Abstract
Signal transduction through epidermal growth factor receptors (EGFRs) is essential for the growth and development of multicellular organisms. A genetic screen for regulators of EGFR signaling has led to the identification of Sprouty, a cell autonomous inhibitor of EGF signaling that is transcriptionally induced by the pathway. However, the molecular mechanisms by which Sprouty exerts its antagonistic effect remain largely unknown. Here we have used transient expression in human cells to investigate the functional properties of human Sprouty (hSpry) proteins. Ectopically expressed full-length hSpry1 and hSpry2 induce the potentiation of EGFR-mediated mitogen-activated protein (MAP) kinase activation. In contrast, truncation mutants of hSpry1 and hSpry2 containing the highly conserved carboxyl-terminal cysteine-rich domain inhibit EGF-induced MAP kinase activation. The potentiating effect of the full-length hSpry2 proteins on EGF signaling is mediated by the amino-terminal domain and results from the sequestration of c-Cbl, which in turn leads to the inhibition of EGFR ubiquitination and degradation. These results indicate that hSpry2 can function both as a negative and positive regulator of EGFR-mediated MAP kinase signaling in a domain-dependent fashion. A dual function of this kind could provide a mechanism for achieving proper balance between the activation and repression of EGFR signaling.
Receptor tyrosine kinase-mediated signaling pathways are used in both invertebrates and vertebrates to control critical aspects of organ morphogenesis, patterning, cellular proliferation, and differentiation (1). These signaling pathways are tightly regulated by positive and negative inputs that ensure the proper cellular fate (2). The development of the Drosophila compound eye is regulated primarily through inductive signaling emanating from the Drosophila homolog of the epidermal growth factor receptor (EGFR) (DER; ref. 3). Recently, a genetic screen for modifiers of DER signaling in the Drosophila eye has identified Sprouty (dSpry) as a negative modulator of this pathway (4). Loss-of-function dSpry mutations were able to overcome the phenotype resulting from the overexpression of another negative regulator of EGFR signaling, Argos, whereas the overexpression of dSpry induced a phenotype similar to that exhibited by EGFR loss-of-function mutants (4). In addition, dSpry function is required for the proper development of other tissues in Drosophila that require DER signaling, including embryonic chordotonal organ precursors, the wing imaginal disk, midline glia, and ovarian follicle cells (5, 6). Overexpression of dSpry in these tissues mimics a DER loss-of-function phenotype, whereas loss of dSpry leads to a hyperactivation of the DER pathway.
The activation of DER by EGF leads to the stimulation of the Ras/mitogen-activated protein (MAP) kinase pathway (7). In Drosophila, dSpry seems to impinge on the DER pathway upstream of MAP kinase as overexpression of dSpry leads to a decrease in activated MAP kinase (6). Epistasis experiments have indicated that during wing development dSpry is acting downstream of the receptor and at or below the level of Raf (6). However, genetic analysis of eye development has placed the level of inhibition by dSpry upstream of Ras (4). The expression of dSpry is induced by DER signaling, indicating the involvement of dSpry in the negative feedback control of DER signaling (4–6). In addition to the inhibitory effects of dSpry on DER signaling, genetic and biochemical studies have shown that it also negatively regulates Drosophila tracheal branching by interfering with Branchless fibroblast growth factor (FGF) signaling (8). Although in the Drosophila tracheal system dSpry acts non-cell-autonomously to repress FGF signaling (8), in the Drosophila eye system, dSpry acts cell autonomously to antagonize EGFR signaling (4, 5).
The Drosophila Sprouty gene encodes a 63-kD membrane-associated protein with a unique carboxyl-terminal cysteine-rich domain that is highly conserved in the four vertebrate Sprouty homologs (Spry1-4) (9, 10). The biological role of vertebrate Sprouty seems to be evolutionarily conserved as murine Spry2 has been shown to interfere with the FGF-mediated processes of lung morphogenesis, and murine Spry2 and Spry4 inhibit FGF-mediated bone outgrowth and limb patterning (9–11). Murine Spry4 has also been shown to inhibit vascular endothelial growth factor (VEGF)-mediated angiogenesis and prevent FGF- or VEGF-induced MAP kinase activation (12). In mammalian cells, EGF stimulation induces the expression of Spry2 and Spry4 (13). However, the role of Sprouty proteins in EGF-mediated signaling in mammalian cells remains unclear. In this study we have investigated the effects of human Spry2 (hSpry2) and Spry1 (hSpry1) on EGF-mediated extracellular signal-regulated kinase 2 (ERK2) MAP kinase activation in human cells. We demonstrate that hSpry2 can exert two opposing effects on EGF-induced ERK2 MAP kinase activation in a domain-dependent manner. Whereas the highly conserved cysteine-rich domain is able to inhibit EGF-induced MAP kinase activation, the amino-terminal domain potentiates EGF-induced MAP kinase activation by interfering with EGFR ubiquitination and down-regulation.
