Abstract
Objective
This study explored the changes in serum neurotrophic factor levels, the status of cognitive function, event-related potential P300, and the relationships between serum neurotrophic factor levels and cognitive functions, as well as event-related potential P300 in male schizophrenia patients with long-term hospitalization.
Methods
A total of 82 male schizophrenia patients with long-term hospitalization and 52 healthy controls were recruited. Cognitive functions were evaluated in all participants, including verbal fluency function, attention function, executive function, and spatial function. Event-related potential P300 latency and amplitude were recorded using the Nicolet Viking Quest evoked potential system (USA). The serum levels of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) were measured in all participants using enzyme-linked immunosorbent assay (ELISA).
Results
The patient group exhibited significantly poorer performance in all cognitive functions compared to the control group (p < 0.05). The patient group exhibited a statistically significant prolongation in P300 latency and a reduction in P300 amplitude compared to the control group (P < 0.01). In addition, serum BDNF levels were significantly lower in the patient group (9.1 ± 2.1 ng/ml) compared to the control group (11.6 ± 2.3 ng/ml, P < 0.01). Serum GDNF levels were 603.4 ± 182.6 pg/ml in the patient group and 610.2 ± 176.3 pg/ml in the control group, showing no statistically significant difference (P > 0.05). Onset levels were significantly correlated with almost all cognitive functions. BDNF levels were correlated to digital cancellation test scores (P < 0.05) and trail making test part B (TMT-part B) scores (P < 0.05). Moreover, a correlation was found between GDNF levels and block design test scores (P < 0.05). The latency of P300 was correlated to digital cancellation test scores (P < 0.01) and trail making test part A (TMT-part A) scores (P < 0.05). The amplitude of P300 was correlated with digital cancellation test scores (P < 0.01).
Conclusions
This study reveals that patients with chronic schizophrenia suffer from notable cognitive deficits during long-term hospitalization, which is linked to specific clinical characteristics. The patient group exhibited significantly lower serum BDNF levels than the control group. Event-related potential P300 testing revealed prolonged latency and reduced amplitude in patients. Serum BDNF and GDNF levels were selectively correlated with specific cognitive function performance.
Keywords: Schizophrenia, Cognitive functions, Neurotrophic factors, Event-related potentials, BDNF, GDNF
Introduction
Schizophrenia is a complex and severe brain disorder characterized by positive, negative, and cognitive symptoms [1]. Schizophrenia impacts approximately 1% of the global population and ranks among the top 10 causes of disability worldwide [2]. Schizophrenia incurs significant societal costs, with 80–90% of individuals affected being unemployed for the majority of their adult lives [3]. Cognitive symptoms in schizophrenia affect various domains, including attention, processing speed, working memory, visual and verbal learning and memory, reasoning and problem-solving, and social cognition [4]. Notably, cognitive symptoms have been observed across all stages of the illness [5] and are regarded as a core characteristic of schizophrenia [6].
Cognitive functions, including learning, memory, attention, executive functions, and working memory, are impaired in individuals with schizophrenia [7]. Individuals with schizophrenia often exhibit cognitive impairments, typically 1–1.5 standard deviations below those of healthy individuals [8]. Changes in developmental trajectory during adolescence are stronger predictors of later schizophrenia onset compared to cognitive performance impairments measured at age 18 [9]. Some cognitive functions are inheritable and linked to schizophrenia, indicating a partially shared genetic basis. Cognitive impairment is associated with decreased treatment adherence, increased hospital admission risk, and extended hospital stays [10, 11]. Cognitive impairments in executive functions, attention, visual memory, and social cognition are linked to depression and anxiety symptoms, which accompany the negative and positive symptoms of schizophrenia. Furthermore, antipsychotic medications and other interventions cannot completely restore normal cognitive performance in patients with schizophrenia [12–14].
