Abstract
Alpha-fetoprotein (AFP), a major oncofetal protein, plays a pivotal role in cancer biology, extending beyond its initial identification in fetal development. Alpha-fetoprotein has multifaceted physiological functions mediated by its’ complex structure interacting with specific receptors and ligands. The involvement of AFP in carcinogenesis encompasses a broad spectrum of pathophysiological mechanisms, including cell proliferation, apoptosis, metastasis, and immune modulation. Alpha-fetoprotein contributes to the pathological landscape of several cancers, especially hepatocellular carcinoma and germ cell tumors, through an intricate network of signaling pathways. Recent studies highlight the expanding clinical applications of AFP, suggesting its potential to improve the diagnosis and treatment of cancer as well as monitoring genetic diseases, such as neural tube defects and Down syndrome. This comprehensive overview of AFP aims to elucidate the complex biological interactions and clinical implications in cancer management.
How to cite this article
Ucdal M, Yazarkan Y, Sonmez G, et al. Alpha-fetoprotein: A Multifaceted Player in Cancer Biology. Euroasian J Hepato-Gastroenterol 2025;15(1):72–82.
Keywords: Alpha-fetoprotein, Cancer biomarker, Hepatocellular carcinoma
The History and Current Clinical Applications of AFP
Alpha-fetoprotein (AFP), initially characterized as a predominant tumor-associated protein in 1963, has established its clinical utility as a significant biomarker. Its applications encompass both oncological diagnostics, particularly in hepatocellular carcinoma (HCC), and prenatal screening protocols for the detection of neural tube defects and chromosomal anomalies.1 Recent research has focused on AFP's immunomodulatory effects, exploring its potential in immunotherapy for HCC, including AFP-specific vaccines and adoptive T-cell therapies.2
The Structure and Physiological Functions of AFP
Alpha-fetoprotein, representing a principal oncofetal protein in mammals, maintains minimal expression in adult tissues. As a constituent of the albuminoid gene superfamily, which encompasses serum albumin, vitamin D binding protein, and alpha-albumin (afamin), it exhibits distinct receptor interactions and ligand-binding properties that facilitate its multifaceted physiological functions.3 Alpha-fetoprotein can bind over 50 ligands, such as bilirubin, fatty acids, and estrogenic compounds, including flavonoids, synthetic steroids, dyes, toxins, retinoids, and thyroid hormones.4
During fetal development, AFP synthesis occurs predominantly in the yolk sac and liver. This protein plays integral roles in multiple developmental processes, including erythropoiesis, histogenesis, organogenesis, and immune system maturation throughout the fetal period.4 Alpha-fetoprotein levels exhibit notable variations in fetus, pregnant women and during the postpartum period. In preeclamptic pregnancies, maternal serum AFP levels are often elevated compared to normal pregnancies, possibly due to placental dysfunction and increased permeability of the fetal-maternal barrier.5
Alpha-fetoprotein synthesis is usually minimal in adults, mostly by the liver and less by the gastrointestinal tract.6 When serum concentrations exceed 50 ng/mL in adult blood, AFP is implicated in tissue regeneration. Alpha-fetoprotein serves as a marker for pregnancy and certain cancers, namely HCC and germ cell cancers in adults. Alpha-fetoprotein has antioxidant effects and can modulate the immune response by inducing T-cell suppressor activity, downregulating dendritic-like cell antigen expression, and impairing macrophage function.2 It provides an advantageous environment for cancer cells regarding nutrient supply and growth stimulation.7
Alpha-fetoprotein is a glycoprotein consisting of a single polypeptide chain of about 590 amino acids, with a molecular weight of approximately 70 kDa. This structure is punctuated by 15 disulfide bridges that are regularly distributed, contributing to a folding architecture which set into three repeating domains. Alpha-fetoprotein's molecular conformation in mammals is basically a cysteine-rich protein exhibiting a V-shaped configuration derived from its three-domain composition.8 The third domain shows interactions with cell cycle regulatory proteins including cyclins, cyclin-dependent kinases, and ubiquitin ligases. These interactions participate in cell cycle control and influence cellular proliferation and differentiation processes. This domain contains regions for binding hydrophobic ligands, receptors, and binding proteins. Research indicates its role in transporting hydrophobic molecules, particularly estrogens, contributing to its inhibitory function in estrogen receptor-positive tumor growth within estrogen-dependent cancers.19
Alpha-fetoprotein presents in two primary forms: naturally occurring AFP (nAFP) produced during fetal development, and tumor-derived AFP (tAFP) which shows elevated levels in HCC and germ cell malignancies. These AFP variants exhibit distinct characteristics in normal physiological conditions vs cancer states, with their key differences outlined in Table 1. A glycosylated pattern of AFP is known as AFP-L3. Alpha-fetoprotein-L3 differs from total AFP in terms of the presence of an α-1,6-linked fucose residue attached to the N-acetylglucosamine of the N-glycan moiety.1 The formation of this modified structure is mediated by fucosyltransferase 8 (FUT8), which shows increased expression in HCC cells,2,4 providing diagnostic value in cancer detection. Alpha-fetoprotein-L3's elevated levels in HCC stem from altered glycosylation pathways in malignant cells. Enhanced FUT8 activity, together with other glycosyltransferases, promotes increased production of fucosylated glycoproteins, particularly AFP-L3.19 Furthermore, the hypoxic tumor microenvironment and activation of oncogenic signaling pathways, such as the wingless-related integration site (Wnt/β-catenin pathway, have been shown to upregulate FUT8 expression and promote AFP-L3 production.20 These patterns serve as differentiation markers between liver cirrhosis and HCC, with AFP-L3 being recognized as a predictive marker for the malignancy risk associated with HCC.
Table 1.
Comparative characteristics of AFP in physiological and neoplastic environments
| Aspect | Physiological environment | Cancer environment | References |
|---|---|---|---|
| AFP levels | Elevated during fetal development, decreasing to low levels after birth | Significantly elevated in HCC and certain other cancers (e.g., germ cell tumors). | 9 |
| Role | Plays a role in fetal growth and development. | Acts as a biomarker for tumor presence, aggressiveness, and treatment monitoring. | 10,11 |
| Expression patterns | Demonstrates elevated expression levels in fetal hepatic tissue, yolk sac, and gastrointestinal structures | Re-expression or increased expression in malignant tissues, especially in liver and germ cell tumors. | 12 |
| Diagnostic utility | Low diagnostic utility in adults under normal physiological conditions. | High diagnostic and prognostic utility in HCC and other AFP-producing cancers; used in surveillance and monitoring. | 11 |
| Treatment monitoring | Not applicable | Changes in AFP levels post-treatment can indicate treatment efficacy or disease recurrence. | 9 |
| Feature | nAFP | CyAFP | |
| Origin | It is derived from fetal cells. | It is derived from tumor cells, specifically HCC and other cancers. | 13 |
| Cellular localization | Primarily secreted into the bloodstream. | Found in the cytoplasm of tumor cells after being endocytosed. | 9 |
| Role in growth promotion | Not directly implicated in promoting the growth of cancer cells. | Facilitates cancer cell proliferation through receptor interaction, subsequent cAMP-PKA pathway activation, and enhanced Ca2+ influx, resulting in increased expression of oncogenic factors including c-fos, c-jun, and Ras. | 14 |
| Effect on apoptosis | Does not directly interfere with apoptosis signaling pathways in cancer cells. | It inhibits apoptosis by binding to caspase-3, preventing apoptotic signal transduction and affecting caspase-3 activation through the mitochondrial apoptosis pathway. | 15 |
| Impact on signaling pathways | Limited direct impact on signaling pathways related to cancer cell proliferation and apoptosis. | After endocytosis, it interacts with cytoplasmic proteins to activate or inhibit signaling pathways, including inhibiting the apoptosis signaling pathway and affecting mitochondrial pathways. | 15 |
| Involvement in endocytosis | It does not undergo endocytosis to exert its biological functions in the context of cancer cell proliferation. | It stimulates its own endocytosis into cells, where it interacts with internal components to promote cancer cell survival and growth. | 16,17 |
| Interaction with receptors | Interaction with specific receptors is not well characterized. | Forms a complex with the RAR-β receptor, preventing nuclear translocation and subsequently enhancing the expression of anti-apoptotic proteins, including survivin. | 18 |
AFP, alpha-fetoprotein; ATRA, all-trans retinoic acid; Ca2+, calcium ion; cAMP-PKA, cyclic adenosine monophosphate-protein kinase A; CyAFP, cytoplasmic AFP; HCC, hepatocellular carcinoma; nAFP, normal AFP; RAR-β, retinoic acid receptor beta
Alpha-fetoprotein-L3's glycosylation pattern provides advantages compared to total AFP measurements in HCC detection. Research demonstrates AFP-L3's enhanced diagnostic specificity for HCC compared to total AFP measurements. While various liver conditions including cirrhosis and hepatitis can elevate total AFP levels, AFP-L3 shows more selective elevation in HCC.3 AFP-L3 demonstrates a correlation between aggressive HCC characteristics and worse clinical outcomes. Studies link elevated AFP-L3 to increased tumor dimensions, vascular invasion, advanced disease stages,5 reduced survival rates, and higher recurrence following surgery or transplantation.6 Additionally, AFP-L3 shows effectiveness in early HCC detection, particularly in identifying cases where total AFP remains normal,7 enabling treatment during more curable stages. Research indicates combining AFP-L3 with other markers like des-gamma-carboxy prothrombin (DCP) and glypican-3 (GPC3) enhances HCC diagnostic precision.8
Alpha-fetoprotein variants can be further classified based on their cellular localization as secreted AFP and cytoplasmic AFP (CyAFP), and points to the complex biology underlying AFP's role in disease pathogenesis and progression. CyAFP is mainly derived from tumors, so it has implications in oncological contexts.20 This distinction can potentially enhance diagnostic accuracy and prognostic assessment in oncology.