Materials and Methods
Cell Culture and Transient Transfection.
HeLa cells were cultured in DMEM supplemented with 10% FBS in a humidified incubator with 5% CO2 at 37°C. Subconfluent HeLa cells were transfected with the indicated plasmids, using the standard calcium phosphate method. The precipitate was left on the cells for 10 h, and cells were allowed to recover for 12 h in DMEM supplemented with 10% FBS. Chinese hamster ovary (CHO) cells were cultured in Ham's F-12 medium supplemented with 10% FCS. For transfection, CHO cells were incubated with Fugene6 (Roche Molecular Biochemicals) for 24 h and then serum-deprived for 16 h.
EGF Stimulation.
Cells were serum-deprived for 2 h and then incubated with EGF stimulation medium [DMEM containing 25 mM Hepes (pH 7.4) and 10 ng/ml recombinant human EGF] at 4°C for 15 min. The stimulation medium was subsequently removed, and the cells were incubated with DMEM at 37°C for the indicated times. To stop stimulation, cells were washed with ice-cold PBS and kept on ice.
EGFR Ubiquitination Assay.
Serum-deprived CHO cells were washed twice with cold PBS and lysed in coimmunoprecipitation buffer (50 mM Tris, pH 7.5/150 mM NaCl/10% glycerol/1% Nonidet P-40/1 mM EDTA/1 mM phenylmethanesulfonyl fluoride/1 mM NaV04/10 μg/ml pepstatin/10 μg/ml aprotinin/10 μg/ml leupeptin/10 mM benzamidine). The lysates were clarified at 14,000 × g for 15 min and then incubated with anti-EGFR mAb for 1 h at 4°C. Subsequently, the immune complexes were incubated with protein A Sepharose beads for 30 min. The immunoprecipitates were washed 3 times in cold lysis buffer and then resuspended in SDS sample buffer. The samples were resolved by SDS/PAGE.
Cloning and Plasmid Construction.
cDNAs encoding human Spry1 and Spry2 were isolated by reverse transcription–PCR. Total RNA was prepared from HeLa cells by the LiCl-urea method (14). All Sprouty constructs and the ERK2 construct were cloned into a hemagglutinin (HA) tag-containing mammalian expression vector (pCGN), and Raf-CAAX and HRasV12 were cloned into a T7 tag-containing mammalian expression vector (pCGT), using standard PCR and molecular cloning techniques. The human PLC-γ1 cDNA was kindly provided by M. Mohammadi (New York University, School of Medicine).
ERK2 MAP Kinase Assay.
ERK2 immunocomplex assay was performed as described by using myelin basic protein (MBP) as a substrate (15). The amount of radiolabeled MBP was quantified with a PhosphorImager (Molecular Dynamics).
Abs and Reagents.
Anti-c-Cbl and anti-EGFR for Western blotting was purchased from Santa Cruz Biotechnology, anti-ERK2 was purchased from Cell Signaling (Beverly, MA), anti-T7 was purchased from Novagen, and the goat anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary Abs were from ICN. Polyclonal anti-Spry Abs were generated by Cocalico Biologicals (Reamstown, PA) with a fragment of hSpry2 corresponding to amino acids 1–172. The anti-EGFR and anti-PLC-γ1 Abs used for immunoprecipitations were kindly provided by Joseph Schlessinger. Human recombinant EGF was obtained from GIBCO. Protein A Sepharose was obtained from Sigma–Aldrich. Mixed monoclonal anti-PLC-γ1 and anti-phosphotyrosine (4G10) were purchased from Upstate Biotechnology (Lake Placid, NY) Ab.
Western Blotting and Coimmunoprecipitation.
HeLa cells were lysed in 1 ml of RIPA buffer (150 mM NaCl/10 mM Tris⋅HCl, pH 7.4/1% Triton X-100/0.5% deoxycholate/0.1% SDS/1 mM EDTA/50 mM NaF), and lysates were incubated with 2 μg/ml of the indicated Abs for 45 min, followed by a 45-min incubation with 60 μl of a 50% slurry protein A Sepharose beads at 4°C. The immunocomplexes were resolved by SDS/PAGE, and the proteins were transferred to nitrocellulose membranes. Membranes were incubated for 30 min at room temperature in blocking buffer containing 5% BSA. The anti-HA Ab (1:10,000), anti-ERK2 (1:1,000), anti-T7 (1:10,000), anti-hSpry (1:2,500), antiphosphotyrosine (1:1000), anti-EGFR (1:200), or anti-PLC-γ1 (1:1000) primary Abs were added to the blocking buffer, and blots were incubated for 1 h at room temperature. The blots were washed in blocking buffer containing 5% milk and then incubated with either goat anti-mouse horseradish peroxidase (HRP)-conjugated (1:10,000) or goat anti-rabbit HRP-conjugated (1:5,000) secondary Abs. Reactive proteins were detected with an enhanced chemiluminescence reagent (NEN).
