Abstract
Introduction
Epidemiological studies have documented the health effects of long-term exposure to fine particulate matter, while there is a growing number of studies looking into associations with one of its main components elemental carbon (EC) and its related metrics such as black carbon (BC), black smoke (BS) or aerosol light absorption coefficient often referred as “PM absorbance”. We performed a systematic review and meta-analysis on the associations between long-term exposure to elemental carbon (EC) and disease incidence.
Methods
We searched for studies published up to April 2025, assessing long-term to EC-related exposure (also including BC, BS, PM absorbance) and incidence of ischemic heart disease (IHD), asthma, chronic obstructive pulmonary disease (COPD) and lung cancer in adults, and asthma and acute lower respiratory infections (ALRI) in children. We pooled effect estimates by random-effects models and investigated heterogeneity by region and risk of bias assessments. The certainty of the evidence was assessed using the Grading of Recommendations Assessment Development approach.
Results
We included 51 studies assessing long-term exposure to EC and disease incidence. The pooled relative risk (RR) for a 1 µg/m3 increase in EC was 1.10 (95% confidence interval (CI): 1.04, 1.17), 1.11 (95% CI: 1.00, 1.05), for incidence of lung cancer and IHD in adults, while a null association was observed for COPD risk. We estimated RR 1.06 (95% CI: 0.94, 1.21) and 1.37 (95% CI: 0.89, 2.04) for asthma and ALRI in children respectively. There was moderate to high heterogeneity in all associations, with the exception of lung cancer incidence for which the certainty of evidence was rated high.
Conclusions
Our meta-analysis supports an increased risk of lung cancer following long term exposure to EC and indicates associations for IHD in adults and respiratory outcomes in children. Although the evidence base on the effects of EC on diseases incidence has been increasing, further research is needed in the associations between long- term exposure to EC and various diseases’ incidence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-025-01209-z.
Keywords: Elemental carbon, Incidence, Long-term exposure, Meta-analysis
Introduction
The health effects of exposure to fine particulate matter (PM2.5; PM ≤ 2.5 μm in aerodynamic diameter) are well documented, with approximately 4.1 million deaths globally attributed to ambient particulate matter pollution in 2019 [1].
Elemental carbon (EC), refers to the refractory carbon existing as graphitic, amorphous carbon or complex structure like fullerenes in ambient PM. It is primarily emitted from incomplete combustion of solid or liquid fuels (fossil fuels or biomass). EC is typically measured using thermal or thermo-optical method, which is a standardized analytical method [2]. Black carbon (BC), is the light-absorbing component of ambient PM which ideally coincides with EC but is measured through optical absorption methods. However, ambient PM rarely consists of pure elemental carbon. It is usually mixed with other materials, which may affect the light absorbing properties of PM when measured by a filter based photometric instrument. According to Petzold et al. (2013), BC is considered a qualitative rather than a quantitative term, therefore, the term “equivalent black carbon” (eBC) was introduced to refer to the mass concentration derived from light absorption measurements [3]. The BC data used in this manuscript from the literature referred as either BC, black smoke (BS) or PM2.5 absorbance are all converted into EC-equivalent estimates in accordance to previous studies [4, 5]. Despite the different measurement methods, EC and BC are often considered interchangeable [6]. Black carbon is considered one of the most toxic constituents of PM2.5 and is hypothesized to be one of the primary contributors to the observed health impacts [6–8]. Furthermore, as EC is used as a proxy to describe near-road environments and urban backgrounds, where tailpipe emissions are the primary source [9], related metrics such as BC, black smoke (BS), and aerosol light absorption coefficient, which is often referred as “PM2.5 absorbance”, are frequently converted into EC-equivalent estimates in order to assess health effects [4, 5]. Given the large number of people exposed to traffic- related air pollution (TRAP) research has also orientated on the investigation of health impacts of EC exposure.
The U.S.A. Health Effects Institute (HEI) conducted a systematic review and meta-analysis including studies published up to 2019 providing evidence for the association between traffic related air pollution and various health outcomes [10]. Notably, the majority of the identified studies have focused on the health effects of long-term exposure to PM2.5 and NO2, while comparatively, fewer studies investigated the impact of EC, especially in terms of incidence of health outcomes. The selection of studies was based on a thorough screening process to identity those that better characterized traffic-related sources. The review indicated high confidence in the evidence for an association between long-term exposure to EC and mortality outcomes, especially for all-cause [11] and circulatory mortality, while there was less certainty for disease incidence both in adults and children. In light of this, the authors underscore the importance of future research especially for cardiorespiratory incidence outcomes [10]. A recent systematic review and meta-analysis by Song et al. (2022) estimated adverse associations between long-term exposure to BC/EC and cardiovascular morbidity [6], while the review by Chen et al. (2024) [12] that investigated the association between PM2.5 constituents and morbidity outcomes supported that long-term exposure to BC was associated with a higher incidence of asthma, type 2 diabetes and stroke. These two reviews, included only cohort studies, hence the results are based on a smaller number of studies compared to the HEI review that allowed for case control and cross sectional studies.
Possible biological pathways linking exposure to EC and health outcomes have been proposed, including oxidative stress and inflammation [6], DNA methylation and histone modification which may lead to cancer development [13, 14] while it is also linked with cardiovascular dysfunction through endothelial molecule alterations and platelet activation [15, 16]. Studies have also indicted that exposure to BC may contribute to diseases related to the respiratory system via the eosinophil and mast cell infiltration [17, 18].
Within the framework of the MI-TRAP (MItigating TRansport-related Air Pollution in Europe), we performed a systematic review and meta-analysis to assess the health effects of long-term exposure to environmental factors, including EC, ultrafine particles (UFP, particulate matter of nanoscale size; less than 0.1 μm or 100 nm in diameter), ambient particles trace elements including Copper (Cu), Zinc (Zn), Silicon (Si), Lead (Pb) and Iron (Fe) and traffic noise, and disease incidence that have most consistently been linked with air pollution exposures to date. Hence we targeted incidence of ischemic heart disease (IHD), chronic obstructive pulmonary disease (COPD), asthma and lung cancer in adults and incidence of asthma and acute lower respiratory infections (ALRI) in children. In this report we present the results of the review on EC and the incidence of health outcomes. Our main objectives were to provide (a) a summary of the up-to-date scientific evidence for the associations; (b) quantitatively pooled effect estimates for each EC-outcome pair informing the meta-analysis; (c) evaluation of the certainty of the evidence for assessed exposure-health outcome pairs and (d) a qualitative summary of the concentration-response relationships.
Methods
The protocol of the systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO, reference number: CRD42024516074). The deviation from the published protocol is that we opted to include all papers assessing incidence of IHD and not restrict the outcome to acute myocardial infarction as stated in the protocol, in order to increase statistical power and better capture the cardiovascular effects of EC exposure. Additionally, we conducted two further subgroup analyses, by the mean levels of the reported EC distribution and by exposure metric, specifically by studies that measured EC versus the rest. Also, we conducted a sensitivity analysis by excluding the study with the shorter follow-up.
The present systematic review uses the following Population, Exposure, Comparison, Outcome, Study Design (PECOS) statement: In the general population, (P), what is the effect of an increase per concentration of long-term elemental carbon (EC)(E)compared to lower exposure(C)in risk of lung cancer (10th International Classification of Diseases codes: ICD-10: C33–C34), IHD (ICD-10: I60–I69), COPD (ICD-10: J40 – J44, J47), asthma (ICD-10: J45–J46) in adults; asthma (up to 16 years old) and adolescents (up to 24 years old) and ALRI (ICD-10: J12–J18, J20–J22) in children(O), as reported in cohort, case-control and case-cohort studies(S)? More details on the inclusion and exclusion criteria that were based on the PECOS are presented in Supplemental Table S1. We excluded studies that did not align with the PECOS criteria or met any of the following criteria: (a) Panel, cross-sectional studies, or studies using ecological design; (b) Studies on occupational or indoor exposure; (c) Qualitative research designs; (d) Systematic reviews and meta-analyses; (e) Non-human studies (e.g., in vivo, in vitro); (f) Methodological papers; (g) Controlled exposure (chamber) studies; (h) Studies from the grey literature; (i) Conference abstracts; (j) Conference papers, editorials, letters, and; k) Publications in languages other than English.
We initially searched the PubMed database for studies published up to April 2024 for related papers according to the MI-TRAP protocol, using free text in combination with Medical Subject Headings (MeSH) terms (Supplemental Table S2). For the results presented in this paper on EC we performed an updated search in April 2025 to identify related articles targeting EC and published after April 2024.
ES and MIK independently assessed all references based on titles and abstracts for potential relevance for full-text review. The full texts of the selected articles, resulting from the title and abstract screening, were assessed independently by the same reviewers to ascertain compliance with the eligibility criteria. In the case of disagreement among the reviewers, a final decision was reached through discussion. In general, we followed an inclusive approach. When two or more cohort studies assessed the same exposure and outcome, we included both if their follow-up periods differed by more than two years. If a single cohort was part of a greater collaborative project and a separate publication presented the pooled results across the multiple cohorts, we selected the publication that reported the pooled effects. Only if the follow up period of the cohort differed by more than two years in the cohort-specific paper in comparison with the follow up period of the cohort contributing to the pooled analysis, the decision was to include both. For case-control studies with same exposure and outcome assessments, we selected the study with the larger sample size.