AFP-related Proteins
Recombinant alpha-fetoprotein (rAFP) is a synthetically produced version of the naturally occurring AFP protein. It is generated using recombinant DNA technology, which involves inserting the AFP gene into a host cell, such as bacteria or yeast, and inducing the host to express the protein.26 There are some rAFP peptide fragments derived from domain 3, and called AFP-inhibiting fragments (AIFs).27 AIFs are designed to prevent the binding of AFP to signal transduction molecules or AFP receptors (AFPRs). The therapeutic potential of AIFs extends beyond mere inhibition, as they can be conjugated with toxins or drugs that allow for targeted delivery for selectively destroying cancer cells while minimizing damage to healthy tissues.27 Table 2 presents a range of AIFs along with their respective characteristics and citations.
Table 2.
Alpha-fetoprotein-related fragments and compounds with potential therapeutic applications in cancer
| Fragment name | Function | Additional notes |
|---|---|---|
| GIP-8 | Inhibits cancer cell growth, especially in estrogen receptor-positive breast cancer cells21 | The core focus of many studies21 |
| GIP-12 | Possibly another bioactive AFP fragment, function unknown16 | Speculative, may need more research16 |
| GIP-34 | Similar, potentially more potent anticancer activity than GIP-821 | Longer peptide21 |
| rhAFP with Taxanes and ACA | Suggests combination therapy with rhAFP for greater efficacy22 | rhAFP = recombinant human alpha-fetoprotein22 |
| FPmAb-PLGA-rhDCN | Targeted drug delivery system, potentially with rhDCN or other therapeutics23 | Advanced concept using monoclonal antibodies23 |
| EGCG | Potential synergy with AFP peptides, enhancing anticancer effects24 | Found in green tea24 |
| AFP 3 BC | Unknown, may refer to a specific AFP section with potential therapeutic use25 | It is highly speculative and needs more context.25 |
ACA, 1’-S-1’-acetoxychavicol acetate; AFP, alpha-fetoprotein; EGCG, epigallocatechin gallate; FPmAb, anti-alpha fetoprotein monoclonal antibody; GIP, growth inhibitory peptide; PLGA, poly (lactic-co-glycolic acid); rhAFP, recombinant human alpha-fetoprotein; rhDCN, recombinant human decorin
The growth inhibitory peptide (GIP), a 34-amino acid segment derived from the AFP molecule, demonstrates significant anti-tumor properties. This peptide effectively modulates oncogenic pathways by interfering with cell cycle progression and tumor angiogenesis. The GIP family comprises three distinct peptides: GIP12, GIP14, and GIP8. Research utilizing both in vivo and in vitro models has revealed notable anti-cancer activity, particularly for GIP-8 and GIP-34 variants.21 GIP-34 has shown significant antiproliferative activity against diverse human malignancies, with documented efficacy in constraining tumor invasion and metastatic progression in mammary neoplasms of both human and mouse origin. The peptide also demonstrated the capability to disrupt cellular adhesion to extracellular matrix protein (ECM) proteins and suppress platelet clustering. Such characteristics indicated its potential role in limiting cell mobility, migration patterns, and tissue infiltration, particularly in pulmonary and pancreatic sites. Studies also revealed GIP-34's regulatory influence on membrane-associated acetylcholinesterase function throughout neoplastic advancement and metastatic development.28
The modeling and rational design approaches of GIP-8 led to the development of a biologically active analog named AFP peptide (AFPep). AFPep has a distinct mechanism of action as an antineoplastic agent that neither exhibits cytotoxicity nor disrupts DNA synthesis. AFPep does not impede estrogen production but controls natural regulatory pathways to diminish estrogen-stimulated growth.29 Following AFPep administration in DU-145 prostate cancer cell lines, researchers observed decreased expression of antiproliferative regulators p18, p21, and p27, alongside reduced levels of pro-apoptotic factors Fas and Bax.30
AFP Receptors
Alpha-fetoprotein receptors play a crucial role in mediating the physiological functions of AFP in normal embryonic processes but are also associated with various pathological conditions, particularly carcinogenesis. Although the structure of AFPR has not been fully understood, in vivo and in vitro studies of AFPR include: (a) MUCIN receptors, (b) Lysophospholipid receptors, (c) Chemokine receptors, (d) Scavenger receptors, (e) Nonselective and selective cation channels, (f) Metastasis related proteins, and (e) intracytoplasmic proteins.14,31
MUCIN receptor proteins, located in cellular membranes, facilitate cell-matrix interactions and communication. Research has identified multiple MUCIN variants as AFP-binding molecules in breast cancer tissue.32 AFP can act as a competitive binding molecule for MUC1 and MUC4 by interacting with their receptor regions. The structural organization of MUC1 and MUC4 encompasses characteristic elements: extracellular portions with extensive O-glycosylation, membrane-traversing segments, and internal domains containing repeated sequences that interact with mitogen-activated protein kinase (MAPK) and JNK/AP-1 signaling components.33 MUC4 binds to ErbB2 receptors on malignant cells via EGF regions, promoting cell growth and movement patterns. The unique absence of MUC4 in normal pancreatic tissue makes it a significant therapeutic candidate.34 A subset of AFPRs belongs to the lysophospholipid receptor family, comprising G protein-coupled receptors that interact with LPA 1-6 and S1P 1-5. Computational analyses indicate potential interactions between these receptors and specific regions of the AFP molecule.35
The Genetic Regulation of AFP: Dual Role in Fetal Development and Carcinogenesis
Alpha-fetoprotein is a multifaceted protein with significant roles in fetal development and adult pathophysiology, and its expression is tightly regulated by various genetic factors. Fetal AFP is synthesized by the yolk sac, fetal liver, and to a lesser extent by other non-hepatic fetal tissues.36 Fetal AFP levels peak around 13 weeks of gestation and gradually decline thereafter, with a sharp decrease near term (Fig. 1).37 Maternal serum AFP levels also rapidly decrease after delivery and typically return to non-pregnant levels within 2–3 weeks.38 However, Elevated AFP levels post-pregnancy can persist due to several pathological conditions. Gestational trophoblastic diseases, including hydatidiform moles, choriocarcinoma, and other trophoblastic tumors or endometrial pathologies such as endometritis and subinvolution of the placental site also cause an elevation in AFP levels. Other pregnancy-related complications, including ectopic pregnancy remnants and missed abortions with retained products or some malignancies like ovarian germ cell tumors or HCC, can be responsible for abnormally high AFP levels.39 The retained placental tissue implies incomplete delivery of placental fragments or tissue adhered to the uterine wall, which is a primary cause of postpartum persistently high AFP levels.
Fig. 1.
