Abstract
Background
Strenuous respiratory effort has been proposed as a second hit in severe acute lung injury (ALI), introducing the concept of “patient self-inflicted lung injury” (P-SILI). In an experimental setting, noninvasive continuous positive airway pressure (CPAP) attenuates lung and diaphragmatic injury, but the underlying mechanisms remains elusive. Here we investigate the effects of noninvasive CPAP on global and regional lung strain and diaphragm velocity of contraction and relaxation in an experimental P-SILI model.
Methods
Lung injury was induced in Sprague Dawley rats through surfactant depletion followed by either three hours of standard oxygen therapy (Control group) or CPAP support (CPAP group). Subjects were assessed through inspiratory and expiratory muscle activation. Regional lung and diaphragmatic deformation amplitude (strain) and the rate of change (strain rate) maps were developed using a micro-computed tomography (µCT) scan. Morphometric tissue assessment was carried out to study biological damage.
Results
Compared with the Control group, the CPAP group resulted in: (1) higher SpO2 and lower respiratory rate, nasal flaring, inspiratory and expiratory muscle activation, and minute ventilation at the end of the study; (2) lower global and regional tidal ventilation at the beginning of the study; (3) lower regional inspiratory and expiratory lung strain rate over time; and (4) higher muscle area in the diaphragm morphometric analysis. Furthermore, intragroup analysis showed that only the CPAP group reduced the inspiratory and expiratory muscle activation, the global and regional expiratory lung strain rate and the regional velocity of relaxation of the diaphragm over time.
Conclusions
Standard oxygen therapy resulted in worse patterns of lung strain rate and diaphragm velocity of relaxation, consistent with P-SILI and load-induced diaphragm injury. CPAP resulted in improved lung function, decreased lung strain rate, and diaphragmatic relaxation velocity throughout the respiratory cycle. We conclude that CPAP promotes biomechanical protection in injured lungs and diaphragm, more noticeably during the expiratory phase.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-025-05536-y.
Keywords: Acute lung injury, Continuous positive airway pressure, Spontaneous breathing, Respiratory effort, Strain, Strain rate, Patient self-inflicted lung injury
Background
Strong respiratory effort can lead to a second hit in severely injured lungs, increasing lung edema and collapse, and exacerbating lung injury [1–3]. This phenomenon has been recently termed “patient-self-inflicted lung injury” (P-SILI) [4, 5]. During strenuous respiratory effort, respiratory muscles generate forces that induce high levels of lung strain, distributed heterogeneously throughout the lungs [6]. Early intervention using controlled low tidal volume mechanical ventilation (MV), the standard of care for moderate-to-severe acute respiratory distress syndrome, effectively prevents harmful negative esophageal pressure swings, regional lung strain progression, and lung injury [3, 6, 7].
Considerable attention has been directed toward minimizing the risks associated with MV. Noninvasive respiratory support has emerged as a promising approach, offering benefits such as reduced sedation requirements, mitigation of diaphragm dysfunction, improved patient mobilization, and prevention of infections and ICU-acquired weakness [8]. The COVID-19 pandemic further expedited the adoption of noninvasive respiratory support strategies. In particular, noninvasive continuous positive airway pressure (CPAP) has become an efficient therapy, enhancing oxygenation and reducing respiratory effort, as reported by multiple healthcare centers worldwide [9–13]. More specifically, experimental studies have shown that noninvasive CPAP attenuates lung and diaphragmatic injury during P-SILI, significantly increasing oxygenation and reducing respiratory rate [14]. However, thoroughly studying the mechanisms involved in reducing tissue damage remains elusive. Given the dynamic nature of lung tissue and diaphragmatic deformation during strenuous breathing, injury progression may depend not only on the deformation amplitude (strain) of tissue, but also on its rate of change (strain rate).
In this study, we investigate the effects of noninvasive CPAP on regional lung strain and diaphragmatic deformation and velocity in an experimental P-SILI model. We hypothesized that reducing respiratory effort by CPAP would result in reduced levels of strain rate and biomechanical improvement of the diaphragmatic kinetics at a regional level throughout the respiratory cycle.
Methods
Animal preparation
This work presents an extensive radiological study developed during an experimental study previously reported by our group [14]. The Universidad Andres Bello Bioethics Committee approved the study protocol (Approval Act ID 024/2021). Nineteen Sprague-Dawley rats (sex balanced) were included in this study. The rats were maintained in a humidity, light, and temperature-controlled environment inside a dedicated animal research facility. Food and water were provided ad libitum. To begin the experiment, the rats underwent a brief inhalatory induction with 2% isoflurane. Subsequently, anesthesia was maintained through intraperitoneal injection of ketamine (30 mg•kg−1) and xylazine (5 mg•kg−1). The adequacy of anesthesia was determined by the absence of the pedal reflex, which was monitored every 15 min. In cases where the reflex persisted, inhalatory anesthesia was briefly increased, and half dose of intraperitoneal anesthetics was administered.
Lung injury model and experimental groups
Based on a previous study, we calculated that a sample size of six animals per group was needed to detect a difference of 368 ± 124 mL/min⋅kg on VE between groups [6], with a significance level of 0.05 and a power of 0.8. Block randomization was used to assign the animals to the Control group (standard oxygen therapy, n = 10), and the CPAP group (n = 9). For comparisons, only those animals with high-quality lung and diaphragmatic tomographic images were considered in this study. Specifically, subjects whose image acquisition was incomplete or had motion artifacts that prevented subsequent registration or reconstruction were excluded. After a larynx instillation with 1% lidocaine (10 µL, Drag Pharma, Santiago, Chile), temporary tracheal intubation was carried out using a 16G BD Angiocath® catheter (Becton Dickinson, Utah, USA) for both groups. To induce acute lung injury, saline lavage was performed following a previously reported surfactant depletion and lung collapse model [6, 14]. Briefly, each animal was placed in a supine position after intubation, and 7.5 ml•kg−1 of warm normal saline was flushed into the trachea, followed by suctioning of the residual fluid from the airway. After lavage, the animals were stabilized for 15 min using a volume-controlled MV in prone position. This was achieved using a VentElite® Small Animal Ventilator (Harvard Apparatus, MA, USA) with the following setting: Tidal volume (Vt) of 6 ml kg−1, positive end-expiratory pressure (PEEP) of 5 cmH2O, I:E ratio of 1:2, respiratory rate (RR) of 90/min−1 and an inspiratory fraction of oxygen (FIO2) of 1.
