Abstract
PURPOSE
The phase III randomized open-label LEAP-015 study (ClinicalTrials.gov identifier: NCT04662710) evaluated first-line lenvatinib plus pembrolizumab and chemotherapy versus chemotherapy for advanced metastatic gastroesophageal adenocarcinoma.
METHODS
Eligible participants 18 years and older with untreated human epidermal growth factor receptor 2–negative locally advanced unresectable or metastatic gastroesophageal adenocarcinoma were randomly assigned 1:1 to induction with oral lenvatinib 8 mg once daily plus pembrolizumab 400 mg intravenously once every 6 weeks (×2) and investigators’ choice of capecitabine and oxaliplatin once every 3 weeks (×4) or fluorouracil, leucovorin, and oxaliplatin once every 2 weeks (×6) and consolidation with lenvatinib plus pembrolizumab, or chemotherapy. Dual primary end points were progression-free survival (PFS) and overall survival (OS) in participants with PD-L1 combined positive score (CPS) ≥1 and all participants. Secondary end points included objective response rate (ORR) and duration of response.
RESULTS
Of 880 participants randomly assigned, 443 received lenvatinib plus pembrolizumab and 437 received chemotherapy. The median follow-ups were 32.2 months (range, 19.0-41.7) in participants with PD-L1 CPS ≥1 and 31.8 months (19.0-41.7) in all participants. At interim analysis, PFS was statistically significant with lenvatinib plus pembrolizumab versus chemotherapy in participants with PD-L1 CPS ≥1 (median, 7.3 v 6.9 months; hazard ratio [HR], 0.75 [95% CI, 0.62 to 0.9]; P = .0012) and all participants (median, 7.2 v 7.0 months; HR, 0.78 [95% CI, 0.66 to 0.92]; P = .0019). The ORR was 59.5% versus 45.4% in participants with PD-L1 CPS ≥1 and 58.0% versus 43.9% in all participants, P < .0001 for both. At final analysis, OS was not statistically significant in participants with PD-L1 CPS ≥1 (median, 12.6 v 12.9 months; HR, 0.84 [95% CI, 0.71 to 1.00]; P = .0244; P value boundary = .0204). Grade ≥3 drug-related adverse event rates were 65% versus 49%.
CONCLUSION
Lenvatinib plus pembrolizumab and chemotherapy versus chemotherapy provided a statistically significant improvement in PFS in advanced unresectable or metastatic gastroesophageal carcinoma at interim analysis although the clinical significance of this difference seems to be limited. No significant improvement occurred in OS in participants with PD-L1 CPS ≥1.
INTRODUCTION
First-line treatment for advanced human epidermal growth factor receptor 2 (HER2)–negative gastric/gastroesophageal junction (G/GEJ) adenocarcinoma has traditionally relied on platinum- and fluoropyrimidine-based chemotherapy. Recently, the combination of immune checkpoint inhibitors and chemotherapy has demonstrated an enhanced clinical benefit, with enriched efficacy noted with increasing levels of PD-L1 expression in selected populations with PD-L1 combined positive score (CPS) 1 or more.1-3 The addition of vascular endothelial growth factor (VEGF) receptor–targeted agents, such as ramucirumab, improved efficacy in the second-line treatment.4 However, the prognosis remains poor, with the median overall survival (OS) ranging from 12 to 14 months in global studies of untreated advanced gastric cancer.1-3
CONTEXT
Key Objective
To evaluate if the addition of lenvatinib and pembrolizumab to standard chemotherapy improves clinical outcomes in participants with previously untreated, human epidermal growth factor receptor 2 (HER2)–negative metastatic gastroesophageal adenocarcinoma.
Knowledge Generated
The LEAP-015 phase III study showed that the triplet regimen of lenvatinib, pembrolizumab, and chemotherapy resulted in a statistically significant improvement in progression-free survival compared with chemotherapy alone. However, the overall survival benefit did not meet the predefined threshold for statistical significance. The combination was associated with higher rates of treatment-related adverse events compared with the control arm.
Relevance (A.H. Ko)
While the mechanistic rationale for evaluating this combination was sound, adding lenvatinib and pembrolizumab to chemotherapy should not be routinely used in patients with HER2-negative metastatic gastroesophageal cancer.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
Multiple studies have shown that targeting VEGF-mediated angiogenesis is associated with antitumor activity.5 Lenvatinib, an oral multikinase inhibitor, demonstrated preclinical synergy with PD-1 inhibitors in an in vivo model.6 In clinical studies, lenvatinib plus pembrolizumab has shown clinical activity across multiple malignancies, including endometrial cancer and renal cell carcinoma,7,8 with preliminary efficacy observed for G/GEJ adenocarcinoma.9,10 In the phase II EPOC1706 study of first- and second-line treatment in advanced gastric cancer, lenvatinib 20 mg once daily plus pembrolizumab provided an objective response rate (ORR) of 69%, with a median progression-free survival (PFS) of 7.1 months.9 This ORR suggested potential synergy of this combination for advanced gastric cancer as the expected response rate is approximately 15% for pembrolizumab monotherapy and <5% for lenvatinib.9 In addition, chemotherapy plus lenvatinib 8 mg once daily plus pembrolizumab had an acceptable safety profile with platinum-based chemotherapy in non–small cell lung cancer.11
The randomized, open-label international phase III LEAP-015 study evaluated the efficacy and safety of first-line lenvatinib plus pembrolizumab and chemotherapy versus chemotherapy in advanced, HER2-negative, untreated G/GEJ adenocarcinoma. We report the results of the interim and final analyses of LEAP-015.
METHODS
Study Design and Participants
Eligible participants were 18 years and older with a histologically or cytologically confirmed HER2-negative unresectable or metastatic G/GEJ adenocarcinoma. Participants were required to have measurable disease per RECIST v1.1 per the investigator, have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-1, provide a tumor tissue sample for PD-L1 analysis, and have no previous systemic therapy for unresectable or metastatic disease and were not expected to require resection during treatment. Participants with a gastrointestinal condition that could affect study drug absorption; with previous treatment with PD-1/PD-L1 inhibitors, VEGF inhibitors, or lenvatinib; and who were eligible for radiotherapy or neoadjuvant therapy were excluded. The Protocol (online only) and all amendments were approved by the relevant institutional review board or the independent ethics committee at each study center. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided informed consent.
Trial Design and Treatment
In part 1 of LEAP-015 (Lead-in Phase), an initial cohort received induction with lenvatinib 8 mg orally once daily plus pembrolizumab 400 mg intravenously once every 6 weeks (×2) and investigator’s choice chemotherapy of capecitabine and oxaliplatin (CAPOX) once every 3 weeks (×4), or modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) once every 2 weeks (×6). This was followed by consolidation with pembrolizumab 400 mg once every 6 weeks up to 16 doses plus lenvatinib, before advancing to part 2. Lenvatinib 8 mg once daily was escalated to 20 mg once daily if the initial 8 mg dose was tolerated with no more than a grade 1 lenvatinib-related adverse event or grade 2 hypothyroidism during induction. In part 1, dose-limiting toxicities (DLTs), defined as selected prespecified grade ≥3 adverse events or any-grade thromboembolic events, were evaluated for 21 days. If two or less DLTs were observed with either CAPOX or mFOLFOX6, enrollment in part 2 was initiated. In part 2 (randomized phase III phase), eligible participants were randomly assigned 1:1 to lenvatinib plus pembrolizumab with chemotherapy as in part 1 for four to six cycles or chemotherapy alone with CAPOX or mFOLFOX6 per investigator's choice in the control arm until progression or per local standards. As the study was planned in 2020, chemotherapy alone was selected as control as nivolumab had not been approved in many regions, and KEYNOTE-859 results were not yet available.
Random assignment was stratified by region (East Asia, North America, and Western Europe v rest of world), ECOG PS (0 v 1), and chemotherapy backbone (CAPOX v mFOLFOX6). Pembrolizumab was administered for up to 2 years, and participants who completed pembrolizumab without disease progression could continue lenvatinib monotherapy per investigator until disease progression, unacceptable toxicity, or withdrawal of consent. Treatment was continued until disease progression, unacceptable toxicity, pregnancy, intercurrent illness, or withdrawal of consent. Full protocol details are provided in the Protocol.
End Points
Dual primary end points were PFS (time from random assignment to disease progression or death from any cause, whichever occurred first) and OS (time from random assignment to death from any cause). Secondary end points included objective response (confirmed complete response [CR] or partial response [PR]), duration of response (DOR, time from first CR or PR to subsequent disease progression, or death from any cause, whichever occurred first), and safety and tolerability. Change from baseline to Week 18 in health-related quality of life as assessed by the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30), EORTC Quality of Life Questionnaire-Stomach cancer module (QLQ-STO22) pain (Gastric), and the EuroQol 5-dimension 5-level (EQ-5D-5L) questionnaires was an exploratory end point.
Assessments
HER2-negative status was assessed locally by immunohistochemistry in situ hybridization or fluorescence in situ hybridization. HER2 status was assessed centrally if local standards were not sufficient as needed by country or site. Tumor response was assessed per RECIST v1.1 by blinded independent central review (BICR) with initial imaging performed at week 6 after random assignment and once every 6 weeks thereafter, until disease progression per investigator verified by BICR. During follow-up, survival was assessed once every 12 weeks. The EORTC QLQ-C30, EORTC QLQ-STO22, and EQ-5D-5L questionnaires were administered once every 6 weeks on day 1 of each pembrolizumab cycle until the completion of 2 years of treatment or discontinuation of both lenvatinib and pembrolizumab, as well as at treatment discontinuation and during safety follow-up. Site staff recorded the reason for any missed completions.
Statistical Analysis
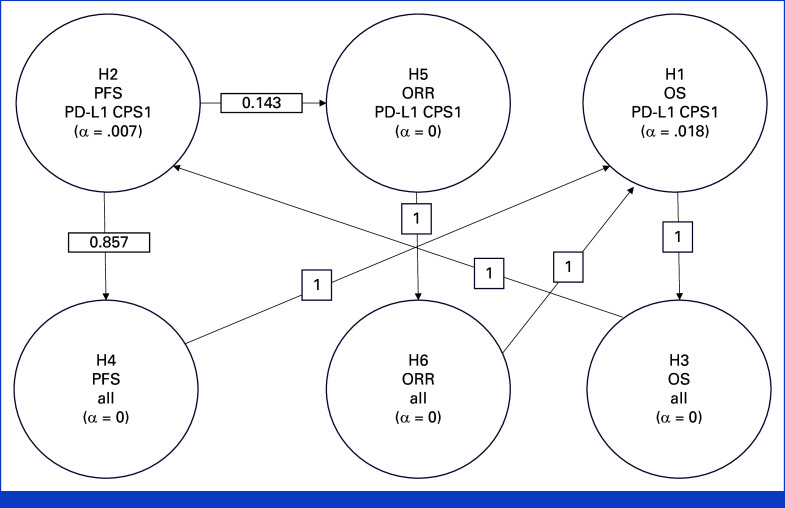
The Kaplan-Meier method was used to estimate OS, PFS, and DOR. Between-group differences in OS and PFS were assessed using a stratified log-rank test. Differences in ORR were assessed using the stratified Miettinen and Nurminen method. A stratified Cox proportional hazards model with Efron's method for tie handling was used to estimate the hazard ratios (HRs) and associated 95% CIs. The final analysis of OS was planned to occur after approximately 537 deaths in participants with PD-L1 CPS ≥1 and approximately 18 months after the last participant was randomly assigned. The overall type 1 error was strongly controlled at a one-sided α of .025 using the graphical method of Maurer and Bretz with 0.018 initially allocated to OS and 0.007 initially allocated to PFS in participants with PD-L1 CPS ≥1 (Appendix Fig A1, online only). Full details of the protocol and statistical analysis plan are provided in the Protocol (see also Appendix 1).
