.
Full-length and truncated forms of APC proteins. (A) Detection of full-length and truncated forms of APC proteins. Cell lysates from normal fibroblasts (6 mg of protein) and cancer cells (2 mg of protein) were incubated with anti-APC polyclonal antibody, and the resultant immunocomplexes were separated on SDS/5% PAGE and probed with monoclonal anti-APC antibody (OP44). Lanes: 1, TIG-3; 2, TIG-7; 3, DLD-1; 4, KMS-4; 5, KMS-8; 6, SW480. Exposure for TIG-3, TIG-7 was about two times longer than that for the other cell lines. (B) Association of protein kinase activity with APC. Cell lysates prepared from TIG-3, SW480, and KMS-8 cells were immunoprecipitated with anti-APC polyclonal antibody and incubated for 15 min in the kinase reaction mixture in the presence (+) or absence (−) of 10 ng/ml heparin. The reaction was stopped by adding sample buffer, and radioactive proteins were separated by SDS/5% PAGE and visualized by autoradiography. (C) The protein kinase utilizes GTP as well as ATP. The phosphorylation activity in the immunoprecipitates from SW480 cells was determined in the presence of either [γ-32P]ATP (left lane) or [γ-32P]GTP (right lane) in the reaction mixtures. Proteins were separated on SDS/5% PAGE, and phosphorylated proteins were visualized by autoradiography. Truncated APC is indicated by an arrowhead.
Protein Kinases in APC Immune Complexes.
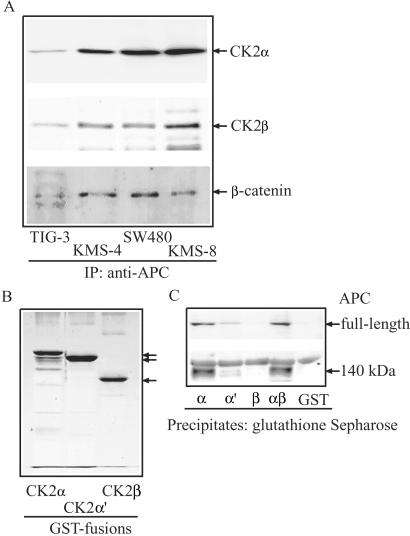
In vitro phosphorylation reactions were performed by using anti-APC immunoprecipitates to prove potential associations with kinase activities. As shown in Fig. 1B, APC precipitated from normal human fibroblast TIG-3 lysates associated with little or no kinase activity, even in the presence of serine/threonine or tyrosine phosphatase inhibitors, okadaic acid, or pervanadate (data not shown). On the other hand, APC as well as other proteins was readily phosphorylated in immunoprecipitates obtained from carcinoma cells SW480 and KMS-8. Most of these phosphoproteins also were observed in kinase reactions by using GTP as a phosphate donor (Fig. 1C). In addition, APC phosphorylation was inhibited strongly upon addition of heparin (10 ng/ml) to the reaction mixture (Fig. 1B). Such behavior suggested a possible involvement of CK2 in the phosphorylation reactions, given that CK2 is known to be inhibited by polyanionic compounds including heparin, and is unusual among protein kinases in using both GTP and ATP as a phosphoryl donor (22). Therefore, anti-APC immunoprecipitates were probed with polyclonal antibodies recognizing α- and β-subunits of the tetrameric CK2 holoenzyme (19). Both α- and β-subunits were readily detected and highest in the immunoprecipitates from KMS-4, KMS-8, and SW480 cells (Fig. 2A). In these cell lines, approximately 15% of total CK2 was found to associate with APC (data not shown). In normal TIG-3 cells that express full-length APC protein, smaller but significant amounts of CK2-α and CK2-β were found in APC immunocomplexes. As reported (23), β-catenin was detected in all APC immunoprecipitates and the amount associated with full-length APC in normal TIG cells was lower than in carcinoma cells, possibly because of accelerated degradation (Fig. 2A).
Figure 2.
