Abstract
Background
This study aims to compare the therapeutic efficacy of whole brain radiotherapy (WBRT) versus WBRT plus simultaneous integrated boost (WBRT + SIB) in patients with brain metastases (BMs) from small cell lung cancer (SCLC).
Methods
A retrospective analysis was conducted on 127 patients with BMs from SCLC who received brain radiotherapy between 2014 and 2023 at the Cancer Hospital of the Chinese Academy of Medical Science. Among them, 71 patients underwent WBRT (25.0–54.0 Gy in 10–21 fractions), while 56 patients received WBRT + SIB (SIB to metastases: 18.0–60.0 Gy in 5–20 fractions). The overall survival (OS), intracranial progression-free survival (iPFS), objective response rate (ORR), and local control rate (LCR) were evaluated to assess the efficacy of the treatments.
Results
With a median follow-up of 14.9 months, the median OS was significantly longer in the WBRT + SIB group compared to the WBRT group (18.0 vs. 11.7 months). Similarly, the iPFS was extended in the WBRT + SIB group (12.2 vs. 7.6 months). Kaplan-Meier analysis revealed that WBRT + SIB significantly improved OS in patients with SCLC of BMs (P = 0.009). Subgroup analysis indicated that male patients, age < 60 years old, and multiple intracranial metastases benefited more from WBRT + SIB. Interaction tests suggested that age significantly influence the efficacy of WBRT + SIB, with patients < 60 years old deriving more benefit (P = 0.049). Concurrent WBRT + SIB with anti-angiogenic targeted therapy significantly improved iPFS (P < 0.001).
Conclusions
WBRT + SIB can prolong the OS in SCLC patients with BMs, with younger age and those receiving anti-angiogenesis therapy potentially achieving additional survival benefits.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14593-z.
Keywords: Small cell lung cancer (SCLC), Brain metastases, Whole brain radiotherapy (WBRT), Simultaneous integrated boost (SIB), Therapeutic efficacy
Background
Small cell lung cancer (SCLC) is a poorly differentiated, high-grade neuroendocrine tumor, comprising about 15% of all lung cancer cases [1]. Brain metastases (BMs) occur in approximately 50% of SCLC patients due to the cancer’s aggressive nature and invasive tendencies. Indeed, more than 18% of SCLC patients present with BMs at the time of primary diagnosis, which indicates a poor prognosis, with a median overall survival (OS) of only about 5 months [2, 3].
Prophylactic cranial irradiation (PCI) is an effective treatment option for patients with limited-stage SCLC, improving OS and reducing the incidence of BMs [4]. For SCLC patients with BMs, the whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS) are efficient and safe strategies for local lesion control. Systemic treatments, such as chemotherapy, immunotherapy, and targeted therapy, are also alternatives for these patients. WBRT, although widely used as a standard treatment, may lead to cognitive disorders and a decline in quality of life [5]. SRS may be more appropriate for patients with fewer intracranial metastases. According to the American Society for Radiation Oncology (ASTRO) clinical practice guideline, SRS is recommended for patients with better performance status and up to 4 intact brain metastases [6]. However, the date was basically all from non-SCLC cohort and maybe not entirely applicable for SCLC. Previously, a study has compared the therapeutic effect of WBRT, WBRT + simultaneous integrated boost (SIB) and SRS [7]. They identified that WBRT + SIB had no overall survival (OS) advantage compared with WBRT or SRS, but prolonged the intracranial progression free survival (iPFS). Another published prospective study showed that SRS combined with WBRT did not significantly improve OS in SCLC patients with BMs and may increase adverse events [8].
WBRT combined with SIB is a promising approach for treating BMs, which have great advantages over other radiotherapeutic strategies. WBRT with SIB can deliver boost dose to multiple metastases with highly conformal distributions, allowing different target areas to receive various dose levels in the same plan. This approach reduces doses to organs at risks (OARs), thereby enhancing the tolerance of radiotherapy. Additionally, SIB dose not prolong the treatment time [9].
Considering there is no unified consensus in radiation therapy for SCLC with BMs, more evidences are needed to find a better therapeutic strategy. Therefore, in this study, we retrospectively analyzed the survival data of SCLC patients with BMs treated with WBRT and WBRT + SIB at our center. We aimed to explore the association between different radiotherapeutic modalities and patient prognosis.