Data extraction
Data from the identified studies were independently extracted by MIK and DS using harmonized predefined Excel spreadsheets. To ascertain the agreement and consistency in the data extraction process a 10% random sample of the studies was subjected to the data extraction process by both researchers and cross-checked.
The extracted information included: publication details (title, authors, date of publication); study characteristics ((e.g., cohort, case-control), location); study population details (Sample size, study period, sex, age); exposure details (exposure metrics; exposure assessment methods; mean and concentration range of the pollutant); outcome assessment (ICD coding); exposure unit of measurement; risk estimate as measure of association with 95% confidence intervals (CI), along with exposure increments. We extracted data from the single-exposure models for each exposure-outcome pair and from models adjusting for co-exposures. When multiple estimates were reported, we selected the model favored by the authors, which was typically the “main” model outlined in the methods section. If the preferred model was not clearly specified, we extracted estimates from (1) the simpler model that included all or as many critical confounders as possible (age, sex, smoking, socioeconomic status (SES), body mass index (BMI) or related measures of obesity/physical activity, while the latter was not critical for respiratory outcomes), and (2) for articles that did not fit into the previous category, a case-by-case assessment was conducted. Studies investigating asthma incidence in children (or children/adolescents), reported effect estimates for different exposure windows. Following the HEI (2022) review and accounting that the early life exposure may be more important, we first selected the results for exposure during pregnancy or birth estimates. If these were not available, we selected estimates for exposure during the first year of life. If neither of these exposure windows were reported, we used an estimate associated with a most recent exposure window.
Risk of bias assessment
All studies included in the meta-analysis were assessed for the risk of bias (RoB), using the tool developed by WHO for studies assessing health effects of air pollution [19]. The RoB tool contains six domains (confounding, selection bias, exposure assessment, outcome measurement, missing data, and selective reporting) and each of them includes at least four sub-domains. Each domain is rated as low, moderate or high risk of bias according to the following rationale: low risk of bias, in cases where all the subdomains are assessed as low risk; moderate risk of bias, in cases when at least one subdomain is evaluated as moderate risk of bias and none of the subdomains are assessed as high risk, and high risk of bias, in cases were at least one of the subdomains is assessed as high risk. No overall risk of bias is assigned across domains.
Meta-analysis
Effect estimates (Hazard Ratios (HRs), Odds Ratios (ORs) or Relative Risks (RRs) and 95% CIs) were extracted from the selected studies and were included in the same meta-analyses on the assumption that when relative risk estimates are close to the null, all those measures approximate the risk ratio (Davies et al. 1998, HEI 2022). Following the methodology of the HEI 2022 review, we converted exposure indicators such as BC, BS, and PM light absorption coefficient (soot), into EC-equivalent estimates (HEI 2022). More specifically, BC in µg/m³ was converted to EC in µg/m³ using a scaling factor of 1.25, BS in µg/m³ was converted to EC in µg/m³ using a scaling factor of 0.11, “PM absorbance” (‘soot’) in 10− 5/m was converted to µg/m³ EC using a scaling factor of 1.10 ([4, 5, 10]). Risk estimates were harmonized to estimate risk per 1 µg/m3 increase in EC, by calculating the natural logarithm of the relative risks (RRs) and their confidence intervals (CIs), then standardizing the estimates to the preferred increments by dividing by the reported increment and multiplying by the preferred one.
In cases where four or more studies were identified, we applied a random-effects meta-analysis using the restricted maximum likelihood (REML) method for the estimation of between studies variability. Heterogeneity between studies was assessed by calculating 80% prediction intervals (80% PI), indicating heterogeneity if the PI includes the null effect. We also used the Cochran’s Q test and the I² statistic to assess heterogeneity. Publication bias was assessed by funnel plots, and the Eggers’ test if 10 studies or more contributed to the meta-analysis. All analyses were performed using the “meta” package (version 6.2-0) in the R statistical software, version 4.3.2 (https://www.r-project.org/). We performed subgroup analysis to investigate heterogeneity by a) World Health Organization (WHO) geographical regions (European Region; Region of the Americas; Western Pacific Region), b) Risk of bias assessment per domain in cases the meta-analyses included studies at high risk of bias for the relevant domain (High vs. Low/Moderate), c) mean levels equal or above versus below the median of the reported EC distribution, and d) by exposure metric originally assessed, and specifically by studies that measured EC versus the rest. For each exposure-outcome pair, we conducted a sensitivity analysis to assess the impact of our decision to include results from the same cohort, if a study contributed with more than two years difference in the follow up of another study from the same cohort, by excluding the study with the shorter follow-up.
Evaluation of certainty of evidence
For each pollutant – outcome pair that informed meta-analyses, we assessed the certainty of evidence using the modified GRADE (Grading of Recommendations Assessment, Development) [20, 21], adapted by a working group of experts under the supervision of the WHO Secretariat. The GRADE instrument includes eight domains, with the starting level of certainty in the evidence to be “moderate” for each domain. In five of the domains, the certainty of evidence may be downgraded for: (1) Limitations in studies, if the pooled estimate of low RoB studies differed from the overall one. The limited influence of high-risk bias studies was not a reason for downgrading; (2) Indirectness, if the studies did not reflect the PECOS (3) Inconsistency, in cases of substantial heterogeneity that was not explained and was indicted by the 80% PI including 1 and being twice the width of the 95% CI; (4) Imprecision, if the number of person years in the included studies was small with inadequate statistical power and (5) Publication bias. Certainty in the evidence may be upgraded when: (1) the impact of potential missing confounders on the pooled effect size was expected to be minimal (2) if all plausible confounding would shift the RR towards the null and the pooled RR remained significant and (3) evidence of linearity or increased risk with increased pollutant levels for the studies that assessed the concentration-response function, with the 95%CI of the RR exceeding 1. Regarding the imprecision domain, previous studies assessing long-term effects of air pollutants exposures on mortality, downgraded the certainty of evidence if the number of person-years was less than 940,000 person years [22–25]. However, as disease rates are higher compared to mortality, we considered a smaller total number of person years (i.e. greater than 40,000) in order to downgrade for imprecision.
Results
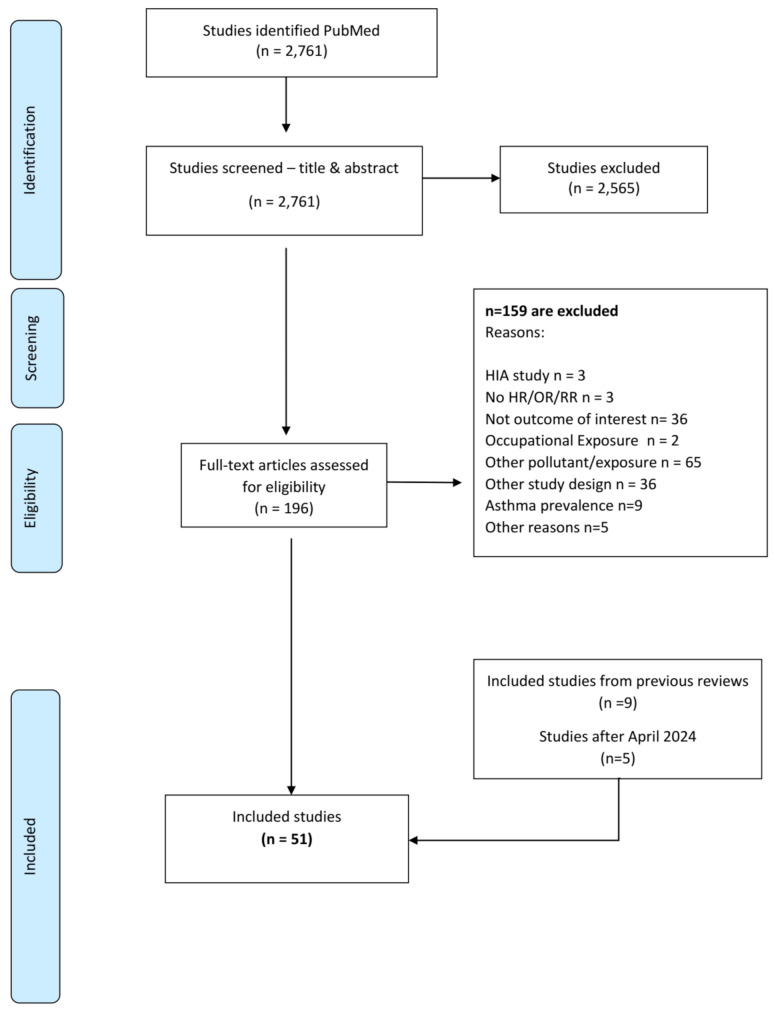
The search strategy resulted in 2,761 studies published until April 2024, while 2,565 were excluded via title and abstract screening (Fig. 1). The remaining 196 articles were retrieved for full text review and assessed for eligibility: three studies were excluded as the focus was on health impact assessment, three did not report HRs, ORs, or RRs, 36 did not assess the outcomes of interest, two were on occupational exposures, 65 assessed exposures to other pollutants, 36 studies were conducted under designs not meeting our inclusion criteria, nine studies assessed (asthma) prevalence while five studies were excluded for other reasons (commentary papers, letter to the editor or not peer-reviewed papers). We further included nine studies that were identified by previous relevant reviews. The updated search for EC related papers published after April 2024 and up to April 2025 initially identified 37 studies. After excluding eight studies via title and abstract screening, five studies were excluded as assessed health effects of other exposures, nine did not investigate the targeted outcomes, seven were conducted using designs that did not meet our criteria, and two were reports presenting results from already identified studies. Therefore, five studies were selected for inclusion in the current review. Finally, a total of 51 studies were selected for the current systematic review (Fig. 1).