Neurotrophic factors play a crucial role in neural development and plasticity. The neurotrophic hypothesis posits that neurotrophic factors participate in the pathophysiology of schizophrenia. Previous research indicates that polymorphisms in brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) are associated with impairments in various cognitive function domains. BDNF is prevalent in the brain and plays a crucial role in memory and learning by enhancing synaptic connectivity and neuroplasticity [15]. BDNF can cross the blood-brain barrier, and serum BDNF concentrations are highly correlated with those in cerebrospinal fluid [16]. Therefore, measuring serum BDNF levels can provide insights into changes in brain BDNF. BDNF is thought to promote neuron survival and differentiation, as well as influence neurotransmission and synaptic plasticity in the central and peripheral nervous systems. Evidence strongly suggests that BDNF is integral to visceral pain and hypersensitivity conditions [17]. GDNF levels were associated with working memory in healthy individuals and attention deficits in schizophrenia patients [18]. In summary, the link between GDNF levels and schizophrenia pathogenesis remains underexplored [19].
Event-related potential P300 (P300) refers to a positive voltage deflection in the stimulus-locked event-related potentials that occurs 300ms post-stimulus, elicited during an oddball target detection task by behaviorally relevant infrequent salient stimuli interspersed among frequent standard stimuli. The P300 amplitude is believed to reflect controlled attentional resource allocation, contextual updating of working memory, and stimulus salience processing [20]. The diminished amplitude of the P300 event-related potential component indicates attention-related processing deficits and is a well-replicated biological finding in schizophrenia, suggesting its potential as a biomarker for psychosis risk. Some studies have shown that P300 amplitude also fluctuates with clinical state and abnormalities worsen with longer illness duration [21].
Accurate assessment of cognitive performance in schizophrenia remains challenging due to time constraints and a lack of formal training [22, 23]. Moreover, a significant proportion of individuals with schizophrenia exhibit severe cognitive impairments but do not report subjective deficits [24]. Therefore, the present study investigated the blood serum levels of BDNF, GDNF, P300, and subjective reports of cognitive functions in long-term male hospitalized patients with schizophrenia and healthy controls. We hypothesize that long-term male hospitalized patients with schizophrenia exhibit significant alterations in BDNF, GDNF, P300, and cognitive function levels, and that there is a certain association between cognitive function and the levels of BDNF, GDNF, and P300.
Subjects and methods
Participants
This study evaluated two groups, including 82 hospitalized schizophrenia patients from WuTaiShan Hospital of Yangzhou University and 52 healthy volunteers as controls.
All patients were diagnosed with schizophrenia based on the DSM-5 criteria [25]. Patients were all male schizophrenia patients who had been hospitalized for more than 10 years and displayed stable symptoms in the recovery phase. The patient’s psychiatric symptoms showed no deterioration over the past five years, and the antipsychotic medication regimen remained unchanged. Patients had no history of modified electroconvulsive therapy. Patients were all right-handed. The exclusion criteria comprised patients with a history of organic mental disorders, mental retardation, alcohol and psychoactive substance dependence, red-green color blindness and color weakness, and hearing impairments. The study exclusively included men to eliminate potential hormonal and gender-related confounding effects.
The control group comprised healthy volunteers matched with the case group patients by age, gender, and education. Individuals without systemic or neurological diseases affecting literacy, no psychiatric diagnoses, and not using psychiatric medication were included in the study. The study was approved by the WuTaiShan Hospital of Yangzhou University’s Ethics Committee, and written consent was secured from all participants or their legal guardians. All methods adhered to the Declaration of Helsinki.
Psychiatric symptoms
The Positive and Negative Syndrome Scale (PANSS) [26] evaluates the severity of positive and negative symptoms, as well as general psychopathology, in individuals with schizophrenia or other psychotic disorders. The structure includes seven positive scales, seven negative scales, and 16 comprehensive psychopathological scales.
Cognitive functions
Cognitive assessments were conducted for all participants as follows: (1) Verbal fluency was evaluated using verbal fluency tests (VFT) for actions and animals; (2) Attention was assessed through the digital cancellation test, trail making test part A (TMT-part A), and Stroop color and word tests; (3) Executive function was measured with the trail making test part B (TMT-part B) and the Stroop interference test; (4) Spatial function was evaluated using the block design test and the Wechsler Memory Scale-III Spatial Span Test (WMS-III SST) [27]. The selected tests were oriented towards verbal fluency, attention and processing speed, attention distribution, working memory, motor speed, and executive function. Detailed descriptions of the cognitive measurement process have been provided in prior studies. An experienced psychiatric specialist conducted the cognitive function assessment in the test laboratory, which took about two hours. Higher test scores generally indicate better cognitive function, except for the TMT.