Methods
Study population
This retrospective study analyzed SCLC patients with BMs who underwent head radiotherapy at the Cancer Hospital Chinese Academy of Medical Sciences from 2014 to 2023. The eligibility criteria were as follows: (a) age ≥ 18 years old; (b) Eastern Cooperative Oncology Group (ECOG) score ≤ 3; (c) pathological confirmed primary SCLC; (d) computed tomography (CT) or magnetic resonance imaging (MRI) examination confirmed BMs; (e) treatment with WBRT or WBRT + SIB. Considering that WBRT + SIB may have survival benefit, clinicians may formulate different treatment plans based on their own experience. Patients were excluded if they had received prior head radiotherapy (including PCI, stereotactic radiotherapy, etc.), undergone craniotomy, had imaging or cytological evidence of meninges metastases, had incomplete clinical information, or had interrupted their radiotherapy schedule for any reason. A total of 127 SCLC patients with BMs were finally enrolled in this study. The baseline characteristics are detailed in Table 1. Informed consent for radiotherapy was obtained from all patients. This research was approved by the Ethics Committee of Cancer Hospital Chinese Academy of Medical Sciences.
Table 1.
Clinical characteristics of SCLC patients by treatment groups
| Characteristics | WBRT (%) (N = 71) |
WBRT + SIB (%) (N = 56) |
P-value |
|---|---|---|---|
| Gender | |||
| Male | 57 (80.3) | 49 (87.5) | 0.277 |
| Female | 14 (19.7) | 7 (12.5) | |
| Mean age, years(± SD) | 61.1(± 9.1) | 61.7(± 8.1) | |
| Age (years) | 0.555 | ||
| <60 | 29 (40.8) | 20 (35.7) | |
| ≥60 | 42 (59.2) | 36 (64.3) | |
| Number of BMs | 0.002 | ||
| Single | 11 (15.5) | 26 (46.4) | |
| ≥2 | 45 (63.4) | 29 (51.8) | |
| Concurrent chemotherapy | 0.066 | ||
| Yes | 5 (7.0) | 0 (0.0) | |
| No | 66 (93.0) | 56 (100.0) | |
| Concurrent targeted therapy | < 0.001 | ||
| Yes | 8 (9.9) | 33 (58.9) | |
| No | 63 (88.7) | 23 (41.1) | |
SCLC Small cell lung cancer, WBRT Whole brain radiotherapy, SIB simultaneous integrated boost, SD Standard Deviation, BMs brain metastases
Radiotherapy strategy
All patients were positioned supine and immobilized with a head-neck-shoulder mask. Enhanced CT scan was performed with a 2 mm slice thickness, covering the region from the top of the skull to the bottom of the thoracic entrance. Images were transferred to the Pinnacle Treatment Planning System and fused with enhanced MRI. Radiotherapy was delivered by 6 MV photon beam linear accelerators.
The gross tumor volume (GTV) encompassed contrast-enhanced BMs on T1-weighted MRI excluding the edema area. The planning gross tumor volume (PGTV) was defined as a 2 mm margin around the GTV. The clinical target volume (CTV) included the whole brain, which expanded 5 mm to generate the PTV. The brain stem, optic nerve, crystals and other OARs were delineated on CT/MRI fused images. The prescribed dose to the PTV ranged from 25.0 to 54.0 Gy in 10–21 fractions, while the simultaneous boost to the PGTV ranged from 18.0 to 60.0 Gy in 5–20 fractions. The prescribed dose covered at least 95% of the target area, with PTV used solely for evaluation purposes. Cone Beam computer tomography (CBCT) was applied for image-guided validation before each treatment fraction. During the radiotherapy, symptomatic treatments were administrated as needed for intracranial hypertension symptoms such as headache, nausea, and vomiting.
Follow-up and outcomes
Clinical examination and brain MRI were performed 1–2 months after the end of radiotherapy with subsequent follow-ups every 3 months in the first year, transiting to annual visits after 3 years if the disease remained stable. Follow-up assessments included enhanced MRI and evaluation of patient symptoms and signs. The RECIST criteria was used to evaluate the therapeutic efficacy: complete response (CR) indicated disappearance of lesions in target area on T1-weighted MRI, partial response (PR) indicated a reduction in the sum of the diameters of the target lesions by at least 30%, progressive disease (PD) signified an increase in lesion size by more than 20% or the appearance of new lesions, and stable disease (SD) was defined when changes did not meet PR or PD criteria [10].
