Abstract
Background
Postoperative pulmonary complications (PPCs) significantly impact the prognosis of elderly patients undergoing coronary artery bypass grafting (CABG), yet the risk factors for PPCs in CABG remain uncertain. The objective of this study is to identify risk factors for PPCs in elderly patients undergoing CABG.
Methods
This study retrospectively analyzed 305 elderly patients who underwent CABG at Xuanwu Hospital, from January 2019 to December 2023. The variables analyzed included patient demographics, comorbidities, anesthesia factors, surgical factors and perioperative laboratory tests. Based on the occurrence of PPCs, patients were divided into the PPC group and the none-PPC group. Univariate analysis and multivariate logistic regression analysis were employed to determine the independent risk factors of PPCs in elderly patients undergoing CABG.
Results
Of the 305 patients, 148 developed PPCs, resulting in an incidence rate of 48.5%. Compared to the none-PPCs group, patients in the PPCs group had significantly longer postoperative lengths of stay and higher perioperative mortality rates. The results indicated that NYHA classification ≥ III (P = 0.024, OR: 1.791, 95%CI: 1.080 ~ 2.972), preoperative P/F ratio (PaO2/FiO2) < 350 mmHg (P = 0.002, OR: 2.363, 95%CI: 1.371 ~ 4.073) and postoperative albumin level (P = 0.020, OR: 0.946, 95%CI: 0.903 ~ 0.991) were independent risk factors for PPCs in elderly patients undergoing CABG.
Conclusion
This study identified NYHA classification ≥ III, preoperative P/F ratio < 350 mmHg and postoperative albumin levels as independent predictors of PPCs in elderly patients undergoing CABG. Further study is needed to validate these findings and explore potential interventions to mitigate the risk of PPCs in elderly CABG patients.
Keywords: Postoperative pulmonary complications, CABG, Elderly patients, Risk factors, P/F ratio
Introduction
In recent years, the incidence of coronary artery disease has been escalating annually, driven by the intensifying trend of population aging and shifts in lifestyle patterns [1]. Coronary artery bypass grafting (CABG) has emerged as a well-established therapeutic approach for complex coronary artery disease involving multiple vessel lesions [2]. Despite significant advancements in surgical techniques and perioperative care, postoperative pulmonary complications (PPCs) remain highly prevalent, occurring in 11 ~ 40% of CABG patients [3–5]. Given the fragile cardiopulmonary function of CABG patients, the occurrence of PPCs significantly hinders their recovery process and perioperative safety, serving as a critical factor contributing to elevated perioperative mortality rates [6, 7]. Consequently, the prevention of PPCs stands as one of the major challenges and priorities in the perioperative management of CABG procedures.
PPCs, such as pleural effusion, respiratory infection and atelectasis etc., are common complications following various major surgeries, particularly in cardiothoracic, abdominal, and neurosurgeries etc [8, 9]. Their high incidence significantly impacts patients’ postoperative recovery process. Previous studies have investigated the perioperative risk factors for PPCs in some surgeries, including crucial elements like age, obesity, Chronic Obstructive Pulmonary Disease (COPD), duration of operation and intraoperative blood loss [4, 5, 10]. However, despite the frequent occurrence of PPCs after CABG surgeries, which severely impedes patients’ postoperative recovery, research focusing on the risk factors specific to CABG remains relatively scarce. In particular, the investigation into the risk factors of PPCs during the perioperative period for elderly CABG patients, who constitute a high-risk population, has yet to be reported.
Currently, studies on preventive measures for PPCs after CABG primarily focuses on preoperative physical training [11], including respiratory training, effective coughing exercises, and aerobic exercises, with the aim of enhancing patients’ preoperative respiratory function [3, 12]. Nevertheless, the occurrence of PPCs is intricately linked to multiple perioperative factors, encompassing both patient-specific conditions and surgical and anesthetic factors. Therefore, there is an urgent need to investigate and analyze the relevant risk factors for PPCs in elderly patients who have undergone CABG, in order to provide a basis for the development of individualized prevention and treatment strategies.
The objective of this study is to investigate the relevant risk factors for PPCs in elderly patients undergoing CABG. By identifying these risk factors, we aim to assist clinicians in optimizing perioperative management strategies to lower the incidence of PPCs.
Methods
Study design
In this study, a retrospective observational analysis was performed aimed at identifying the risk factors of PPCs in 305 elderly patients who underwent CABG under general anesthesia at Xuanwu Hospital, between January 2019 and December 2023. This study was formally approved by the Ethics Committee of Xuanwu Hospital (approval number: 2024 No.277-001), ensuring that all study procedures adhered to ethical norms and legal requirements. Informed consent from patients was waived by the ethics committee of Xuanwu Hospital, since this is a retrospective study.
