Abstract
Background
Data in Ethiopia on the epidemiology of Respiratory Syncytial Virus (RSV) and subtypes among influenza-like illness (ILI) and severe acute respiratory infections (SARI) cases is limited. Here, we assessed the epidemiology of RSV and its subtypes among the pediatric and adult ILI /SARI cases in Ethiopia.
Methods
We conducted prospective, multicenter facility-based study from May 2023 to April 2024. Respiratory swab specimens, epidemiological and clinical data were collected from participants of all age groups, and both hospitalized and non-hospitalized individuals who met the World Health Organization (WHO) case definition for ILI/SARI and provided informed consent. Laboratory investigation was performed using reverse transcription polymerase chain reaction (RT-PCR). Data were analyzed using SPSS V29. Descriptive statistics were used to summarize frequencies and ratios, and multivariable logistic regression model was employed to assess factors associated with RSV positivity.
Results
In total, 4170 participants were enrolled, the majority (57.9%) of whom were cases from children under five and SARI (76.5%). RSV was detected in 654 cases (15.7%; 95% CI: 14.6–16.8). RSV subtyping was carried out for 475 (72.6%) of 654 RSV positive samples. The finding revealed that both RSV-A and RSV-B subtypes were co-circulating in Ethiopia, with predominance of RSV-B (68.8%). Age group, season and timing of sample collection were factors independently associated with RSV positivity. Accordingly, children aged < 2 years (AOR: 8.20, 95% CI: 3.57–18.81) and 2–4 years (AOR: 5.01, 95% CI: 2.15–11.67), autumn (AOR: 5.89, 95% CI: 3.79–9.17) and winter (AOR: 3.27, 95% CI: 2.07–5.16) seasons, and case whose samples were collected within three days of symptom onset (AOR: 1.76, 95% CI: 1.09–2.84) were significantly associated with RSV positivity.
Conclusions
The study provides evidence of RSV circulation among ILI/SARI cases in Ethiopia. It also revealed that RSV-B was the predominant subtype circulating in the country. The age-specific and temporal patterns of RSV positivity identified in this study contribute to the understanding of RSV and its subtypes epidemiology in Ethiopia. The findings provide valuable evidence to inform implementation of RSV vaccine introduction programs, particularly targeting high-risk populations during periods of peak transmission. Future research focusing on RSV genomic analysis and disease burden is needed to better understand RSV viral evolution, transmission dynamics and public health impacts in Ethiopia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11330-6.
Keywords: Epidemiology, RSV, RSV subtypes, ILI/SARI, RT-PCR, Ethiopia
Background
Human Respiratory Syncytial Virus (RSV) is one of the most significant respiratory viral pathogens causing acute respiratory tract infections (ARI) in children and adults worldwide [1, 2]. Globally, RSV is commonly associated with childhood acute lower respiratory tract infections (ALRI) and hospital admissions, which result in a substantial burden on the health care system and economy [2, 3]. More importantly, the majority of RSV associated ALRI and deaths across all age groups were reported in low and middle income countries (LMICs) [4].
The RSV virus is classified into two genetically and antigenically distinct subtypes, RSV-A and RSV-B [5]. This categorization into specific subtypes is useful as the variability of RSV is believed to be responsible for its ability to infect hosts repeatedly [6]. These two subtypes can co-circulate during the same season, with one subtype often predominating over the other [7]. This predominance of one strain over the other in an alternating pattern can challenge the immune barriers of individuals and can sometimes cause local epidemics [8]. With respect to disease severity, studies reported that RSV-A is associated with an increased disease severity [9] while others reported that either RSV-B is more severe [7] or that the two subtypes have equivalent severity [10].
People infected with RSV can be asymptomatic or presenting with mild or severe diseases, and in some cases, result in death [1]. While RSV can affect individuals of all ages, it has a heightened impact on young children, older adults and adults with pre-existing health conditions [1, 11]. Underlying health conditions such as chronic lung disease, congenital heart disease and others can significantly increase the risk of hospitalization due to RSV infection [12]. After decades of RSV vaccine development effort, the US Food and Drug Administration (FDA) approved the first RSV maternal vaccine, Abrysvo, in 2023; marking a significant milestone in protecting these early age children and high-risk older age groups [13]. In addition, Nirsevimab, a recently approved long acting monoclonal antibody, administered as a single dose to infants and neonates for immediate protection against RSV infections [14]. In many countries, RSV circulation can happen throughout the year with peaks during a particular season. In tropical and subtropical African countries; RSV peaks in autumn in Egypt, end of the rainy seasons in Mozambique. In some other scenarios, RSV seasonality might be distinct for different geographic areas within a country or may not have clear seasonal patterns [15].
Epidemiologic studies on RSV have reported in varying positivity rates globally. In Ethiopia, a study reported an RSV positivity rate of 16.2% among children under five years of age [16]. Another study from a tertiary hospital in Addis Ababa also reported RSV positivity rate of 22.2% among children [17]. Similarly, studies from other countries reported RSV positivity rates of 31.2% in Thailand [18], 16.0% in Senegal [19], 16.2% in Uganda [20], 19.7% in Morocco [21] and 14% in France [22]. A meta-analysis conducted in Africa also indicated that RSV test positivity rates ranging from 9 to 30% across different subregions of Africa [23].
Epidemiology of RSV and its subtypes among children and adult populations with SARI and ILI is not well studied in Ethiopia. Although a few studies were conducted previously, they only included limited clinical data, restricted to few age groups. Furthermore, no prior studies have examined RSV subtypes across all age groups in Ethiopia. This study aimed to fill this gap by describing the the epidemiology of RSV and its subtypes distribution, and associated risk factors among children and adults with ILI/SARI in selected facilities in Ethiopia.