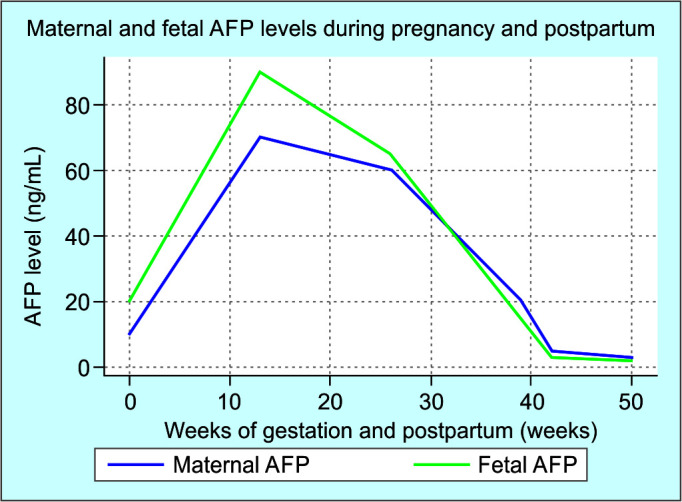
The dynamic changes in AFP levels throughout fetal development, pregnancy, and the postpartum period
AFP, alpha-fetoprotein; PP, postpartum
Alpha-fetoprotein is a major plasma protein produced by the yolk sac and the liver during fetal development that is normally silenced in the adult liver but can be reactivated during the process of HCC.40 The regulation of AFP expression involves a complex network of transcription factors, signaling pathways, and non-coding RNAs that play distinct roles across prenatal development, and postnatal life, as illustrated in Figures 2 and 3. Alpha-fetoprotein transcriptional regulation during fetal development involves complex coordination among multiple regulatory elements, including cis-regulatory sequences, trans-acting factors, epigenetic modifications, and developmental signaling pathways. A critical regulatory domain within the first intron of the AFP gene contains a 44 bp sequence centered on a CACCC motif. This sequence serves as a binding site for transcription factors BKLF and YY1, where BKLF functions as an activator while YY1 acts as a repressor. Alterations in the CACCC sequence can reduce BKLF binding while enhancing YY1 interaction, resulting in AFP overexpression. Additionally, a secondary promoter designated P2, positioned downstream of the enhancer element, contributes to initial AFP expression in both the yolk sac and fetal liver tissues.41
Fig. 2.
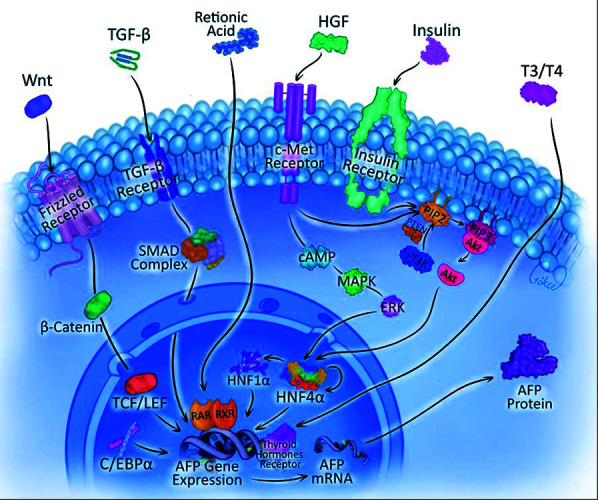
The complex molecular mechanisms governing AFP gene expression regulation. The cascade initiates with membrane-bound receptor activation by diverse extracellular ligands (Wnt, TGF-β, HGF, insulin, and thyroid hormones). These receptor-ligand interactions trigger distinct intracellular signaling pathways: Wnt/β-catenin pathway through the frizzled receptor, TGF-β/suppressor of mothers against decapentaplegic (SMAD) complex formation, HGF/c-Met signaling via MAPK/extracellular signal-regulated kinase (ERK) activation, and insulin receptor-mediated PI3K/protein kinase B (Akt) cascade. The convergence of these pathways leads to the activation of key hepatic transcription factors (HNF1α, HNF4α, and C/EBPα), which subsequently bind to specific regulatory elements within the AFP gene promoter. This coordinated transcriptional regulation ultimately results in AFP mRNA synthesis and subsequent protein production
Fig. 3.
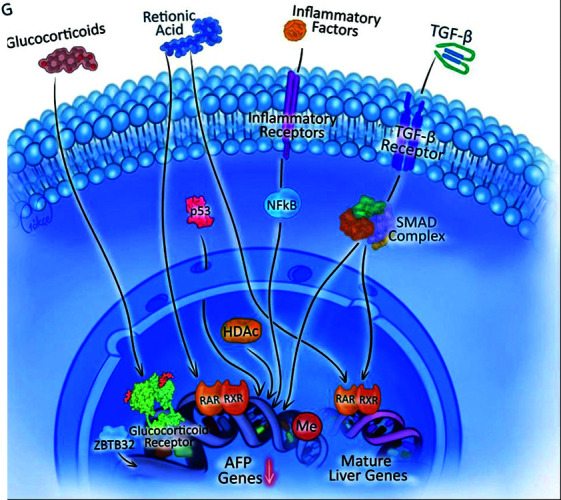
This figure illustrates the regulatory pathways involved in the suppression of AFP gene expression and the activation of mature liver-specific genes. Various extracellular factors, including glucocorticoids, retinoic acid, inflammatory factors, and TGF-β, interact with specific receptors, triggering intracellular signaling cascades that converge on transcriptional regulators like nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), p53, and the SMAD complex. These factors, along with epigenetic modifiers such as histone deacetylase (HDAC) and methylation (Me), shift transcriptional activity from AFP genes toward mature liver gene expression, indicating hepatic differentiation and reduced AFP production
Postnatally, the AFP gene undergoes regulatory control through the AFP regulator 1 (Afr1) region, mediated by the CCAAT-enhancer-binding protein alpha (C/EBPα) transcription factor, which plays key roles in hepatic developmental processes and cellular differentiation pathways.42,43 Alpha-fetoprotein gene transcriptional control is mediated by Nkx2.8, which exerts its regulatory effects via interaction with the upstream promoter-coupling element within the AFP promoter region.44
The genes Zinc-fingers and homeoboxes 2 (Zhx2) and zbtb20 control postnatal AFP repression and are linked to elevated AFP levels in HCC and germ cell tumors. ZBTB20 interacts directly with specific regions of the AFP gene promoter and activation of ZBTB20 after birth mediates the repression of AFP.40,45 Furthermore, ZBTB20 expression in the liver is developmentally regulated and inversely correlated with AFP gene expression, suggesting that the activation of ZBTB20 after birth mediates the repression of AFP.40 The binding site for ZBTB20 is highly conserved across species and is distinct from the albumin gene, which helps to explain the differential regulation of these two genes postnatally.45 Studies also have demonstrated that ZHX2 overexpression in human hepatoma cell lines leads to a significant decrease in AFP secretion in a dose-dependent manner.46 When ZHX2 levels are reduced, either through siRNA inhibition or due to promoter methylation, AFP is de-repressed. Thus, ZHX2 directly represses AFP expression via the AFP promoter and requires intact hepatocyte nuclear factor 1 (HNF1) binding sites.46,47 The relationship between ZHX2 and AFP is particularly relevant in HCC, where the loss of ZHX2 expression is often observed and is associated with the reactivation of AFP. This reactivation of AFP in HCC can be linked to the methylation status of the ZHX2 promoter, with methylation correlating with higher AFP expression.47 Hepatocyte nuclear factor 3 (HNF3/FOXA) functions as a far-upstream enhancer for the rat AFP gene.48 The regulatory relationship between HNF3 and AFP exhibits a complex dual mechanism, encompassing both activating and repressive functions that vary based on the cellular environment and specific HNF3 isoforms. Investigations using HepG2 hepatoma cells have revealed HNF3's inhibitory influence on AFP promoter function, which is mediated through a specific promoter segment positioned between -205 and -150 base pairs upstream of the transcription initiation site.49 This suppressive effect does not require direct HNF3-DNA interaction within the specified region but is hypothesized to operate through indirect pathways that modulate the binding or function of alternative liver-enriched factors that interact with the AFP promoter.49 HNF3, along with AFP and HNF1A, exhibits elevated expression levels in hepatoblastoma tissues compared to adjacent normal tissues [Xiaoyun Zhan and Alan Zhao, published in the Journal of Clinical Laboratory Analysis (2021)], in contrast to reduced FHX3. The experimental approach involved the knockdown of HNF3 using specific siRNAs in HB cell lines, which resulted in a marked reduction in both cell viability, colony formation capabilities, and cellular metabolism.50 HNF3 has been demonstrated to activate AFP expression by relieving chromatin-mediated repression of the AFP gene. HNF3 can activate AFP expression in non-liver cellular environments, highlighting its pivotal role in establishing hepatic-specific gene expression.51
AFP in Carcinogenesis
Molecular mechanisms of AFP involve several vital aspects in carcinogenesis, including its role in cellular proliferation, apoptosis, and modulation of immune responses. The effects of AFP on a cancer cell are summarized in Figure 4. Understanding these mechanisms provides insight into the potential of AFP as a biomarker for diagnosis and prognosis and a novel therapeutic target. Indeed, AFP has transcended the role of a mere diagnostic marker for HCC, actively engaged in the modulation of tumor dynamics through a spectrum of molecular mechanisms. In addition to HCC, AFP's utility as a biomarker in the diagnosis and monitoring of germ cell tumors, especially non-seminomatous variants, has been well-established. However, the exploration into its broader oncogenic or tumor-suppressing roles in other cancer types remains a fertile ground for scientific inquiry.
Fig. 4.