Assessment of BDNF and GDNF
Venous blood was collected from fasted subjects into anticoagulant-free tubes between 06:30 and 07:30 AM, followed by centrifugation at 3000×g for 15 min. Serum was subsequently aliquoted and stored at −80 °C until analysis. BDNF and GDNF concentrations were quantified using commercial sandwich ELISA kits, strictly adhering to the manufacturer’s protocols. All assays were performed in triplicate. BDNF concentrations were expressed in ng/mL, while GDNF concentrations were expressed in pg/mL. Both intra- and inter-assay coefficients of variation were below 5%. Sample collection and biomarker analysis were conducted by investigators blinded to participant group assignments.
P300
The Oddball auditory paradigm was utilized, delivering stimuli binaurally via soundproof headphones. Non-target stimuli: probability 80%, intensity 85dB, frequency 1000 Hz; target stimuli: probability 20%, intensity 95dB, frequency 2000 Hz. Electrode impedance was kept under 5KΩ, using a filter range of 0.5–100 Hz and an analysis duration of 600 ms. Participants were seated in a relaxed yet attentive state and were instructed to press a button for target stimuli and disregard non-target stimuli. The instrument automatically recorded reaction times and hit rates, with each test repeated twice and the average taken. If a subject’s hit rate was less than 80%, the test was considered invalid. The P300 latency and amplitude were recorded for each subject.
Data analysis
The data were analyzed using SPSS 21.0 software. For continuous variables conforming to a normal distribution, data are shown as means ± standard deviation (SD) and analyzed using independent sample t-tests. The Kolmogorov-Smirnov test was performed to assess whether the data followed a normal distribution, while chi-squared analysis was used to evaluate relationships between dichotomous variables. In addition, correlation analysis between two variables was conducted using Pearson’s correlation analysis. A stepwise regression analysis was employed to explore the potential influence of confounding variables on cognitive functions. Statistical tests were two-tailed, with significance set at P < 0.05.
Results
Demographic data and cognitive functions
The average age was 52.0 ± 7.5 for patients and 52.9 ± 5.7 for controls. No statistically significant difference was found between the two groups in terms of mean age (p > 0.05). The patient group had an average education of 8.9 ± 3.0 years, while the control group averaged 9.8 ± 2.9 years. No statistically significant difference was observed between the two groups (p > 0.05).
In the patient group, the average age of disease onset was 28.4 ± 6.9 years, and the mean score on the PANSS positive subscale was 10.8 ± 4.8. The average score of the PANSS negative subscale was 18.3 ± 8.7, while the PANSS general subscale showed an average score of 29.7 ± 8.8.
Moreover, the cognitive functions (verbal fluency function, attention function, executive function, and spatial function) of the patient group were compared to those of the control group. The patient group exhibited significantly poorer performance across all cognitive functions compared to the control group (p < 0.05) (Table 1).