In terms of the curative effect evaluation, the local control rate (LCR) was defined as the proportion of patients achieving CR, PR, or SD, while objective response rate (ORR) was defined as the proportion of patients achieving CR or PR. IPFS was defined as the time from radiotherapy to intracranial progression or death. OS was defined as the time from radiotherapy to death or the last follow-up.
Statistical analysis
The Kaplan-Meier (K-M) analysis and log-rank test were used to analyze the survival between the two radiotherapy modalities. Chi-square test or Fisher’s exact test was applied to compare clinical information, ORR, and LCR among different groups. Univariable Cox regression analysis was performed for potential prognostic factors of iPFS and OS and factors with P < 0.10 were included in multivariable analysis. Statistical analyses and visualizations were performed using SPSS 29.0 (IBM, Chicago, IL, USA), GraphPad Prism 10.2.0 (GraphPad Software, La Jolla, CA), and R language version 4.3.0 (R development Core Team, Vienna, Austria), including the “survival” and “survminer” packages. The cut-off value of different factors in WBRT + SIB group was calculated by R language software. A P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 127 SCLC patients with BMs were retrospectively enrolled in the study, including 106 males and 21 females. The median age was 62 years old (range 38–78 years old). Among the patients, 71 received WBRT, While the remaining 56 patients received WBRT + SIB. The chi-square test results showed no statistically significant differences in gender, age, and concurrent chemotherapy between the WBRT and WBRT + SIB groups (Table 1). Patients with single BM and those receiving targeted therapy were relatively more prevalent in the WBRT + SIB group. In patients with concurrent targeted therapy, nearly all patients received Anlotinib, except one with Osimertinib.
Survival analysis
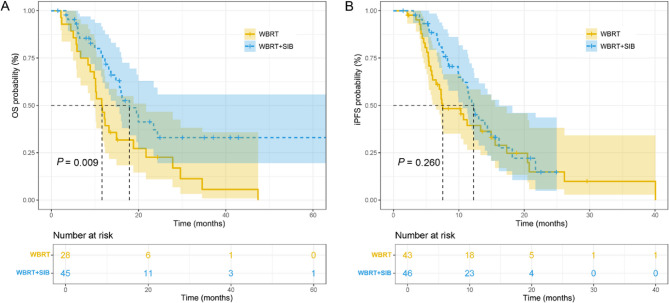
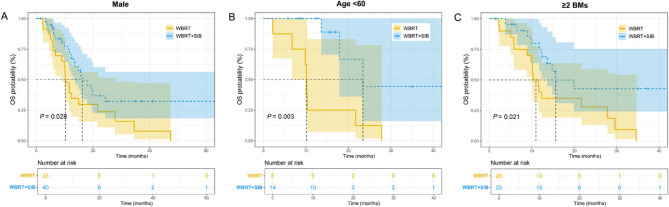
The median follow-up was 14.9 months (range: 1.5–63.2 months). The 1-year and 2-year cumulative survival rate were 46.4% and 22.7% in the WBRT group, and 74.5% and 33.0% in the WBRT + SIB group, respectively. The 1-year cumulative survival rate was statistically different between the two groups (P = 0.023). The 1-year and 2-year cumulative iPFS rates were 39.4% and 14.9% in the WBRT group, and 50.8% and 14.7% in the SIB group, respectively, with no statistical significance. The median OS (18.0 months and 11.7 months) and iPFS (12.2 months and 7.6 months) in the SIB group were higher than those in the WBRT group. The K-M survival curve analysis showed that WBRT + SIB significantly prolonged the OS of SCLC patients with BMs compared to WBRT alone (P = 0.009; Fig. 1A). However, there was no significant difference in iPFS between the two groups (P = 0.260; Fig. 1B). In the subsequent subgroup analyses, the K-M analyses were performed according to age, gender, number of BMs and other factors of patients. The results demonstrated that male patients (P = 0.028), patients younger than 60 years old (P = 0.003), and patients with the number of BMs ≥ 2 (P = 0.021) were prone to have favorable OS after undergoing WBRT + SIB compared to WBRT (Fig. 2). The statistical significance in OS and iPFS among other subgroups was not achieved (Fig. S1).