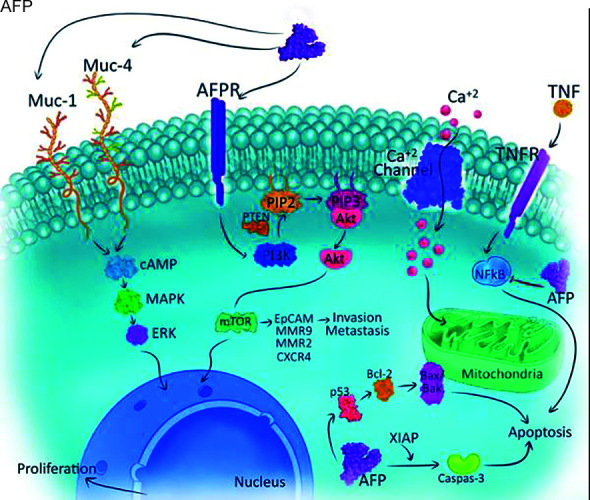
Alpha-fetoprotein exerts complex regulatory effects on cancer cell pathways through its interaction with AFPR. This binding initiates diverse signaling cascades, notably triggering PI3K-mediated conversion of PIP2 to PIP3, leading to Akt pathway activation. Subsequent downstream signaling through mTOR enhances cellular survival mechanisms, while EpCAM, MMP9, MMP2, and CXCR4 mediate invasive and metastatic processes. Cellular calcium dynamics are modulated through ion channel regulation, affecting NFkB signaling, while AFP's interaction with TNF/TNFR system influences inflammatory and immune responses. Additionally, AFP modulates apoptotic pathways through regulation of Bcl-2, Bax, Bak, and XIAP proteins, ultimately affecting Caspase-3 activation and cell survival
1. The Role of AFP in Cell Cycle
Recent studies have elucidated the connection between AFP expression and the control of crucial cell cycle checkpoints. Alpha-fetoprotein production synchronizes with key cell cycle transitions, occurring from late G1 through the S phase and ceasing before the M phase. Research shows AFP interacts with FOXM1, a cell cycle-regulating transcription factor that shows increased expression in AFP-positive HCC. Studies demonstrate that FOXM1 reduction leads to decreased AFP expression and G2/M phase arrest.52 RNA interference targeting AFP impedes G1 to S phase progression. Similar findings using Modified Stealth RNAi duplexes showed cell accumulation in G0/G1 and reduced S and G2 phase populations 48 h post-transfection. These findings indicate AFP promotes cellular proliferation by facilitating G1 to S phase transition and modulating cyclin expression.53 Secreted AFP interacts with specific membrane AFPRs, activating the expression of oncogenes including c-fos, c-jun, and Ras, and triggering cascades monophosphate (cAMP) signaling. Furthermore, AFP facilitates the transmission of extracellular signals for promoting cell proliferation through intracellular signaling pathways. Notably, AFP has been reported to interact with phosphatase and tensin homolog (PTEN), resulting in increased ubiquitination and accelerated degradation of PTEN, subsequently inhibiting its phosphoinositide phosphatase activity. This essential molecular association activates the AKT cascade and amplifies phosphoinositide 3-kinase (PI3K)/AKT signaling, resulting in enhanced proliferative activity of HCC cells.54 Experimental reduction of AFP levels in HepG2 cells demonstrated diminished expression of transforming growth factor-beta (TGF-β) and mutant P53 proteins, highlighting AFP's intricate regulatory influence on cell cycle mechanisms.55 The interaction of AFP with cell cycle proteins is facilitated by its third domain which is extensively put forward by in silico analysis. AFP's third domain also harbors cation channel interaction sites. The effect of cation channels on G1 events and G to S phase transition is shown in MCF-7 cells by changing membrane potentials.56 Alpha-fetoprotein GIP also engages with cellular cation channel proteins and has a direct impact on the expressions of cell cycle regulators.57
2. The Role of AFP in Cancer Metastasis
In general, AFP levels are related to the metastasis potential of HCC, although, a propensity analysis has indicated a lack of supporting evidence for a correlation between AFP cut-off levels and the occurrence of metastasis in less than 5 cm tumors measuring.58
Cancer stem cell (CSC) populations play a key role in HCC metastasis. Research identifies EpCAM, CD90, CD44, and CD133 as CSC markers. Studies show a correlation between EpCAM, CD133, and elevated AFP levels in HCC.59,60 EpCAM-positive cells frequently express AFP and exhibit poor differentiation characteristics. CD90-positive cells similarly show poor differentiation and a higher risk of distant metastasis within 2 years post-surgery.61 However, CD44 shows no significant correlation with cell differentiation, AFP levels, or disease-free survival (DFS).37 Research demonstrates AFP inhibits PTEN activity, activating PI3K/AKT pathways that increase RAS, SRC, and CXCR4 expression along with metastasis-associated proteins including EpCAM, K19, MMP2/9, and CXCR4.62,63 These findings indicate AFP promotes HCC metastasis through PI3K/AKT pathway activation. Recent studies reveal AFP's interaction with focal adhesion kinase pathways, enhancing tumor cell mobility and invasion.64 This finding opens new possibilities for targeted therapies aimed at disrupting AFP-FAK interactions.
3. The Role of AFP on Apoptosis
Alpha-fetoprotein demonstrates the capacity to bind with caspase-3, a crucial proteolytic enzyme in apoptotic signaling, resulting in the suppression of its enzymatic function. This molecular interaction impedes caspase-3-mediated apoptotic cascade progression, thus enhancing HCC cell survival against programmed cell death.65 Studies have revealed that AFP acts as an antagonistic factor against XIAP's anti-caspase properties. Through this mechanism, AFP disrupts the XIAP-caspase complex formation, leading to caspase-3 liberation from inhibitory control, which suggests its direct involvement in the dysregulation of apoptotic mechanisms within neoplastic cells.13
Studies have elucidated AFP's regulatory role in the tumor protein (p53)/Bax/cytochrome c/caspase-3 signaling cascade. Experimental suppression of AFP expression in HCC cells results in elevated Bax/Bcl-2 ratios, augmented mitochondrial cytochrome c release, and enhanced caspase-3 activation, indicating that AFP facilitates cellular proliferation by conferring resistance to apoptotic mechanisms.66 Moreover, AFP has been identified as a downstream target of nuclear factor-kappaB (NF-kappaB), and it has been suggested that AFP may inhibit TNF-alpha-induced cell death of murine HCCs, through association with TNF-alpha and inhibition of TNFRI signaling.65
4. The Role of AFP in Immune Modulation
Alpha-fetoprotein demonstrates immunological modifying capabilities through its inhibitory effects on the survival and function of diverse immune cell types, encompassing natural killer cells, monocytes, and dendritic cells (DCs). Investigations reveal that DCs altered by AFP influence naïve T cell differentiation toward regulatory T cell phenotypes, suggesting a mechanism for tumor immune escape.2 Additionally, AFP has been reported to exert selective immunosuppressive activity on T-dependent immune responses, particularly affecting T lymphocytes responding against histocompatibility-associated alloantigens.67 Alpha-fetoprotein also affects the differentiation of myeloid suppressor cells (MDSCs), increasing the number of monocytic MDSCs in culture.68 Alpha-fetoprotein may also regulate the expression of immune-related proteins through the NF-kappaB (P65) pathway, potentially affecting tumor immunity.69 These findings suggest that AFP has complex roles in immune modulation, which may have implications for immunotherapeutic strategies targeting AFP-expressing tumors.