Trial Oversight
The study was designed by academic investigators and employees of the sponsor (Merck Sharp & Dohme, LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ). All the authors had access to the data, were involved in reviewing and editing the manuscript, and approved the submitted draft and vouch for the accuracy of the data reported. Assistance in the preparation of the manuscript was provided by a medical writer employed by the sponsor.
RESULTS
Participants and Treatment
In part 1, 15 participants enrolled between December 30, 2020, and January 27, 2021, to receive induction therapy. A total of two DLTs occurred (one DLT of grade 3 asthenia with CAPOX and one DLT of grade 4 neutropenia with mFOLFOX6). This met the criteria for enrollment in part 2. In part 2, between May 11, 2021, and March 31, 2023, 880 participants from 157 sites in 24 countries were randomly assigned to lenvatinib plus pembrolizumab and chemotherapy (443 participants [lenvatinib group]) or chemotherapy alone (437 participants [chemotherapy group]; Fig 1). Baseline participant characteristics and demographics were generally well balanced between groups. Participants had a median age of 61.0 years (range, 21-84), 689 (78%) had PD-L1 CPS ≥1, 172 (20%) had PD-L1 CPS <1, and 662 (75%) had primary gastric adenocarcinoma (Table 1). At final analysis (data cutoff date October 29, 2024), the median time from random assignment was 32.2 months (range, 19.0-41.7) in participants with PD-L1 CPS ≥1 and 31.8 months (range, 19.0-41.7) in all participants. A total of 33 (4%) participants completed study treatment (25 [6%] in the lenvatinib group and eight [2%] in the chemotherapy group), and 41 (5%) remain on treatment (35 [8%] in the lenvatinib group and six [1%] in the chemotherapy group). A total of 796 (92%) participants discontinued treatment, 381 (86%) and 415 (97%) in the lenvatinib and chemotherapy groups, respectively. This was largely due to progressive disease ([n = 543], 254 [58%] in the lenvatinib group and 289 [67%] in the chemotherapy group) and adverse events ([n = 97], 61 [14%] in the lenvatinib group and 36 [8%] in the chemotherapy group).
FIG 1.
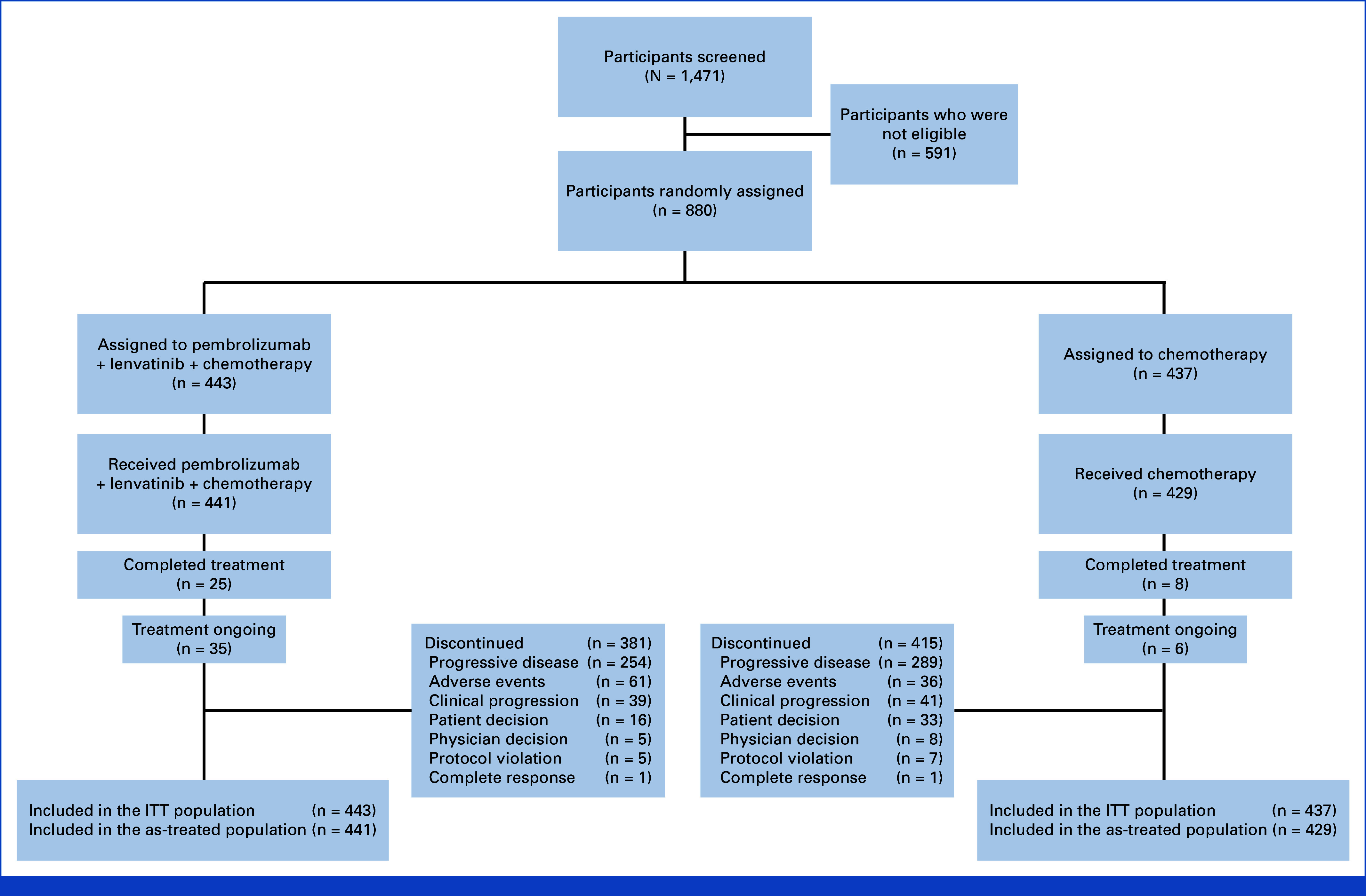
CONSORT diagram. ITT, intention-to-treat.
TABLE 1.
Demographics and Participant Characteristics at Baseline in the Intention-to-Treat Population
| Characteristic | Lenvatinib + Pembrolizumab + Chemotherapy (n = 443) | Chemotherapy (n = 437) |
|---|---|---|
| Age, years, median (range) | 62 (21-84) | 61 (24-84) |
| ≥65 years, No. (%) | 178 (40) | 157 (36) |
| Male, No. (%) | 292 (66) | 306 (70) |
| Race, No. (%) | ||
| American Indian/Alaska Native | 11 (2) | 15 (3) |
| Asian | 164 (37) | 161 (37) |
| African American/Black | 1 (<1) | 1 (<1) |
| White | 235 (53) | 223 (51) |
| Multiple/missing | 32 (7) | 37 (8) |
| Region, No. (%) | ||
| East Asia | 161 (36) | 161 (37) |
| North America/Western Europe/Israel/Australia | 125 (28) | 122 (28) |
| Rest of world | 157 (35) | 155 (35) |
| ECOG performance status, No. (%) | ||
| 0 | 204 (46) | 204 (47) |
| 1 | 239 (54) | 233 (53) |
| PD-L1 status, No. (%) | ||
| PD-L1 CPS ≥1 | 334 (75) | 355 (81) |
| PD-L1 CPS <1 | 101 (23) | 71 (16) |
| Unknown | 8 (2) | 11 (3) |
| MSI-H status, No. (%) | ||
| Non–MSI-H | 346 (78) | 316 (72) |
| MSI-H | 14 (3) | 8 (2) |
| Missing | 83 (19) | 113 (26) |
| Disease status, No. (%) | ||
| Locally advanced | 8 (2) | 6 (1) |
| Metastatic | 435 (98) | 431 (99) |
| Primary location, No. (%) | ||
| Gastric adenocarcinoma | 340 (77) | 322 (74) |
| Gastroesophageal junction adenocarcinoma | 91 (21) | 97 (22) |
| Esophagus adenocarcinoma | 12 (3) | 18 (4) |
| Histology subtype, No. (%) | ||
| Diffuse | 134 (30) | 113 (26) |
| Indeterminate | 157 (35) | 166 (38) |
| Intestinal | 152 (34) | 158 (36) |
| Tumor burden, No. (%) | ||
| ≥Median | 210 (47) | 220 (50) |
| <Median | 219 (49) | 204 (47) |
| Previous gastrectomy/esophagectomy, No. (%) | ||
| No | 351 (79) | 347 (79) |
| Yes | 92 (21) | 90 (21) |
| Chemotherapy, No. (%) | ||
| CAPOX | 232 (52) | 225 (51) |
| mFOLFOX6 | 209 (47) | 204 (47) |
| Missing | 2 (<1) | 8 (2) |
Abbreviations: CAPOX, capecitabine and oxaliplatin; CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; mFOLFOX6, fluorouracil, leucovorin, and oxaliplatin; MSI-H, microsatellite instability-high.
PFS
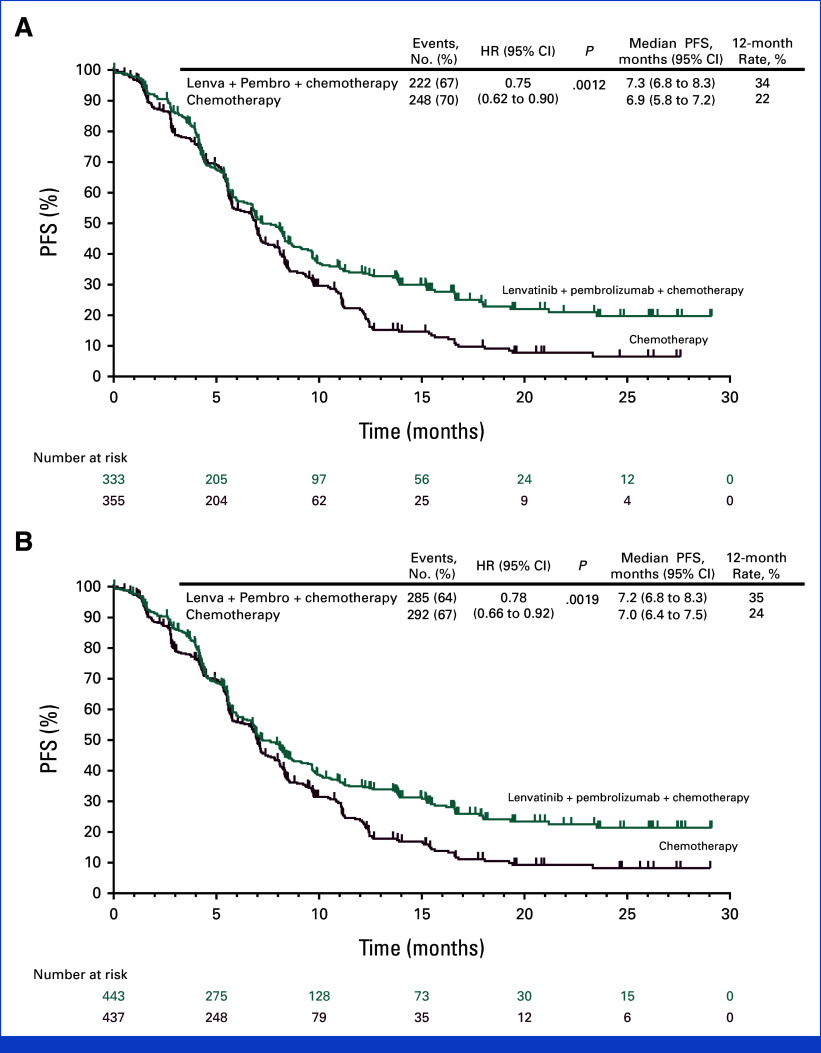
At interim analysis (data cutoff date November 16, 2023), with the median follow-up of 20.8 months (range, 7.6-30.2) in participants with PD-L1 CPS ≥1 and 20.3 months (range, 7.6-30.2) in all participants, PFS per RECIST v.1 by BICR met the prespecified criteria for statistical significance with lenvatinib plus pembrolizumab versus chemotherapy in participants with PD-L1 CPS ≥1 (median, 7.3 v 6.9 months; HR, 0.75 [95% CI, 0.62 to 0.9]; P = .0012), with a 24-month PFS rate of 20% versus 7%, and in all participants (median, 7.2 v 7.0 months; HR, 0.78 [95% CI, 0.66 to 0.92]; P = .0019), with a 24-month PFS rate of 21% versus 8%, respectively (Fig 2). PFS was generally consistent across the subgroups evaluated, including in participants with PD-L1 CPS ≥1 and PD-L1 CPS <1 (Appendix Fig A2).