Inclusion and exclusion criteria
The inclusion criteria included elderly patients (≥ 65 years old) who underwent CABG at Xuanwu Hospital, during the period from January 2019 to December 2023. The exclusion criteria include patients who had pre-existing severe pulmonary complications according to PPCs standards, prior to the CABG surgery. Additionally, patients who had undergone thoracic or cardiac surgery within 30 days prior to this CABG surgery were also excluded. Furthermore, patients who had concurrent CABG and heart valve replacement surgery were excluded. Patients with thoracic deformities were also excluded. Lastly, patients with missing or incomplete critical clinical data necessary for the analysis were excluded.
Outcome definition
In this study, the primary outcome was to assess the occurrence of PPCs within 7 days post-surgery. Despite PPCs being a widely discussed topic in medical literature, there exists variability in the specific diagnostic criteria employed across different studies. This study has adopted the European Perioperative Clinical Outcome (EPCO) definitions, jointly proposed by ESA and ESICM in 2015 [8]. The EPCO standard defines PPCs as a comprehensive set of adverse pulmonary events that may arise after surgery. These encompass pleural effusion, respiratory infections, pneumothorax, atelectasis, respiratory failure, bronchospasm, and aspiration pneumonitis. This constellation of complications poses a significant threat to surgical patients’ perioperative survival, notably elevating their risk of mortality. The EPCO definition, as a widely accepted standard, provides a standardized approach for identifying and classifying PPCs, thereby facilitating the standardization and comparability of data collection in clinical studies. Two reviewers independently assessed the occurrence of PPCs in each patient included in the study by reviewing relevant examinations, laboratory tests, and medical records in the medical record system based on the EPCO criteria. Any discrepancies were resolved through discussion between the two reviewers, and if necessary, they could seek assistance from the entire review team.
The secondary outcomes include postoperative length of hospital stay (LOS) and perioperative mortality. Postoperative LOS is defined as the time from surgery to discharge, measured in days. Perioperative mortality refers to the rate of death occurring during the hospital stay.
Data collection
The demographic, comorbidity, laboratory examination, anesthesia information, surgical information and postoperative outcome data of all patients were gathered from the anesthesia electronic record system and the electronic medical record system. The demographic data collected in this study include age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) classification and New York Heart Association (NYHA) functional classification. The preoperative comorbidity variables consist of hypertension, diabetes, hyperlipidemia, myocardial infarction, atrial fibrillation, smoking, alcohol drinking, pulmonary hypertension, percutaneous coronary intervention, cerebral infarction, cerebral vascular stenosis (defined as moderate or more severe narrowing of intracranial and cervical arteries), liver insufficiency, renal insufficiency, chronic bronchitis and COPD. The preoperative laboratory tests and examination-related variables encompass the left ventricular ejection fraction (LVEF) obtained from echocardiography and the P/F ratio (PaO2/ FiO2), which is the ratio of the arterial partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2), derived from blood gas analysis (Table 1).
Table 1.
Univariate analysis of preoperative characteristics of patients with or without PPCs
| Characteristics | none-PPCs group n = 157, (%) |
PPCs group n = 148, (%) |
P-value |
|---|---|---|---|
| Age, years (median, IQR) | 69 (66,72) | 70 (67,73) | 0.016 |
| Sex | 0.330 | ||
| female | 49 (31.2) | 54 (36.5) | |
| male | 108 (68.8) | 94 (63.5) | |
| BMI | 25.7 (24.0, 27.9) | 25.4 (23.4, 27.6) | 0.355 |
| ASA classification | 0.037 | ||
| < IV | 131 (83.4) | 109 (73.6) | |
| ≥IV | 26 (16.6) | 39 (26.4) | |
| NYHA classification | 0.002 | ||
| < III | 90 (57.3) | 58 (39.2) | |
| ≥III | 67 (42.7) | 90 (60.8) | |
| Hb (g/L) | 132.8 ± 18.0 | 131.7 ± 16.2 | 0.550 |
| Hct (%) | 39.9 ± 4.5 | 39.3 ± 4.8 | 0.310 |
| Smoking | 50 (31.8) | 60 (40.5) | 0.114 |
| Drinking | 46 (29.3) | 34 (23.0) | 0.209 |
| Hypertension | 116 (73.9) | 120 (81.8) | 0.133 |
| Diabetes mellitus | 65 (41.4) | 73 (49.3) | 0.165 |
| Hyperlipidemia | 65 (41.4) | 71 (48.0) | 0.249 |
| Myocardial infarction | 61 (38.9) | 47 (31.8) | 0.195 |
| Preoperative atrial fibrillation | 15 (9.6) | 17 (11.5) | 0.582 |
| Pulmonary hypertension | 2 (1.3) | 6 (4.1) | 0.246 |
| PCI | 27 (17.2) | 28 (18.9) | 0.696 |
| LVEF | 62.0 (54.0, 67.5) | 62.0 (56.0, 67.0) | 0.830 |
| Cerebral infarction | 45 (28.7) | 48 (32.4) | 0.475 |
| Cerebrovascular stenosis | 37 (23.6) | 38 (25.7) | 0.669 |
| Renal insufficiency | 6 (3.8) | 10 (6.8) | 0.251 |
| Hepatic insufficiency | 1 (0.6) | 7 (4.7) | 0.061 |
| Preoperative P/F ratio (mmHg) | 0.005 | ||
| ≥ 350 | 121 (77.1) | 92 (62.2) | |
| < 350 | 36 (22.9) | 56 (37.8) | |
| Chronic bronchitis | 13 (8.3) | 21 (14.2) | 0.101 |
| COPD | 3 (1.9) | 10 (6.8) | 0.036 |
Note: PPCs, postoperative pulmonary complications; BMI, body mass index; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; Hb, hemoglobin; Hct, hematocrit; PCI, Percutaneous Coronary Intervention; LVEF, Left ventricular ejection fraction; P/F ratio, PaO2/FiO2 ratio; COPD, chronic obstructive pulmonary disease
The intraoperative and postoperative analytical variables included in this study are anesthesia method, duration of surgery, duration of anesthesia, intraoperative fluid infusion volume, cardiopulmonary bypass (CPB), number of bypass grafts, application of left internal mammary artery (LIMA) and postoperative albumin level. The postoperative albumin level refers to the concentration of albumin measured during the patient’s first assessment upon admission to the Intensive Care Unit following surgery (reflecting the albumin level status at the end of the surgical procedure). The net intraoperative fluid infusion volume is calculated as the total fluid infusion volume minus the total fluid output during the surgery (Table 2).
