Abstract
Background
Non-typhoidal Salmonella (NTS) typically cause self-limiting enterocolitis, but can lead to life-threatening invasive diseases, particularly in sub-Saharan Africa. In Kenya, multidrug-resistant (MDR) NTS strains with increasing non-susceptibility to third-generation cephalosporins pose a growing public health threat. As traditional antimicrobial treatments become less effective, bacteriophages are emerging as a potential alternative. This study aimed to isolate and characterize bacteriophages targeting MDR and extended spectrum-β-lactamase (ESBL)-producing non-typhoidal Salmonella (NTS).
Methods
Environmental samples were collected from seven sites in Nairobi City County, Kenya. Four NTS bacterial strains were used for phage enrichment, screening, and purification via spot tests and plaque assays. Phage efficacy was assessed in vitro by testing host range and efficiency of plating (EOP) against 12 Salmonella strains isolated in Kenya over different years. Ten selected broad-host-range phages were evaluated for thermal and pH stability and their ability to disrupt pre-formed NTS biofilms. Phage genomes were sequenced using the Illumina sequencing platform, and analyzed with bioinformatics tools to screen for antimicrobial resistance (AMR), lysogeny, virulence, and allergenic genes. The morphological characteristics of four representative phages were examined using Transmission Electron Microscopy.
Results
Thirty-one phages were isolated, with host ranges varying from lysing one strains to all 12 strains. Ten phages lysed more than 80% of the Salmonella strains and were selected for further characterization. Most phages exhibited high production EOP on at least one bacterial strain, except KE26 and KE28. All phages were stable from − 80 °C to 40 °C and pH 5 to 11, with noticeable but statistically insignificant biofilm disruption. Genome sizes ranged from 23,215 bp to 159,981 bp, and were free of known AMR, lysogeny, or virulence genes. Allergenicity screening identified no allergenic hits across most phages, with exception of KE23, which showed potential allergenic regions in its tail fiber and endolysin proteins. All phages belonged to class Caudoviricetes, with KE23, KE26, and KE28 exhibiting a myovirus-like morphotype, and KE15 displaying a siphovirus morphotype.
Conclusion
This study identified phages with desirable safety and stability profiles for potential usage against MDR and ESBL-producing NTS infections. Further in vivo studies are recommended to evaluate their therapeutic potential.
Importance
Non-typhoidal Salmonella (NTS) typically cause self-limiting enterocolitis but can lead to life-threatening invasive diseases. In Kenya, multidrug-resistant (MDR) NTS strains with increasing nonsusceptibility to third-generation cephalosporins have been reported, posing a significant public health concern that requires urgent attention. Bacteriophages are increasingly being considered as an alternative treatment for MDR bacterial infections because of the growing ineffectiveness of conventional antibiotics. Our study reports the isolation and characterization of lytic Salmonella phages devoid of detectable antimicrobial resistance (AMR) genes, lysogeny potential, allergens or virulence factors. These attributes position them as promising candidates for therapeutic interventions against MDR NTS infections. These findings highlight the potential of our study phages as a therapy for drug-resistant NTS and underscore the need for further investigation into their clinical application against MDR strains.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11325-3.
Keywords: Non-typhoidal Salmonella, Multidrug-resistant, And phage
Introduction
Globally, non-typhoidal Salmonella (NTS) causes 93 million enteric infections and 155,000 diarrheal deaths yearly [1]. The most common implicated serovars are Salmonella enterica serovars Enteritidis and Salmonella enterica serovars Typhimurium (S. Enteritidis and S. Typhimurium). While NTS is typically associated with self-limiting enterocolitis in humans, novel lineages of serovars Typhimurium and Enteritidis have been increasingly implicated in invasive disease, especially among children under five years in sub-Saharan Africa (SSA). In SSA, NTS ranks among the three most prevalent bacteria associated with bloodstream infections [2], with the region disproportionately bearing 79% of cases and 85% of deaths from invasive NTS (iNTS)- 535,000 cases and 77,500 deaths annually [3].
According to the Centers for Disease Control (CDC), AMR in NTS is a significant public health concern [4, 5]. In Kenya, a high prevalence of MDR NTS, 77.2%, has been reported. These strains show resistance to the first-line antimicrobials such as ampicillin, chloramphenicol, and sulpha-trimethoprim, as well as reduced susceptibility to the last resort drugs, including ciprofloxacin and ceftriaxone [6, 7]. Similar findings of MDR NTS, including the novel S. Typhimurium sequence type (ST) 313, have been reported in several other studies in SSA [8, 9].
The increasing trend of drug resistance in bacteria is mainly due to the overuse and misuse of antibiotics, driving bacterial evolution. Additionally, global interconnectedness has facilitated the spread of drug-resistant pathogens and their resistance genes across borders [10]. The development of new antibiotics has not kept pace due to the high cost of research and development of new molecules, posing a public health threat from emerging drug-resistant pathogens and the risk of returning the world to the pre-chemotherapy era, with increased deaths occurring due to common bacterial infections [11]. AMR is associated with heightened morbidity, mortality, and healthcare costs [12]. To mitigate the drug-resistance crisis, the World Health Organization (WHO) has developed a global action plan on AMR, which aims, among others to strengthen knowledge and promote evidence-based alternatives to antibiotic therapy [13].
Bacteriophage (phage) therapy is a promising alternative to conventional antimicrobial treatment for bacterial infections [14]. Some phage-based products, such as those developed by Intralytix, have received the “generally recognized as safe (GRAS)” status by the United States Food and Drug Administration (FDA) [15–17]. Phages are highly specific to their bacterial targets, which helps preserve commensal bacteria and maintain a healthy microbiome. They are also effective in low dosages due to their ability to replicate at the site of infection. In addition, phage therapy has shown minimal or no severe side effects, such as allergic reactions or toxicity, and can be used in combination with antibiotics, potentially enhancing treatment efficacy [18, 19]. Phages also exhibit the ability to disrupt bacterial biofilms, which often serve as protective mechanisms enabling bacteria to resist conventional antibiotics [20]. Although phage therapy is generally considered safe, there is a notable gap in the guidelines outlining the genetic safety of phages before their clinical use [21]. Essential considerations for evaluating phage safety include screening for lysogeny-encoding genes that could convert phages into prophages, identifying virulence, and AMR genes that might be transferred to bacteria, and assessing potential allergenicity [22]. Whole genome sequence analysis is important for understanding their genetic makeup, taxonomic classification, and comparative genomics [23]. Surprisingly, the functionality of 65% of phage genes remains uncharacterized [24].
Several studies have isolated and characterized NTS phages [25–28], but only a few have been tested in vivo [29, 30]. However, due to the diversity of Salmonella serovars, as well as phages, susceptibility may vary between different phages and targeted strains [31]. Phage diversity can be beneficial in preparing phage cocktails for therapy, as it increases the range of receptors available for attachment to the targeted bacteria [32]. There is significant variation in phage genome sizes, with some phage genomes being less than 50 kb, while others are jumbo phages greater than 200kb [26]. While phages with smaller genomes are often considered more virulent and less likely to carry undesirable genes, genome size alone is not definitive. Some large-genome phages also exhibit strictly lytic behavior, underscoring the importance of genome analysis when assessing their biocontrol potential [33, 34]. Despite the vast diversity of phage genomes and the enormous number of phages (over 10³¹), the number of phage genomes available in public databases is still quite limited [35]. This is partly due to the evolving nature of phage taxonomy. The International Committee on Taxonomy of Viruses (ICTV) has recently adopted a genome-based classification system, replacing the traditional morphology-based classification of families such as Myoviridae, Siphoviridae, and Podoviridae [36]. To support phage classification and improve our understanding of phage diversity, more phage genomes need to be deposited into public databases. In Africa, only a few studies have focused on phages with lytic activity against NTS, including work by Rodwell et al. [25] and Ismael et al. [28],. In Kenya, Mhone et al.. isolated S. Enteritidis phages for application in poultry [37]. To the best of our knowledge, there has been no exploration of phages as a treatment option for MDR and extended spectrum-beta-lactamase (ESBL)-producing NTS infections in Kenya. This study aimed to fill this gap by isolating and characterizing phages with therapeutic potential against these resistant NTS infections.
Materials and methods
Non-typhoidal Salmonella strains used in phage isolation
In this study, we utilized archived non-typhoidal Salmonella (NTS) isolates from previous research in Kenya [6], including a panel of four MDR Salmonella enterica serovars Typhimurium and Enteritidis (NCBI/Bioproject No. PRJEB19289 and Biosample accessions ERS4397787, ERS3403399, ERS3403411, and ERS4397849) with varying AMR patterns (Table 1). The strains were revived from a −80 °C freezer using tryptic soy agar (TSA) (Oxoid Ltd., Basingstoke, UK), and overnight colonies were sub-cultured into tryptic soy broth (TSB) (Oxoid Ltd., Basingstoke, UK), for overnight incubation. Antimicrobial susceptibility testing (AST) was repeated to confirm the MDR phenotype of the strains, using Kirby Bauer’s disc diffusion method following the Clinical Laboratory Standards Institute (CLSI) guidelines 2022 [38].
Table 1.
The NTS strains used for phage isolation in this study
| NTS strains Serovar | NCBI Accession No. | Lab No |
|---|---|---|
| Salmonella Enteritidis | ERS4397787 | MR 2829 |
| Salmonella Typhimurium | ERS3403399 | MR 3372 |
| Salmonella Enteritidis | ERS4397849 | MCC 1462 |
| Salmonella Typhimurium | ERS3403411 | MB 1102 |
A 0.5 McFarland-equivalent suspension of the NTS bacterial strain was spread evenly on Mueller-Hinton Agar (MHA) (Oxoid Ltd., Basingstoke, UK), and allowed to air dry. Antibiotic disks were then placed on the bacterial lawn, and the plates were incubated overnight at 37 °C. The zones of inhibition were measured, and susceptibility was interpreted according to the 2022 CLSI guidelines. ESBL production was tested using a double-disc diffusion test, where cefotaxime/clavulanic acid (30/10 µg) and ceftazidime/clavulanic acid (30/10 µg) discs (BD, Franklin Lakes, NJ, USA), along with cefotaxime (30 µg) and ceftazidime (30 µg) discs without clavulanic acid (Oxoid Ltd., Basingstoke, UK), were used for AST as previously described [39, 40]. ESBL production was confirmed using the Phenotypic Confirmatory Disc Diffusion Test (PCDDT). An isolate was considered an ESBL producer if there was a > 5 mm difference in the zone of inhibition between cefotaxime with clavulanic acid and cefotaxime without clavulanic acid, or between ceftazidime with and without clavulanic acid. Conversely, isolates with a < 5 mm difference in the zones of inhibition were classified as non-ESBL producers. Escherichia coli (E. coli) NCTC 13,351 and E. coli ATCC 25,922 were the positive controls for ESBL and non-ESBL production, respectively.
The study used a panel of 13 antimicrobial agents from different classes, including penicillin (ampicillin (10 µg)), cephalosporins (ceftriaxone (30 µg), cefotaxime (30 µg), ceftazidime (30 µg), cefpodoxime (10 µg)), β-lactam-β-lactamase inhibitor (amoxicillin/clavulanic acid (20/10 µg)), quinolones (ciprofloxacin (5 µg)), nalidixic acid (30 µg)), aminoglycosides (gentamicin (10 µg), kanamycin (30 µg)), sulfonamides (trimethoprim-sulfamethoxazole (1.25/23.75 µg)), tetracyclines (tetracycline (30 µg)), and phenicol (chloramphenicol (30 µg)) all from Oxoid Ltd., Basingstoke, UK.
Collection of environmental samples
A one-time environmental sampling was conducted between April and October 2022 at seven locations in Nairobi City County, including open drains, rivers, and a dam. At each site, five samples were collected and pooled to form one composite sample, yielding in a total of seven composite samples, one from each location. The drains in Nairobi’s informal settlements are polluted with raw sewage and household waste, creating an ideal breeding ground for bacteriophages [41]. Four sampling points were from informal settlements, including Kamukunji (Majengo), Mukuru slums (River Ngong), Njiru River, and Kibera (Nairobi Dam), two points were selected from the Nairobi Wastewater Treatment Plant at Ruai (influent and effluent), and the other sampling point was at an open drain at Dagoretti Market (Supplementary Fig S1). The samples (100 mL) were collected in sterile Whirl-Pak bags (Whirl-Pak® Sample Bag, Nasco, USA) and immediately transported in cool boxes to the laboratory at the Centre for Microbiology Research (CMR) in KEMRI for processing the same day after collection.