Association of CK2 with APC in vivo and in vitro. (A) Detection of CK2 in APC immunocomplex. Lysates (2 mg each) obtained from TIG-3 (lane 1), KMS-4 (lane 2), SW480 (lane 3), or KMS-8 (lane 4) cells were immunoprecipitated with polyclonal anti-APC antibody and analyzed by SDS/12% PAGE, followed by immunoblotting with anti-CK2α, anti-CK2β, and β-catenin. (B) Recombinant GST-CK2α, GST-CK2α′, and GST-CK2β were separated by SDS/12% PAGE, and proteins were visualized by Coomassie blue staining. (C) CK2 pull-down assay. Cell lysates (≈2 mg) were prepared from TIG-3 (Upper) and SW480 (Lower) and incubated with GST-CK2α, GST-CK2α′, GST-CK2β, a 1:1 mixture of GST-CK2α and GST-CK2β, or GST alone (≈1 μg), each immobilized on glutathione-Sepharose beads. After washing thoroughly, binding proteins were solubilized and separated by SDS/5% PAGE, followed by immunoblotting with anti-APC mAb.
Binding of Full-Length and Truncated-APC to CK2.
In vitro binding of full-length vs. truncated APC with CK2 was assayed by incubating purified fusions of GST-CK2α, α′, and β (Fig. 2B) with cell lysates obtained from logarithmically growing TIG-3 and SW480 cells and analyzing bound proteins by immunoblotting with monoclonal anti-APC antibody. As shown in Fig. 2C, full-length APC protein bound best to CK2 holoenzyme formed by mixing GST-CK2α and β, and also to CK2α, but did not bind CK2β or GST alone. Similar behavior was observed with the 140-kDa APC derived from SW480 cells (Fig. 2C). Both forms recognized CK2α preferentially to CK2α′ subunit. These results indicated that CK2α likely binds directly to APC and that the binding site is contained within the N-terminal 140-kDa region of the APC protein. On the other hand, CK2β does not directly bind APC but binds only via its association with α and/or α′ in the tetrameric CK2 holoenzyme.
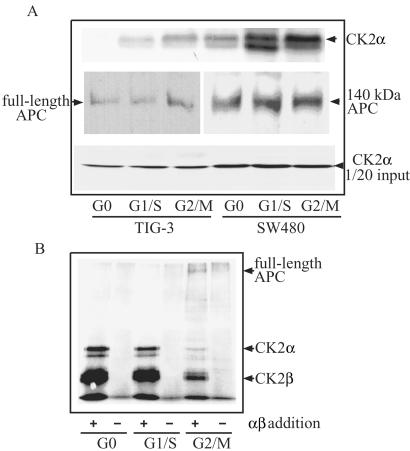
Full-length APC is a tumor suppressor protein that down-regulates proliferative cell-cycle signaling but up-regulates proliferation when inactivated by deletion or mutation. We therefore hypothesize that if association of APC and CK2 functions to regulate proliferation, the association may occur in a cell cycle-dependent manner. This was tested by synchronizing TIG-3 and SW480 in G0, G1/S, and G2/M phase and analyzing anti-APC immunoprecipitates for association with endogenous CK2. In TIG-3 cells, the association of APC with CK2α was highest in G2/M and also in G1/S, but not in G0 (Fig. 3A). In contrast, in SW480 cells, APC-CK2 interactions were observed at all phases in the cell cycle, although its highest level occurred in G2/M. Cellular levels of full-length or truncated APC and CK2α were constant during the cell cycle in both TIG-3 and SW480 cells. The results suggest that APC and CK2α interactions are cell cycle-regulated.
Figure 3.
Association with CK2 and phosphorylation of APC during the cell cycle. (A) Cell cycle-dependent association of CK2α with APC in vivo. Cell lysates were prepared from TIG-3 and SW480 cells synchronized in G0, G1/S, or G2/M phase of the cell cycle and immunoprecipitated with anti-APC polyclonal antibodies. Proteins were solubilized and separated by SDS/12% PAGE for α or by 5% gels for APC and blotted with anti-CK2α or anti-APC antibodies. Total lysates were analyzed in parallel for the content of CK2α. (B) Phosphorylation of full-length APC from G2/M-arrested cells by CK2. Anti-APC immunoprecipitates prepared from synchronized TIG-3 cells were combined with (+) or without (−) recombinant CK2α and CK2β to analyze in vitro phosphorylation activity. Samples were separated by SDS/7.5% PAGE and autoradiographed.
We next examined whether phosphorylation of full-length APC occurred in cell cycle-arrested cell populations. As shown in Fig. 3B, phosphorylation of immunoprecipitated full-length APC protein from TIG-3 cells was observed by autoradiography when cells were synchronized in G2/M phase, in which the association of APC with endogenous CK2 holoenzyme is maximal. Phosphorylation was enhanced further by adding exogenous CK2α/β holoenzyme. Importantly, autophosphorylation of exogenous CK2α and CK2β was attenuated in G2/M compared with other phases of cell cycle. These results suggested that the cell cycle-dependent binding between APC and CK2α, in which the higher binding of APC to CK2 in G2/M occurs, may suppress CK2 activity.