Fig. 1.
Flowchart of the systematic review on long-term exposure to elemental carbon and disease incidence, including ischemic heart disease (IHD), chronic obstructive pulmonary disease (COPD), asthma, lung cancer in adults and asthma and acute lower respiratory infections (ALRI) in children
Description of included studies
Main characteristics of the 51 studies are presented in Supplemental Table S3 [26–76]. Among the studies focusing on adult population, sixteen studies reported risk estimates for incidence of IHD, 10 for lung cancer incidence, five for COPD, and 4 for asthma. Four and 12 studies assessed the association between EC-related exposure and ALRI and asthma incidence in children (until the age of 16 years), respectively, while four studies evaluated asthma onset in children and adolescents (up to 24 years of age). Regarding exposure metrics, 26 studies reported effect estimates considering long-term exposure to BC, 3 to BS, 7 to EC and 16 to PM absorbance (‘soot’). Most of the studies were conducted in the European Region (n = 36), nine were in the Region of the Americas, one in South-East Asian Region, while five studies were in the Western Pacific Region. The sample sizes of the selected studies varied from 186 [33] to 7,657,323 [72] (Supplemental Table S3).
We conducted separate meta-analyses for pairs with at least four studies according to the protocol, namely for the association between long-term exposure to EC and incidence of lung cancer, IHD and COPD in adult population, as well as asthma in children/adolescents and ALRI incidence in children. No meta-analysis was conducted for the association between EC and asthma incidence in adults. Although four relevant studies were initially identified [27, 45, 55, 76], the study of Liu et al. (2021) [55] reported asthma results from the ELAPSE project that were replicated in the project report by Brunekreef et al. (2021) [27] (Supplemental Table S3), decreasing the final number to three studies.
Risk of bias
RoB assessment was performed for EC-disease incidence pairs that were informed by at least four studies in order to inform the meta-analysis and is presented in Supplemental Tables S4–S8. For the domains regarding selection bias, exposure assessment, outcome measurement, missing data and selective reporting the studies were characterized as low/moderate RoB. Regarding the confounding domain, the majority of the studies were also characterized as low or moderate RoB, except for Gan et al., 2011, Gan et al., 2013, Sbihi et al., 2016 and Beelen et al., 2008 [30, 37, 38, 66] that were assessed as high RoB as not all the critical confounders were included (body mass or smoking) without reporting any rationale (e.g., exploratory analysis) of minimal risk due to residual confounding.
Meta-analysis results
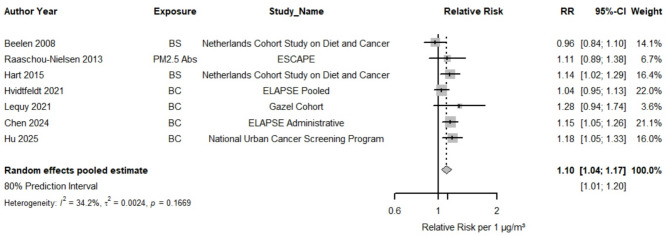
Seven studies were included in the meta-analysis for EC and lung cancer incidence indicating that 1 µg/m3 increase was associated with a RR 1.10 (95% CI: 1.04, 1.17, Figs. 2 and 3) with low heterogeneity (80% PI: 1.01, 1.20 and I2 = 34.2%). There was no indication of small study bias and funnel plot asymmetry (Supplemental Figure S1). RRs were consistently > 1 for almost all studies but one. As all studies but one originated from the WHO European region the subgroup analysis by WHO region (Table 1, Supplemental Figure S2) estimated 1.09 (95% CI: 1.02, 1.16) for the European Region (n = 6) vs. 1.18 (95% CI: 1.05, 1.33) in one study conducted in the Western Pacific Region. The RR for low/moderate RoB studies in the confounding domain was 1.12 (95% CI: 1.06, 1.19) in the studies that were evaluated as low or moderate RoB in the confounding domain (Table 2, Supplemental Figure S3) vs. 0.96 (95% CI: 0.84, 1.10) in high RoB. Studies with mean levels of lower than 1.82 µg/m3 (n = 5) estimated an RR of 1.08 versus 1.19 for those with levels equal to or above 1.82 µg/m3 (n = 2) (p for difference = 0.14, Supplemental Figure S4).
Fig. 2.
Forest plot for the relative risk (RR) in lung cancer incidence associated with 1 µg/m3 increase in EC
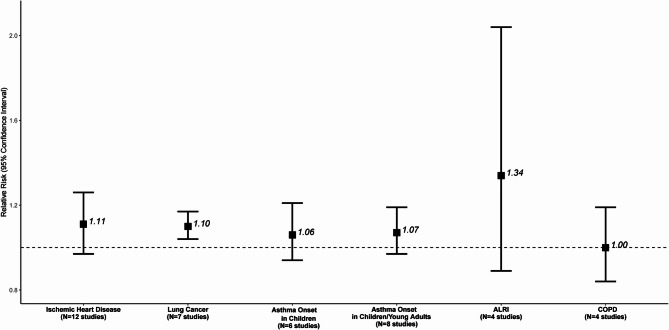
Fig. 3.
Meta-analysis results for the associations between EC- long-term exposure and disease incidence
The associations are expressed per 1 µg/m3 increase in EC
Table 1.
Meta-analysis results for the associations between EC- long-term exposure and disease incidence by WHO region
| Outcome (Incidence) |
Age Group | WHO Region | Number of Studies in meta-analysis | Pooled RR (95% CI) |
|---|---|---|---|---|
| IHD | Adults |
European Region Region of the Americas Western Pacific Region |
8 2 2 |
1.03 (1.00, 1.05) 1.01 (1.00, 1.02) 1.48 (0.71, 3.07) |
| Lung cancer | Adults |
European Region Western Pacific Region |
6 1 |
1.09 (1.02, 1.16) 1.18 (1.05, 1.33) |
| Asthma Onset | Children |
European Region Region of the Americas |
4 2 |
1.13 (0.91, 1.39) 0.99 (0.95, 1.03) |
| Asthma Onset | Children/ adolescents |
European Region Region of the Americas |
6 2 |
1.11 (0.96, 1.28) 0.99 (0.95, 1.03) |
| ALRI | Children |
European Region Region of the Americas South-East Asian Region |
2 1 1 |
1.82 (1.37, 2.42) 0.99 (0.96, 1.02) 1.59 (0.63, 4.01) |
| COPD | Adults |
European Region Region of the Americas Western Pacific Region |
2 1 1 |
0.97 (0.59, 1.58) 1.06 (1.02, 1.09) 0.92 (0.84, 1.19) |
IHD: Ischemic Heart Disease; ALRI: Acute Lower Respiratory Infections; COPD: Chronic obstructive pulmonary disease. The associations are expressed per 1 µg/m3 increase in EC
Table 2.
Meta-analysis results for the associations between EC- long-term exposure and disease incidence by risk of Bias (RoB) assessment in the confounding domain
| Outcome (Incidence) |
Age Group | RoB | Number of Studies in meta-analysis | Pooled RR (95% CI) |
|---|---|---|---|---|
| IHD | Adults |
High Low/Moderate |
1 11 |
1.01 (1.00, 1.02) 1.12 (0.96, 1.31) |
| Lung cancer | Adults |
High Low/Moderate |
1 6 |
0.96 (0.84, 1.10) 1.12 (1.06, 1.19) |
| Asthma Onset | Children |
High Low/Moderate |
1 6 |
0.99 (0.95, 1.03) 1.11 (0.93, 1.33) |
| Asthma Onset | Children/ adolescents |
High Low/Moderate |
1 7 |
0.99 (0.95, 1.03) 1.10 (0.97, 1.26) |
| ALRI | Children | All Low/Moderate | ||
| COPD | Adults |
High Low/Moderate |
1 3 |
1.06 (1.02, 1.09) 0.96 (0.74, 1.26) |
IHD: Ischemic Heart Disease; ALRI: Acute Lower Respiratory Infection; COPD: Chronic obstructive pulmonary disease. The associations are expressed per 1 µg/m3 increase in EC
Fifteen studies reported on EC and incidence of IHD, out of which 12 were included in the meta-analysis. Of the 12 studies included in the meta-analysis, more than half (n = 7) assessed IHD [32, 34, 48, 52, 56, 68, 76]. The majority of these studies (n = 5) were conducted in Europe, and all but one assessed long- term exposure to BC. The results from the 12 studies, supported that a 1 µg/m3 increase in EC was associated with a RR 1.11 (95% CI: 0.97, 1.26), while there was high heterogeneity (80% PI: 0.82, 1.49 and I2 = 79.5%) (Supplemental Figure S5) and no indication of asymmetry according to the Egger’s test (p = 0.15, Supplemental Figure S6). Meta-analysis by WHO region (Table 1, Supplemental Figure S7) estimated a statistically significant RR of 1.03 (95% CI: 1.00, 1.05) for the European Region (n = 8), 1.01 (95% CI: 1.00, 1.02) for the Region of the Americas (n = 2), and 1.48 (95% CI: 0.71, 3.07) in the Western Pacific Region (n = 2). Eleven out of the 12 studies were evaluated low/moderate RoB in the confounding domain providing a RR of 1.12 (95% CI: 0.96, 1.31, Table 2, Supplemental Figure S8). Studies with levels below 1.40 µg/m3 (n = 6) estimated a RR of 1.01 versus 1.17 for those equal or above 1.40 µg/m3 (n = 6) with no statistically significant difference (p = 0.19, Supplemental Figure S9). For the 10 studies assessing exposure to BC or PM2.5 abs, we estimated a RR of 1.09 versus 1.32 for those that evaluated directly the exposure to EC (p for difference = 0.55, Supplemental Figure S10).