Phage isolation
We isolated phages as previously described [28, 42], with slight modification. The water samples were centrifuged at 10,000 × g for 10 min to decant the solid particles, and the supernatant was filtered through a 0.45 μm PES syringe filter (Scientific Laboratory Supplies Ltd, Nottingham, UK). To enrich phages, 10 mL of the filtrate was combined with 10 mL of TSB and 100 µl of exponentially growing MDR NTS strain (Table 1) subsequently added. The mixture was incubated overnight at 37 °C in an Eppendorf New Brunswick Innova 40 shaker incubator (Eppendorf SE, Hamburg, Germany) at 150 rpm to allow amplification of host-specific lytic phages.
Screening for phage by spot assay
We followed the previously described spot assay protocol [28, 42] for phage screening. The enriched culture was centrifuged at 10,000 × g for 10 min and filtered through 0.45 μm filters. To prepare the bacterial lawn, 100 µL of an overnight culture of the respective NTS host bacteria (Table 1.) was added to tryptic soy broth (TSB) containing 0.7% agar at 45 °C and poured onto tryptic soy agar (TSA) (Oxoid Ltd., Basingstoke, UK) to form a lawn. The lawn was allowed to cool, and 10 µl of the enriched filtrate was spotted and incubated overnight at 37 °C. Clear zones (plaques) indicated the presence of phages.
Phage purification
To purify the phage, we followed a procedure described by Kazibwe et al. [42]., where the filtrate containing phage, after spot assay, was serially diluted (ten-folds) using sterile saline magnesium (SM) buffer (100 mM sodium chloride, 10 mM magnesium sulfate, 50 mM Tris-HCl, pH 7.5 and 0.01% weight by volume gelatin). The spot assay was repeated by spotting 2 µl of each dilution on a lawn of bacteria on a TSA plate labeled with dilutions ranging from 10−1 to 10−8. The plates were allowed to air dry and incubated overnight at 37 °C. The plaque assay was then performed by mixing 100 µl of bacteria strain with 100 µl of phage from the highest dilution in 5mL of 0.7% soft agar. The soft agar was spread on TSA, allowed to gel at room temperature, and incubated overnight at 37 °C. The plaques were examined based on morphology, and the distinct single plaques were picked using a sterile pipette tip for further propagation.
Purification was conducted in five rounds of plaque assays, picking individual plaques each round. After purification, phages with uniform and distinct plaques were harvested in 1 mL sterile SM buffer at room temperature for 30 min before centrifuging at 4000 × g for 5 min. The phages were filtered through 0.22 μm PES syringe filters (Scientific Laboratory Supplies Ltd, Nottingham, UK) and stored at 4 °C as working stock, with small aliquots in 20% glycerol stored at −80 °C for further analysis. All purified phages were named according to the system described by Adriaenssens and Rodney Brister [43]. The phage name starts with the word Salmonella, followed by the word phage, and then a unique identifier, which is a serial number that begins with the prefix KE (Kenya). For instance, the first purified phage was named Salmonella phage vB_SenST11_KE01. The third part of the name vB_SenST11_KE01 denotes ‘virus of Bacteria’, infecting Salmonella Enteritidis Sequence Type 11 and then the unique identifier. In the subsequent processes during phage characterization, the unique identifier has been used in phage labeling (e.g. KE01).
Phage titer determination
The concentration of the phages was determined following the agar overlay method [42], with serial dilutions (10−1 to 10−8) of the purified phages prepared using SM buffer. A 100 µl of respective NTS host bacteria was inoculated into 5 mL soft agar and poured on a gridded TSA plate. We performed a spot test for all dilution factors to determine the highest dilution that showed lysing. Subsequently, we conducted plaque-forming assay using the highest dilution, counted the number of plaques formed, and expressed phage titer in plaque-forming units per milliliter (PFUs/mL) as follows;
 [44].
[44].
Phage host range determination
We determined the phage host range by spot test as described by Esmael et al. [28], using 12 Salmonella strains - MDR S. Typhimurium, ESBL-producing S. Typhimurium, MDR S. Enteritidis, ciprofloxacin-resistant S. Enteritidis, recently isolated S. Typhimurium and S. Enteritidis (from an ongoing study (2022)), ATCC 13,076 S. Enteritidis, NCTC 3048 S. Typhimurium, S. Arizonae, S. Dublin, S. Heidelberg, and S. Typhi. A bacterial lawn was prepared on TSA by spreading 100 µL of each tested strain. Once the lawn had air-dried, 2 µL of each individual phage stock was spotted onto the surface and allowed to air dry. The plates were then incubated overnight at 37 °C. The study considered spot tests showing a clear zone as susceptibility and the absence of a clear zone or plaque as resistance of a bacterial strain to the tested phages, respectively. Although the term “broad host range” is commonly used to describe phages capable of lysing multiple strains or species, there is currently no universally accepted numerical threshold for defining this based solely on intra-species lytic activity [45, 46]. Here, we considered phages that lysed more than 80% of the study bacterial strains as having a broad host range.
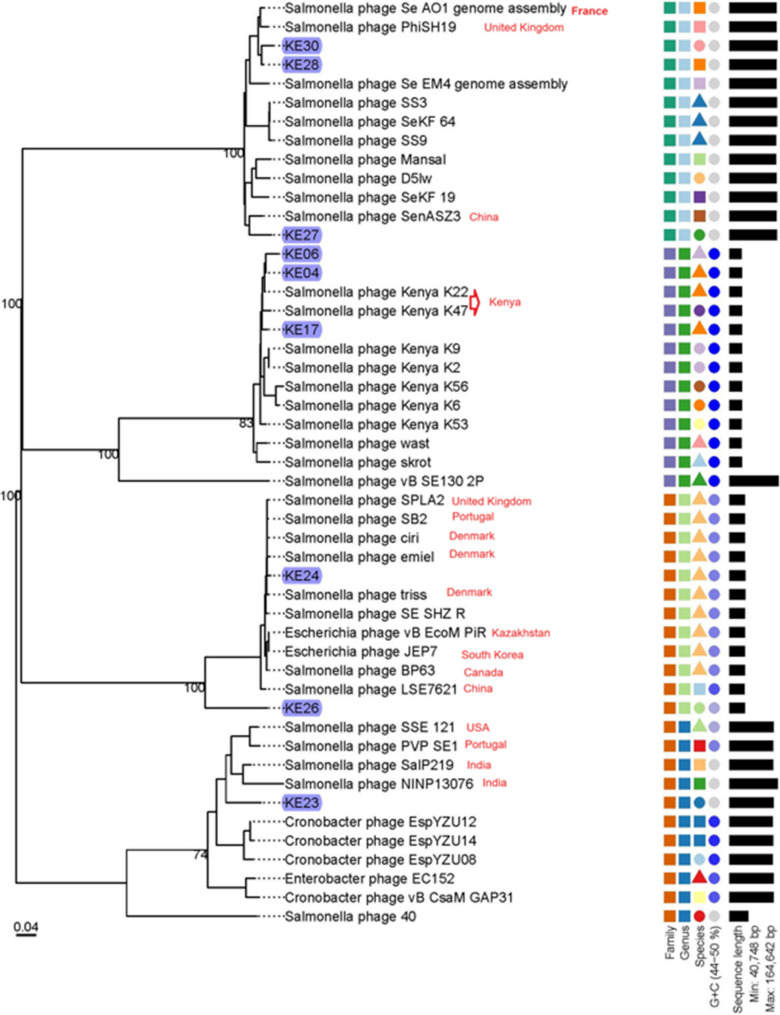
Determining the efficiency of plating (EOP)
We evaluated the efficiency of plating (EOP) of phages that showed > 80% host range following Kotter’s protocol with slight modifications [47]. Phage titer was determined using all the susceptible bacteria strains and the titer obtained from its isolation host, with the EOP calculated as follows;
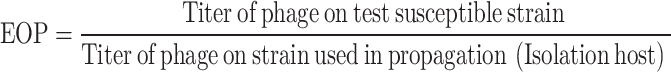 |
We interpreted the EOP ratio as follows: ≥ 0.5 as high production efficiency, ≥ 0.1 to < 0.5 as medium production efficiency, 0.001 to 0.1 as low production efficiency, and ≤ 0.001 as inefficient [48–50].
Determination of thermal and pH stability
Phages with > 80% host range were analyzed further for thermal and pH stability, as described by Bao et al. [51]. Phage lysates stored at 4 °C were used as the reference titer to establish the baseline for thermal stability testing. Aliquots of each phage were then incubated at various temperatures, including − 80 °C, −20 °C, 4 °C, 20 °C, 30 °C, 40 °C, 50 °C, 60 °C, 70 °C, and 80 °C. These temperature ranges covered freezing and fridge temperatures, the phage storage conditions [52], and room temperatures between 20 °C and 30 °C in Kenya for the better part of the year [53]. It also included body temperatures, critical in phage application for therapy ranging from 30 °C to 40 °C [54], with high temperatures of 50 °C to 80 °C studied to inform phage packaging during phage therapy production [55].
A volume of 50 µl phage was aliquoted in PCR tubes and incubated for 60 min in respective temperatures in a freezer or thermal cycler. After incubation, we held the phages at room temperature for 30 min and determined their titer. For pH stability testing, SM buffer was adjusted to pH values of 1, 3, 7, 9, 11, and 13 by adding 1 M NaOH or 1 M HCl drop by drop until the desired pH was reached, as measured with a pH meter (Thermo Scientific, Roskilde, Denmark). Phages were then incubated in the adjusted SM buffers at 37 °C for 60 min, and titers were subsequently evaluated. Phages incubated at pH 7 served as the control to assess stability across the pH range. All experiments for phage titer followed the double-layer agar plate method using 0.7% soft agar and TSA.
Effects of phages on NTS biofilms
The effectiveness of the selected phages on biofilms formed by their respective NTS host strains (MR2829, MCC1462, and MB1102) was quantitatively determined as previously described [28, 56]. Single colonies of the NTS host strain (Table 1.) were cultured in TSB at 37 °C, 200 rpm for 24 h. Following incubation, the bacterial culture was diluted 1:100 in fresh TSB. Then, 100 µl of the diluted NTS strain culture was aliquoted into 96-well microplates, in triplicates for two sets, and incubated at 30 °C for 72 h. The TSB was carefully replaced every 24 h to avoid disturbing the biofilm layer by gently aspirating the media and replenishing it with fresh TSB. For negative control, three wells contained TSB media with no bacteria. We treated one set of the wells containing bacteria with their respective phages and the second set with PBS and incubated at 37 °C for 24 h. After incubation, we washed the wells five times to remove planktonic cells and then air-dried them. We added 98% methanol into wells for 10 min, discarded methanol, and air-dried the plates before staining wells with 1% crystal violet for 45 min and eluting with 33% acetic acid, and Microplate Reader (ELx808 Bio Tek Instruments, Winooski, USA) used to read the optical densities (OD) of the wells at 630 nm wavelength.
Phage genomic DNA extraction and sequencing
Phages were propagated using host bacteria strains to achieve titers exceeding 1 × 10¹⁰ plaque-forming units per milliliter (PFUs/mL) as described by Jakočiūnė & Moodley [57]. Before phage DNA extraction, we treated 1 mL of the phage suspension with 2.5 U/ml of DNase I (Thermo Fisher Scientific, USA) and 0.07 mg/ml of RNase A (Thermo Fisher Scientific, USA) to degrade bacterial DNA and RNA respectively, and utilized the Phage DNA Isolation Kit (Norgen Biotek Corp., Thorold, ON, Canada) following the manufacturer’s instructions [58]. The quality and quantity of the extracted DNA were assessed using the Nanodrop One spectrophotometer and the Invitrogen™ Qubit™ 4 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). DNA library preparation was performed using Nextera XT Library protocol (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. The genomes were sequenced using the Illumina NextSeq 2000 sequencing platform using 2 × 150 bp paired-end reads.