Materials and methods
Study design, setting and period
We employed multicenter facility-based cross-sectional study design in 21 governmental health facilities participating in influenza surveillance program in Ethiopia. These study sites included 17 hospitals and 4 health centers which are designated as SARI and ILI surveillance sites, respectively. These sites were selected based on the WHO ILI/SARI surveillance site selection criteria [24]. They are located in the capital city, Addis Ababa and across various regions of Ethiopia. Each region was represented by at least one study site except Harari and the newly established South West Ethiopia region. Due to logistic and specimen transportation constraints (direct air transport system not available), no ILI/SARI surveillance site has been operation in the two regions.
The study was conducted within the context of broader ILI/SARI surveillance program, which has been implemented in Ethiopia since 2008. However, RSV surveillance project in which its subtypes testing and montoring included, was formaly integrated in to this program since 2023. This study was conducted from May 2023 to April 2024.
Study population
The study population coprised patients presenting with ILI/SARI at selected health facilities during the study period. Among these, study participants were recruited each week through systematic random sampling of five ILI, and all SARI cases provided informed consent. We included participants of all age groups, both hospitalized and non hospitalized cases, and ILI/SARI cases visiting sentinel surveillance sites located in the capital city Addis Ababa and selected regional and zonal towns. Data were collected from study participants and RSV tests were also done for all of them and all were included in the analysis.
Study variables
The RSV test status of the study participants was the outcome or dependent variable of the study, determined using RT-PCR test. The result was categorized as either positive or negative. The independent variables included demographic, clinical, regional and temporal characteristics of the study participants. The demographic characterstics assessed were age and sex; while case classification (ILI/SARI), signs and symptoms of study participants and duration from date of symptom onset to case enrolment at study sites were the clinical characterstics considered. Season and region of case enrolment were temporal and geographic variables assessed in the study. For the variable season, we used a four season classification of a year and defined as follows: (1) Autumn (September-November), marks transition from rainy to dry season); (2) Winter (December-February), a dry season; (3) Spring (March-May), characterized by light and intermittent rain; and (4) Summer (June-August), representing the main and heavy rainy season in most areas of Ethiopia [25]. In our study, the season was determined based on date of data and respiratory swab specimen collection for each study participant.
Sampling and data collection procedures
The standard WHO case definitions of ILI/SARI (2023 WHO surveillance protocol) were used to enroll participants into the study during their visit at the selected facilities. Accordingly, ILI is defined as an ARI with measured temperature of ≥ 38 °C and cough, with onset within the last 10 days. SARI is defined as ARI with a history of fever or measured fever of ≥ 38 °C, cough, with disease onset within the past 10 days and requiring hospitalization [26]. Each day, consecutively, the first five ILI and all SARI cases who fulfilled the inclusion criteria and provided consent were included from each site. Cases who were on life support (oxygen supplementation or mechanical ventilation) and unable to provide swab samples were excluded from the study.
Well-structured case reporting formats tailored for ILI and SARI were used to collect socio-demographic information, clinical and epidemiological data. The data was also electronically captured using the District Health Information System (DHIS II), parallelly with paper-based data to enhance data quality and data archival. Nasopharyngeal swabs were collected from each participant using 3 ml viral transport media (VTM), temporarily stored at 4 °C at sentinel sites and transported for testing by trained professionals every week using triple packaging system at 2–8 °C. Upon receipt, the swab samples were processed for RT-PCR testing at the laboratory or temporarily stored at 4 °C for 72 h until testing. Leftover samples were preserved at −80 °C for long-term storage.
Laboratory analysis: RNA extraction, detection and subtyping
Nucleic acid extraction was performed from these samples using N32 extraction kit and automated nucleic acid extractor (MGISP-NE32, Wuhan China) following the manufacturer’s instructions. A volume of 300 µl of swab sample was used yielding a final volume of 50 µl elute.
Two phases of RT-PCR run were conducted: one for RSV detection and another for RSV subtyping. During RSV testing, 5 µl of the 50 µl eluted RNA was mixed with 20 µl of master mix prepared from Flu-SC-2-and RSV triplex kit of Thermo Fisher and loaded onto ABI7500FAST RT-PCR machines with appropriate run program. Then RSV subtyping from RSV positive samples was followed to identify the circulating subtype using CDC RSV multiplex real-time RT-PCR kit (Catalog: GR-1365). Results showing sigmoid amplification curves and cycle threshold (CT) value < 37 for detection and < 35 for the subtyping was considered as PCR positive, in accordance with the manufacturer’s instructions.
Data Quality Assurance and Statistical Analysis
To ensure data quality, both paper-based and electronic data were collected from each health facility. Necessary training on ILI/SARI suspect case identification and enrolment procedure, data collection, respiratory swab sample collection, storage and transportation and associated biosafety implementation was provided to clinicians/epidemiologsts and laboratory experts who were involved in specimen and data collection. Dedicated data managers supported and regularly followed up on data collection and reporting at each site. With this close support, any missing data elements were promptly tracked and completed without delay. Moreover, each study site had dedicated refrigerators to store samples at appropriate temperature until they were transported for testing. Laboratory testing and result interpretations were performed by trained personnel, following standard operating procedure (SOPs) and manufacturer’s instructions. Both negative and positive controls were included in each RT-PCR run to monitor the quality of test results.
Statistical analyses were conducted using SPSS statistical software (IBM SPSS Statistics, version 29.0). Descriptive summary measures, such as frequencies and proportions were used to analyze the demographic and clinical characteristics, as well as RSV and RSV subtype positivity rates. Associations between each independent variable and RSV test results were assessed using bi-variable and multi-variable logistic regression model. Variables with a p-value < 0.20 in bivariable analysis were included in the multivariable model. Odds ratios (OR) with the 95% confidence interval (CI) were used to estimate the strength of associations. Statistical significance was determined at a P-value < 0.05. Multicollinearity was assessed using the Variance Inflation Factor (VIF) in SPSS V29.0 and found VIF values ranging from 1.00 to 1.01, indicating no significant multicollinearity between the predictor variables(VIF < 5).
