Abstract
A key mechanism underlying physiological angiogenesis of the human endometrium is its ability to regenerate the vascular capillary network and to perform vascular remodeling (i.e., development of spiral arteries). Vascular endothelial growth factor (VEGF) is associated with angiogenesis and capillary permeability in this tissue. VEGF is expressed as several spliced variants, its main human isoforms contain 121 and 165 aa; 17β-estradiol (E2) increases endometrial VEGF, possibly in all isoforms. Here we show that progesterone (P) selectively increases the expression of the VEGF189 (V189) isoform in the human uterus. V189 is identified in the conditioned medium of stromal cells treated with E2 + P; its presence in this in vitro model of decidual stromal cells is detected after 6–8 days, using ELISA, and after 8–10 days, using Western blot analysis with different antibodies, including one specific for V189. The secretion pattern of V189 parallels that of the decidual protein IGFBP-1. V189 is secreted as a native isoform, as compared with the migration of recombinant V189 by SDS/PAGE. In situ hybridization and immunocytochemistry, performed on the same biopsies, suggest that decidual cells express V189 during the mid-late secretory phase of the menstrual cycle and early gestation. Finally, using an in vivo permeability assay, we show that native V189 increases capillary permeability. These observations demonstrate that P regulates V189 expression in decidual cells, which could have important implications for understanding uterine vascular remodeling and implantation, and may be relevant in a range of disease states such as edema and irregular bleeding.
As a tissue that exhibits rapid cyclic changes (1), the human endometrium is a good model for the study of physiological angiogenesis, which is under the control of 17β estradiol (E2), progesterone (P), and vascular endothelial growth factor (VEGF) (2–7).
VEGF is a protein with angiogenic activity and is a potent stimulator of microvascular permeability (8, 9); it plays an important role in physiological and pathological neovascularization, via its receptors Flt-1/VEGFR1 and Flk-1/VEGFR2 (10–13). Molecular cloning of the cDNA for VEGF revealed that differential exon splicing generates several isoforms containing 121, 165, 189, and 206 aa (V121, V165, V189, and V206) (14, 15). In most systems V121 and V165 are the major species expressed, whereas V189 is only minimally present and V206 is limited to embryonic tissue (9, 15). The different isoforms appear to have similar functions, but differ in their binding affinity for extracellular matrix (ECM). In contrast to VEGF121, which is secreted and found freely soluble in the culture medium, the bioavailability of VEGF165 and to a greater extent V189 appears to be regulated by binding to heparan sulfate proteoglycans (HSPG) in the ECM (9, 16). Bound VEGF isoforms could provide a reserve of growth factors available after cleavage by heparinase or urokinase-type plasminogen activator (uPA) (17, 18).
Previous studies have reported cycle-dependent changes in VEGF expression in the human uterus (5–7). V121 and V165 mRNA predominate in the uterus; E2 increases VEGF expression, possibly of all VEGF isoforms, in the endometrium and isolated endometrial cells (2, 4–7, 19, 20). In contrast, the role of P in endometrial angiogenesis and stromal swelling necessary for embryo implantation has not been elucidated. However, the possibility exists that progestins can affect VEGF expression (6, 7, 21, 22) or bioavailability. Whether the three VEGF isoforms have identical biological functions in the uterus is not clear at present.
In this study, we show that P increases endometrial V189 during the secretory phase of the menstrual cycle. By immunocytochemistry and in situ hybridization we localize the V189 isoform in the decidual cells of the secretory and pregnant endometrium. Using an in vitro model of stromal cells treated with E2 + P for 14 days to mimic the secretory phase, we demonstrate that the secretion of native V189 is correlated with decidualization. In vivo studies demonstrate that V189 modulates capillary permeability (CP). These findings suggest that P by its action on decidual cells modulates the vascular permeabilization necessary for implantation and possibly for the maintenance of pregnancy.
Experimental Procedures
Hormones and Reagents.
E2 and P were provided by Sigma and EGF by PreproTech (Rocky Hill, NJ). Recombinant VEGF proteins (V165 and V189) and antibodies against V189 (P2 directed to the sequence corresponding to exon 6) have been described (18). A mouse-specific anti-V189 antibody was also prepared by immunization with three s.c. injections of 5 μg V189 in Freund's adjuvant. IgG were purified by protein A Sepharose (Pharmacia), chromatographed on a V165 affinity column (CnBr Sepharose, Pharmacia), and the flow-through was dialyzed against PBS. The specificity of the antibody for V189 was confirmed by ELISA on V165 and V189 coated plates and Western blot analysis (not shown).
Human Biopsies.