Table 1.
Demographics, clinical characteristics, and cognitive performance of the sample cohort
| Patient group (n=82) | Control group (n=52) | t | |
|---|---|---|---|
| Age (years) | 52.0±7.5 | 52.9±5.7 | -0.678 |
| Education (years) | 8.9±3.0 | 9.8±2.9 | -1.772 |
| Onset (years) | 28.4±6.9 | ||
| PANSS positive subscale | 10.8±4.8 | ||
| PANSS negative subscale | 18.3±8.7 | ||
| PANSS general subscale | 29.7±8.8 | ||
| PANSS total score | 58.5±18.9 | ||
| Verbal fluency function | |||
| VFT-actions | 11.1±5.5 | 16.3±3.2 | -6.446** |
| VFT-animals | 5.7±4.0 | 11.2±3.4 | -7.514** |
| Attention function | |||
| Digital cancellation test (s) | 313.6±298.8 | 170.9±58.1 | 3.744** |
| TMT-part A (s) | 107.7±60.0 | 69.9±33.8 | 4.203** |
| Stroop word | 49.6±19.8 | 70.1±16.6 | -5.651** |
| Stroop color | 30.4±13.3 | 43.7±13.9 | -5.022** |
| Executive function | |||
| Stroop interference | 17.5±9.1 | 23.9±9.4 | -3.526** |
| TMT-part B(s) | 236.5±93.0 | 151.5±66.8 | 4.576** |
| Spatial function | |||
| Block design test | 18.0±8.4 | 29.4±6.1 | -8.355** |
| WMS-III SST | 12.2±3.8 | 16.6±7.1 | -4.269** |
**P<0.01
Levels of serum BDNF and GDNF
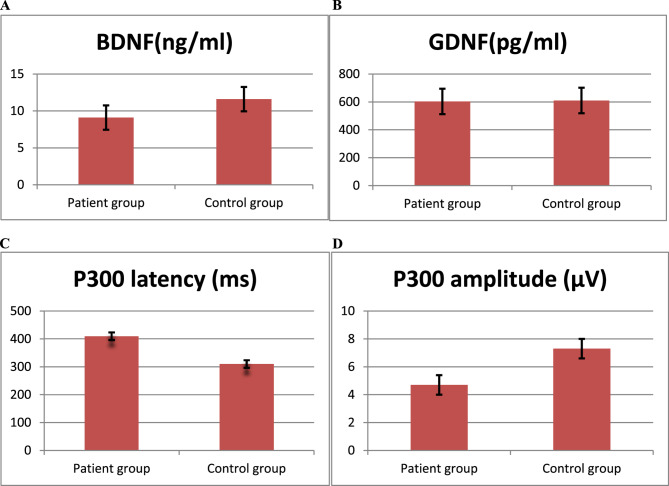
The serum BDNF levels in the patient group and the control group were 9.1±2.1 ng/ml and 11.6±2.3 ng/ml, respectively, with a statistically significant difference between the two groups (t = −6.186, P < 0.01). The serum GDNF levels were 603.4 ± 182.6 pg/ml in the patient group and 610.2 ± 176.3 pg/ml in the control group, showing no statistically significant difference (t = 0.201, P > 0.05) (Fig. 1A and B).
Fig. 1.
A Serum BDNF levels in patient group and control group. B Serum GDNF levels in patients group and control group. C P300 latency in patient group and control group. D P300 amplitude in patient group and control group
P300 latency and amplitude
The P300 latency was 409.4 ± 27.3 ms in the patient group and 309.7 ± 22.4 ms in the control group. The patient group exhibited a significantly longer P300 latency compared to the control group (t = 22.990, P < 0.01). Furthermore, the P300 amplitude was 4.7 ± 1.0 µV in the patient group and 7.3 ± 1.8 µV in the control group. The patient group exhibited a significantly reduced P300 amplitude compared to the control group (t=−9.699, P < 0.01). (Fig. 1C and D)
Association between cognitive functions and clinical characteristics in the patient group
Multivariate regression analyses were performed to examine the relationships between cognitive functions and clinical characteristics within the patient group (Table 2). The onset levels significantly correlated with cognitive performance tests, while PANSS positive subscale scores showed no correlation with any cognitive performance tests. The onset levels were correlated to VFT-actions scores (r=−0.256, P < 0.05), VFT-animals scores (r = 0.459, P < 0.01), digital cancellation test scores (r = 0.293, P < 0.05), TMT part A scores (r = 0.508, P < 0.01) Stroop word test scores (r = 0.481, P < 0.01), Stroop color test scores (r=−0.342, P < 0.01), TMT part B scores (r = 0.456, P < 0.01) and WMS-III SST scores (r=−0.305, P < 0.01).
Table 2.
