Abstract
Some ants have an extraordinary social organization, called unicoloniality, whereby individuals mix freely among physically separated nests. This type of social organization is not only a key attribute responsible for the ecological domination of these ants, but also an evolutionary paradox and a potential problem for kin selection theory because relatedness between nest mates is effectively zero. The introduction of the Argentine ant in Europe was apparently accompanied by a dramatic loss of inter-nest aggression and the formation of two immense supercolonies (which effectively are two unicolonial populations). Introduced populations experienced only limited loss of genetic diversity at neutral markers, indicating that the breakdown of recognition ability is unlikely to be merely due to a genetic bottleneck. Rather, we suggest that a “genetic cleansing” of recognition cues occurred after introduction. Indeed workers of the same supercolony are never aggressive to each other despite the large geographical distance and considerable genetic differentiation between sampling sites. By contrast, aggression is invariably extremely high between the two supercolonies, indicating that they have become fixed for different recognition alleles. The main supercolony, which ranges over 6,000 km from Italy to the Spanish Atlantic coast, effectively forms the largest cooperative unit ever recorded.
Since its inadvertent introduction from South America into all other continents with a Mediterranean climate, the Argentine ant Linepithema humile (formerly Iridomyrmex humilis) has invaded vast areas, becoming a major pest species. The Argentine ant displaces or disrupts the local arthropod fauna (1–3), protects insects that devastate plants, destroys fruits and buds (4), and even invades human houses (5). The ecological domination of this species is thought to stem from its unusual social structure, called unicoloniality, whereby individuals mix freely among physically separated nests (6). In contrast, in its native habitats in South America, L. humile exhibits a more common social system, multicoloniality, with systematic aggression between workers from different nests (7). By reducing costs associated with territoriality, unicoloniality allows high worker densities and interspecific dominance in invaded habitats.
Although unicoloniality is a key attribute responsible for the ecological domination of several ant species (2, 6), it is also an evolutionary paradox and a potential problem for kin selection theory because relatedness between nestmates is effectively zero (5, 8–12). Despite the ecological and evolutionary significance of unicolonial ants, the origin of their extreme social organization has remained a mystery for more than 30 years (5, 9, 11, 12). Recently, it has been suggested (7) that unicoloniality in L. humile arose from a genetic bottleneck when invasive populations were found outside South America at the beginning of last century (2, 13). If kin recognition is based on similarity of heritable cues (14–17), a bottleneck may lead to reduced genetic diversity at the recognition locus (loci) and to a loss of aggression between colonies having the same recognition allele(s). Unicoloniality would arise when the bottleneck has been so severe that all variation at the recognition loci was lost (7). A prediction of this hypothesis is that the loss of genetic diversity should be similar at recognition loci and other loci that are not involved in recognition.
To determine patterns of aggression and quantify the loss of genetic diversity in populations of the Argentine ant introduced in Europe, we collected populations distributed over 6,004 km along the Mediterranean and Atlantic coasts in Southern Europe. We conducted aggression tests and genetic analyses, and compared these results with results obtained for native South American populations.
Material and Methods
Field Collection.
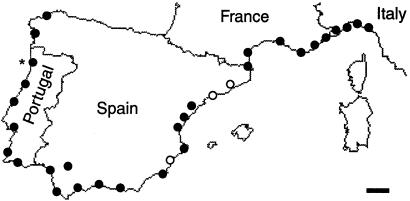
We collected 33 Argentine ant populations along the Mediterranean and Atlantic coasts in Southern Europe between March 12 and April 13, 2000 (Fig. 1). Approximately 5,000 workers were collected from one nest of each population and kept in 750-ml glass jars filled to one-third with original nest material; these ants were fed with a standard diet (18). The geographic distance between each pair of populations was estimated from the coastal distance separating them. This distance was determined from a digitized map with pixels separated by 5.35 km.
Figure 1.
Map of 33 European populations of L. humile sampled. Populations were assigned to one of two distinct groups based on the aggression tests: the main supercolony (●) and the Catalonian supercolony (○). In the population marked with asterisk, workers were heavily infected with mites, which affected the behavioral interactions. Consequently, this population was not included in the analysis of behavioral data. (Scale bar is 100 km.)
Aggression Tests.