Results
Expression of hSpry1 and hSpry2 Potentiates EGF-Induced MAP Kinase Activation.
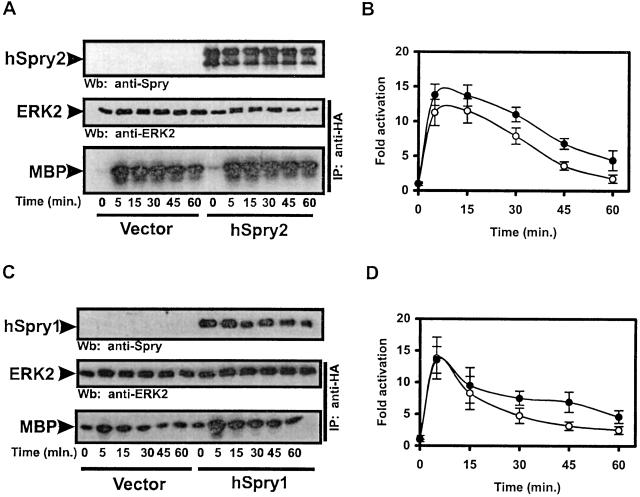
To test the effect of hSpry2 on EGF signaling, we investigated the effects of ectopically expressed hSpry2 on EGF-induced ERK2 MAP kinase activation, hereafter referred to as MAP kinase. HeLa cells were transiently transfected with HA-tagged ERK2 and HA-tagged hSpry2 constructs, and MAP kinase activity was measured by an immunocomplex kinase assay with MBP as a substrate. In the absence of hSpry2, MAP kinase activation induced by EGF reached a peak 5 min after the addition of the growth factor and returned to basal levels within 1 h (Fig. 1 A and B). The expression of hSpry2 had no effect on the magnitude of ERK2 activation by EGF. However, a small but reproducible increase in the duration of EGF-induced MAP kinase activation was observed in hSpry2-expressing cells. These observations differ from the genetic data implicating dSpry in the negative regulation of EGF-mediated MAP kinase activation (4, 6). That this difference does not reflect a unique aspect of the experimental system used is indicated by the fact that the lack of an inhibitory effect by hSpry2 expression on MAP kinase activation was observed in a number of different cell types including COS1, 293T, and NIH 3T3 cells (data not shown). Moreover, the expression of a different isoform of human Sprouty, hSpry1, had the same effect on the kinetics of EGF-induced MAP kinase activation as hSpry2 (Fig. 1 C and D). Thus, the expression of hSpry1 and hSpry2 protein in mammalian cells does not interfere with the activation of MAP kinase by EGF.
Figure 1.
The expression of hSpry1 and hSpry2 potentiates EGF-induced MAP kinase activation. Serum-deprived HeLa cells coexpressing HA-ERK2 and vector, HA-hSpry2 (A and B), or HA-hSpry1 (C and D) were stimulated with 10 ng/ml of EGF for the indicated times. MAP kinase activation was measured by an immunocomplex kinase assay with MBP as a substrate. (A and C) Expression levels of hSpry proteins and ERK2 were determined by Western blotting (Wb), using anti-Spry and anti-ERK2 Abs, respectively. (B and D) Quantitation of the kinetics of MAP kinase activation by EGF in the absence (open circles) or presence (closed circles) of hSpry proteins. The amount of phosphorylated MBP was determined with a PhosphorImager and is presented in arbitrary units as the fold activation over unstimulated vector-expressing cells. Shown are the averages of five independent experiments ± SDs.
The Carboxyl-Terminal Cysteine-Rich Domain and the Amino-Terminal Domain of hSpry2 Have Opposing Effects on EGF-Induced MAP Kinase Activation.
There are two main regions of homology between hSpry2 and dSpry proteins. The most conserved region corresponds to a carboxyl-terminal cysteine-rich domain (amino acids 179–301 in hSpry2 and 380–503 in dSpry) where the degree of homology is 51%. The second region resides in the amino-terminal domain of the proteins and corresponds to amino acids 36–60 in hSpry2 and amino acids 179–205 in dSpry (8). To investigate the possible role of these two regions of homology in EGF signaling, HA-tagged truncation mutants were constructed that contained either the amino-terminal homology region (residues 1–172, hSpry2-172) or the cysteine-rich domain (residues 176–315, hSpry2-Δ175). These constructs were assayed for their ability to influence the activation of MAP kinase by EGF. Transiently transfected HeLa cells expressing hSpry2-172 displayed an enhancement in the level of MAP kinase activation in response to EGF stimulation at all time points measured (Fig. 2 A and B). Conversely, when hSpry2-Δ175 was ectopically expressed there was a marked attenuation of EGF-induced MAP kinase activation (Fig. 2 C and D). Similar results were obtained after the expression of the cysteine-rich domain of hSpry1 (residues 177–319 hSpry1-Δ177, data not shown). These observations suggest that an inhibitory activity, with regards to EGFR signaling, resides in the carboxyl terminal of hSpry1 and hSpry2, whereas an opposing stimulatory activity is located within the amino terminal. The mutual antagonism between the amino- and carboxyl-terminal domains could explain the lack of an inhibitory effect of the full-length hSpry proteins on EGF-induced MAP kinase activation. Indeed, the inhibitory effect of the cysteine-rich domain on MAP kinase activation could be suppressed by the coexpression of the amino-terminal domain (data not shown).