Table 2.
Univariate analysis of intraoperative and postoperative variables of patients with or without PPCs
| Variables | None-PPCs group n = 157, (%) |
PPCs group n = 148, (%) |
P-value |
|---|---|---|---|
| Anesthesia method | 0.126 | ||
| Intravenous anesthesia | 84 (53.5) | 92 (62.2) | |
| Intravenous combined with respiration | 73 (46.5) | 56 (37.8) | |
| Duration of operation (hour) | 3.80 (3.28, 4.43) | 4.00 (3.43, 4.48) | 0.025 |
| Duration of anesthesia (hour) | 5.15 (4.59, 5.69) | 5.37 (4.69, 6.16) | 0.017 |
| Net intraoperative fluid infusion volume (ml) | 1365 (900, 1960) | 1400 (900, 2068) | 0.788 |
| Total amount of intraoperative fluids (ml) | 3400 (2835, 3844) | 3200 (2800, 3900) | 0.415 |
| Intraoperative blood loss(ml) | 400 (280, 600) | 500 (300, 600) | 0.063 |
| Intraoperative blood transfusion | 43 (27.4) | 54 (36.5) | 0.088 |
| CPB | 3 (1.9) | 11 (7.4) | 0.021 |
| Number of bypass grafts | 0.101 | ||
| < 4 | 74 (47.1) | 56 (37.8) | |
| ≥ 4 | 83 (52.9) | 92 (62.2) | |
| Application of LIMA | 106 (67.5) | 106 (71.6) | 0.436 |
| Small-incision surgery | 12 (7.6) | 6 (4.1) | 0.184 |
| Postoperative albumin (g/L) | 35.72 ± 4.99 | 34.05 ± 5.95 | 0.009 |
Note: PPCs, postoperative pulmonary complications; CPB, Cardiopulmonary bypass; LIMA, left internal mammary artery
Statistical analysis
SPSS (version 23.0) was utilized for data analysis. Depending on whether the continuous variables adhered to a normal distribution, they were represented as mean ± SD or median (interquartile range, IQR). Categorical variables were presented using numbers and proportions. Patients were categorized into two groups based on the occurrence of PPCs. Differences in continuous variables were assessed using either Student’s t-test or the Mann-Whitney U test. Comparisons of categorical variables were conducted using Chi-square tests or Fisher’s exact tests where applicable. P < 0.05 was considered statistically significant. Variables that were shown to be statistically significant in univariate analyses or considered clinically significant were further evaluated with multivariate logistic regression.
Results
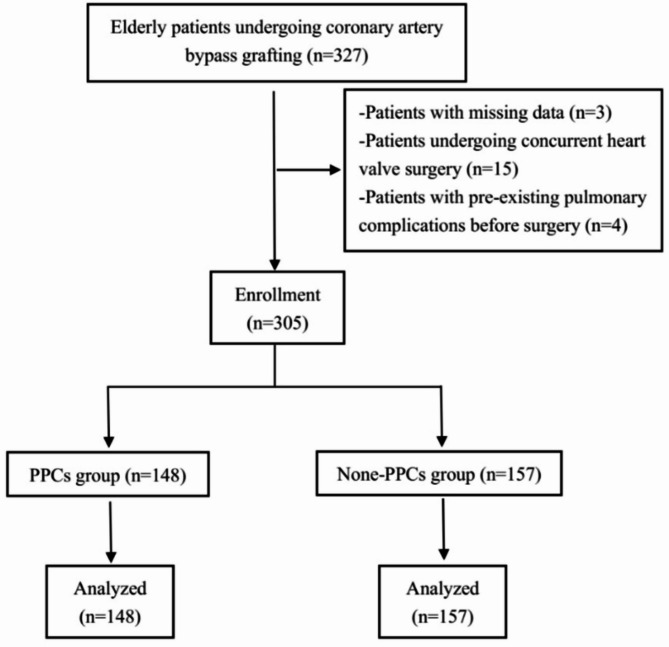
This study retrospectively reviewed a total of 305 elderly patients (aged 65 to 86) who underwent CABG, consisting of 103 females and 202 males (Fig. 1). The results showed that a total of 236 PPCs were documented in 148 patients, resulting in an incidence rate of 48.5%. Of the 148 patients, 78 had one type of PPCs and 70 had two or more types of PPCs. Among these PPCs, pleural effusion was the most common, occurring in 76.4% (113/148) of the affected patients, followed by respiratory infection in 48.6% (72/148), and atelectasis in 16.2% (24/148). Other PPCs included respiratory failure in 9.5% (14/148), pneumothorax in 6.8% (10/148), and bronchospasm in 2.0% (3/148) of the patients. No cases of aspiration pneumonia were observed (Table 3).
