Abstract
Chronic ultraviolet (UV) exposure is the primary cause of skin photoaging, leading to wrinkles, pigmentation changes, and loss of dermal elasticity. This systematic review and network meta-analysis evaluated the efficacy and safety of topical compounds for treating skin photoaging. A comprehensive search identified 23 RCTs with 3905 participants, comparing anti-aging agents. Bayesian network meta-analysis showed isotretinoin, retinol, and tretinoin significantly improved fine wrinkles, with isotretinoin ranked highest. Tazarotene was most effective for coarse wrinkles, while glycolic acid reduced roughness. Tretinoin and retinol were superior for hyperpigmentation. Safety analysis indicated tretinoin had the most favorable profile, whereas tazarotene and glycolic acid had higher adverse event risks. Isotretinoin and tretinoin emerged as the most balanced treatments across efficacy and safety. These findings provide evidence-based guidance for clinical decision-making in anti-photoaging therapy and underscore the potential for these agents to be integrated into routine dermatologic practice, particularly for patients seeking effective and well-tolerated topical interventions. However, limitations included limited racial diversity, potential commercial bias, and variability in dermatological assessments. These findings provide evidence-based guidance for clinical decision-making in anti-photoaging therapy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-12597-0.
Keywords: Skin photoaging, Topical compounds, Network meta-analysis, Efficacy, Safety
Subject terms: Public health, Drug discovery, Medical research
Introduction
Skin photoaging is the premature aging of the skin caused by long-term exposure to sunlight. It reflects how environmental damage interacts with the natural aging process. Common signs of photoaging include fine lines, deep wrinkles, uneven pigmentation, and reduced skin elasticity. Unlike intrinsic aging—which is mainly influenced by genetics and time—photoaging is primarily caused by ultraviolet (UV) radiation. UVA rays penetrate deep into the dermis, while UVB rays, although less penetrating, can cause sunburn, DNA damage, and even skin cancer. Together, these effects-sunburn, phototoxic reactions, sensitivity to light, and photoaging—are forms of “skin photodamage” that affect both appearance and skin health1.
Preventive measures like sunscreen and UV-protective clothing are important, but they cannot reverse existing photoaging. This highlights the need for effective topical treatments that can repair or slow down sun- and pollution-related skin damage2,3. However, the growing number of anti-photoaging products has led to inconsistent results in studies. There is currently no standard method to evaluate how well these treatments work, making it hard to compare findings across different studies. In addition, many studies face challenges such as confounding factors like participant age, lifestyle, and environmental exposure. These can affect how well a treatment seems to work. There are also safety concerns, as the long-term effects of some topical treatments remain unclear4.
In light of the aforementioned context, there is a clear need for a systematic and thorough review of the available evidence. This systematic review and network meta-analysis aims to fill that gap by comparing the effectiveness and safety of different topical treatments for photoaging.
Methods
Search strategy and selection criteria
A comprehensive search of PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials was conducted from database inception to June 1, 2025 to identify all randomised controlled trials of anti-ageing skin care ingredients compared with a negative control or another anti-ageing skin care ingredient for daily prevention of ageing. Details of the search strategy are provided in the Appendix (p.7). We also searched the reference lists of the included studies and relevant systematic reviews to identify additional potentially eligible studies.
This systematic review and network meta-analysis programme follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and is registered with PROSPERO (CRD42024528500).
Eligibility criteria
This study focused on evaluating the efficacy of topical monotherapies for facial photoageing. Facial photoageing refers to skin damage and visible ageing primarily caused by chronic sun exposure, and sometimes exacerbated by lifestyle factors. To ensure comparability, the control groups received either a placebo (an inactive topical substance) or an alternative anti-ageing topical agent. Only trials specifically reporting outcomes on facial photodamage or photoageing were included; studies focusing on other skin conditions were excluded unless they presented separate results for facial photoageing.
Inclusion criteria
Participants with facial photodamage: Individuals exhibiting signs of photoageing on the face, such as fine lines, wrinkles, uneven pigmentation, and skin roughness, primarily resulting from sun exposure and lifestyle. It is worth noting that participants with genetic disorders associated with premature skin ageing (e.g., xeroderma pigmentosum, pigmented xerodermoid, progeria) were excluded.
Topical monotherapy: The intervention consisted of a single topical compound (e.g., cream or lotion) applied to the human face to treat photoageing. Topical agents were excluded if they appeared only once across all included treatment or control groups, to avoid data heterogeneity.
Human studies only: Only studies involving human participants were included. Data from animal or in vitro (cell-based) experiments were excluded.
Daily application of treatment: Included participants received daily topical treatment, whether the intervention or placebo/control compound.
Exclusion criteria
Non-photodamage skin conditions: Trials involving skin conditions other than photoageing were excluded unless they provided separate outcome data specific to facial photodamage.
Non-topical or procedural treatments: Studies using photoelectric, laser, injectable treatments, or uncommon systemic medications were excluded to ensure a focus on topical therapy.
Genetic disorders related to skin ageing: Participants with conditions such as xeroderma pigmentosum, pigmented xerodermoid, or progeria were excluded to avoid confounding effects from atypical ageing pathways.
Small sample size: To reduce statistical bias and improve the reliability of effect estimates, studies with fewer than 30 participants were excluded. Small studies often lack statistical power, have wider confidence intervals, and are more prone to random error and publication bias. This decision was based on an analysis of the sample size distribution across all initially considered studies, which revealed that 40% had fewer than 30 participants. Setting the threshold at 30 therefore ensured adequate statistical power while excluding studies with very small sample sizes.
Trials involving combination therapies or systemic treatments were excluded.
Outcomes’ measures
The primary outcomes were the number of participants who demonstrated improvement in specific clinical indicators of skin photoaging, including: fine wrinkles, coarse wrinkles, skin roughness, hyperpigmentation, and safety/tolerability.
Clinical improvements were assessed by trained dermatologists using validated scales, such as:
Fitzpatrick Wrinkle Classification System: A widely used scale that grades facial wrinkles on a scale from 1 (fine wrinkles) to 9 (deep wrinkles and redundant folds), based on wrinkle depth and anatomical location.