Four studies that reported effect estimates for EC and COPD incidence estimated a RR of 1.00 (95% CI: 0.84, 1.19) with high heterogeneity (Fig. 3, Supplemental Figure S11) and no evidence of funnel plot asymmetry (Supplemental Figure S12). The RR for the European Region (n = 2) was 0.97 (95% CI: 0.59, 1.58), vs. 1.06 (95% CI: 1.02, 1.09), for one study in the Region of the Americas and 0.92 (95% CI: 0.84, 1.19) for on study in the Western Pacific Region (Table 1, Supplemental Figure S13). Low/ moderate RoB studies estimated a RR 0.96 (95% CI: 0.74, 1.26, Table 2, Supplemental Figure S14). Studies with mean levels of lower than 1.75 µg/m3 (n = 2) estimated a RR of 1.13 versus 0.87 for those with levels equal to or above 1.75 µg/m3 (n = 2) (p for difference = 0.03, Supplemental Figure S15).
No meta-analysis was conducted for the association between EC and asthma incidence in adults. Liu et al. (2021) assessed the association between long-term exposure to BC and asthma incidence providing a RR of 1.15 (95% CI: 1.08, 1.23) per 0.5 10–5/m increase; Jacquemin et al., (2015) [45] reported from the collaborative ESCAPE project, a RR 1.06 (95% CI: 0.95, 1.19) per 1 10–5/m increase in PM2.5 absorbance. Higher effect estimate was estimated in a study from China with RR 2.12 (95% CI: 1.29, 3.48) per 1.51 µg/m3 increase in BC [76] (Supplemental Table S3).
We conducted two separate analyses on asthma incidence, one for children until the age of 16 years old, and one including adolescents until the age of 24 years. For children, 1 µg/m3 increase in EC was associated with a RR of 1.06 (95% CI: 0.94, 1.21) derived from 6 studies (Fig. 3, Supplemental Figure S16) with moderate heterogeneity (80% PI: 0.88, 1.28 and I2 = 45.7%, Supplemental Figure S16). No evidence of small study bias/funnel plot asymmetry was detected (Supplemental Figure S17). Stratification by WHO region supported a higher effect estimate in the studies conducted in the European Region based on four studies (RR: 1.13, 95% CI: 0.91, 1.39), while the RR for the studies from the Region of the Americas (n = 2) was 0.99 (95% CI: 0.95, 1.03) (Table 1, Supplemental Figure S18). Excluding one study assessed as high risk of bias the RR was 1.11 (95% CI: 0.93, 1.33, Table 2, Supplemental Figure S19). The two studies with levels of lower than 1.76 µg/m3 presented a RR 1.06 versus 1.07 derived from the four studies with levels equal to or above 1.76 µg/m3 (p for difference = 0.96, Supplemental Figure S20). In the sensitivity analysis for asthma onset in children, we excluded Gehring et al. (2010) [39], on PIAMA cohort from 1996 to 2006, and Krämer et al. (2009) [47] from the GINIplus/LISAplus cohorts from 1995 to 2005, as both studies where included in the ESCAPE study by Gehring et al. (2015) [40]. The revised RR is 1.01 (95% CI: 0.90, 1.14) per 1 µg/m3 increase in EC versus 1.06 (95% CI: 0.94, 1.21) for the same increment in the main analysis (Supplemental Figure S21). The meta-analysis of studies that also included adolescents estimated a RR for asthma incidence 1.07 (95% CI: 0.97, 1.19) per 1 µg/m3 increase in EC, derived from eight studies (Fig. 3, Supplemental Figure S22). There was high heterogeneity (80% PI: 0.91, 1.27 and I2 = 81%, Supplemental Figure S22) and no evidence of funnel plot asymmetry (Supplemental Figure S23). The six studies from European Region had RR 1.11 (95% CI: 0.96, 1.28) while the two studies form the Region of the Americas had RR 0.99 (95% CI: 0.95. 1.03, Table 1, Supplemental Figure S24). Excluding one high RoB study the RR was 1.10 (95% CI: 0.97, 1.26) derived from seven studies (Table 2, Supplemental Figure S25). For the four studies with mean levels of lower than 1.34 µg/m3 we estimated a RR of 1.05 versus 1.14 for the four with levels equal or above 1.34 µg/m3 (p for difference = 0.50, Supplemental Figure S26). Studies assessing exposure to BC or PM2.5 abs (n = 7) estimated a RR of 1.07 versus 1.10 derived from the one study evaluating directly the exposure to EC with no statistically significant difference (p = 0.71, Supplemental Figure S27). Similarly, for asthma onset in children/young adults, we excluded the study by Krämer et al. (2009), and retained the ESCAPE (Gehring et al., 2020) [41] and the results remained robust (RR: 1.07, 95% CI: 0.96, 1.19 per 1 µg/m³ in EC, Supplemental Figure S28).
Four studies associated a 1 µg/m3 increase in EC with a RR 1.34 (95% CI: 0.89, 2.04) for ALRI incidence in children with high heterogeneity (Fig. 3, Supplemental Figure S29) and no indication of publication bias (Supplemental Figure S30). Subgroup analysis by WHO region (Table 1, Supplemental Figure S31) estimated a RR of 1.82 (95% CI: 1.37, 2.42) for the European Region (n = 2), 0.99 (95% CI: 0.96, 1.02) for the Region of the Americas (n = 1), and 1.59 (95% CI: 0.63, 4.01) in the South-East Asian Region (n = 1). All the studies were rated as low/moderate RoB. Studies with levels below 1.65 µg/m3 (n = 2) estimated a RR of 1.33 versus 1.44 for those equal or above 1.65 µg/m3 (n = 2) with no statistically significant difference (p = 0.88, Supplemental Figure S32).
We extracted information on the shape of the concentration-response for each EC and outcome pair assessed in meta-analyses. Almost half of the studies provided details regarding the assessment of linearity the majority of them used splines (natural or penalized splines) with varying degrees of freedom indicating linear shapes (Table S9), while all the reported results supported linear associations [29, 37, 38, 42, 44, 48, 51, 59, 60, 64, 66, 68, 72, 73].
Certainty of evidence assessments for EC and incidence outcomes are presented in Tables S10–S15. The association between EC and lung cancer incidence was assessed as high certainty, considering that six out of seven studies presented adverse associations while the CI and the PI excluded unity and there was support for linearity. The associations were assessed as low certainty for IHD and ALRI incidence, while very low for COPD incidence as the summary RR decreased after the exclusion of the high risk of bias study (RR = 1.00, 95% CI: 0.84, 1.19 for all studies vs. RR = 0.96, 95% CI: 0.74, 1.26 after the exclusion of the study rated as high risk) and the high unexplained heterogeneity (I2 = 87.8%, 80% PI: 0.74, 1.35). Asthma incidence in children/adolescents was rated as moderate. More specifically, the association with asthma was not downgraded for heterogeneity as even though the prediction interval included unity (80% PI: 0.88, 1.28), its width was not considerably wider than the confidence interval (95% CI: 0.94, 1.21) and heterogeneity was moderate or explained by subgroup analysis).
Co-pollutant adjustment
Eight studies reported on estimates adjusted from other co-exposures, mostly for PM2.5 and NO2.
Chen et al. (2024) assessed the association with lung cancer incidence adjusting for PM2.5, NO2 and warm period ozone (O3) separately, reporting that incidence risks remained statistically significant after controlling PM2.5 and positive but not statistically significant after the adjustment for warm period O3. Adjustment with NO2 resulted to an inverse but not statistically significant association, while the authors mention the high correlation between the two pollutants [72].
For IHD incidence, risk estimates reduced to or below the null in all models including UFP, PM2.5 or NO2 in Poulsen et al. (2024) [63] and after UFP in Downard et al. (2018) [36]. In Wolf et al. (2024) [68] the estimate remained robust to PM2.5, but not to NO2 or warm season O3. Simultaneous adjustment for PM2.5 and NO2, increased the IHD effect estimate in Gan et al. (2011) [38].
For COPD incidence, Liu et al. (2021) [54] noted that in two-pollutant models the HRs remained robust after adjusting for PM2.5 and slightly increased after adjustment for annual O3, while attenuated following NO2 adjustment. Nevertheless, Gan et al. (2013) [37] reported stable estimates after controlling for PM2.5 and NO2 (simultaneously).
Regarding asthma incidence, the results became lower and not statistically significant after adjusting for PM coarse in Gehring et al. (2020) increased after controlling for PM2.5 or PM10 and attenuated after adjusting for NO2, but the authors noted that these results are not valid due to multi-collinearity [41].