Human endometrial biopsies were obtained from consenting women investigated for infertility (23, 24) without any apparent endocrinological problems or local organic pathology. The specimens were in the proliferative (n = 10) and secretory (n = 15) phases of the cycle. A few samples were obtained from women undergoing voluntary termination of pregnancy. Tissues were either frozen in isopentane precooled in liquid nitrogen, or fixed in 4% paraformaldehyde and paraffin-embedded. Biopsies were also obtained from premenopausal cycling women undergoing hysterectomy for benign non-endometrial pathology (7). Tissues were immediately placed into DMEM containing 10% FCS and 1% antibiotic-antimycotic solution, and processed for stromal cell preparation.
Isolation and Treatment of Human Endometrial Cells.
Stromal cell isolation was performed as described (7, 25). The purity of the cell preparation was verified at passage 3–4 by using an antivimentin antibody (7). Stromal cells were grown in DMEM/10% FCS until confluent. Before steroid stimulation, cells were cultured for 2 days in phenol red-free DMEM/3% stripped FCS, and overnight in 0.3% stripped FCS. Hormones (E2, 10−8 M; P, 10−6 M) were added in phenol red-free DMEM in the presence of 5% apo-transferrin, 0.1% ascorbate, and 20 ng/ml EGF for 1–14 days (23). Control cells were incubated in the same medium without hormone.
Extraction of RNA from Cells and Semiquantitative Reverse Transcription (RT)-PCR.
Confluent cells were scraped into lysis buffer by using the TRIzol reagent kit (GIBCO/BRL). RT was performed as described (7). RT-PCR was performed using primers derived from the human VEGF sequence (positions 335 and 698, ref. 21) as described (26), using an annealing temperature of 56°C. The relative concentrations of VEGF transcripts in stromal cells from different groups was determined using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA as internal standard and [γ-33P]ATP as described (26). Bands were visualized and quantified on a Instant Imager (Hewlett–Packard). Two RT and two PCR/RT were performed for each experiment. Data were analyzed from six independent experiments and statistical analysis was performed using a two-way ANOVA test.
Immunocytochemistry.
VEGF immunocytochemistry (ICC) was performed on paraffin sections by using a rabbit antibody raised against the first 20 aa of human VEGF (VEGF A20, Santa Cruz Biotechnology; 1/250 dilution), followed by incubation with biotinylated anti-rabbit IgG and streptavidin peroxidase (Dakopatts, Glostrup, Denmark) (7). The latter was reacted with amino-ethylcarbazole. Antibodies against smooth α-actin (Dakopatts; refs. 27 and 28) and TIMP-3 metalloproteinase inhibitor (Oncogene Science) were used to detect specific decidual cell components. VEGF189 was detected on frozen sections by using a mouse-specific anti-V189 antibody (10 μg/ml). Controls included incubation of sections with irrelevant rabbit or mouse IgGs.
Immunoprecipitation.
The culture medium was collected every 48 h from confluent stromal cells stimulated with E2 + P and used after concentration (Amicon) or immunoprecipitation with magnetic beads coated with sheep anti-rabbit IgGs (Dynabeads M-280, Dynal, Great Neck, NY) (29). Beads were incubated overnight at 4°C with VEGF antibody (A-20; P2 antibody; 1 μg antibody for 4 × 107 beads) in PBS/1% skim milk, followed by overnight incubation at 4°C with 50 μg proteins from different conditioned media, in the presence of protease inhibitors (pepstatin 1 μg/ml, leupeptin 5 μg/ml, antipain 5 μg/ml, and aprotinin 1 μg/ml). Bound proteins were eluted from beads by boiling in 25 μl of SDS/PAGE sample buffer.
Immunoblotting of VEGF.
Proteins were separated by 12% SDS/PAGE under reducing conditions (DTT, 100 mM; iodoacetamide, 50 mM) and transferred onto polyvinylidene difluoride membrane (Polyscreen NEN Life Science Products). Rabbit anti-VEGF antibodies (A-20, 2 μg/ml; P2, 1 μg/ml) were used to detect VEGF. In some experiments, membranes were incubated with mouse anti-VEGF antibody (clone 26503, R & D Systems; 5 μg/ml) and rabbit anti-IGFBP-1 antibody (Upjohn Pharmacia, 1/1,000 dilution).
Enzyme Immunoassay for VEGF.
All VEGF isoforms were measured using Quantikine ELISA kit (R & D Systems). The presence of V189 was also specifically detected using P2 antibody in an ELISA assay (18).
In Situ Hybridization.
In situ hybridization was performed on endometrial paraffin sections as described (30). The slides were hybridized overnight at 42°C with sense or antisense oligonucleotide probes specific for all VEGF transcripts (sequence corresponding to exon 4) or only V189 transcripts (sequence corresponding to exon 6) (15); exon 4 sense probe, 5′-CTCACCAAGGCCAGCACATAGGAGAGATGA-3′; antisense probe, 5′-TCATCTCTCCTATGTGCTGGCCTTGGTGAG-3′; exon 6 sense probe, 5′-GCAAGAAATCCCGGTATAAGTCCTGGAGCG-3′; antisense probe, 5′-CGCTCCAGGACTTATACCGGGATTTCTTGC-3′; these probes were labeled with [35S]ATP at the 3′ end by using terminal deoxynucleotidyl transferase. After hybridization (30), the slides were exposed with photographic emulsion for 3–5 weeks.