Full-Length but Not Truncated APC Inhibits CK2 Activity.
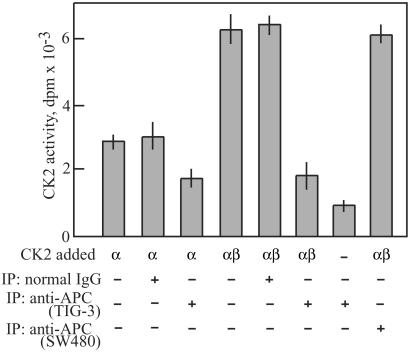
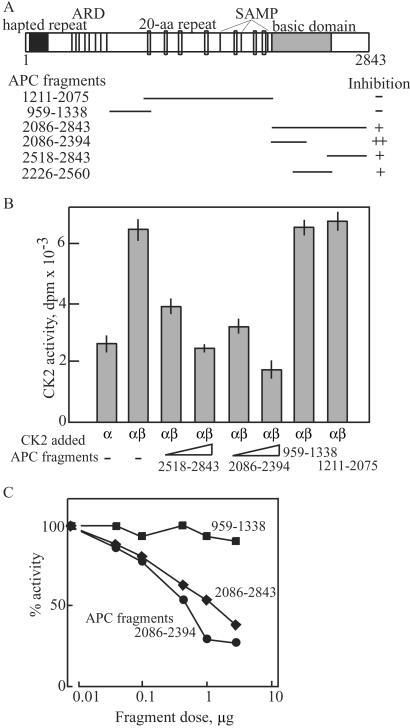
To examine further the biological significance of APC-CK2 interactions, we measured the catalytic activity of CK2 bound to APC. Anti-APC immunoprecipitates or control IgG precipitates were prepared from TIG-3 cells synchronized in G2/M and were added to reaction mixtures containing purified recombinant CK2. In this assay, the catalytic activity of recombinant CK2α protein was enhanced by the addition of β-subunit as shown in Fig. 4. Addition of full-length APC inhibited the catalytic activity of CK2α and CK2α/β by 45% and 70%, respectively. In marked contrast, addition of truncated APC derived from SW480 cells did not affect CK2 activity, indicating large differences between the ability of full-length and C-terminal truncated forms of APC to regulate CK2. To verify that the inhibition of CK2 activity specifically was from APC protein itself, we tested full-length APC partially purified from pig brains by sequential column chromatography. This APC-containing preparation also inhibited CK2α by 20% (data not shown), suggesting that APC most likely attenuates CK2 by direct binding. This was examined further by preparing recombinant APC fragments overlapping the C-terminal half of the 300-kDa protein (Fig. 5A) and examining their effects toward CK2 activity. As shown in Fig. 5B, C-terminal fragments corresponding to residues 2518–2843 and 2086–2394 exhibited strong inhibition compared with other fragments corresponding to 959–1338 or 1211–2075. Dose-response curves showed that the inhibition was dramatically dependent on the concentration (Fig. 5C) and the incubation period (data not shown). In particular, complete suppression of CK2 activity was observed at approximately equimolar levels of APC fragments. These results suggest that the truncation of the C-terminal region of APC by mutation causes a loss of regulation of CK2, which may be involved in tumorigenesis.
Figure 4.
Inhibitory effect of APC on CK2 activity. The kinase activities of GST-CK2α (≈0.5 μg) and a 1:1 mixture of GST-CK2α (≈0.5 μg) and GST-CK2β (≈0.5 μg) were measured against substrate peptide in the presence or absence of anti-APC or human IgG immunoprecipitates from TIG-3 or SW480 cells arrested at G2/M with nocodazole. Results were expressed as mean ± SD of three independent experiments.
Figure 5.
C terminus of APC is responsible for inhibition of CK2 activity. (A) APC fragments used in this study with a schematic structure of APC are shown. APC contains various domains including heptad repeat, armadillo repeat (ARD), 20-aa repeat, SAMP, and basic domain. The portion of APC fragments used in this study was indicated as bars with amino acid number. (B) Inhibition of CK2 activity by C-terminal fragment of APC. The kinase activity of GST-CK2α (≈0.5 μg) and a 1:1 mixture of GST-CK2α and GST-CK2β (≈0.5 μg each) were measured against substrate peptide in the presence or absence of recombinant APC fragments (0.1 or 0.5 μg) as indicated. Inhibitory effect of each fragment was summarized in A. (C) Dose-dependent inhibition of CK2 activity by APC. The kinase activity of a 1:1 mixture of GST-CK2α and GST-CK2β (≈0.5 μg each) was measured against substrate peptide in the presence of various concentrations of recombinant APC fragments as indicated. Results were expressed as means ± SD of three independent experiments.