The Clinical Role of AFP: Its Diagnostic, Prognostic, and Therapeutic Implications
1. Screening and Diagnosis
Alpha-fetoprotein is the first oncoprotein discovered and remains a widely used biomarker in both screening and monitoring of HCC.70 Since AFP is not elevated in many HCC cases, an approach combining AFP with ultrasound (USG) enhances the sensitivity of HCC screening, compared to either one alone.71,72 The utility of AFP as an individual screening tool is limited due to its variable sensitivity and specificity. This statement depends on the AFP cut-off values, and the currently used cut-off of 20 ng/mL yields a sensitivity of about 60% with a variable positive predictive value depending on HCC prevalence.73 Guidelines from the Asia-Pacific Association for the Study of the Liver advise against using AFP independently for early HCC detection, instead recommending combined USG and AFP screening with 200 ng/mL as the threshold value.74 These guidelines suggest lower AFP thresholds may be appropriate in populations with controlled or eliminated hepatitis virus.74 Surveillance protocols endorsed by the NCCN advocate for the combined application of serum AFP measurement and ultrasonography, as this dual approach demonstrates superior efficacy in HCC detection compared to isolated AFP screening.80 Notably, when utilizing a threshold of 100 ng/mL, AFP exhibits marked specificity despite limited sensitivity.80 In contemporary HCC management, AFP continues to serve as a crucial biomarker, particularly in prognostic assessment and therapeutic response evaluation. Clinical evidence suggests that elevated baseline AFP concentrations correlate with diminished overall and DFS metrics among HCC patients, underscoring its value in outcome prediction.81 Furthermore, AFP quantification proves instrumental in assessing liver transplantation candidacy and monitoring therapeutic response in patients receiving targeted molecular interventions such as sorafenib therapy.82
The utilization of AFP alone as a screening biomarker demonstrates inherent diagnostic constraints regarding sensitivity and specificity, underscoring the requirement for complementary markers and refined detection methodologies for effective HCC identification. Protein Induced by Vitamin K Absence-II (PIVKA-II), alternatively known as DCP, originates from defective carboxylation of the prothrombin precursor molecule during states of vitamin K insufficiency or impairment of vitamin K-dependent carboxylase activity, circumstances frequently encountered in liver pathologies. As an abnormal prothrombin form, PIVKA-II deviates from standard physiological protein variants.83 In HCC pathogenesis, malignant hepatocytes generate PIVKA-II, leading to elevated serum levels that serve as a valuable diagnostic and monitoring tool, particularly in cases where AFP remains within normal parameters. Research by Kim and colleagues advocates for the combined utilization of AFP and PIVKA-II to improve HCC surveillance efficacy, specifically addressing the needs of Asia-Pacific populations where normal AFP levels are frequently encountered.83 Additionally, AFP-L3 is an AFP-like biomarker that has emerged in recent years to improve diagnostic accuracy in patients with low AFP levels. AFP-L3 has also a role in the staging and risk stratification of HCC. Elevated levels of AFP-L3, particularly when they exceed 10% of the total AFP, are associated with a worse prognosis, including lower survival rates and higher recurrence rates following treatment.84,85 Research indicates that AFP-L3 demonstrates a correlation with aggressive tumor characteristics, including increased tumor dimensions, vascular infiltration, and enhanced metastatic potential. This property establishes AFP-L3 as an important diagnostic indicator for identifying advanced disease states, particularly in cases where conventional serum AFP measurements remain within normal ranges.86 AFP-L3 percentage demonstrates superior predictive value for HCC recurrence in post-transplantation patients, particularly among those who have undergone transarterial chemoembolization therapy.87
Alpha-fetoprotein receptors offers new insights into their potential diagnostic and therapeutic applications in the context of HCC. AFPR is a potential early indicator of malignant transformation in hepatocytes since it is upregulated and expressed on the membranes of HCC cells, prior to the expression of other oncogenic markers.88 The expression and upregulated of AFPR at early stages of hepatocarcinogenesis is driven by the hepatitis B virus X protein, even before other markers like RAS, SRC, and CXCR4.89 Additionally, AFPR has been explored for its potential as a tool for monitoring disease progression and metastasis.89
2. Prognosis
Alpha-fetoprotein-based prognostic models and scoring systems are used to predict outcomes in HCC, highlighting the importance of AFP in conjunction with other clinical parameters for assessing prognosis and guiding treatment decisions (Table 3). The GALAD score represents a biomarker-based model incorporating multiple factors including gender, age, AFP, AFP-L3, and DCP for predicting outcomes and treatment guidance in HCC patients.90 Studies demonstrate the GALAD score's high diagnostic precision with AUC values between 0.88 and 0.98.90 Studies demonstrate that implementing a GALAD score threshold of −0.76 yields 91% sensitivity and 85% specificity in identifying HCC.91 Clinical data suggest that utilizing AFP-L3 in conjunction with DCP provides enhanced prognostic accuracy for HCC recurrence following liver transplantation, surpassing the predictive capabilities of isolated AFP measurements.92 Tayob et al. analyzed the GALAD score for detecting HCC and concluded that it shows increased sensitivity, although its overall efficacy aligns with that of AFP-L3.93 This calls for further enhancement of surveillance methodologies.
Table 3.
AFP-based prognostic models for hepatocellular carcinoma
| Prognostic model | Components | Clinical significance | References |
|---|---|---|---|
| Fucosylation Index (FI) of AFP | Fucosylated AFP, AFP | Persistent high FI (>18%) before and after treatment indicates a poorer prognosis. | 75 |
| Preoperative AFP Levels | AFP l | Higher preoperative AFP levels are linked to worse OS and DFS patients undergoing curative hepatectomy. | 81 |
| AFP-TBS-ALBI score | AFP, TBS, ALBI | Predicts OS and PFS in HCC patients RFA. | 76 |
| AFP score model | AFP, tumor size, tumor number | Serves as a prognostic indicator for patients with hepatitis B virus-associated HCC following curative hepatic resection. Higher scores correlate with poorer OS and DFS. | 77 |
| A-A score | Preoperative serum AFP, ALP levels | Higher A-A scores are associated with worse outcomes in patients with ruptured HCC after hepatectomy. | 78 |
| ADV score | AFP, DCP, tumor volume | The assessment serves as a prognostic indicator for both HCC recurrence patterns and survival outcomes following LDLT | 79 |
AFP, alpha-fetoprotein; ALBI, albumin-bilirubin; ALP, alkaline phosphatase; DCP, des-γ-carboxyprothrombin; DFS, disease-free survival; HCC, hepatocellular carcinoma; LDLT, living donor liver transplantation; OS, overall survival; PFS, progression-free survival; RFA, radiofrequency ablation; TBS, tumor burden score
Therapeutically, AFP's role is expanding, with Mizejewski uncovering the anti-tumoral potential of an AFP-derived peptide, GIP, offering a new therapeutic avenue. Furthermore, Sherman et al. discussed the conjugation of AFP with maytansine, which illustrates a creative approach to targeted therapy, aiming for tumor regression in ovarian cancer models while mitigating toxicity.94,95
3. Treatment
The cancerous cells frequently exploit the immune tolerance state as a mechanism to evade detection by the immune system. AFP is transcending its traditional role as a biomarker in oncology and is being strategically incorporated into the domain of vaccine development, exemplifying its capability to counteract immune tolerance. By incorporating AFP epitopes into diverse vaccine platforms, encompassing DCs, DNA, peptide, and genetic vaccines, researchers have devised innovative strategies to stimulate the immune system to recognize and eliminate tumor cells that express AFP on their surface. These vaccine constructs are meticulously designed to induce cytotoxic T lymphocytes (CTLs), which are pivotal components of the immune system, to actively search for and destroy cancer cells that display AFP. For example, experimental evidence has demonstrated that a DNA vaccine encoding the murine AFP gene, in conjunction with the co-expression of the heat shock protein 70 gene, elicits a pronounced AFP-specific CTL response and extends survival in tumor-bearing mice. In a parallel clinical context, a prime-boost regimen involving AFP DNA and an adenovirus expressing AFP has been subjected to clinical trials in patients with HCC. The results from these trials have revealed immunogenic properties and suggest a potential enhancement in progression-free survival among patients who develop AFP-specific T cell-mediated immunity.96,97
Clinical trials have been pivotal in uncovering the efficacy of AFP-centric vaccines. They have documented that peptides hailing from AFP can elicit tangible immune reactions, culminating in results that span from halting tumor growth to achieving complete tumor regression in some cases. This success underscores not only the therapeutic promise of AFP-derived vaccines but also serves as an impetus for ongoing vaccine improvement endeavors.86,97 To enhance the immune response against HCC, researchers are refining AFP's structure through epitope enhancement and combining AFP-expressing DC vaccines with agents that inhibit regulatory T cells. While AFP-based vaccination alone induces a significant CD8+ T cell response but upregulates exhaustion markers like PD1, LAG3, and Tim3—diminishing effectiveness against established tumors—combining the vaccine with anti–PD-L1 treatment markedly inhibits HCC progression by overcoming immune escape mechanisms, offering a promising therapeutic avenue for AFP-positive HCC.98
AFP-siRNA, designed to suppress AFP expression, offers a promising therapeutic approach for HCC by inhibiting cell proliferation and promoting apoptosis. Its impact is enhanced by delivery mechanisms such as liposomes, nanoparticles, and polymers that improve its stability and uptake. The specifically incorporated AFP-siRNA into poly(lactic-co-glycolic) acid (PLGA) nanoparticles shows synergistic effects with chemotherapy agents like doxorubicin and is associated with cell cycle arrest and increased caspase-3 expression, indicating efficacy against HCC.53,99,100
The collective progress in AFP research encourages a transition towards more precise and personalized medical approaches, focusing on AFP's applications in diagnostics and therapeutics. This interplay between clinical research, computational biology, and materials science is not only enhancing our knowledge but also leading us toward a new era of personalized medicine, poised to influence patient outcomes in oncology and beyond profoundly.