Fig. 1.
Flow diagram of the study populations
Table 3.
Frequency of postoperative pulmonary complications
| PPCs | Number of patients (%) |
|---|---|
| Pleural effusion | 113(76.4) |
| Respiratory infection | 72(48.6) |
| Atelectasis | 24(16.2) |
| Respiratory failure | 14(9.5) |
| Pneumothorax | 10(6.8) |
| Bronchospasm | 3(2.0) |
| Number of patients with one PPC | 78 (25.6) |
| Number of patients with two or more PPCs | 70 (23.0) |
| Total number of patients with PPCs | 148(48.5) |
Note: PPCs, postoperative pulmonary complications
All patients were categorized into the PPCs and the none-PPCs groups, with the distinction based on the presence or absence of PPCs. The median postoperative LOS among patients in the PPCs group was 10 (8,13) days, whereas for those in the none-PPCs group, it was 8 (7,11) days. The postoperative LOS was significantly prolonged in the PPCs group compared to the none-PPCs group (P < 0.001, Table 4). In this study, a total of 9 patients died postoperatively, with 8 deaths occurring in the PPCs group, resulting in a mortality rate of 5.41%, and 1 death in the none-PPCs group, yielding a mortality rate of 0.64%. The mortality rate in the PPCs group was significantly higher than that in the none-PPCs group (P = 0.034, Table 4).
Table 4.
Clinical outcomes of patients
| Outcomes | None-PPCs group n = 157, (%) |
PPCs group n = 148, (%) |
P-value |
|---|---|---|---|
| Death | 1 (0.64) | 8 (5.41) | 0.034 |
| Postoperative LOS (day) | 8 (7,11) | 10 (8,13) | <0.001 |
Note: PPCs, postoperative pulmonary complications; LOS, length of hospital stay
As depicted in Tables 1 and 2, the univariate analysis was conducted to identify potential risk factors for PPCs after CABG in elderly patients. The results showed statistically significant differences between the two groups in the following variables: ASA classification (P = 0.037), age (P = 0.016), NYHA classification (P = 0.002), COPD (P = 0.036), P/F ratio (P = 0.005), CPB (P = 0.021), duration of operation (P = 0.025), duration of anesthesia (P = 0.017) and postoperative albumin levels (P = 0.009).
Since the duration of anesthesia was positively correlated with the duration of operation (P < 0.001, r = 0.959), duration of anesthesia was excluded from subsequent multivariate logistic regression analysis. Furthermore, smoking, which has been established as a risk factor for PPCs in previous study [13, 14], was included in the multivariate analysis. Ultimately, the following 9 variables including age, ASA classification, NYHA classification, smoking, COPD, preoperative P/F ratio, CPB, duration of operation and postoperative albumin levels were included for multivariate logistic regression analysis. The results indicated that NYHA classification ≥ III (P = 0.024, OR: 1.791, 95%CI: 1.080 ~ 2.972), preoperative P/F ratio < 350 mmHg (P = 0.002, OR: 2.363, 95%CI: 1.371 ~ 4.073) and postoperative albumin level (P = 0.020, OR: 0.946, 95%CI: 0.903 ~ 0.991) were identified as independent risk factors for PPCs in elderly patients undergoing CABG (Table 5).
Table 5.
