Abstract
We present the case of a 74-year-old woman with behavioral variant frontotemporal dementia (bvFTD) linked to a pathogenic TANK-binding kinase 1 (TBK1) mutation (c.1349_1352del; p.Ile450Lysfs*15). During clinical workup, the patient underwent comprehensive biomarker analysis, including tau positron emission tomography (PET) and cerebrospinal fluid (CSF) seed amplification assay (SAA) for α-synuclein (αSyn). While CSF biomarkers for Alzheimer’s disease were normal, the αSyn SAA was clearly positive, indicating misfolded αSyn aggregates. Tau PET revealed increased [18F]PI-2620 uptake in the basal ganglia. Genetic testing confirmed autosomal dominant TBK1-associated FTD. The patient’s condition deteriorated over the following year, with rapid cognitive decline and the emergence of cortical signs. Post-mortem neuropathological analysis confirmed multiple proteinopathies: FTLD-TDP43 (subtype A), Lewy body disease (limbic type, Braak stage 5), argyrophilic grain disease (AGD), aging-related tau astrogliopathy (ARTAG), and primary age-related tauopathy (PART). This is the first reported TBK1-FTD case with in vivo detection of αSyn pathology via SAA and in vivo monitoring of tau pathology. The case expands the clinical and neuropathological spectrum of TBK1-associated FTD. Our findings support a broader interpretation of TBK1-associated neurodegeneration and highlight the importance of multimodal diagnostic approaches that integrate molecular, genetic, imaging, and neuropathological tools. This case also underscores the utility of αSyn SAA and tau PET in detecting co-pathologies that may otherwise remain clinically silent and illustrates the need for further studies exploring the molecular cross-talk between TBK1, tau, and αSyn pathologies.
Introduction
We report a 74-year-old female with frontotemporal dementia (FTD) linked to a pathogenic TANK-binding kinase 1 (TBK1) mutation (c.1349_1352del (p.Ile450Lysfs*15)). At autopsy, this case featured multiple neurodegenerative pathologies: frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP-43), Lewy-body disease (LBD), argyrophilic grain disease (AGD) and Primary Age-Related Tauopathy (PART). This is the first documented FTD case due to a TBK-1 mutation with in vivo detection of Lewy-body co-pathology using an α-synuclein (αSyn) seed amplification assay (SAA), alongside in vivo longitudinal tau pathology monitoring using tau PET.
Case presentation
At diagnosis, the patient, a right-handed 74-year-old woman, was assessed following a two-year history of behavioral changes including increased apathy, neglect of self-care, and substance use, consistent with behavioral variant FTD [1]. Motor function was intact with no signs of upper motor neuron disease or extrapyramidal/parkinsonian features (no rigidity, tremor, or bradykinesia).
Neuropsychological testing including the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery showed normal processing speed and attention, slight backward digit span impairment, and significant deficits in verbal learning, though recognition remained intact. Executive functions were mildly to moderately impaired, especially in inhibition and flexibility, with increased impulsivity. Language and visuo-constructive skills were within normal limits (see Table 1 for full scores).
Table 1.