Conclusion
Alpha-fetoprotein is a significant oncofetal protein with a profound impact on the biology of various cancers, especially HCC and germ cell tumors. Its structural complexity allows it to play critical roles in cellular proliferation, apoptosis, metastasis, and immune system modulation. AFP's diagnostic value is highlighted by its use in distinguishing between benign and malignant liver conditions, with specific attention to its variants AFP-L3.
The protein's ability to interact with a myriad of receptors and ligands further emphasizes its biological significance in carcinogenesis and so treatment. The leveraging binding properties of AFP to drug delivery systems aimed at minimizing damage to healthy tissues while effectively combating cancer cells. Innovations in AFP-related therapy, including recombinant AFP fragments and conjugated drugs create a potential for AFP as a key molecule in the development of novel cancer therapies. The ongoing research will unveil the multifaceted roles of AFP in oncology and will pave the way for enhanced diagnostic techniques, improved treatment monitoring, and advance personalized cancer treatment.
Orcid
Basak Celtikci https://orcid.org/0000-0002-3242-978X
Hatice Y Balaban https://orcid.org/0000-0002-0901-9192
Footnotes
Source of support: Nil
Conflict of interest: Dr Hatice Y Balaban is associated as Editor-in-Chief of this journal and this manuscript was subjected to this journal's standard review procedures, with this peer review handled independently of the Editor-in-Chief and his research group.
References
- 1.Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: A renaissance. Tumour Biol. 2013;34(4):2075–2091. doi: 10.1007/s13277-013-0904-y. [DOI] [PubMed] [Google Scholar]
- 2.Munson PV, Adamik J, Butterfield LH. Immunomodulatory impact of α-fetoprotein. Trends Immunol. 2022;43(6):438–448. doi: 10.1016/j.it.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Terentiev AA, Moldogazieva NT. Structural and functional mapping of α-fetoprotein. Biochemistry (Mosc) 2006;71(2):120–132. doi: 10.1134/s0006297906020027. [DOI] [PubMed] [Google Scholar]
- 4.Mizejewski GJ. Biological roles of alpha-fetoprotein during pregnancy and perinatal development. Exp Biol Med (Maywood) 2004;229(6):439–463. doi: 10.1177/153537020422900602. [DOI] [PubMed] [Google Scholar]
- 5.Androutsopoulos G, Gkogkos P, Decavalas G. Mid-trimester maternal serum HCG and alpha fetal protein levels: Clinical significance and prediction of adverse pregnancy outcome. Int J Endocrinol Metab. 2013;11(2):102–106. doi: 10.5812/ijem.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pak VN. Alpha-Fetoprotein: A revolutionary anti-cancer drug. Med Res Arch. 2023;11(7.1) doi: 10.18103/mra.v11i7.1.4125. [DOI] [Google Scholar]
- 7.Pak VN. The use of alpha-fetoprotein for the treatment of autoimmune diseases and cancer. Ther Deliv. 2018;9(1):37–46. doi: 10.4155/tde-2017-0073. [DOI] [PubMed] [Google Scholar]
- 8.Morinaga T, Sakai M, Wegmann TG, et al. Primary structures of human alpha-fetoprotein and its mRNA. Proc Natl Acad Sci U S A. 1983;80(15):4604–4608. doi: 10.1073/pnas.80.15.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Głowska-Ciemny J, Szymański M, Kuszerska A, et al. The role of alpha-fetoprotein (AFP) in contemporary oncology: The path from a diagnostic biomarker to an anticancer drug. Int J Mol Sci. 2023;24(3):2539. doi: 10.3390/ijms24032539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Głowska-Ciemny J, Szymanski M, Kuszerska A, et al. Role of alpha-fetoprotein (AFP) in diagnosing childhood cancers and genetic-related chronic diseases. Cancers (Basel) 2023;15(17):4302. doi: 10.3390/cancers15174302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma C, Jin Y, Wang Y, et al. Beyond liver cancer, more application scenarios for alpha-fetoprotein in clinical practice. Front Oncol. 2023;13:1231420. doi: 10.3389/fonc.2023.1231420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Wang L, Zhang S, et al. Alpha-fetoprotein predicts the treatment efficacy of immune checkpoint inhibitors for gastric cancer patients. BMC Cancer. 2024;24(1):266. doi: 10.1186/s12885-024-11999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudich E, Semenkova L, Dudich I, et al. Alpha-fetoprotein antagonizes X-linked inhibitor of apoptosis protein anticaspase activity and disrupts XIAP-caspase interaction. Febs J. 2006;273(16):3837–3849. doi: 10.1111/j.1742-4658.2006.05391.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin B, Wang Q, Liu K, et al. Alpha-fetoprotein binding mucin and scavenger receptors: An available bio-target for treating cancer. Front Oncol. 2021;11:625936. doi: 10.3389/fonc.2021.625936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin B, Zhu M, Wang W, et al. Structural basis for alpha fetoprotein-mediated inhibition of caspase-3 activity in hepatocellular carcinoma cells. Int J Cancer. 2017;141(7):1413–1421. doi: 10.1002/ijc.30850. [DOI] [PubMed] [Google Scholar]
- 16.Mizejewski GJ. Review of the putative cell-surface receptors for alpha-fetoprotein: Identification of a candidate receptor protein family. Tumour Biol. 2011;32(2):241–258. doi: 10.1007/s13277-010-0134-5. [DOI] [PubMed] [Google Scholar]
- 17.Kong M, Tian S, Shi H, et al. The effect of alpha-fetoprotein on the activation and phagocytosis of granulocytes and monocytes. Hepatogastroenterology. 2012;59(120):2385–2388. doi: 10.5754/hge12296. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M, Li W, Lu Y, et al. Alpha fetoprotein antagonizes apoptosis induced by paclitaxel in hepatoma cells in vitro. Sci Rep. 2016;6:26472. doi: 10.1038/srep26472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moldogazieva NT, Ostroverkhova DS, Kuzmich NN, et al. Elucidating binding sites and affinities of ERα agonists and antagonists to human alpha-fetoprotein by in silico modeling and point mutagenesis. Int J Mol Sci. 2020;21(3):893. doi: 10.3390/ijms21030893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizejewski GJ. Nonsecreted cytoplasmic alpha-fetoprotein: A newly discovered role in intracellular signaling and regulation. An update and commentary. Tumour Biol. 2015;36(12):9857–9864. doi: 10.1007/s13277-015-3736-0. [DOI] [PubMed] [Google Scholar]
- 21.Mizejewski GJ, Mirowski M, Garnuszek P, et al. Targeted delivery of anti-cancer growth inhibitory peptides derived from human alpha-fetoprotein: Review of an international multi-center collaborative study. J Drug Target. 2010;18(8):575–588. doi: 10.3109/10611861003587243. [DOI] [PubMed] [Google Scholar]
- 22.Arshad NM, In LL, Soh TL, et al. Recombinant human alpha fetoprotein synergistically potentiates the anti-cancer effects of 1’-S-1’-acetoxychavicol acetate when used as a complex against human tumours harbouring AFP-receptors. Oncotarget. 2015;6(18):16151–16167. doi: 10.18632/oncotarget.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Wang S, Wang Y, et al. Decorin-loaded poly lactic-co-glycolic acid nanoparticles modified by anti-alpha fetoprotein antibody: preparation, proliferation inhibition and induced apoptosis effects on HepG2 cells in vitro. J Pharm Pharmacol. 2017;69(6):633–641. doi: 10.1111/jphp.12695. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Liu S, Xu J, et al. A new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2 cells. Cell Death Dis. 2017;8(11):e3160. doi: 10.1038/cddis.2017.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posypanova GA, Gorokhovets NV, Makarov VA, et al. Recombinant alpha-fetoprotein C-terminal fragment: The new recombinant vector for targeted delivery. J Drug Target. 2008;16(4):321–328. doi: 10.1080/10611860801927721. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie JR, Uversky VN. Structure and function of alpha-fetoprotein: A biophysical overview. Biochim Biophys Acta. 2000;1480(1–2):41–56. doi: 10.1016/s0167-4838(00)00104-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin B, Dong X, Wang Q, et al. AFP-inhibiting fragments for drug delivery: The promise and challenges of targeting therapeutics to cancers. Front Cell Dev Biol. 2021;9:635476. doi: 10.3389/fcell.2021.635476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizejewski GJ. Mechanism of cancer growth suppression of alpha-fetoprotein derived growth inhibitory peptides (GIP): Comparison of GIP-34 versus GIP-8 (AFPep). Updates and prospects. Cancers (Basel) 2011;3(2):2709–2733. doi: 10.3390/cancers3022709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansouri W, Fordyce SB, Wu M, et al. Efficacy and tolerability of AFPep, a cyclic peptide with anti-breast cancer properties. Toxicol Appl Pharmacol. 2018;345:10–18. doi: 10.1016/j.taap.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Z, West GR, Wang DC, et al. AFP peptide (AFPep) as a potential growth factor for prostate cancer. Med Oncol. 2021;39(1):2. doi: 10.1007/s12032-021-01598-4. [DOI] [PubMed] [Google Scholar]