Vascular Permeability Assay.
The Miles vascular permeability assay (31, 32) was adapted to Wistar rats as follows. Adult male Wistar rats (body weight 300–500 g) (CERJ Janvier, Le Genest St. Isle, France) were anesthetized with pentobarbital (50 mg/kg i.p.) and their backs closely shaved. Animals were then given filtered Evans-Blue solution (0.5%) via the left jugular vein. Twenty minutes later, native and cleaved recombinant V189 (18) or recombinant V165 were injected at 1–50 ng per injection (0.1 ml) intradermally into the back of the rat. Saline solution and 100 μM histamine were used as controls. The antiprotease aprotinin (100 nM) and a polyclonal anti-uPA antibody (gift from E. Angles Cano, INSERM, Paris; 50 μg/ml) were added to native V189 in some experiments. The increase in CP was assessed by the leakage of dye bound to the serum proteins, giving an intense blue spot surrounding the injection site. The size of the different spots was measured 15 min after injection.
Results
We have previously described the modulation of VEGF expression in the human endometrial functional layer throughout the menstrual cycle and its induction by sex steroid hormones in isolated human stromal cells (7). The steady-state expression of VEGF transcripts was increased by E2 within 1–3 h and persisted for 12 days, whereas P for 1–24 h did not modify VEGF mRNA levels. To further analyze the role of P on VEGF expression, subconfluent cultures of endometrial stromal cells were treated for 14 days with E2 + P; these cells express estradiol and progesterone receptors (7, 34).
Human Endometrial Stromal Cells Treated with Estradiol and Progesterone Express V189 mRNA in Vitro.
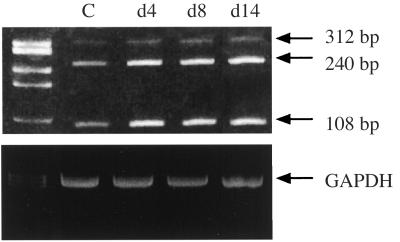
Northern blot analysis of RNA extracted from E2 + P-treated cells detected no significant changes in VEGF transcripts during the time course of stimulation (1–14 days) (data not shown). Using RT-PCR and oligonucleotides derived from exons shared by every VEGF mRNA species, 108-, 240-, and 312-bp fragments, corresponding to the mRNA encoding V121, V165, and V189, were amplified. The major amplified species corresponded to V121 and V165 (Fig. 1). Using a semiquantitative RT-PCR assay (see Experimental Procedures), the level of expression of V189 transcripts was usually not increased in stromal cells incubated with EGF + E2 + P for 1–14 days (Fig. 1) (n = 4), except in one experiment where a small increase (×2, P < 0.01) was observed at 14 days. Altogether, these data indicate no significant change in V189 mRNA levels in stromal cells incubated with E2 + P for 14 days.
Figure 1.
Expression level of VEGF transcripts in stromal cells incubated with both estradiol and progesterone. Total RNA was extracted every 2 days from cells subjected to EGF + E2 + P for 14 days. This figure shows an ethidium bromide gel of RT-PCR analysis from stromal cells incubated with hormones for 4, 8, and 14 days.
In Vitro Decidualized Cells Express V189 Protein.
To analyze the production of VEGF isoforms by stromal cells during the E2 + P treatment, the conditioned medium was analyzed by Western blot and ELISA. Analysis by ELISA using anti-VEGF antibody that recognizes all isoforms revealed that VEGF was secreted throughout the duration of culture. Using a specific anti-V189 recognizing antibody (P2), the presence of V189 was detected in the conditioned medium after 6–8 days of cell treatment with EGF + E2 + P (Table 1) or E2 + P (data not shown); in contrast, it was not secreted by cells in the absence of P. Western blot analysis with two different anti-VEGF antibodies (A-20 and clone 26503) reproducibly detected a major protein band, approximately 29–30 kDa, in the conditioned medium from cells treated with EGF + E2 + P or E2 + P for 10-12 days, corresponding to native V189 (Fig. 2 A and C). V189 was detectable over 4–6 days, starting on day 8 or 10 depending on the endometrium; it was not detected before day 8, or was present in small amounts under E2 treatment (Fig. 2A). Two minor bands, 22–23 and 18 kDa corresponding to V165 and V121, were strongly detected under E2 treatment but were present in smaller amounts under E2 + P treatment (Fig. 2A Upper). The specificity of the 30-kDa band was ascertained when proteins of the conditioned medium were immunoprecipitated using anti-V189 antibody (P2), then immunoblotted with anti-VEGF antibodies (Fig. 2B).
Table 1.