Figure 2.
The carboxyl-terminal cysteine-rich and the amino-terminal domains of hSpry2 have opposing effects on EGF-induced MAP kinase activation. Serum-deprived HeLa cells coexpressing HA-ERK2 and vector, HA-hSpry2-172 (A and B),or HA-hSpry-Δ175 (C and D) were stimulated with 10 ng/ml of EGF for the indicated times. MAP kinase activation was measured by an immunocomplex kinase assay with MBP as a substrate. (A and C) Expression levels of HA-hSpry2-172, HA-hSpry-Δ175, or HA-ERK2 were determined by Wb, using anti-hSpry, anti-HA, and anti-ERK2 Abs, respectively. (B and D) Quantitation of the kinetics of MAP kinase activation by EGF in the absence (open circles) or presence (closed circles) of hSpry proteins. The amount of phosphorylated MBP was determined with a PhosphorImager and is presented in arbitrary units as the fold activation over unstimulated vector-expressing cells. Shown are the averages of five independent experiments ± standard deviations.
The Cysteine-Rich Domain of hSpry2 Acts Upstream of Ras.
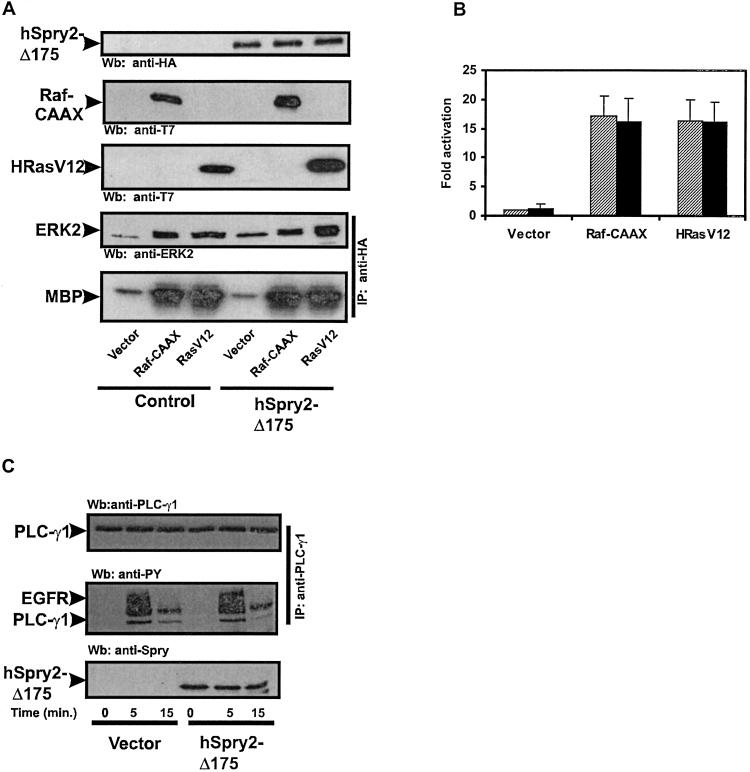
It has been shown that during Drosophila compound eye development, dSpry inhibits MAP kinase activation upstream of Ras and downstream of the receptor (4). To determine the level at which the cysteine-rich domain of hSpry2 is inhibiting EGF-mediated MAP kinase activation, constitutively active forms of Ras (HRasV12) or Raf (Raf-CAAX) were coexpressed with the cysteine-rich domain of hSpry2 and MAP kinase activation was measured. As illustrated in Fig. 3 A and B, the cysteine-rich domain of hSpry2 was unable to inhibit either the Raf-CAAX- or HRasV12-mediated activation of MAP kinase. Similar results were obtained with the cysteine-rich domain of hSpry1-Δ177 (data not shown). These data place the level of inhibition by the cysteine-rich domain of hSpry2 downstream of the EGFR and upstream of both Raf and Ras. To investigate whether hSpry2-Δ175 can influence other downstream effectors of EGFR signaling, this construct was assayed for its ability to effect EGF-mediated activation of PLC-γ1. HeLa cells were transiently transfected with either vector or hSpry2-Δ175 and human PLC-γ1. The transfection efficiency under the conditions used was greater than 90%. The cells were then stimulated with EGF, and PLC-γ1 activation was monitored by assessing its tyrosine phosphorylation state by means of immunoprecipitating with anti-PLC-γ1 Ab and immunoblotting with antiphosphotyrosine Ab. The expression of hSpry2-Δ175 had no effect on EGF-mediated tyrosine phosphorylation of PLC-γ1 (Fig. 3C), indicating that the cysteine-rich domain is not acting in a broad, indiscriminate manner to block EGF signaling but rather interferes specifically with EGFR-mediated activation of the Ras/MAP kinase pathway.