- 31.Mizejewski JG. Review of the third domain receptor binding fragment of alphafetoprotein (AFP): Plausible binding of AFP to lysophospholipid receptor targets. Curr Drug Targets. 2017;18(7):874–886. doi: 10.2174/1389450117666160201105245. [DOI] [PubMed] [Google Scholar]
- 32.Mizejewski GJ. Review of the adenocarcinoma cell surface receptor for human alpha-fetoprotein; proposed identification of a widespread mucin as the tumor cell receptor. Tumour Biol. 2013;34(3):1317–1336. doi: 10.1007/s13277-013-0704-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Liu G, Li Q, et al. Mucin1 promotes the migration and invasion of hepatocellular carcinoma cells via JNK-mediated phosphorylation of Smad2 at the C-terminal and linker regions. Oncotarget. 2015;6(22):19264–19278. doi: 10.18632/oncotarget.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoup N, Liberelle M, Schulz C, et al. The EGF domains of MUC4 oncomucin mediate HER2 binding affinity and promote pancreatic cancer cell tumorigenesis. Cancers (Basel) 2021;13(22):5746. doi: 10.3390/cancers13225746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaho VA, Chun J. ‘Crystal’ clear? Lysophospholipid receptor structure insights and controversies. Trends Pharmacol Sci. 2018;39(11):953–966. doi: 10.1016/j.tips.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deutsch HF. In: Vande Woude GF, Klein G, editors. Advances in Cancer Research. 56: Academic Press; 1991. Chemistry and Biology of α-Fetoprotein. pp. 253–312. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Tan Y. Prognostic value of CD44 expression in patients with hepatocellular carcinoma: Meta-analysis. Cancer Cell Int. 2016;16:47. doi: 10.1186/s12935-016-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glowska-Ciemny J, Pankiewicz J, Malewski Z, et al. Alpha-fetoprotein (AFP) — new aspects of a well-known marker in perinatology. Ginekologia Polska. 2022;93(1):70–75. doi: 10.5603/GP.a2021.0226. [DOI] [PubMed] [Google Scholar]
- 39.Kupferminc MJ, Tamura RK, Wigton TR, et al. Placenta accreta is associated with elevated maternal serum alpha-fetoprotein. Obstet Gynecol. 1993;82(2):266–269. 7687756 [PubMed] [Google Scholar]
- 40.Peterson ML, Ma C, Spear BT. Zhx2 and Zbtb20: Novel regulators of postnatal alpha-fetoprotein repression and their potential role in gene reactivation during liver cancer. Semin Cancer Biol. 2011;21(1):21–27. doi: 10.1016/j.semcancer.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scohy S, Gabant P, Szpirer C, et al. Identification of an enhancer and an alternative promoter in the first intron of the α-fetoprotein gene. Nucleic Acids Res. 2000;28(19):3743–3751. doi: 10.1093/nar/28.19.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerlach JC, Over P, Foka HG, et al. Role of transcription factor CCAAT/enhancer-binding protein alpha in human fetal liver cell types in vitro. Hepatol Res. 2015;45(8):919–932. doi: 10.1111/hepr.12420. [DOI] [PubMed] [Google Scholar]
- 43.Peyton DK, Huang MC, Giglia MA, et al. The alpha-fetoprotein promoter is the target of Afr1-mediated postnatal repression. Genomics. 2000;63(2):173–180. doi: 10.1006/geno.1999.6073. [DOI] [PubMed] [Google Scholar]
- 44.Kajiyama Y, Tian J, Locker J. Regulation of alpha-fetoprotein expression by Nkx2.8. Mol Cell Biol. 2002;22(17):6122–6130. doi: 10.1128/MCB.22.17.6122-6130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Cao D, Zhou L, et al. ZBTB20 is a sequence-specific transcriptional repressor of alpha-fetoprotein gene. Sci Rep. 2015;5:11979. doi: 10.1038/srep11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen H, Luan F, Liu H, et al. ZHX2 is a repressor of alpha-fetoprotein expression in human hepatoma cell lines. J Cell Mol Med. 2008;12(6b):2772–2780. doi: 10.1111/j.1582-4934.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lv Z, Du Y, Wen J. [The methylation of ZHX2 gene promoter enhances AFP gene expression in hepatocellular carcinoma] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29(7):706–709. 23837980 [PubMed] [Google Scholar]
- 48.Thomassin H, Bois-Joyeux B, Delille R, et al. Chicken ovalbumin upstream promoter-transcription factor, hepatocyte nuclear factor 3, and CCAAT/enhancer binding protein control the far-upstream enhancer of the rat α-fetoprotein gene. DNA Cell Biol. 1996;15(12):1063–1074. doi: 10.1089/dna.1996.15.1063. [DOI] [PubMed] [Google Scholar]
- 49.Huang MC, Li KK, Spear BT. The mouse alpha-fetoprotein promoter is repressed in HepG2 hepatoma cells by hepatocyte nuclear factor-3 (FOXA) DNA Cell Biol. 2002;21(8):561–569. doi: 10.1089/104454902320308933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhan X, Zhao A. Transcription factor FOXA3 promotes the development of Hepatoblastoma via regulating HNF1A, AFP, and ZFHX3 expression. J Clin Lab Anal. 2021;35(3):e23686. doi: 10.1002/jcla.23686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crowe AJ, Sang L, Li KK, et al. Hepatocyte nuclear factor 3 relieves chromatin-mediated repression of the alpha-fetoprotein gene. J Biol Chem. 1999;274(35):25113–25120. doi: 10.1074/jbc.274.35.25113. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Okada H, Yamashita T, et al. FOXM1 is a novel molecular target of AFP-positive hepatocellular carcinoma abrogated by proteasome inhibition. Int J Mol Sci. 2022;23(15):8305. doi: 10.3390/ijms23158305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, He T, Cui H, et al. Effects of AFP gene silencing on apoptosis and proliferation of a hepatocellular carcinoma cell line. Discov Med. 2012;14(75):115–124. 22935208 [PubMed] [Google Scholar]
- 54.Wang S, Zhu M, Wang Q, et al. Alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2018;9(10):1027. doi: 10.1038/s41419-018-1036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Chen L, Liang Y, et al. Knockdown of alpha-fetoprotein expression inhibits HepG2 cell growth and induces apoptosis. J Cancer Res Ther. 2018;14(Supplement):S634–s43. doi: 10.4103/0973-1482.180681. [DOI] [PubMed] [Google Scholar]
- 56.Mizejewski GJ. The alpha-fetoprotein (AFP) third domain: A search for AFP interaction sites of cell cycle proteins. Tumour Biol. 2016;37(9):12697–12711. doi: 10.1007/s13277-016-5131-x. [DOI] [PubMed] [Google Scholar]
- 57.Mizejewskia GJ, Butterstein G. Survey of functional activities of alpha-fetoprotein derived growth inhibitory peptides: review and prospects. Curr Protein Pept Sci. 2006;7(1):73–100. doi: 10.2174/138920306775474130. [DOI] [PubMed] [Google Scholar]
- 58.Wang YQ, Wang AJ, Zhang TT, et al. Association of alpha-fetoprotein and metastasis for small hepatocellular carcinoma: A propensity-matched analysis. Sci Rep. 2022;12(1):15676. doi: 10.1038/s41598-022-19531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulte LA, López-Gil JC, Sainz B, Jr.,, et al. The cancer stem cell in hepatocellular carcinoma. Cancers (Basel) 2020;12(3):684. doi: 10.3390/cancers12030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeng K-S, Chang C-F, Sheen I-S, et al. Cellular and molecular biology of cancer stem cells of hepatocellular carcinoma. Int J Mol Sci. 2023;24(2):1417. doi: 10.3390/ijms24021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romano M, De Francesco F, Pirozzi G, et al. Expression of cancer stem cell biomarkers as a tool for a correct therapeutic approach to hepatocellular carcinoma. Oncoscience. 2015;2(5):443–456. doi: 10.18632/oncoscience.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu M, Guo J, Xia H, et al. Alpha-fetoprotein activates AKT/mTOR signaling to promote CXCR4 expression and migration of hepatoma cells. Oncoscience. 2015;2(1):59–70. doi: 10.18632/oncoscience.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Y, Zhu M, Li W, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med. 2016;20(3):549–558. doi: 10.1111/jcmm.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braghini MR, De Stefanis C, Tiano F, et al. Focal adhesion kinase and its epigenetic interactors as diagnostic and therapeutic hints for pediatric hepatoblastoma. Front Oncol. 2024;14:1397647. doi: 10.3389/fonc.2024.1397647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tiegs G, Horst AK. TNF in the liver: Targeting a central player in inflammation. Semin Immunopathol. 2022;44(4):445–459. doi: 10.1007/s00281-022-00910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y, Guo Q, Wei L. The emerging influences of alpha-fetoprotein in the tumorigenesis and progression of hepatocellular carcinoma. Cancers (Basel) 2021;13(20):5096. doi: 10.3390/cancers13205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linson EA, Hanauer SB. More than a tumor marker…a potential role for alpha-feto protein in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(7):1271–1276. doi: 10.1093/ibd/izy394. [DOI] [PubMed] [Google Scholar]