Early extubation was performed following stabilization and detection of spontaneous respiratory effort, and all animals were supported with an FIO2 of 1. The CPAP group was supported with a CPAP device (ResMed H5i®, San Diego, USA) using a Total-Face Anesthesia Masks (VetFlo™, Kent Scientific, USA) to maintain a constant pressure of 6 cmH2O.
Target rectal temperature (38 °C), heart rate, RR, and peripheral oxygen saturation (SpO2) were monitored using the Small Animal Physiological Monitoring System (Harvard Apparatus, MA, USA). Before lung injury (baseline) and after 15 min of clinical stability (T0) in both groups, the vital signs were registered, and the first set of thoracic tomographic images was acquired using the SkyScan 1278® in vivo µCT scanner (Bruker microCT, Kontich, Belgium). Then, the animals were observed and monitored every hour for 3 h (T1, T2, and T3). During these time instants, nasal flaring, inspiratory, and expiratory muscle activation were assessed and registered. Each variable received a score between 0 (absence) and 3 (maximum intensity): nasal flaring was visually determined by noticing the widening of the nostrils during inspiration, inspiratory muscle activation was determined by gentle palpation of the sternocleidomastoid clavicular insertion during inspiration, and expiratory muscles activation was determined by gentle palpation of the abdomen during expiration.
Micro-computed tomography imaging
Micro-computed tomography (µCT) images at end-of-expiration (EE) and end-of-inspiration (EI) were acquired at the beginning (T0) and at the end (T3) of the ventilation stage. The scanner includes a physiological monitoring system to track the breathing of the animal, enabling time-resolved four-dimensional (4D) image sequences, and delivering time series data from which respiratory rate, inspiratory and expiratory times were determined. Respiratory gating based on the thorax movement was employed to reduce the effect of motion artifacts [15]. Scans were performed using a source voltage of 39 kV and a source current of 970 µA. The isotropic voxel resolution was 51.5 μm. The retrospectively synchronized “listmode” scan was performed with an exposure time of 32 ms, a scan rotation of 360°, and a step of 0.25°. The entire scanning procedure took approximately 30 min. µCT images were then post-processed using the software provided by Bruker (NRecon, Tsort, DataViewer, and CTan) to increase the signal-to-noise ratio and enhance the contrast. Ring artifact and hardening filters were employed to improve the quality of the acquired images. Median and unsharp mask filters were applied to reduce the noise and enhance the definition of boundaries in the images. Lung images were segmented using the active-contour method implemented in the ITK-Snap software (University of Pennsylvania, Philadelphia, USA) [15, 16]. Whole lung masks for EE and EI images were generated during the segmentation step. These segmentations were used to compute the end-of-inspiration lung volume (EILV) and end-of-expiration lung volume (EELV). Tidal volume was defined as Vt = EILV-EELV. Minute ventilation (VE) was determined as VE = RR x Vt. Lung strain was calculated as the ratio between Vt and EELV. Inspiratory and expiratory strain rate were calculated as the ratio between strain and inspiratory or expiratory time, respectively.
Lung biomechanical analysis and regional volumetric strain and strain rate maps
Image-based biomechanical deformation analysis was performed following the approach introduced by our group [17, 18]. In brief, the NiftyReg library was employed to perform image registration using segmented EE and EI images to obtain the displacement field between the expiratory and inspiratory states of the lung [19]. A 3D tetrahedral finite-element mesh was created from the whole lung mask at EE. The mesh displacement from EE to EI allowed for the calculation of local volumetric strain. The biomechanical approach used in this work has been summarized in non-technical terms elsewhere [20]. 3D regional lung strain maps indicate local parenchymal stretching [21, 22]. Regional tidal ventilation was assessed through the change in gas fraction on each ROI, 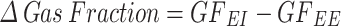 . A value of
. A value of  = 0.1 is equivalent to a difference of 100 HU [22]. To allow for regional comparison between groups, the lungs in each subject were divided into ten segments with approximately equal volumes along the apical-basal (AB) direction and into ten segments along the dorsal-ventral (DV) direction [6]. By intersecting all AB and DV segments, we constructed a matrix of 10 × 10 regions of interest (ROIs) that are independent of one another. During this procedure, some AB and DV segments did not intersect; therefore, some ROIs were considered void. Weighted mean and standard deviation values of regional volumetric strain were computed for each ROI, where the sample includes tetrahedra contained in each ROI, and weighting is performed according to each tetrahedron volume. Regional inspiratory and expiratory lung strain rate were assessed as the ratio between regional strain and inspiratory or expiratory time, respectively, in the matrix of 10 × 10 ROIs.
= 0.1 is equivalent to a difference of 100 HU [22]. To allow for regional comparison between groups, the lungs in each subject were divided into ten segments with approximately equal volumes along the apical-basal (AB) direction and into ten segments along the dorsal-ventral (DV) direction [6]. By intersecting all AB and DV segments, we constructed a matrix of 10 × 10 regions of interest (ROIs) that are independent of one another. During this procedure, some AB and DV segments did not intersect; therefore, some ROIs were considered void. Weighted mean and standard deviation values of regional volumetric strain were computed for each ROI, where the sample includes tetrahedra contained in each ROI, and weighting is performed according to each tetrahedron volume. Regional inspiratory and expiratory lung strain rate were assessed as the ratio between regional strain and inspiratory or expiratory time, respectively, in the matrix of 10 × 10 ROIs.
Global diaphragmatic motion
At T3, and before µCT acquisition, diaphragm function was evaluated using diaphragmatic ultrasound (US) in the right hemithorax to maintain prone positioning. The fur was removed on the right hemithorax, and the depth was set to 3 cm. The broadband linear array probe was set at 10 MHz and placed at the junction of the anterior and posterior axillary lines and the lower edge of the right costal arch (Wireless Ultrasound Probe Double Head Linear + Convex, ATL Milano, Italy). The liver was set as the diaphragm acoustic window. The probe was pointed to the head and back, and diaphragmatic excursion, inspiratory and expiratory time, and I/E ratio were displayed under M-mode (time-motion mode). Diaphragmatic excursion reflects the amplitude of the movement of the diaphragm during the respiratory cycle. Velocity of contraction was measured as the ratio between diaphragmatic excursion and inspiratory time. Velocity of relaxation was measured as the ratio between diaphragmatic excursion and expiratory time. The mean of two valid measurements were recorded, defined as those with < 10% difference. At T3, after US, a second set of tomographic images were obtained.