Results
Demographic and clinical characteristics of enrolled cases
A total of 4,170 cases were enrolled in this study, with more than half (54.1%) being male. The majority (57.9%) of the study participants were children under five years of age. The mean age was 13 years (standard deviation (SD): 19.9), while the median age was 3 years (Interquartile Range (IQR): 1.0–20.0). Most of the enrolled cases were SARI cases (76.5%), and from study sites located outside Addis Ababa (58.7%). Diving deep into the regions, higher numbers of cases were enrolled from Oromia (15.8%), South Ethiopia (8.7%), Amhara (8.0%) and Sidama (7.9%) regions. Enrolment was conducted during all four seasons of the year with the highest number of cases recorded in autumn (42.9%). Most participants (94.5%) provided respiratory samples within the first week of symptom onset (Table 1 and Supplementary Table 1). A higher number of the enrolled cases presented with clinical symptoms such as difficulty of breathing (62.5%), runny nose (52.4%). Underlying health conditions including chronic lung diseases, asthma and heart diseases were reported among 5.5%, 3.6% and 2.6% of the study participants, respectively (Supplementary Table 2).
Table 1.
Demographic characteristics, and RSV test positivity rates(n=4170) and its subtype distribution (n=475) among children and adults with ILI/SARI in selected health facilities in Ethiopia, May 2023-April 2024
| Description | RSV Status (n=4170) | RSV Subtype Status (n=475) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total tests (%) | Positive (%) | Subtyping tests (%) | RSV-A positive (%) | RSV-B positive (%) | RSV-A and B positive (%)* | Un-subtypeable (%)# | ||
| Sex | Male | 2256(54.1) | 362(16.0) | 277(58.3) | 75(27.1) | 198(71.5) | - | 4(1.4) |
| Female | 1914(45.9) | 292(15.3) | 198(41.7) | 65(32.8) | 129(65.2) | 2(1.0) | 2(1.0) | |
| Age-group (years) | <2 | 1675(40.2) | 446(26.6) | 343(72.2) | 105(30.6) | 234(68.2) | 2(0.6) | 2(0.6) |
| 2-4 | 739(17.7) | 135(18.3) | 89(18.7) | 22(24.7) | 64(71.9) | - | 3(3.4) | |
| 5-14 | 576(13.8) | 47(8.2%) | 31(6.5) | 8(25.8) | 22(71.0) | - | 1(3.2) | |
| 15-49 | 806(19.3) | 14(1.7) | 7(1.5) | 2(28.6) | 5(71.4) | - | - | |
| 50-64 | 192(4.6) | 6(3.1) | 2(0.4) | 1(50.0) | 1(50.0) | - | - | |
| >65 | 182(4.4) | 6(3.3) | 3(66.7) | 2(1.4) | 1(33.3) | - | - | |
| Admission Type | SARI | 3190(76.5) | 532(16.6) | 421(88.6) | 136(32.3) | 277(65.8) | 2(0.5) | 6(1.4) |
| ILI | 980(23.5) | 123(12.6) | 54(11.4) | 4(7.4) | 50(92.6) | - | - | |
| Location | Addis Ababa | 1722(41.3) | 294(17.1) | 178(37.5) | 41(23.0) | 136(76.4) | 1(0.6) | - |
| Regions | 2448(58.7) | 360(14.7) | 297(62.5) | 99(33.3) | 191(64.3) | 1(0.3) | 6(2.0) | |
| Season (Months) | Autumn (Sep, Oct, Nov) | 1788(42.9) | 425(23.8) | 325(68.4) | 85(26.2) | 234(72.0) | 1(0.3) | 5(1.5) |
| Spring (Mar, Apr, May) | 722(17.3) | 28(3.9) | 18(3.8) | 9(50.0) | 9(50.0) | - | - | |
| Winter (Dec, Jan, Feb) | 1187(28.5) | 178(15.0) | 112(23.6) | 34(30.4) | 76(67.9) | 1(0.9) | 1(0.9) | |
| Summer (Jun, Jul, Aug) | 473(11.3) | 23(4.9) | 20(4.2) | 12(60.0) | 8(40.0) | - | - | |
| DUR (days) | ≤3 | 2329(55.9) | 405(17.4) | 268(56.4) | 58(21.6) | 207(77.2) | - | 3(1.1) |
| 4-7 | 1611(38.6) | 226(14.0) | 189(39.8) | 74(39.2) | 110(58.2) | 2(1.1) | 3(1.6) | |
| 8-10 | 230(5.5) | 23(10.0) | 18(3.8) | 8(44.4) | 10(55.6) | - | - | |
| Total | 4170(100.0) | 654(15.7) | 475(100.0) | 140(29.5) | 327(68.8) | 2(0.4) | 6(1.3) | |
Key: DUR Duration in days from date of diseases onset to date of sample collection, n number of cases tested for RSV detection and RSV subtyping, SARI Severe acute respiratory illness, ILI Influenza like illness
*both RSV-A and RSV-B detected in the same sample
#Subtyping test was conducted for the RSV positive samples but it’s subtype couldn’t be determined using the employed RT-PCR assay
RSV and subtype detection rates
RSV was detected among 15.7% (95%CI: 14.6–16.8) of study participants. Children under two years of age had the highest positivity rate of 26.6% followed by the age group of 2–5 years at 18.3%. SARI cases had an RSV positivity rate of 16.6%. With respect to geographic location, a positivity rate of 17.1% was reported among cases enrolled from sites in Addis Ababa and 14.7% among those from regional sites (14.7%). Moreover, RSV test positivity rate of 23.8% in autumn and 15.0% in winter were reported. Additionally, 17.4% positivity rate was reported from the cases enrolled within the first three days after symptom onset (Table 1).