Inhibition of Tumor Growth by CK2 Inhibitor Polypeptide.
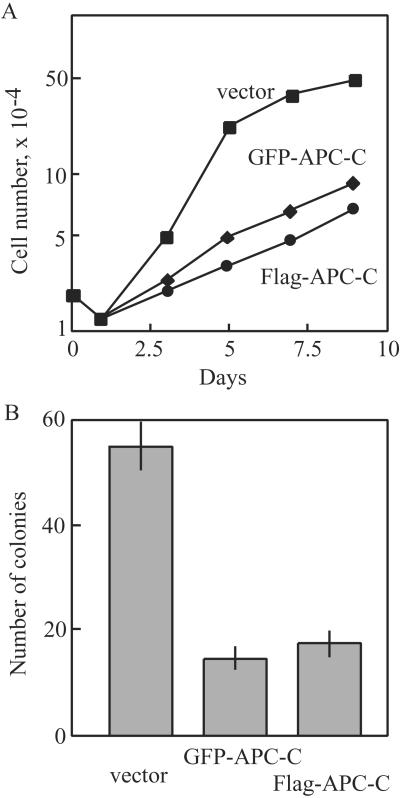
Finally, the in vivo significance of the interaction between APC and CK2 for regulating cell proliferation was examined. Stable 293 or SW480 cells expressing the inhibitory C-terminal fragment 2086–2394 (APC-C) were isolated and monitored in growth and transformation assays. As shown in Fig. 6A, 293 cells constitutively expressing the C-terminal fragment from either FLAG- or GFP-vector strongly suppressed growth rates, increasing doubling times by 2-fold compared with control cells. Likewise, assays for colony formation on soft agar in SW480 showed that expression of the C-terminal reduced outgrowth of cells by about 70% (Fig. 6B). These results are consistent with a model in which inhibitory interactions between APC and CK2 perturb cell proliferation as well as cellular transformation in a manner correlated with inhibition of catalytic activity of CK2.
Figure 6.
Effect of APC fragments on growth and transformation properties. (A) 293 cells (2 × 104) constitutively expressing an APC fragment 2086–2394 (APC-C) in pEGFP vector (○) or in pCDNA3-FLAG (♦) vs. wild-type cells expressing empty pEGFP (■) were split to 35-mm plates, and cell counts were monitored at each time point. (B) Triplicate plates of SW480 cells (1 × 106) were transfected with 0.3 μg of either pEGFP-APC-C (column 2), pCDNA3-FLAG-APC-C (column 3), or empty pEGFP (column 1), plated in soft agar, and assayed for colony formation after 3 weeks. Results were expressed as means ± SD of three independent experiments.
Discussion
The tumor suppressor APC is linked to the initiation of sporadic human colorectal cancer. Full-length APC protein negatively regulates signaling pathways for cell growth and tumorigenesis. APC is inactivated by mutation, frequently through C-terminal truncations. This demonstrates an important role of the C-terminal half of the molecule in the normal tumor suppressor functions of APC. Our results revealed a cellular target controlled by APC, providing the evidence that APC binds to and functionally interacts with CK2. We demonstrated that CK2 binds to APC through the direct interaction of CK2α-subunit and an N-terminal region of APC in a cell cycle-dependent manner and that full-length APC, but not the C-terminal truncated forms of APC, inhibits the kinase activity of CK2. We further confirmed the suppressive effect of polypeptide identical to a C-terminal region of APC on cell growth and anchorage-independent colony formation. Because CK2 is known to be an important regulator for cell proliferation (22, 24, 25), our findings reveal a mechanism for APC in tumor and growth suppression through interference with CK2.
One mechanism underlying the malignant cell growth and tumorigenesis of the C-terminal truncation of APC proposes loss of inhibitory effects of APC toward the β-catenin pathway. When APC is inactivated by truncation, the Wnt pathway is activated, allowing β-catenin to accumulate and translocate into the nucleus, complex with TCF, and activate transcription of genes such as myc, cyclin D1, and TCF-1 (3), which, in turn, produce continuous signals for proliferation. Our results provide evidence for an additional function of APC in binding CK2 and suppressing its activity.