Independent risk factors for postoperative pulmonary complications after CABG in the multivariate logistic regression analysis
| Parameters | OR | 95% CI | P-value |
|---|---|---|---|
| Age | 1.060 | 0.996 ~ 1.129 | 0.066 |
| ASA classification | 1.217 | 0.651 ~ 2.275 | 0.539 |
| NYHA classification | 1.791 | 1.080 ~ 2.972 | 0.024 |
| Smoking | 1.437 | 0.860 ~ 2.399 | 0.166 |
| COPD | 3.199 | 0.817 ~ 12.530 | 0.095 |
| Postoperative albumin | 0.946 | 0.903 ~ 0.991 | 0.020 |
| P/F ratio | 2.363 | 1.371 ~ 4.073 | 0.002 |
| CPB | 3.402 | 0.840 ~ 13.779 | 0.086 |
| Duration of operation | 1.206 | 0.941 ~ 1.547 | 0.140 |
Note: CABG, Coronary Artery Bypass Grafting; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; P/F ratio, PaO2/FiO2 ratio; CPB, Cardiopulmonary bypass
Discussion
This study retrospectively reviewed 305 elderly patients who underwent CABG. Patients in the PPCs group had significantly longer postoperative LOS compared to those in the none-PPCs group, and the mortality rate was also significantly higher in the PPCs group. The results revealed that NYHA classification ≥III, preoperative P/F ratio < 350 mmHg and postoperative albumin levels were independent predictors of PPCs in elderly CABG patients.
Elderly patients constitute a high-risk population for the development of PPCs following CABG. However, there is a scarcity of research in the existing literature focusing on the risk factors for PPCs in elderly CABG patients. This study has reported for the first time that preoperative P/F ratio < 350 mmHg as an independent risk factor for PPCs in elderly CABG patients. This parameter may aid clinicians in the early detection of potential pulmonary function decline in elderly CABG patients, identification of high-risk individuals. Additionally, in clinical practice, particularly for major surgeries like CABG, clinicians often attach great importance to adjusting patients’ preoperative albumin levels in order to reduce the perioperative risks. With the current advancement in preoperative preparation, the incidence of preoperative hypoalbuminemia in CABG patients has become extremely low. In contrast, monitoring of albumin levels during surgery is often neglected. This study found that postoperative albumin levels, as measured for the first time upon admission to the intensive care unit after surgery, are an independent risk factor for PPCs in elderly CABG patients. Since this measurement generally reflects the albumin status at the end of the surgical procedure, it suggests that enhanced monitoring and management of albumin levels during the intraoperative period may be beneficial in preventing the occurrence of PPCs.
Among the total 305 patients, 240 patients were classified as ASA III and 65 patients as ASA IV, with no patients belonging to other ASA grades. Since the common presence of impaired cardiac function among elderly CABG patients [15], NYHA classification was utilized in this study to evaluate cardiac function prior to the operation. Univariate analysis revealed significant differences in both ASA and NYHA classification between the PPC and none-PPC groups. Previous studies have reported close relationships between preoperative ASA classification and PPCs in certain types of surgeries [16, 17]. However, multivariate logistic regression analysis in this study identified NYHA classification ≥III, rather than ASA classification ≥IV, as an independent predictor of PPCs. The differences in such study findings may be attributed to the varying types of surgeries involved. Many elderly CABG patients suffer from preoperative cardiac insufficiency. The preoperative cardiac function status of elderly CABG patients has multifaceted and significant impacts on all aspects of the surgery and prognosis. The NYHA classification, by more accurately reflecting the patients’ actual cardiac function level, appears to have greater value in predicting PPCs. In contrast, while ASA classification also encompasses cardiac function assessment, its independent predictive power for PPCs of CABG may be relatively weaker when considering multiple factors simultaneously. This result emphasizes the importance of maintaining preoperative cardiac function in elderly CABG patients. Clinicians must actively adjust the preoperative cardiac function of these patients to lower the risk of PPCs.
Previous studies have reported close relationships between advanced age and the occurrence of PPCs [5, 18]. There was a statistically significant difference in age between the two groups in the univariate analysis. Despite the results of the multivariate analysis not supporting age as an independent predictor of PPCs in elderly patients undergoing CABG, age remains a high-risk factor that warrants vigilance. The significant decline in cardiorespiratory reserve and the increase in preoperative comorbidities in elderly patients are both potential factors that may increase the risk of PPCs. To further elucidate the relationship between age and PPCs in the elderly CABG population, studies with larger sample sizes may be required.
In this study, the principle for variable selection in the multivariate logistic regression analysis is primarily based on the results of univariate analysis. Variables that show statistical significance in the univariate analysis are further evaluated with multivariate logistic regression. In addition, previous studies have identified significant associations between smoking and PPCs [13, 14]. Preoperative smoking cessation has been proven to be an important measure in effectively reducing PPCs risks [14]. Smoking impairs respiratory mucous membranes and reduces lung defense capabilities, thereby increasing the likelihood of postoperative lung infections [19]. However, the univariate analysis in this study did not reveal the statistically significant difference in smoking between the two groups. The discrepancy in the association between smoking and PPCs between this study and previous studies may stem from differences in surgical types. Additionally, smoking may interact with other risk factors, such as age, comorbidities, and cardiopulmonary function etc., to jointly influence the occurrence of PPCs. This interaction may not have been adequately recognized in the univariate analysis, resulting in the underestimation or obscuring of the effect of smoking. Given smoking’s well-documented impact on respiratory function, it was included in the multivariate analysis as a clinically relevant factor in this study.