Figure 3.
The cysteine-rich domain of hSpry2 acts upstream of Ras. (A) HeLa cells cotransfected with HA-ERK2 in the absence or presence of HA-hSpry-Δ175, and vector, T7-Raf-CAAX, or T7-HRasV12. Transfected cells were serum-deprived for 16 h, and MAP kinase activation was measured by an immunocomplex kinase assay with MBP as a substrate. Expression levels of ectopically expressed proteins were determined by the Wb of cell lysates with the indicated Abs. (B) Quantitation of the level of MAP kinase activity induced by the transfection of vector, T7-Raf-CAAX, or T7-HRasV12 in the absence (shaded bars) or presence of HA-hSpry2-Δ175 (solid bars). The amount of phosphorylated MBP was determined with a PhosphorImager and is presented in arbitrary units as the fold activation over serum-deprived vector-expressing cells. Shown are the averages of five independent experiments ± SDs. (C) Serum-starved HeLa cells coexpressing either vector or HA-hSpry2-Δ175 and human PLC-γ1 were stimulated with 10 ng/ml of EGF for the indicated times. PLC-γ1 was immunoprecipitated (IP) with anti- PLC-γ1 Ab and detected by Wb with antiphosphotyrosine Ab (PY). The slower migrating tyrosine-phosphorylated band corresponds to the activated EGFR that was coprecipitated with PLC-γ1. Expression levels of HA-hSpry2-Δ175 or PLC-γ1 were determined by using anti-HA and anti-PLC-γ1 Abs, respectively. The results shown are from a single experiment that is representative of four independent experiments.
The Interaction of hSpry2 with c-Cbl Influences its Ability to Negatively Modulate EGF-Induced MAP Kinase Activation.
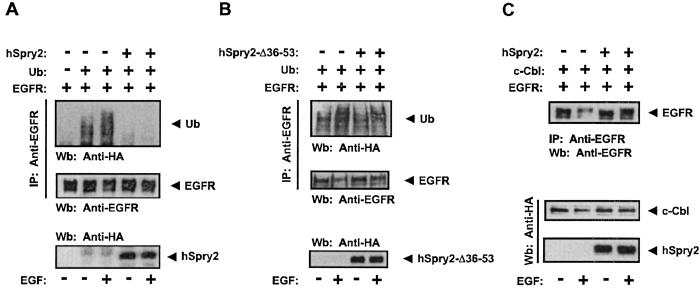
Both hSpry2 and dSpry have been shown to bind constitutively to the protooncogene c-Cbl. This interaction is mediated by the amino-terminal conserved region of hSpry2 and dSpry (residues 36–53 in hSpry2 and 179–205 in dSpry) and the RING finger domain of c-Cbl (16). c-Cbl functions as a negative regulator of receptor tyrosine kinases by targeting activated receptors for ubiquitination and lysosomal degradation (17, 18). To investigate the functional significance of hSpry2 binding to c-Cbl, we constructed an hSpry2 deletion mutant (hSpry2-Δ36-53) that lacks the c-Cbl binding domain. In agreement with an earlier study (16), this deletion mutant was defective in binding to ectopically expressed c-Cbl (Fig. 4A) as well as endogenous c-Cbl (data not shown). In contrast to the full-length hSpry2, the hSpry2-Δ36-53 construct was able to inhibit MAP kinase activation by approximately 50% (Fig. 4 B and C). These results suggest that the interaction of hSpry2 with c-Cbl interferes with the ability of hSpry2 to inhibit EGF signaling. Furthermore, these observations illustrate that the negative regulatory function of the cysteine-rich domain of hSpry2 can be exerted in the context of the full-length protein.
Figure 4.