It is noteworthy that the full-length and C-terminal truncated forms of APC protein had distinct effects on CK2. Whereas the full-length form suppressed CK2 activity, the truncated 140-kDa product, commonly observed in colon cancer, had no effect on CK2, although it was able to interact with CK2. Thus, the suppressive effect of the C-terminal region of APC on CK2 correlates well with the antitumor activity of APC. On the other hand, when APC is inactivated by its truncation, the inhibition of CK2 is attenuated, which could enable sustained activation of CK2. In analogy with previous results using antibody microinjection or antisense oligonucleotides to ablate CK2 activity (26), derepression of CK2 by APC mutations may well promote constitutive cell-cycle progression.
CK2 is a pleiotropic, ubiquitous, and constitutively active protein kinase, with both cytosolic and nuclear localization in most mammalian cells. CK2 has many cellular targets and forms different signaling complexes in different locations, which reflect the multifunctional nature of CK2. CK2 is believed to promote tumorigenesis, because its activity and protein content are enhanced in many human tumors, transformed cell lines, growth-stimulated cells, and rapidly proliferating tissues (27–29). APC is the first endogenous protein found to suppress the kinase activity of CK2. The kinase activity of catalytic α- and α′-subunits is enhanced by association with β-subunit, most likely by stabilization of α and α′. In our study, APC inhibited the activity of CK2α/β holoenzyme to a much larger extent than the catalytic α-subunit, although the binding interaction occurred exclusively through the α-subunit. Importantly, almost complete inhibition was observed at 1:1 stoichiometries of CK2 and APC. Thus, it is conceivable that APC protein reduces the stability of the α-subunit by direct inactivation of its catalytic center.
We also found that the association of CK2 with APC is cell cycle-dependent, reaching its highest levels during G2/M in normal cells. Although the mechanism is obscure, several aspects of APC and CK2 signaling may reflect this cell-cycle dependence. A number of cellular proteins including cell cycle-dependent kinases and nuclear factors interact with CK2. In particular, p34cdc2 phosphorylates and activates CK2 (30). Alternatively, inhibition of CK2 accelerates the degradation of β-catenin and Dvl proteins in Wnt signaling, which causes cell-cycle arrest in mammalian cells (31), and inactivation of CK2 in the temperature-sensitive yeast strains results in cell-cycle arrest in G1/S or G2/M (24). This implies an important role for regulation of CK2 activity for maintaining normal properties of cell division. Our results raise the possibility that CK2 activity is negatively regulated in G2/M by interaction with APC.
Likewise in mammalian cells, introduction of neutralizing antibodies or antisense oligonucleotides directed against CK2 inhibits cell-cycle progression (26). In analogy with these strategies to attenuate CK2 activity, suppression of CK2 by introduction of APC fragments would control cell growth of aberrant cells. Our study localized the region within APC required for the CK2 inhibition; the 34-kDa fragment corresponding to residues 2086–2394 was the smallest domain among fragments tested so far. Expression of this fragment in transformed cells prevented cell growth and colony formation on soft agar. Additional studies are needed to determine the minimum inhibitory region and kinetics of the inhibition. Thus, small molecule or peptide agonists based on APC interference of CK2 eventually may prove useful for colon cancer therapeutics.
Acknowledgments
We greatly thank Drs. Natalie G. Ahn (University of Colorado at Boulder), Randy Moon (University of Washington, Seattle) and Yumiko Saito (Saitama Medical College) for critical reading of the manuscript and continuous encouragement, and Dr. Akira Kikuchi (University of Hiroshima) for providing two types of APC fragments. We thank Dr. Masayuki Sekimata and Ms. Junko Yamaki for technical assistance. This work was supported in part by the Yamada Science Foundation (Osaka).