Discussion
We assessed the association between long-term to EC- related exposure (by further including BS, BC, PM absorbance converted to EC) and specific disease. Our meta-analysis estimated a statistically significant increased risk of lung cancer, while adverse associations were observed with IHD, asthma onset in children or children and adolescents and ALRI in children but did not achieve the nominal level of significance. A null association with COPD risk in adults was observed. The certainty in the evidence was high for lung cancer incidence and moderate for asthma onset in the younger age groups.
We observed a statistically significant association of long-term exposure to EC and lung cancer incidence with low heterogeneity (RR = 1.10, 95% CI: 1.04, 1.17). To the best of our knowledge, no previous meta-analyses assessed the exposure to EC and lung cancer incidence as previous reviews focused on the lung cancer mortality [10, 77]. One recent meta-analysis [77] presented an effect estimate for mortality 1.54 (95% CI: 1.09; 2.19) per 10 µg/m3 increase in BC that corresponds to 1.05 (95% CI: 1.01 to 1.10) per 1 µg/m3 increase in EC. Our results estimated a higher effect estimate (RR = 1.10 per 1 µg/m3) with lung cancer incidence than mortality, although with overlapping the confidence intervals with the reported mortality pooled estimate that may reflect the high case fatality rate of lung cancer [72]. The magnitude of the reported effect estimate is higher (if we consider the reported increments in each case as representative also of the pollutants’ variation) compared to the ones previously estimated from exposure to regulated pollutants and specifically PM2.5 and NO2 for which there is evidence for an association. Previous meta-analyses have evaluated the effect of long-term exposure to regulated pollutants such as PM2.5 and NO2 and lung cancer incidence. Huang et al. (2017) assessed the association between long-term exposure to PM2.5 and lung cancer incidence where a RR of 1.08 (95% CI: 1.03, 1.12) per 10 µg/m3 was observed, derived from 17 studies [78], while Hamra et al. (2017) estimated that 10 µg/m3 increase in exposure to NO2 was associated with 4% (95% CI: 1%, 8%) risk of lung cancer [79].
The review by Song et al. (2022) assessed long-term exposure to EC and IHD morbidity, and reported an adverse association (RR 1.07, 95% CI 1.01 to 1.13 per 1 µg/m3) based on three cohort studies [6], while the HEI 2022 review estimates 1.01 (95% CI: 0.99–1.03) from five studies, that is considerably lower to the estimate reported in our review (1.11, 95% CI: 0.97, 1.26) for the same increment. The difference in the magnitude of the pooled effect estimates is attributed to the inclusion of two recent studies conducted in Western Pacific Region. The exclusion of these studies, provided a similar effect estimate to the HEI review and further achieved statistical significance association (RR = 1.01, 95% CI: 1.00–1.03 per 1 µg/m3 increase). Furthermore, the restriction of our analysis in the studies conducted in the European Region and in the Region of the Americas presented statistically significant and comparable results (RR = 1.03, 95% CI: 1.00, 1.05, n = 8 studies; RR = 1.01, 95%CI: 1.00, 1.02, n = 2 studies, respectively). In the HEI review (2022) the association between long-term exposure to PM2.5 and NO2 with IHD morbidity evaluated a RR 1.09 (95% CI: 0.86, 1.39) per 5 µg/m3 in PM2.5 derived from four studies, while a null association with NO2 was observed (RR = 0.99, 95% CI: 0.94, 1.05 per 10 µg/m3 increase). For COPD incidence a null association was observed while to the best of our knowledge no other meta-analysis has been conducted due to the small number of available studies. However, the levels of the EC partly explained the heterogeneity in the association between EC and COPD incidence indicating with reduced effects in increasing levels of the pollutant’ levels that may indicate a supra-linear shape, although the number of studies in the subgroup analyses and overall is extremely small to provide confidence. For regulated pollutants the HEI traffic review (2022) estimated a RR 1.03 (95% CI: 0.94, 1.13 per 5 µg/m3 increase) for PM2.5 and an inverse but not statistically significant association with NO2 (RR = 0.91, 95% CI: 0.62, 1.36 per 10 µg/m3 increase).
No meta-analysis was conducted for the association between EC and asthma incidence in adults, due to the limited number of related studies. The three studies identified in our review indicated an increased risk of EC exposure on asthma onset in adult population ([45, 55, 76]). Even though previous research has presented positive associations for long-term exposure to air pollutants such as NO2 or PM2.5 and adult-onset asthma [45, 80–83], the literature remains limited, mainly due to the lack of cohorts with information on asthma incidence in adults. Identifying the exact time of onset is challenging, as adult asthma is considered a chronic disease accompanied by complex phenotype and recurring symptoms. Furthermore, asthma definitions based on self-reports are prone to recall bias which may lead to less precise definitions and maybe to an overestimation of true disease burden [55].
For asthma incidence in children, the HEI review (2022) estimated a RR 1.11 (95% CI: 0.94, 1.31), consistent with our meta-analysis (RR = 1.06, 95% CI: 0.94, 1.21) that identified only one additional study. Long-term exposure to PM2.5 and NO2 was associated with increased risk of the asthma onset in children in the HEI review (2022), but none of the effect estimates reached the nominal level of statistical significance (RR = 1.33, 95% CI: 0.90, 1.98 per 5 µg/m3 increase in PM2.5; RR = 1.05, 95% CI: 0.99, 1.12 per 10 µg/m3 increase in NO2). Slightly higher pooled effect estimate was presented in the analysis focused on asthma onset in also adolescents (RR = 1.09, 95% CI: 0.97, 1.22). Considering the great variability in the reporting for asthma associations in children and the potential for underdiagnoses, the HEI traffic review (2022) also assessed the association between EC and prevalence of asthma (asthma ever) and prevalence of active asthma and supported not statistically significant associations with wide confidence intervals derived from a small number of studies (RR = 1.30, 95% CI: 0.56, 3.04 and RR = 1.25, 95% CI: 0.98, 1.59 from three studies, respectively). However, in our review we focused on asthma incidence, as the most important outcome is the first diagnosis of the disease during the life course (HEI 2022). Regarding asthma incidence in children, the sensitivity analysis after exclusion of the studies with the shorter follow-up in cases where studies with same cohort were used, resulted to a lower effect estimate compared to the main analysis (RR = 1.01, 95% CI: 0.90, 1.14 per 1 µg/m3 versus RR: 1.07, 95% CI: 0.96, 1.19, respectively). The results remained not statistically significant while the smaller number of studies included, resulted in reduced statisitical power to detect an association. The results from the sensitivity analysis regarding asthma onset in children/ young adults remained robust. The association between EC and ALRI in children in the HEI (2022) review yielded a RR 1.30 (95% CI: 0.78, 2.18) per 1 µg/m3 similar to our 1.34 (95% CI: 0.89, 2.04) for the same increment. Our difference with the HEI report is the exclusion of cross-sectional study of Janssen et al. (2003) [84], as studies under cross-sectional design did not meet our inclusion criteria, and the inclusion of a recent cohort study conducted in the South-East Asian Region [74]. The wide confidence intervals in both cases are due to the smaller number of studies and greater variability among these studies’ results. Finally, for ALRI incidence in children in the HEI review, a statistically significant association was observed after long-term exposure to NO2 (RR = 1.09, 95% CI: 1.03, 1.16 per 10 µg/m3 increase) derived from 11 studies.
A potential limitation of our systematic review is the use of the estimates derived from single exposure models, under the rationale of the possible dependent effects of EC exposure. However, the pooling of the effects derived from multi-pollutant models is challenging due to the lack of information provided by the individual studies regarding the variance-covariance structure among the pollutants in order to reflect the varying correlations across different geographic locations. Nevertheless, this limitation may be of greater importance in the current work compared to previous regulated pollutants’ meta analyses, considering the high correlations of EC with NO2 and the potential confounding effects with PM2.5. There were few studies that reported on multi exposure models that provided inconsistent results on the stability of the single exposure model estimates. Nonetheless an overarching indication is that results remain rather stable following PM or O3 adjustment and attenuated following NO2 control. This indicates the impact on the model’s effect estimates of the high correlation with NO2, supporting that the main source of EC in the assessed studies is traffic related and indicating the difficulty in disentangling the independent effect of these two exposures. Future epidemiological studies should extensively report results from multi exposure models, particularly following adjustment for regulated pollutants as PM2.5 and NO2 for which there is overwhelming evidence of causal impacts on health. Future reports should also consider including the variance-covariance matrices of targeted exposures to facilitate future multivariate meta-analyses to account for underlying correlations. We acknowledge that ORs, HRs, and RRs represent distinct metrics, while their differences may become more pronounced under conditions of higher event rates, extended follow-up durations, and larger effect sizes. Nevertheless, given that the included studies were among the general population with low outcome prevalence, the OR approximates the RR. Additionally, the small effect estimates observed justifying the use of HR as RR. Even though the HRs are not precisely equivalent to RRs and ORs, they are traditionally used interchangeably in meta-analysis as their differences are generally considered minimal.