The interaction of hSpry2 with c-Cbl influences its ability to negatively modulate EGF-induced MAP kinase activation. (A) HeLa cells transfected with HA-c-Cbl and vector, HA-hSpry2, HA-hSpry2-172, or HA-hSpry2-Δ36-53 were lysed and analyzed by immunoprecipitation with anti-c-Cbl Ab (IP) and Wb with the indicated Abs. (B) HeLa cells transfected with HA-ERK2 and either vector or HA-hSpry2-Δ36-53 were stimulated with 10 ng/ml of EGF for the indicated times. MAP kinase activation was measured by an immunocomplex kinase assay with MBP as a substrate. Expression levels of HA-hSpry2-Δ36-53 and HA-ERK2 were determined by Wb, using anti-Spry or anti-ERK2 Abs, respectively. (C) Quantitation of the kinetics of MAP kinase activation by EGF in the absence (open circles) or presence (closed circles) of HA-hSpry2-Δ36-53 protein. The amount of phosphorylated MBP was determined with a PhosphorImager and is presented in arbitrary units as the fold activation over unstimulated vector-expressing cells. Shown are the averages of three independent experiments ± SDs.
hSpry2 Inhibits EGFR Ubiquitination.
The targeting of activated EGFR for ubiquitination by c-Cbl is mediated by its RING finger domain that acts as an E3 protein ubiquitin ligase (19–21). Because hSpry2 has been shown to bind directly to the RING finger domain of c-Cbl it would be predicted to interfere with c-Cbl-dependent EGFR ubiquitination. To test this idea, we investigated the effects of hSpry2 expression on EGFR ubiquitination. CHO cells, which lack endogenous EGFR, were cotransfected with the EGFR and HA-ubiquitin with or without hSpry2. The EGFR was immunoprecipitated, and ubiquitination was assessed by immunoblotting with anti-HA Ab. Consistent with earlier findings (17), the ubiquitination of EGFR was enhanced after addition of EGF (Fig. 5A). The expression of hSpry2 induced a marked inhibitory effect on EGFR ubiquitination. In contrast, the hSpry2-Δ36-53 deletion mutant that does not interact with c-Cbl had no effect on EGFR ubiquitination (Fig. 5B), indicating that the antagonistic effect of hSpry2 on EGFR ubiquitination is a result of its interaction with c-Cbl. The inhibitory effect of hSpry2 on EGFR ubiquitination was accompanied by the inhibition of receptor degradation, which could be clearly observed in the presence of overexpressed c-Cbl (Fig. 5C). The interference of hSpry2 with ligand-induced EGFR down-regulation provides a mechanistic explanation for the inability of full-length hSpry2 to exert an inhibitory effect on EGF-mediated MAP kinase activation and for the enhancement of EGF-mediated MAP kinase activation induced by the expression of hSpry2 amino-terminal region.
Figure 5.
hSpry inhibits EGFR ubiquitination and degradation through its c-Cbl binding domain. (A and B) CHO cells were transiently transfected with expression vectors encoding the EGFR, HA-ubiquitin, and either HA-hSpry2 (A) or HA-hSpry2-Δ36-53 (B). Forty-eight hours after transfection, the cells were incubated with or without EGF (10 ng/ml) for 10 min. Subsequently, the cells were lysed and analyzed by IP and Wb with the indicated Abs. (C) CHO cells were transiently transfected with expression vectors encoding EGFR, HA-c-Cbl, and HA-hSpry2. The cells were stimulated with EGF as described in A and B and analyzed by IP and Wb with the indicated Abs.
Discussion
EGFR-mediated signaling is critical for the growth and development of multicellular organisms (22). Genetic studies in Drosophila suggest that dSpry negatively regulates EGFR signaling pathways in multiple tissues by interfering with EGF-mediated Ras/MAP kinase activation (4, 6). However, the precise mechanisms by which dSpry inhibits EGFR signaling are poorly understood. In mammalian cells, murine Sprouty proteins (mSpry) and hSpry2 have been shown to inhibit EGF-induced cell migration and proliferation (23, 24), but the biochemical basis for these effects is unknown. In the present study we have found that ectopic expression of either hSpry1 or hSpry2 did not interfere with EGF-mediated activation of MAP kinase in mammalian cells. Although these observations seem to be contradictory to the genetic evidence for the function of dSpry, they are in agreement with a recent study documenting the lack of an inhibitory effect of either mSpry1 or mSpry2 on EGF-induced MAP kinase activation in endothelial cells (24).