Results from in vitro experiments (EGF + E2 + P)
| Days | VEGF189
|
IGFBP-1 WB | |
|---|---|---|---|
| ELISA | WB | ||
| 1–4 | − | − | − |
| 4–8 | −/+ | − | − |
| 8–12 | + | + | + |
| 12–14 | −/+ | − | + |
The conditioned medium from stromal cells was analyzed every 2 days for the presence of V189 and IGFBP-1, using Western blot (WB) and ELISA assay.
Figure 2.
VEGF189 expression in the conditioned media from decidual stromal cells. (A) Western blot analysis of proteins (100 μg) from conditioned media after 2, 4, and 6 days of cell treatment with E2, or after 8, 10, 12, and 14 days with E2 + P (15 μg proteins). Antibodies to human VEGF (A-20) (Upper) or to IGFBP-1 (Lower) were used for immunoblotting of proteins. Treatment with E2 + P markedly increases the synthesis of a 29–30 kDa VEGF isoform after 10–12 days (maximum on day 12 in this experiment) and of IGFBP-1. Depending on the human sample, the expression of V189 can be observed over 4 days, starting on day 8–10. (B) Western blot analysis of proteins (50 μg) immunoprecipitated with anti-V189 antibody (P2) and blotted with the anti-VEGF A-20 antibody. (C) SDS/PAGE analysis of recombinant V189, native (5 ng), or cleaved (10 ng) (18), and proteins (35 μg) of conditioned medium from E2 + P-treated stromal cells (12 days treatment) using A-20 antibody. The 29–30-kDa protein seen at day 12 is comparable to that of recombinant native V189.
We then investigated whether the V189-expressing stromal cells are decidual cells and secrete IGFBP-1, as decidual cells are known to secrete this protein in the secretory phase and under P treatment (33). The increase in V189 in the conditioned medium of stromal cells treated with EGF + E2 + P for 8–12 days was parallel to the increase in IGFBP-1 (Fig. 2A). Thus, endometrial stromal cells decidualized in vitro under P treatment secrete V189 mRNA and protein (Table 1).
V189 Is Expressed in the Human Endometrium During the Luteal Phase and Pregnancy.
Immunostaining for VEGF correlates with total production and bioavailability when commercially VEGF antibodies that cannot distinguish between the different isoforms are used. During the proliferative and early-secretory periods a faint staining for VEGF was observed in endometrial stromal cells, along with diffuse interstitial staining (Fig. 3A and refs. 5–7). During the late secretory period, although not previously described (5–7), VEGF labeling was strongly present in the round perivascular stromal cells as shown in Fig. 3B. These cells express smooth α-actin (Fig. 3C), typical of predecidual cells. To confirm the localization of V189 in the luteal phase, endometrial sections were incubated with a mouse-specific anti-V189 antibody. As shown in Fig. 3E, V189 isoform was localized in decidual cells during the luteal phase.
Figure 3.
In situ expression of VEGF by decidual cells. Sections from early (A, day 20) and mid-late (B–D, day 24; E, day 26) secretory endometrium were immunostained with rabbit anti-VEGF (A-20) (A and B), anti-smooth muscle α-actin (C), or anti-VEGF189 (E) antibodies. Stromal cells are weakly stained for VEGF in the early secretory phase (A); note in B the strong immunolabeling for total VEGF in stromal cells having a decidual-like appearance, located close to vessels (v), and positive for smooth α-actin (C, arrow). Note in E the strong pericellular labeling for V189 isoform in decidual cells. Hematoxylin/eosin section in D. Light counterstaining with hematoxylin/eosin in A and B. a, arteriole. (Magnification, ×400.)
We next used in situ hybridization to confirm V189 mRNA expression in human decidual stromal cells; endometrial sections were incubated with oligonucleotide probes covering the exon 6 specific to V189, or the exon 4 present in all VEGF transcripts. As shown in Fig. 4 A–D, V189 transcripts were observed during the mid-late secretory period and early gestation in perivascular decidualized cells; control studies using sense probe (Fig. 4D Inset) indicated that the hybridization signal seen in Fig. 4 A–D represents specific binding to V189 transcripts. VEGF mRNAs corresponding to all isoforms were expressed at moderate levels in stromal cells throughout the menstrual cycle (Fig. 4 E and F). Altogether, immunocytochemistry (ICC) and in situ hybridization on the same biopsies demonstrate that the enhanced expression of V189 mRNA during the mid-late secretory period (days 24–28) corresponds to a high level of VEGF, probably the 189 isoform, produced in groups of perivascular decidual cells (Table 2).
Figure 4.
In situ hybridization for VEGF189 mRNA in human endometrium. Sections were hybridized with a radiolabeled antisense V189 probe (A–D) or a probe for all VEGF transcripts (E and F). (A and B) Late secretory phase (day 28), strong V189 expression in decidual cells. (C and D) Early pregnancy, stromal cells near a blood vessel (v); dark-field in A and C and bright-field in B and D. (Inset) No staining is detected with the corresponding sense probe on sections adjacent to those shown in panels C and D. (E and F) Proliferative (day 11) and secretory (day 25) phase, expression of all VEGF mRNA by using exon 4 antisense probe is moderate in stromal cells. Hematoxylin/eosin counterstaining. (Magnification, ×400.)