Genome annotation and comparative genome analysis
Bioinformatics analyses were as described by Shen and Millard [59]. We assessed the quality of sequence raw reads using FastQC v0.12.1, with adapters, overrepresented sequences, and poor-quality bases trimmed off using Fastp v0.20.1. Seqtk version 1.4-r122 was used for read subsampling to attain 50-100x genome coverage. Genome assembly was performed using Shovill v1.1.0 with default settings, applying SPAdes as the assembler. Genome completeness was checked using checkv version 1.0.3. Phage genome annotation was performed using Pharokka v1.7.1, which uses PHANOTATE as the default gene caller and integrates tRNAscan-SE to predict tRNA genes. The linear genome map was constructed using Proksee (https://proksee.ca/, accessed on 30 June 2025). PhageLead online tool (https://phageleads.dk/, accessed on 06 June 2024) used to screen for lysogeny, AMR, and virulence genes to assess the suitability of the phages for therapeutic use. Additionally, phages lifestyle was determined using PhaTYP2, a lifecycle prediction tool in PhaBOX2 (https://phage.ee.cityu.edu.hk/ accessed on 01 May 2025). Presence of AMR genes was further assessed using Resistance Gene Identifier (https://card.mcmaster.ca/analyze/rgi accessed on 01 May 2025) as well as abricate version 1.0.1 (https://github.com/tseemann/abricate accessed on 01 May 2025). The allergenic potential of phage proteins was evaluated using AllerCatPro 2.0 (https://allercatpro.bii.a-star.edu.sg/ accessed on 28 April 2025), which predicts allergenicity based on sequence similarity, structural features, and epitope matching [60].
To assess the genetic relatedness of the phages, a phylogenetic tree was constructed using the Molecular Evolutionary Genetics Analysis (MEGA11) program. Multiple sequence alignment of the major head protein nucleotide sequences was performed with the ClustalW algorithm using default settings. The phylogenetic tree was generated using the neighbor-joining method with 1000 bootstrap replicates [61]. An online tool, the Virus Intergenomic Distance Calculator (VIRIDIC) (https://rhea.icbm.uni-oldenburg.de/viridic, accessed on 10 June 2024), was used to assess the phage’s inter-genomic similarities, Clinker [62] was used to compare phages proteins, while the Virus Classification and Tree Building Online Resource (VICTOR) (https://ggdc.dsmz.de/victor.php, accessed on 10 June 2024) was used to assess the relatedness of our study phages with those reported in other studies using whole genome sequences. Roary was employed to analyze and compare the presence or absence of various genes across different phage genomes, as described by Page et al. [63].
Morphological characterization of phages
The morphology of the study phages was characterized using Transmission Electron Microscopy (TEM). Phages were propagated to achieve high titers exceeding 1 × 10¹⁰ PFU/mL and inactivated using paraformaldehyde following the protocol described by Möller et al. [64]. Briefly, 500 µL of phage suspension was mixed with 55 µL of a concentrated paraformaldehyde solution to achieve a final concentration of 2% in 0.05 M HEPES buffer (pH 7.2). The mixture was incubated for 30 min at 25 °C followed by an additional 30 min at 37 °C with shaking. Samples were then shipped at room temperature to the Advanced Light and Electron Microscopy Unit (ZBS 4) at the Robert Koch Institute, Berlin, for negative-staining TEM analysis.
For sample preparation, 10 µL of the inactivated phage suspension was applied to a pre-treated electron microscopy grid (coated with Alcian blue) and incubated at room temperature for 10 min as described by Laue, M [65]. The grid was washed three times distilled water, stained with 0.5% uranyl acetate for a short incubation, and dried using filter paper. Phage particles were visualized using a Tecnai Spirit transmission electron microscope (Thermo Fisher) operated at 120 kV. Images were captured using a side-mounted CMOS camera (Phurona, EMSIS) at a resolution of 4100 × 3000 pixels.
Data analysis
We conducted all the experiments in triplicates and the data entry was done on Microsoft Excel. Phage titer for each experiment was converted to log10 PFUs/mL. The effect of phage on biofilms was assessed by comparing the optical density readings of bacterial wells treated with PBS to those treated with phage. Statistical significance was determined using GraphPad Prism 8.0.2 (GraphPad Software, Inc., San Diego, CA, USA) by performing Mann-Whitney U test with confidence intervals set at 95% and a statistical significance at p < 0.05.
Results
Phenotypic AST of NTS strains used for phage isolation
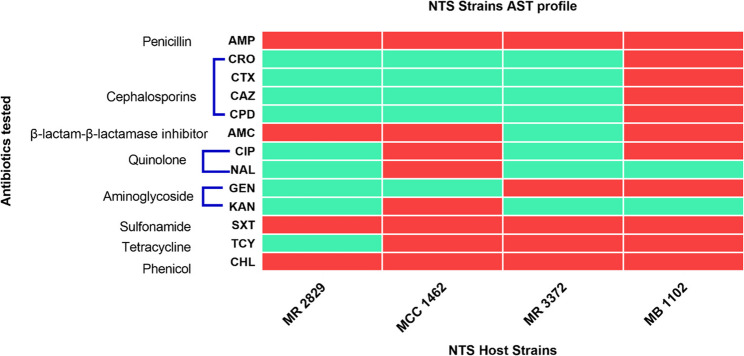
All NTS strains used for phage isolation were MDR, exhibiting resistance to at least one antimicrobial in three classes of antibiotics, namely penicillin (ampicillin), sulfonamides (trimethoprim/sulfamethoxazole), and chloramphenicol (Fig. 1). S. Enteritidis MCC1462 and S. Typhimurium MB 1102 were non-susceptible to ciprofloxacin and recognized as high-priority pathogens per the WHO global priority pathogen list of antibiotic-resistant bacteria. Additionally, S. Typhimurium MB1102 tested positive for ESBL production.
Fig. 1.
Heatmap illustrating AST patterns. The color red indicates non-susceptibility, while green denotes susceptibility. The strains shown are S. Enteritidis MR 2829 and MCC 1462 and S. Typhimurium MR 3372 and MB 1102. Antibiotics used were, Ampicillin (AMP), ceftriaxone (CRO), cefotaxime (CTX), ceftazidime (CAZ), cefpodoxime (CPD), amoxicillin/clavulanic acid (AMC), ciprofloxacin (CIP), nalidixic acid (NAL), gentamicin (GEN), kanamycin (KAN), trimethoprim-sulfamethoxazole (SXT), tetracycline (TCY), and chloramphenicol (CHL)
Isolated phages
Thirty-one phages were isolated from seven locations in Nairobi City County, including Kamukunji (Manjengo drains), Mukuru slums (River Ngong), Ruai sewage treatment plant (both inlet and outlet points), Njiru area, Nairobi dam, and a point at Dagoretti Marke (Table 2 and Supplementary Fig S1).
Table 2.
Distribution of phages isolated from various locations within Nairobi City County
| Isolation Hosts | |||||
|---|---|---|---|---|---|
| Sampling location | MR 2829 | MR 3372 | MCC1462 | MB1102 | Total |
| Kamukunji (Manjengo) | 2 | 1 | 1 | 1 | 5 |
| Mukuru (River Ngong) | 1 | 2 | 1 | 1 | 5 |
| Ruai sewage treatment inlet | 2 | 4 | 1 | 1 | 8 |
| Ruai sewage treatment outlet | 0 | 0 | 0 | 0 | 0 |
| Njiru river | 1 | 1 | 1 | 1 | 4 |
| Nairobi Dam | 2 | 3 | 1 | 1 | 7 |
| Dagoretti Market | 2 | 0 | 0 | 0 | 2 |
| Total | 10 | 11 | 5 | 5 | 31 |
Host range and efficiency of plating
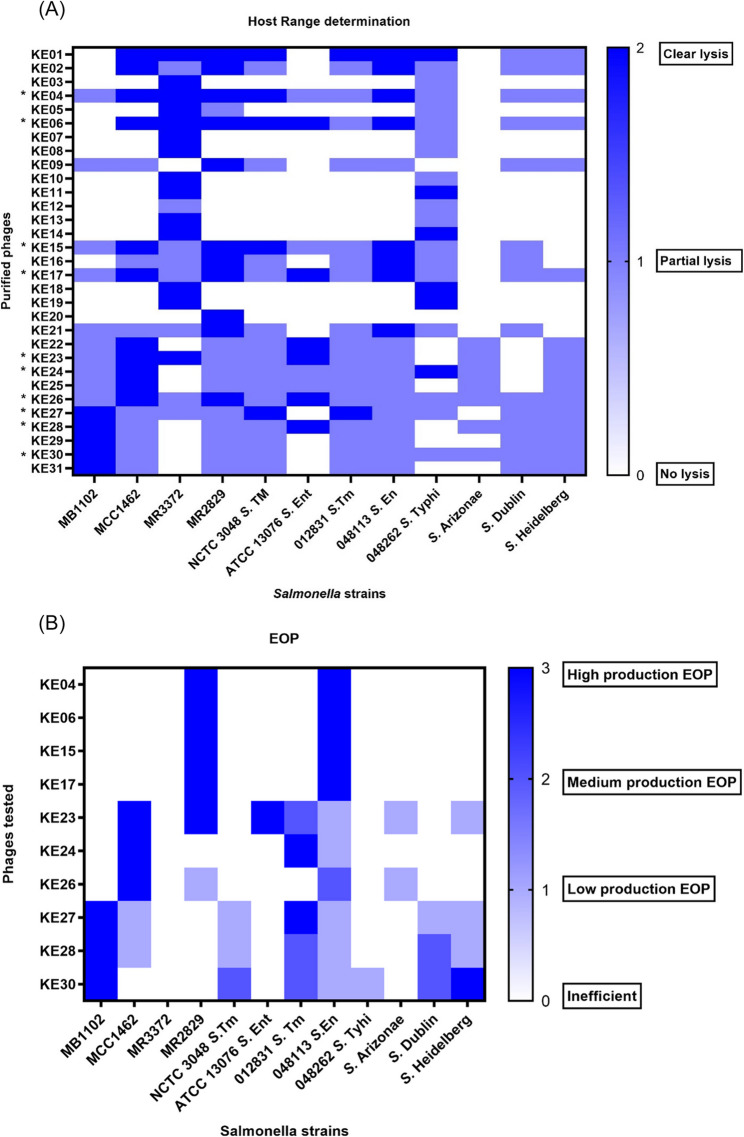
All purified phages were examined for their host range across 12 Salmonella strains with different phenotypic characteristics. The panel included the four NTS strains used in phage isolation, a clinical S. Typhi strain (hereafter 048262 S. Typhi), recently isolated clinical S. Typhimurium (hereafter 012831 S. Tm), and S. Enteritidis (hereafter 048113 S. En) from an ongoing study (2022), ATCC 13,076 S. Enteritidis (hereafter ATCC 13076 S. En), NCTC 3048 S. Typhimurium (hereafter NCTC 3048 S. Tm), S. Arizonae, S. Dublin, and S. Heidelberg, (Fig. 2A).
Fig. 2.
Phage Host range and EOP. A A heatmap showing host range assay for 31 phages against 12 Salmonella strains. Dark blue color indicates clear plaque formation; light blue represents partial plaque formation and white denotes no lysis. Phages selected for further characterization are marked with an asterisk (*). B Heatmap depicting the EOP of the ten selected phages. EOP scoring ranges from high phage production efficiency with the Dark blue color to clear regions representing inefficiency in phage production
We used host range analysis to choose phages for further characterization, selecting 32% (10/31) that lysed more than 10 of the 12 Salmonella strains. This included phage KE26, which lysed all (100%) of the strains tested (Fig. 2A). Phages capable of lysing the majority of the tested Salmonella strains were prioritized, as they demonstrate broader activity against Salmonella infections and hold greater therapeutic potential. Further, we assessed the efficiency of platting for the selected phages against all the strains that showed lysis, with eight (80%) of them showing high phage production EOP in at least one strain (Fig. 2B). Phages KE26 and KE28 did not exhibit high phage production EOP in any of the tested strains, while KE26 showed medium phage production EOP on 048113 S. En and KE28 on 012831 S. Tm and S. Dublin. Phage KE23 had the best EOP, showing high phage production EOP on MR2829 and ATCC 13,076 S. En and a medium production EOP in 012831 S. Tm strain. Phage KE30 scored medium phage production on three strains, 012831 S. Tm, NCTC 3048 S. Tm and S. Dublin, and a high phage production on S. Heidelberg. High phage production EOP of most phages was observed on 012831 S. Tm and 048113 S. En. Three phages, KE4, KE6, and KE15, showed high phage production EOP in 048113 S. En. For 012831 S. Tm, two phages had high phage production EOP, with three phages scoring medium phage production EOP (Fig. 2B).