Fig. 1.
The K-M survival curves of OS (A) and iPFS (B) between the WBRT and WBRT + SIB groups. K-M, kaplan-Meier; OS, overall survival; iPFS, intracranial progression-free survival; WBRT, whole brain radiotherapy; SIB, simultaneous integrated boost
Fig. 2.
The K-M survival curves of OS in different subgroups. (A) OS in male patients, (B) OS in patients < 60 years old. (C) OS in patients with ≥ 2 BMs. K-M, kaplan-Meier; OS, overall survival; BMs, brain metastases
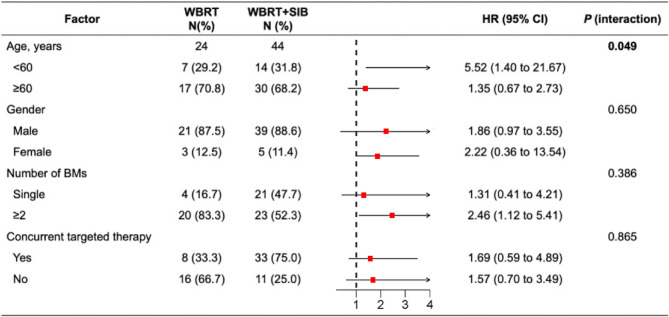
Univariate and multivariate Cox regression analyses were conducted on the entire cohort. The results suggested that concurrent anti-angiogenesis targeted therapy was an independent OS prognostic factor for SCLC patients with BMs (HR = 0.486, 95%CI 0.256–0.924; P = 0.028) (Table 2). Subgroup analysis further demonstrated that male patients, those < 60 years old, and patients with two or more BMs derived greater benefit from the WBRT + SIB radiotherapy. Additionally, the interaction test revealed that patients < 60 years old had a significantly greater benefit from the WBRT + SIB treatment strategy (P = 0.049; Fig. 3).
Table 2.
Univariable and multivariable Cox regression analysis in SCLC patients with BMs
| Characteristics | Categories | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | P-value | HR | 95%CI | P-value | ||
| Gender | Male vs. Female | 1.274 | 0.569–2.853 | 0.556 | |||
| Age (years) | ≥ 60 vs. <60 | 1.383 | 0.701–2.727 | 0.349 | |||
| Number of BMs | ≥ 2 vs. Single | 1.192 | 0.623–2.278 | 0.596 | |||
| Concurrent targeted therapy | Yes vs. No | 0.410 | 0.223–0.755 | 0.004 | 0.486 | 0.256–0.924 | 0.028 |
| Radiation type | WBRT + SIB vs. WBRT | 0.471 | 0.265–0.837 | 0.010 | 0.592 | 0.322–1.085 | 0.090 |
SCLC small cell lung cancer, BMs brain metastases, HR hazard ratio, CI confidence interval, WBRT whole brain radiotherapy, SIB simultaneous integrated boost
Fig. 3.
The forest plot of factors associated with OS in patients treated with WBRT versus WBRT + SIB. OS, overall survival; WBRT, whole brain radiotherapy; SIB, simultaneous integrated boost
Curative effect evaluation
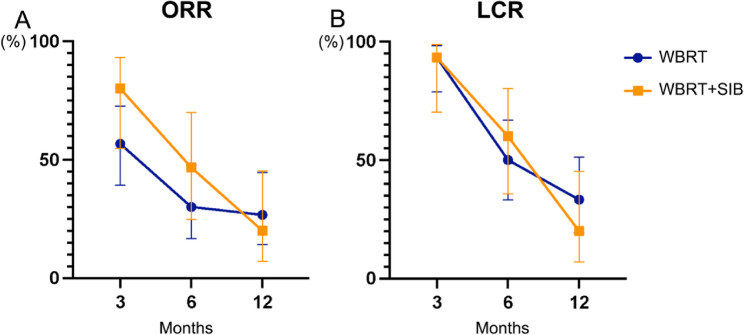
A total of 45 patients had complete information available for curative effect evaluation. The ORR and LCR at 3, 6, and 12 months after radiotherapy were the main evaluation indicators. In the WBRT + SIB group, the ORR at 3 and 6 months was higher than WBRT group (Table 3; Fig. 4A). While the LCR within a year between the two groups showed little differences (Fig. 4B). In general, there was no statistical significance in the comparison of ORR and LCR between WBRT and WBRT + SIB group (Table 3).