Neuropsychological test results at baseline and one-year follow-up. Summary of raw scores and corresponding Z-scores (or percentile ranks) for key cognitive domains, demonstrating progression of memory, executive, language, and visuospatial impairments over one year. N/A indicates that the test was not administered at follow-up because the patient was too cognitively impaired to complete it. Word list recognition Z-score at follow‐up could not be calculated due to false positives. Abbreviations: WMS-R = Wechsler Memory Scale-Revised
| Test | Baseline | One-Year Follow-Up |
|---|---|---|
| Mini-Mental State Examination (MMSE) | 25/30 | 24/30 |
| Word List Learning | 13 (3/5/5) (Z-score=-2.64) | 7 (1/3/3) (Z-score=-4.49) |
| Word List Recall | 5 (Z-score=-1.21) | 1/10 (Z-score=-3.18) |
| Word List Savings | 100% (Z-score = 0.93) | 34% (Z-score=-2.71) |
| Word List Intrusions | 1 (Z-score=-0.82) | 7 (Z-score=-2.61) |
| Word List Recognition |
10 correct, 0 false positives (Z-score = 0.68) |
7/10 correct, 2 false positives |
| Discriminability | 100% | 75% (Z-score=-3.00) |
| Constructional Praxis | 11 (Z-score=-1.35) | 10 (Z-score=-0.36) |
| Constructional Praxis Recall | 8 (Z-score=-0.13) | 3 (Z-score=-2.03) |
| Constructional Praxis Savings | 73% (Z-score=-0.43) | 30% (Z-score=-1.97) |
| Semantic Fluency (Animals) | 17 (Z-score=-0.59) | 11 (Z-score=-1.82) |
| Phonemic Fluency (S-words) | 8 (Z-score=-0.29) | 6 (Z-score=-1.35) |
| Naming (Boston Naming Test − 15 items) | 14/15 (Z-score = 0.13) | 12/15 (Z-score=-1.24) |
| Trail-Making Test A | 43 s (Z-score = 0.31) | 61 s (Z-score=-0.76) |
| Trail-Making Test B | 98 s (Z-score = 0.56) | N/A |
| Trail-Making Test B/A | 2.3 (Z-score=-0.31) | N/A |
| Memory: Digit Span (WMS-R) - forward | 5 (Percentile Rank = 15) | N/A |
| Memory: Digit Span (WMS-R) - backward | 3 (Percentile Rank = 5) | N/A |
| Executive Function: Color-Word Interference Test (Stroop Test) - processing speed (III) | 31 s (Percentile Rank = 27–35) | N/A |
| Color-Word Interference Test (Stroop Test) - interference condition (III) | 76 s (Percentile Rank = 9–13) | N/A |
| Color-Word Interference Test (Stroop Test) - interference control (III-II) | 46 s (Percentile Rank = 9–13) | N/A |
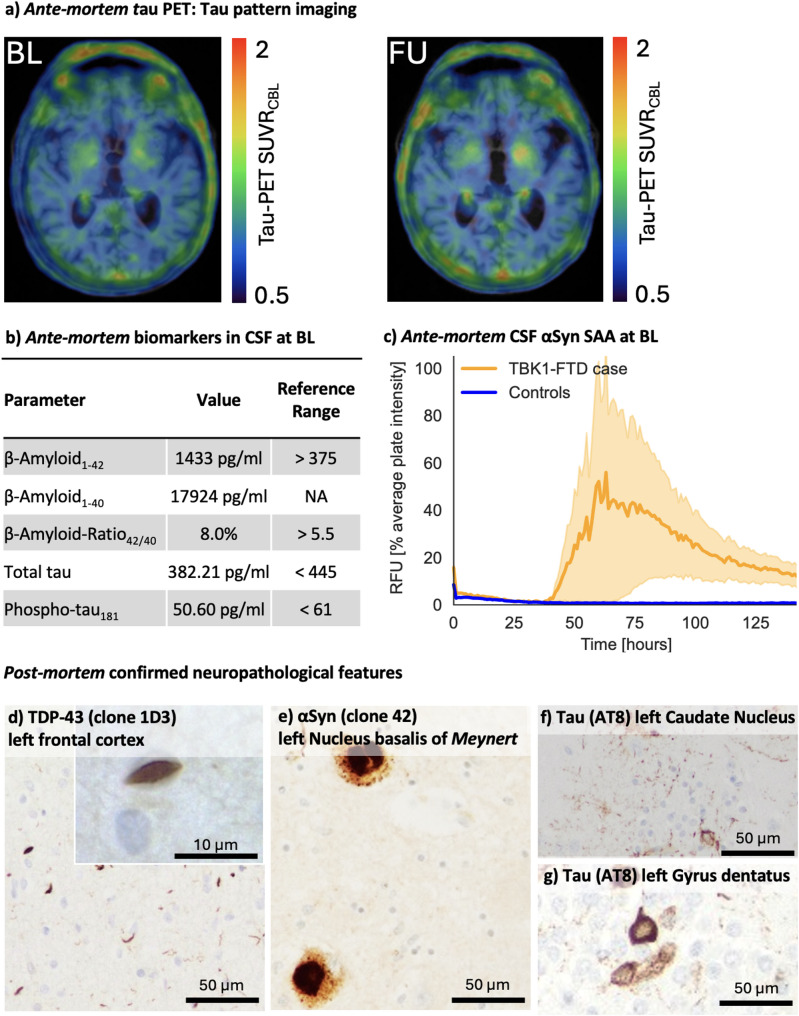
Cerebrospinal fluid (CSF) analysis was performed using the INNOTEST® ELISA platform (Fujirebio Europe N.V., Belgium) out of clinical routine CSF examination, revealing normal β-amyloid, phosphorylated tau181, and total tau levels. A positive αSyn SAA indicated the presence of misfolded aSyn aggregates suggestive of Lewy-body pathology. Using our previously validated αSyn SAA [2], CSF from 20 age- and sex-matched individuals without any clinical or biomarker evidence of neurodegenerative disease showed no seeding activity. Other CSF parameters were normal, with no evidence of infection or inflammation. The initial tau PET scan at diagnosis revealed increased [18F]PI-2620 tracer uptake in the basal ganglia, particularly on the left side with a quantitative standard uptake value ratio SUVR of 1.28, while cortical areas did not exhibit significant [18F]PI-2620 tracer uptake (Fig. 1a). Genetic analysis used a