Temperatures and pH stability of the study phages
We further characterized the selected phages to assess their stability at temperatures ranging from − 80 °C to 80 °C (Fig. 3A). After one hour of incubation at different temperatures, phages demonstrated relatively constant stability from − 80 °C to 40 °C, with a slight titer reduction of approximately two logs at 50 °C for phages KE6, KE26, KE27, KE28, and KE30. There was a steep decline in titer for all phages at 70 °C, with phages KE4, KE6, KE15, and KE17 showing relatively low stability. There was no detectable phage titer at temperatures beyond 70 °C for all the phages. The highest titers were observed between 20 °C and 40 °C, indicating that these temperatures are optimal for the stability of tested phages.
Fig. 3.
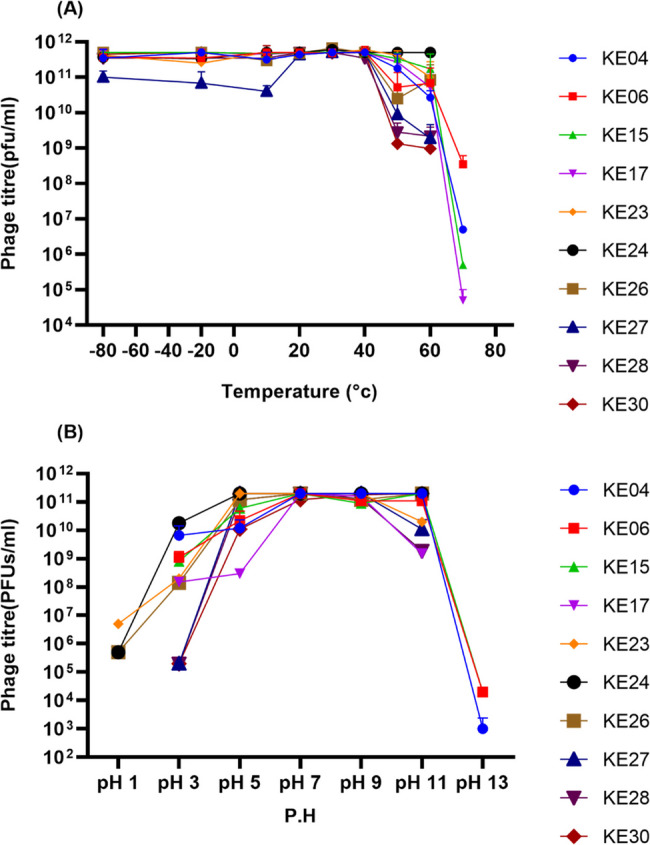
Phage Thermal and pH stability. A Phage viability at different temperatures after one-hour incubation performed in triplicate. B Effect of pH on phage viability after one hour of incubation at 37 °C performed in triplicate
Similarly, a change in phage titer was observed after incubation at pH 1 to 13 for one hour at 37 °C (Fig. 3B). All phages were stable between pH 3 to 11, with phages KE23, KE24, and KE26 showing hazy lysing up to 10−3 dilution at pH 1, and phages KE4, KE6 and KE15 demonstrating reduced stability at pH 13. All phages recorded the highest titers at pH 7 to 9, approximately 2.0 × 10¹¹ PFUs/mL, suggesting the optimal pH range for all our study phages (Fig. 3B).
Effects of phages on NTS biofilms
Although there was no significant disruption by the phages, we observed 56%, 70%, 54%, 27%, 18%, 9%, and 5% biofilm disruption activity by phages KE04, KE06, KE15, KE17, KE26, KE24, and KE23, respectively. There was no noticeable biofilm disruption by Phage KE27, KE28, and KE30 (Fig. 4).
Fig. 4.
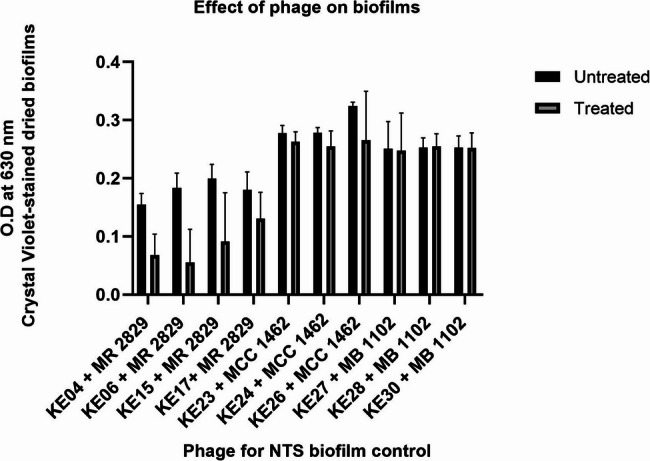
Effects of phages on host bacteria biofilms performed in triplicate. Mann-Whitney U test was used to compare phage-treated and untreated NTS biofilms. No significant differences (p > 0.05) were observed
Genomic characterization of phages
The genomes of all the phages were linear double-stranded DNA, with sizes ranging from 23,215 bp to 159,981 bp and GC content ranging from 44 to 50% (Table 3). Genome annotation revealed that the number of coding sequences (CDSs) varied from 35 to 283. However, the 35 CDSs belong to phage KE15 with a partial genome and might not accurately reflect the total number of CDSs or the exact genome of this phage. The coding density varied from 93.92 to 95.75%. The functions assigned to the putative proteins encoded by the predicted CDSs varied between phages, ranging from 34.63 to 68.57%. Additionally, the percentage of proteins with unassigned functions was high, ranging from 31.43 to 65.37% across different phages. Phages KE23 and KE27 encoded for 24 and 4 transfer RNAs (tRNAs), respectively, whereas KE28 and KE30 had six tRNAs each.
Table 3.
Genome annotations of phages using Pharokka
| Phage Name | Accession numbers | Host Strain Sequence type (ST) | Phage genome length (bp) | GC % | CDS | CDSs coding density | lysis | tRNAs | CDSs assigned function % | Hypothetical proteins % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella phage vB_SEnST11_KE04 | PP856710 | S. Enteritidis ST11 | 42,863 | 50 | 79 | 95.56 | 6 | 0 | 50.63 | 49.37 | ||
| Salmonella phage vB_SEnST11_KE06 | PP856712 | S. Enteritidis ST11 | 42,863 | 50 | 79 | 95.55 | 6 | 0 | 51.90 | 48.10 | ||
| Salmonella phage vB_SEnST11_KE15 | PP856717 | S. Enteritidis ST11 | 23,215 | 50 | 35 | 95.75 | 2 | 0 | 68.57 | 31.43 | ||
| Salmonella phage vB_SEnST11_KE17 | PP856719 | S. Enteritidis ST11 | 42,179 | 50 | 75 | 94.47 | 6 | 0 | 54.67 | 45.33 | ||
| Salmonella phage vB_SEnST11_KE23 | PP856722 | S. Enteritidis ST11 | 148,527 | 44 | 283 | 93.92 | 7 | 24 | 34.63 | 65.37 | ||
| Salmonella phage vB_SEnST11_KE24 | PP856723 | S. Enteritidis ST11 | 53,795 | 46 | 88 | 95.07 | 4 | 0 | 50.00 | 50.00 | ||
| Salmonella phage vB_SEnST11_KE26 | PP856725 | S. Enteritidis ST11 | 52,838 | 45 | 87 | 94.82 | 3 | 0 | 45.98 | 54.02 | ||
| Salmonella phage vB_STmST313_KE27 | PP856726 | S.Typhimurium ST313 | 158,493 | 45 | 237 | 95.3 | 3 | 4 | 39.66 | 60.34 | ||
| Salmonella phage vB_STmST313_KE28 | PP856727 | S.Typhimurium ST313 | 157,588 | 45 | 235 | 95.18 | 3 | 6 | 37.87 | 62.13 | ||
| Salmonella phage vB_STmST313_KE30 | PP856728 | S.Typhimurium ST313 | 159,981 | 45 | 241 | 95.13 | 3 | 6 | 39.42 | 60.58 | ||
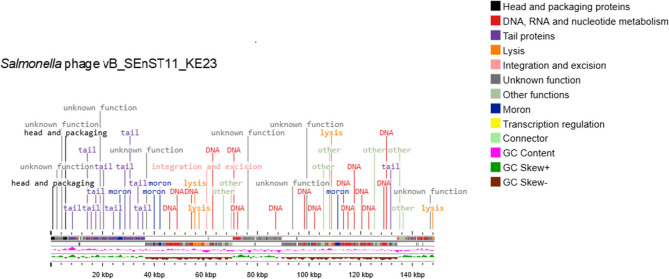
The putative CDSs coded for structural (major head protein, tail spike protein), host cell lysis (Rz-like spanin, endolysin), replication and regulatory (DNA polymerase and replication initiation protein), packaging-associated (terminase large subunit), moron, auxiliary metabolic genes, and host takeover proteins (Fig. 5). Phage KE23, with the most CDSs, was used as a representative genome map of the study phages. No known genes for antibiotic resistance, phage virulence, or bacterial virulence were detected in all phage genomes as assessed using the Antibiotic Resistance Gene Databases and Virulence Factor Databases. In silico screening revealed allergenic proteins only in KE23 phage genome, which contained two proteins with potential allergenic signals. The tail fiber protein (CDS_0172) of KE23 showed strong evidence of allergenicity, with a Win80Lin similarity score exceeding 35% to the known allergen Par ol alpha2I. The endolysin, CDS_0254, exhibited weak similarity (3D epitope match ≤ 93%) to Vit v 5. All phages exhibited a lytic life cycle based on PhageLead analysis. Overall, the bioinformatics analysis indicated that most of the characterized phages are promising candidates for further in vivo studies aimed at developing phage therapy for NTS infections. However, due to predicted allergenicity in two of its proteins, phage KE23 should be deprioritized for therapeutic use unless its safety is confirmed through in vitro and in vivo allergenicity assessments.
Fig. 5.
Linear genome map of Salmonella phage vB_SEnST11_KE23 constructed using Proksee. The CDSs are color-coded and labeled based on predicted protein function categories
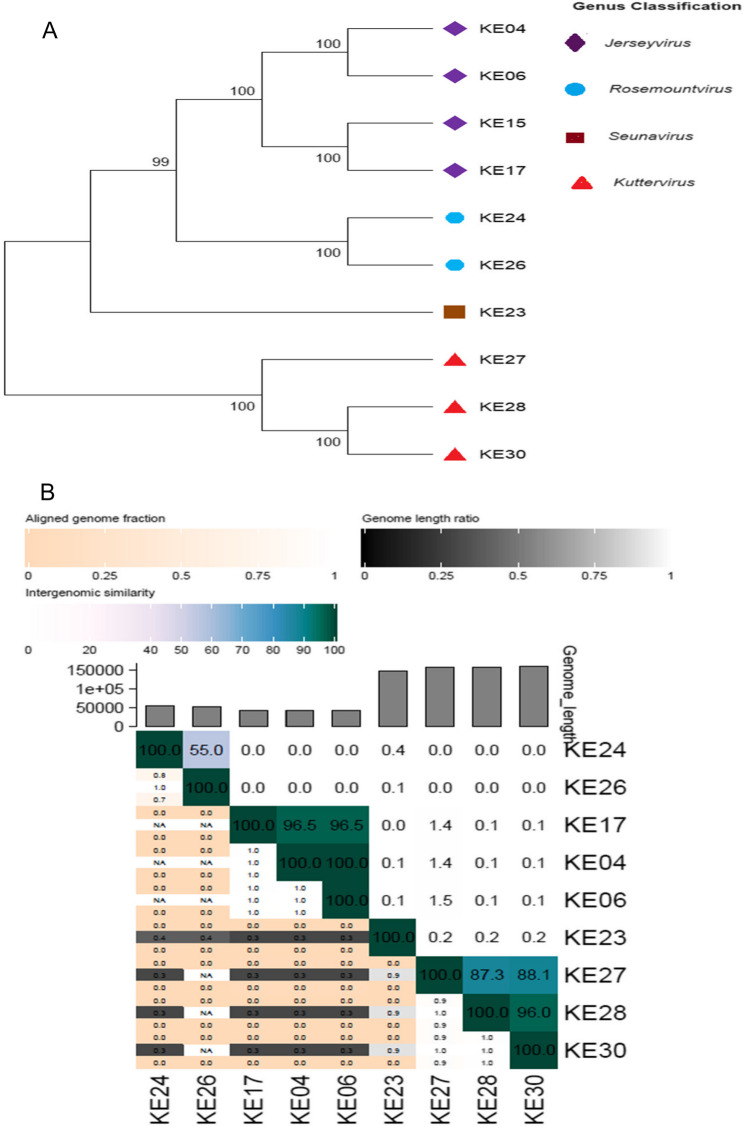
Phylogeny and genomic comparative analysis
The phylogenetic trees derived from the major head protein analysis showed that the ten phages belonged to four clades, each corresponding to a different genus. This classification was consistent with the Pharokka predictions, where phages KE04, KE06, KE15, and KE17 clustered under the Jerseyvirus; phages KE24 and KE26 belonged to Rosemountvirus; phages KE27, KE28, and KE30 fell under the genus Kuttervirus; and phage KE23 was a singleton, belonging to the genus Seunavirus (Fig. 6A). Further, we assessed the phages’ inter-genomic similarities using VIRIDIC, where phages with 95% or more whole genome similarity clustered under the same species, following the species demarcation criterion for bacterial and archaeal viruses [43], which suggests that viruses belonging to the same species differ by less than 5% in their nucleotide sequences. Phage KE15 was not included in the VIRIDIC inter-genomic similarity analysis because it had a partial genome, whereas the other phages had complete genomes. The phages belonged to six species, with phages KE04, KE06, and KE17 belonging to the same species, as did phages KE28 and KE30, but phages KE23, KE24, KE26, and KE27 belonged to different species (Fig. 6B).