5-point Global Dermatologist Assessment Scale: A semi-quantitative scale in which dermatologists rate the severity of skin aging signs (e.g., wrinkles, pigmentation, texture) from 0 (none) to 4 (severe).
Evaluations were conducted at baseline and at the end of treatment. A participant was defined as a positive case (i.e., showing improvement) if they exhibited a clinically and statistically significant reduction in at least one of the measured aging indicators (e.g., a ≥ 1-point reduction on either scale, confirmed by statistical testing). Those who did not meet this criterion were classified as negative cases.
Safety outcomes were assessed by recording adverse events (e.g., irritation, redness, dryness) throughout the study period, and rated using a standardized dermatological adverse event checklist.
Study selection
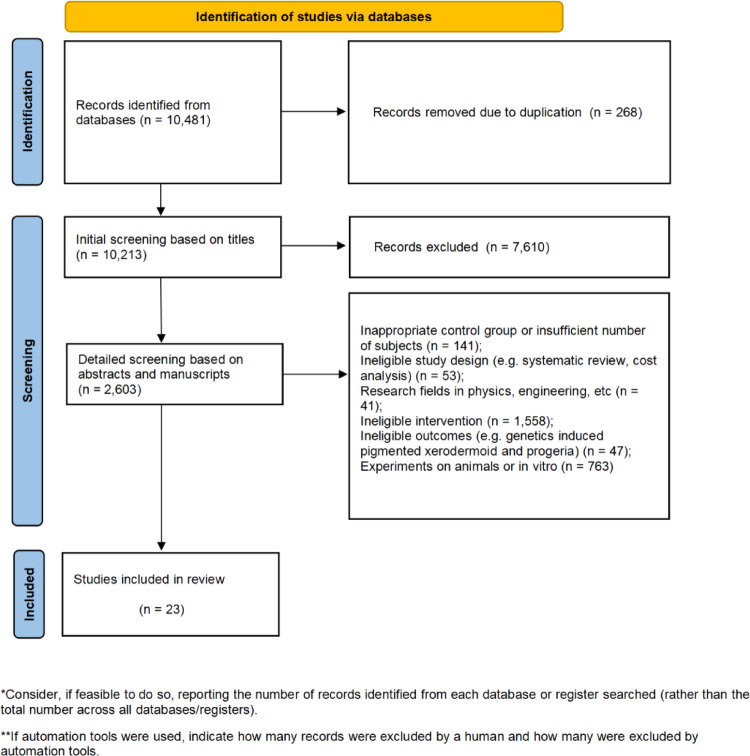
After duplicate studies have been removed, two authors independently screen titles and abstracts for eligibility. For studies that both authors agree meet the inclusion criteria, the full text will be obtained and reviewed for inclusion. Disagreements about inclusion in the final review will be resolved by consensus. If consensus cannot be reached, a third author is consulted. The study screening process is documented using the PRISMA flowchart (Fig. 1).
Fig. 1.
PRISMA flowchart.
Data extraction
Data extraction was carried out for all included studies. Two reviewers independently extracted information on study design, study size, and intervention details. Intervention details considered in this paper included route, dose, frequency, and first author, year of publication, study population, sample size, duration of follow-up, control group, measurements, and original unit value. A pre-prepared electronic data collection form Microsoft Excel spreadsheet was used to retrieve information from eligible studies. If data from included studies were missing or incomplete, the corresponding author was contacted by e-mail.
Quality assessment
Two reviewers independently assessed the risk of bias in included studies using the criteria in the Cochrane Handbook for Systematic Reviews of Interventions. For randomised controlled trials, bias due to the randomisation process, bias due to departures from the intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported outcome were assessed and graded as ‘low risk of bias’, ‘some concerns’, or ‘high risk of bias’. The quality of evidence for the outcomes reported in the included studies was assessed using the Grading of Recommendations Assessment, Development and Evaluations (GRADE) framework. Outcomes were assigned to one of four levels of evidence - high, moderate, low, or very low. Discrepancies in quality ratings were resolved by discussion or consensus with a third author.
Data synthesis and analysis
Random effects model was adopted to investigate comparisons providing only direct evidence of compounds versus placebo. Network meta-analyses was the used to explore comparisons contributing both direct and indirect evidence. Network meta-analyses were performed within a Bayesian framework using a Markov chain Monte Carlo method. The number of chains was set to 4. Gibbs sampling was based on 250,000 iterations, with 50,000 iterations removed during the burin-in phase. The Brooks-Gelman-Rubin statistic was used to measure model convergence5 (Appendix p. 13). Rankograms were employed to visually represent the probability distribution of each intervention across all potential rankings, thus facilitating the evaluation of their relative performance and associated uncertainty. The use of rankograms provides insights into the probability of an intervention being the optimal or suboptimal choice, thus facilitating the identification of its relative advantages and disadvantages within the network. By comparing the probability distributions, we were able to gain a deeper understanding of the comparative effectiveness of different interventions in the network meta-analysis6.
Heterogeneity between studies was assessed using the I2 statistic. 25%, 50% and 75% were considered low, medium and high heterogeneity. Overall inconsistency was assessed by comparing network meta-analytic inconsistency models with network meta-analytic consistency models. Divergence Information Criterion (DIC) values 5 units lower indicate significant inconsistency. Local inconsistency was assessed using the node splitting technique5.
We performed a sensitivity analysis based on the results of commercial and academic studies. By comparing results from the two types of studies, we were able to identify potential bias and heterogeneity raised by the commercial interests.
All data were analysed using R statistics (version 4.3.1), ‘gemtc’ package, the ‘rjags’ package and ‘netmeta’ package. Alpha was set to 0.05 bilaterally.