Limitations can also be raised regarding specific domains in the Risk of Bias tool. The domain for the exposure assessment includes the subdomain for the evaluation of the assessment of exposure contrast, an information that could not be assessed due to the lack of the information in the studies regarding whether the between-subject’ variability is larger than within-subject variability. Furthermore, in the confounding domain, the lack of spatial confounding factors in the proposed list of critical confounders, does not allow the detection of high risk of bias studies that are based on country wide populations but lack adjustment for regional differences, an important confounder due to the growing body of literature using country-wide assessments. Similar concerns were also raised in previous meta-analyses that assessed the health effects of long-term exposure to ambient particles [25] and gases [24] that were conducted to update the evidence base that informed 2021 WHO Air Quality Guidelines. The authors of the aforementioned reviews [24, 25] have also commented on the limitations regarding the use of the modified GRADE under the rationale that the risks in air pollution epidemiology are considerably smaller than other risk factors and may be more sensitive to the heterogeneity introduced by several factors. In the same context, the HEI review panel [10, 85] noted the certainty of the evidence should not be weakened due to the heterogeneity in the magnitude of effect estimates as applied in the GRADE domain on inconsistency but it should be strengthened in cases when the preceding evidence largely follow the same direction of results. We followed this rationale if part of the heterogeneity could be explained by our analysis and also all evidence was consistent in direction. However, as this condition did not arise in any of the associations under investigation, we were rather conservative in our evaluations as most were based on a rather small number of studies. Furthermore, the high heterogeneity in our results may be partly attributed to the different exposure assessment methods applied in the selected studies. Previous studies, have reported that different exposure assessment methods varied the magnitude of the effect estimates of the association between air pollutants and health outcomes, although not the direction and significance of the results [86–88]. Additionally, measurement error introduced by using modelled estimates generally leads to underestimation of results with different levels of bias to the null [87]. The vast majority of the studies used modelled based exposure assessment methods such as spatio (-temporal) land use regression (LUR) models, hybrid models in combination of methods, or Chemical Transport Models. The great variety of exposure assessment methods does not allow for a subgroup analysis to further assess the impact on the observed heterogeneity. Finally, there is ongoing progress on the optimal conversion factors between different metrics. The factors used for the conversion of BC, BS and “PM absorbance” (‘soot’) to EC depend on the measurement instruments that have changed over the years. As the studies in our meta-analysis published up to 2019 were also part of the HEI (2022) review, we applied the same conversion factors. However, as these conversion factors are approximate, derived from specific instrument comparisons and may vary depending on regional conditions, source and particle composition, these conversions may introduce additional uncertainty, which may not be adequately reflected in the pooled effect estimates [10]. The establishment of updated, standardized and validated conversion factors under harmonized protocols are of utmost importance in order to strengthen and expand the certainty in EC related health effects.
The main advantage of the study is the long time period of our search providing up-to-date scientific evidence on the incidence of the health outcomes that have received less attention so far. However, the small number of studies available related to one of the potentially most toxic components of particles and disease incidence highlights the importance of further investigation and especially in areas outside Europe, the United States and Canada. Further research is needed in order to increase the certainty of the evidence for the association between long-term exposure to EC and health effects, as TRAP is of utmost public health significance considering the large number of people exposed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
M.I.K contributed to data extraction, statistical analysis, interpretation of data, writing original draft and manuscript review, D.S contributed to data extraction and manuscript review, A.A, K.K, M.L, M.I.G, K.E conducted extensive review and editing of the manuscript, E.S contributed to conceptualization, supervision, interpretation, and reviewing and editing draft. All authors have reviewed and approved the final manuscript.
Funding
This project has received funding from the European Union’s Horizon Europe programme under grant agreement No 101138449 — MI-TRAP.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sang S, Chu C, Zhang T, Chen H, Yang X. The global burden of disease attributable to ambient fine particulate matter in 204 countries and territories, 1990–2019: A systematic analysis of the global burden of disease study 2019. Ecotoxicol Environ Saf. 2022;238:113588. [DOI] [PubMed] [Google Scholar]
- 2.16909 E. Ambient air – Measurement of elemental carbon (EC) and organic carbon (OC) collected on filters. Brussels: CEN; 2017.
- 3.Petzold A, Ogren JA, Fiebig M, Laj P, Li SM, Baltensperger U, Holzer-Popp T, Kinne S, Pappalardo G, Sugimoto N, et al. Recommendations for reporting black carbon measurements. Atmos Chem Phys. 2013;13(16):8365–79. [Google Scholar]
- 4.Cyrys J, Heinrich J, Hoek G, Meliefste K, Lewné M, Gehring U, Bellander T, Fischer P, van Vliet P, Brauer M, et al. Comparison between different traffic-related particle indicators: elemental carbon (EC), PM2.5 mass, and absorbance. J Expo Anal Environ Epidemiol. 2003;13(2):134–43. [DOI] [PubMed] [Google Scholar]
- 5.Janssen NA, Hoek G, Simic-Lawson M, Fischer P, van Bree L, ten Brink H, Keuken M, Atkinson RW, Anderson HR, Brunekreef B, et al. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ Health Perspect. 2011;119(12):1691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song X, Hu Y, Ma Y, Jiang L, Wang X, Shi A, Zhao J, Liu Y, Liu Y, Tang J, et al. Is short-term and long-term exposure to black carbon associated with cardiovascular and respiratory diseases? A systematic review and meta-analysis based on evidence reliability. BMJ Open. 2022;12(5):e049516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and Temporal variation in PM2. 5 chemical composition in the united States for health effects studies. Environ Health Perspect. 2007;115(7):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, Gold DR, Mittleman MA. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol. 2012;176(4):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briggs NL, Long CM. Critical review of black carbon and elemental carbon source apportionment in Europe and the united States. Atmos Environ. 2016;144:409–27. [Google Scholar]
- 10.HEI. Health effects institute.systematic review and Meta-analysis of selected health effects of Long-Term exposure to Traffic-Related air pollution, Special Report 23. In.; 2022.
- 11.Boogaard H, Samoli E, Patton AP, Atkinson RW, Brook JR, Chang HH, Hoffmann B, Kutlar Joss M, Sagiv SK, Smargiassi A, et al. Long-term exposure to traffic-related air pollution and non-accidental mortality: A systematic review and meta-analysis. Environ Int. 2023;176:107916. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Liu D, Huang L, Guo C, Gao X, Xu Z, Yang Z, Chen Y, Li M, Yang J. Global associations between long-term exposure to PM(2.5) constituents and health: A systematic review and meta-analysis of cohort studies. J Hazard Mater. 2024;474:134715. [DOI] [PubMed] [Google Scholar]
- 13.Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roller M. In vitro genotoxicity data of nanomaterials compared to carcinogenic potency of inorganic substances after inhalational exposure. Mutat Research/Reviews Mutat Res. 2011;727(3):72–85. [DOI] [PubMed] [Google Scholar]
- 15.Folkmann JK, Vesterdal LK, Sheykhzade M, Loft S, Møller P. Endothelial dysfunction in normal and prediabetic rats with metabolic syndrome exposed by oral Gavage to carbon black nanoparticles. Toxicol Sci. 2012;129(1):98–107. [DOI] [PubMed] [Google Scholar]
- 16.Frikke-Schmidt H, Roursgaard M, Lykkesfeldt J, Loft S, Nøjgaard JK, Møller P. Effect of vitamin C and iron chelation on diesel exhaust particle and carbon black induced oxidative damage and cell adhesion molecule expression in human endothelial cells. Toxicol Lett. 2011;203(3):181–9. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Zimra M, Bachelet I, Seaf M, Gleich G, Levi‐Schaffer F. Eosinophil major basic protein activates human cord blood mast cells primed with fibroblast membranes by integrin‐β1. Allergy. 2013;68(10):1259–68. [DOI] [PubMed] [Google Scholar]
- 18.Pawelczyk T, Sakowicz-Burkiewicz M, Wesserling M, Grden M, Kuczkowski J. Altered response of fibroblasts from human tympanosclerotic membrane to interacting mast cells: implication for tissue remodeling. Int J Biochem Cell Biol. 2014;57:35–44. [DOI] [PubMed] [Google Scholar]
- 19.WHO Global Air Quality Guidelines Working Group on Risk of Bias Assessment. 2020. Risk of bias assessment instrument for systematic reviews informing WHO global air quality guidelines. Copenhagen: WHO Regional Office for Europe [WWW Document]. URL https://apps.who.int/iris/handle/10665/341717 (accessed 29.06.25).
- 20.Schünemann H, Brożek J, Guyatt G, Oxman A. The GRADE handbook. In. Cochrane Collaboration London, UK; 2013.
- 21.WHO Global Air Quality Guidelines Working Group on Certainty of Evidence Assessment. 2020. Approach to assessing the certainty of evidence from systematic reviews informing WHO global air quality guidelines. [WWW Document]. URL https://ars.els-cdn.com/content/image/1-s2.0-S0160412020318316-mmc4.pdf (accessed 29.06.25).