Diaphragm biomechanical analysis and regional strain and strain rate maps
Whole tomographic diaphragmatic deformation and velocity were assessed by studying the basal region of the thorax. The whole-lung surface was initially isolated from the tetrahedral mesh, resulting in a triangular mesh clipped to retain the basal surface. Then, the normal for each triangle was computed, and a threshold operation based on the longitudinal component of the normal was applied such that the bottom of the lung was appropriately isolated from the rest. Finally, the displacement fields that stemmed from a previous biomechanical analysis were then analyzed, and the maximum longitudinal displacement from each animal was assessed.
The regional analysis was performed by defining ten equidistant internal points of interest in the ventro-dorsal direction on the diaphragm surface. Each of these was used to define a left-to-right line where the fields of interest were sampled along at multiple equidistant points. This results in a collection of measurements, which were then characterized by their median values for each point of interest. We characterize the end-expiratory and end-inspiratory position of the diaphragm, quantifying its shape at both states, and its displacement field, indicating the diaphragmatic strain pattern. Velocity of contraction and relaxation were assessed as the ratio between displacement and inspiratory or expiratory time, in 10 dorsal-to-ventral ROIs.
Lung and diaphragmatic computationally assisted morphometric assessments
At the end of the study, animals were placed in supine position. Next, euthanasia was done by a lethal intraperitoneal dose of thiopental (200 mg/kg). Subsequently, the chest cavity was opened, and lungs and diaphragms were removed. The right lung and diaphragm were fixated in PBS-4% formaldehyde for 24 h. The lungs were cross-sectioned every 1 mm from apex to base (22–24 slices per lung), analyzing the twentieth (basal) lung slices. Computer-assisted morphometric evaluations were performed (ImageProPlus®, ver. 7.0.1.658, Media Cybernetics, Rockville, MD, USA) to determine lung aeration (expressed as µm2), and muscular and interstitial areas in diaphragms (expressed as µm2). 100x magnification field was employed to report the mean of 5 random fields. A schematic depicting the experimental protocol can be found in Figure E1 (Additional file 1).
Statistical analysis
All statistical analyses were performed in Python using SciPy v1.11.1. Normality of the data was evaluated using the Shapiro-Wilk test. Statistical differences between T0 and T3 in ROI-matrix data (Inspiratory and Expiratory Strain Rate) were assessed using the Wilcoxon signed-rank test. Intergroup comparisons between Control and CPAP groups at T0 and T3 (EE Intensity, Tidal Ventilation, Volumetric Strain, and Inspiratory and Expiratory Strain Rate) were evaluated using the Mann-Whitney U-test. Welch’s t-test was used for intergroup comparisons and Student’s t-test for intragroup comparisons of diaphragm displacement-derived data and morphometric analysis. Statistical significance was set at p < 0.05. Data were presented as median (IQR).
Results
High-quality whole 4D lung and diaphragm µCT reconstructions were achieved in thirteen animals: six in the CPAP group and seven in the Control group. Three subjects were excluded from further analysis from the CPAP group due to movement artifacts, and three from the Control group (two due to movement artifacts and one due to death prior to completion of the second image acquisition). There was no difference in total dose and number of repetitions of anesthetics between groups.
Physiological variables
Table 1 reports the physiological parameters for both groups. At baseline, no significant differences were observed between groups in terms of weight, sex, and physiological variables. Surfactant depletion resulted in a SpO2 < 90% in both groups post injury (p < 0.05). At the end of the study (T3), the CPAP group had a significantly higher SpO2 and lower RR, nasal flaring, inspiratory and expiratory muscle activation, and VE than the Control group (all p < 0.05). There was no difference in Vt between the Control and CPAP groups at T0 nor at T3. Intragroup analysis showed a reduction of inspiratory and expiratory muscle activation in the CPAP group at T2 and T3 (p < 0.05) (Table 1).
Table 1.
Physiologic data for the experimental groups at baseline and during the ventilation