Association between cognitive functions and clinical characteristics in the patient group (r)
| Age (years) | Education (years) | Onset (years) | PANSSpositive subscale | PANSS negative subscale | PANSS general subscale | PANSS total score | |
|---|---|---|---|---|---|---|---|
| Verbal fluency function | |||||||
| VFT-actions | -0.133 | 0.083 | -0.256* | -0.045 | -0.330** | -0.385** | -0.322* |
| VFT-animals | -0.288** | 0.194* | 0.459** | 0.089 | -0.129 | -0.171 | -0.097 |
| Attention function | |||||||
| Digital cancellation test (s) | 0.199* | -0.179 | 0.293* | 0.036 | 0.234 | 0.271* | 0.190 |
| TMT-part A (s) | 0.389** | -0.161 | 0.508** | 0.142 | 0.332** | 0.338** | 0.318** |
| Stroop word | 0.274* | 0.059 | 0.481** | 0.022 | -0.130 | -0.190 | -0.140 |
| Stroop color | -0.185 | 0.164 | -0.342** | 0.093 | -0.008 | 0.011 | 0.023 |
| Executive function | |||||||
| Stroop interference | -0.184 | 0.206* | -0.249 | 0.144 | -0.164 | -0.02 | -0.045 |
| TMT-part B(s) | 0.163 | -0.097 | 0.456** | 0.275 | 0.167 | 0.178 | 0.271 |
| Spatial function | |||||||
| Block design test | -0.024 | 0.314** | 0.144 | -0.123 | -0.192 | -0.205 | -0.203 |
| WMS-III SST | 0.280* | 0.072 | -0.305** | 0.140 | -0.223* | -0.137 | -0.109 |
*P<0.05, **P<0.01
Association between cognitive functions and BDNF, GDNF, and P300 in the patient group
Multivariate regression analyses were conducted to examine the relationships between cognitive functions and BDNF, GDNF, and P300 in the patient group (Table 3). BDNF levels were correlated to digital cancellation test scores (r=−0.281, P < 0.05) and TMT-part B scores (r=−0.355, P < 0.05). GDNF levels were correlated to block design test scores (r = 0.272, P < 0.05). P300 latency levels were correlated to digital cancellation test scores (r=−0.481, P < 0.01) and TMT-part A scores (r = 0.245, P < 0.05). Additionally, P300 amplitude levels were correlated to digital cancellation test scores (r=−0.338, P < 0.01).
Table 3.
Association between cognitive functions and BDNF, GDNF, and P300 in the patient group (r)
| BDNF | GDNF | P300 latency | P300 amplitude | |
|---|---|---|---|---|
| Verbal fluency function | ||||
| VFT-actions | 0.160 | 0.030 | -0.104 | 0.124 |
| VFT-animals | 0.213 | 0.031 | -0.231 | 0.137 |
| Attention function | ||||
| Digital cancellation test(s) | -0.281* | 0.156 | 0.481** | -0.338** |
| TMT-part A(s) | -0.016 | -0.185 | 0.245* | 0.046 |
| Stroop word | 0.127 | 0.078 | -0.164 | 0.178 |
| Stroop color | 0.046 | 0.057 | -0.081 | 0.034 |
| Executive function | ||||
| Stroop interference | -0.102 | 0.108 | 0.044 | -0.023 |
| TMT-part B(s) | -0.355* | -0.192 | 0.317 | -0.126 |
| Spatial function | ||||
| Block design test | 0.014 | 0.272* | -0.004 | 0.072 |
| WMS-III SST | 0.051 | 0.002 | -0.219 | 0.183 |
*P<0.05, **P<0.01
Discussion
This study reveals that chronic schizophrenia patients develop cognitive deficits during long-term hospitalization, which are linked to specific clinical features. The patient group exhibited significantly lower serum BDNF levels than healthy controls, showing no significant difference in GDNF levels. P300 testing revealed prolonged latency and reduced amplitude in the patient group. Notably, serum neurotrophic factor levels and P300 indicated selective correlations with performance on specific cognitive function assessments. Cognitive impairment has recently been added to the set of clinical features of schizophrenia [28]. Patients in the early stages of schizophrenia exhibit significantly poorer performance than healthy controls across nearly all cognitive function tests, including processing speed, attention, and executive function [29–33]. Moreover, antipsychotic medications affect the cognitive function of schizophrenia patients. Clozapine exerted a more positive impact on cognitive functions in patients who were younger, had higher education levels, and showed greater clinical improvement. Therefore, cognitive function in older patients, a vulnerable group, should be closely monitored, and clozapine dosage should be adjusted as necessary [34]. Male schizophrenia patients with long-term hospitalization performed significantly worse than healthy controls in all cognitive function tests. Antipsychotic medications seem to yield minimal cognitive improvement in chronically hospitalized schizophrenia patients. This study found relatively higher scores on the negative symptom subscale of the PANSS, which may be related to the characteristics of our research sample. The schizophrenia patients in this study had average illness duration of 28.4 years, with hospitalization periods all exceeding 10 years, and poor social support. Consequently, despite long-term stability in medication regimens, these patients exhibited declined social functioning and more prominent negative symptoms. In China, such cases of schizophrenia are not uncommon. These patients may present distinct features in terms of cognitive function and neurotrophic factors, which prompted us to conduct this analytical study on this specific subgroup of schizophrenia patients.