- 22.Chen J, Hoek G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ Int. 2020;143:105974. [DOI] [PubMed] [Google Scholar]
- 23.Huangfu P, Atkinson R. Long-term exposure to NO(2) and O(3) and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ Int. 2020;144:105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasdagli M-I, Orellano P, Pérez Velasco R, Samoli E. Long-Term exposure to nitrogen dioxide and Ozone and mortality: update of the WHO air quality guidelines systematic review and Meta-Analysis. Int J Public Health 2024, 69. [DOI] [PMC free article] [PubMed]
- 25.Orellano P, Kasdagli M-I, Pérez Velasco R, Samoli E. Long-Term exposure to particulate matter and mortality: an update of the WHO global air quality guidelines systematic review and Meta-Analysis. Int J Public Health 2024, 69. [DOI] [PMC free article] [PubMed]
- 26.Bert Brunekreef RB, Hoek G, Schouten L, Bausch-Goldbohm S, Fischer P, Armstrong B, Hughes E. Michael jerrett, and Piet Van Den brandt: effects of Long-Term exposure to Traffic-Related air pollution on respiratory and cardiovascular mortality in the Netherlands. The NLCS-AIR Study; 2009. [PubMed]
- 27.Brunekreef M, Strak JC, Zorana J, Andersen R, Atkinson M, Bauwelinck T, Bellander M-C, Boutron Jørgen, Brandt I, Carey G, Cesaroni F, Forastiere D, Fecht J, Gulliver O, Hertel BH. Kees de Hoogh, Danny Houthuijs, Ulla Hvidtfeldt, Nicole Janssen, Jeanette Jørgensen, Klea Katsouyanni, Matthias Ketzel, Jochem Klompmaker, Norun Hjertager Krog, Shuo Liu, Petter Ljungman, Amar Mehta, Gabriele Nagel, Bente Oftedal, Göran ershagen, Annette Peters, Ole Raaschou-Nielsen, Matteo Renzi, Sophia Rodopoulou, Evi Samoli, Per Schwarze, Torben Sigsgaard, Massimo Stafoggia, Danielle Vienneau, Gudrun Weinmayr, Kathrin Wolf, and Gerard Hoek: Mortality and Morbidity Effects of Long Term Exposure to Low-Level PM2.5, BC, NO2, and O3: An Analysis of European Cohorts in the ELAPSE Project. 2021. [PMC free article] [PubMed]
- 28.Ulrike Gehring RB, de Hoogh JC, de Jongste M, Keuken, Gerard H, Koppelman KM, Marieke Oldenwening DS, Postma LvR, Wang M, Smit HA. Brunekreef aB: Particulate Matter Composition and Respiratory Health The PIAMA Birth Cohort Study. 2015. [DOI] [PubMed]
- 29.Alexeeff SE, Roy A, Shan J, Liu X, Messier K, Apte JS, Portier C, Sidney S, Van Den Eeden SK. High-resolution mapping of traffic related air pollution with Google street view cars and incidence of cardiovascular events within neighborhoods in oakland, CA. Environ Health. 2018;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, Armstrong B, Brunekreef B. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology. 2008;19(5):702–10. [DOI] [PubMed] [Google Scholar]
- 31.Brunst KJ, Ryan PH, Brokamp C, Bernstein D, Reponen T, Lockey J, Khurana Hershey GK, Levin L, Grinshpun SA, LeMasters G. Timing and duration of Traffic-related air pollution exposure and the risk for childhood wheeze and asthma. Am J Respir Crit Care Med. 2015;192(4):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlsen HK, Andersson EM, Molnár P, Oudin A, Xu Y, Wichmann J, Spanne M, Stroh E, Engström G, Stockfelt L. Incident cardiovascular disease and long-term exposure to source-specific air pollutants in a Swedish cohort. Environ Res. 2022;209:112698. [DOI] [PubMed] [Google Scholar]
- 33.Carlsten C, Dybuncio A, Becker A, Chan-Yeung M, Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68(4):291–5. [DOI] [PubMed] [Google Scholar]
- 34.Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, de Faire U, Erbel R, Eriksen KT, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE project. BMJ. 2014;348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118(2):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downward GS, van Nunen E, Kerckhoffs J, Vineis P, Brunekreef B, Boer JMA, Messier KP, Roy A, Verschuren WMM, van der Schouw YT, et al. Long-Term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch cohort. Environ Health Perspect. 2018;126(12):127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187(7):721–7. [DOI] [PubMed] [Google Scholar]
- 38.Gan WQ, Koehoorn M, Davies HW, Demers PA, Tamburic L, Brauer M. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ Health Perspect. 2011;119(4):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, Oldenwening M, Smit HA, Brunekreef B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181(6):596–603. [DOI] [PubMed] [Google Scholar]
- 40.Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, Fuertes E, Gruzieva O, Heinrich J, Hoffmann B, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3(12):933–42. [DOI] [PubMed] [Google Scholar]
- 41.Gehring U, Wijga AH, Koppelman GH, Vonk JM, Smit HA, Brunekreef B. Air pollution and the development of asthma from birth until young adulthood. Eur Respir J 2020, 56(1). [DOI] [PubMed]
- 42.Hart JE, Spiegelman D, Beelen R, Hoek G, Brunekreef B, Schouten LJ, van den Brandt P. Long-Term ambient residential Traffic-Related exposures and measurement Error-Adjusted risk of incident lung Cancer in the Netherlands cohort study on diet and Cancer. Environ Health Perspect. 2015;123(9):860–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann B, Weinmayr G, Hennig F, Fuks K, Moebus S, Weimar C, Dragano N, Hermann DM, Kälsch H, Mahabadi AA, et al. Air quality, stroke, and coronary events: results of the heinz Nixdorf recall study from the Ruhr region. Dtsch Arztebl Int. 2015;112(12):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hvidtfeldt UA, Severi G, Andersen ZJ, Atkinson R, Bauwelinck M, Bellander T, Boutron-Ruault MC, Brandt J, Brunekreef B, Cesaroni G, et al. Long-term low-level ambient air pollution exposure and risk of lung cancer - A pooled analysis of 7 European cohorts. Environ Int. 2021;146:106249. [DOI] [PubMed] [Google Scholar]
- 45.Jacquemin B, Siroux V, Sanchez M, Carsin AE, Schikowski T, Adam M, Bellisario V, Buschka A, Bono R, Brunekreef B, et al. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE). Environ Health Perspect. 2015;123(6):613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karr CJ, Demers PA, Koehoorn MW, Lencar CC, Tamburic L, Brauer M. Influence of ambient air pollutant sources on clinical encounters for infant bronchiolitis. Am J Respir Crit Care Med. 2009;180(10):995–1001. [DOI] [PubMed] [Google Scholar]
- 47.Krämer U, Sugiri D, Ranft U, Krutmann J, von Berg A, Berdel D, Behrendt H, Kuhlbusch T, Hochadel M, Wichmann HE, et al. Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J Dermatol Sci. 2009;56(2):99–105. [DOI] [PubMed] [Google Scholar]
- 48.Kriit HK, Andersson EM, Carlsen HK, Andersson N, Ljungman PLS, Pershagen G, Segersson D, Eneroth K, Gidhagen L, Spanne M et al. Using distributed lag Non-Linear models to estimate exposure lag-Response associations between Long-Term air pollution exposure and incidence of cardiovascular disease. Int J Environ Res Public Health 2022, 19(5). [DOI] [PMC free article] [PubMed]
- 49.Kuiper IN, Markevych I, Accordini S, Bertelsen RJ, Bråbäck L, Christensen JH, Forsberg B, Halvorsen T, Heinrich J, Hertel O et al. Associations of preconception exposure to air pollution and greenness with offspring asthma and hay fever. Int J Environ Res Public Health 2020, 17(16). [DOI] [PMC free article] [PubMed]
- 50.Lavigne É, Talarico R, van Donkelaar A, Martin RV, Stieb DM, Crighton E, Weichenthal S, Smith-Doiron M, Burnett RT, Chen H. Fine particulate matter concentration and composition and the incidence of childhood asthma. Environ Int. 2021;152:106486. [DOI] [PubMed] [Google Scholar]
- 51.Lequy E, Siemiatycki J, de Hoogh K, Vienneau D, Dupuy JF, Gares V, Hertel O, Christensen JH, Zhivin S, Goldberg M, et al. Contribution of Long-Term exposure to outdoor black carbon to the carcinogenicity of air pollution: evidence regarding risk of Cancer in the Gazel cohort. Environ Health Perspect. 2021;129(3):37005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B, Wen F, Liu K, Xie Y, Zhang F, Li P, Sun Y, Qu A, Yang X, Zhang L. The mediation effect of lipids, blood pressure and BMI between air pollutant mixture and atherosclerotic cardiovascular disease: the CHCN-BTH cohort study. Ecotoxicol Environ Saf. 2023;264:115491. [DOI] [PubMed] [Google Scholar]
- 53.Lim EH, Franklin P, Trevenen ML, Nieuwenhuijsen M, Yeap BB, Almeida OP, Hankey GJ, Golledge J, Etherton-Beer C, Flicker L, et al. Exposure to low-level ambient air pollution and the relationship with lung and bladder cancer in older men, in perth, Western Australia. Br J Cancer. 2023;129(9):1500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu S, Jørgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, Magnusson PKE, Rizzuto D, Hvidtfeldt UA, Raaschou-Nielsen O, et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: the ELAPSE project. Environ Int. 2021;146:106267. [DOI] [PubMed] [Google Scholar]
- 55.Liu, Jørgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, Magnusson PKE, Rizzuto D, Hvidtfeldt UA, Raaschou-Nielsen O et al. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J 2021, 57(6). [DOI] [PubMed]