| Control group | CPAP group | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SpO2 | ||||||||||
| Baseline | 100 (100–100) | 99 (99–100) | 0.05 | |||||||
| Post injury | 83 (82–86) | 83 (80–86) | 0.67 | |||||||
| T0 | 90 (84–91) | 88 (86–89) | 0.34 | |||||||
| T1 | 92 (89–94) | 88 (88–90) | 0.16 | |||||||
| T2 | 95† (92–97) | 94 (94–97) † | 1 | |||||||
| T3 | 91 (88–92) | 96 (93–99) *† | < 0.01 | |||||||
| Respiratory rate | ||||||||||
| Baseline | 60 (56–71) | 60 (52–72) | 1 | |||||||
| Post injury | 68 (66–90) | 79 (68–84) | 0.54 | |||||||
| T0 | 78 (51–90) | 71 (62–80) | 0.93 | |||||||
| T1 | 70 (62–89) | 70 (58–90) | 1 | |||||||
| T2 | 70 (66–99) | 78 (77–81) | 0.67 | |||||||
| T3 | 92 (82–108) | 76 (68–82) * | 0.02 | |||||||
| Nasal flaring | ||||||||||
| Baseline | 0 (0–0) | 0 (0–0) | 1 | |||||||
| Post injury | 2 (1.5–2.5) | 2 (2-2.75) | 0.38 | |||||||
| T0 | 2 (1.5-2) | 2 (2–2) | 0.49 | |||||||
| T1 | 2 (1.5-2) | 2 (2–2) | 0.49 | |||||||
| T2 | 2 (1.5-2) | 1 (1-1.75) | 0.14 | |||||||
| T3 | 2 (2–2) | 1 (1-1.75) * | 0.01 | |||||||
| Inspiratory muscle activation | ||||||||||
| Baseline | 0 (0–0) | 0 (0–0) | 1 | |||||||
| Post-injury | 2 (1.5-3) | 3 (3–3) | 0.16 | |||||||
| T0 | 2 (1.5–2.5) | 2.5 (2–3) | 0.19 | |||||||
| T1 | 2 (2-2.5) | 2.5 (2–3) | 0.54 | |||||||
| T2 | 2 (1–3) | 1 (1-1.75) † | 0.25 | |||||||
| T3 | 3 (1.5-3) | 1 (1–1) *† | 0.01 | |||||||
| Expiratory muscle activation | ||||||||||
| Baseline | 0 (0–0) | 0 (0–0) | 1 | |||||||
| Post injury | 3 (1.5-3) | 3 (2.3-3) | 0.62 | |||||||
| T0 | 3 (1.5-3) | 3 (3–3) | 0.44 | |||||||
| T1 | 3 (1.5-3) | 2.5 (2–3) | 0.94 | |||||||
| T2 | 3 (2–3) | 2 (2–2) † | 0.07 | |||||||
| T3 | 3 (3–3) | 2 (1.3-2) *† | < 0.01 | |||||||
| Vt (ml/kg) | ||||||||||
| T0 | 6.1 (5.8–6.5) | 5.5 (4.9-6.0) | 0.42 | |||||||
| T3 | 6.6 (5.5–7.5) | 5.8 (5.4–6.3) | 0.31 | |||||||
| VE (ml/kg·min−1) | ||||||||||
| T0 | 497 (310–581) | 403 (303–506) | 0.57 | |||||||
| T3 | 639 (449–745) | 419 (378–490) * | 0.04 | |||||||
Data are expressed as median (interquartile range)
Significant within-group differences are denoted by P < 0.05
Intergroup analysis: *Significant difference comparing CPAP and control groups
Time-dependent analysis: †Significant changes were detected between T0 and T1, T2, or T3
CPAP: Continuous positive airway pressure; SpO2: Oxygen saturation as measured by pulse oximetry; Vt: Tidal volume; VE: Minute ventilation
Global biomechanical lung analysis
At a global level, there were no differences between the Control and CPAP groups in terms of EELV, lung strain, inspiratory and expiratory strain rate (Table E1, Additional file 2). The CPAP group resulted in lower tidal ventilation at T0 (0.05 [0.04–0.05]) vs. 0.02 [0.01–0.03], p = 0.01). Intragroup analysis shows that only the CPAP group reduced the expiratory strain rate as time progressed (1.46 [1.14–1.81] to 0.60 [0.52–0.75] s−1, p = 0.03)
Global diaphragmatic motion
No differences were detected between the Control and CPAP groups in diaphragmatic excursion (assessed by both US and CT), inspiratory time, and I: E ratio. The CPAP group displayed a trend towards longer expiratory times (0.56 [0.53–0.64] vs. 0.47 [0.32–0.52]) s, p = 0.1), slower velocity of diaphragmatic contraction (6.9 [5.7-7.0] vs. 7.8 [7.3-8.0]) mm/s, p = 0.07) and slower velocity of diaphragmatic relaxation (3.2 [2.7–3.6] vs. 4.1 [2.9-5.0]) mm/s, p = 0.10) than the Control group (Table E2, Additional file 3).
Regional biomechanical lung analysis
In the 10 × 10 ROIs lung matrix, 95 ROIs achieve the conditions to be used for the regional biomechanical analysis.
At a regional level, the CPAP group showed lower regional tidal ventilation in 21 ROIs and regional strain in 15 ROIs at T0 (Fig. 1).
Fig. 1.
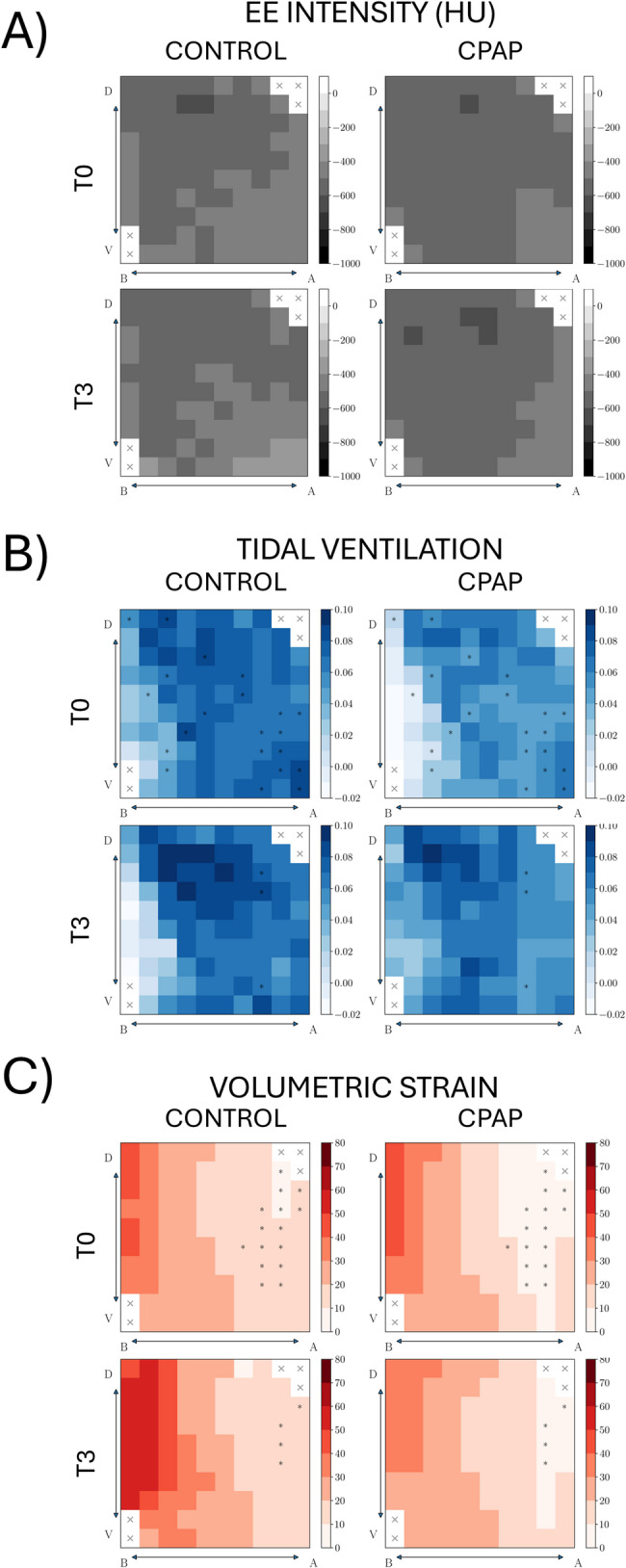
Regions-of-interest (ROI) array heat maps in the apical–basal (A-B) and ventral–dorsal (V-D) directions at the beginning (T0) and the ending (T3) of the study (A) End-expiratory intensity. (B) Tidal ventilation. (C) Volumetric strain Significant within-group differences are denoted by P < 0.05 *Significant difference comparing CPAP and control groups
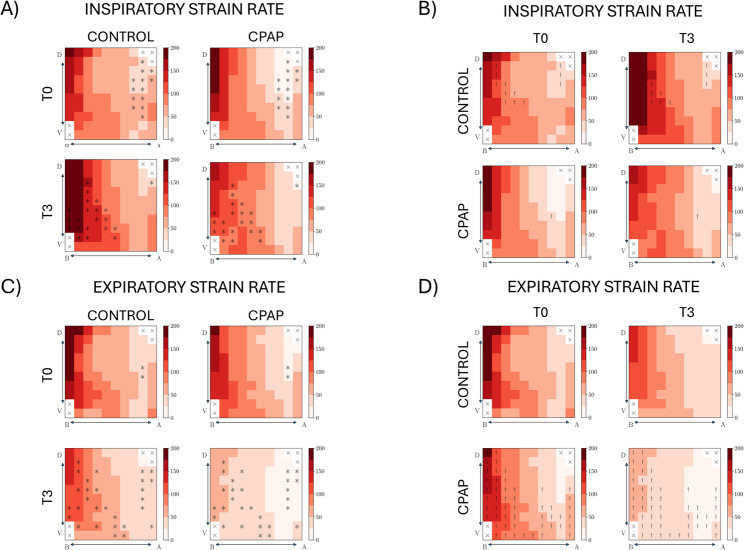
Regarding the inspiratory phase, the CPAP group resulted in a lower regional strain rate at T0 (13 ROIs) and T3 (21 ROIs) (Fig. 2A). Intragroup analysis showed that the Control group increased regional inspiratory strain rate in 12 ROIs, predominantly in the basal regions, with differences in only 1 ROI in the CPAP group (Fig. 2B).