targeted dementia/FTD-ALS gene panel (APOE, APP, CHMP2B, CSF1R, DNAJC5, DNMT1, EPM2A, GRN, ITM2B, MAPT, NHLRC1, NOTCH3, OPTN, PRNP, PSEN1, PSEN2, SQSTM1, TARDBP, TBK1, TREM2, TUBA4A, TYROBP, UBQLN2, VCP), plus C9orf72 repeat expansion and APOE4 genotyping. This identified a pathogenic TBK1 mutation (c.1349_1352del (p.Ile450Lysfs*15)), consistent with autosomal dominant inheritance and resulting in TBK1 haploinsufficiency [3] - and revealed no other pathogenic or likely pathogenic variants. The patient’s three-year-younger sister was also diagnosed with a behavioral variant FTD carrying the same pathogenic TBK1 mutation, while no other family members had a history of neurodegenerative diseases.
Fig. 1.
Ante-mortem detection of post-mortem confirmed pathologies as well as immunohistochemistry of presented case. (a) Ante-mortem tau PET at baseline (BL) and one year later at follow-up (FU): tau pattern imaging with standard uptake value ratios (SUVR). (b) Ante-mortem biomarkers in cerebrospinal fluid (CSF) at diagnosis indicating the absence of Alzheimer pathology (c) Ante-mortem CSF αSyn Seed Amplification Assay (SAA) at BL indicating the presence of Lewy-body (LB) pathology in the TBK1-FTD case (yellow curve) versus absence of signal in controls without clinical and biomarker evidence of neurodegenerative disease (blue curve, n = 20). Relative fluorescence unit (RFU) values are normalized to the maximum intensity of fluorescence of the respective experimental plate. Each curve represents the average of the fours replicates ± standard error of the mean. (d) TDP-43 pathology (clone 1D3) of the left frontal cortex (e) Moderate number of Lewy bodies and Lewy neurites (clone 42) in the left Nucleus basalis of Meynert (f) AT8 positive, mainly glial tau aggregates of the left Caudate Nucleus (g) AT8 positive, neuronal tau aggregates in the left Gyrus dentatus consistent with argyrophilic grain disease (AGD) pathology
One year later, the patient exhibited marked cognitive decline, dysphagia, and significant weight loss (BMI 17.0). Neurological assessment showed brisk symmetric reflexes and ideomotor apraxia, indicative of cortical dysfunction. No parkinsonian features (rigidity, tremor, bradykinesia) emerged. At one-year follow‐up, neuropsychological reassessment documented decline across all cognitive domains. Verbal memory was severely reduced: learning and delayed recall scores were markedly lower, and recognition accuracy decreased substantially. Both semantic and phonemic fluencies were significantly lower. Executive tasks requiring cognitive flexibility, inhibition, and working memory exhibited pronounced slowing, increased errors, and failure to complete certain measures due to cognitive fatigue. Visuo‐constructive copying remained largely intact, but delayed recall of visuospatial designs was significantly impaired. These findings indicate progressive, multifocal cognitive deterioration with relative preservation of basic visuoconstruction (see Table 1). A follow-up tau PET at the one-year mark showed a slight increase in basal ganglia [18F]PI-2620 uptake to an SUVR of 1.32, with cortical tracer binding remaining within normal limits (Fig. 1b).