Results
A total of 23 studies with 3905 participants met eligibility criteria and were included in the analysis. The average age of participants was 49.6 years (ranging from 24.0 to 75.2 years). The average proportion of female participants was 93.29% (ranging from 60.0 to 100.0%). The average treatment period lasted 8 weeks (ranging from 4 to 36 weeks). 17 studies7–22, had an all-Caucasian trial population, three studies23–25 included Caucasian, Hispanic, and Asian, two studies26,27, had an all-Asian trial population, and one study28, had a Caucasian, Hispanic, Black, and Asian trial population. It is evident that the accumulated data on photodamage experiments are predominantly focused on the white population, while data on other races or ethnicities remain relatively scarce.
Improvement of fine wrinkle
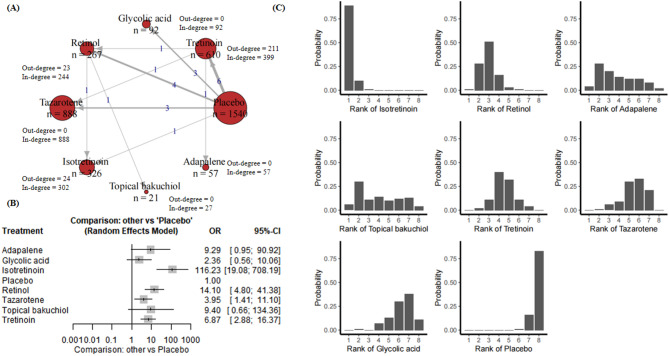
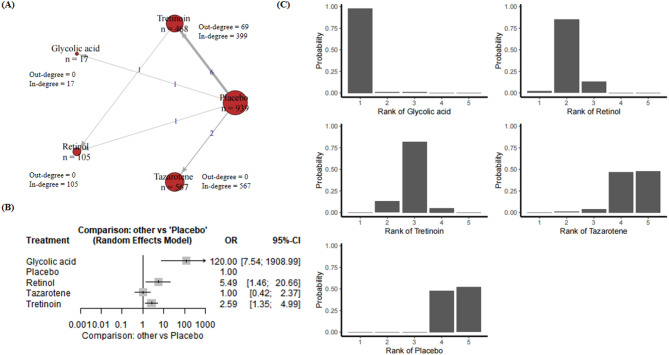
22 RCTs presented results relevant to fine wrinkle. These studies include 3801 subjects, 7 compounds, and placebo (Appendix p. 18). Figure 2A presents a network plot for the fine wrinkle improvement outcome. Twenty-two RCTs including 5750 participants were included in the NMA. Figure 2A demonstrated the treatment-control relationships using a weighted directed network. The thickness of the edge corresponds to the number of trials in direct comparison between the two treatments. The direction of the link indicates control group (starting node) and treatment group (end node). Note that such number is the addition of participants for trials that the node is involved, whichever control group or treatment group the participant is in. Therefore, we further calculate the in-degree and out-degree for each node to clarify the specific number for this node as control group and treatment group. Similar explanations to the treatment comparison network can be applied to other skin aging indicators, therefore, we will not repeat clarifying the meanings of edges and nodes in the rest of this paper.
Fig. 2.
(A) Network plot for fine wrinkle. Nodes are weighted by the addition of participants for trials that the node is involved; (B) Forest plot for fine wrinkle; (C) Rankograms for the effectiveness of interventions on fine wrinkle improvement.
Forest plot in Fig. 2B demonstrates comparisons providing only direct evidence of compounds versus placebo. For fine wrinkle, compared with the placebo group, adapalene was associated with an odds ratio (OR) of 9.29 (95% CI [0.95, 90.92], P = 0.0554), indicating an effect that was close to significance but not statistically significant. Glycolic acid showed an OR of 2.36 (95% CI [0.56, 10.06], P = 0.2441), indicating no significant efficacy. Isotretinoin showed a significant effect with an OR of 116.23 (95% CI [19.08, 708.19], P < 0.0001). Retinol also showed a significant effect with an OR of 14.10 (95% CI [4.80, 41.38], P < 0.0001). Tazarotene was associated with an OR of 3.95 (95% CI [1.41, 11.10], P = 0.0090), indicating significant efficacy. In contrast, topical bakuchiol had an OR of 9.40 (95% CI [0.66, 134.36]), which did not reach statistical significance. Tretinoin showed significant efficacy with an OR of 6.87 (95% CI [2.88, 16.37], P < 0.0001). According to the results, isotretinoin, retinol and tretinoin showed significant effects under the random effects model. Adapalene and tazarotene showed some efficacy, although adapalene was only significant in the fixed effects model. Glycolic acid and topical bakuchiol did not reach significance in either model.
Results of network meta-analyses explored comparisons contributing both direct and indirect evidence, which further reinforced the effectiveness of isotretinoin in treating fine wrinkle, with retinol demonstrating comparable efficacy, ranking as a close second to isotretinoin. Adapalene and topical bakuchiol demonstrated moderate efficacy, with cumulative probabilities situated in the mid-range. In contrast, tretinoin, tazarotene and glycolic acid demonstrated lower cumulative probabilities, indicating a diminished efficacy in the improvement of fine wrinkles. The control group exhibited the lowest cumulative probability, indicating a lack of significant improvement in efficacy (Fig. 2C).