Fig. 2.
Regional volumetric strain rate in the dorsoventral (D‹—›V) and basoapical (B‹—›A) axes of the lung. (A, C) Group-wise comparisons between control and CPAP at the beginning (T0) and end (T3) of the study, during the inspiratory phase (A) and expiratory phase (C). (B, D) Time-wise comparisons between T0 and T3 for each group during the inspiratory phase (B) and expiratory phase (D). † denotes P < 0.05 for significant differences between T0 and T3. * denotes P < 0.05 for significant differences between CPAP and control groups.
Regarding the expiratory phase, the CPAP group resulted in a lower regional strain rate at T0 (2 ROIs), and T3 (28 ROIs) (Fig. 2C). Intragroup analysis showed that the CPAP group decreased regional strain rate in 50 ROIs, with no differences in any ROI in the Control group (Fig. 2D).
Regional biomechanical diaphragm analysis
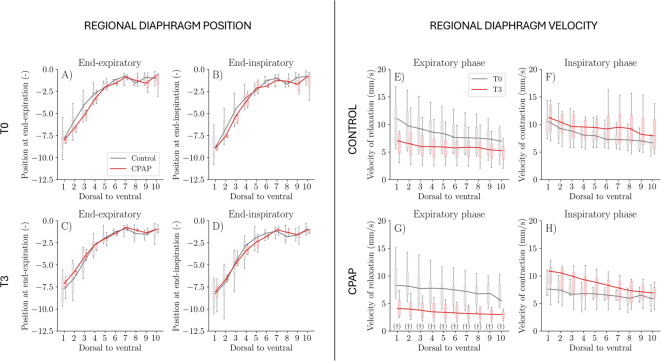
Ten equidistant points of interest in the ventro-dorsal direction on the diaphragm surface were used for the regional biomechanical analysis. No significant differences were observed between groups in terms of regional diaphragm position (Fig. 3A-D) and regional displacement (data not shown), both at the end of expiration and at the end of inspiration.
Fig. 3.
Regional diaphragmatic biomechanics along the ventro-dorsal axis. Left panels: Diaphragm position at end-expiration (A, C) and end-inspiration (B, D) at the beginning (T0; A, B) and end (T3; C, D) of the study for control and CPAP groups. Right panels: Regional diaphragm velocity of contraction (E, F) and relaxation (G, H) over time for control (E, G) and CPAP (F, H) groups. Ten equidistant points of interest are shown from ventral to dorsal regions. Solid lines indicate group medians; boxplots represent intersubject variability. † denotes P< 0.05 for significant differences between T0 and T3. * denotes P < 0.05 for significant differences between CPAP and control groups.
No significant differences were observed between groups in terms of regional diaphragm velocity of contraction and relaxation (Fig. 3E-H). Intragroup analysis in the CPAP group shows a reduction in the velocity of relaxation in all points of interest (Fig. 3G).
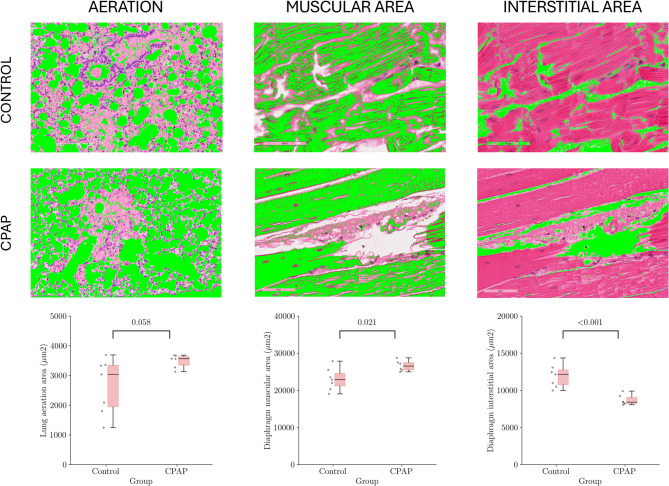
Morphometric analysis
The CPAP group displayed a trend towards higher lung aeration than the Control group (3563 [3344–3640] vs. 3039 [1944–3344] µm2, p = 0.06). The diaphragmatic morphometric analysis showed higher muscular area in the CPAP group (26611 [25467–27451] vs. 23024 [21181–24534] µm2, p = 0.02), and lower interstitial area in the CPAP group when compared to the Control group (8697 [8223–9042] vs. 11926 [10748–12754] µm2, p < 0.001). Comparisons and representative morphometric images are shown in Fig. 4.
Fig. 4.