Table 3.
Summary of confirmed objective response
| Variables | Follow-up time (months) | WBRT (N = 30) |
WBRT + SIB (N = 15) |
P-value |
|---|---|---|---|---|
| ORR, % (95% CI) | 3 | 56.7 (39.2 to 72.6) | 80.0 (54.8 to 93.0) | 0.123 |
| 6 | 30.0 (16.7 to 47.9) | 46.7 (24.8 to 69.9) | 0.271 | |
| 12 | 26.7 (14.2 to 44.5) | 20.0 (7.1 to 45.2) | 0.624 | |
| LCR, % (95% CI) | 3 | 93.3 (78.7 to 98.2) | 93.3 (70.2 to 98.8) | > 0.999 |
| 6 | 50.0 (33.2 to 66.9) | 60.0 (35.8 to 60.0) | 0.526 | |
| 12 | 33.3 (19.2 to 51.2) | 20.0 (7.1 to 45.2) | 0.352 |
WBRT whole brain radiotherapy, SIB simultaneous integrated boost, ORR objective response rate, CI confidence interval, LCR local control rate
Fig. 4.
The curative effect evaluation of the whole cohort. (A) ORR. (B) LCR. ORR, objective response rate; LCR, local control rate
Analysis within the WBRT + SIB cohort
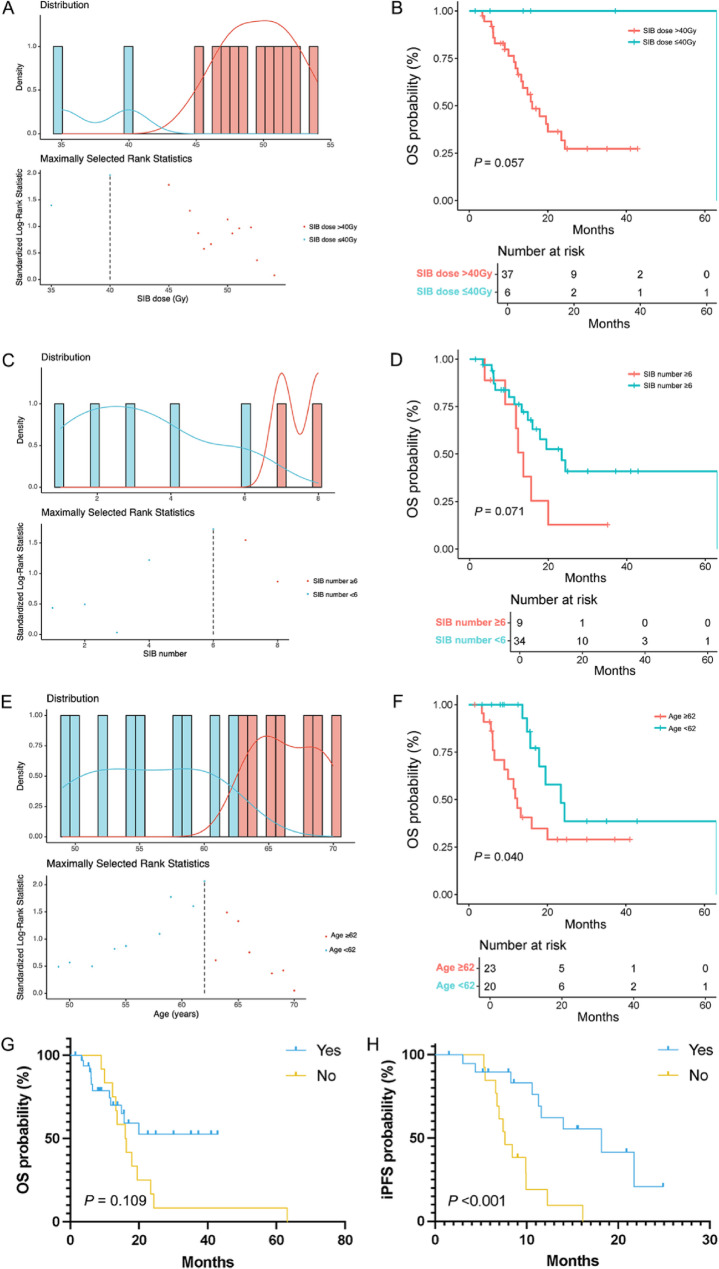
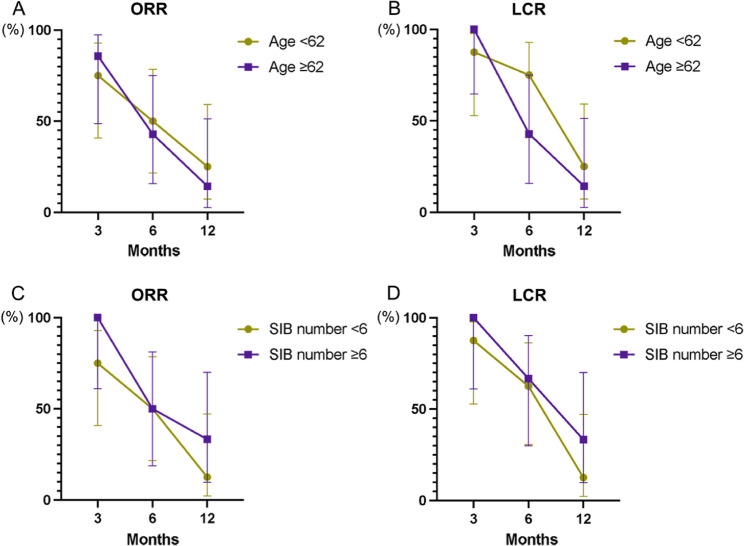
Fifty-six patients received WBRT + SIB treatment. Two patients were excluded for the subsequent analysis because of lacking the number of BMs and the SIB dose (Table S1). The impact of SIB dose, age, the number of BMs, and concurrent anti-angiogenesis therapy on survival in the WBRT + SIB group is shown in Fig. 5A-F. The median whole brain irradiation dose was 34.2 Gy (range 25.0–52.5 Gy). The median SIB dose was 50.4 Gy (range 18.0–60.0 Gy). And the median number of BMs was 2.5 (range 1–34). The result showed that the SIB dose cut-off was 40 Gy, the number of BMs cut-off was 6, and the age cut-off was 62 years old (Fig. 5A, C, E). The K-M curves (Fig. 5B, D, F) demonstrated that the OS was relatively shorter in patients with SIB > 40 Gy (P = 0.057), ≥ 6 BMs (P = 0.071) and ≥ 62 years old (P = 0.040). Moreover, concurrent anti-angiogenesis therapy with WBRT + SIB improved the iPFS (P < 0.001), but not the OS (P = 0.109) (Fig. 5G-H). The therapeutic effect evaluation results showed that there was no statistical significance in ORR or LCR according to the analyses of different subgroups (Fig. 6). Due to the whole patients with complete objective response information received more than 40 Gy of SIB dose, the influence of SIB dose on ORR and LCR was not evaluated.