Aggression tests between pairs of workers were conducted blindly and in a random order between each pair of populations, 5 to 15 days after the last field collection. We randomly selected a single worker from each of two laboratory colonies and placed them together for 10 min in a 5.5-cm diameter vial with fluon-coated sides. The behavioral assay was adapted from standard protocols (19) with interactions scored as follows: 0 = Ignore, physical contact in which neither ant showed any interest; 1 = Antennation, repeated tapping the antennae somewhere on the other ant; 2 = Avoidance, one or both ants retreating in opposite directions after contact; 3 = Dorsal flexion, gaster raised to vertical position as escalation to chemical defense; 4 = Aggression, biting or pulling extremities or head, or deposition of venom; 5 = Fight, prolonged aggression, often involving locking the mandibles onto a body part of the other ant or carrying it. Levels 0–2 are referred to as nonaggressive behavior and levels 3–5 are referred to as aggressive behavior. Different workers were used for each trial. One trial was conducted for each combination of all 33 populations. These experiments revealed the presence of two supercolonies (the main and Catalonian supercolonies, see Results) with no aggression between colonies of the same supercolony but strong aggression between individuals of the two supercolonies. Three replicate trials were obtained for 20 populations chosen as being the most evenly distributed along the coast and including the three populations of the Catalonian supercolony. For each population pair, these data allowed us to calculate the average maximum level of aggression and the average frequency of antennations.
Genetic Analyses.
DNA was extracted from 20 workers of each population by using the PUREGENE DNA Isolation kit (Gentra Systems) and analyzed at eight microsatellite loci: Lhum-11, Lhum-13, Lhum-19, Lhum-35, Lhum-62 (20), Lihu-M1, Lihu-S3, and Lihu-T1 (7). Additionally, a sample of 60 workers from an Argentinean reference population (20) and from a population of each supercolony were analyzed at 14 more loci (20). PCR products were separated in polyacrylamide gels and visualized by autoradiography.
Statistical Analyses.
To avoid pseudoreplication, we used a simulation model to test the hypothesis that ants from different populations antennate each other at a higher frequency than ants from the same population. For each population, we calculated the difference between the within-population antennation frequency and the antennation frequency observed in tests between workers of this population and a randomly drawn other population. Subsequently, the mean difference across populations could be found. By running this simulation 2,000 times, we obtained a simulated distribution of the mean difference in antennation frequency, which was used to estimate the P value of the hypothesis test.
F statistics and exact tests of genetic population differentiation were calculated by using FSTAT 2.9.1 (available at http://www.unil.ch/izea/softwares/fstat.html; ref. 21). Partial matrix correlation coefficients with associated Mantel tests for data on the main supercolony were calculated by the program ARLEQUIN 2.000 (available at http://anthropologie.unige.ch/arlequin; ref. 22).
Results
Patterns of Aggression Between Colonies.
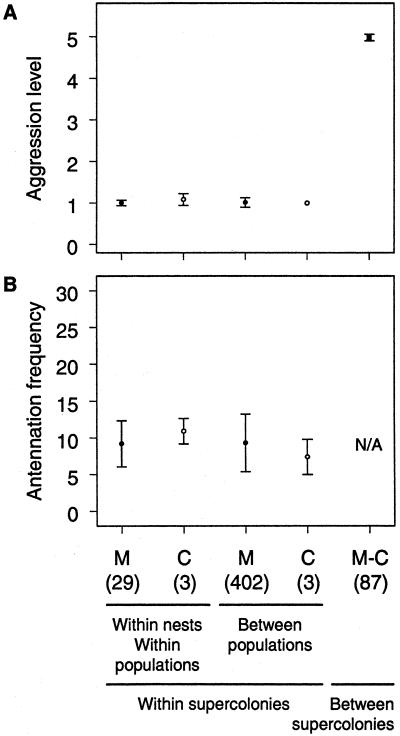
Aggression tests were conducted between all pairs of populations (Fig. 1). In the vast majority (1,131 of 1,151, or 98%) of the 10-min trials, workers either fought vigorously or showed no sign of antagonism. These experiments revealed the existence of two distinct supercolonies, the Catalonian supercolony with three populations in eastern Spain, and the main supercolony including all other populations (Fig. 1). Aggression was always severe between workers from the two supercolonies, leading to worker death in 98% (235 of 241) of the trials. By contrast, aggression never occurred between individuals from the same supercolony (Fig. 2A).
Figure 2.