Morphometric analysis of lung aeration, muscular and interstitial areas of the diaphragm Data are expressed as median (IQR). Significant within-groups differences are denoted by p < 0.05. Representative images of lung and diaphragm morphometry for each study group (hematoxylin and eosin, 100x). Findings of interest were highlighted in green Data are expressed as median (interquartile range). Significant within-group differences are denoted by P < 0.05. *Significant difference comparing CPAP and control groups
Discussion
In a severe ALI model, our research revealed significant findings related to the biomechanical impact of noninvasive CPAP on lungs and diaphragm. In contrast with the Control group, the CPAP group resulted in: (1) higher oxygenation and lower respiratory rate, nasal flaring, inspiratory and expiratory muscle activation, and VE at T3; (2) lower global and regional tidal ventilation at T0; (3) lower regional volumetric strain at T0; (4) lower regional lung strain rate at T0 and T3 during the inspiratory phase; (5) lower regional lung strain rate at T3 during the expiratory phase; (6) higher muscle area in the diaphragm morphometric analysis; and (7) a marked trend to higher aeration in the lung morphometric analysis. In addition, intragroup analysis showed that the CPAP group reduced the inspiratory and expiratory muscle activation at T2 and T3, global and regional expiratory lung strain rate, and regional velocity of relaxation of the diaphragm as time progressed. In contrast, the Control group increased regional lung strain rate during the inspiratory phase. Although CPAP induced regional biomechanical changes throughout the respiratory cycle, these were most noticeable during expiration.
The harmful effects of respiratory effort as a promoter of lung injury have been supported by experimental studies and by indirect clinical evidence. Studies on sheep have shown that pharmacologically induced hyperventilation causes lung injury in previously healthy lungs [23]. In severely injured lungs, respiratory effort induced higher esophageal pressure swings, uneven ventilation distribution, more profound hypoxemia, and higher lung injury [3]. Likewise, in unsupported ALI animals, strenuous respiratory effort caused regional lung strain and heterogeneity as time progressed, and severe vascular injury in the juxta-diaphragmatic regions [6, 7]. We proposed that it could be caused by large blood flow oscillations resulting from cyclic inspiratory negative pressures. Early use of controlled MV prevented hypoxemia, progression of lung injury, and the related mechanobiological phenomena [6, 7].
More recently, noninvasive CPAP also prevented hypoxemia and lung injury compared to standard oxygen therapy on a murine P-SILI model, mainly in the vascular side of the blood-gas barrier [14]. It is well-known that PEEP can prevent ventilator-induced lung injury and improve lung physiology in animals with injured lungs [24]. Furthermore, additional protective effects can be attributed to decreased energy dissipation in the lungs at both global (lower RR and VE) and regional levels, due to a lower strain rate throughout the respiratory cycle [25–28], which reinforces the crucial role of inspiratory strain rate in lung injury [29]. However, it is important to highlight that the application of positive end-expiratory pressure improved several parameters related to expiratory physiology, such as lung and diaphragmatic deformation velocities during expiration, and expiratory muscle activation, in addition to prevent lung and diaphragm injury. Overall, this suggests that active expiration could also be a promoter of P-SILI.
In our study, CPAP increased EELV and oxygenation, in addition to prevent pulmonary vascular injury, decreasing all these afferent inputs to the respiratory center [30], thereby reducing expiratory effort and allowing exhalation to proceed more passively. The marked attenuation of regional expiratory strain rate induced by CPAP was likely prompted by a redistribution of EELV due to PEEP, resulting in a more homogeneous strain distribution.
It is interesting to note that we had a discrepancy between the EELV measured by histology and tomography. In the former, there was a clear trend toward higher aeration in the CPAP group, a predictable consequence of PEEP use [25]. In the case of high lung recruitability (expected in a surfactant depletion model), PEEP should have increased EELV due to lung recruitment. On the contrary, PEEP should have also increased EELV due to overdistension. Surprisingly, there were no differences between groups in tomographic EELV. We speculate that the presence of dynamic air trapping in the Control group counteracted the predictable effect of CPAP on EELV. Air trapping can be intuited in the presence of a higher respiratory rate and a marked tendency towards shorter expiratory time, avoiding full deflation. This should have disappeared when the lungs were removed for morphological analysis.
The harmful effects of strong respiratory effort on the diaphragm are less known. Strenuous respiratory effort induces diaphragm edema, inflammation, fiber fragmentation, sarcolemma fragility, and even necrosis, in accordance with load-induced diaphragm injury and underassistance myotrauma [14, 31, 34]. Diaphragm inflammation and proteolysis have been reported under conditions of increased static transpulmonary driving pressure, even with mild ALI and physiological Vt [35]. In line with previous studies, we found that noninvasive CPAP regionally modified the velocity of relaxation, better preserving its morphology. These anatomical and functional consequences were associated with an attenuation of compensatory respiratory symptoms [30, 36–46]. PEEP induces shortening of the diaphragm (modifying the force-length relationship), thus limiting its ability to generate force [25, 26, 30].
A recent experimental study reported that, compared to standard oxygen therapy, CPAP reduced damage to both the lungs and diaphragm, supporting a common pathophysiological trigger for P-SILI and load-induced diaphragm injury [14]. It is interesting to note that the magnitude of diaphragm injury (necrosis and fragmentation of muscle fibers) is disproportionately greater than the concomitant lung injury (edema, hyperemia, and hemorrhage), despite sharing the same mechanical origin [14]. Since tissue stress is directly related to strain rate and viscosity, the higher viscosity of the diaphragm muscle is a possible explanation.
Our study has several limitations: (1) We did not measure esophageal pressure, which is the gold-standard method for monitoring respiratory effort. Operational restrictions and scanning-time demand imposed by the µCT (over 30 min for any full image acquisition) prevent its use; (2) We also did not measure diaphragm electromyography, which is the reference method for quantifying respiratory drive under spontaneous breathing, due to technical restrictions (disproportionate size of conventional EAdi catheter or surface electrodes for rodents); (3) Anesthetic drugs could modify the respiratory pattern; (4) Dynamic but not static strain was quantified. This prevented quantification of strain related to PEEP volume; (5) Lung image registration and biomechanical analysis can only be computed in aerated regions. This technical limitation prevented deformation assessment in collapsed lung tissue; (6) Animals were in prone position, which may affect the respiratory pattern, and limiting extrapolation to subjects in supine position; (7) The small number of subjects might lead to type II error; and (8) Finally, caution should be exercised when extrapolating experimental findings to the clinical setting. Despite these limitations, our study highlights global and regional biomechanical phenomena, both in the lungs and diaphragm, throughout the respiratory cycle, which explain how CPAP attenuates tissue damage in experimental self-inflicted lung injury. The approach used will allow studying at a mechanobiological level the effect of other noninvasive respiratory support therapies in the future, specifically regional strain rate and the kinetics of the diaphragm displacement.