FIG 2.
PFS in participants with advanced metastatic HER2-negative gastric and gastroesophageal junction adenocarcinoma. Kaplan-Meier estimate of PFS at interim analysis in (A) participants with PD-L1 CPS ≥1 (H2; P value boundary for significance = .007000) and (B) all participants (H4; P value boundary for significance = .005999). PFS was assessed per the RECIST version 1.1 by blinded, independent central review. Tick marks represent data censored at the time of last imaging assessment. CPS, combined positive score; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; Lenva, lenvatinib; Pembro, pembrolizumab; PFS, progression-free survival.
OS
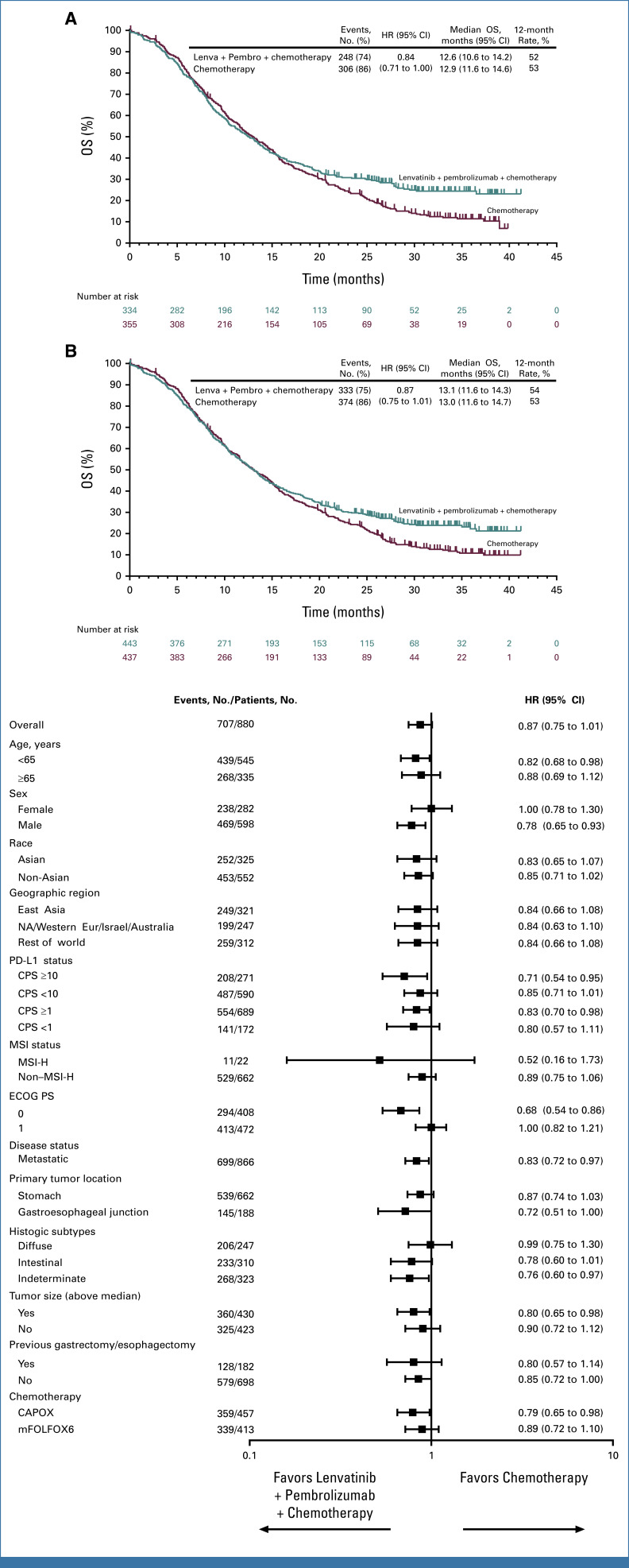
At final analysis (data cutoff date October 29, 2024), OS in participants with PD-L1 CPS ≥1 was not statistically significant with lenvatinib plus pembrolizumab versus chemotherapy (median, 12.6 v 12.9 months; HR, 0.84 [95% CI, 0.71 to 1.00]; P = .0244), with a 24-month OS rate of 31% versus 23%. The P value boundary for significance was .0204 (Figs 3A and 3B). OS in all participants was not tested for significance per the multiplicity strategy (median, 13.1 v 13.0 months; HR, 0.87 [95% CI, 0.75 to 1.01]). OS was generally consistent across subgroups evaluated, including in participants with PD-L1 CPS ≥1 and PD-L1 CPS <1 (Fig 3C). A total of 202 (46%) participants in the lenvatinib group versus 273 (63%) in the chemotherapy group received subsequent anticancer therapy. This included 196 (44%) versus 255 (58%) participants who received subsequent chemotherapy and 20 (5%) versus 83 (19%) participants who received subsequent immunotherapy in the lenvatinib versus chemotherapy groups, respectively (Appendix Table A1).
FIG 3.
OS in participants with advanced metastatic HER2-negative gastric and gastroesophageal junction adenocarcinoma. Kaplan-Meier estimate of OS at final analysis in (A) participants with PD-L1 CPS ≥1 (H1) and (B) all participants (H3). Tick marks represent data censored at the time of last imaging assessment. (C) Forest plot of OS at final analysis in prespecified subgroups. The unstratified Cox regression model with Efron's method of tie handling with treatment as a covariate was used to assess the magnitude of the treatment difference between arms. CAPOX, capecitabine and oxaliplatin; CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; Lenva, lenvatinib; mFOLFOX6, fluorouracil, leucovorin, oxaliplatin; MSI, microsatellite instability; MSI-H, microsatellite instability-high; NA, North America; OS, overall survival; Pembro, pembrolizumab.
Antitumor Response
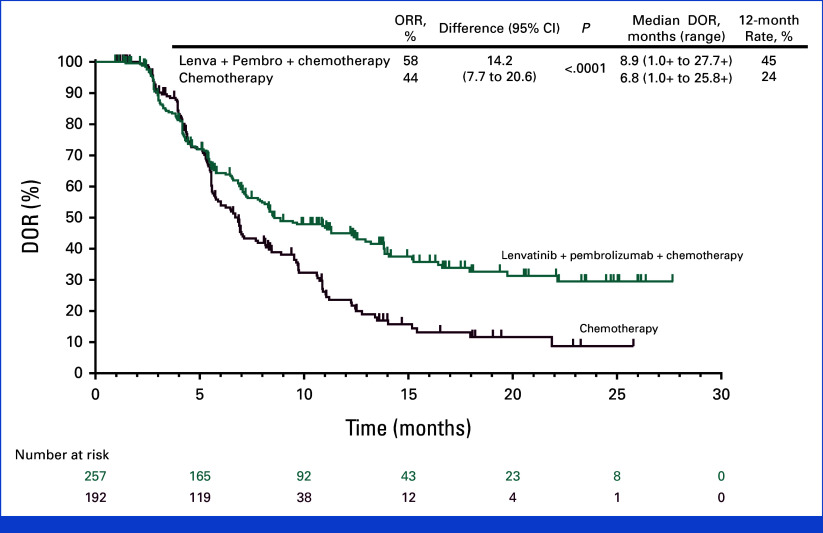
At interim analysis, the ORR was 59.5% (198 of 333 [95% CI, 54.0 to 64.8]) in the lenvatinib group versus 45.4% (161 of 355 [95% CI, 40.1 to 50.7]) in the chemotherapy group in participants with PD-L1 CPS ≥1 and 58.0% (257 of 443 [95% CI, 53.3 to 62.7]) versus 43.9% (192 of 437 [95% CI, 39.2 to 48.7]), respectively, in all participants (P < .0001 for both). The median DOR was 8.5 months (range, 1.0+ to 27.7+) in the lenvatinib group versus 6.5 months (range, 1.0+ to 25.8+) in the chemotherapy group in participants with PD-L1 CPS ≥1 and 8.9 months (range, 1.0+ to 27.7+) and 6.8 months (range, 1.0+ to 25.8+), respectively, in all participants (Table 2; Appendix Fig A3).
TABLE 2.
Antitumor Activity in the Intention-to-Treat Population at Interim Analysis
| Response | Lenvatinib + Pembrolizumab + Chemotherapy (n = 443) | Chemotherapy (n = 437) |
|---|---|---|
| Objective response rate, No. (%) 95% CIa |
257 (58.0) 53.3 to 62.7 |
192 (43.9) 39.2 to 48.7 |
| Difference, % (95% CI); P | 14.2 (7.7 to 20.6);b P < .0001c | |
| Best overall response, No. (%) | ||
| Complete response | 38 (8.6) | 22 (5.0) |
| PR | 219 (49.4) | 170 (38.9) |
| Stable disease | 140 (31.6) | 168 (38.4) |
| Progressive disease | 18 (4.1) | 39 (8.9) |
| Not evaluable/no assessment | 28 (6.3) | 38 (8.7) |
| Response duration, months, median (range) | 8.9 (1.0+ to 27.7+) | 6.8 (1.0+ to 25.8+) |
| Response duration ≥24 months,d % | 29.7 | 8.7 |
NOTE. + indicates no progressive disease by the time of last assessment.
Abbreviations: BICR, blinded independent central review; PR, partial response.
Participants with confirmed complete response or PR by BICR per RECIST v1.1. Percentages were calculated using all randomly assigned participants.
Based on the Miettinen and Nurminen method stratified by region, performance status, and chemotherapy.
One-sided P value for testing.
From the Kaplan-Meier method for censored data.