Abbreviations
- APC
adenomatous polyposis coli
- CK2
casein kinase 2
- GST
glutathione S-transferase
References
- 1.Kinzler K W, Nilnert M C, Su L-K, Vogelstein B, Bryan T, Levy D B, Smith K J, Preisinger A C, Hedge P, McKechnie D, et al. Science. 1991;253:661–664. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 2.Fearon E R, Vogelstein B. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 3.VanEs J H, Giles R H, Clevers H C. Exp Cell Res. 2001;264:126–134. doi: 10.1006/excr.2000.5142. [DOI] [PubMed] [Google Scholar]
- 4.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 5.Rubinfeld B, Albers I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 6.Rubinfeld B, Tice D A, Polakis P. J Biol Chem. 2001;278:39037–39045. doi: 10.1074/jbc.M105148200. [DOI] [PubMed] [Google Scholar]
- 7.Homma M K, Homma Y, Yamasaki M, Ohmi-Imajoh S, Yuasa Y. Cell Growth Differ. 1996;7:281–288. [PubMed] [Google Scholar]
- 8.Homma M K, Homma Y, Namba M, Yuasa Y. J Cell Biochem. 1994;5:477–485. doi: 10.1002/jcb.240550407. [DOI] [PubMed] [Google Scholar]
- 9.Oshima M, Dinchuk J E, Kargman S, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M. Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 10.Hong K H, Bonventre J C, O'Leary E, Bonventre J V, Lander E S. Proc Natl Acad Sci USA. 2001;98:3935–3939. doi: 10.1073/pnas.051635898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata J E, Li M, Suzuki A, Bouchard D, Ho A, Redston M, et al. Nature (London) 2000;406:897–902. doi: 10.1038/35022585. [DOI] [PubMed] [Google Scholar]
- 12.Trzepacz C, Lowy A M, Kordich J J, Groden J. J Biol Chem. 1997;72:21681–21684. doi: 10.1074/jbc.272.35.21681. [DOI] [PubMed] [Google Scholar]
- 13.Norris A L, Clissold P M, Askham J M, Morrison E E, Moncur P, McCall S H, Coletta P L, Meredith D M, Markham A F. Eur J Cancer. 2000;36:525–532. doi: 10.1016/s0959-8049(99)00305-6. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharjee R N, Hamada F, Toyoshima K, Akiyama T. Biochem Biophys Res Commun. 1996;220:192–195. doi: 10.1006/bbrc.1996.0379. [DOI] [PubMed] [Google Scholar]
- 15.Namba M, Miyamoto K, Hyodo F, Iwama T, Utsunomiya J, Fukushima F, Kimoto T. J Cancer. 1983;32:697–702. doi: 10.1002/ijc.2910320608. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Natalie G A, Sager R, Bratlien R L, Diltz D D, Tonks N K, Krebs E G. J Biol Chem. 1991;266:4220–4227. [PubMed] [Google Scholar]
- 18.Lozeman F J, Litchfield D W, Piening C, Takio K, Walsh K A, Krebs E G. Biochemistry. 1990;29:8436–8447. doi: 10.1021/bi00488a034. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Dobrowolska G, Krebs E G. J Biol Chem. 1996;271:15662–15668. doi: 10.1074/jbc.271.26.15662. [DOI] [PubMed] [Google Scholar]
- 20.Narayan S, Jaiswal A S. J Biol Chem. 1997;272:30619–30622. doi: 10.1074/jbc.272.49.30619. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto Y, Kitazawa R, Maeda S, Kitazawa S. J Cell Biol. 2001;80:415–423. [PubMed] [Google Scholar]
- 22.Guerra B. Electrophoresis. 1999;20:391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Su L-K, Vogelstein B, Kinzler K W. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 24.Hanna D E, Rethinaswamy A, Glover C V C. J Biol Chem. 1995;270:25905–25914. doi: 10.1074/jbc.270.43.25905. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Dobrowolska G, Aicher L D, Chen M, Wright J H, Drueckes P, Dunphy E, Munar E S, Krebs E G. J Biol Chem. 1999;274:32988–32996. doi: 10.1074/jbc.274.46.32988. [DOI] [PubMed] [Google Scholar]
- 26.Faust R A, Tawfic S, Davis A T, Bubash L A, Ahmed K. Head and Neck. 2000;22:341–346. doi: 10.1002/1097-0347(200007)22:4<341::aid-hed5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Gruppuso P, Boylan J M. J Cell Biochem. 1995;58:65–72. doi: 10.1002/jcb.240580109. [DOI] [PubMed] [Google Scholar]
- 28.Guo C, Yu S, Davis A T, Ahmed K. Cancer Res. 1999;59:1146–1151. [PubMed] [Google Scholar]
- 29.Sommercorn J, Mulligan J A, Lozeman F J, Krebs E G. Proc Natl Acad Sci USA. 1987;84:8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slominski E, Sichler C, Litchfield D W. J Biol Chem. 1995;270:25872–25878. doi: 10.1074/jbc.270.43.25872. [DOI] [PubMed] [Google Scholar]
- 31.Song D H, Sussman D J, Seldin D C. J Biol Chem. 2000;275:23790–23797. doi: 10.1074/jbc.M909107199. [DOI] [PubMed] [Google Scholar]