Conclusions
Standard oxygen therapy in ALI subjects leads to a significant increase in regional inspiratory and expiratory lung strain rate and regional velocity of relaxation of the diaphragm, consistent with P-SILI and load-induced diaphragm injury. CPAP can be effective in attenuating these biomechanical effects and diaphragm injury. CPAP not only reduces compensatory symptoms and VE but also improves oxygenation, diaphragm regional kinetics, and prevents the regional lung strain rate during the whole respiratory cycle, particularly during expiration. These findings suggest that CPAP can be an integrated lung- and diaphragm-protective approach in this setting. Further research is needed to explore in depth the underlying mechanobiological pathways involved, the long-term effects of different ventilatory support strategies, and the lung and diaphragm injury induced by active expiration on experimental P-SILI.
Electronic supplementary material
Supplementary Material 1. Figure E1. Schematic flowchart and timeline of the experimental protocol
Supplementary Material 2. Table E1. Global lung biomechanical parameters for Control and CPAP groups at the beginning (T0) and at the end (T3) of the study
Supplementary Material 3. Table E2. Global diaphragmatic motion for the experimental groups at the ending (T3) of the study, either for Control, or CPAP groups
Acknowledgements
Authors acknowledge the financial support of the Agencia Nacional de Investigación y Desarrollo ANID Chile through grants Fondecyt Regular 1220322, Fondecyt Regular 1220465, and intramural grant DI-07-20/CB by Universidad Andrés Bello. Authors further acknowledge the support of Plataforma Experimental Bio-CT, Faculty of Dentistry from Universidad de Chile (FONDEQUIP EQM150010) in performing the micro-CT image acquisition.
Abbreviations
- ALI
Acute lung injury
- CPAP
Continuous positive airway pressure
- µCT
Micro Computed tomography
- EELV
End-of-expiration lung volume
- MV
Mechanical ventilation
- PEEP
Positive end-expiratory pressure
- P-SILI
Patient self-inflicted lung injury
- ROI
Region of interest
- RR
Respiratory rate
- SpO2
Oxygen saturation
- VE
Minute ventilation
- Vt
Tidal volume
Author contributions
AP, PC, BE and DEH conceived the research idea. PC and BE secured funding. PC, BE and SR designed and performed the animal experiments and acquired data; AP, NAR and DEH performed the biomechanical analysis. CG analyzed and wrote the methods and results of histology; all authors analyzed and interpreted the experimental, biomechanical and histological results, drafted the manuscript, approved the submitted version of the manuscript and have agreed both to be personally accountable for their contributions and integrity of this work.
Funding
This work received funding from Agencia Nacional de Investigación y Desarrollo, ANID Chile, through grants Fondecyt Regular 1220322, Fondecyt Regular 1220465, and Proyectos en Ciencias Biomédicas y Clínicas 2021 Universidad Andrés Bello DI-07-20/CB.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Universidad Andrés Bello Bioethics Committee, approval ID #05/2016 and #020/2017.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med. 2013;41:536–45. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort May worsen lung injury. Crit Care Med. 2012;40:1578–85. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann MC, Cruces P, Díaz F, et al. Spontaneous breathing promotes lung injury in an experimental model of alveolar collapse. Sci Rep. 2022;12:12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–42. [DOI] [PubMed] [Google Scholar]
- 5.Cruces P, Retamal J, Hurtado DE, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. 2020;24:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurtado DE, Erranz B, Lillo F, et al. Progression of regional lung strain and heterogeneity in lung injury: assessing the evolution under spontaneous breathing and mechanical ventilation. Ann Intensive Care. 2020;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruces P, Erranz B, González C, Diaz F. Morphological differences between patient Self-inflicted and Ventilator-induced lung injury: an experimental study. Am J Respir Crit Care Med. 2023;207:780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on noninvasive support. Minerva Anestesiol. 2019;85:1014–23. [DOI] [PubMed] [Google Scholar]
- 9.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315:2435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliberti S, Radovanovic D, Billi F, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. 2020;56(4):2001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosentini R, Brambilla AM, Aliberti S, et al. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Chest. 2010;138:114–20. [DOI] [PubMed] [Google Scholar]
- 12.Brambilla AM, Aliberti S, Prina E, et al. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014;40:942–9. [DOI] [PubMed] [Google Scholar]
- 13.Ashish A, Unsworth A, Martindale J, et al. CPAP management of COVID-19 respiratory failure: a first quantitative analysis from an inpatient service evaluation. BMJ Open Respir Res. 2020;7:e000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruces P, Erranz B, Pérez A, et al. Noninvasive CPAP is a lung- and Diaphragm-protective approach in Self-inflicted lung injury. Am J Respir Crit Care Med. 2024;209:1022–5. 10.1164/rccm.202309-1629LE. [DOI] [PubMed] [Google Scholar]
- 15.Cruces P, Erranz B, Lillo F, et al. Mapping regional strain in anesthetised healthy subjects during spontaneous ventilation. BMJ Open Respir Res. 2019;6:e000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yushkevich PA, Piven J, Hazlett HC, et al. User- guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–28. [DOI] [PubMed] [Google Scholar]
- 17.Hurtado DE, Villarroel N, Retamal J, Bugedo G, Bruhn A. Improving the accuracy of registration-based Biomechanical analysis: a finite element approach to lung regional strain quantification. IEEE Trans Med Imaging. 2016;35:580–8. [DOI] [PubMed] [Google Scholar]
- 18.Hurtado DE, Villarroel N, Andrade C, Retamal J, Bugedo G, Bruhn A. Spatial patterns and frequency distributions of regional deformation in the healthy human lung. Biomech Model Mechanobiol. 2017;16:1413–23. [DOI] [PubMed] [Google Scholar]
- 19.Modat M, Ridgway GR, Taylor ZA, et al. Fast free-form deformation using graphics processing units. Comput Methods Programs Biomed. 2010;98:278–84. [DOI] [PubMed] [Google Scholar]