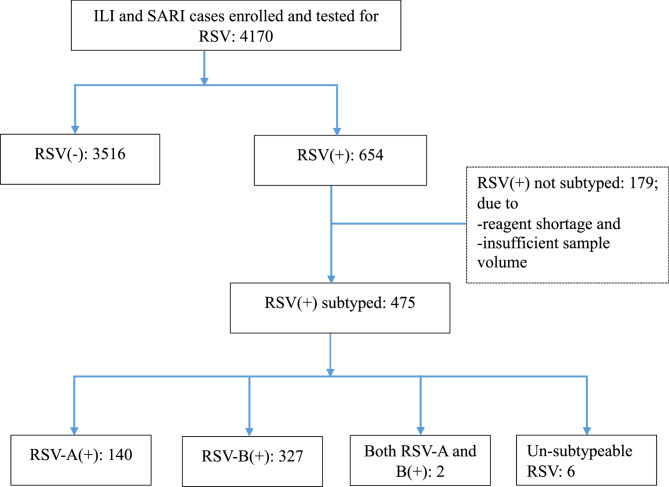
RSV subtyping was performed on 475 (72.6%) of 654 RSV positive samples; subtyping could not be conducted on the rest of the positive samples due to reagent shortage and insufficient sample volume. Of the total subtyped samples, males accounted for 58.3%. Most of the tests were from children under five years old (90.9%), individuals with SARI cases (86.6%), and those enrolled from study sites outside Addis Ababa (62.5%). Subtyping analysis included samples collected throughout different seasons, with a positivity rate of 68.4% in autumn. Among the subtyped samples, 327 (68.8%) were identified as RSV-B, while 140 (29.5%) were RSV-A. Both RSV-A and RSV-B were co-detected in two (0.4%) samples collected from female SARI cases aged bellow 2 years of age, and six samples (1.3%) could not be subtyped with the employed PCR test (Table 1 and Fig. 1).
Fig. 1.
Flowchart depicting testing and results of RSV and its subtypes among individuals with ILI/SARI. The (+) and (–) signs in the figure represent RT-PCR positive and negative results for RSV or its subtypes, respectively. The numbers in each box represent the number of individuals tested or their respective test outcomes. RSV subtyping was not performed for 179 positive cases due to reasons such as reagent shortages and insufficient sample volume
Association of RSV positivity with study participants’ characteristics
In the bivariable analysis, the following variables showed a statistically significant association with RSV positivity: age group, case category, location and season of case enrollment, and duration from symptom onset to sample collection. However, in the multivariable logistic regression analysis only the age category, timing of sample collection from symptom onset and season of case enrolment remained significantly associated with RSV positivity.
From the multivariable logistic regression analysis, case category and location of enrollment were not significantly associated with RSV test positivity. Gender was not included in the multivariable model as it did not meet the selection criteria in the bivariable analysis (COR: 1.1, 95% CI: 0.91–1.25, p: 0.49). In contrast, age group showed a strong association with RSV positivity. Compared to ≥ 65 years old study participants, those aged < 2 and 2–5 had 8.2 (95%CI: 3.6–18.8, p < 0.001) and 5.0 times (95%CI: 2.2–11.7, p < 0.001) higher odds of testing positive, respectively.
In the current study, we found that RSV positivity was significantly higher in the autumn season compared to summer (AOR: 5.9, 95%CI: 3.8–9.2, p < 0.001) and also elevated in winter (AOR 3.3, 95% CI 2.1–5.2, p < 0.001). This likely reflects higher circulation of RSV in these seasons. Moreover, the timing of sample collection played a significant role. Study participants who provided samples within the first three days of symptom onset had significantly higher positivity rate compared to those who were sampled 8–10 days after symptom onset (AOR:1.8, 95%CI: 1.1–2.8, p: 0.02) (Table 2).
Table 2.
Association of RSV test result with characteristics of study patients in selected health facilities in Ethiopia, May 2023-April 2024
| Description | RSV test result | COR (95%CI) | P-value | AOR (95%CI) | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Total | ||||||
| Sex | Male | 1896(84.0) | 362(16.0) | 2256 | 1.06(0.90–1.26) | 0.490 | N/A | |
| Female | 1622(84.7) | 292(15.3) | 1914 | Ref | ||||
| Age-group(years) | <2 | 1231(73.4) | 446(26.6) | 1675 | 10.64(4.68-24.19) | <0.001 | 8.20(3.57-18.81) | <0.001* |
| 2-4 | 604(81.7) | 135(18.3) | 739 | 6.56(2.85-15.11) | <0.001 | 5.01(2.15-11.67) | <0.001* | |
| 5-14 | 529(91.8) | 47(8.2) | 576 | 2.61(1.10-6.20) | 0.030 | 1.91(0.79-4.60) | 0.150 | |
| 15-49 | 792(98.3) | 14(1.7) | 806 | 0.52(0.20–1.37) | 0.185 | 0.42(0.16-1.13) | 0.090 | |
| 50-64 | 186(96.9) | 6(3.1) | 192 | 0.95(0.30-2.99) | 0.925 | 0.84(0.27-2.69) | 0.77 | |
| >65 | 176(96.7) | 6(3.3) | 182 | Ref | ||||
| Admission Type | SARI | 2661(83.4) | 531(16.6) | 3190 | 1.39(1.13–1.72) | 0.002 | 1.18(0.90-1.55) | 0.242 |
| ILI | 857(87.4) | 123(12.6) | 980 | Ref | ||||
| Location | Addis Ababa | 1428(83.0) | 294(17.1) | 1722 | 1.19(1.01–1.41) | 0.039 | 1.06(0.85-1.31) | 0.632 |
| Regions | 2090(85.3) | 360(14.7) | 2450 | Ref | ||||
| Season (months) | Autumn (Sep, Oct, Nov) | 1356(76.2) | 425(23.8) | 1788 | 6.08(3.96-9.41) | <0.001 | 5.89(3.79-9.17) | <0.001* |
| Spring (Mar, Apr, May) | 693(96.1) | 28(3.9) | 722 | 0.79(0.45-1.39) | 0.411 | 0.80(0.47-1.42) | 0.440 | |
| Winter (Dec, Jan, Feb) | 1018(85.0) | 178(15.0) | 1187 | 3.45(2.21-5.40) | <0.001 | 3.27(2.07-5.16) | <0.001* | |
| Summer (Jun, Jul, Aug) | 451(95.1) | 23(4.9) | 473 | Ref | ||||
| DUR† (days) | ≤3 | 1924(82.6) | 405(17.4) | 2329 | 1.89(1.22-2.95) | 0.005 | 1.76(1.09-2.84) | 0.020* |
| 4-7 | 1385(86.0) | 226(14.0) | 1611 | 1.47(0.93-2.31) | 0.096 | 1.37(0.84-2.22) | 0.200 | |
| 8-10 | 207(90.0) | 23(10.0) | 230 | Ref | ||||
| Total** | 3518(84.3) | 654(15.7) | 4170 | - | ||||
Key: SARI Severe acute respiratory illness, ILI Influenza like illness, COR Crude Odds Ratio, AOR Adjusted odds ratio, 95%CI 95% Confidence interval, DUR Duration in days from date of symptom onset to data of sample collection, N/A Not applicable