Results of behavioral tests within and across populations. M and C denote populations assigned to the main and Catalonian supercolony, respectively. Sample size in number of population pairs is given in parentheses. (A) Average maximum aggression level: 0 = ignore; 1 = antennation; 2 = avoidance; 3 = dorsal flexion; 4 = aggression; 5 = fight. (B) Average antennation frequency for pairs within the same supercolony. Bars show SD.
These data suggest that nestmate recognition has a strong genetic basis. This finding was confirmed by a set of aggression tests conducted 6 and 18 months after colonies had been collected and maintained under similar conditions in the laboratory. Because the average and maximum life spans of workers are 4.8 and 11.2 months, respectively (L.K., unpublished results), most of the workers of the 6-month aggression test and all workers of the 18-month aggression test had eclosed in the laboratory and always experienced the same environment. Yet, the level of aggression between workers of the two supercolonies remained maximum, and they invariably killed each other in trials conducted after 6 and 18 months (aggression level 5 for all trials, n = 30). By contrast, the level of aggression between workers of the same supercolony remained minimal (mean ± SE = 1.17 ± 0.12, n = 30) and significantly lower than between workers of the two supercolonies (P < 0.0001, Mann–Whitney U test). Also, in sharp contrast to tests performed between colonies of the two supercolonies, no injuries were observed during interactions of workers from nests from the same supercolony.
The complete lack of discrimination within each of the supercolonies was further demonstrated by observations of worker antennation frequency. Workers from different populations of the same supercolony did not antennate each other more often (Fig. 2B; mean number of antennations in 10 min ± SE = 9.14 ± 0.19, n = 32) than workers from the same nest (9.38 ± 0.54 antennations; permutation test, P = 0.66). This test was powerful, as it would have detected a difference of only 0.85 antennation per 10 min with 95% probability. It is thus very unlikely that any biologically significant difference in antennation frequency has gone undetected.
Loss of Genetic Diversity.
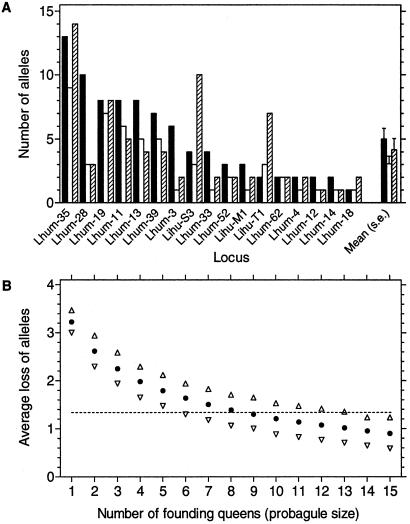
Evidence that the emergence of unicoloniality in Europe is not merely due to a genetic bottleneck came from the examination of allelic diversity at 17 polymorphic microsatellite loci in a native reference population (20) and populations of the two supercolonies. Although the European populations harbored significantly fewer alleles, indicating that the colonizers went through a genetic bottleneck, the reduction was only 28% or 1.4 ± 0.6 (SE) alleles on average per locus (Fig. 3A). A simulation study estimated that at least 6–13 unrelated mated queens must have been introduced in Europe to account for the observed diversity (conservatively assuming that there has been no further loss of genetic diversity after the founding stage; Fig. 3B). This means that at least 18–39 random haploid genomes were introduced in Europe, and thus that the bottleneck was not very severe.
Figure 3.
(A) Genetic diversity measured as number of alleles at 17 microsatellite loci in an Argentinean reference population (filled bars) compared with a population of the main supercolony (open bars) and the Catalonian supercolony (hatched bars). Sample size is 60 workers for all loci and populations. Five additional loci with no variation in the present sample are not included. The introduced populations hold significantly fewer alleles than the native population (two-way ANOVA with locus and population type as factors; population type F1,17 = 7.4, P = 0.014). Introduced populations harbored no alleles that were not present in this or other native populations studied. (B) Average number of alleles lost across loci in 2,000 simulations of a genetic bottleneck by a limited number of founding queens taken from the native reference population (each queen representing three haploid genomes caused by mating). Open triangles give the 95% confidence intervals. The horizontal line shows the observed reduction in allele number (see A). The simulated reduction is conservative (i.e., possibly downward biased) as it is assumed that no alleles in the founding generation are subsequently lost from the new population or from other populations descending from this first population introduced.
Population Genetic Structure.