Improvement of coarse wrinkle
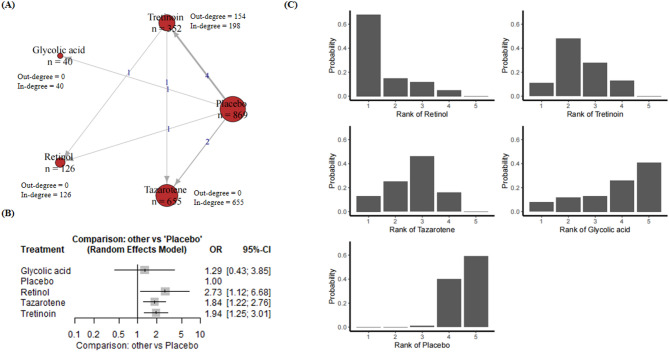
The results regarding coarse wrinkle included 10 RCTs, 2042 people, five compounds, and placebo (Appendix p. 20). Figure 3A presents a network plot for the coarse wrinkle improvement outcome. Ten RCTs including 2042 participants were included in the NMA. Figure 3A demonstrated the treatment-control relationships using a weighted directed network. As the results shown in Fig. 3B, glycolic acid showed an odds ratio (OR) of 12.44 (95% CI [0.66, 234.37], P = 0.0923). Although this indicates a significant effect, the wide confidence interval and a p-value slightly above 0.05 indicate a lack of statistical significance. Isotretinoin had an OR of 5.10 (95% CI [0.59, 43.92], P = 0.1380), showing some effect, but it was also not significant. Retinol had an OR of 3.78 (95% CI [0.90, 15.87], P = 0.0689), which was close to significance but did not meet the 0.05 threshold. Tazarotene was significantly effective with an OR of 4.78 (95% CI [1.84, 12.41], P = 0.0013), indicating a stable effect. Tretinoin had an OR of 1.50 (95% CI [0.76, 2.93], P = 0.2413), which was not significant. Overall, tazarotene showed significant efficacy compared to the control group, while the other treatments showed potential but did not reach statistical significance, suggesting a need for more data or more precise estimates. The heterogeneity between trials was low, indicating good consistency of results.
Fig. 3.
(A) Network plot for coarse wrinkle. Nodes are weighted by the addition of participants for trials that the node is involved; (B) Forest plot for coarse wrinkle; (C) Rankograms for the effectiveness of interventions on coarse wrinkle improvement.
According to rankogram’s ranking of treatments to improve coarse wrinkle, retinol consistently leads the pack with a superior cumulative probability and is undoubtedly the most effective treatment. Tretinoin follows as the next best choice, also showing strong results in reducing the appearance of coarse wrinkle. On the other hand, tazarotene, although behind retinol and tretinoin, remained moderately effective and significantly better than the other treatments. Glycolic acid, although showing some potential for improvement, ranked relatively low compared to other highly effective treatments. In summary, retinol and tretinoin showed the most promise and potential for reducing coarse wrinkle, closely followed by tazarotene with a moderate effect. Glycolic acid, on the other hand, had relatively limited efficacy (Fig. 3C).
Improvement of hyperpigmentation
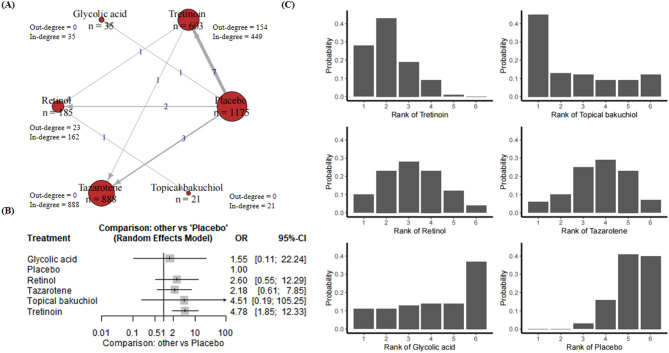
A total of 16 RCTs with 2907 participants were included with regard to the treatment of hyperpigmentation (Appendix p. 23). These studies include six compounds, and placebo (Appendix p. 23). Figure 4A presents a network plot for the hyperpigmentation improvement outcome. Figure 4A demonstrated the treatment-control relationships using a weighted directed network. In the fixed-effects model, retinol (OR 2.03; 95% CI [1.19, 3.45], P = 0.0089 < 0.05), tazarotene (OR 1.94; 95% CI [1.53, 2.47], P < 0.0001) and tretinoin (OR 2.50; 95% CI [1.90, 3.29], P < 0.0001) showed significant efficacy compared to the control group, while glycolic acid (OR 1.55; 95% CI [0.56, 4.24], P = 0.0089) and topical bakuchiol (OR 3.52; 95% CI [0.95, 13.00], P = 0.0592) did not demonstrate significant effects. In the random-effects model, tretinoin (OR 4.78; 95% CI [1.85, 12.33], P = 0.0012 < 0.05) maintained its significant effectiveness, but the effects of the other treatments remained uncertain (Fig. 4B).
Fig. 4.
(A) Network plot for hyperpigmentation. Nodes are weighted by the addition of participants for trials that the node is involved; (B) Forest plot for hyperpigmentation; (C) Rankograms for the effectiveness of interventions on hyperpigmentation improvement.
The analysis of the rankogram for hyperpigmentation revealed that tretinoin exhibited a relatively high cumulative probability, indicating that it may be one of the most effective treatments and has a strong likelihood of ranking among the top options. Furthermore, retinol exhibited a rapid increase in cumulative probability, particularly within the upper echelons of the rankings, thereby indicating its efficacy as a treatment modality. Topical bakuchiol demonstrated a gradual increase in cumulative probability, indicating a position slightly above the mean in terms of effectiveness. The increase in efficacy of glycolic acid was less pronounced in comparison to the other treatments, indicating that its efficacy may be somewhat lower. Tazarotene demonstrated a consistent performance with a moderate cumulative probability, ranking in the middle range. The control group exhibited the lowest cumulative probability, indicating the least effectiveness. In conclusion, tretinoin and topical bakuchiol are the most efficacious treatments for the reduction of hyperpigmentation, while retinol and tazarotene demonstrate moderate efficacy, and glycolic acid is average. It is evident that the control group exhibits the least efficacy (Fig. 4C).
Improvement of roughness
Eleven RCT trails and 2096 participants were included (Appendix p. 26). These studies include five compounds, and placebo (Appendix p. 26). Figure 5A presents a network plot for the roughness improvement outcome. Eleven RCTs including 2096 participants were included in the NMA. Figure 5A demonstrated the treatment-control relationships using a weighted directed network. The fixed effects model shows that glycolic acid (OR 120.00; 95% CI [7.54, 1908.99], P = 0.0007 < 0.05), retinol (OR 5.49; 95% CI [1.46, 20.66], P = 0.0076 < 0.05) and tretinoin (OR 1.91; 95% CI [1.37, 2.66], P < 0.0001) have significant efficacy compared to the control group, and the same statistically significant results were also shown in the fixed effects model (Fig. 5B).