- 56.Ljungman PLS, Andersson N, Stockfelt L, Andersson EM, Nilsson Sommar J, Eneroth K, Gidhagen L, Johansson C, Lager A, Leander K, et al. Long-Term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect. 2019;127(10):107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacIntyre EA, Gehring U, Molter A, Fuertes E, Klumper C, Kramer U, Quass U, Hoffmann B, Gascon M, Brunekreef B, et al. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE project. Environ Health Perspect. 2014;122(1):107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Gehring U, Koletzko S, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med. 2007;64(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olsson D, Forsberg B, Bråbäck L, Geels C, Brandt J, Christensen JH, Frohn LM, Oudin A. Early childhood exposure to ambient air pollution is associated with increased risk of paediatric asthma: an administrative cohort study from stockholm, Sweden. Environ Int. 2021;155:106667. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen M, Liu S, Zhang J, Jovanovic Andersen Z, Brandt J, Budtz-Jorgensen E, Bonnelykke K, Frohn LM, Nybo Andersen AM, Ketzel M, et al. Early-Life exposure to ambient air pollution from multiple sources and asthma incidence in children: A nationwide birth cohort study from Denmark. Environ Health Perspect. 2023;131(5):57003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poulsen AH, Sorensen M, Hvidtfeldt UA, Christensen JH, Brandt J, Frohn LM, Ketzel M, Andersen C, Raaschou-Nielsen O. Source-Specific air pollution including ultrafine particles and risk of myocardial infarction: A nationwide cohort study from Denmark. Environ Health Perspect. 2023;131(5):57010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulsen AH, Sørensen M, Hvidtfeldt UA, Frohn LM, Ketzel M, Christensen JH, Brandt J, Massling A, Khan J, Lassen CF, et al. Air pollution and myocardial infarction; effect modification by sociodemographic and environmental factors. A cohort study from Denmark. Environ Res. 2023;229:115905. [DOI] [PubMed] [Google Scholar]
- 63.Poulsen AH, Sørensen M, Hvidtfeldt UA, Ketzel M, Christensen JH, Brandt J, Frohn LM, Massling A, Khan J, Münzel T, et al. Concomitant exposure to air pollution, green space and noise, and risk of myocardial infarction: a cohort study from Denmark. Eur J Prev Cardiol. 2024;31(1):131–41. [DOI] [PubMed] [Google Scholar]
- 64.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European study of cohorts for air pollution effects (ESCAPE). Lancet Oncol. 2013;14(9):813–22. [DOI] [PubMed] [Google Scholar]
- 65.Rodins V, Lucht S, Ohlwein S, Hennig F, Soppa V, Erbel R, Jöckel KH, Weimar C, Hermann DM, Schramm S, et al. Long-term exposure to ambient source-specific particulate matter and its components and incidence of cardiovascular events - The heinz Nixdorf recall study. Environ Int. 2020;142:105854. [DOI] [PubMed] [Google Scholar]
- 66.Sbihi H, Tamburic L, Koehoorn M, Brauer M. Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur Respir J. 2016;47(4):1062–71. [DOI] [PubMed] [Google Scholar]
- 67.Schikowski T, Adam M, Marcon A, Cai Y, Vierkotter A, Carsin AE, Jacquemin B, Al Kanani Z, Beelen R, Birk M, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J. 2014;44(3):614–26. [DOI] [PubMed] [Google Scholar]
- 68.Wolf K, Hoffmann B, Andersen ZJ, Atkinson RW, Bauwelinck M, Bellander T, Brandt J, Brunekreef B, Cesaroni G, Chen J, et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet Health. 2021;5(9):e620–32. [DOI] [PubMed] [Google Scholar]
- 69.Yamazaki S, Shima M, Nakadate T, Ohara T, Omori T, Ono M, Sato T, Nitta H. Association between traffic-related air pollution and development of asthma in school children: cohort study in Japan. J Expo Sci Environ Epidemiol. 2014;24(4):372–9. [DOI] [PubMed] [Google Scholar]
- 70.Yang A, Janssen NA, Brunekreef B, Cassee FR, Hoek G, Gehring U. Children’s respiratory health and oxidative potential of PM2.5: the PIAMA birth cohort study. Occup Environ Med. 2016;73(3):154–60. [DOI] [PubMed] [Google Scholar]
- 71.Yu Z, Koppelman GH, Boer JMA, Hoek G, Kerckhoffs J, Vonk JM, Vermeulen R, Gehring U. Ambient ultrafine particles and asthma onset until age 20: the PIAMA birth cohort. Environ Res. 2022;214(Pt 1):113770. [DOI] [PubMed] [Google Scholar]
- 72.Chen, Atkinson RW, Andersen ZJ, Oftedal B, Stafoggia M, Lim YH, Bekkevold T, Krog NH, Renzi M, Zhang J, et al. Long-term exposure to ambient air pollution and risk of lung cancer - A comparative analysis of incidence and mortality in four administrative cohorts in the ELAPSE study. Environ Res. 2024;263(Pt 3):120236. [DOI] [PubMed] [Google Scholar]
- 73.Hu J, Yang L, Kang N, Wang N, Shen L, Zhang X, Liu S, Li H, Xue T, Ma S, et al. Associations between long-term exposure to fine particulate matter and its constituents with lung cancer incidence: evidence from a prospective cohort study in beijing, China. Environ Pollut. 2025;368:125686. [DOI] [PubMed] [Google Scholar]
- 74.Soesanti F, Hoek G, Brunekreef B, Meliefste K, Chen J, Idris NS, Putri ND, Uiterwaal C, Grobbee DE, Klipstein-Grobusch K. Perinatal exposure to traffic related air pollutants and the risk of infection in the first six months of life: a cohort study from a low-middle income country. Int Arch Occup Environ Health. 2024;97(5):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu Z, Kebede Merid S, Bellander T, Bergström A, Eneroth K, Merritt AS, Ödling M, Kull I, Ljungman P, Klevebro S, et al. Improved air quality and asthma incidence from school age to young adulthood: A Population-based prospective cohort study. Ann Am Thorac Soc. 2024;21(10):1432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou H, Hong F, Wang L, Tang X, Guo B, Luo Y, Yu H, Mao D, Liu T, Feng Y, et al. Air pollution and risk of 32 health conditions: outcome-wide analyses in a population-based prospective cohort in Southwest China. BMC Med. 2024;22(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu X, Liu B, Guo C, Li Z, Cheng M, Zhu X, Wei Y. Short and long-term association of exposure to ambient black carbon with all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ Pollut. 2023;324:121086. [DOI] [PubMed] [Google Scholar]
- 78.Huang F, Pan B, Wu J, Chen E, Chen L. Relationship between exposure to PM2.5 and lung cancer incidence and mortality: A meta-analysis. Oncotarget. 2017;8(26):43322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamra GB, Laden F, Cohen AJ, Raaschou-Nielsen O, Brauer M, Loomis D. Lung Cancer and exposure to nitrogen dioxide and traffic: A systematic review and Meta-Analysis. Environ Health Perspect. 2015;123(11):1107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen ZJ, Bønnelykke K, Hvidberg M, Jensen SS, Ketzel M, Loft S, Sørensen M, Tjønneland A, Overvad K, Raaschou-Nielsen O. Long-term exposure to air pollution and asthma hospitalisations in older adults: a cohort study. Thorax. 2012;67(1):6–11. [DOI] [PubMed] [Google Scholar]
- 81.Salimi F, Morgan G, Rolfe M, Samoli E, Cowie CT, Hanigan I, Knibbs L, Cope M, Johnston FH, Guo Y, et al. Long-term exposure to low concentrations of air pollutants and hospitalisation for respiratory diseases: A prospective cohort study in Australia. Environ Int. 2018;121(Pt 1):415–20. [DOI] [PubMed] [Google Scholar]
- 82.Weichenthal S, Bai L, Hatzopoulou M, Van Ryswyk K, Kwong JC, Jerrett M, van Donkelaar A, Martin RV, Burnett RT, Lu H, et al. Long-term exposure to ambient ultrafine particles and respiratory disease incidence in in toronto, canada: a cohort study. Environ Health. 2017;16(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. Women. Am J Respir Crit Care Med. 2014;190(8):914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janssen NA, Brunekreef B, van Vliet P, Aarts F, Meliefste K, Harssema H, Fischer P. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect. 2003;111(12):1512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boogaard H, Atkinson RW, Brook JR, Chang HH, Hoek G, Hoffmann B, Sagiv SK, Samoli E, Smargiassi A, Szpiro AA, et al. Evidence synthesis of observational studies in environmental health: lessons learned from a systematic review on Traffic-Related air pollution. Environ Health Perspect. 2023;131(11):115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jerrett M, Turner MC, Beckerman BS, Pope CA, van Donkelaar A, Martin RV, Serre M, Crouse D, Gapstur SM, Krewski D, et al. Comparing the health effects of ambient particulate matter estimated using Ground-Based versus remote sensing exposure estimates. Environ Health Perspect. 2017;125(4):552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klompmaker JO, Janssen N, Andersen ZJ, Atkinson R, Bauwelinck M, Chen J, de Hoogh K, Houthuijs D, Katsouyanni K, Marra M, et al. Comparison of associations between mortality and air pollution exposure estimated with a hybrid, a land-use regression and a dispersion model. Environ Int. 2021;146:106306. [DOI] [PubMed] [Google Scholar]
- 88.Samoli E, Butland BK, Rodopoulou S, Atkinson RW, Barratt B, Beevers SD, Beddows A, Dimakopoulou K, Schwartz JD, Yazdi MD, et al. The impact of measurement error in modeled ambient particles exposures on health effect estimates in multilevel analysis: A simulation study. Environ Epidemiol. 2020;4(3):e094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon request.