Fig. 5.
The cut-off value calculation and the survival analysis within the WBRT + SIB group. (A, B) SIB dose. (C, D) the number of BMs. (E, F) Age. (G) The K-M survival curve of OS based on the targeted therapy status. (H) The K-M survival curve of iPFS based on the targeted status. WBRT, whole brain radiotherapy; SIB, simultaneous integrated boost; BMs, brain metastases; K-M, kaplan-Meier; OS, overall survival; iPFS, intracranial progression-free survival
Fig. 6.
The curative effect evaluation of the WBRT + SIB group. Grouped by the age (A, B) and the number of BMs (C, D). (A, C) ORR. (B, D) LCR. WBRT, whole brain radiotherapy; SIB, simultaneous integrated boost; BMs, brain metastases; ORR, objective response rate; LCR, local control rate
Discussion
Despite the sensitivity to systemic cytotoxic chemotherapy, the metastases and drug resistance are inevitable for SCLC patients, which played a major part in treatment failure. Approximately two third of the patients developed distant metastases in the early stage, particularly inclined to BMs [11]. The optional treatment strategies of SCLC with BMs include WBRT, SRS, targeted therapy, immune therapy, and other combination treatments. WBRT could improve the local control of intracranial lesions but limited for the OS. For patients with few BMs, SRS was considered as an effective method that could improve the OS significantly with lower neurotoxicity. SRS also played an important role in salvage therapy, especially for the relapse after WBRT treatment [12]. However, SRS combined with WBRT was not currently recommended in view of the increased neurotoxicity. To improve both OS and local control, WBRT + SIB seemed to be a beneficial way for the treatment of SCLC patients with BMs.