Post-mortem examination (three years after first symptom onset) included semiautomated immunohistochemistry on a Ventana BenchMark XT platform (Ventana/Roche, Basel, Switzerland) using primary antibodies at the following dilutions: α-synuclein (mouse monoclonal clone 42, Abcam, Cambridge, UK; 1:2,000), TDP-43 (rat monoclonal clone 1D3, in-house; 1:50), phospho-tau (AT8; mouse monoclonal pSer202/pThr205, Invitrogen/Thermo Fisher, Carlsbad, CA, USA; 1:200), RD3 (3R phospho-tau, mouse monoclonal clone 8E6/C11, Millipore; 1:800) and RD4 (four-repeat (4R) phospho-tau, mouse monoclonal clone 1E1/A6, Millipore; 1:4,000). The examination revealed:
FTLD-TDP43 (Subtype A): Widespread TDP-43 protein aggregates, including neuronal cytoplasmic inclusions and short dystrophic neurites, primarily in the frontal and temporal cortices, with involvement of subcortical regions such as the caudate nucleus, putamen, and nucleus accumbens, ventromedial thalamus, amygdala, entorhinal cortex.
LBD (McKeith: limbic-stage, Braak stage 5): mild αSyn pathology according to Braak-stage 5.
Tau-pathology: neuronal and glial pathology regarding shape and distribution pattern characteristic of AGD (Saito 4), ARTAG, PART (Braak&Braak II of VI).
No detectable β-amyloid plaques (Thal-Phase 0).
Secondary findings included mild atherosclerosis and scattered microinfarcts. A summary of the ante-mortem biomarker and imaging results alongside representative post-mortem immunohistochemical findings is shown in Fig. 1.
Discussion and conclusions
The exploration of αSyn co-pathology in FTD is minimal. In one study [4], all FTLD-TDP-43 cases with concurrent LBD pathology had genetic mutations– specifically, two with progranulin mutations and one with a C9orf72 repeat expansion. The clinical phenotype of TBK1-associated FTD is still being elucidated, where some patients show significant early memory impairment, while others may present with extrapyramidal features or cerebellar signs– ranging from PSP-like rigidity and supranuclear gaze palsy to progressive cerebellar ataxia and imaging evidence of mesencephalic or cerebellar atrophy [5, 6]. However, the absence of motor neuron disease (MND) in the current case is noteworthy, given that TBK1 mutations are often associated with FTD-MND.
While TBK1-FTD is primarily associated with TDP-43 (FTLD (subtype B) or MND) pathologies [7], the presence of both AGD, ARTAG and PART in this case adds complexity to the clinical picture. The interpretation of increased tracer uptake, particularly in non-Alzheimer tauopathies such as AGD, ARTAG and PART, requires further investigation, as the exact clinical relevance of these pathologies is not yet fully understood. In our TBK1-FTD case, basal ganglia SUVRs of 1.28 at baseline and 1.32 at follow-up indicate a mild but measurable tau accumulation, intermediate between cognitively healthy controls and classical PSP-RS, demonstrating that [18F]PI-2620 can also detect subtle non-cortical tau pathologies [8]. Post-mortem AT8 immunohistochemistry confirmed tau pathology in the globus pallidus of the TBK1 carrier, but quantitative AT8 occupancy (%N/Area) was only 0.57, markedly lower than the 4.06 ± 2.45 observed in a reference cohort of seven PSP patients, consistent with a copathological rather than a primary 4R tauopathy [9]. Previous work indicates that neuronal and oligodendroglial tau deposits drive the [¹⁸F]PI-2620 PET signal more strongly than astroglial inclusions; a relatively higher proportion of astroglial tau in our case may thus underlie the lower AT8 burden despite positive PET findings. Importantly, [¹⁸F]PI-2620 exhibits high affinity for both 3/4R and 4R tau isoforms, with particular selectivity for aggregated 4R species– making it well suited to capture mixed or non-Alzheimer tau pathologies as seen here [10].