In this study, the postoperative albumin levels we collected were the results of the first biochemical test conducted after the patients were admitted to the ICU following surgery. This result generally reflects the albumin level status at the end of the surgical procedure. This albumin level status was present prior to the occurrence of PPCs in the patients included in this study. Albumin serves as the primary component maintaining plasma colloidal osmotic pressure [20]. When serum albumin levels decline, plasma colloidal osmotic pressure significantly drops. Hypoalbuminemia increases the risk of postoperative complications through various mechanisms, including reduced plasma colloidal osmotic pressure, compromised immune function, impaired respiratory muscle function, induction of pulmonary tissue edema and fluid retention, and exacerbation of inflammatory responses [20, 21]. Recent studies have reported serum albumin as an independent risk factor for pulmonary complications in patients with multiple rib fractures [22], and another recent investigation has confirmed that postoperative albumin concentration is an independent predictor for pulmonary complications following esophageal resection surgery [23]. In this study, the results of the multivariate analysis also verified that postoperative albumin level is an independent predictor of PPCs in elderly patients undergoing CABG. Currently, anesthesiologists and surgeons attach great importance to preoperative hypoalbuminemia in CABG patients and actively correct it before surgery. However, due to the lack of routine intraoperative monitoring of serum albumin levels, clinicians often overlook the occurrence of intraoperative hypoalbuminemia. These findings suggest that intraoperative monitoring of patients’ serum albumin level may be beneficial for major surgeries, emphasizing the importance of timely correction of hypoalbuminemia during the perioperative period to reduce PPCs and improve patients’ postoperative recovery outcomes.
Defined as PaO2 to FiO2, the P/F ratio in blood gas analysis serves as a crucial physiological parameter for assessing patients’ lung oxygenation capacity and respiratory function [24]. The normal range of P/F ratio generally falls between 400 and 500 mmHg. A decreased P/F ratio below this range may indicate some degree of lung dysfunction or reduced oxygen exchange efficiency. Previous studies have utilized P/F ratio to evaluate the severity of respiratory failure in patients with ARDS [25], where it has been intimately correlated with mortality rates [26, 27]. In the study of H1N1 influenza, B. Morton et al. discovered that P/F ratio serves as a predictive factor for the need for mechanical ventilation and admission to intensive care units [28]. Moreover, during the COVID-19 pandemic, P/F ratio has been proposed as a prognostic indicator for the disease [29].
This study identified P/F ratio < 350 mmHg as an independent risk factor of PPCs in elderly patients undergoing CABG, marking the first instance where this parameter has been utilized for predicting PPCs. As a simple and easily obtainable monitoring parameter, the P/F ratio has wide applicability and convenience in clinical practice. Preoperative decline in the P/F ratio may serve as an early indicator of underlying pulmonary issues, including ventilation-perfusion mismatch, alveolar damage, and reduced lung compliance [30, 31]. Importantly, the decrease in the P/F ratio may precede overt clinical symptoms of pulmonary complications, which allows clinicians to promptly identify potential lung injury and implement preventive measures or early interventions.
Although the results of the multivariate logistic analysis in this study do not support CPB as an independent risk factor for PPCs, the univariate analysis still suggests that CPB may increase the risk of PPCs in elderly patients undergoing CABG. Ischemia-reperfusion (I/R) injury is likely a key contributor to pulmonary complications following CPB [32]. During CPB, the pulmonary tissue experiences ischemia, leading to the generation of reactive oxygen species (ROS) and inflammatory factors. Upon reperfusion, oxygen-rich blood promotes the massive production of ROS, which activates the inflammatory response and exacerbates tissue damage [33]. I/R injury disrupts pulmonary function by damaging alveolar epithelial cells and pulmonary capillary endothelial cells, resulting in hypoxemia, pulmonary edema, and aggravated respiratory impairment. It also disturbs the immune system, increasing the risk of infection [34]. Elderly patients have poor cardiopulmonary reserve and low tolerance to I/R injury, making them more prone to severe complications such as acute respiratory distress syndrome after surgery, which elevates the mortality risk. Moreover, the interaction between underlying diseases and complications complicates treatment in elderly patients. Therefore, close monitoring of pulmonary function in elderly patients is necessary in CPB clinical practice.
There are some limitations to this study. Firstly, the retrospective nature of the study means that some data may be subject to bias, particularly in terms of unmeasured or inadequately controlled confounding variables. Future prospective studies with rigorous data collection protocols and comprehensive confounder adjustment will strengthen the evidence base. Secondly, due to the study’s single-center design and relatively small sample size (305 cases), some uncertainty may exist. Thirdly, the focus on short-term outcomes may have overlooked longer-term impacts on patient quality of life and survival. Longer follow-up periods are warranted to assess the full consequences of PPCs in this patient population. Finally, this study did not explore potential interventions to mitigate PPCs risk. Future work should aim to develop and evaluate targeted prevention and management strategies based on the identified risk factors, ultimately aiming to improve outcomes for elderly CABG patients.