Table 2.
VEGF expression during the secretory phase (in situ)
| Period | VEGF189 mRNA | VEGF stained cells | Type IV collagen |
|---|---|---|---|
| Early (days 14–19) | − | − | − |
| Mid (days 20–24) | + | ++ | −/+ |
| Late (days 25–28) | + | + | + |
The development of a stromal basement membrane [consisting of laminin, fibronectin, heparan sulfate proteoglycans (HSPG), and type IV collagen] at the end of decidualization (days 24–25, refs. 35 and 36) suggests that V189 could anchor at it during this period. As expected, expression of type IV collagen and HSPG was observed in predecidual cells at day 25, whereas it was mainly confined to the vascular subendothelial basement membrane before days 22–23 (data not shown). The development of a basement membrane by decidual cells in vitro, as deduced from the immunostaining of the decidual marker TIMP-3 (data not shown), correlates with the absence of V189 secretion observed after day 14 (Fig. 2A).
V189 Increases CP.
The strong expression of V189 by perivascular decidual cells suggests that P and V189 play a role in the physiological differentiation and function of the endometrial vessels during the mid-late secretory phase, as previously suggested (7). Different expression of VEGF receptors during the implantation or mid-late secretory periods (24) supports a physiological role for endometrial V189. We next tested whether native V189 secreted by decidual cells could alter CP, as previously described for VEGF in rat uterus (19).
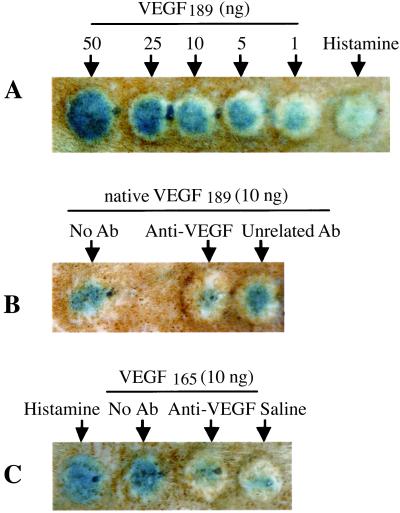
VEGF effect on CP was analyzed using the Miles assay (31, 32) adapted for use in the rat. Intradermal injection of V189 (1–50 ng) produced a large increase in CP compared with the control vehicle saline (Fig. 5); dose–response studies indicated that maximum induction occurs with 50 ng, as assessed by the area of diffusion of blue dye in the bleb. Both native (Fig. 5A) and cleaved (data not shown) V189 increased CP at 1 ng. V165 and histamine also strongly increased CP. Preincubation of V189 or V165 with neutralizing anti-VEGF antibody inhibited VEGF-mediated CP, as compared with V189 or V165 alone used at the same dose (Fig. 5 B and C). Addition of aprotinin or an anti-uPA antibody with native V189 did not significantly change the CP increase (data not shown), indicating that cleavage by endogenous rat proteases is not required for the CP effect.
Figure 5.
VEGF189 increases capillary permeability. (A) V189 increases CP in a dose-dependent manner. No effect is detected with saline. Histamine (100 μM) as a positive control increases CP. (B and C) Preincubation of V189 (B) or V165 (C) with neutralizing anti-VEGF antibody (clone 26503) (1 μg IgG/10 ng VEGF) completely inhibits V189 (or V165)-mediated CP; preincubation of VEGF with an unrelated antibody (used in the same conditions) does not inhibit VEGF-mediated CP.
Discussion
Although previous studies have shown that E2 regulates the expression of all VEGF transcripts in endometrial cells (3–7, 20), the expression and modulation of VEGF isoforms during human implantation and decidualization is not yet elucidated. The present study demonstrates considerable expression of the V189 isoform in the human endometrium (functional layer) during the mid-late secretory phase. Using various approaches we have shown that V189 is up-regulated by P in decidual (stromal) cells, both in vivo and in vitro, supporting an important role of this isoform in the control of angiogenesis. Functional studies demonstrate that V189 is a potent permeability factor in vivo.
V189 Expression in the Human Endometrium.
In situ hybridization in endometrial samples indicates the presence of V189 mRNA in a subset of perivascular stromal cells during the mid-late secretory phase, suggesting that the expression of V189 mRNA may be temporarily and spatially increased. This result extends previous reports showing that V121 and V165 are the predominant forms produced at the mRNA level in the uterus (3, 5, 7). Consistent with our in situ observations, relative expression levels of immunoreactive VEGF isoforms and specific V189 increase in perivascular stromal cells during the same period, in contrast to the diffuse staining in the stroma observed in proliferative and early-mid secretory endometrium (5–7); the difference between faint stromal staining and presence of VEGF transcripts in stromal cells during the menstrual cycle was initially interpreted to be a consequence of differences in the sensitivity between immunocytochemistry (ICC) and RT-PCR analysis or in situ hybridization. However, a less-defined localization pattern with ICC could result from the production by the stromal cells of the more diffusible variants of VEGF, V121 and V165 (9). Our data clearly indicate that the strong V189 immunostaining observed in clusters of decidual cells during the mid-late secretory phase corresponds to a local high level of expression of V189 mRNA, suggesting that these mRNA are indeed translated into proteins in these cells.