Additional evidence that the loss of nestmate recognition ability is not merely due to a bottleneck comes from the lack of association between antagonism of workers and genetic differentiation between populations. The genetic differentiation between populations at eight microsatellite loci was considerable (main supercolony Fst ± SE = 0.12 ± 0.02, Catalonian supercolony Fst = 0.10 ± 0.04, P < 0.0002 for both), yet there was no association within the main supercolony between the genetic distance and the maximum level of aggression (partial matrix correlation controlling for geographic distance, r = −0.07, n = 402 pairs, P = 0.90), nor with the frequency of antennations (r = 0.01, P = 0.44). Thus, the level of aggression between workers strongly depends on whether they are from the same supercolony, but not on their overall genetic dissimilarity.
Discussion
A surprising finding of the aggression tests was that there are two enormous and distinct supercolonies in Southern Europe, the Catalonian supercolony and the main supercolony. Aggression never occurred between individuals from the same supercolony, even when taken from very distant nests. By contrast, aggression was invariably severe between supercolonies. This pattern of aggression remained unchanged after colonies had been maintained under similar conditions in the laboratory during several months, demonstrating that the level of aggression is primarily influenced by the genetic background of workers, and not by whether or not they have experienced the same environment. That is, workers in natural populations do not use environmental cues to any significant extent to discriminate nestmates from non-nestmates. A recent laboratory study (23) suggested a role of food in nestmate recognition of L. humile, but it is unclear whether this is an artifact because of the type of food provided. The two laboratory colonies were fed exclusively with one of two species of cockroaches, whereas field colonies have a generalist diet (2).
The lack of discrimination within each of the supercolonies was further demonstrated by observations of worker antennation frequency. We found that workers from different populations of the same supercolony did not antennate each other more often than workers from the same nest. Antennation frequency is often used as a sensitive measure of nestmate discrimination ability because ant workers typically spend more time inspecting non-nestmates than nestmates (24). Also consistent with the finding that workers fail to discriminate between nestmates and non-nestmates from the same supercolony, we found no evidence of an increase of the frequency of antennations with geographical distance between nests. Thus, workers from the same supercolony treat each other in a similar manner, whether or not they are from the same nest and whatever the distance between nests. This complete lack of discrimination is particularly striking because workers came from populations up to 6,000 km apart, which encompass a wide range of environmental conditions.
The finding that the two supercolonies are extremely large and encompass millions of nests is interesting because it demonstrates that there is no clear distinction between supercoloniality and unicoloniality. The term supercolony has been generally used for large aggregations of nests that are nonaggressive to each other in species where aggression between nests can occur (e.g., Formica paralugubris and Formica yessensis; ref. 25). By contrast, unicoloniality is restricted to species in which all workers are thought to be amicable, whatever their nest of origin (2, 11). Argentine ants have been used as a perfect example of unicoloniality (2, 26), but our data show that even in introduced European populations aggression may occur between nests when these are from different supercolonies. Thus, supercoloniality and unicoloniality probably represent points on a continuum along which the proportion of colonies that are nonaggressive to each other varies. Multicoloniality with all colonies being aggressive to each other would represent one extreme, and unicoloniality would represent the other extreme of this continuum (see also refs. 11 and 27).
Evolution of Unicoloniality.
Evidence that the lack of aggression between colonies of the same supercolony and hence the emergence of two unicolonial populations in Europe is not merely due to a genetic bottleneck comes from an examination of neutral allelic diversity in a native reference population and populations of the two supercolonies. Although the European populations harbored significantly fewer alleles, indicating that the colonizers indeed went through a genetic bottleneck, the reduction was weak. Our simulation, indeed, showed that a conservative estimate of at least 18–39 random haploid genomes should have been introduced in Europe to account for the observed diversity. Hence, the bottleneck was not very severe and is unlikely (28, 29) to explain the shift from an effective kin-recognition system in native populations (7, 30) to the almost complete breakdown of nestmate recognition in Europe and other introduced populations.
Our data also show that the evolution of unicoloniality is not simply due to a decrease of the overall level of aggression and/or loss of discrimination ability of workers. Workers were invariably very aggressive to workers from the other supercolony. Moreover, high aggression between supercolonies remained very high even when individuals had been kept under similar laboratory conditions for several months.