Conclusions
In summary, this study indicated that NYHA classification ≥III, preoperative P/F ratio < 350 mmHg and postoperative albumin levels are independent predictors of PPCs. These findings have enhanced the understanding of the risk of PPCs in elderly CABG patients and provided a basis for implementing individualized interventions targeting high-risk populations. Further multi-center studies are needed to validate these risk factors and to explore more effective prevention and treatment strategies for PPCs.
Acknowledgements
We extend our gratitude to Mr. Wei Gao for his assistance throughout the data collection process.
Abbreviations
- CABG
Coronary artery bypass grafting
- PPCs
Postoperative pulmonary complications
- COPD
Chronic obstructive pulmonary disease
- EPCO
European Perioperative Clinical Outcome
- LOS
Length of hospital stay
- BMI
Body mass index
- ASA
American Society of Anesthesiologists
- NYHA
New York Heart Association
- LVEF
Left ventricular ejection fraction
- P/F ratio
PaO2/FiO2 ratio
- CPB
Cardiopulmonary bypass
- LIMA
Left internal mammary artery
- I/R
Ischemia-reperfusion
- ROS
Reactive oxygen species
Author contributions
Study design: TW and GF. Data acquisition and validation: GF and GZ. Statistical analysis and data management: GF and YJ. Manuscript editing: GF, YZ and TW. Manuscript reviewing: YJ, YZ and TW. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Post-subsidy funds for National Clinical Research Center, Ministry of Science and Technology of China (Tianlong Wang: 303-01-001-0272-03) and the Hui Zhi talent project of Xuanwu Hospital, Capital Medical University (2021).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Xuanwu Hospital, Capital Medical University (2024 No.277-001). Informed consent from patients was waived by the ethics committee of Xuanwu Hospital, since this is a retrospective study.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guang Feng and Yitong Jia contributed equally to this work.
References
- 1.Bottardi A, Prado GFA, Lunardi M, Fezzi S, Pesarini G, Tavella D, Scarsini R, Ribichini F. Clinical updates in coronary artery disease: A comprehensive review. J Clin Med 2024, 13(16). [DOI] [PMC free article] [PubMed]
- 2.Alzghari T, Sandner S, Di Franco A, Harik L, Perezgorvas-Olaria R, Soletti G Jr., Dimagli A, Cancelli G, Demetres M, Lau C, et al. Coronary artery bypass surgery to treat anomalous origin of coronary arteries in adults: A systematic review. Heart Lung Circ. 2023;32(12):1500–11. [DOI] [PubMed] [Google Scholar]
- 3.Xiang Y, Zhao Q, Luo T, Zeng L. Inspiratory muscle training to reduce risk of pulmonary complications after coronary artery bypass grafting: a systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:1223619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lusquinhos J, Tavares M, Abelha F. Postoperative pulmonary complications and perioperative strategies: A systematic review. Cureus. 2023;15(5):e38786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–34. [DOI] [PubMed] [Google Scholar]
- 6.Pooria A, Pourya A, Gheini A. Postoperative complications associated with coronary artery bypass graft surgery and their therapeutic interventions. Future Cardiol. 2020;16(5):481–96. [DOI] [PubMed] [Google Scholar]
- 7.Souza AV, da Cunha Carvalho R, da Cruz Dias D, Santana DGT, de Cassia Mascarenhas H, Cordeiro ALL, Guimaraes ARF. Clinical and functional outcomes associated with pulmonary complications after coronary artery bypass grafting. J Cardiothorac Surg. 2024;19(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, Leva B, Rhodes A, Hoeft A, Walder B, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European perioperative clinical outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32(2):88–105. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon G, Buddhavarapu VS, Grewal H, Munjal R, Verma RK, Surani S, Kashyap R. Evidence-based practice interventions for reducing postoperative pulmonary complications: A narrative review. Open Respiratory Med J. 2023;17:e18743064271499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odor PM, Bampoe S, Gilhooly D, Creagh-Brown B, Moonesinghe SR. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ. 2020;368:m540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perelló-Díez M, Paz-Lourido B. Prevention of postoperative pulmonary complications through preoperative physiotherapy interventions in patients undergoing coronary artery bypass graft: literature review. J Phys Ther Sci. 2018;30(8):1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweity EM, Alkaissi AA, Othman W, Salahat A. Preoperative incentive spirometry for preventing postoperative pulmonary complications in patients undergoing coronary artery bypass graft surgery: a prospective, randomized controlled trial. J Cardiothorac Surg. 2021;16(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative ST, Collaborative T. Evaluation of prognostic risk models for postoperative pulmonary complications in adult patients undergoing major abdominal surgery: a systematic review and international external validation cohort study. Lancet Digit Health. 2022;4(7):e520–31. [DOI] [PubMed] [Google Scholar]