*RSV positivity is significantly associated with independent variables
**Overall RSV test positivity was 15.7% (95%CI: 14.6-16.8)
†DUR: adjusted for age
Discussion
To the best of our knowledge, this is the first study to comprehensively investigate the epidemiology of RSV and its subtypes among both pediatric and adult populations in Ethiopia. It provides insights on factors influencing RSV positivity across various demographic and clinical parameters. Our study revealed significant variations in RSV positivity rates among different age groups, with children under 2 years showing the highest positivity rate (26.6%), particularly highlighting their vulnerability. Seasonal patterns were also observed, with the autumn (September to November) demonstrating widespread circulation of RSV among ILI/SARI cases (23.7%). The duration of symptom onset also played a role, with patients presenting to study sites within three days of symptom onset showing a higher likelihood of RSV positivity emphasizing the importance of early testing and diagnosis for effective interventions. Subtyping analysis also revealed higher positivity rate of RSV-B than RSV-A.
In the current study, we identified RSV among 15.7% (95%CI: 14.6–16.8) of study participants. This overall positivity rate align with findings from other regions, such as reports of 16.0% in Senegal [19], 16.2% in Uganda [20] and 15.8% in China [27]. However, it was lower than 19.7% reported in Morocco [21] but slightly higher than 12.9% in Italy [28]. The long study period they employed could contribute to the variations. Moreover, the study in Italy included data of ILI cases and older age groups (median age of 36 years) compared to ours which could contribute for lower rates.
We attempted RSV subtyping for 475 (72.6%) of all RSV positive samples collected during the study period. The subtyping results revealed that RSV-B (68.8%) was predominately circulating over RSV-A (29.5%) in Ethiopia. Co-detection of RSV-A and B was observed in 0.4% of the samples, while 1.3% of them could not be subtyped using the employed test, possibly due to the inherent limitation of the PCR primer kit in covering the targeted sequence regions of the RSV genome or RSV genomic mutations. Similar patterns of higher RSV-B positivity rates over RSV-A reported in other countries including Morocco (61% vs. 35%) [21], Senegal (54.8% vs. 45.2) [12] and Italy (63.6 vs. 36.4) [29]. However, studies spanning multiple years have shown that RSV-A and RSV-B often co-circulate with alternating predominance across different seasons or years [30]; suggesting that one RSV subtype may evade the herd immunity developed previously against the other [21]. Furthermore, the predominance of RSV-B may imply the need for subtype-specific vaccine design that match circulating subtypes and subsquent monitoring of its effectiveness [31].
Although not statistically significantly, males, SARI cases and study participants from regional sites had higher RSV positivity rates than their counter parts. In contrast, a statistically significant variation in RSV positivity was observed across age groups, with the highest rates among children under 2 years (26.6%, AOR: 8.2, 95% CI: 3.6–18.8, p < 0.001) and those aged 2–5 years (18.3%, AOR: 5.0, 95% CI: 2.2–11.7, p < 0.001), compared to study participants of 65 years and above. This finding was consistent with the study findings of 26.5% among infants in China [32] and pooled estimates of 23.0% [23] and 25.0% [33] in Africa. The heightened susceptibility of younger children is likely due to their immature immune defense mechanisms [37]. Its worthy to not that low RSV positivity in adults aged ≥ 50 should be interpreted cautiously, as this age groupp made up only 10.0% of the cohort in our study and the finding may reflect under-representation or differential health-seeking behaviors rather than a true lower risk. Recently approved RSV preventive tools including Abrysvo (maternal vaccines) and Nirsevimab (monoclonal antibody) offer promising strategy to reduce RSV burden in young children and other high risk adults [13, 14].
We found that RSV positivity was significantly associated with the season of case enrollment. The highest RSV positivity rate was reported in autumn (23.8%, AOR:5.9, 95%CI: 3.8–9.2, p < 0.001) followed by winter (15.0%, AOR: 3.3, 95%CI: 2.1–5.2, p < 0.001%), compared to summer. This seasonal trend pattern aligns with previous reports from Ethiopia, particularly among under five years of age children [16, 34]. Our results are also consistent with studies from other regions such as Mozambique where RSV activity started toward the end of the rainy season [34]. In most areas of Ethiopia, autumn is a season of transition from rainy to dry season, while winter is typically a dry season and both are regarded as colder seasons [25] which creates favorable condition for RSV transmission and seasonal peaks [35]. In tropical and subtropical regions, RSV positivity typically peak during colder seasons although fluctuations and variations exist among countries and regions due to differences in climate, population, dynamics and local epidemiological factors [36].