Safety
A total of 870 participants received at least one dose of study treatment (441 in the lenvatinib group and 429 in the chemotherapy group). The median (range) duration of treatment was 6.5 months (0-41) versus 5.6 (0-41), respectively. In the lenvatinib group, the median (range) duration of treatment was 6.0 months (0-41) for lenvatinib, 5.6 (0-30) for pembrolizumab, 2.6 (0-7) for CAPOX, and 2.4 (0-5) for mFOLFOX6. In the chemotherapy group, the median duration of treatment was 5.1 months (0-41) for CAPOX and 6.1 (0-37) for mFOLFOX6. Of note, in the lenvatinib group, chemotherapy was restricted to four cycles for CAPOX and six cycles for mFOLFOX6 in the induction phase only, whereas in the control group, chemotherapy continued until progression or per local standards. Overall, participants received lenvatinib at a median dose intensity of 8 mg/d (range, 2-19), with 359 (81%) participants receiving lenvatinib at a median dose intensity of 7.9 mg/d (range, 2-10) during induction and 343 (78%) receiving lenvatinib at a median dose intensity of 11.5 mg/d (range, 2-20) during consolidation. In total, 200 of 441 (46%) treated participants in the lenvatinib group had lenvatinib dose escalated to 20 mg once daily during consolidation.
Adverse events of any cause occurred in 439 (99%) participants in the lenvatinib group and 414 (97%) in the chemotherapy group. Grade ≥3 events occurred in 350 (79%) versus 276 (64%) participants in the lenvatinib versus chemotherapy groups, with neutrophil count decreased (25% v 24%), hypertension (12% v 1%), anemia (8% v 10%), and diarrhea (5% v 3%) being most common. Serious adverse events occurred in 226 (51%) versus 137 (32%) participants in the lenvatinib versus chemotherapy groups, respectively, with discontinuation of any drug because of adverse events occurring in 146 (33%) versus 116 (27%) participants, respectively. In the lenvatinib group, 106 participants (24%) discontinued lenvatinib, 79 (18%) discontinued pembrolizumab, and 54 (12%) discontinued both drugs. Drug-related adverse events occurred in 430 (98%) versus 394 (92%) participants in the lenvatinib versus chemotherapy groups, respectively. Grade ≥3 drug-related events occurred in 288 (65%) versus 208 (48%) participants, respectively. Discontinuations because of a drug-related adverse event occurred in 119 (27%) versus 99 (23%) participants. Grade 5 drug-related adverse events occurred in 24 (5%) versus two (<1%) participants in the lenvatinib versus chemotherapy groups, respectively (Table 3). Immune-mediated adverse events of special interest occurred in 202 (46%) participants in the lenvatinib group and 51 (12%) participants in the chemotherapy group. Grade ≥3 events occurred in 44 (10%) versus six (1%) participants, respectively (Appendix Table A2).
TABLE 3.
Treatment-Related Adverse Events Occurring in ≥5% of All the Treated Participants
| Adverse Event | Lenvatinib + Pembrolizumab + Chemotherapy (n = 441), No. (%) | Chemotherapy (n = 429), No. (%) | ||
|---|---|---|---|---|
| Any | Grade ≥3 | Any | Grade ≥3 | |
| Treatment-related eventsa | 430 (97) | 288 (65) | 394 (92) | 208 (48) |
| Neutrophil count decreased | 203 (46) | 105 (24) | 194 (45) | 97 (23) |
| Nausea | 171 (39) | 10 (2) | 173 (40) | 5 (1) |
| Diarrhea | 169 (38) | 20 (5) | 109 (25) | 9 (2) |
| Hypertension | 140 (32) | 49 (11) | 0 | 0 |
| Decreased appetite | 130 (29) | 11 (2) | 79 (18) | 4 (1) |
| Hypothyroidism | 121 (27) | 2 (<1) | 0 | 0 |
| Platelet count decreased | 117 (27) | 21 (5) | 172 (40) | 35 (8) |
| Fatigue | 103 (23) | 14 (3) | 61 (14) | 6 (1) |
| WBC count decreased | 102 (23) | 15 (3) | 89 (21) | 14 (3) |
| Anemia | 101 (23) | 17 (4) | 109 (25) | 25 (6) |
| PPES | 95 (22) | 14 (3) | 59 (14) | 7 (2) |
| Proteinuria | 92 (21) | 9 (2) | 1 (<1) | 0 |
| AST increased | 81 (18) | 11 (2) | 75 (17) | 5 (1) |
| Vomiting | 79 (18) | 10 (2) | 92 (21) | 8 (2) |
| ALT increased | 70 (16) | 16 (4) | 57 (13) | 5 (1) |
| Weight decreased | 70 (16) | 9 (2) | 43 (10) | 6 (1) |
| Peripheral neuropathy | 69 (16) | 1 (<1) | 100 (23) | 13 (3) |
| Stomatitis | 64 (15) | 7 (2) | 40 (9) | 2 (<1) |
| Peripheral sensory neuropathy | 54 (12) | 2 (<1) | 81 (19) | 6 (1) |
| Asthenia | 51 (12) | 6 (1) | 54 (13) | 1 (<1) |
| Mucosal inflammation | 51 (12) | 9 (2) | 26 (6) | 2 (<1) |
| Lipase increased | 46 (10) | 12 (3) | 27 (6) | 8 (2) |
| Rash | 44 (10) | 1 (<1) | 10 (2) | 0 |
| Pruritus | 42 (10) | 1 (<1) | 6 (1) | 0 |
| Constipation | 41 (9) | 0 | 40 (9) | 0 |
| Amylase increased | 40 (9) | 6 (1) | 19 (4) | 1 (<1) |
| Blood thyroid-stimulating hormone increased | 37 (8) | 0 | 2 (<1) | 0 |
| Blood bilirubin increased | 35 (8) | 6 (1) | 32 (7) | 6 (1) |
| Abdominal pain | 32 (7) | 3 (1) | 19 (4) | 3 (1) |
| Hyperthyroidism | 30 (7) | 1 (<1) | 4 (1) | 0 |
| Hypokalemia | 27 (6) | 6 (1) | 16 (4) | 3 (1) |
| Paraesthesia | 26 (6) | 1 (<1) | 36 (8) | 3 (1) |
| Dysgeusia | 25 (6) | 0 | 38 (9) | 0 |
| Blood alkaline phosphatase increased | 24 (5) | 4 (1) | 28 (7) | 3 (1) |
| Dry mouth | 24 (5) | 0 | 5 (1) | 0 |
| Arthralgia | 23 (5) | 3 (1) | 4 (1) | 0 |
| Dysphonia | 22 (5) | 0 | 3 (1) | 0 |
Abbreviation: PPES, palmar-plantar erythrodysesthesia syndrome.
Treatment-related events with incidence ≥5% in any group. Treatment-related grade 5 events included autoimmune hemolytic anemia, cardiac arrest, myocarditis, gastric hemorrhage (n = 2), gastric perforation (n = 3), gastric ulcer perforation, gastrointestinal hemorrhage, immune-mediated enterocolitis, intestinal ischemia, acute pancreatitis, upper gastrointestinal hemorrhage (n = 3), death, aseptic meningitis, sepsis, urosepsis, pulmonary embolism, intracranial hemorrhage, and malignant neoplasm progression and encephalitis in the lenvatinib plus pembrolizumab and chemotherapy group in one participant each unless otherwise indicated and gastrointestinal hemorrhage and hepatic failure in one participant each in the chemotherapy group.
Health-Related Quality of Life
A total of 850 participants were enrolled in the PRO population (430 lenvatinib plus pembrolizumab; 420 chemotherapy). At baseline, the observed completion rates were 97% for the QLQ-C30 and QLQ-STO22 questionnaires for both treatment groups and 94% and 93% for the EQ-5D-5L questionnaire in the lenvatinib and chemotherapy groups, respectively. At baseline, compliance rates were 100% for all questionnaires in both groups. At week 18, the observed completion rates were 70% and 57% for QLQ-C30 and QLQ-STO22 and 68% and 56% for the EQ-5D-5L questionnaire with lenvatinib plus pembrolizumab and chemotherapy, respectively. Compliance rates were 97% for all questionnaires with lenvatinib and pembrolizumab and ranged from 99% to 100% for chemotherapy. No meaningful differences in least square mean change from baseline to Week 18 were observed between groups for the prespecified QLQ-C30 Global Health Status/Quality of Life, QLQ-STO22, or EQ-5D-5L visual analog scale questionnaires (Appendix Table A3).
DISCUSSION
In this phase III study, lenvatinib plus pembrolizumab and chemotherapy demonstrated a statistically significant improvement in PFS and ORR versus chemotherapy as first-line treatment for advanced, unresectable, or metastatic HER2-negative G/GEJ adenocarcinoma. The study did not meet the prespecified threshold for OS significance in participants with PD-L1 CPS ≥1, and OS in the overall population was not tested per the multiplicity strategy. The safety profile in both arms was consistent with the known adverse events of the therapeutic agents although higher rates of treatment-related adverse events were observed with the combination. Despite the limitations inherent in analysis of self-reported patient outcomes, there were no meaningful differences in quality-of-life measures between arms, potentially reflecting both treatment-related toxicity and tumor-related symptom improvement from disease control.
In LEAP-015, chemotherapy induction was designed to reduce potential for early disease progression as observed in previous global studies of first-line treatment in advanced G/GEJ cancer.12,13 The 12-week duration of induction chemotherapy in the LEAP-015 study was similar to that in the JAVELIN-100 study of induction chemotherapy and maintenance avelumab in urothelial carcinoma and selected for maximizing initial response with chemotherapy.14 Of note, the control arm of chemotherapy alone was selected as the standard of care at study initiation preceding approval of nivolumab and pembrolizumab in many countries and publication of CheckMate-649 and KEYNOTE-859 results, respectively.2 The study design was based on preliminary results from the EPOC1706 phase II study, which demonstrated the activity of lenvatinib plus pembrolizumab in G/GEJ adenocarcinoma in a Japanese population.9 Moreover, data from the LEAP-015 safety lead-in phase showed a low incidence of DLT, no drug-related deaths, and a preliminary ORR of 73% in 11 of 15 participants who received at least one dose of study therapy, suggesting the feasibility of this combination.15
In part 2 of LEAP-015, lenvatinib plus pembrolizumab and chemotherapy versus chemotherapy provided a significant improvement in PFS (median, 7.2 v 7.0 months) in all participants and in participants with PD-L1 CPS ≥1 (median, 7.3 v 6.9 months) although the magnitude of this difference is minimal, suggesting a limited clinical value. This benefit in PFS is supported by the extended tail observed in the PFS Kaplan-Meier curves. However, the lack of early separation until approximately 4 months suggests limited early benefit of lenvatinib plus pembrolizumab. Despite these results, the OS benefit in LEAP-015 in participants with PD-L1 CPS ≥1 (HR, 0.84 [95% CI, 0.71 to 1.00]) was not statistically significant. A similar lack of OS benefit with VEGF inhibition plus first-line fluoropyrimidines and platinum-based chemotherapy was previously reported in the RAINFALL16 and AVAGAST17 phase III, randomized studies, despite the positive OS benefit reported in the second line in the RAINBOW study.4 These outcomes suggest a potential lack of synergy with VEGF inhibition and platinum-based chemotherapy in this indication.