Fig. 5.
(A) Network plot for roughness. Nodes are weighted by the addition of participants for trials that the node is involved; (B) Forest plot for roughness; (C) Rankograms for the effectiveness of interventions on roughness improvement.
The rankogram of roughness improvement showed glycolic acid to be one of the most effective treatments, with a rapid increase in cumulative probability, suggesting that it is likely to be one of the top treatments. Retinol followed with steady growth, especially in the top three rankings, suggesting good efficacy. Tretinoin grew more slowly, suggesting moderate efficacy and a lower ranking compared to the top treatments. Tazarotene and control had the lowest cumulative probabilities and control consistently ranked last, suggesting the lowest efficacy. In summary, glycolic acid and retinol were the most effective treatments for improving skin roughness, with tretinoin showing moderate efficacy. Tazarotene and the control were the least effective and the control was consistently the least effective (Fig. 5C).
Safety
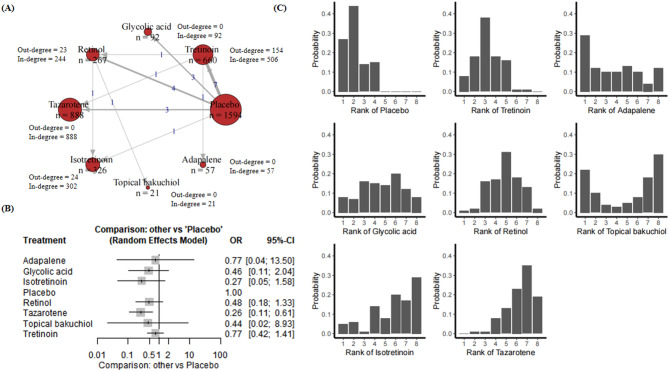
23 RCTs including 3905 participants were included in the NMA. These studies include eight compounds, and placebo (Appendix p. 26). Figure 6A presents a network plot for the safety improvement outcome. Figure 6A demonstrated the treatment-control relationships using a weighted directed network. As the results shown in Appendix p. 29, the odds ratio (OR) was 0.0804 (95% CI [0.0043, 1.5138], P = 0.0923) for glycolic acid and 0.1960 (95% CI [0.0228, 1.6880], P = 0.1380) for isotretinoin, neither of which was significant for safety. The OR were 0.2644 (95% CI [0.0630, 1.1088], P = 0.0689) for retinol and 0.6685 (95% CI [0.3408, 1.3113], P = 0.2413) for tretinoin, suggesting no significant difference in safety. In contrast, tazarotene was statistically significant with an OR of 0.2093 (95% CI [0.0806, 0.5437], P = 0.0013), indicating a worse safety profile compared to the control group. The random effects model confirmed the significance of tazarotene (P = 0.0013), while the other treatments remained non-significant. Tazarotene was the only treatment that showed a significant difference in safety compared to the control group, while the other treatments did not reach statistical significance in terms of adverse events (Fig. 6B).
Fig. 6.
(A) Network plot for safety. Nodes are weighted by the addition of participants for trials that the node is involved; (B) Forest plot for safety; (C) Rankograms for the effectiveness of interventions on safety improvement.
Rankogram (Fig. 6C) was employed to evaluate the prevalence of adverse reactions associated with a diverse array of therapeutic interventions. The findings demonstrated that the control group exhibited the lowest incidence of adverse reactions, thereby establishing the most favourable safety profile. Tretinoin demonstrated a relatively low rate of adverse reactions and a positive safety profile. In contrast, both topical bakuchiol and retinol exhibited an intermediate risk of adverse reactions. Isotretinoin demonstrated a higher likelihood of adverse reactions, while tazarotene exhibited the greatest. In conclusion, from a safety perspective, the control and tretinoin treatments demonstrated the most favourable therapeutic outcomes with the lowest incidence of adverse reactions. Conversely, tazarotene and isotretinoin were found to have an elevated risk of adverse reactions, with tazarotene exhibiting the most unfavourable safety profile. Table 1 summarizes the results of all compound comparisons.
Table 1.
Summary table of compound comparisons.
| Treatment | Improvement of fine wrinkle (OR, 95% CI) | Improvement of coarse wrinkle (OR, 95% CI) | Improvement of hyperpigmentation (OR, 95% CI) | Improvement of roughness (OR, 95% CI) | Safety (OR, 95% CI) |
|---|---|---|---|---|---|
| Adapalene | 9.29 (0.95, 90.92) | – | – | – | 0.77 (0.04, 13.5) |
| Glycolic acid | 2.36 (0.56, 10.06) | 1.29 (0.43, 3.85) | 1.55 (0.11, 22.24) | – | 0.46 (0.11, 2.04) |
| Isotretinoin | 116.23 (19.08, 708.19) | – | – | – | 0.27 (0.05, 1.58) |
| Retinol | 14.10 (4.80, 41.38) | 2.73 (1.12, 6.68) | 2.60 (0.55, 12.29) | 5.49 (1.46, 20.66) | 0.48 (0.18, 1.33) |
| Tazarotene | 3.95 (1.41, 11.10) | 1.84 (1.22, 2.76) | 2.18 (0.61, 7.85) | 1.00 (0.42, 2.37) | 0.26 (0.11, 0.61) |
| Topical bakuchiol | 9.40 (0.66, 134.36) | – | – | – | 0.44 (0.02, 8.93) |
| Tretinoin | 6.87 (2.88, 15.37) | 1.94 (1.25, 3.01) | 4.78 (1.85, 12.33) | 2.59 (1.35, 4.99) | 0.77 (0.42,1.41) |
Quality of included studies
The publication bias funnel plots of the included studies showed overall symmetry (Appendix p. 34). 82.61% and 17.39% of the studies had an overall low and unclear risk of bias. The risk of bias of studies is shown in Appendix p. 37.