This study retrospectively analyzed 127 SCLC patients with BMs treated with WBRT or WBRT + SIB. The intracranial tumor burden was relatively high in the WBRT group. Survival analysis revealed that the median OS in the SIB group was 18.0 months, 6.3 months higher than that in the WBRT group. The 1- and 2-year cumulative survival rate was higher in SIB group, and the 1-year cumulative survival rate of SIB group was 23.7% higher than that of WBRT group (P = 0.023). The K-M survival curve showed that WBRT + SIB could significantly prolong the OS in SCLC patients with BM (P = 0.009). There was no statistical significance in the iPFS between two groups. Patients with male (P = 0.028), < 60 years old (P = 0.003), and ≥ 2 BMs (P = 0.021) benefitted more from WBRT + SIB compared to WBRT alone. The univariate and multivariate analysis further verified the result that male, younger age and two more BMs indicated longer OS after receiving WBRT + SIB treatment. Among those prognostic factors, age could significantly affect the prognosis of WBRT + SIB (P = 0.049).
In the subsequent exploration of the cut-off value in WBRT + SIB group, we identified that SIB dose > 40 Gy (P = 0.057), ≥ 6 BMs (P = 0.071), and ≥ 62 years old (P = 0.040) had a relatively poor prognosis. Previously reported studies on radiotherapy for SCLC with BMs mentioned that the simultaneous boost with biological effective dose greater than 58.35 Gy remarkably improved the OS (23 months and 17 months; P = 0.002) [13]. Targeted therapy was a non-independent prognostic factor for patients receiving WBRT + SIB therapy, and it could prolong iPFS (HR = 4.006, 95%CI 1.747–9.187; P = 0.001). In the past decade, targeted therapies had made great progress in lung cancer treatment. However, the efficacy of targeted therapy in SCLC is unsatisfied because of the low incidence of epidermal growth factor receptor (EGFR) gene mutation. Many anti-angiogenesis agents that used as first-line or maintenance treatment in non-SCLC failed to show survival benefit in SCLC patients [14]. While, as a new orally administrated, multi-targeted tyrosine kinase inhibitor (TKI), Anlotinib demonstrated substantial antitumor effects via anti-angiogenetic signaling, and showed satisfactory intracranial control rate in SCLC with BMs [15]. In a case report, a combination of Anlotinib, WBRT and Atezolizumab was administrated to a 55-year-old man with brain metastatic SCLC whose OS reached over 41months, and after a full 3-year course of Atezolizumab, only Anlotinib as maintenance [16]. However, the therapeutic efficacy of the combination of radiotherapy with anti-angiogenesis in brain metastatic SCLC seems insufficient based on the current studies and is needed to be explored in the future. The result of therapeutic effect evaluation showed no statistical significance among age and the number of BM in the WBRT + SIB group.
As a single-center retrospective study, there are some inevitable limitations. The sample size was relatively small, as well as the incomplete follow-up data of some patients, which led to the bias to a certain extent, as well as limited the more detailed subgroup analyses. Moreover, we do not have patient quality of life data. There was also a lack of data on tumor size, previous treatment history and the toxicity. After undergoing initial head radiotherapy in our center, the determination of subsequent treatment strategy against the progressive or recurrent disease could also influence the prognosis. In the future, it is necessary to expand the sample size, and more prospective data are needed to evaluate the efficacy of WBRT + SIB for SCLC brain metastases.
In conclusion, WBRT + SIB can improve the prognosis of SCLC patients with BMs. The combination with anti-angiogenic targeted therapy may be appropriate for young patients who can gain more survival benefit.