Recent studies suggest that TBK1 may influence tau phosphorylation and aggregation, in addition to its role in TDP-43 proteinopathy [11]. The co-occurrence of AGD, ARTAG and PART in this case– with the first described already by Koriath et al., 2017 - raises the question of whether TBK1 mutations could predispose to or exacerbate tau-driven neurodegeneration. Further research is needed to clarify these molecular links and their clinical implications.
This case underscores the critical need for comprehensive diagnostic approaches that integrate molecular, imaging, and neuropathological assessments. The findings suggest that TBK1 mutations may predispose patients to a multifaceted neurodegenerative process, highlighting the potential and need for personalized targeted therapeutic strategies.
Abbreviations
- 4R
four–repeat
- AGD
Argyrophilic grain disease
- ARTAG
Aging–related tau astrogliopathy
- αSyn
Alpha–synuclein
- BMI
Body mass index
- bvFTD
Behavioral variant frontotemporal dementia
- CSF
Cerebrospinal fluid
- FTD
Frontotemporal dementia
- FTLD
Frontotemporal lobar degeneration
- LBD
Lewy body disease
- PART
Primary age–related tauopathy
- PET
Positron emission tomography
- SAA
Seed amplification assay
- SUVR
standard uptake value ratio
- TDP
43–TAR DNA–binding protein 43
- TBK1
TANK–binding kinase 1
Author contributions
A.M.B.: Conceptualization, Clinical assessment and diagnosis, Recruitment and clinical characterization, Investigation, Writing—Original Draft, Writing—Review & Editing, Project administration (ethics approval), Data curation, Visualization. S.R.: Neuropathological examination, Immunohistochemistry, Data curation, Visualization, Writing—Review & Editing. V.R.: Neuropathological examination, Immunohistochemistry, Data curation, Visualization, Writing—Review & Editing. E.W.: Clinical data collection, Writing—Review & Editing. S.L.: Writing—Review & Editing.S.V.T.: Writing—Review & Editing. J.G.: Tau PET analysis, Data interpretation, Visualization, Writing—Review & Editing. M.B.: Tau PET analysis, Data interpretation, Visualization, Writing—Review & Editing. J.H.: Neuropathological examination, Immunohistochemistry, Data curation, Visualization, Writing—Review & Editing. A.G.: Conceptualization, Supervision, Writing—Review & Editing. G.U.H.: Conceptualization, Clinical assessment and diagnosis, Supervision, Supervision, Writing—Review & Editing, Visualization. J.L.: Conceptualization, Clinical assessment and diagnosis, Supervision, Writing—Review & Editing, Visualization.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. This project was also supported by the German Center for Neurodegenerative Diseases (DZNE, DescribeFTD Study). C.P., G.U.H, J.L. and M.B. were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy: ID 390857198). J.L. was supported by the German Ministry of Research and Education (Förderkennzeichen: FKZ161L0214B, FKZ161L0214C CLINSPECT-M).
Data availability
Due to the nature of this case report and the inclusion of detailed clinical, genetic, and imaging data, the dataset is not publicly available to protect patient confidentiality. Anonymized data may be shared by the corresponding author upon reasonable request, provided appropriate institutional and ethical approvals are in place.
Declarations
Ethics approval and consent to participate
This study was approved by our Institutional Review Board and patient gave consent.