Assumption of transitivity, heterogeneity, and inconsistency
The characteristics of participants and treatments were distributed in a similar patterns across studies, as illustrated in the Appendix p. 9. This finding suggests that the transitivity assumption was acceptable. The heterogeneity test indicated varying levels of heterogeneity, with low to moderate levels observed in fine wrinkle (I2 = 2%), coarse wrinkle (I2 = 2%), hyperpigmentation (I2 = 5%), roughness (I2 = 7%) and safety (I2 = 0%). No significant global or local inconsistency was identified (see Appendix p. 28).
Sensitivity analysis
A sensitivity analysis was conducted to evaluate the robustness of commercially sponsored research and purely academic research in terms of drug safety. The heterogeneity across studies ranged from 10 to 25%, indicating a moderate level of variability that, while potentially affecting the precision of pooled estimates, remains within generally acceptable limits for meta-analytical interpretation.
Importantly, subgroup analyses revealed a trend in which commercially sponsored trials more frequently reported lower levels of irritation or adverse events. This raises the possibility of reporting bias associated with funding sources. Previous literature has noted that industry-sponsored trials may be more likely to present favorable safety profiles29. Therefore, potential funding-related bias should be considered when interpreting the safety outcomes of included studies.
Discussion
This meta-analysis evaluated the effectiveness of multiple treatments in improving fine wrinkle, coarse wrinkle, hyperpigmentation, and roughness based on the results of 22 RCTs and 3801 participants. The analysis demonstrated that isotretinoin exhibited a notable efficacy in the treatment of fine wrinkle, with an odds ratio of 116.23, indicative of a pronounced therapeutic impact. This result is consistent with existing studies30, that have demonstrated that isotretinoin improves skin texture by promoting cellular skin renewal and collagen synthesis. Clinically, isotretinoin may be particularly suited for patients with generalized photoaging, as it exerts anti-aging effects by modulating epidermal differentiation and inhibiting matrix metalloproteinases31. Additionally, retinol (OR = 14.10) and tretinoin (OR = 6.87) demonstrated favourable outcomes, further substantiating their prevalent utilisation in anti-ageing therapies32. Retinol acts as a precursor to retinoic acid and provides milder stimulation of fibroblast activity and dermal matrix production, making it a good option for patients with sensitive skin32.
In comparison, adapalene (OR = 9.29) exhibited a tendency towards significance under the fixed-effects model, indicating a potential effect. However, the variability of the results suggests that further investigation may be required to ascertain the stability of this effect. This is consistent with the findings of some studies which suggest that adapalene may have a limited effect in certain patient groups33. Given its selective affinity for retinoic acid receptors, adapalene may offer a lower irritation profile, but its clinical effectiveness for photoaging appears less robust34. The statistical analysis revealed that glycolic acid and topical bakuchiol did not achieve a statistically significant result, indicating that further validation is required to ascertain the efficacy of these two treatments in improving fine wrinkle.
In the context of coarse wrinkle, tazarotene was identified as the most efficacious treatment option, with an OR of 4.78, thereby substantiating its capacity to stimulate skin renewal. In accordance with the findings of numerous studies, the potential of tazarotene in addressing skin dryness was underscored. Tazarotene modulates gene expression involved in keratinocyte differentiation and proliferation, thereby improving skin architecture28. Tretinoin (OR = 1.50) and retinol (OR = 3.78) also demonstrated some degree of improvement, whereas glycolic acid did not reach statistical significance, emphasizing the need for further investigation.
With regard to hyperpigmentation, tretinoin, tazarotene and retinol demonstrated notable efficacy, with tretinoin exhibiting the highest odds ratio (OR = 4.78) in the random-effects model. This highlights its particular effectiveness in the treatment of hyperpigmentation. This finding is consistent with the existing literature on the efficacy of retinol in improving skin hyperpigmentation35. Mechanistically, retinoids accelerate epidermal turnover and reduce melanin transfer, leading to visible brightening14. It is notable that glycolic acid and topical bakuchiol did not demonstrate a significant improvement, which suggests that their efficacy in this domain may be constrained.
Regarding the improvement of skin roughness, glycolic acid demonstrated an OR of 120.00, thereby indicating its superior efficacy in this regard. In addition, retinol and tretinoin exhibited notable therapeutic effects. The exfoliating properties of glycolic acid have been demonstrated in existing studies to significantly enhance the smoothness and evenness of the skin36, a finding that is consistent with the results of our analysis. As an alpha-hydroxy acid (AHA), glycolic acid dissolves intercellular cohesion, promoting desquamation and dermal remodeling. Clinically, it may be best utilized for patients with rough texture or early signs of photodamage37.
Tazarotene has been observed to exhibit a significantly higher prevalence of adverse effects in comparison to glycolic acid, thereby underscoring the necessity for heightened caution when contemplating its clinical utilisation. In contrast, isotretinoin and retinol exhibited more favourable safety profiles, with fewer reported adverse events, supporting their suitability for long-term application. These findings carry important clinical implications, as they suggest a need to balance efficacy with tolerability when selecting treatment options. This highlights the necessity for personalized treatment strategies that account not only for efficacy but also for the patient’s tolerance and skin type. Clinicians can use this evidence to tailor topical therapies based on individual skin aging profiles (e.g., prioritizing tretinoin for pigmentation, glycolic acid for roughness, and isotretinoin for overall wrinkle improvement).
Compared with prior systematic reviews38, our study offers a more comprehensive analysis by incorporating network meta-analytic techniques, allowing for indirect comparisons across multiple interventions. This provides stronger guidance for clinical decision-making where head-to-head trials may be lacking39. Furthermore, the analysis indicated the presence of bias in the reporting of safety outcomes associated with the funding sources of the trials. This emphasises the necessity for independent, unbiased research to validate these safety profiles and inform evidence-based clinical decisions.