In Vitro Decidualized Cells Express V189 Protein.
V189 was shown by Western blot and ELISA to be secreted in vitro during the E2 + P-dependent differentiation of stromal into decidual cells secreting IGFBP-1, as deduced from the analysis of both proteins from the same medium and the use of specific antibodies. In vitro studies on stromal cells also indicate that V189 is secreted as an uncleaved isoform (28–30-kDa monomer) in response to E2 + P. This finding correlates with a low level of uPA expression that has been shown to cleave V189 (17, 18), and with the increase in the expression of plasminogen activator inhibitor-1 (PAI-1) in the same cells under the same conditions (38).
Although V189 was originally described as an isoform anchored to the basement membrane (17, 18), the secretion of V189 by endometrial stromal cells is not surprising, because these cells do not produce a pericellular basement membrane until complete decidual transformation (ref. 1 and unpublished observations). Stromal decidualization begins by day 22 or 23 in the perivascular areas of the functional layer, increases on day 24, and is achieved by day 25. During their differentiation from mid-secretory phase to the end of pregnancy, predecidual cells sequentially express and secrete relaxin, prolactin, and IGFBP-1 (33), change their morphology in association with the appearance of smooth muscle α-actin (ref. 28 and this study), and express progesterone and estrogen (ERβ) receptors (27, 34). The presence of type IV collagen, heparan sulfate proteoglycans (HSPG), and TIMP-3 during the mid-late secretory phase (days 24–26) (unpublished observations) reflects the production and assembly of a distinct pericellular basement membrane by differentiated decidual cells (35–37). The decrease in V189 secreted by decidual cells observed around 14 days of culture with E2 + P, while IGFBP-1 was still secreted (Fig. 2A), argues in favor of progressive anchoring of V189 to the basement membrane at the end of the cycle (see Tables 1 and 2). Thus, V189 could modulate vascular function according to its release or sequestration in the cell basement membrane. In addition, it is possible that E2 or P may modulate the expression of proteins and proteoglycans that sequester V189.
Progesterone Regulation of V189.
There are a few reports that progestins can modulate VEGF production by human endometrial and breast cancer cells (6, 7, 39, 40), and these effects have been less studied than those of estrogens (41). Results on monkey endometrium treated for 20 days with levonorgestrol support the hypothesis that progestins can modulate V189 gene expression in primate endometrium (21). In the present study, we consistently observed an increase in V189 protein expression in long-term E2 + P-treated human endometrial stromal cells (with no consistent increase in V189 mRNA levels). Although substantial differences may exist between cell types and in different species, the strong increase in V189 protein secreted from endometrial decidual cells, as compared with V121 and V165 and irrespective of their relative transcript levels, suggests another mechanism of regulation, possibly at the level of translation.
Physiologic Implication of V189 Expression in Endometrium.
Neovascularization in normal endometrium is indirectly modulated by ovarian steroids, via the production of locally active angiogenic factors such as VEGF (6, 7), which is mitogenic for endothelial cells (9, 10), through its receptors (10–13, 24, 42). E2 and E2 + progestin (such as P or medroxyprogesterone acetate) cause a marked increase in VEGF mRNA accumulation, correlating with a high vascular density during the late proliferative phase and between cycle days 18–22 (i.e., during the implantation window) (6, 7), where it is associated with a high level of expression of VEGFR1 and VEGFR2 (24). However, some evidence exists to support a role of certain progestins as antiangiogenic factors in vitro (reviewed in ref. 40). P-induced V189 expression by perivascular decidual cells correlates with its binding to endometrial vessels and with a switch of endothelial VEGF receptors (decreased ratio of VEGFR2/VEGFR1) and ER subtype (increase in ERβ) during the mid-late secretory phase (24, 27), suggesting that V189 plays a role in the physiological differentiation and function of the endometrial vessels. Human endometrial decidualization is associated with vascular remodeling such as development of spiral arteries, inhibition of angiogenesis, and sustained CP (1). Also, a marked increase in the expression of PAI-1 and an inhibition of uPA expression accompany progestin-induced decidualization in vivo and in vitro. Our results suggest that V189 could function either in its native form, which binds to VEGFR1, or in its cleaved form, which binds to both VEGFR1 and VEGFR2 (the latter mediating the VEGF-dependent mitogenic effect), depending of the presence of proteases (18). Our results further suggest that secretion of native V189 during the mid-late secretory phase in the absence of uPA can alter CP (and possibly other functions) without inducing angiogenesis. In contrast, V189 could be cleaved by uPA during steroid hormone removal resulting in menstruation. Also, the increase in CP induced by V189 provides an explanation for the episodes of endometrial capillary rupture and irregular bleeding associated with progestin-based contraceptives and hormonal substitutive treatments (43).