- 68.Zamorina SA, Shardina KY, Timganova VP, et al. Effect of alpha-fetoprotein on differentiation of myeloid supressor cells. Dokl Biochem Biophys. 2021;501(1):434–437. doi: 10.1134/S1607672921060077. [DOI] [PubMed] [Google Scholar]
- 69.Li QT, Qiu MJ, Yang SL, et al. Alpha-fetoprotein regulates the expression of immune-related proteins through the NF-κB (P65) pathway in hepatocellular carcinoma cells. J Oncol. 2020;2020:9327512. doi: 10.1155/2020/9327512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeo YH, Lee YT, Tseng HR, et al. Alpha-fetoprotein: Past, present, and future. Hepatol Commun. 2024;8(5):e0422. doi: 10.1097/HC9.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo P, Wu S, Yu Y, et al. Current status and perspective biomarkers in AFP negative HCC: Towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol Oncol Res. 2020;26(2):599–603. doi: 10.1007/s12253-019-00585-5. [DOI] [PubMed] [Google Scholar]
- 72.Kim MN, Kim BK, Kim SU, et al. Longitudinal assessment of alpha-fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis. Scand J Gastroenterol. 2019;54(10):1283–1290. doi: 10.1080/00365521.2019.1673478. [DOI] [PubMed] [Google Scholar]
- 73.Daniele B, Bencivenga A, Megna AS, et al. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aoyagi Y, Mita Y, Suda T, et al. The fucosylation index of serum α-fetoprotein as useful prognostic factor in patients with hepatocellular carcinoma in special reference to chronological changes. Hepatol Res. 2002;23(4):287–295. doi: 10.1016/s1386-6346(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 76.Huang J, Cui W, Xie X, et al. A novel prognostic model based on AFP, tumor burden score and Albumin-Bilirubin grade for patients with hepatocellular carcinoma undergoing radiofrequency ablation. Int J Hyperthermia. 2023;40(1):2256498. doi: 10.1080/02656736.2023.2256498. [DOI] [PubMed] [Google Scholar]
- 77.Wei B, Chen J-S, Yang S-L, et al. A scoring model combining serum alpha-fetoprotein and tumor size and number predicts prognosis in hepatitis B virus-related hepatocellular carcinoma patients after curative hepatectomy. Transl Cancer Res. 2019;8(4):1438–1448. doi: 10.21037/tcr.2019.07.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xia F, Ndhlovu E, Liu Z, et al. Alpha-fetoprotein+alkaline phosphatase (A-A) score can predict the prognosis of patients with ruptured hepatocellular carcinoma underwent hepatectomy. Dis Markers. 2022;2022:9934189. doi: 10.1155/2022/9934189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehta N, Kotwani P, Norman J, et al. AFP-L3 and DCP are superior to AFP in predicting waitlist dropout in HCC patients: Results of a prospective study. Liver Transpl. 2023;29(10):1041–1049. doi: 10.1097/LVT.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benson AB, D‘Angelica MI, Abbott DE, et al. Hepatobiliary cancers, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 81.Chan MY, She WH, Dai WC, et al. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy- an analysis of 1,182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52. doi: 10.21037/tgh.2019.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Personeni N, Bozzarelli S, Pressiani T, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57(1):101–107. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 83.Kim DY, Toan BN, Tan CK, et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin Mol Hepatol. 2023;29(2):277–292. doi: 10.3350/cmh.2022.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nouso K, Kobayashi Y, Nakamura S, et al. Prognostic importance of fucosylated alpha-fetoprotein in hepatocellular carcinoma patients with low alpha-fetoprotein. J Gastroenterol Hepatol. 2011;26(7):1195–1200. doi: 10.1111/j.1440-1746.2011.06720.x. [DOI] [PubMed] [Google Scholar]
- 85.Yamao T, Yamashita YI, Imai K, et al. Clinical significance of preoperative hepatocellular carcinoma with high lens culinaris agglutinin-reactive fraction of alpha-fetoprotein, But low alpha-fetoprotein. Anticancer Res. 2019;39(2):883–839. doi: 10.21873/anticanres.13189. [DOI] [PubMed] [Google Scholar]
- 86.Force M, Park G, Chalikonda D, et al. Alpha-fetoprotein (AFP) and AFP-L3 is most useful in detection of recurrence of hepatocellular carcinoma in patients after tumor ablation and with low AFP level. Viruses. 2022;14(4):775. doi: 10.3390/v14040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Zhang Y, Yang N, et al. Evaluation of the combined application of AFP, AFP-L3%, and DCP for hepatocellular carcinoma diagnosis: A meta-analysis. BioMed Res Int. 2020;2020:5087643. doi: 10.1155/2020/5087643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Omar MA, Omran MM, Farid K, et al. Biomarkers for hepatocellular carcinoma: From origin to clinical diagnosis. Biomedicines. 2023;11(7):1852. doi: 10.3390/biomedicines11071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li M, Zhu M, Li W, et al. Alpha-fetoprotein receptor as an early indicator of HBx-driven hepatocarcinogenesis and its applications in tracing cancer cell metastasis. Cancer Lett. 2013;330(2):170–180. doi: 10.1016/j.canlet.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 90.Guan MC, Zhang SY, Ding Q, et al. The performance of GALAD score for diagnosing hepatocellular carcinoma in patients with chronic liver diseases: A systematic review and meta-analysis. J Clin Med. 2023;12(3):949. doi: 10.3390/jcm12030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang JD, Addissie BD, Mara KC, et al. GALAD score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score. Cancer Epidemiol Biomarkers Prev. 2019;28(3):531–538. doi: 10.1158/1055-9965.EPI-18-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Norman JS, Li PJ, Kotwani P, et al. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J Hepatol. 2023;79(6):1469–1477. doi: 10.1016/j.jhep.2023.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tayob N, Kanwal F, Alsarraj A, et al. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): A phase 3 biomarker study in the United States. Clin Gastroenterol Hepatol. 2023;21(2):415–423.e4. doi: 10.1016/j.cgh.2022.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mizejewski DGJ. An alpha-fetoprotein derived peptide suppresses growth in breast cancer and other malignancies: A review and prospectus. Med Res Arch. 2023;11(7.2) doi: 10.18103/mra.v11i7.2.4147. [DOI] [Google Scholar]
- 95.Sherman IA, Hill W, Griffin P, et al. Efficacy and safety study of a novel alpha-fetoprotein (AFP)-maytansine conjugate in an ovarian xenograft model. J Clin Oncol. 2023;41(16_suppl):e14570. doi: 10.1200/JCO.2023.41.16_suppl.e14570. [DOI] [Google Scholar]
- 96.Lan YH, Li YG, Liang ZW, et al. A DNA vaccine against chimeric AFP enhanced by HSP70 suppresses growth of hepatocellular carcinoma. Cancer Immunol Immunother. 2007;56(7):1009–1016. doi: 10.1007/s00262-006-0254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pak VN. Selective targeting of myeloid-derived suppressor cells in cancer patients through AFP-binding receptors. Future Sci OA. 2019;5(1):Fso321. doi: 10.4155/fsoa-2018-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu X, Deng S, Xu J, et al. Combination of AFP vaccine and immune checkpoint inhibitors slows hepatocellular carcinoma progression in preclinical models. J Clin Invest. 2023;133(11):e163291. doi: 10.1172/JCI163291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang YS, Ma XL, Qi TG, et al. Downregulation of alpha-fetoprotein siRNA inhibits proliferation of SMMC-7721 cells. World J Gastroenterol. 2005;11(38):6053–6055. doi: 10.3748/wjg.v11.i38.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pho-Iam T, Punnakitikashem P, Somboonyosdech C, et al. PLGA nanoparticles containing α-fetoprotein siRNA induce apoptosis and enhance the cytotoxic effects of doxorubicin in human liver cancer cell line. Biochem Biophys Res Commun. 2021;553:191–197. doi: 10.1016/j.bbrc.2021.03.086. [DOI] [PubMed] [Google Scholar]