In contrast to the full-length forms of hSpry1 and hSpry2, their truncated forms encompassing the carboxyl-terminal cysteine-rich domain exerts a pronounced inhibitory effect on EGF-mediated MAP kinase activation. The cysteine-rich domain comprises the most highly conserved region between vertebrate and invertebrate Sprouty proteins (8) and has been implicated in the membrane targeting of both Drosophila and human Sprouty proteins (4, 25). Therefore, it is likely that the capacity of this domain to interfere with EGF-induced MAP kinase activation in mammalian cells represents a conserved inhibitory mechanism. This possibility is also supported by our observation that, like dSpry, the cysteine-rich domain of hSpry2 acts downstream of the EGFR and upstream of Ras. Moreover, the cysteine-rich domain is necessary for the inhibition of cellular migration in response to serum, EGF, FGF, and platelet-derived growth factor by hSpry2 (23), and the cysteine-rich domain of Xenopus Sprouty is sufficient to block FGF-mediated PLC-γ activation (26). Taken together, these findings indicate that the inhibitory activity of Sprouty on receptor tyrosine kinase-signaling proteins is conferred by the highly conserved cysteine-rich domain. Although the molecular basis for the inhibitory effect of the cysteine-rich domain remains to be determined, it is noteworthy that this effect is exerted only in the context of EGF-induced MAP kinase activation, suggesting that the cysteine-rich domain of hSpry proteins targets regulatory events that specifically control the coupling of activated EGFR with the Ras/MAP kinase signaling pathway.
The amino-terminal domains of Sprouty proteins are highly divergent not only between the invertebrate and vertebrate proteins, but also within the four vertebrate proteins themselves (9). Our data with the amino-terminal domain of hSpry2 have demonstrated that this region is a positive modulator of EGF-dependent MAP kinase activation. This effect results from the inhibition of EGFR ubiquitination and down-regulation. That the potentiating effect of the amino-terminal domain of hSpry2 on EGF-induced MAP kinase activation is antagonistic to the inhibitory effect of the cysteine-rich domain suggests that the negative regulation of EGFR signaling by Sprouty might be best accomplished when receptor levels are limiting. In support of this idea, it has been shown that in Drosophila the EGFR signaling defect induced by dSpry can be rescued by the overexpression of the EGFR (4).
We have demonstrated that the potentiating effects of hSpry2 on EGF-induced MAP kinase activation can be attributed to its interaction with c-Cbl. Because this interaction occurs through the binding of a small amino-terminal region of hSpry2 to the RING finger domain of c-Cbl (16), it is presumed to interfere with the E3 enzymatic activity of the RING finger. The c-Cbl binding region of hSpry is conserved in dSpry, and the latter has been shown to also interact with Drosophila Cbl (dCbl) (16). Yet genetically, dSpry seems to function solely as a negative regulator of EGFR/MAP kinase signaling. What could account for the apparent differences between the effects of Cbl binding on the activities of mammalian and Drosophila Sprouty proteins? One possibility is that in the EGFR-dependent developmental processes that are influenced by Sprouty, the level of EGFR activity is limiting even in the presence of dSpry. Indeed, the influence of dCbl on EGFR signaling is extremely sensitive to the dosage of genes that participate in this pathway, and, on a wild-type background, overexpression of dCbl has no visible effect on EGFR signaling in the eye (27, 28). Another possibility is that the interaction of Cbl with Sprouty is differentially regulated in Drosophila and mammalian cells. The diverse sequences in the amino-terminal domains of mammalian and Drosophila Sprouty proteins may direct different protein–protein interactions or posttranslational modifications that in turn could influence Cbl binding. Finally, the functional consequences of changes in receptor ubiquitination might be different in Drosophila and mammalian systems. Whereas in mammalian cells c-Cbl-mediated ubiquitination promotes EGFR down-regulation (17, 29), a recent study has demonstrated a role for deubiquitination in stimulating receptor internalization in Drosophila (30). In this study, the Drosophila Fat facet protein, which is a member of the UBP class of deubiquitinating enzymes and plays a critical role in Drosophila eye development, has been found to activate endocytosis (30). Biochemical analysis of the effects of dSpry on the ubiquitination and endocytic sorting of the EGFR in Drosophila will be required to distinguish between these possibilities.
Our observations indicate that, depending on its interactions with c-Cbl, hSpry2 can function as a positive or negative regulator of EGFR/MAP kinase signaling. A dual function of this kind could be used to set a temporal limit to the inhibitory effect of Sprouty on EGF signaling. For example, by analogy to dSpry, high levels of EGFR signaling in mammalian cells would induce the expression of Sprouty proteins, which could act initially as inhibitors and subsequently as potentiators of EGFR signaling. The nature of the effects exerted by Sprouty and their sequential order would depend on the availability of proteins that interact with Sprouty and regulate its function. In this manner, cells can regain the capacity to be activated by EGF while Sprouty is present. This mechanism would be of particular importance in biological systems where there is a requirement for the repeated use of EGF signaling.
Acknowledgments
We thank all members of the laboratory for helpful advice and discussions and James Keller for the generation of anti-hSpry Abs. A.B.H. is supported by National Institutes of Health Training Grant T32DK07521-14. D.B.S. acknowledges support by National Institutes of Health Grants CA55360 and CA28146.