Fig. 6.
Phylogenetic tree and phage classification. A Phylogenetic analysis of phages based on the major head protein using MEGA11 and following the neighbor-joining method with the number of bootstrap replicates set at 1000 (B) Heat map showing the inter-genomic similarity of the phages based on the nucleotide sequences using VIRDIC
The Clinker analysis of the linear global genomic comparison showed significant protein similarities among phages KE04, KE06, KE15, and KE17. Phages KE24 and KE26 also demonstrated substantial protein similarities, as did phages KE27, KE28, and KE30. However, phage KE23 did not share any similar proteins with the other phages when the cutoff was set at > 50% (Fig. 7A). Despite this, phage KE23 exhibited 39% and 42% similarity in one of its membrane proteins and one of its tail proteins, respectively, with phages KE24 and KE26 (Fig. 7B).
Fig. 7.
Genome comparison of the ten phages using Clinker (A). The grey-shaded regions between the genome maps indicate the level of protein similarity with a similarity cutoff set at > 50%. The degree of similarity is indicated by the intensity of the grey shading. B Phage KE23 shows similarity in two proteins with phage KE24 and KE26 when the similarity cutoff was set at > 30%
Assessing the relatedness of the study phages with other Salmonella phages
To perform a comparative analysis of our phages with those in the GeneBank database, we selected a representative phage from each genus among our ten phages, prioritizing the phage with the largest genome size and excluding phage KE15 due to its partial genome. Specifically, we chose KE04 for the genus Jerseyvirus, KE30 for Kuttervirus, KE24 for Rosemountvirus, and KE23 as the sole representative for the genus Seunavirus. We subjected the representative phages to BLASTn analysis on the NCBI platform, identifying the top ten non-study phages with the highest sequence similarity to each representative phage, and subsequently downloaded them for comprehensive comparative analysis against our study phages (Fig. 8).
Fig. 8.
Phylogenetic analysis of the nine NTS phages from this study, alongside other studies’ phages with the highest sequence similarity from NCBI. The current study phages are highlighted in blue color, while other studies’ phages that clustered near our study phages are labeled per country of isolation in red. The tree also includes a color-coded legend that provides the following information: the first column indicates the phages’ families, the second column highlights the genus, the third column corresponds to the species, the fourth column shows the GC content and the fifth column represents the genome length
The analysis revealed distinct clustering patterns among the NTS phages with phages from diverse geographical regions, including France, the United Kingdom, China, Kenya, Portugal, Denmark, Canada, South Korea, Kazakhstan, the USA, and India. Phages KE04, KE06, and KE17 grouped within the same clade as Salmonella phages K22 and K47 isolated previously in Kenya. This phylogenetic grouping was further supported by VIRIDIC analysis, which confirmed that all five phages belong to the same species within the genus Jerseyvirus. The recurrence of this species among independently isolated phages suggests it may be endemic to Kenya (Supplementary Fig S2). Similarly, phages KE28 and KE30 clustered closely with Salmonella phages Se_AO1 and PhiSH19 from France and the United Kingdom, respectively. Intergenomic similarity analysis confirmed that these phages belong to the same species, suggesting a potential international distribution of this phage lineage. Phage KE27 clustered closely with SenASZ3 from China, although intergenomic analysis indicated that they do not belong to the same species. Phages KE28, KE27, KE30, Se_AO1, PhiSH19 and SenASZ3 belong to the genus Kuttervirus. Phage KE24 clustered with Salmonella phages SPLA2 (UK), SB2 (Portugal), ciri, emiel, and triss (Denmark), BP63 (Canada), and SHZ-R (Belgium), with intergenomic analysis confirming that they all belong to the same species. These phages, including KE24, were classified within the genus Rosemountvirus. KE26, also assigned to Rosemountvirus, formed a singleton, indicating distinct genomic divergence within the genus. Although phage KE23 was a singleton, it clustered within a major clade alongside other Seunavirus phages, including Salmonella phages SalP219 and NINP13076 from India, SSE121 from the USA, and PVP SE1 from Portugal. Phages clustering within the same clade exhibited a notable overlap in shared genes, as illustrated in (Supplementary Fig S3).
Phage morphology by TEM
Transmission electron microscopy analysis was performed on four representative phages, KE15, KE23, KE26 and KE28, each selected from one of the four genera identified among the ten phages. All phages belong to the class Caudoviricetes. Phages KE23, KE26, and KE28 exhibited myovirus-like morphologies, characterized by icosahedral heads and thicker, ridged tails resembling those of contractile-tailed phages (Fig. 9). Phage KE15 displayed a siphovirus-like morphology, with a long, slightly bent, non-contractile tail. The approximate morphological measurements of these phages ranged from 63 nm to 109 nm in capsid diameter, with tail lengths between approximately 100 nm and 129 nm. Specifically, Phage KE15 had an approximate capsid diameter of 63 nm and the longest tail, measuring around 129 nm; KE23 had a capsid diameter of approximately 90 nm with a 124 nm tail; KE26 exhibited the largest capsid at approximately 109 nm with a tail length of 100 nm; while KE28 had a capsid diameter of about 98 nm and a tail length of approximately 126 nm. These observations underscore the morphological diversity among the phages, suggesting potential variations in their mechanisms of host interaction.
Fig. 9.
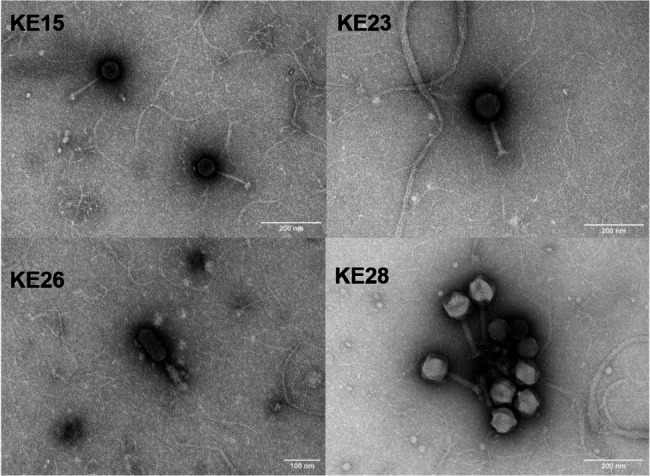
Transmission electron microscopy of phages. KE15 exhibits a siphovirus morphotype, while KE23, KE26, and KE28 display myovirus-like morphotypes. (scale bar = 100 nm for KE26 and 200 nm for KE15, KE23 and KE28)
Discussion
Non-typhoidal Salmonella infections are among the leading causes of diarrheal enterocolitis, and some strains are associated with invasive infections in sterile body sites. The emergence of MDR NTS strains poses a significant threat to the management of severe invasive infections caused by NTS (iNTS), especially among young children in sub-Saharan Africa (SSA) [3, 66]. In this study, the NTS strains used for phage hunting were MDR. S. Enteritidis MCC1462 and S. Typhimurium MB 1102 were ciprofloxacin non-susceptible. Additionally, S. Typhimurium MB 1102 was resistant to third-generation cephalosporins. These strains have limited treatment options and are among the World Health Organization (WHO) high-priority global pathogens requiring urgent development of newer antimicrobials [67].
Phages are considered a feasible option for the treatment of MDR infections. Yen et al.. demonstrated the ability of bacteriophages to prevent cholera-like diarrhea by reducing the Vibrio cholerae intestinal tract colonization in an animal model [68]. Previous studies have used host range and the efficiency of plating analysis to select the most efficient phages with broad host ranges as the best candidates to be studied in the subsequent phage characterization [48, 69]. The development of phage therapy necessitates rigorous screening and characterization of newly isolated phages to ensure their safety and efficacy before proceeding to in vivo studies and therapeutic applications. Phage genome analysis, including toxins, antimicrobial resistance, and virulence genes screening [16], is crucial in the early stages of evaluating phages to ensure that only safe and effective phages are studied further for therapeutic applications.
In this study, we isolated phages showing variation in host range. The phage with the broadest host range lysed all Salmonella strains, while the one with the narrowest host range lysed only one of the strains. We found that ten phages had a broad host range, lysing over 10 strains, while 11 phages lysed as few as two strains. This suggests that some of the Salmonella strains used in this study may share similar surface receptors with the host strains, while others were more specific to their original hosts. Phages isolated and purified using a single host may exhibit a narrow host range, with high specificity to its host and without lysing untargeted bacteria, but the host range can broaden when cocktails of phages with different host ranges are constituted [70]. Although the host range was tested against only 12 strains, these included clinically relevant isolates of S. Enteritidis and S. Typhimurium, the dominant NTS serovars implicated in invasive disease in Kenya and wider SSA [2, 71]. Our study may not have included a sufficiently diverse range of Salmonella strains and serovars in phage hunting. Therefore, while our findings are informative, they may not fully capture the broader strain diversity of NTS in the region. Additional researches are necessary to develop clear guidelines for choosing representative bacterial panels to support robust and standardized host range assessments.
Except for phages KE26 and KE28, most of the phages exhibited high phage production in at least one strain. Phage KE26 showed a broad host range by lysing all stains (100%) but medium phage production EOP in only one strain. Phage KE23 exhibited high phage production EOP in two strains, making it the most effective phage compared to the rest. Our findings were comparable to those of Mirzaei and Nilsson [48] and suggest that a phage can exhibit a broad host range but show no high production EOP to the same strains lysed during the host range analysis. Myers et al.. observed that host range assay by spot test overestimates the effectiveness of a phage compared to the EOP method [72]. Poor phage adsorption to the bacterial cell or host resistance mechanism that prevents the replication of the phages intracellularly could be associated with reduced EOP [73].
Physiochemical characteristics, such as temperature and pH, are crucial in determining phage optimal conditions for survival, adsorption, penetration, and replication [74, 75]. In our study, all phages maintained high titer at temperatures ranging from − 80 °C to 40 °C, with an approximately two-logs drop in phage titer at 50 °C for phages KE06, KE26, KE27, KE28, and KE30. Similar reductions in phage titer at 50 °C have been reported in previous studies [37, 42, 76]. Phages KE04, KE15, KE17, KE23, and KE24 sustained relatively high titer up to 5.0 × 1011 PFUs/mL at 60 °C, with phage KE04, KE06, KE15, and KE17 still showing stability at 70 °C though with sharp titer decline of approximately 8 logs. The findings are agreeable with those of Islam et al.. (Salmonella phages) and Litt et al. (E.coli phages) who reported phage stability at 60 °C for one hour [61, 77]. Thermal stability of therapeutic phages remains a key hurdle for phage stability during packaging and application. Prolonged exposure to high temperatures could reduce phage viability, a challenge further exacerbated by global climate change. Recent advancements have shown that encapsulation in alginate microspheres followed by lyophilization, especially when combined with stabilizers such as trehalose, skim milk, or mannitol, can significantly improve thermal resistance and storage stability [78, 79]. These agents likely confer thermal stability by acting as water substitutes and forming protective matrices. Their high glass transition temperatures, 60 °C for sucrose, 108 °C for lactose, and 115 °C for trehalose, are particularly important in maintaining phage integrity at elevated temperatures [80] Combining encapsulation with sugar based excipients may allow the development of temperature resilient phage therapeutics, enabling broader application in tropical climates [81, 82].