Our study revealed that cases who provided samples within the first three days of disease symptom onset had a significantly higher positivity rate compared to those who provided samples within 8–10 days of symptom onset (17.4%, AOR: 1.8, 95%CI: 1.1–2.8, p: 0.02). The majority of study participants (57.9%) in our study were children under five years old. Studies in Ethiopia indicated that lower age children are more likely to get health services more promptly [37] and exhibit RSV positivity rates [16] compared to older age groups. This tendency of earlier presentation to facilities increases the likelihood of detecting RSV within the optimal diagnostic window. As such, age can act as a confounder when analyzing the association between the duration from symptom onset to sampling and RSV positivity. To account for this, after adjusting for age in our multivariable analysis, the association between RSV positivity and sampling within three days of symptom onset remained significant, independent of the patient’s age. Our study also aligns with a report from Kenya indicating a mean duration of RSV viral shedding of 4 days [38]. However, longer viral shedding duration of 6.5 days in South Africa [39] and 13.5 days of other report in Kenya [40] were reported. The studies from South Africa and Kenya employed prospective cohort designs, included different study cohorts (such as asymptomatic, household contacts), and sample sizes, which could contribute to the longer shedding durations reported. The WHO and CDC recommend earlier diagnosis of RSV to reduce further transmission and enhance protection of high risk groups [1, 24].
The most commonly observed clinical characteristics among the enrolled participants included difficulty of breathing, runny nose, headache, sore throat, and vomiting. Higher rates of RSV positivity were observed among cases with difficulty of breathing (17.4% versus 12.9%), runny nose (vs. 16.6% versus 14.6%) and vomiting (18.1% versus 15.1%) compared to their counter parts. As many of the enrolled cases were children under 5 years of age, taking clinical pictures more objectively might be difficult. The WHO format used for this study and broader ILI/SARI uses fever as criterion for case identification and enrolment [26]. However, some RSV cases particularly children under 2 years old with RSV, may not have fever and instead, they may show additional clinical characteristics [24]. Moreover, RSV positivity rates in the current study was not positively associated with underlying health conditions, possibly due to the larger proportion of the positive cases being children under five years of age while many cases with underlying health conditions were from the elderly age groups (Supplementary Table 1).
The study has certain limitations. This study was conducted as part of a broader ILI/SARI surveillance effort in Ethiopia based on the 2023 WHO guideline, and this may have limitations in fully capturing the spectrum of RSV cases. The reliance on fever-based WHO criteria may underestimate RSV cases, particularly in afebrile infants; a known limitation in RSV case detection. Children infected with RSV particularly < 2 year of age might not be presented with fever and could be missed from enrolment considering the utilized case enrolment criteria. The different ILI and SARI case enrolment approaches employed might affect the proportion of ILI and SARI cases enrolled and overall RSV positivity rates. Additionally, some eligible ILI/SARI cases may not have been enrolled during weekends coverage gaps, facility staff workload, emergencies, competing priorities and resource constraints. Together, these factors may have contributed to an underestimation of RSV positivity in our study. Lastly, due to resource constraint and insufficient volume, RSV subtyping could not be conducted for all RSV positive samples, limiting the study to include subtype distribution and associated characteristics for all the RSV positive cases.
Conclusion
The study provides evidence of RSV circulation among ILI/SARI cases in Ethiopia, with the highest positivity rate observed among children under five years of age and during autumn. It also revealed that RSV-B was the predominant subtype circulating in the country. The age-specific and temporal patterns of RSV positivity identified in this study contribute to the understanding of RSV and its subtype epidemiology in Ethiopia. The findings provide valuable evidence to inform the planning and implementation of RSV vaccine introduction and piloting programs, particularly targeting high-risk populations such as infants and young children during periods of peak transmission. Future research focusing on RSV genomic analysis and disease burden is needed to better understand RSV viral evolution, transmission dynamics and public health impacts in Ethiopia.
Supplementary Information
Acknowledgements
We would like to acknowledge EPHI for the support and leadership and EPHI colleagues for the lab works and all other supports. We express our gratitude to AAU ALIPB for technical support and guidance in doing this project. The authors acknowledge the U.S. CDC, OSU-GOHi and WHO for technical, financial and logistic supports. The RSV detection kits were obtained through OSU-GOHi support, and the CDC RSV Multiplex Real-Time RT-PCR Kit, GR-1365, was obtained through the International Reagent Resource, Coronavirus and Other Respiratory Viruses Division, CDC, Atlanta, GA, USA. We would also extend our heartfelt acknowledgement to regions, sites and study participants involved in and supported the work.
Abbreviations
- AAU
Addis Ababa University
- ALRI
Acute lower respiratory tract infection
- ARI
Acute respiratory infection
- CDC
Centers for Disease Control and Prevention
- EPHI
Ethiopian Public Health Institute
- HCW
Health care workers
- ILI
Influenza like illness
- LMIC
Low- and middle-income countries
- RNA
Ribonucleic acid
- RSV
Respiratory syncytial viruses
- RT-PCR
Reverse transcription polymerase chain reaction
- SARI
Severe acute respiratory infection
- VIF
Variance Inflation Factor
- WHO
World Health Organization
Authors’ contributions
A.T, Z.M. and N.K. conceived and designed work; A.T, A.G, T.B performed the data analysis; A.T, W.S, M.G involved in laboratory work; AT, ZM, MW, MA, MH, GT, MK, DM, NB, PM and NK supervised the work; and AT, DA and PM drafted original manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Support was provided by the Ethiopian Public Health Institute, Addis Ababa University (through Swedish International Development Cooperation Agency/SIDA) and Österlund Foundation, Malmö, Sweden and Swedish Research Council (grant number 2020–02344), the Österlund Foundation, and a donation through the Medical Faculty of Lund. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. The contents are solely the responsibilities of the authors and do not represent or reflect the views of the funders.