Furthermore, the analysis indicated the presence of bias in the reporting of safety outcomes associated with the funding sources of the trials. This emphasises the necessity for independent, unbiased research to validate these safety profiles and inform evidence-based clinical decisions. Notably, this study excluded sunscreens, hyaluronic acid, and vitamin C. Sunscreens, acting as photoprotectants, prevent new UV damage but don’t reverse existing photoaging, so they were omitted as our focus was on therapeutic interventions. Hyaluronic acid and vitamin C are often studied in combination with other compounds, making it hard to isolate their independent effects. Thus, we prioritized single-agent studies for analytical rigor. It is also worth noting that industry-sponsored trials may be more likely to present favorable safety profiles29. Given this concern, we conducted sensitivity analyses to evaluate the robustness of commercially sponsored research and purely academic research in terms of drug safety. The heterogeneity across studies ranged from 10 to 25%, indicating a moderate level of variability that, while potentially affecting the precision of pooled estimates, remains within generally acceptable limits for meta-analytical interpretation.
This study has several limitations. Firstly, the results may be subject to bias from external confounding factors, such as ambient temperature and humidity during follow-up, which are known to induce skin physiological changes independently. Secondly, the concentrations of the topical treatments were not considered in this study. We found that some studies reported specific concentrations while others did not. Therefore, subgrouping by concentration would substantially reduce the number of comparable studies. Thus, we prioritized maintaining a robust sample size to enhance statistical power, at the cost of omitting concentration details.
Conclusions
In conclusion, the findings of this study demonstrated that isotretinoin, tretinoin and retinol were effective in improving fine wrinkles, while tazarotene was found to have a significant advantage in the improvement of coarse wrinkles. This network meta-analysis addresses a gap in the existing literature by synthesizing the findings of comparative studies of multiple topical compounds against skin ageing. Importantly, the evidence provided has direct clinical utility, offering dermatologists a comparative framework to guide therapeutic choices based on both efficacy and safety. The results of the data collection suggest that trials of compounds against skin ageing still need to adhere to recognized and uniform standards of reporting results to ensure the rigour of the trials and results. Furthermore, the safety profiles of the treatments were also fully discussed in our analyses, which provide an important reference point for clinical decision-making. It is recommended that future studies continue to explore the efficacy and safety of these treatments in different populations and conditions, with a view to enhancing overall patient care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- UVA
Ultraviolet A
- UVB
Ultraviolet B
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO
International prospective register of systematic reviews
- GRADE
The grading of recommendations assessment, development and evaluations
- SUCRA
Surface under the cumulative ranking curve
- DIC
Divergence information criterion
- OR
Odds ratio
- RCTs
Randomized controlled trials
Author contributions
L.L. and X.C. contributed equally to this work. L.L. conducted data analysis and interpretation, and was involved in drafting the manuscript. X.C. was responsible for data collection and preliminary analysis. C.L. assisted with data collection and literature review. Q.W. provided valuable insights and feedback during the manuscript development process. W.L., X.X., and H.Z. supported the data analysis and interpretation. J.Z. and N.Z. contributed to the conceptualization and design of the study. W.C., P.S. and Z.X. were the corresponding authors and were responsible for overseeing the study, ensuring its accuracy and integrity, and finalizing the manuscript for submission. All authors read and approved the final manuscript.
Funding
This study was funded in part by the Science and Technology Projects in Guangzhou, China (No.: 2024A04J4621); in part by the Guangdong Basic and Applied Basic Research Foundation, China (No.: 2025A1515012013); and in part by the Noncommunicable Chronic Diseases-National Science and Technology Major Project, China (No.: 2024ZD0543300).
Data availability
All data generated or analyzed during this study are included in this published article. For further inquiries, please contact the corresponding author, Zhongzhi Xu, at xuzhzh26@mail.sysu.edu.cn.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and statement
This meta-analysis has not obtained written patient consent for publication,therefore ethical approval and an ethics statement regarding patient consentfor publication are not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lulu Lin and Xueqing Chen contributed equally to this work.
Contributor Information
Weibin Cheng, Email: chwb817@gmail.com.
Peng Shu, Email: shupeng@hbn.cn.
Zhongzhi Xu, Email: xuzhzh26@mail.sysu.edu.cn.
References
- 1.Wu, P. S., Lee, Y. C., Kuo, Y. C. & Lin, C. C. Development of octyl methoxy cinnamates (OMC)/silicon dioxide (SiO2) nanoparticles by sol-gel emulsion method. Nanomaterials. 7, (2017). [DOI] [PMC free article] [PubMed]
- 2.Tang, Z. et al. Research progress of keratinocyte-programmed cell death in UV-induced skin photodamage. Photodermatol. Photoimmunol. Photomed.37, 442–448 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Gilchrest, B. A. A review of skin ageing and its medical therapy. Br. J. Dermatol. 1996. 135, 7–75 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Tang, Z. et al. Research progress of keratinocyte-programmed cell death in UV-induced Skin photodamage. Photodermatol. Photoimmunol. Photomed.37, 442–448. 10.1111/phpp.12679 (2021). [DOI] [PubMed]