Further, all phages in this study showed relative stability across pH ranges of 5 to 11, with the highest titers recorded at pH 7 to 9. These results are consistent with findings by Jung et al.. on S. Typhimurium lytic phage [83]. The observed phage stability within the pH range of 7–9 aligns well with the physiological pH of blood and body fluids (approximately 7.2–7.4). This supports their potential suitability for intravenous administration, which is one of the routes used for phage delivery, especially in invasive infections [84]. We observed reduced phage stability at very low pH (below pH 3) and high pH (above pH 11). Nevertheless, phages KE23, KE24, and KE26 exhibited turbid lysis at pH 1, while phages KE04, KE06, and KE15 showed some turbid lysis at pH 13. The resistance of phage at pH 13 was also observed in Salmonella phage LPSE1 in a study by Huang et al. [85]. Reduced phage stability at low pH, similar to conditions in the gastrointestinal tract, may be attributed to the denaturation of the phage protein coat. Nobrega et al.. demonstrated that coating phages with phospholipids could improve their stability at low pH, achieving a higher survival rate of 102–107 PFUs/mL compared to wild-type phages [86].
Biofilms represent a significant public health challenge due to their ability to resist antibiotics. Phages KE04, KE06, KE15, KE17, KE26, KE24, and KE23 showed biofilm disruption of 56%, 70%, 54%, 27%, 18%, 9%, and 5%, respectively, though these effects were not statistically significant. Previous studies have shown similar results where phage cocktails achieved a 44–74% reduction of S. Typhimurium and S. Enteritidis biofilms [28, 56]. Phages can eradicate biofilms through various mechanisms, including the production of biofilm-degrading enzymes [87, 88].
All ten phages have linear double-stranded DNA, with genome sizes ranging from 23 kb to 160 kb. This size range is consistent with the characteristics of Salmonella phages reported in previous studies [89–91]. The predicted CDSs were attributable to a diverse array of functional proteins including structural, host cell lysis, replication and regulatory, packaging, morons, auxiliary metabolic, and host takeover mechanisms proteins. The predicted structural proteins included those associated with the phage tail, which plays a significant role in recognizing and binding to host bacterial receptors [92]. The main tail-associated proteins identified included tail fiber proteins, tail tube, tail connector, tail sheath, tail competition, and baseplate structural proteins, all essential for the assembly and functionality of the phage tail [93]. Another significant structural protein identified was the major head protein utilized in this study to construct the phage phylogenetic tree. These proteins are highly conserved and are commonly used in taxonomic classification [94]. Phage host cell lysis proteins, such as Rz-like spanin, endolysin, amidase, and holin, were also identified. As previously described, phage lysins demonstrate effectiveness as potential therapeutic agents for treating bacterial infections independently [95]. Terminase enzymes are crucial for protein packaging and assembly in phages. The large subunit of terminase is a versatile enzyme that plays a central role in packaging, while the small subunit guides the process [96]. These enzymes were predicted in all phages except KE15, which was identified as a partial phage. It is notable that phages KE23, KE27, KE28, and KE30 were found to encode tRNAs. Transfer RNAs are more commonly associated with lytic phages than lysogenic ones, and it has been hypothesized that the acquisition of tRNAs may enhance phage virulence [97]. Additionally, it is suggested that phage-encoded tRNAs might evade host tRNAases by inducing mutations within the tRNA cleavage and recognition sites, potentially enabling the engineering of phages to infect hosts with anticodon nucleases [98]. A high number of tRNAs could also assist phages in synthesizing their own proteins without relying on the host’s machinery [94]. Notably, a substantial proportion of the predicted CDSs, from 31.43 to 65.37%, were classified as having unknown functions. Bacteriophage genomes exhibit mosaic patterns, indicating that their genetic material is derived from various sources. This vast diversity results in up to 80% of phage-encoded proteins lacking homology with known proteins, thereby representing some of the most genetically novel elements in the biological world [99]. Future studies incorporating functional genomics approaches, such as transcriptomics or proteomics, will be essential in elucidating the roles of these hypothetical proteins and uncovering novel phage biology.
Analysis of the genomes from all ten phages revealed no known virulence genes, antibiotic resistance genes, or lysogeny genes was detected, indicating these phages may be safe and suitable candidates for phage therapy against MDR NTS infections. In silico phage screening identified no potential allergenic proteins in the majority of phages, suggesting a low risk of immunogenic complications. However, two proteins in phage KE23 were flagged for potential allergenicity. The tail fiber protein (CDS_0172) showed strong sequence similarity to Par o 1 alpha2I, a well-characterized airborne pollen allergen from Parietaria officinalis, which is known to trigger respiratory allergic reactions, such as rhinitis and asthma [100]. The endolysin (CDS_0254) exhibited weak similarity to Vit v 5, a profilin allergen from Vitis vinifera (grapevine), which has been associated with pollen-related oral allergy [101, 102]. Although these findings are computational predictions and do not confirm clinical allergenicity, they highlight the importance of allergenicity screening as part of preclinical phage evaluation. Phages lacking allergen-like domains may be prioritized for therapeutic development. Nonetheless, further immunological assessments, including in vitro and in vivo studies, are warranted for phage KE23 before it can be considered for clinical application.
Four phages, each representing one of the four genera identified in our collection, were examined to characterize their morphology. TEM analysis placed the phages within the class Caudoviricetes, with KE23, KE26, and KE28 exhibiting myovirus-like morphologies and KE15 displaying a siphovirus-like morphology. Phages with similar morphotypes have been described previously, including Salmonella phage T156 (siphovirus-like) and phages VSe11 and VSe102 (myovirus-like) [103, 104]. Myovirus-like phages typically possess thick, contractile tails and are often associated with strictly lytic life cycles, making them promising candidates for therapeutic applications [105, 106]. In contrast, siphovirus-like phages, characterized by long, non-contractile tails, are commonly temperate; however, strictly lytic siphoviruses have also been identified and explored for antimicrobial use [106, 107]. The structural differences between these morphotypes reflect variations in infection mechanisms and host interactions, which are important considerations when selecting phages for therapeutic use [108].
Comparative genomic analysis revealed diverse relationships among the study phages. Phages KE04, KE06, KE15, and KE17 exhibited high sequence similarity, with KE04, KE06, and KE17 clustering alongside previously reported Kenyan phages K22 and K47 within the Jerseyvirus genus. This close genetic relationship, supported by VIRIDIC analysis, suggests that the phage species are endemic to Kenya. In contrast, phages KE28 and KE30 clustered with Se_AO1 and PhiSH19 from France and the United Kingdom, respectively, indicating a shared international lineage. Phage KE24 clustered with a group of phages from geographically diverse locations SPLA2 (UK), SB2 (Portugal), ciri, emiel, and triss (Denmark), BP63 (Canada), and SHZ-R (Belgium), and all were confirmed to belong to the same species within the Rosemountvirus genus. Interestingly, KE26, although from the same genus, formed a singleton and shared only 55% intergenomic similarity with KE24, below the 70% genus-level threshold [43]. Phages KE27, KE28, and KE30 also exhibited high similarity among themselves, however, KE27, despite clustering closely with SenASZ3 from China, did not belong to the same species, highlighting possible evolutionary divergence. KE23 stood out as highly divergent, not clustering with any of the other phages, a finding supported by Clinker based genome organization analysis. These findings reveal a complex genomic landscape comprising potentially endemic, globally distributed, and unique phages. The broad phylogeographic clustering, including phages from countries such as Kenya, France, the UK, China, Portugal, Denmark, Canada, South Korea, Kazakhstan, the USA, and India, underscores the ecological and evolutionary diversity of Salmonella phages circulating in different environments.
Conclusion
In conclusion, our study successfully isolated thirty-one bacteriophages from environmental samples in Nairobi City County, with notable lytic activity against multi-drug-resistant S. Enteritidis and S. Typhimurium. Phages KE04, KE06, KE15, KE17, KE23, KE24, KE26, KE27, KE28, and KE30 lysed over 80% of strains tested for host range and characterized phenotypically and genotypically. While trends toward biofilm disruption were observed, these did not reach statistical significance and require further validation. The phages belong to four genera and six distinct species, with a notable portion of their proteins remaining functionally unannotated. Importantly, these phages do not possess AMR, lysogeny, or virulence factors, and represent promising candidates for further preclinical evaluation, pending immunogenicity assessments. Allergenicity analysis further supports the safety of most candidate phages. However, phage KE23 contains two proteins (tail fiber and endolysin) with predicted similarity to known allergens. Based on these findings, KE23 should be deprioritized for therapeutic use unless subsequent in vitro and in vivo allergenicity studies confirm its safety. Given the limited number of public phage genome sequences, this study contributes valuable new genomes to public databases, offering a significant resource for future research efforts globally.
Study limitations
The study did not include in vivo studies using animal models, which would provide a more accurate representation of phages’ efficacy in clearing NTS infections and allergic reactions in humans. This limits the ability to translate our findings directly into real-world clinical or therapeutic applications. Further research is needed to evaluate the effectiveness of these phages for a comprehensive understanding of their potential for therapeutic use.
Supplementary Information
Acknowledgements
We gratefully acknowledge the Genome Competence Centre (MF1) at the Robert Koch Institute, Berlin, Germany, for their support with phage sequencing, supported by the German Research Foundation (DFG) grant, as part of the project “Multidrug-resistant invasive non-typhoidal Salmonella disease in children: The role of carriage in humans and environmental contamination in an endemic setting in Kenya (MISSIoN).” We also thank the Advanced Light and Electron Microscopy Unit (ZBS 4) at the Robert Koch Institute for capturing the transmission electron microscopy (TEM) images. Our sincere appreciation goes to the KEMRI phage team for their support and for sharing resources during the data collection phase.
Authors’ contributions
Conceptualization and study designing MM, SK, AM2 AM3. Acquiring of MDR NTS strains SK and CM. Phage Isolation and laboratory experiments KK, IM, PM, CK and MM, AM3. Phage sequencing TP. Bioinformatics analysis MM and OD. Data analysis MM, AM2, and AM3. Transmission electron microscopy TH. Manuscript writing MM, AM2, AM3, IM, KK, PM, CK, KW, MP, TP, OD, CM, AF, and SK. Funding acquisition was done by AF, LW and SK. All authors reviewed and edited the manuscript.
Funding
This study was supported by grants from the German Research Foundation (DFG): Ref. DG: WI 1436/13 − 1 to S. Kariuki and L. Wieler; FL 359/9 − 1 to A. Flieger; TH 2521/1–1 to A. Thürmer; and SE 2030/1–1 to T. Semmler.
Data availability
The whole genome sequence data was submitted to the National Center for Biotechnology Information (NCBI) GenBank under the following accession numbers PP856710, PP856712, PP856717, PP856719, PP856722, PP856723, PP856725, PP856726, PP856727 and PP856728.
Declarations
Ethical approval and consent to participate
There are no human or animal participants in this study, hence no consent requirement. However, approval to conduct the study was granted by Kenyatta University graduate school (Ref No: Pl 54/20269/2020). Ethical approval was obtained from the Kenya Medical Research Institute (KEMRI) through the Scientific and Ethics Review Unit (study No., KEMRI/SERU/CMR/P00185/4448). Additionally, a research permit was issued by the National Commission for Science, Technology, and Innovation (License No, NACOSTI/P/22/18422). Permit to ship DNA samples for sequencing to Robert Koch Institute, Berlin, Germany was obtained from KEMRI and National Environment Management Authority (NEMA), Kenya, (Ref No: NEMA/AGR/182/2023).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael Mugo, Email: mikemugom@gmail.com, Email: mmuraya@kemri.go.ke.
Samuel Kariuki, Email: skariuki@kemri.go.ke, Email: samkariuki2@gmail.com.