On the basis of our findings and the biology of L. humile and other successful invasive species, we propose an explanation for the evolution of unicoloniality in ants. The introduction into new habitats with relaxed ecological constraints (e.g., the release from native parasites and competitors; refs. 30 and 31) typically leads to high nest density (31). This, in turn, may select for a loss of genetic diversity at recognition loci because high nest density induces increased rates of encounter between workers from foreign colonies with the effect that the costs of defending a territory increase, possibly outweighing the potential benefits of territory defense. Several studies have indeed shown that nest density of introduced populations of Argentine ants and of fire ants is much higher than in native populations (30, 31). Experimental studies on the Argentine ant have further demonstrated that nonaggressive neighbor colonies attain a higher worker number (19), and thus a greater competitive ability (32), than aggressive neighbors. Consequently, colonies harboring the most common recognition cues will experience a selective advantage because they fight less often with neighbors and are more productive. Such selection against rare recognition alleles has previously been discussed in the context of colonial marine invertebrates, and models showed that it would lead to a selective loss of diversity of heritable recognition cues (16, 33, 34). The expected outcome of this “genetic cleansing” during the stage of initial population growth under relaxed ecological constraints is the emergence of large supercolonies effectively composed of nonaggressive colonies sharing identical recognition alleles.
In contrast to the bottleneck hypothesis, the genetic cleansing hypothesis does not require a severe bottleneck to account for the evolution of unicoloniality. Furthermore, in contrast to the bottleneck hypothesis, the genetic cleansing hypothesis predicts that there should be no direct association between geographic distribution of genetic variability at neutral markers and at loci involved in recognition. This difference is because the genetic cleansing hypothesis explicitly assumes different selection regimes acting on neutral markers and recognition loci, with frequency-dependent selection acting on recognition loci only. Consistent with this prediction of the genetic cleansing hypothesis, we found no significant association between antagonism of workers and genetic differentiation between populations. The genetic differentiation between populations at eight microsatellite loci was considerable, yet there was no association within the main supercolony between the genetic distance and the maximum level of aggression, nor with the frequency of antennations. Thus, the level of aggression between workers strongly depends on whether they are from the same supercolony, but not on their overall genetic dissimilarity.
In conclusion, this study demonstrates that the introduction of Argentine ants into Europe was accompanied by a complete breakdown of nestmate recognition ability and a drastic shift in social organization that is unlikely to be merely the result of a genetic bottleneck. Rather, it is more likely that a selective cleansing of genetic diversity at the recognition locus (loci) lead to the formation of two supercolonies presumably fixed for different recognition loci. This may be a general mechanism that allows the Argentine ant and other introduced ant species (2, 6) to form very large supercolonies. The significance of this type of organization lays not only in the tremendous ecological success it confers to invasive ants, but also in the fact that it leads to the emergence of a new cooperative biological unit (35, 36). In the case of the European populations of Argentine ants, the size of this cooperative unit is truly astonishing, as the main supercolony extends at least 6,000 km and consists of millions of nests comprising billions of workers. Incidentally, such a unicolonial system is expected to be unstable because workers help raising unrelated brood. Thus, selfish mutants inducing larvae to develop into queens rather than workers should spread (5, 12), with the effect that unicoloniality might be a transient social system doomed to failure.
Acknowledgments