Data availability
All the data is available within the manuscript and its supplemental materials.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with ethical standards of the Declaration of Helsinki for medical research involving human subjects. Ethical approval was obtained from the Research and Ethical Review Committee of Addis Ababa University, Aklilu Lemma Institute of Pathobiology (Ref. No: ALIPB IRERC/110/2015/23) and the Institutional Review Board of Ethiopian Public Health Institute (Protocol No: EPHI-IRB-511-2023). Appropriate training was provided to data and sample collectors and study participants were recruited only after informed consent was obtained from the participants themselves, or from parents, guardians, or caregivers of children under the age of 18 years. Additionally, an assent was obtained for children between 12 and 17 years of age. Confidentiality of the data was maintained throughout the study using unique and anonymous identifier. Test results were shared back to health facilities and regions to support proper case management and surveillance efforts.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CDC. Respiratory Syncytial Virus Infection (RSV). 2024. Available from: https://www.cdc.gov/rsv/about/index.html. Cited 2024 Oct 15.
- 2.Domnich A, Calabrò GE. Epidemiology and burden of respiratory syncytial virus in Italian adults: A systematic review and meta-analysis. PLoS ONE. 2024;19(3 March):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–58. Available from:https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(17)30938-8.pdf. [DOI] [PMC free article] [PubMed]
- 4.Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and National disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calaor-Morin J, Arguelles VL, Foronda JL, Tan A, Lagamayo E, Dapat C, et al. Genotyping of respiratory syncytial virus among influenza-like illness and severe acute respiratory infection cases of children in the Philippines from 2006 to 2016. Influenza Other Respi Viruses. 2022;16:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HN, Hwang J, Yoon SY, Lim CS, Cho Y, Lee CK et al. Molecular characterization of human respiratory syncytial virus in Seoul, South Korea, during 10 consecutive years, 2010–2019. PLoS One. 2023;18(4 April):1–21. Available from: 10.1371/journal.pone.0283873. [DOI] [PMC free article] [PubMed]
- 7.Bender W, Zhang Y, Corbett A, Chu C, Grier A, Wang L et al. Association of disease severity and genetic variation during primary Respiratory Syncytial Virus infections. BMC Med Genomics. 2024;17(1):1–11. Available from: 10.1186/s12920-024-01930-7. [DOI] [PMC free article] [PubMed]
- 8.Nuttens C, Moyersoen J, Curcio D, Aponte-Torres Z, Baay M, Vroling H et al. Differences Between RSV A and RSV B Subgroups and Implications for Pharmaceutical Preventive Measures. Infect Dis Ther. 2024;13(8):1725–42. Available from: 10.1007/s40121-024-01012-2. [DOI] [PMC free article] [PubMed]
- 9.Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infections is related to virus strain. J Infect Dis. 1997;175(4):814–20. [DOI] [PubMed] [Google Scholar]
- 10.Martinello RA, Chen MD, Weibel C, Kahn JS. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis. 2002;186(6):839–42. [DOI] [PubMed] [Google Scholar]
- 11.Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: A systematic review and meta-analysis. J Infect Dis. 2022;222(Suppl 7):S577–83. [DOI] [PubMed] [Google Scholar]
- 12.Fall A, Dia N, Cisse el HA, Kiori DE, Sarr FD, Sy S et al. Epidemiology and Molecular Characterization of Human Respiratory Syncytial Virus in Senegal after Four Consecutive Years of Surveillance, 2012–2015. PLoS One. 2016;11(6):e0157163. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27315120. [DOI] [PMC free article] [PubMed]
- 13.Dieussaert I, Hyung Kim J, Luik S, Seidl C, Pu W, Stegmann J-U, et al. RSV prefusion F Protein–Based maternal Vaccine — Preterm birth and other outcomes. N Engl J Med. 2024;390(11):1009–21. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, et al. Single-Dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–25. [DOI] [PubMed] [Google Scholar]
- 15.Rose EB, Nyawanda BO, Munywoki PK, Murunga N, Bigogo GM, Otieno NA, et al. Respiratory syncytial virus seasonality in three epidemiological zones of Kenya. Influenza Other Respi Viruses. 2021;15(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tayachew A, Teka G, Gebeyehu A, Shure W, Biru M, Chekol L, et al. Prevalence of respiratory syncytial virus infection and associated factors in children aged under five years with severe acute respiratory illness and influenza-like illness in Ethiopia. IJID Reg. 2024;10(January):191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weldetsadik AY, Riedel F. Respiratory syncytial virus in severe lower respiratory infections in previously healthy young Ethiopian infants. BMC Pediatr. 2021; 21(201):1–9. Available from: 10.1186/s12887-021-02675-3. [DOI] [PMC free article] [PubMed]
- 18.Wanlapakorn N, Thongpan I, Sarawanangkoor N, Vichaiwattana P, Auphimai C, Srimuan D et al. Epidemiology and clinical characteristics of severe acute respiratory infections among hospitalized children under 5 years of age in a tertiary care center in Bangkok, Thailand, 2019–2020. Heliyon. 2023;9(11). Available from: 10.1016/j.heliyon.2023.e22300. [DOI] [PMC free article] [PubMed]
- 19.Jallow MM, Diagne MM, Sagne SN, Tall F, Diouf JBN, Boiro D et al. Respiratory syncytial virus in pediatric patients with severe acute respiratory infections in Senegal: findings from the 2022 sentinel surveillance season. Sci Rep. 2023;13(1):1–12. Available from: 10.1038/s41598-023-47015-w. [DOI] [PMC free article] [PubMed]
- 20.Simusika P, Okamoto M, Dapat C, Muleya W, Malisheni M, Azam S et al. Characterization of human respiratory syncytial virus in children with severe acute respiratory infection before and during the COVID-19 pandemic. IJID Reg. 2024;11(March):100354. Available from: 10.1016/j.ijregi.2024.03.009. [DOI] [PMC free article] [PubMed]
- 21.Bimouhen A, Regragui Z, El Falaki F, Ihazmade H, Benkerroum S, Barakat A, et al. Circulation patterns and molecular epidemiology of human respiratory syncytial virus over five consecutive seasons in Morocco. Influenza Other Respi Viruses. 2023;17(10):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaymard A, Bouscambert-Duchamp M, Pichon M, Frobert E, Vallee J, Lina B, et al. Genetic characterization of respiratory syncytial virus highlights a new BA genotype and emergence of the ON1 genotype in lyon, france, between 2010 and 2014. J Clin Virol. 2018;102(February):12–8. [DOI] [PubMed] [Google Scholar]
- 23.Regassa BT, Gebrewold LA, Mekuria WT, Kassa NA. Molecular epidemiology of respiratory syncytial virus in children with acute respiratory illnesses in africa: A systematic review and meta-analysis. J Glob Health. 2023;13(04001). Available from: https://jogh.org/2023/jogh-13-04001. [DOI] [PMC free article] [PubMed]
- 24.World Health Organization (WHO). Implementing the integrated sentinel surveillance of epidemic and pandemic potential respiratory viruses of influenza and other by the Global Influenza Surveillance and Response System: Standards and operational guidance. 2024. Available from: https://www.who.int/publications/i/item/9789240101432.