By contrast, a statistically significant OS benefit was observed in KEYNOTE-859 in all participants (HR, 0.78) and in participants with PD-L1 CPS ≥1 (HR, 0.74) and PD-L1 CPS ≥10 (HR, 0.65). Potential factors that may account for this difference in OS outcomes between the LEAP-015 and KEYNOTE-859 studies include the potential impact of chemotherapy discontinuation after 3 months in the experimental versus control group of LEAP-015 as studies have suggested that immunotherapy alone does not prolong OS compared with chemotherapy maintenance with or without immunotherapy.14,18 Another factor may be the higher rate of drug-related grade ≥3 events (65% v 49%) and serious events (51% v 32%) in the lenvatinib versus chemotherapy groups, respectively. In addition, treatment discontinuation because of adverse events associated with lenvatinib dose escalation might have influenced long-term outcomes, possibly reducing treatment exposure. A lower lenvatinib dose escalation may offer a better balance between efficacy and tolerability.
The performance of the control group in LEAP-015 exceeded expectations, with median OS and PFS comparable with that of pembrolizumab plus chemotherapy in KEYNOTE-859. The lower rate of subsequent anticancer therapy in the experimental versus control group in LEAP-015 (46% v 63%), with 44% versus 58% receiving subsequent chemotherapy and 5% versus 19% receiving subsequent immunotherapy, might have influenced survival outcomes in the LEAP-015 control arm. By contrast, subsequent anticancer therapy was more balanced between the pembrolizumab and placebo groups (45% v 47%) in KEYNOTE-859. Notably, in KEYNOTE-859, the OS benefit was enriched at higher PD-L1 CPS levels, with an OS HR of 0.65 and a median OS of 15.7 versus 11.8 months for PD-L1 CPS ≥10 tumors.2 A consistent outcome was observed in LEAP-015 for participants with PD-L1 CPS ≥10 for OS (HR, 0.71; median OS, 14.7 v 13.9 months) and PFS (HR, 0.56; median PFS, 8.5 v 6.7 months) although these differences were likely not clinically significant.
Beyond efficacy, the feasibility of multikinase inhibitors in combination with pembrolizumab and chemotherapy requires further exploration. While this regimen has demonstrated clinical activity across multiple tumor types, recent studies11,19-22 have failed to show an OS benefit with similar combination strategies. The various on-target and off-target toxicities of multikinase inhibitors remain an important consideration as higher rates of adverse events and treatment discontinuation in the experimental group might have compromised treatment compliance. Of note, the rate of treatment-related grade 5 adverse events in the experimental versus control arm of LEAP-015 (5% v 1%) was higher than that in the CheckMate 649 (2% v 1%) and KEYNOTE-859 (1% v 2%) studies.1,2 Alternative strategies that offer improved tolerability while maintaining efficacy should be considered.
In conclusion, in LEAP-015, lenvatinib plus pembrolizumab and chemotherapy versus chemotherapy provided a significant improvement in PFS and ORR, but not OS in participants with PD-L1 CPS ≥1. These findings could not confirm the role of anti-VEGF and anti–PD-1 combination therapies while raising critical questions regarding sequencing, chemotherapy maintenance, and toxicity management.
ACKNOWLEDGMENT
This study and assistance with medical writing were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD). We thank the participants and their families and caregivers for participating in the study, all primary investigators, and their site personnel, Chie-Schin Shih (MSD), Amanda Cartwright (MSD), Jodi Carillo (MSD), and Yanfen Guan (MSD) for clinical study support; M. Catherine Pietanza (MSD) for critical review; and Luana Atherly-Henderson, PhD, CMPP (MSD) for medical writing assistance. A complete list of investigators who participated in the LEAP-015 study is provided in Appendix Table A4.
APPENDIX 1. METHODS
Assessments
PD-L1 expression was centrally assessed during screening using the PD-L1 immunohistochemistry 22C3 assay (Agilent Technologies, Carpinteria, CA). PD-L1 combined positive score (CPS) was calculated as the number of PD-L1–staining cells (tumor cells, macrophages, and lymphocytes) divided by the total number of viable tumor cells, multiplied by 100. A prespecified validated cutoff of PD-L1 CPS of 1 or higher was used in the study. Microsatellite instability status was assessed in tumor tissue at a central laboratory by polymerase chain reaction (Almac Diagnostics, Armagh, United Kingdom). Adverse events were evaluated throughout the study and at 30 days (90 days for serious adverse events and events of interest to pembrolizumab) after treatment discontinuation and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Statistical Analysis
Efficacy was assessed in the intention-to-treat population of all randomly assigned participants. Safety was assessed in the as-treated population of all randomly assigned participants who received at least one dose of study treatment. Participant-reported outcomes (PROs) were assessed in the PRO full analysis set (FAS) population of all randomly assigned participants with at least one PRO assessment available for the specific end point and who had received at least one dose of study treatment.
Completion and compliance rates of European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, Quality of Life Questionnaire-Stomach cancer module, and EuroQoL 5-dimension, 5-level questionnaire by visit and by treatment are described. Completion rate is defined as the number of treated participants who complete at least one item/number of participants in the PRO FAS population. Compliance rate is defined as the number of treated participants who complete at least one item/the number of eligible participants who are expected to complete. The protocol specified four primary and two secondary hypotheses. One interim analysis and a final analysis were planned. The interim analysis (final analysis of objective response rate and progression-free survival (PFS) and interim analysis of overall survival in participants with PD-L1 CPS ≥1 and all participants) were planned to occur after approximately 494 PFS events were observed in participants with PD-L1 CPS ≥1 and approximately 8 months after the last participant was randomly assigned.
APPENDIX 2
TABLE A1.
Postdiscontinuation Anticancer and Immunotherapy in All Treated Participants
| Therapy | Lenvatinib + Pembrolizumab + Chemotherapy (n = 443), No. (%) | Chemotherapy (n = 437), No. (%) |
|---|---|---|
| Received any subsequent systemic anticancer therapy | 202 (46) | 273 (62) |
| Subsequent systemic therapy by type | ||
| Chemotherapy | 196 (44) | 255 (58) |
| Any PD-1/PD-L1 | 20 (5) | 83 (19) |
| Any VEGF | 85 (19) | 126 (29) |
| Other | 93 (21) | 108 (25) |
| Other immunotherapy | 2 (1) | 8 (2) |
Abbreviation: VEGF, vascular endothelial growth factor.
TABLE A2.
Potentially Immune-Mediated Adverse Events in All Treated Participants
| Adverse Event | Lenvatinib + Pembrolizumab + Chemotherapy (n = 441), No. (%) | Chemotherapy (n = 429), No. (%) | ||
|---|---|---|---|---|
| Any | Grade ≥3 | Any | Grade ≥3 | |
| Any | 202 (46) | 44 (10) | 51 (12) | 6 (1) |
| Adrenal insufficiency | 13 (3) | 4 (1) | 1 (<1) | 0 |
| Arthritis | 1 (<1) | 0 | 0 | 0 |
| Cholangitis sclerosing | 1 (<1) | 0 | 0 | 0 |
| Colitis | 11 (2) | 6 (1) | 1 (<1) | 1 (<1) |
| Encephalitis | 1 (<1) | 1 (<1) | 0 | 0 |
| Exocrine pancreatic insufficiency | 0 | 0 | 1 (<1) | 0 |
| Gastritis | 9 (2) | 1 (<1) | 4 (1) | 1 (<1) |
| Hemolytic anemia | 1 (<1) | 1 (<1) | 0 | 0 |
| Hepatitis | 8 (2) | 5 (1) | 1 (<1) | 0 |
| Hyperthyroidism | 33 (7) | 1 (<1) | 7 (2) | 0 |
| Hypophysitis | 5 (1) | 1 (<1) | 0 | 0 |
| Hypothyroidism | 128 (29) | 3 (1) | 5 (1) | 0 |
| Infusion reactions | 24 (5) | 5 (1) | 34 (8) | 3 (1) |
| Myelitis | 0 | 0 | 1 (<1) | 0 |
| Myositis | 1 (<1) | 1 (<1) | 0 | 0 |
| Myocarditis | 2 (<1) | 1 (<1) | 0 | 0 |
| Nephritis | 5 (1) | 2 (<1) | 0 | 0 |
| Pancreatitis | 6 (1) | 3 (1) | 1 (<1) | 0 |
| Pneumonitis | 15 (3) | 1 (<1) | 2 (<1) | 1 (<1) |
| Severe skin reactions | 15 (3) | 12 (3) | 0 | 0 |
| Thyroiditis | 2 (<1) | 0 | 0 | 0 |
| Type 1 diabetes mellitus | 2 (<1) | 2 (<1) | 0 | 0 |
| Vasculitis | 3 (1) | 1 (<1) | 1 (<1) | 0 |
TABLE A3.
Change From Baseline to Week 18 in EORTC QLQ-C30 GHS/QoL, QLQ-STO22 Pain, and EQ-5D-5L Scores in the Total PRO FAS Population
| Questionnaire | Lenvatinib + Pembrolizumab + Chemotherapy, LSM (95% CI) | Chemotherapy, LSM (95% CI) | Difference in LSM Changea (95% CI) |
|---|---|---|---|
| QLQ-C30 GHS/QoL | n = 430 | n = 420 | –4.15 (–7.18 to –1.12) |
| –1.72 (–3.95 to 0.50) | 2.42 (–0.01 to 4.86) | ||
| QLQ-STO22 pain scale | n = 429 | n = 418 | 3.07 (0.21 to 5.93) |
| –5.55 (–7.73 to –3.38) | –8.62 (–10.97 to –6.27) | ||
| EQ-5D-5L VAS | n = 427 | n = 416 | –0.78 (–3.43 to 1.86) |
| –0.80 (–2.76 to 1.15) | –0.02 (–2.14 to 2.10) |
Abbreviations: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EORTC QLQ-STO22, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Stomach cancer module; EQ-5D-5L VAS, EuroQoL 5-dimension, 5-level visual analog scale; GHS/QoL, Global Health Status/Quality of Life; FAS, full analysis set; LSM, least squares mean; PRO, participant-reported outcome.
For EORTC QLQ-C30 GHS/QoL and EQ-5D-5L VAS, a higher score indicates better HR QoL; for EORTC-QLQ STO22 pain scale, a higher score indicates worsened symptoms.
TABLE A4.