- 5.Hu, D., O’Connor, A. M., Wang, C., Sargeant, J. M. & Winder, C. B. How to conduct a bayesian network meta-analysis. Front. Vet. Sci.7, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salanti, G., Ades, A. E. & Ioannidis, J. P. A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol.64, 163–171 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Andreano, J. M., Bergfeld, W. F. & Vanderbrug Medendorp, S. Tretinoin emollient cream 0.01% for the treatment of photoaged skin. Cleve Clin. J. Med.60, 49–55 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Bertin, C. et al. Combined retinol-lactose-glycolic acid effects on photoaged skin: a double-blind placebo-controlled study. Int. J. Cosmet. Sci.30, 175–182 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Kafi, R. et al. Improvement of naturally aged skin with vitamin A (Retinol). Arch. Dermatol.143, 606–612 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Kang, S. et al. Tazarotene cream for the treatment of facial photodamage. Arch. Dermatol.137, (2001). [DOI] [PubMed]
- 11.Leyden, J. J. et al. Treatment of photodamaged facial skin with topical tretinoin. J. Am. Acad. Dermatol.21, 638–644 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Lowe, P. M., Woods, J., Lewis, A., Davies, A. & Cooper, A. N. J. Topical tretinoin improves the appearance of photo damaged skin. Aust. J. Dermatol35 (1994). [DOI] [PubMed]
- 13.Nathan Newman et al. Clinical improvement of photoaged skin with 50% glycolic acid a double-blind vehicle-controlled study. Am. Soc. Dermatol. Surg.22, 455–460 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Elyse, S. R. et al. Topical tretinoin treatment for liver spots associated with photodamage. N. Engl. J. Med.326, 368–374 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann-La, F. et al. Topical isotretinoin for photodamaged skin. J. Am. Acad. Dermatol.27, 15–18 (1992). [DOI] [PubMed] [Google Scholar]
- 16.Thibault, P. K., Wlodarczyk, J. & Wenck, A. A double-blind randomized clinical trial on the effectiveness of a daily glycolic acid 5% formulation in the treatment of photoaging. Dermatol. Surg.24, 573–578 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Tucker-Samaras, S. et al. A stabilized 0.1% retinol facial moisturizer improves the appearance of photodamaged skin in an eight-week, double-blind, vehicle-controlled study original articles journal of drugs in dermatology. J. Drugs Dermatol.8, (2009). [PubMed]
- 18.Weinstein, G. D. et al. Topical tretinoin for treatment of photodamaged skin A multicenter study. Arch. Dermatol.127, 659–665 (1991). [PubMed] [Google Scholar]
- 19.Jonathan, S. & Weiss, J. S. Tretinoin microsphere gel 0.1% for photodamaged facial skin: A placebo-controlled trial. Ther. Clin. 78, 426–432 (2006). [PubMed] [Google Scholar]
- 20.Bagatin, E., Gonçalves, H. S., Sato, M., Almeida, L. M. C. & Miot, H. A. Comparable efficacy of adapalene 0.3% gel and tretinoin 0.05% cream as treatment for cutaneous photoaging. Eur. J. Dermatol. 28, 343–350 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Chien, A. L. et al. Induction of Procollagen I May not correlate well with clinical improvement in photoaging by retinoids. J. Investig. Dermatol. 133, S159–S190 (2013). [Google Scholar]
- 22.Dhaliwal, S. et al. Prospective, randomized, double-blind assessment of topical Bakuchiol and retinol for facial photoageing. Br. J. Dermatol.180, 289–296 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Kang, S. et al. A multicenter, randomized, double-blind trial of Tazarotene 0.1% cream in the treatment of photodamage. J. Am. Acad. Dermatol.52, 268–274 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Phillips, T. J. et al. Efficacy of 0.1% tazarotene cream for the treatment of photodamage a 12-month multicenter, randomized trial. Arch Dermatol.138. http://archderm.jamanetwork.com/ (2002). [DOI] [PubMed]
- 25.Kang, S. et al. Long-Term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin a two-year, randomized, placebo-controlled trial. Am. J. Clin. Dermatol. 6 (2005). [DOI] [PubMed]
- 26.Kikuchi, K., Suetake, T., Kumasaka, N. & Tagami, H. Improvement of photoaged facial skin in middle-aged Japanese females by topical retinol (vitamin A alcohol): A vehicle-controlled, double-blind study. J. Dermatol. Treat.20, 276–281 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Kim, H. et al. Improvement in skin wrinkles from the use of photostable retinyl retinoate: A randomized controlled trial. Br. J. Dermatol.162, 497–502 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Lowe, N. J. et al. Tazarotene 0.1% cream versus tretinion 0.05% emollient cream in the treatment of photodamaged facial skin: A multicenter, double-blind, randomized, parallel-group study. J. Cosmet. Laser Ther. 6, 79–85 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Lundh, A., Lexchin, J., Mintzes, B., Schroll, J. B. & Bero, L. Industry sponsorship and research outcome (Review). Cochrane Database Syst. Rev.2017. 10.1002/14651858.MR000033.PUB3 (2017). [DOI] [PMC free article] [PubMed]
- 30.Fisher, G. J. et al. Mechanisms of photoaging and chronological skin aging. JAMA Dermatol.138, 1462–1470 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Zasada, M., Budzisz, E. & Retinoids Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatologii i Alergologii. 36, 392–397. 10.5114/ada.2019.87443 (2019). [DOI] [PMC free article] [PubMed]
- 32.Mukherjee, S. et al. Retinoids in the treatment of skin aging: an overview of clinical Effi Cacy and safety. Clin. Interv. Aging. 327–348 (2006). [DOI] [PMC free article] [PubMed]
- 33.Rusu, A., Tanase, C. & Pascu, G. A. Todoran, N. Recent advances regarding the therapeutic potential of adapalene. Pharmaceuticals. 13, 1–22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaenglein, A. L. Topical retinoids in the treatment of acne vulgaris. Semin. Cutaneous Med. Surg.27, 177–182. 10.1016/j.sder.2008.06.001 (2008). [DOI] [PubMed]
- 35.Thawabteh, A. M., Jibreen, A., Karaman, D., Thawabteh, A. & Karaman, R. Skin pigmentation types, causes and treatment—a review. Molecules. 10.3390/molecules28124839 (2023). [DOI] [PMC free article] [PubMed]
- 36.Sharad, J. Glycolic acid peel therapy—A current review. Clin. Cosmet. Investig. Dermatol.6, 281–288. 10.2147/CCID.S34029 (2013). [DOI] [PMC free article] [PubMed]
- 37.Ditre, C. M. et al. Clinical and laboratory studies effects of OL-hydroxy acids on photoaged skin: a pilot clinical, histologic, and ultrastructural study. (1996). [DOI] [PubMed]
- 38.Samuel, M., Brooke, R., Hollis, S. & Griffiths, C. E. Interventions for photodamaged skin. In Cochrane Database of Systematic Reviews. 10.1002/14651858.cd001782.pub2 (Wiley, 2005). [DOI] [PubMed]
- 39.Salanti, G., Giovane, C., Del, Chaimani, A., Caldwell, D. M. & Higgins, J. P. T. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 9 (2014). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. For further inquiries, please contact the corresponding author, Zhongzhi Xu, at xuzhzh26@mail.sysu.edu.cn.