Competing interests
Alexander Bernhardt is inventor of a patent application related to a quantitative Lewy-fold specific alpha-synuclein seed amplification assay. Sebastian Longen reports full-time employment by AESKU.Diagnostics GmbH. In addition, he is inventor of a patent application related to a quantitative Lewy-fold specific alpha-synuclein seed amplification assay. Svenja V. Trossbach reports full-time employment by MODAG GmbH. In addition, she is inventor of a patent application related to a quantitative Lewy-fold specific alpha-synuclein seed amplification assay. Günter Höglinger is inventor of patents related to the treatment of synucleinopathies (United States Patent No.: US 10,918,628 B2, European Patent Patent No.: EP 17 787 904.6–1109 / 3 525 788). Armin Giese reports employment by and being a shareholder of MODAG GmbH. He is inventor of a patent application related to a quantitative Lewy-fold specific alpha-synuclein seed amplification assay. Johannes Levin reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA and Roche, consulting fees from Axon Neuroscience and Biogen, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers. In addition, he reports compensation for serving as chief medical officer for MODAG GmbH, is beneficiary of the phantom share program of MODAG GmbH. He is inventor of a patent application related to a quantitative Lewy-fold specific alpha-synuclein seed amplification assay. All other authors have no other competing interests to report.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhardt AM, Longen S, Trossbach SV, Rossi M, Weckbecker D, Schmidt F et al (2025) A quantitative Lewy-fold-specific alpha-synuclein seed amplification assay as a progression marker for parkinson’s disease. Acta Neuropathol 149:20. 10.1007/s00401-025-02853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Müller K et al (2015) Haploinsufficiency of TBK1 causes Familial ALS and fronto-temporal dementia. Nat Neurosci 18:631–636. 10.1038/nn.4000 [DOI] [PubMed] [Google Scholar]
- 4.Forrest SL, Crockford DR, Sizemova A, McCann H, Shepherd CE, McGeachie AB et al (2019) Coexisting lewy body disease and clinical parkinsonism in frontotemporal Lobar degeneration. Neurology 92:e2472–e2482. 10.1212/WNL.0000000000007530 [DOI] [PubMed] [Google Scholar]
- 5.Koriath CAM, Bocchetta M, Brotherhood E, Woollacott IOC, Norsworthy P, Simón-Sánchez J et al (2017) The clinical, neuroanatomical, and neuropathologic phenotype of TBK1-associated frontotemporal dementia: A longitudinal case report. Alzheimer’s Dementia: Diagnosis Assess Disease Monit 6:75–81. 10.1016/j.dadm.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilke C, Baets J, De Bleecker JL, Deconinck T, Biskup S, Hayer SN et al (2018) Beyond ALS and FTD: the phenotypic spectrum of TBK1 mutations includes PSP-like and cerebellar phenotypes. Neurobiol Aging 62. 10.1016/j.neurobiolaging.2017.10.010.:244.e9-244.e13 [DOI] [PubMed]
- 7.van der Zee J, Gijselinck I, Van Mossevelde S, Perrone F, Dillen L, Heeman B et al (2017) TBK1 mutation spectrum in an extended European patient cohort with frontotemporal dementia and amyotrophic lateral sclerosis. Hum Mutat 38:297–309. 10.1002/humu.23161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazhenets G, Soleimani-Meigooni DN, Thomas W, Mundada N, Brendel M, Vento S et al (2023) [18F]PI-2620 binding patterns in patients with suspected alzheimer disease and frontotemporal Lobar degeneration. J Nucl Med 64:1980–1989. 10.2967/jnumed.123.265856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slemann L, Gnörich J, Hummel S, Bartos LM, Klaus C, Kling A et al (2024) Neuronal and oligodendroglial, but not astroglial, Tau translates to in vivo Tau PET signals in individuals with primary Tauopathies. Acta Neuropathol 148:70. 10.1007/s00401-024-02834-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brendel M, Barthel H, van Eimeren T, Marek K, Beyer L, Song M et al (2020) Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol. 10.1001/jamaneurol.2020.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abreha MH, Ojelade S, Dammer EB, McEachin ZT, Duong DM, Gearing M et al (2021) TBK1 interacts with Tau and enhances neurodegeneration in Tauopathy. J Biol Chem 296:100760. 10.1016/j.jbc.2021.100760 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this case report and the inclusion of detailed clinical, genetic, and imaging data, the dataset is not publicly available to protect patient confidentiality. Anonymized data may be shared by the corresponding author upon reasonable request, provided appropriate institutional and ethical approvals are in place.