References
- 1.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal salmonella disease, 2010. Emerg Infect Dis. 2015;21(6):941–9. 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phoba MF, Barbé B, Ley B, et al. High genetic similarity between non-typhoidal salmonella isolated from paired blood and stool samples of children in the Democratic Republic of the congo. PLoS Negl Trop Dis. 2020;14(7):1–15. 10.1371/journal.pntd.0008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tack B, Vanaenrode J, Verbakel JY, Toelen J, Jacobs J. Invasive non-typhoidal Salmonella infections in sub-Saharan africa: A systematic review on antimicrobial resistance and treatment. BMC Med. 2020;18(1):1–22. 10.1186/s12916-020-01652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrivastava SR, Shrivastava PS, Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. JMS - J Med Soc. 2018;32(1):76–7. 10.4103/jms.jms_25_17. [Google Scholar]
- 5.US Department of Health and Human Services. CDC. Antibiotic resistance threats in the united States. Centers Dis Control Prev. Published online 2019:1–113. https://www.cdc.gov/drugresistance/biggest_threats.html
- 6.Kariuki S, Mbae C, Van Puyvelde S, et al. High relatedness of invasive multi-drug resistant non-typhoidal salmonella genotypes among patients and asymptomatic carriers in endemic informal settlements in Kenya. PLoS Negl Trop Dis. 2020;14(8):1–14. 10.1371/journal.pntd.0008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kariuki S, Mbae C, Onsare R, et al. Multidrug-resistant nontyphoidal Salmonella hotspots as targets for vaccine use in management of infections in endemic settings. Clin Infect Dis. 2019;68(Suppl 1):S10–5. 10.1093/cid/ciy898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feasey NA, Archer BN, Heyderman RS, et al. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis. 2010;16(9):1448–51. 10.3201/eid1609.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalonji LM, Post A, Phoba MF, et al. Invasive salmonella infections at multiple surveillance sites in the Democratic Republic of the congo, 2011–2014. Clin Infect Dis. 2015;61(Suppl 4):S346–53. 10.1093/cid/civ713. [DOI] [PubMed] [Google Scholar]
- 10.Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Heal. 2014;2(SEP):1–8. 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piddock LJ V, Alimi Y, Anderson J, et al. Advancing global antibiotic research, development and access. Nat Med. 2024;30(9):2432–43. 10.1038/s41591-024-03218-w. [DOI] [PubMed]
- 12.Dougan G, Kariuki S. Antibacterial resistance in sub-Saharan africa: an underestimated emergency. Ann N Y Acad Sci. 2014;1323(1):43–55. 10.1111/nyas.12380.Antibacterial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assembly S, eighth WH. Global action plan on antimicrobial resistance. Microbe Mag. 2015;10(9):354–5. 10.1128/microbe.10.354.1. [Google Scholar]
- 14.Golkar Z, Bagasra O, Gene Pace D. Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014;8(2):129–36. 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- 15.Kawacka I, Olejnik-Schmidt A, Schmidt M, Sip A. Effectiveness of Phage-Based Inhibition of Listeria monocytogenes in Food Products and Food Processing Environments. Microorganisms. 2020;8(11). 10.3390/microorganisms8111764. [DOI] [PMC free article] [PubMed]
- 16.Hitchcock NM, Devequi Gomes Nunes D, Shiach J, et al. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses. 2023;15(4). 10.3390/v15041020. [DOI] [PMC free article] [PubMed]
- 17.Dhulipalla H, Basavegowda N, Haldar D, et al. Integrating phage biocontrol in food production: industrial implications and regulatory overview. Discov Appl Sci. 2025;7(4). 10.1007/s42452-025-06754-3.
- 18.Barman RK, Chakrabarti AK, Dutta S. Screening of Potential Vibrio cholerae Bacteriophages for Cholera Therapy: A Comparative Genomic Approach. Front Microbiol. 2022;13. 10.3389/fmicb.2022.803933. [DOI] [PMC free article] [PubMed]
- 19.Abedon ST. How simple maths can inform our basic Understanding of phage therapy. Clin Infect Dis. 2023;77:S401–6. 10.1093/cid/ciad480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alisigwe CV, Ikpa CS, Otuonye UJ. Examining alternative approaches to antibiotic utilisation: A critical evaluation of phage therapy and antimicrobial peptides combination as potential alternatives. Microbe (Netherlands). 2025;6(January):100254. 10.1016/j.microb.2025.100254. [Google Scholar]
- 21.Philipson CW, Voegtly LJ, Lueder MR, et al. Characterizing phage genomes for therapeutic applications. Viruses. 2018;10(4):1–20. 10.3390/v10040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fayez MS, Hakim TA, Zaki BM et al. Correction: Morphological, biological, and genomic characterization of Klebsiella pneumoniae phage vB_Kpn_ZC2 (Virology Journal, (2023), 20, 1, (86), 10.1186/s12985-023-02034-x). Virol J. 2023;20(1):1–16. 10.1186/s12985-023-02083-2
- 23.Hyman P. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals. 2019;12(1). 10.3390/ph12010035. [DOI] [PMC free article] [PubMed]
- 24.Grigson SR, Giles SK, Edwards RA, Papudeshi B. Knowing and naming: phage annotation and nomenclature for phage therapy. Clin Infect Dis. 2023;77:S352–9. 10.1093/cid/ciad539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodwell EV, Wenner N, Pulford CV, et al. Isolation and characterisation of bacteriophages with activity against invasive non-typhoidal salmonella causing bloodstream infection in Malawi. Viruses. 2021;13(3). 10.3390/v13030478. [DOI] [PMC free article] [PubMed]
- 26.Thanki AM, Brown N, Millard AD, Clokie MRJ. Genomic characterization of Jumbo Salmonella phages that effectively target united Kingdom pig-associated Salmonella serotypes. Front Microbiol. 2019;10(JUL):1–14. 10.3389/fmicb.2019.01491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunathilake KMD, Makumi A, Loignon S, et al. Diversity of Salmonella enterica phages isolated from chicken farms in Kenya. Microbiol Spectr. 2024;12(1):e0272923. 10.1128/spectrum.02729-23. [DOI] [PMC free article] [PubMed]
- 28.Esmael A, Azab E, Gobouri AA, et al. Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant salmonella typhimurium and their efficacy to combat salmonellosis in ready‐to‐use foods. Microorganisms. 2021;9(2):1–19. 10.3390/microorganisms9020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clavijo V, Baquero D, Hernandez S, et al. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult Sci. 2019;98(10):5054–63. 10.3382/ps/pez251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phothaworn P, Supokaivanich R, Lim J, et al. Development of a broad-spectrum Salmonella phage cocktail containing viunalike and jerseylike viruses isolated from Thailand. Food Microbiol. 2020;92(March):103586. 10.1016/j.fm.2020.103586. [DOI] [PubMed] [Google Scholar]
- 31.Bernasconi OJ, Donà V, Tinguely R, Endimiani A. In vitro activity of 3 commercial bacteriophage cocktails against salmonella and shigella spp. Isolates of human origin. Pathog Immun. 2018;3(1):72–81. 10.20411/pai.v3i1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abedon ST, Danis-Wlodarczyk KM, Wozniak DJ. Phage cocktail development for bacteriophage therapy: toward improving spectrum of activity breadth and depth. Pharmaceuticals. 2021;14(10). 10.3390/ph14101019. [DOI] [PMC free article] [PubMed]
- 33.John R, Giudicessi BAMJA. 2013. Bacteriophages and their Genomes Graham. Bone. 2008;23(1):1–7. 10.1038/jid.2014.371
- 34.Aworh MK, Lawal OU, Egyir B, Hendriksen RS. In Silico genomic insights into bacteriophages infecting ESBL-producing Escherichia coli from human, animal, and environmental sources. BMC Microbiol. 2025;25(1). 10.1186/s12866-025-03913-9. [DOI] [PMC free article] [PubMed]
- 35.Ye Y, Tong G, Chen G, et al. The characterization and genome analysis of a novel phage phiA034 targeting multiple species of Aeromonas. Virus Res. 2023;336(August):199193. 10.1016/j.virusres.2023.199193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner D, Kropinski AM, Adriaenssens EM. A roadmap for genome-based phage taxonomy. Viruses. 2021;13(3):1–10. 10.3390/v13030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mhone AL, Makumi A, Odaba J, et al. Salmonella Enteritidis bacteriophages isolated from Kenyan poultry farms demonstrate Time-Dependent stability in environments mimicking the chicken Gastrointestinal tract. Viruses. 2022;14(8). 10.3390/v14081788. [DOI] [PMC free article] [PubMed]
- 38.Weinstein M. M100 Performance Standards for Antimicrobial Susceptibility Testing. Vol 8.; 2021.
- 39.Tornberg-Belanger SN, Rwigi D, Mugo M, et al. Antimicrobial resistance including extended spectrum beta lactamases (ESBL) among E. coli isolated from Kenyan children at hospital discharge. PLoS Negl Trop Dis. 2022;16(3):1–16. 10.1371/journal.pntd.0010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutua JM, Njeru JM, Musyoki AM. Extended-spectrum β-lactamase- producing gram-negative bacterial infections in severely ill COVID-19 patients admitted in a National referral hospital, Kenya. Ann Clin Microbiol Antimicrob. 2023;22(1):1–11. 10.1186/s12941-023-00641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vane CH, Kim AW, Lopes dos Santos RA, et al. Impact of organic pollutants from urban slum informal settlements on sustainable development goals and river sediment quality, nairobi, kenya, Africa. Appl Geochem. 2022;146(September):105468. 10.1016/j.apgeochem.2022.105468. [Google Scholar]
- 42.Kazibwe G, Katami P, Alinaitwe R, Alafi S, Nanteza A, Nakavuma JL. Bacteriophage activity against and characterisation of avian pathogenic Escherichia coli isolated from colibacillosis cases in Uganda. PLoS ONE. 2020;15(12 December):1–16. 10.1371/journal.pone.0239107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adriaenssens EM, Rodney Brister J. How to name and classify your phage: an informal guide. Viruses. 2017;9(4):1–9. 10.3390/v9040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gooch JW. Plaque assay. Encycl Dict Polym. 2011;8915–915. 10.1007/978-1-4419-6247-8_14509.
- 45.Fong K, Wong CWY, Wang S, Delaquis P. How broad is enough: the host range of bacteriophages and its impact on the Agri-Food sector. PHAGE Ther Appl Res. 2021;2(2):83–91. 10.1089/phage.2020.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross A, Ward S, Hyman P. More is better: selecting for broad host range bacteriophages. Front Microbiol. 2016;7(SEP):1–6. 10.3389/fmicb.2016.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kutter E. Chapter 14 - Phage host range and efficiency of plating. Bacteriophages Methods Protoc Vol 1 Isol Charact Interact. 2009;501(November):141–149. 10.1007/978-1-60327-164-6 [DOI] [PubMed]
- 48.Mirzaei MK, Nilsson AS. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE. 2015;10(3):1–13. 10.1371/journal.pone.0118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdo Ahmad TA, El Houjeiry SA, Abou Fayad A, Kanj SS, Matar GM, Saba ES. Isolation and genomic analysis of Escherichia coli phage AUBRB02: implications for phage therapy in Lebanon. Antibiotics. 2025;14(5):1–17. 10.3390/antibiotics14050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karthika C, Malligarjunan N, Hari Prasath N, Karutha Pandian S, Gowrishankar S. Phage (cocktail)-antibiotic synergism: a new frontier in addressing Klebsiella pneumoniae resistance. Front Microbiol. 2025;16(May):1–20. 10.3389/fmicb.2025.1588472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bao H, Zhang H, Wang R. Isolation and characterization of bacteriophages of Salmonella enterica serovar pullorum. Poult Sci. 2011;90(10):2370–7. 10.3382/ps.2011-01496. [DOI] [PubMed] [Google Scholar]
- 52.Zhai X, Castro-Mejía JL, Gobbi A, et al. The impact of storage buffer and storage conditions on fecal samples for bacteriophage infectivity and metavirome analyses. Microbiome. 2023;11(1):1–12. 10.1186/s40168-023-01632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogoli DM. Predicting indoor temperatures in closed buildings with high thermal mass. Energy Build. 2003;35(9):851–62. 10.1016/S0378-7788(02)00246-3.