We thank Xavier Espalader, Xim Cerda, Yves Hurand, Jacqueline Giraud, and Thierry Darras for helping collecting ants, and Laetitia Giraud, Patrick Presi, and Raoul Vega for assistance in the laboratory. We thank François Balloux, Koos Boomsma, Michel Chapuisat, Philippe Christe, Jérôme Goudet, Cathy Liautard, Joel and Karen Parker, Nicolas Perrin, Dave Queller, Francis Ratnieks, Jacqui Shykoff, Lotta Sundström, and two anonymous referees for helpful comments on the manuscript. This research was funded by the Carlsberg Foundation, the Fondation Fyssen, the Fondation du 450ème anniversaire of the University of Lausanne, the Roche Fondation, the Singer–Polignac Fondation, the Swiss Academy of Sciences, the European Community's Improving Human Potential Programme under contract HPRN-CT-2000-00052 “INSECTS,” and several grants from the Swiss National Science Foundation.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bolger D T, Suarez A V, Crooks K R, Morrison S A, Case T J. Ecol Appl. 2000;10:1230–1248. [Google Scholar]
- 2.Passera L. In: Exotic Ants: Biology, Impact, and Control of Introduced Species. Williams D F, editor. Boulder, CO: Westview; 1994. pp. 23–43. [Google Scholar]
- 3.Human K G, Gordon D M. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- 4.Visser D, Wright M G, Giliomee J H. African Entomol. 1996;4:285–287. [Google Scholar]
- 5.Bourke A F G, Franks N R. Social Evolution in Ants. Princeton: Princeton Univ. Press; 1995. [Google Scholar]
- 6.Chapman R E, Bourke A F G. Ecol Lett. 2001;4:650–662. [Google Scholar]
- 7.Tsutsui N D, Suarez A V, Holway D A, Case T J. Proc Natl Acad Sci USA. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton W D. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 9.Crozier R H. In: Social Insects. Hermann H R, editor. New York: Academic; 1979. pp. 223–286. [Google Scholar]
- 10.Keller L. Trends Ecol Evol. 1995;10:355–360. doi: 10.1016/s0169-5347(00)89133-8. [DOI] [PubMed] [Google Scholar]
- 11.Crozier R H, Pamilo P. Evolution of Social Insect Colonies: Sex Allocation and Kin Selection. Oxford: Oxford Univ. Press; 1996. [Google Scholar]
- 12.Queller D C, Strassmann J E. Bioscience. 1998;48:165–175. [Google Scholar]
- 13.Suarez A V, Holway D A, Case T J. Proc Natl Acad Sci USA. 2001;98:1095–1100. doi: 10.1073/pnas.98.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher D J C, Michener C D. Kin Recognition in Animals. Chichester, U.K.: Wiley; 1987. [Google Scholar]
- 15.Hepper P G. Kin Recognition. Cambridge, U.K.: Cambridge Univ. Press; 1991. [Google Scholar]
- 16.Ratnieks F L W. Am Nat. 1991;137:202–226. [Google Scholar]
- 17.Vander Meer R K, Breed M D, Winston M L, Espilie K E. Pheromone Communication in Social Insects: Ants, Wasps, Bees, and Termites. Boulder, CO: Westview; 1998. [Google Scholar]
- 18.Keller L, Cherix D, Ulloa Chacón P. Insectes Sociaux. 1989;36:348–352. [Google Scholar]
- 19.Holway D A, Suarez A V, Case T D. Science. 1998;282:949–952. doi: 10.1126/science.282.5390.949. [DOI] [PubMed] [Google Scholar]
- 20.Krieger M J B, Keller L. Mol Ecol. 1999;8:1078–1080. [Google Scholar]
- 21.Goudet J. J Hered. 1995;86:485–486. [Google Scholar]
- 22. Schneider, S., Kueffer, J.-M., Roessli, D. & Excoffier, L. (2000) ARLEQUIN V. 2.000. A software for population genetics analysis. http://lgb.unige.ch/arlequin/about.php3.
- 23.Liang D, Silverman J. Naturwissenschaften. 2000;87:412–416. doi: 10.1007/s001140050752. [DOI] [PubMed] [Google Scholar]
- 24.Hölldobler B, Wilson E O. The Ants. New York: Springer; 1990. [Google Scholar]
- 25.Chapuisat M, Goudet J, Keller L. Evolution (Lawrence, Kans) 1997;51:475–482. doi: 10.1111/j.1558-5646.1997.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilson E O. The Insect Societies. Cambridge, MA: Harvard Univ. Press; 1971. [Google Scholar]
- 27.Rissing S W, Pollock G B. In: Interindividual Behavioral Variability in Social Insects. Jeanne R L, editor. Boulder, CO: Westview; 1988. pp. 179–222. [Google Scholar]
- 28.Getz W M. J Theor Biol. 1981;92:209–226. [Google Scholar]
- 29.Lacy R C, Sherman P W. Am Nat. 1983;121:489–512. [Google Scholar]
- 30.Suarez A V, Tsutsui N D, Holway D A, Case T J. Biol Inv. 1999;1:43–53. [Google Scholar]
- 31.Porter S D, Williams D F, Patterson R S, Fowler H G. Environ Entomol. 1997;26:373–384. [Google Scholar]
- 32.Holway D A. Ecology. 1999;80:238–251. [Google Scholar]
- 33.Crozier R H. Evolution (Lawrence, Kans) 1986;40:1100–1101. doi: 10.1111/j.1558-5646.1986.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 34.Grosberg R K, Quinn J F. Evolution (Lawrence, Kans) 1989;43:504–515. doi: 10.1111/j.1558-5646.1989.tb04248.x. [DOI] [PubMed] [Google Scholar]
- 35.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford: Freeman; 1995. [Google Scholar]
- 36.Queller D C. Nature (London) 2000;405:519–520. doi: 10.1038/35014705. [DOI] [PubMed] [Google Scholar]