Thus, endometrial development in the primate represents a physiological model for differential expression and function of VEGF isoforms (especially V189) and their receptors. Our data also suggest that VEGF isoforms derived from specific splice variants can have unique functions, depending on their tissue origin. Also, the possibility remains that other unknown tissue-specific vascular factors might be involved in human reproduction biology, such as the endocrine-gland-derived VEGF (EG-VEGF) specifically reported in fenestrated capillary endothelial cells from endocrine glands (44). Another model utilizes transformed cells nullizygous for VEGF to specifically express each of these isoforms and determine their role in tumorigenic neovascularization (45). Mouse V188-expressing tumors failed to grow, because of little or no recruitment of the host vasculature. Further studies are in progress in our laboratory to analyze other tissue localizations and functions of the V189 isoform.
Acknowledgments
We thank S. Dumas (CNRS, Paris) for her kind assistance in in situ hybridization, F. Cavaillé (INSERM, Paris) for help in IGFBP-1 experiments, and M. Trombe (CNRS, Toulouse, France) for anti-V189 antibody preparation. This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, the Association pour la Recherche sur le Cancer, the Ligue Nationale contre le Cancer, and the Foundation pour la Recherche Médicale.
Abbreviations
- VEGF
vascular endothelial growth factor
- Vn
VEGFn
- E2
17β-estradiol
- P
progesterone
- CP
capillary permeability
- RT
reverse transcription
References
- 1.Giudice L. In: Reproductive Endocrinology, Surgery and Technology. Adashi E Y, Rock J A, Rosenwaks Z, editors. Philadelphia: Lippincott–Raven; 1996. pp. 272–300. [Google Scholar]
- 2.Perrot-Applanat M. In: Encyclopedic Reference of Vascular Biology and Pathology. Bikfalvi A, editor. Berlin: Springer-Verlag; 2000. pp. 157–162. [Google Scholar]
- 3.Charnock-Jones D S, Sharkey A M, Rajput-Williams J, Burch D, Schofield J P, Foutain S A, Boocock C A, Smith S K. Biol Reprod. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Rees M C P, Bicknell R. J Cell Sci. 1995;108:323–331. doi: 10.1242/jcs.108.1.323. [DOI] [PubMed] [Google Scholar]
- 5.Torry D S, Holt V J, Keenan J A, Harris G, Caulde M R, Torry R J. Fertil Steril. 1996;66:72–80. [PubMed] [Google Scholar]
- 6.Shifren J L, Tseng J F, Zaloudek C J, Ryan I P, Meng Y G, Ferrara N, Jaffe R B, Taylor R N. J Clin Endocrinol Metab. 1996;81:3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 7.Bausero P, Cavaillé F, Meduri G, Freitas S, Perrot-Applanat M. Angiogenesis. 1998;2:167–182. doi: 10.1023/a:1009292506879. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak H F, Brown L F, Detmar M, Dvorak A M. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N, Davis-Smith T. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 10.Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Moller N, Risau W, Ullrich A. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 11.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 12.De Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 13.Terman B I, Dougher-Vermozen M, Carrion M E, Dimitrov D, Armellino D C, Gospodarowicz D, Böhlen P. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 14.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes J C, Abraham J A. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 15.Houck K, Ferrara N, Winer J, Cachianes G, Li W, Leung D W. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 16.Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. J Biol Chem. 1992;267:6093–6098. [PubMed] [Google Scholar]
- 17.Houck K A, Leung D W, Rowland A M, Winer A, Ferrara N. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 18.Plouet J, Moro F, Bertagnoli S, Coldeboeuf N, Mazarguil H, Clamens S, Bayard F. J Biol Chem. 1997;272:13390–13396. doi: 10.1074/jbc.272.20.13390. [DOI] [PubMed] [Google Scholar]
- 19.Cullinan-Bove K, Koos R D. Endocrinology. 1993;133:829–837. doi: 10.1210/endo.133.2.8344219. [DOI] [PubMed] [Google Scholar]