- 54.Grabow WOK, Bacteriophages. Update on application as models for viruses in water. Water SA. 2001;27(2):251–68. 10.4314/wsa.v27i2.4999. [Google Scholar]
- 55.Malik DJ. Bacteriophage encapsulation using spray drying for phage therapy. Curr Issues Mol Biol. 2021;40:303–16. 10.21775/cimb.040.303. [DOI] [PubMed] [Google Scholar]
- 56.Islam MS, Zhou Y, Liang L, et al. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses. 2019;11(9). 10.3390/v11090841. [DOI] [PMC free article] [PubMed]
- 57.Jakočiūnė D, Moodley A. A rapid bacteriophage Dna extraction method. Methods Protoc. 2018;1(3):1–5. 10.3390/mps1030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, Liu LH, Wang Z, Jiang A, Wu YR. A gradient phage-assisted continuous evolution method for screening suppressor tRNAs in Escherichia coli. N Biotechnol. 2024;82:85–91. 10.1016/j.nbt.2024.05.005. [DOI] [PubMed]
- 59.Shen A, Millard A. Phage genome annotation: where to begin and end. PHAGE Ther Appl Res. 2021;2(4):183–93. 10.1089/phage.2021.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen MN, Krutz NL, Limviphuvadh V, Lopata AL, Gerberick GF, Maurer-Stroh S. AllerCatPro 2.0: a web server for predicting protein allergenicity potential. Nucleic Acids Res. 2022;50(W1):W36–43. 10.1093/nar/gkac446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Islam MS, Hu Y, Mizan MFR, et al. Characterization of salmonella phage LPST153 that effectively targets most prevalent salmonella serovars. Microorganisms. 2020;8(7):1–18. 10.3390/microorganisms8071089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilchrist CLM, Chooi YH. Clinker & clustermap.js: automatic generation of gene cluster comparison figures. Bioinformatics. 2021;37(16):2473–5. 10.1093/bioinformatics/btab007. [DOI] [PubMed] [Google Scholar]
- 63.Page AJ, Cummins CA, Hunt M, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31(22):3691–3. 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Möller L, Schünadel L, Nitsche A, Schwebke I, Hanisch M, Laue M. Evaluation of virus inactivation by formaldehyde to enhance biosafety of diagnostic electron microscopy. Viruses. 2015;7(2):666–79. 10.3390/v7020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laue M. Electron microscopy of viruses. Volume 96. Elsevier Inc.; 2010. 10.1016/S0091-679X(10)96001-9.
- 66.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379(9835):2489–99. 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sati H, Carrara E, Savoldi A, et al. The WHO Bacterial Priority Pathogens List 2024: a prioritisation study to guide research, development, and public health strategies against antimicrobial resistance. Lancet Infect Dis. Published online July 16, 2025. 10.1016/S1473-3099(25)00118-5. [DOI] [PubMed]
- 68.Yen M, Cairns LS, Camilli A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nat Commun. 2017;8:1–7. 10.1038/ncomms14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duc HM, Son HM, Honjoh K, ichi, Miyamoto T. Isolation and application of bacteriophages to reduce Salmonella contamination in Raw chicken meat. Lwt. 2018;91(January):353–60. 10.1016/j.lwt.2018.01.072. [Google Scholar]
- 70.Artawinata PC, Lorraine S, Waturangi DE. Isolation and characterization of bacteriophages from soil against food spoilage and foodborne pathogenic bacteria. Sci Rep. 2023;13(1):1–10. 10.1038/s41598-023-36591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kering K, Njaanake K, Wairimu C, et al. Shedding of nontyphoidal Salmonella by asymptomatic convalescing children under 5 years as a risk factor for invasive disease in Mukuru informal settlement in nairobi, Kenya. J Clin Microbiol. 2024;62(11):e0075024. 10.1128/jcm.00750-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Myers J, Davis J, Lollo M, Hudec G, Hyman P. More’s the Same—Multiple hosts do not select for broader host range phages. Viruses. 2023;15(2). 10.3390/v15020518. [DOI] [PMC free article] [PubMed]
- 73.Azeredo J,(role)edt (role), Sillankorva S, editors. (role)edt (role)http://id.loc.gov/vocabulary/relators/ed. Bacteriophage Therapy From Lab to Clinical Practice. 1st ed. 20.; 2018. http://lib.ugent.be/catalog/ebk01:4100000001115020
- 74.Pradeep AN, Ramasamy S, Veniemilda JK, Kumar CSV, Effect of PH & Temperature Variations on Phage Stability - A Crucial Prerequisite for Successful Phage Therapy Introduction. Effect of PH and Temperature variations on phage stability-A crucial prerequisite for phage therapy: Phage therapy was a popular t. 2022;13(December). 10.13040/IJPSR.0975-8232.13(12).5178-82
- 75.Jończyk E, Kłak M, Międzybrodzki R, Górski A. The influence of external factors on bacteriophages-review. Folia Microbiol (Praha). 2011;56(3):191–200. 10.1007/s12223-011-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mutai IJ, Juma AA, Inyimili MI, Nyachieo A, Nyamache AK. Efficacy of diversely isolated lytic phages against multi-drug resistant Enterobacter cloacae isolates in Kenya. Afr J Lab Med. 2022;11(1):1–9. 10.4102/ajlm.v11i1.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Litt PK, Saha J, Jaroni D. Characterization of bacteriophages targeting non-O157 Shiga toxigenic Escherichia coli. J Food Prot. 2018;81(5):785–94. 10.4315/0362-028X.JFP-17-460. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Menendez E, Fernandez L, Gutierrez D, Rodríguez A, Martínez B, GarcíaI P. Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS ONE. 2018;13(10):1–14. 10.1371/journal.pone.0205728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wdowiak M, Paczesny J, Raza S. Enhancing the stability of bacteriophages using physical, chemical, and Nano-Based approaches: A review. Pharmaceutics. 2022;14(9). 10.3390/pharmaceutics14091936. [DOI] [PMC free article] [PubMed]
- 80.Leung SSY, Parumasivam T, Nguyen A, et al. Effect of storage temperature on the stability of spray dried bacteriophage powders. Eur J Pharm Biopharm. 2018;127(February):213–22. 10.1016/j.ejpb.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loh B, Gondil VS, Manohar P, Khan FM, Yang H, Leptihn S. Encapsulation and delivery of therapeutic phages. Appl Environ Microbiol. 2021;87(5):1–13. 10.1128/AEM.01979-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Śliwka P, Skaradziński G, Dusza I, Grzywacz A, Skaradzińska A. Freeze-Drying of encapsulated bacteriophage T4 to obtain Shelf-Stable dry preparations for oral application. Pharmaceutics. 2023;15(12). 10.3390/pharmaceutics15122792. [DOI] [PMC free article] [PubMed]
- 83.Jung Lseung, Ding T, Ahn J. Evaluation of lytic bacteriophages for control of multidrug-resistant Salmonella Typhimurium. Ann Clin Microbiol Antimicrob. 2017;16(1):1–9. 10.1186/s12941-017-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nang SC, Lin YW, Petrovic Fabijan A, et al. Pharmacokinetics/pharmacodynamics of phage therapy: a major hurdle to clinical translation. Clin Microbiol Infect. 2023;29(6):702–9. 10.1016/j.cmi.2023.01.021. [DOI] [PubMed] [Google Scholar]
- 85.Huang C, Virk SM, Shi J, et al. Isolation, characterization, and application of bacteriophage LPSE1 against Salmonella enterica in ready to eat (RTE) foods. Front Microbiol. 2018;9(MAY):1–11. 10.3389/fmicb.2018.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nobrega FL, Costa AR, Santos JF, et al. Genetically manipulated phages with improved pH resistance for oral administration in veterinary medicine. Sci Rep. 2016;6(December):1–12. 10.1038/srep39235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu S, Lu H, Zhang S, Shi Y, Chen Q. Phages against pathogenic bacterial biofilms and Biofilm-Based infections: A review. Pharmaceutics. 2022;14(2). 10.3390/pharmaceutics14020427. [DOI] [PMC free article] [PubMed]
- 88.Chang C, Yu X, Guo W, et al. Bacteriophage-Mediated control of biofilm: A promising new dawn for the future. Front Microbiol. 2022;13(April):1–14. 10.3389/fmicb.2022.825828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karpe YA, Kanade GD, Pingale KD, Arankalle VA, Banerjee K. Genomic characterization of Salmonella bacteriophages isolated from India. Virus Genes. 2016;52(1):117–26. 10.1007/s11262-015-1269-7. [DOI] [PubMed] [Google Scholar]
- 90.Schatten H, Eisenstark A. Salmonella: Methods and Protocols: Second Edition. Vol 1225.; 2014. 10.1007/978-1-4939-1625-2
- 91.Gao R, Naushad S, Moineau S, Levesque R, Goodridge L, Ogunremi D. Comparative genomic analysis of 142 bacteriophages infecting Salmonella enterica subsp. enterica. BMC Genomics. 2020;21(1):1–13. 10.1186/s12864-020-6765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalatzis PG, Rørbo N, Castillo D, et al. Stumbling across the same phage: comparative genomics of widespread temperate phages infecting the fish pathogen Vibrio anguillarum. Viruses. 2017;9(5):1–19. 10.3390/v9050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang L, Xiang Y. Structures of the tailed bacteriophages that infect Gram-positive bacteria. Curr Opin Virol. 2020;45:65–74. 10.1016/j.coviro.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Fu J, Li Y, Zhao L, Wu C, He Z. Characterization and genomic analysis of a bacteriophage with potential in Lysing Vibrio alginolyticus. Viruses. 2023;15(1). 10.3390/v15010135. [DOI] [PMC free article] [PubMed]
- 95.O’Flaherty S, Ross RP, Coffey A. Bacteriophage and their lysins for elimination of infectious bacteria: review Article. FEMS Microbiol Rev. 2009;33(4):801–19. 10.1111/j.1574-6976.2009.00176.x. [DOI] [PubMed] [Google Scholar]
- 96.Lokareddy RK, Hou CFD, Li F, Yang R, Cingolani G. Viral small terminase: A divergent structural framework for a conserved biological function. Viruses. 2022;14(10). 10.3390/v14102215. [DOI] [PMC free article] [PubMed]
- 97.Bailly-Bechet M, Vergassola M, Rocha E. Causes for the intriguing presence of tRNAs in phages. Genome Res. 2007;17(10):1486–95. 10.1101/gr.6649807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van den Berg DF, van der Steen BA, Costa AR, Brouns SJJ. Phage tRNAs evade tRNA-targeting host defenses through anticodon loop mutations. Elife. 2023;12:1–7. 10.7554/eLife.85183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hatfull GF, Hendrix RW. Bacteriophages and their genomes. Curr Opin Virol. 2011;1(4):298–303. 10.1016/j.coviro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ciprandi G, Puccinelli P, Incorvaia C, Masieri S. Parietaria allergy: an intriguing challenge for the allergist. Med. 2018;54(6):1–10. 10.3390/MEDICINA54060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barre A, Benoist H, Rougé P. An overview of fruit allergens: structural, functional, phylogenetical, and clinical aspects. Allergies. 2023;3(3):134–76. 10.3390/allergies3030010. [Google Scholar]
- 102.Hassan AKG, Venkatesh YP. An overview of fruit allergy and the causative allergens. Eur Ann Allergy Clin Immunol. 2015;47(6):180–7. [PubMed] [Google Scholar]
- 103.Volozhantsev NV, Denisenko EA, Kislichkina AA, et al. Complete genome sequences of two Salmonella viruses, VSe11 and VSe102 (family myoviridae, subfamily Ounavirinae), with a very high degree of similarity. Genome Announc. 2018;6(21):11–2. 10.1128/genomeA.00398-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J, Li Y, Ding Y, et al. Characterization of a novel siphoviridae Salmonella bacteriophage T156 and its microencapsulation application in food matrix. Food Res Int. 2021;140(September 2020):110004. 10.1016/j.foodres.2020.110004. [DOI] [PubMed] [Google Scholar]
- 105.Kutter E, De Vos D, Gvasalia G, et al. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol. 2010;11(1):69–86. 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 106.Albarella D, Dall’Ara P, Rossi L, Turin L. Bacteriophage therapy in freshwater and saltwater aquaculture species. Microorganisms. 2025;13(4):1–32. 10.3390/microorganisms13040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hatoum-Aslan A. The phages of staphylococci: critical catalysts in health and disease. Trends Microbiol. 2021;29(12):1117–29. 10.1016/j.tim.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veesler D, Cambillau C. A common evolutionary origin for Tailed-Bacteriophage functional modules and bacterial machineries. Microbiol Mol Biol Rev. 2011;75(3):423–33. 10.1128/mmbr.00014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The whole genome sequence data was submitted to the National Center for Biotechnology Information (NCBI) GenBank under the following accession numbers PP856710, PP856712, PP856717, PP856719, PP856722, PP856723, PP856725, PP856726, PP856727 and PP856728.