Abbreviations
- MAP
mitogen-activated protein
- ERK2
extracellular signal-regulated kinase 2
- EGF
epidermal growth factor
- EGFR
EGF receptor
- HA
hemagglutinin
- MBP
myelin basic protein
- DER
Drosophila homolog of EGFR
- CHO
Chinese hamster ovary
- IP
immunoprecipitated/immunoprecipitation
- Wb
Western blotting
References
- 1.Schlessinger J. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Freeman M. Nature (London) 2000;408:313–319. doi: 10.1038/35042500. [DOI] [PubMed] [Google Scholar]
- 3.Freeman M. Development (Cambridge, UK) 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- 4.Casci T, Vinos J, Freeman M. Cell. 1999;96:655–665. doi: 10.1016/s0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- 5.Kramer S, Okabe M, Hacohen N, Krasnow M, Hiromi Y. Development (Cambridge, UK) 1999;126:2515–2525. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- 6.Reich A, Sapir A, Shilo B-Z. Development (Cambridge, UK) 1999;126:4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- 7.Chang H, Karim F, O'Neill E, Rebay I, Solomon N, Therrien M, Wassarman D, Wolff T, Rubin G. Cold Spring Harbor Symp Quant Biol. 1994;59:147–153. doi: 10.1101/sqb.1994.059.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow M. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 9.Minowada G, Jarvis L, Chi C L, Neubuser A, Sun X, Hacohen N, Krasnow M, Martin G R. Development (Cambridge, UK) 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 10.Tefft J D, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Crowe D L, Warburton D. Curr Biol. 1999;9:219–222. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- 11.Mailleux A, Tefft D, Ndiaye D, Itoh N, Thiery J P, Warburton D, Bellusci S. Mech Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee S H, Schloss D J, Jarvis L, Krasnow M A, Swain J L. J Biol Chem. 2001;276:4128–4133. doi: 10.1074/jbc.M006922200. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki A, Taketomi T, Wakioka T, Kato R, Yoshimura A. J Biol Chem. 2001;276:36804–36808. doi: 10.1074/jbc.C100386200. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 15.Walsh A B, Bar-Sagi D. J Biol Chem. 2001;276:15609–15615. doi: 10.1074/jbc.M010573200. [DOI] [PubMed] [Google Scholar]
- 16.Wong E S M, Lim J, Low B C, Chen Q, Guy G R. J Biol Chem. 2001;276:5866–5875. doi: 10.1074/jbc.M006945200. [DOI] [PubMed] [Google Scholar]
- 17.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon W Y, Beguinot L, Geiger B, Yarden Y. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake S, Lupher M L, Jr, Druker B, Band H. Proc Natl Acad Sci USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joazeiro C A n P, Wing S S, Huang H-k, Leverson J D, Hunter T, Liu Y-C. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 20.Yokouchi M, Kondo T, Houghton A, Bartkiewicz M, Horne W C, Zhang H, Yoshimura A, Baron R. J Biol Chem. 1999;274:31707–31712. doi: 10.1074/jbc.274.44.31707. [DOI] [PubMed] [Google Scholar]
- 21.Waterman H, Levkowitz G, Alroy I, Yarden Y. J Biol Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- 22.Moghal N, Sternberg P W. Curr Opin Cell Biol. 1999;11:190–196. doi: 10.1016/s0955-0674(99)80025-8. [DOI] [PubMed] [Google Scholar]
- 23.Yigzaw Y, Cartin L, Pierre S, Scholich K, Patel T B. J Biol Chem. 2001;276:22742–22747. doi: 10.1074/jbc.M100123200. [DOI] [PubMed] [Google Scholar]
- 24.Impagnatiello M-A, Weitzer S, Gannon G, Compagni A, Cotten M, Christofori G. J Cell Biol. 2001;152:1087–1098. doi: 10.1083/jcb.152.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim J, Wong E S M, Ong S H, Yusoff P, Low B C, Guy G R. J Biol Chem. 2000;275:32837–32845. doi: 10.1074/jbc.M002156200. [DOI] [PubMed] [Google Scholar]
- 26.Nutt S, Dingwell K, Holt C, Amaya E. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai L-M, Barcelo G, Schupbach T. Cell. 2000;103:51–61. doi: 10.1016/s0092-8674(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 28.Meisner H, Daga A, Buxton J, Fernandez B, Chawla A, Banerjee U, Czech M. Mol Cell Biol. 1997;17:2217–2225. doi: 10.1128/mcb.17.4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lill N L, Douillard P, Awwad R A, Ota S, Lupher M L, Jr, Miyake S, Meissner-Lula N, Hsu V W, Band H. J Biol Chem. 2000;275:367–377. doi: 10.1074/jbc.275.1.367. [DOI] [PubMed] [Google Scholar]
- 30.Cadavid A, Ginzel A, Fischer J. Development (Cambridge, UK) 2000;127:1727–1736. doi: 10.1242/dev.127.8.1727. [DOI] [PubMed] [Google Scholar]