- 20.Retamal J, Hurtado D, Villarroel N, et al. Does regional lung strain correlate with regional inflammation in acute respiratory distress syndrome during nonprotective ventilation? An experimental Porcine study. Crit Care Med. 2018;46:e591–9. [DOI] [PubMed] [Google Scholar]
- 21.Bodduluri S, Newell JD, Hoffman EA, Reinhardt JM. Registration-based lung mechanical analysis of chronic obstructive pulmonary disease (COPD) using a supervised machine learning framework. Acad Radiol. 2013;20:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–6. [DOI] [PubMed] [Google Scholar]
- 23.Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D. Acute respiratory failure following Pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15:8–14. [DOI] [PubMed] [Google Scholar]
- 24.Algera AG, Pisani L, Chaves RCF, et al. PROVE network investigators. Effects of peep on lung injury, pulmonary function, systemic circulation and mortality in animals with uninjured lungs-a systematic review. Ann Transl Med. 2018;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morais CCA, Koyama Y, Yoshida T, et al. High positive End-Expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med. 2018;197:1285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellegrini M, Hedenstierna G, Larsson AS, Perchiazzi G. Inspiratory efforts, positive End-Expiratory pressure, and external resistances influence intraparenchymal gas redistribution in mechanically ventilated injured lungs. Front Physiol. 2021;11:618640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widing H, Chiodaroli E, Liggieri F, Mariotti PS, Hallén K, Perchiazzi G. Homogenizing effect of PEEP on tidal volume distribution during neurally adjusted ventilatory assist: study of an animal model of acute respiratory distress syndrome. Respir Res. 2022;23:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widing CH, Pellegrini M, Larsson A, Perchiazzi G. The effects of positive end-expiratory pressure on transpulmonary pressure and recruitment–derecruitment during neurally adjusted ventilator assist: a continuous computed tomography study in an animal model of acute respiratory distress syndrome. Front Physiol. 2019;10:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Protti A, Maraffi T, Milesi M, et al. Role of strain rate in the pathogenesis of Ventilator-Induced lung edema. Crit Care Med. 2016;44:e838–845. [DOI] [PubMed] [Google Scholar]
- 30.Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically ill patients. Pathophysiology and clinical implications. Am J Resp Crit Care. 2020;201:20–32. [DOI] [PubMed] [Google Scholar]
- 31.Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1734–9. [DOI] [PubMed] [Google Scholar]
- 32.Jiang TX, Reid WD, Belcastro A, Road JD. Load dependence of secondary diaphragm inflammation and injury after acute inspiratory loading. Am J Respir Crit Care Med. 1998;157:230–6. [DOI] [PubMed] [Google Scholar]
- 33.Pinochet R, Escobar M, Díaz F, Cruces P. Trabajo Resistivo de La ventilación: efectos En Comportamiento locomotor y regulación mTOR. Reem. 2021;8:8–18. [Google Scholar]
- 34.Ebihara S, Hussain SN, Danialou G, Cho WK, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165:221–8. [DOI] [PubMed] [Google Scholar]
- 35.Pinto EF, Santos RS, Antunes MA, et al. Static and dynamic transpulmonary driving pressures affect lung and diaphragm injury during pressure-controlled versus pressure-support ventilation in experimental mild lung injury in rats. Anesthesiology. 2020;132:307–20. [DOI] [PubMed] [Google Scholar]
- 36.Plens GM, Droghi MT, Alcala GC, et al. Expiratory muscle activity counteracts positive End-Expiratory pressure and is associated with Fentanyl dose in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2024;209:563–72. [DOI] [PubMed] [Google Scholar]
- 37.Road JD, Leevers AM. Inspiratory and expiratory muscle function during continuous positive airway pressure in dogs. J Appl Physiol. 1990;68(3):1092–100. [DOI] [PubMed] [Google Scholar]
- 38.Torres A, Kacmarek RM, Kimball WR, Qvist J, Stanek K, Whyte R, Zapol WM. Regional diaphragmatic length and EMG activity during inspiratory pressure support and CPAP in awake sheep. J Appl Physiol (1985). 1993;74(2):695–703. [DOI] [PubMed] [Google Scholar]
- 39.Soilemezi E, Koco E, Tsimpos C, Tsagourias M, Savvidou S, Matamis D. Effects of continuous positive airway pressure on diaphragmatic kinetics and breathing pattern in healthy individuals. Respirology. 2016;21(7):1262–9. [DOI] [PubMed] [Google Scholar]
- 40.Sassoon CS, Zhu E, Fang L, Sieck GC, Powers SK. Positive end-expiratory airway pressure does not aggravate ventilator-induced diaphragmatic dysfunction in rabbits. Crit Care. 2014;18(5):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haberthür C, Guttmann J. Short-term effects of positive end-expiratory pressure on breathing pattern: an interventional study in adult intensive care patients. Crit Care. 2005;9(4):R407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widing H, Pellegrini M, Chiodaroli E, Persson P, Hallén K, Perchiazzi G. Positive end-expiratory pressure limits inspiratory effort through modulation of the effort-to-drive ratio: an experimental crossover study. Intensive Care Med Exp. 2024;12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen D, Jonkman AH, De VHJ, et al. Positive end-expiratory pressure affects geometry and function of the human diaphragm. J Appl Physiol. 2021;131:1328–39. [DOI] [PubMed] [Google Scholar]
- 44.Firstiogusran AMF, Yoshida T, Hashimoto H, Iwata H, Fujino Y. Positive end-expiratory pressure and prone position alter the capacity of force generation from diaphragm in acute respiratory distress syndrome: an animal experiment. BMC Anesthesiol. 2022;22:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Schans CP, de Jong W, de Vries G, Postma DS, Koëter GH, van der Mark TW. Effect of positive expiratory pressure on breathing pattern in healthy subjects. Eur Respir J. 1993;6:60–6. [PubMed] [Google Scholar]
- 46.Pellegrini M, Hedenstierna G, Roneus A, Segelsjö M, Larsson A, Perchiazzi G. The diaphragm acts as a brake during expiration to prevent lung collapse. Am J Respir Crit Care Med. 2017;195:1608–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Figure E1. Schematic flowchart and timeline of the experimental protocol
Supplementary Material 2. Table E1. Global lung biomechanical parameters for Control and CPAP groups at the beginning (T0) and at the end (T3) of the study
Supplementary Material 3. Table E2. Global diaphragmatic motion for the experimental groups at the ending (T3) of the study, either for Control, or CPAP groups
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.