LEAP-015 Investigators
| Country | Investigator |
|---|---|
| Argentina | Ezequiel Heman Slutsky |
| Juan Cundom | |
| Andrea Gabriela Soria | |
| Marcela Alejandro Carballido | |
| Juan Manuel O'Connor | |
| Julieta Grasseli | |
| Australia | Matthew Burge |
| Daniel Paul Brungs | |
| Muhammad Adnan Khattak | |
| Belgium | Karen Paula Jozefa Geboes |
| Eric Van Cutsem/Jeroen Dekervel | |
| Lionel D. Hondt | |
| Canada | Frederic Lemay |
| Rosalyn Anne Juergens | |
| Chile | Felipe Reyes |
| Gonzalo Pizarro Brito | |
| Maria Alejandra Ojeda | |
| Herman Adolfo Araya | |
| Patricio Eduardo Yanez | |
| China | Jianwei Yang |
| Xi Chen | |
| Yuxian Bai | |
| Hongming Pan | |
| Nong Xu | |
| Yueyin Pan | |
| Qinghong Guo | |
| Baorui Liu | |
| Feng Ye | |
| Xin Wang | |
| Qi Li | |
| Yong Tang | |
| Huiting Xu | |
| Haichuan Su | |
| Ying Cheng | |
| Xianli Yin | |
| Qun Zhao | |
| Ning Li | |
| Jun You | |
| Yi Ba | |
| Jinsheng We | |
| Lin Shen | |
| Jin Li | |
| Wangjun Liao | |
| Zhen Li | |
| Lei Yang | |
| Colombia | Yovany Eduardo Rodriguez Pena |
| Ivan Jose Bustillo | |
| Carlos Jose Narvaez | |
| Manuel Enrique Gonzalez Fernandez | |
| Raimundo Manneh | |
| Andres Fernando Arenas Arias | |
| Costa Rica | Luis Corrales |
| Andres Wiernik Rodriguez | |
| France | Thomas Aparicio |
| Yuan Touchefeu | |
| Helene Boussion-Desloges | |
| Laurent Mineur | |
| Christophe Tournigand | |
| Francois Ghiringhelli | |
| Marie Pierre Galais | |
| Eric Terrebonne | |
| Thomas Walter | |
| Mathieu Baconnier | |
| Germany | Sylvie Lorenzen |
| Eray Goekkurt | |
| Annika Kurreck | |
| Arne Kandulski | |
| Thorsten Goetze | |
| Florian Lordick | |
| Guatemala | Mynor Aguilar |
| Karla Alejandra Lopez | |
| Rixci Augusto Lenin Ramirez Fallas | |
| Pier Anyelo Ramos Elias | |
| Juan Pablo Moreira | |
| Hong Kong | Wing Lok Wendy Chan |
| Ashely Cheng | |
| Winnie Yeo | |
| Ireland | Maeve Lowery |
| Adrian Murphy | |
| Israel | Sharon Pelles Avraham |
| Ayala Hubert | |
| Irit Ben-Aharon | |
| Valeriya Semenisty | |
| Gali Perl | |
| Wael Hozaeel | |
| Italy | Armando Santoro |
| Elena Aurelia Mazza | |
| Ferdinando de Vita | |
| Guiseppe Aprile | |
| Giovanni Gerardo Cardellino | |
| Filippo Pietrantonio | |
| Pierfrancesco Tassone | |
| Japan | Kohei Shitara |
| Hirokazu Shoji | |
| Yukiya Narita | |
| Hiroki Hara | |
| Kensei Yamaguchi | |
| Naotoshi Sugimoto | |
| Nozomu Machida | |
| Tomohiro Nishina | |
| Akihito Tsuji | |
| Yasuhiro Choda | |
| Kenji Amagai | |
| Masahiro Tsuda | |
| Shogen Boku | |
| Poland | Boguslawa Karaszewska |
| Kamil Stanislaw Kuc | |
| Jacek Mackiewicz | |
| Lucjan Stanislaw Wyrwicz | |
| Lukasz Hajac | |
| Russia | Alexy Alexandrovich Tryakin |
| Dmitry Aleksandrovich Nosov | |
| Rashida Orlova | |
| Alexey Mikhailovich Karachun | |
| Michael Osipov | |
| Mikhail Valerievich Kopp | |
| Natalia Vladimirovna Fadeeva | |
| Nikolay Viktorovich Kislov | |
| South Korea | Sun Young Rha |
| Seung Tae Kim | |
| Min-Hee Ryu | |
| Do-Youn Oh | |
| Keun-Wook Lee | |
| Hei-Cheul Jeung | |
| Jonggwon Choi | |
| Sang Cheul Oh | |
| Spain | Paula Jimenez Fonseca |
| Fernando Rivera Herrero | |
| Pilar Aitana Calvo Ferrandiz | |
| Daniel Acosta Eyzaguirre | |
| Taiwan | Kun-Huei Yeh |
| Jen-Shi Chen | |
| Li-Yuan Bai | |
| Chia-Jui Yen | |
| Turkey | Bulent Erdogan |
| Pinar Gursoy | |
| Mustafa Ozguroglu | |
| Suayib Yalcin | |
| Umut Demirci | |
| Mehmet Bilici | |
| Ilhan Hacibekiroglu | |
| United Kingdom | Hugo Ford |
| Kai-Keen Shiu | |
| Martin Scott-Brown | |
| Russell Petty | |
| Wasat Mansoor | |
| United States | Zev A. Wainberg |
| Marcus Noel | |
| Peter Enzinger | |
| Sreenivasa Chandana | |
| Geoffrey Ku | |
| Dierdre Cohen | |
| Vincent Lam |
NOTE. Investigators with at least one participant randomly assigned and enrolled.
FIG A1.
Multiplicity strategy for α reallocation. Hypotheses (H) are indicated in order of α reallocation. CPS, combined positive score; PFS, progression-free survival; ORR, objective response rate; OS, overall survival.
FIG A2.
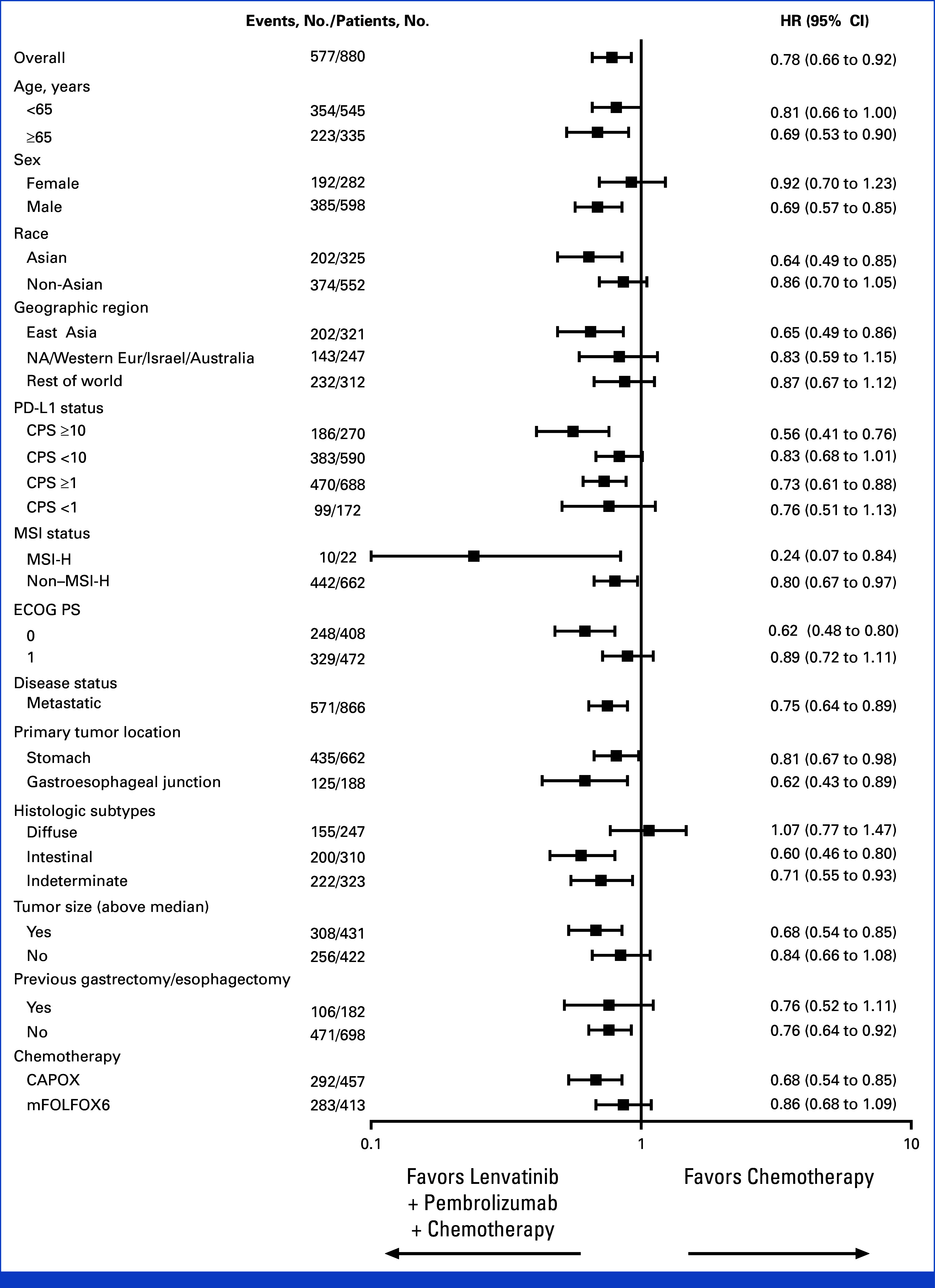
Forest plot of progression-free survival at interim analysis in participants with advanced metastatic HER2-negative gastric and gastroesophageal junction adenocarcinoma. The unstratified Cox regression model with Efron's method of tie handling with treatment as a covariate was used to assess the magnitude of the treatment difference between arms. CAPOX, capecitabine and oxaliplatin; CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; mFOLFOX6, fluorouracil, leucovorin, and oxaliplatin; MSI, microsatellite instability; MSI-H, microsatellite instability-high; NA, North America.
FIG A3.
DOR in participants with advanced metastatic HER2-negative gastric and gastroesophageal junction adenocarcinoma. Kaplan-Meier estimate of DOR at interim analysis. Tick marks represent data censored at the time of last imaging assessment. DOR, duration of response; HER2, human epidermal growth factor receptor 2; Lenva, lenvatinib; Pembro, pembrolizumab; ORR, objective response rate.