- 25.Alemu GT, Desta SA, Tareke KA. Characterize and analysis of meteorological and hydrological drought trends under future climate change conditions in South Wollo, North Wollo, and Oromia Zones, in Ethiopia. Heliyon. 2024;10(8):e29694. Available from: 10.1016/j.heliyon.2024.e29694. [DOI] [PMC free article] [PubMed]
- 26.World Health Organization. End-to-end integration of SARS-CoV-2 and influenza sentinel surveillance [Internet]. 2023. Available from: https://www.who.int/publications/i/item/9789240056701
- 27.Cui G, Zhu R, Qian Y, Deng J, Zhao L, Sun Y, et al. Genetic variation in attachment glycoprotein genes of human respiratory syncytial virus subgroups A and B in children in recent five consecutive years. PLoS ONE. 2013;8(9):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.C LP. Respiratory syncytial virus in influenza-like illness cases.pdf. J Med Virol. 2020;92:2999–3006. [DOI] [PubMed] [Google Scholar]
- 29.Panatto D, Domnich A, Lai PL, Ogliastro M, Bruzzone B, Galli C, et al. Epidemiology and molecular characteristics of respiratory syncytial virus (RSV) among Italian community-dwelling adults, 2021/22 season. BMC Infect Dis. 2023;23(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsh S, Hindiyeh M, Kolet L, Regev L, Sherbany H, Yaary K, et al. Epidemiological changes of respiratory syncytial virus (RSV) infections in Israel. PLoS ONE. 2014;9(3):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korsun N, Trifonova I, Madzharova I, Alexiev I, Uzunova I, Ivanov I, et al. Resurgence of respiratory syncytial virus with dominance of RSV-B during the 2022–2023 season. Front Microbiol. 2024;15(April):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Yuan L, Zhang Y, Zhang X, Zheng M, Kyaw MH. Burden of respiratory syncytial virus infections in China: Systematic review and meta-analysis. J Glob Heal. 2015/12/19. 2015;5(2):20417. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4676581/pdf/jogh-05-020417.pdf. [DOI] [PMC free article] [PubMed]
- 33.Wada FW, Tadesse Boltena M, Howe R, Solomon FB, Feleke A, Seyoum T et al. Burden of respiratory syncytial virus diseases among under 5 children in Sub-Saharan Africa: A systematic review and meta-analysis. Heliyon. 2023;9(12):e22211. Available from: 10.1016/j.heliyon.2023.e22211. [DOI] [PMC free article] [PubMed]
- 34.Chadha M, Hirve S, Bancej C, Barr I, Baumeister E, Caetano B, et al. Human respiratory syncytial virus and influenza seasonality patterns—Early findings from the WHO global respiratory syncytial virus surveillance. Influenza Other Respi Viruses. 2020;14(6):638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie LY, Wang T, Yu T, Hu X, Yang L, Zhong LL, et al. Seasonality of respiratory syncytial virus infection in children hospitalized with acute lower respiratory tract infections in hunan, china, 2013–2022. Virol J. 2024;21(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staadegaard L, Caini S, Wangchuk S, Thapa B, de Almeida WAF, de Carvalho FC, et al. Defining the seasonality of respiratory syncytial virus around the world: National and subnational surveillance data from 12 countries. Influenza Other Respi Viruses. 2021;15(6):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abegaz NT, Berhe H, Gebretekle GB. Mothers/caregivers healthcare seeking behavior towards childhood illness in selected health centers in addis ababa, ethiopia: A facility-based cross-sectional study. BMC Pediatr. 2019;19(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okiro EA, White LJ, Ngama M, Cane PA, Medley GF, Nokes DJ. Duration of shedding of respiratory syncytial virus in a community study of Kenyan children. BMC Infect Dis. 2010;10(15):1–7. [DOI] [PMC free article] [PubMed]
- 39.Cohen C, Kleynhans J, Moyes J, McMorrow ML, Treurnicht FK, Hellferscee O, et al. Incidence and transmission of respiratory syncytial virus in urban and rural South africa, 2017–2018. Nat Commun. 2024;15(1):2017–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munywoki PK, Koech DC, Agoti CN, Kibirige N, Kipkoech J, Cane PA, et al. Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding. Epidemiol Infect. 2014;143(4):804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data is available within the manuscript and its supplemental materials.