- 20.Hyder S M, Stancel G M, Chiappetta C, Murthy L, Boettger-Tong H L, Makela S. Cancer Res. 1996;56:3954–3960. [PubMed] [Google Scholar]
- 21.Greb R R, Heikinheimo O, Williams R F, Hodgen G D, Goodman A L. Human Reprod. 1997;12:1280–1292. doi: 10.1093/humrep/12.6.1280. [DOI] [PubMed] [Google Scholar]
- 22.Hyder S M, Stancel G M. Mol Endocrinol. 1999;6:806–811. doi: 10.1210/mend.13.6.0308. [DOI] [PubMed] [Google Scholar]
- 23.Freitas S, Meduri G, Le Nestour E, Bausero P, Perrot-Applanat M. Biol Reprod. 1999;61:1070–1082. doi: 10.1095/biolreprod61.4.1070. [DOI] [PubMed] [Google Scholar]
- 24.Meduri G, Bausero P, Perrot-Applanat M. Biol Reprod. 2000;62:439–447. doi: 10.1095/biolreprod62.2.439. [DOI] [PubMed] [Google Scholar]
- 25.Osteen K G, Hill G A, Hargrove J T, Gorstein F. Fertil Steril. 1989;52:965–972. doi: 10.1016/s0015-0282(16)53160-4. [DOI] [PubMed] [Google Scholar]
- 26.Bausero P, Ben-Mahdi M-H, Mazucatelli J-P, Bloy C, Perrot-Applanat M. Am J Physiol. 2000;279:H2033–H2042. doi: 10.1152/ajpheart.2000.279.5.H2033. [DOI] [PubMed] [Google Scholar]
- 27.Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M. J Clin Endocrinol Metab. 2001;86:1379–1386. doi: 10.1210/jcem.86.3.7322. [DOI] [PubMed] [Google Scholar]
- 28.Oliver C, Montes M J, Galindo J A, Ruiz C, Olivares E G. Human Reprod. 1999;14:1599–1605. doi: 10.1093/humrep/14.6.1599. [DOI] [PubMed] [Google Scholar]
- 29.Biswas B, Vemulapalli R, Dutta S K. J Clin Microbiol. 1994;32:2147–2151. doi: 10.1128/jcm.32.9.2147-2151.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanièce P, Le Hir H, Bodeau-Péan S, Charon Y, Valentin L, Thermes C, Mallet J, Dumas S. J Neurochem. 1996;6:1819–1825. doi: 10.1046/j.1471-4159.1996.66051819.x. [DOI] [PubMed] [Google Scholar]
- 31.Miles A A, Miles E M. J Physiol. 1952;118:228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murohara T, Horowitz J R, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner J M. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- 33.Bryant-Greenwood G D, Rutanen E M, Partanen S, Coelho T K, Yamamoto S Y. Mol Cell Endocrinol. 1993;95:23–29. doi: 10.1016/0303-7207(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 34.Garcia E, Bouchard P, De Brux J, Berdha J, Frydman R, Schaison G, Milgrom E, Perrot-Applanat M. J Clin Endocrinol Metab. 1988;67:80–87. doi: 10.1210/jcem-67-1-80. [DOI] [PubMed] [Google Scholar]
- 35.Aplin J D, Charlton A K, Ayad S. Cell Tissue Res. 1988;253:231–240. doi: 10.1007/BF00221758. [DOI] [PubMed] [Google Scholar]
- 36.Wewer U M, Faber M, Liotta L A, Albrechtsen R. Lab Invest. 1985;53:624–633. [PubMed] [Google Scholar]
- 37.Higuchi T, Kanzaki H, Nakayama H, Fujimoto T, Hatayama H, Kojima K, Iwai M, Mori T, Fujita J. Endocrinology. 1995;136:4973–4981. doi: 10.1210/endo.136.11.7588231. [DOI] [PubMed] [Google Scholar]
- 38.Casslén B, Nordengren J, Gustavsson B, Nilbert M, Lund L R. J Clin Endocrinol Metab. 1995;80:2776–2784. doi: 10.1210/jcem.80.9.7673423. [DOI] [PubMed] [Google Scholar]
- 39.Hyder S, Murthy L, Stancel G M. Cancer Res. 1998;58:392–395. [PubMed] [Google Scholar]
- 40.Lebovic D I, Shifren J L, Ryan I P, Mueller M D, Korn A P, Darney P D, Taylor R N. Human Reprod. 2000;15:67–77. doi: 10.1093/humrep/15.suppl_3.67. [DOI] [PubMed] [Google Scholar]
- 41.Mueller M D, Vigne J-L, Minchenko A, Lebovic D I, Leitman D C, Taylor R N. Proc Natl Acad Sci USA. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das S K, Chakraborty I, Wang J, Dey S K, Hoffman L H. Biol Reprod. 1997;56:1390–1399. doi: 10.1095/biolreprod56.6.1390. [DOI] [PubMed] [Google Scholar]
- 43.Vincent A J, Malakooti N, Zhang J, Rogers P A, Affandi B, Salamonsen L A. Human Reprod. 1999;14:807–815. doi: 10.1093/humrep/14.3.807. [DOI] [PubMed] [Google Scholar]
- 44.Lecouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telms L, Frantz G, Rangell L, DeGuzman L, Keller G-A, et al. Nature (London) 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 45.Grunstein J, Masbad J J, Hickey R, Giordano F, Johnson R S. Mol Cell Biol. 2000;20:7282–7291. doi: 10.1128/mcb.20.19.7282-7291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]