- 14.Varga JT. Smoking and pulmonary complications: respiratory prehabilitation. J Thorac Dis. 2019;11(Suppl 5):S639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim FY, Yap J, Gao F, Teo LL, Lam CSP, Yeo KK. Correlation of the new York heart association classification and the cardiopulmonary exercise test: A systematic review. Int J Cardiol. 2018;263:88–93. [DOI] [PubMed] [Google Scholar]
- 16.Bevilacqua Filho CT, Schmidt AP, Felix EA, Bianchi F, Guerra FM, Andrade CF. Risk factors for postoperative pulmonary complications and prolonged hospital stay in pulmonary resection patients: a retrospective study. Braz J Anesthesiol. 2021;71(4):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canet J, Gallart L. Predicting postoperative pulmonary complications in the general population. Curr Opin Anaesthesiol. 2013;26(2):107–15. [DOI] [PubMed] [Google Scholar]
- 18.Smetana GW, Pfeifer KJ, Slawski BA, Jaffer AK, Dutta S, Cohn SL. Risk factors for postoperative pulmonary complications: an update of the literature. Hosp Pract (1995). 2014;42(5):126–31. [DOI] [PubMed] [Google Scholar]
- 19.Kotlyarov S. The role of smoking in the mechanisms of development of chronic obstructive pulmonary disease and atherosclerosis. Int J Mol Sci 2023, 24(10). [DOI] [PMC free article] [PubMed]
- 20.Kumar M, Jain K, Chauhan R, Meena SC, Luthra A, Thakur H, Singh A, Nair R, Gupta R. Hypoalbuminemia: incidence and its impact on acute respiratory distress syndrome and 28-day outcome in trauma patients. Eur J Trauma Emerg Surg. 2023;49(5):2305–14. [DOI] [PubMed] [Google Scholar]
- 21.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enter Nutr. 2019;43(2):181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Ge H, Gong A. Construction and validation study of a model for predicting the risk of pulmonary complications in elderly patients with multiple rib fractures. Altern Ther Health Med 2024. [PubMed]
- 23.Li X, Yu L, Fu M, Yang J, Tan H. Perioperative risk factors for postoperative pulmonary complications after minimally invasive esophagectomy. Int J Gen Med. 2024;17:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho EB, Leite TRS, Sacramento RFM, Nascimento P, Samary CDS, Rocco PRM, Silva PL. Rationale and limitations of the SpO2/FiO2 as a possible substitute for PaO2/FiO2 in different preclinical and clinical scenarios. Rev Bras Ter Intensiva. 2022;34(1):185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 26.Sakr Y, Francois B, Sole-Violan J, Kotfis K, Jaschinski U, Estella A, Leone M, Jakob SM, Wittebole X, Fontes LE, et al. Temporal changes in the epidemiology, management, and outcome from acute respiratory distress syndrome in European intensive care units: a comparison of two large cohorts. Crit Care. 2021;25(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiumello D, Fioccola A. Recent advances in cardiorespiratory monitoring in acute respiratory distress syndrome patients. J Intensive Care. 2024;12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton B, Tang L, Gale R, Kelly M, Robertson H, Mogk M, Robin N, Welters I. Performance of influenza-specific triage tools in an H1N1-positive cohort: P/F ratio better predicts the need for mechanical ventilation and critical care admission. Br J Anaesth. 2015;114(6):927–33. [DOI] [PubMed] [Google Scholar]
- 29.Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet. 2021;397(10270):173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HY, Cho J, Kwak N, Choi SM, Lee J, Park YS, Lee CH, Yoo CG, Kim YW, Lee SM. Improved oxygenation after prone positioning May be a predictor of survival in patients with acute respiratory distress syndrome. Crit Care Med. 2020;48(12):1729–36. [DOI] [PubMed] [Google Scholar]
- 31.Fridman SE, Di Giampietro P, Sensoli A, Beleffi M, Bucce C, Salvatore V, Giostra F, Gianstefani A. Prediction of conventional oxygen therapy failure in COVID-19 patients with acute respiratory failure by assessing serum lactate concentration, PaO2/FiO2 ratio, and body temperature. Cureus. 2022;14(2):e21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorjipour F, Dehaki MG, Totonchi Z, Hajimiresmaiel SJ, Azarfarin R, Pazoki-Toroudi H, Mahdavi M, Korbi M, Dehaki MG, Soltani B, et al. Inflammatory cytokine response and cardiac troponin I changes in cardiopulmonary bypass using two cardioplegia solutions; Del Nido and modified st. Thomas’: a randomized controlled trial. Perfusion. 2017;32(5):394–402. [DOI] [PubMed] [Google Scholar]
- 33.Gorjipour F, Saeedzadeh T, Toloueitabar Y, Kachoueian N, Bahlouli Ghashghaei S, Mortazian M, Dehghani Firoozabadi M, Jadbabaie A, Tirgarfakheri K, Motamednejad A, et al. Remote ischemic preconditioning effects on inflammatory markers and myocardial protection in coronary artery bypass graft surgery. Perfusion. 2022;37(1):56–61. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Li X, Xie W, Wang Y, Xu Z, Bai YX, Zhou Q, Wu Q. Risk factors and Short-Term outcomes of postoperative pulmonary complications in elderly patients after cardiopulmonary bypass. Clin Interv Aging. 2024;19:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.