Supplementary Information
Authors’ contributions
L.D., Z.Z. and N.B. designed and guided the research. X.S. and W.W. performed most of the follow-up work and analyzed the data. X.S. wrote the manuscript. L.D. contributed methodology support. Z.Z., L.D. and N.B. helped with revised work. T.Z., W.L., J.W., X.W., Y.W., Z.X., J.L. and Q.F. participated in the diagnosis and treatment of all included patients. All authors have reviewed and approved the manuscript.
Funding
This work was supported by the Clinical and Translational Research Special Project of Medical and Health Science and Technology Innovation Engineering of Chinese Academy of Medical Sciences (2020-I2M-C&T-B-076). The National High Level Hospital Clinical Research Funding (2022-CICAMS-80102022203). And Beijing Xisike Clinical Oncology Research Foundation (Y-HR2020MS-0671).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All aspects of this study involving human subjects were performed in an ethical manner, in compliance with the Helsinki Declaration and approved by the Ethics Committee of Cancer Hospital Chinese Academy of Medical Sciences. Informed consent was waived by the Ethics Committee of Cancer Hospital Chinese Academy of Medical Sciences since our study was retrospective, and all the data were deidentified.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xia Shan and Wenqing Wang contributed equally to this work.
Contributor Information
Nan Bi, binan_email@163.com.
Zongmei Zhou, Email: zhouzongmei2013@163.com.
Lei Deng, Email: dengleipumc@163.com.
References
- 1.Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D’Amico TA, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(12):1441–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalemkerian GP. Small cell lung cancer. Semin Respir Crit Care Med. 2016;37(5):783–96. [DOI] [PubMed] [Google Scholar]
- 3.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–55. [DOI] [PubMed] [Google Scholar]
- 4.Maroufi SF, Fallahi MS, Kankam SB, Sheehan JP. Prophylactic cranial irradiation effect on survival in patients with small cell lung cancer: a comprehensive systematic review and meta-analysis. Neurosurg Focus. 2023;55(2):E4. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Zhao Y, Ma T, Shao H, Wang T, Jin S, et al. Radiotherapy for extensive-stage small-cell lung cancer in the immunotherapy era. Front Immunol. 2023;14:1132482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gondi V, Bauman G, Bradfield L, Berri SH, Cabrera AR, Cunningham DA, et al. Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12(4):265–82. [DOI] [PubMed] [Google Scholar]
- 7.Ni M, Jiang A, Liu W, Sheng Y, Zeng H, Liu N, et al. Whole brain radiation therapy plus focal boost may be a suitable strategy for brain metastases in SCLC patients: a multi-center study. Radiat Oncol. 2020;15(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levegrun S, Pottgen C, Wittig A, Lubcke W, Abu Jawad J, Stuschke M. Helical tomotherapy for whole-brain irradiation with integrated boost to multiple brain metastases: evaluation of dose distribution characteristics and comparison with alternative techniques. Int J Radiat Oncol Biol Phys. 2013;86(4):734–42. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 11.Cittolin-Santos GF, Knapp B, Ganesh B, Gao F, Waqar S, Stinchcombe TE, et al. The changing landscape of small cell lung cancer. Cancer. 2024;130(14):2453–61. [DOI] [PubMed] [Google Scholar]
- 12.Viani GA, Gouveia AG, Louie AV, Moraes FY. Stereotactic radiosurgery for brain metastases from small cell lung cancer without prior whole-brain radiotherapy: a meta-analysis. Radiother Oncol. 2021;162:45–51. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Li W, Qi C, Zhou L, Wen F, Qu Y, et al. Optimizing whole brain radiotherapy treatment and dose for patients with brain metastases from small cell lung cancer. Front Oncol. 2021;11: 726613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Zhong H, Shi C, Gao Z, Zhong R, Gu A, et al. Rationale and design of a multicenter, randomized phase II trial of durvalumab with or without multitarget tyrosine kinase inhibitor as maintenance treatment in extensive-stage small-cell lung cancer patients (DURABLE study). Clin Respir J. 2023;17(12):1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third- or further- line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled phase 2 study. Br J Cancer. 2021;125(3):366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long YY, Chen J, Xie Y, Wang Y, Wu YZ, Xv Y, et al. Long-term survival with a combination of immunotherapy, anti-angiogenesis, and traditional radiotherapy in brain metastatic small cell lung cancer: a case report. Front Oncol. 2023;13: 1209758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.