Kohei Shitara
Honoraria: Bristol Myers Squibb, Janssen, AstraZeneca, Lilly, Ono Pharmaceutical, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, Takeda, Ono Pharmaceutical, MSD, Novartis, Daiichi Sankyo, Amgen, Astellas Pharma, Guardant Health, Bayer, Zymeworks, AstraZeneca, ALX Oncology, GlaxoSmithKline K.K, Janssen, Healios, Moderna Inc, Arcus Biosciences Inc
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst), PRA Health Sciences (Inst), AstraZeneca (Inst), PPD-SNBL (Inst), Toray Industries (Inst)
Sylvie Lorenzen
Consulting or Advisory Role: MSD, Lilly, BMS GmbH & Co KG
Hirokazu Shoji
Consulting or Advisory Role: Astellas Pharma
Research Funding: Ono Pharmaceutical (Inst), Takeda (Inst), MSD (Inst), Astellas Pharma (Inst), Amgen (Inst), Daiichi Sankyo Company, Limited (Inst), AstraZeneca (Inst), AbbVie (Inst), Elevation Oncology (Inst)
Felipe Reyes-Cosmelli
Travel, Accommodations, Expenses: AstraZeneca
Yovany Rodriguez Peña
Research Funding: MSD
Expert Testimony: Amgen—Local PI
Travel, Accommodations, Expenses: Amgen
Luis Corrales
Consulting or Advisory Role: AstraZeneca, Roche, MSD Oncology, Pfizer, BMS, Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Roche, AstraZeneca, MSD Oncology, Pfizer, Merck Serono
Lucjan Wyrwicz
Honoraria: BeiGene, BMS, MSD, Servier
Consulting or Advisory Role: GlaxoSmithKline, Servier, AstraZeneca, Agenus
Speakers' Bureau: BMS, Servier, MSD
Travel, Accommodations, Expenses: Servier, Amgen
Daniel Acosta Eyzaguirre
Honoraria: BMS GmbH & Co KG, MSD
Consulting or Advisory Role: BMS GmbH & Co KG
Speakers' Bureau: BMS GmbH & Co KG, MSD Oncology
Travel, Accommodations, Expenses: BMS GmbH & Co KG, MSD
Min-Hee Ryu
Honoraria: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca, Astellas Pharma
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca, Astellas Pharma
Research Funding: Ono Pharmaceutical (Inst), AstraZeneca, Bristol Myers Squibb (Inst)
Deirdre J. Cohen
Consulting or Advisory Role: Astellas Pharma, Fosun Pharma, Summit Therapeutics
Speakers' Bureau: Guardant Health
Research Funding: Eisai (Inst), Merck (Inst), Viome (Inst)
Zev A. Wainberg
Consulting or Advisory Role: Novartis, Lilly, Merck, Merck KGaA, Bristol Myers Squibb, Bayer, AstraZeneca/MedImmune, Ipsen, Amgen, Daiichi Sankyo/AstraZeneca, Arcus Biosciences, Pfizer, Seagen, Alligator Bioscience, Astellas Pharma, EMD Serono, Janssen Oncology, Revolution Medicines, Phanes Therapeutics, Bridgebio
Research Funding: Novartis (Inst), Plexxikon (Inst), Pfizer (Inst), Merck (Inst), Five Prime Therapeutics (Inst)
Travel, Accommodations, Expenses: Lilly, Merck, Bayer, Amgen
Geoffrey Ku
Consulting or Advisory Role: Merck Sharp & Dohme, Pieris Pharmaceuticals, Bristol Myers Squibb, Apexigen, I-Mab, AstraZeneca/Daiichi Sankyo, Jazz Pharmaceuticals
Research Funding: Merck (Inst), Arog (Inst), Bristol Myers Squibb (Inst), Pieris Pharmaceuticals (Inst), Oncolys BioPharma (Inst), Zymeworks (Inst), Daiichi Sankyo (Inst), AstraZeneca/MedImmune (Inst), CARsgen Therapeutics (Inst), Adaptimmune (Inst), Triumvira Immunologics, Inc (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Merck Sharp & Dohme, Bristol Myers Squibb, Aduro Biotech, Pieris Pharmaceuticals, ImaBiotech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1023944
Josep Tabernero
Stock and Other Ownership Interests: Oniria Therapeutics, Alentis Therapeutics, 1TRIALSP, Pangaea Oncology
Consulting or Advisory Role: Boehringer Ingelheim, Lilly, MSD, Novartis, Taiho Pharmaceutical, Peptomyc, Chugai Pharma, Pfizer, AstraZeneca, Genentech, Menarini, Servier, F. Hoffmann LaRoche, Pierre Fabre, Daiichi Sankyo, Merus, Scandion Oncology, Sotio, Scorpion Therapeutics, Tolremo, Takeda Pharmaceuticals International AG, Alentis Therapeutics, Quantro Therapeutics, Accent Therapeutics, Ono Pharmaceutical, Bristol Myers Squibb, Cartography Biosciences, Carina Biotech, Johnson & Johnson/Janssen
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Servier, Bristol Myers Squibb, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Daiichi Sankyo, Pierre Fabre, Taiho Pharmaceutical, Astellas Pharma, GlaxoSmithKline, Nordic Group, Pfizer, Takeda, ALX Oncology, AbbVie, BeiGene, Boehringer Ingelheim, Mirati Therapeutics, Seagen, Ipsen, Agenus, Amgen, Arcus Biosciences, BioNTech SE, Debiopharm Group, ElmediX, Eisai, Simcere, Bexon Clinical Consulting, Cantargia AB, Fosum, Galapagos NV, ITeos Therapeutics, Microbial Machines, Novocure, Sanofi, Trishula Therapeutics
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho Pharmaceutical, ASLAN Pharmaceuticals, Halozyme, Zymeworks, Celgene, Basilea, BeiGene, Turning Point Therapeutics, Yuhan, Arcus Biosciences, IQVIA, MSD Oncology, LG Chem, Astellas Pharma, AbbVie, J-Pharma, Mirati Therapeutics, Eutilex, Moderna Therapeutics, Idience, Alligtor Bioscience AB, Hana Pharm, Revolution Medicines, Tallac Therapeutics
Research Funding: AstraZeneca, Novartis, Array BioPharma, Lilly, Servier, BeiGene, MSD, Handok
Li Wen Liang
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Merck
Travel, Accommodations, Expenses: Merck
Sonal Bordia
Employment: Merck
Pooja Bhagia
Employment: Merck
Stock and Other Ownership Interests: Merck
Sun Young Rha
Consulting or Advisory Role: MSD Oncology, Daiichi Sankyo, Eisai, LG Chem, Astellas Pharma, Indivumed, AstraZeneca, Ono Pharmaceutical, Amgen, Toray Industries, Arcus Biosciences
Speakers' Bureau: Eisai, MSD Oncology, BMS/Ono, Amgen, Daiichi Sankyo/UCB Japan, AstraZeneca, Astellas Pharma, Arcus Biosciences
Research Funding: MSD Oncology, Bristol Myers Squibb, Eisai, Roche/Genentech, ASLAN Pharmaceuticals, Sillajen, Bayer, Daiichi Sankyo, Lilly, AstraZeneca, BeiGene, Zymeworks, Astellas Pharma, Indivumed, Amgen (Inst)
No other potential conflicts of interest were reported.
Listen to the podcast by Dr Li and Dr Ko at https://ascopubs.org/do/jco-asco-annual-meeting-lenvatinib-plus-pembrolizumab-and-chemotherapy-gastric-cancer
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting, Chicago, IL, May 31-June 3, 2025.
SUPPORT
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and Eisai Inc, Nutley, NJ.
CLINICAL TRIAL INFORMATION
NCT04662710 (LEAP-015)
Contributor Information
Collaborators: Ezequiel Heman Slutsky, Juan Cundom, Andrea Gabriela Soria, Marcela Alejandro Carballido, Juan Manuel O'Connor, Julieta Grasseli, Matthew Burge, Daniel Paul Brungs, Muhammad Adnan Khattak, Karen Paula Jozefa Geboes, Eric Van Cutsem, Jeroen Dekervel, Lionel D. Hondt, Frederic Lemay, Rosalyn Anne Juergens, Felipe Reyes, Gonzalo Pizarro Brito, Maria Alejandra Ojeda, Herman Adolfo Araya, Patricio Eduardo Yanez, Jianwei Yang, Xi Chen, Yuxian Bai, Hongming Pan, Nong Xu, Yueyin Pan, Qinghong Guo, Baorui Liu, Feng Ye, Xin Wang, Qi Li, Yong Tang, Huiting Xu, Haichuan Su, Ying Cheng, Xianli Yin, Qun Zhao, Ning Li, Jun You, Yi Ba, Jinsheng We, Lin Shen, Jin Li, Wangjun Liao, Zhen Li, Lei Yang, Yovany Eduardo Rodriguez Pena, Ivan Jose Bustillo, Carlos Jose Narvaez, Manuel Enrique Gonzalez Fernandez, Raimundo Manneh, Andres Fernando Arenas Arias, Luis Corrales, Andres Wiernik Rodriguez, Thomas Aparicio, Yuan Touchefeu, Helene Boussion-Desloges, Laurent Mineur, Christophe Tournigand, Francois Ghiringhelli, Marie Pierre Galais, Eric Terrebonne, Thomas Walter, Mathieu Baconnier, Sylvie Lorenzen, Eray Goekkurt, Annika Kurreck, Arne Kandulski, Thorsten Goetze, Florian Lordick, Mynor Aguilar, Karla Alejandra Lopez, Rixci Augusto Lenin Ramirez Fallas, Pier Anyelo Ramos Elias, Juan Pablo Moreira, Wing Lok Wendy Chan, Ashely Cheng, Winnie Yeo, Maeve Lowery, Adrian Murphy, Sharon Pelles Avraham, Ayala Hubert, Irit Ben-Aharon, Valeriya Semenisty, Gali Perl, Wael Hozaeel, Armando Santoro, Elena Aurelia Mazza, Ferdinando de Vita, Guiseppe Aprile, Giovanni Gerardo Cardellino, Filippo Pietrantonio, Pierfrancesco Tassone, Kohei Shitara, Hirokazu Shoji, Yukiya Narita, Hiroki Hara, Kensei Yamaguchi, Naotoshi Sugimoto, Nozomu Machida, Tomohiro Nishina, Akihito Tsuji, Yasuhiro Choda, Kenji Amagai, Masahiro Tsuda, Shogen Boku, Boguslawa Karaszewska, Kamil Stanislaw Kuc, Jacek Mackiewicz, Lucjan Stanislaw Wyrwicz, Lukasz Hajac, Alexy Alexandrovich Tryakin, Dmitry Aleksandrovich Nosov, Rashida Orlova, Alexey Mikhailovich Karachun, Michael Osipov, Mikhail Valerievich Kopp, Natalia Vladimirovna Fadeeva, Nikolay Viktorovich Kislov, Sun Young Rha, Seung Tae Kim, Min-Hee Ryu, Do-Youn Oh, Keun-Wook Lee, Hei-Cheul Jeung, Jonggwon Choi, Sang Cheul Oh, Paula Jimenez Fonseca, Fernando Rivera Herrero, Pilar Aitana Calvo Ferrandiz, Daniel Acosta Eyzaguirre, Kun-Huei Yeh, Jen-Shi Chen, Li-Yuan Bai, Chia-Jui Yen, Bulent Erdogan, Pinar Gursoy, Mustafa Ozguroglu, Suayib Yalcin, Umut Demirci, Mehmet Bilici, Ilhan Hacibekiroglu, Hugo Ford, Kai-Keen Shiu, Martin Scott-Brown, Russell Petty, Wasat Mansoor, Zev A. Wainberg, Marcus Noel, Peter Enzinger, Sreenivasa Chandana, Geoffrey Ku, Dierdre Cohen, and Vincent Lam
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO-25-00748. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of study participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical study data with qualified external scientific researchers. The MSD data sharing website (available at https://externaldatasharing-msd.com/) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Kohei Shitara, Josep Tabernero, Eric Van Cutsem, Shu-Kui Qin, Sonal Bordia, Pooja Bhagia
Provision of study materials or patients: Kohei Shitara, Sylvie Lorenzen, Jin Li, Yuxian Bai, Yueyin Pan, Min-Hee Ryu, Deirdre J. Cohen, Zev A. Wainberg, Geoffrey Ku, Josep Tabernero, Eric Van Cutsem, Shu-Kui Qin
Collection and assembly of data: Kohei Shitara, Jin Li, Yuxian Bai, Manuel González Fernández, Mynor Aguilar, Felipe Reyes-Cosmelli, Yovany Rodriguez Peña, Lucjan Wyrwicz, Daniel Acosta Eyzaguirre, Yueyin Pan, Min-Hee Ryu, Deirdre J. Cohen, Zev A. Wainberg, Josep Tabernero, Eric Van Cutsem, Shu-Kui Qin, Do-Youn Oh, Sonal Bordia, Sun Young Rha
Data analysis and interpretation: Kohei Shitara, Sylvie Lorenzen, Hirokazu Shoji, Luis Corrales, Lucjan Wyrwicz, Daniel Acosta Eyzaguirre, Min-Hee Ryu, Deirdre J. Cohen, Zev A. Wainberg, Geoffrey Ku, Josep Tabernero, Eric Van Cutsem, Do-Youn Oh, Jianming Xu, Li Wen Liang, Sonal Bordia, Pooja Bhagia, Sun Young Rha
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lenvatinib Plus Pembrolizumab and Chemotherapy Versus Chemotherapy in Advanced Metastatic Gastroesophageal Adenocarcinoma: The Phase III, Randomized LEAP-015 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kohei Shitara
Honoraria: Bristol Myers Squibb, Janssen, AstraZeneca, Lilly, Ono Pharmaceutical, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, Takeda, Ono Pharmaceutical, MSD, Novartis, Daiichi Sankyo, Amgen, Astellas Pharma, Guardant Health, Bayer, Zymeworks, AstraZeneca, ALX Oncology, GlaxoSmithKline K.K, Janssen, Healios, Moderna Inc, Arcus Biosciences Inc
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst), PRA Health Sciences (Inst), AstraZeneca (Inst), PPD-SNBL (Inst), Toray Industries (Inst)
Sylvie Lorenzen
Consulting or Advisory Role: MSD, Lilly, BMS GmbH & Co KG
Hirokazu Shoji
Consulting or Advisory Role: Astellas Pharma
Research Funding: Ono Pharmaceutical (Inst), Takeda (Inst), MSD (Inst), Astellas Pharma (Inst), Amgen (Inst), Daiichi Sankyo Company, Limited (Inst), AstraZeneca (Inst), AbbVie (Inst), Elevation Oncology (Inst)
Felipe Reyes-Cosmelli
Travel, Accommodations, Expenses: AstraZeneca
Yovany Rodriguez Peña
Research Funding: MSD
Expert Testimony: Amgen—Local PI
Travel, Accommodations, Expenses: Amgen
Luis Corrales
Consulting or Advisory Role: AstraZeneca, Roche, MSD Oncology, Pfizer, BMS, Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Roche, AstraZeneca, MSD Oncology, Pfizer, Merck Serono
Lucjan Wyrwicz
Honoraria: BeiGene, BMS, MSD, Servier
Consulting or Advisory Role: GlaxoSmithKline, Servier, AstraZeneca, Agenus
Speakers' Bureau: BMS, Servier, MSD
Travel, Accommodations, Expenses: Servier, Amgen
Daniel Acosta Eyzaguirre
Honoraria: BMS GmbH & Co KG, MSD
Consulting or Advisory Role: BMS GmbH & Co KG
Speakers' Bureau: BMS GmbH & Co KG, MSD Oncology
Travel, Accommodations, Expenses: BMS GmbH & Co KG, MSD
Min-Hee Ryu
Honoraria: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca, Astellas Pharma
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca, Astellas Pharma
Research Funding: Ono Pharmaceutical (Inst), AstraZeneca, Bristol Myers Squibb (Inst)
Deirdre J. Cohen
Consulting or Advisory Role: Astellas Pharma, Fosun Pharma, Summit Therapeutics
Speakers' Bureau: Guardant Health
Research Funding: Eisai (Inst), Merck (Inst), Viome (Inst)
Zev A. Wainberg
Consulting or Advisory Role: Novartis, Lilly, Merck, Merck KGaA, Bristol Myers Squibb, Bayer, AstraZeneca/MedImmune, Ipsen, Amgen, Daiichi Sankyo/AstraZeneca, Arcus Biosciences, Pfizer, Seagen, Alligator Bioscience, Astellas Pharma, EMD Serono, Janssen Oncology, Revolution Medicines, Phanes Therapeutics, Bridgebio
Research Funding: Novartis (Inst), Plexxikon (Inst), Pfizer (Inst), Merck (Inst), Five Prime Therapeutics (Inst)
Travel, Accommodations, Expenses: Lilly, Merck, Bayer, Amgen
Geoffrey Ku
Consulting or Advisory Role: Merck Sharp & Dohme, Pieris Pharmaceuticals, Bristol Myers Squibb, Apexigen, I-Mab, AstraZeneca/Daiichi Sankyo, Jazz Pharmaceuticals
Research Funding: Merck (Inst), Arog (Inst), Bristol Myers Squibb (Inst), Pieris Pharmaceuticals (Inst), Oncolys BioPharma (Inst), Zymeworks (Inst), Daiichi Sankyo (Inst), AstraZeneca/MedImmune (Inst), CARsgen Therapeutics (Inst), Adaptimmune (Inst), Triumvira Immunologics, Inc (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Merck Sharp & Dohme, Bristol Myers Squibb, Aduro Biotech, Pieris Pharmaceuticals, ImaBiotech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1023944
Josep Tabernero
Stock and Other Ownership Interests: Oniria Therapeutics, Alentis Therapeutics, 1TRIALSP, Pangaea Oncology
Consulting or Advisory Role: Boehringer Ingelheim, Lilly, MSD, Novartis, Taiho Pharmaceutical, Peptomyc, Chugai Pharma, Pfizer, AstraZeneca, Genentech, Menarini, Servier, F. Hoffmann LaRoche, Pierre Fabre, Daiichi Sankyo, Merus, Scandion Oncology, Sotio, Scorpion Therapeutics, Tolremo, Takeda Pharmaceuticals International AG, Alentis Therapeutics, Quantro Therapeutics, Accent Therapeutics, Ono Pharmaceutical, Bristol Myers Squibb, Cartography Biosciences, Carina Biotech, Johnson & Johnson/Janssen
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Servier, Bristol Myers Squibb, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Daiichi Sankyo, Pierre Fabre, Taiho Pharmaceutical, Astellas Pharma, GlaxoSmithKline, Nordic Group, Pfizer, Takeda, ALX Oncology, AbbVie, BeiGene, Boehringer Ingelheim, Mirati Therapeutics, Seagen, Ipsen, Agenus, Amgen, Arcus Biosciences, BioNTech SE, Debiopharm Group, ElmediX, Eisai, Simcere, Bexon Clinical Consulting, Cantargia AB, Fosum, Galapagos NV, ITeos Therapeutics, Microbial Machines, Novocure, Sanofi, Trishula Therapeutics
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho Pharmaceutical, ASLAN Pharmaceuticals, Halozyme, Zymeworks, Celgene, Basilea, BeiGene, Turning Point Therapeutics, Yuhan, Arcus Biosciences, IQVIA, MSD Oncology, LG Chem, Astellas Pharma, AbbVie, J-Pharma, Mirati Therapeutics, Eutilex, Moderna Therapeutics, Idience, Alligtor Bioscience AB, Hana Pharm, Revolution Medicines, Tallac Therapeutics
Research Funding: AstraZeneca, Novartis, Array BioPharma, Lilly, Servier, BeiGene, MSD, Handok
Li Wen Liang
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Merck
Travel, Accommodations, Expenses: Merck
Sonal Bordia
Employment: Merck
Pooja Bhagia
Employment: Merck
Stock and Other Ownership Interests: Merck
Sun Young Rha
Consulting or Advisory Role: MSD Oncology, Daiichi Sankyo, Eisai, LG Chem, Astellas Pharma, Indivumed, AstraZeneca, Ono Pharmaceutical, Amgen, Toray Industries, Arcus Biosciences
Speakers' Bureau: Eisai, MSD Oncology, BMS/Ono, Amgen, Daiichi Sankyo/UCB Japan, AstraZeneca, Astellas Pharma, Arcus Biosciences
Research Funding: MSD Oncology, Bristol Myers Squibb, Eisai, Roche/Genentech, ASLAN Pharmaceuticals, Sillajen, Bayer, Daiichi Sankyo, Lilly, AstraZeneca, BeiGene, Zymeworks, Astellas Pharma, Indivumed, Amgen (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rha SY, Oh DY, Yañez P, et al. : Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 24:1181-1195, 2023 [DOI] [PubMed] [Google Scholar]
- 3.Qiu MZ, Oh DY, Kato K, et al. : Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ 385:e078876, 2024 [DOI] [PubMed] [Google Scholar]
- 4.Wilke H, Muro K, Van Cutsem E, et al. : Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol 15:1224-1235, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Ellis LM, Hicklin DJ: VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer 8:579-591, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Tabata K, Kimura T, et al. : Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One 14:e0212513, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Makker V, Colombo N, Casado Herráez A, et al. : Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386:437-448, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawazoe A, Fukuoka S, Nakamura Y, et al. : Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): An open-label, single-arm, phase 2 trial. Lancet Oncol 21:1057-1065, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Chung HC, Lwin Z, Gomez-Roca C, et al. : LEAP-005: A phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the gastric cancer cohort. J Clin Oncol 39, 2021. (suppl 3; abstr 230) [Google Scholar]
- 11.Herbst RS, Cho BC, Zhou C, et al. : 64O Lenvatinib plus pembrolizumab, pemetrexed, and a platinum (len + pembro + chemo) as first-line therapy for metastatic non squamous non-small cell lung cancer (NSCLC): Phase III LEAP-006 study. Immunooncol Technol 20:100536, 2023 [Google Scholar]
- 12.Shitara K, Van Cutsem E, Bang YJ, et al. : Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6:1571-1580, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shitara K, Ajani JA, Moehler M, et al. : Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 603:942-948, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powles T, Park SH, Voog E, et al. : Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218-1230, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Yanez PE, Ben-Aharon I, Rojas C, et al. : First-line lenvatinib plus pembrolizumab plus chemotherapy versus chemotherapy in advanced/metastatic gastroesophageal adenocarcinoma (LEAP-015): Safety run-in results. J Clin Oncol 41, 2023. (suppl 4; abstr 411) [Google Scholar]
- 16.Fuchs CS, Shitara K, Di Bartolomeo M, et al. : Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 20:420-435, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Ohtsu A, Shah MA, Van Cutsem E, et al. : Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29:3968-3976, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Lorenzen S, Goetze TO, Thuss-Patience PC, et al. : LBA59 Modified FOLFOX plus/minus nivolumab and ipilimumab vs FLOT plus nivolumab in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction: Final results of the IKF-AIO-Moonlight trial. Ann Oncol 35:S1249-S1250, 2024 [Google Scholar]
- 19.Kawazoe A, Xu RH, García-Alfonso P, et al. : Lenvatinib plus pembrolizumab versus standard of care for previously treated metastatic colorectal cancer: Final analysis of the randomized, open-label, phase III LEAP-017 study. J Clin Oncol 42:2918-2927, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llovet JM, Kudo M, Merle P, et al. : Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol 24:1399-1410, 2023 [DOI] [PubMed] [Google Scholar]
- 21.Matsubara N, de Wit R, Balar AV, et al. : Pembrolizumab with or without lenvatinib as first-line therapy for patients with advanced urothelial carcinoma (LEAP-011): A phase 3, randomized, double-blind trial. Eur Urol 85:229-238, 2024 [DOI] [PubMed] [Google Scholar]
- 22.Yang JC, Han B, De La Mora Jiménez E, et al. : Pembrolizumab with or without lenvatinib for first-line metastatic NSCLC with programmed cell death-ligand 1 tumor proportion score of at least 1% (LEAP-007): A randomized, double-blind, phase 3 trial. J Thorac Oncol 19:941-953, 2024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO-25-00748. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of study participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical study data with qualified external scientific researchers. The MSD data sharing website (available at https://externaldatasharing-msd.com/) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.