This study’s results revealed increased latency and decreased amplitude in patients compared to healthy controls, which aligns with earlier research [35]. However, research on the effects of antipsychotic medications on P300 remains inconsistent. Some studies have reported increased P300 amplitude in schizophrenia patients after antipsychotic treatment, while others found no significant changes in P300 amplitude post-treatment [36]. These discrepancies may stem from differences in the mechanisms of action among antipsychotic drugs, leading to varying effects on P300 latency and amplitude.
This study found lower serum BDNF levels in chronic schizophrenia patients compared to healthy controls, aligning with previous findings [37–39]. However, compared with some previous studies, our findings showed lower serum BDNF levels in schizophrenia patients, which might be related to the fact that our study subjects were long-term hospitalized patients. Decreased BDNF levels may serve as a trait marker for schizophrenia. Despite symptom improvement with treatment, the serum BDNF levels in schizophrenia patients did not recover to normal levels, suggesting that antipsychotic drugs may not significantly alter serum BDNF levels. Downregulation of BDNF expression may directly or indirectly contribute to neuronal atrophy and death. However, the decline in serum BDNF might not be directly linked to long-term antipsychotic use but rather associated with deteriorating brain function and the chronic disease course. Research suggests that antipsychotics may not alter serum BDNF levels in individuals with schizophrenia, with abnormal BDNF levels potentially indicating inherent neuronal damage [40]. A comprehensive meta-analysis revealed that neither the type nor the dosage of antipsychotics affects BDNF levels in individuals with schizophrenia [41].
The study indicates that serum GDNF levels demonstrate no significant difference between chronic schizophrenia patients and healthy controls, implying that GDNF may not serve as a specific biomarker for schizophrenia. This aligns with Niitsu [18], who reported comparable serum GDNF levels between 63 chronic schizophrenia patients and 52 healthy controls. Conversely, Xiao [42] observed significantly lower GDNF levels in acute-phase schizophrenia patients, which gradually increased after 2–8 weeks of antipsychotic treatment. These findings imply that antipsychotics may elevate serum GDNF levels. Since our cohort consisted of medicated, long-term hospitalized patients, the antipsychotic effects on GDNF levels cannot be excluded. Furthermore, Tunca [43] reported reduced GDNF levels in 33 schizophrenia patients compared to controls, possibly reflecting disease state heterogeneity. Previous studies have indicated that untreated schizophrenia patients exhibit decreased serum GDNF levels, while those who respond to treatment show restoration of serum GDNF levels. GDNF, unlike BDNF, is a large protein molecule that cannot cross the blood-brain barrier. Although serum GDNF levels might not accurately represent those in the central nervous system, experiments in rats indicate that administering recombinant human GDNF to the striatum increases antioxidant enzyme activity, such as superoxide dismutase, catalase, and glutathione peroxidase [44]. Peripheral GDNF may exert neuroprotective effects by boosting antioxidant defenses, mitigating oxidative damage—a mechanism potentially relevant to CNS pathophysiology.
The study revealed a correlation between serum BDNF levels and performance on the Digit Cancellation Test and TMT-part B in schizophrenia patients, indicating a possible link between serum BDNF levels and cognitive functions like executive function and attention. Zhang [45] evaluated cognitive function in chronic schizophrenia patients with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and assessed serum BDNF levels. The study revealed that patients exhibited significantly lower cognitive scores and serum BDNF levels than healthy controls, with a notable positive correlation between serum BDNF levels and immediate memory function within the patient group. Postmortem analyses of schizophrenia patients revealed significantly decreased BDNF protein and mRNA levels in the dorsolateral prefrontal cortex and hippocampus, which are areas essential for memory, learning, and attention [46, 47]. Reduced BDNF expression in these regions may lead to cognitive deficits in schizophrenia. Moreover, BDNF has been shown to enhance acetylcholine release in the septo-hippocampal pathway in rats, with evidence of a positive feedback loop between BDNF and cholinergic activity [48]. Considering the established role of central cholinergic systems and basal forebrain cholinergic neurons in learning and memory, BDNF may mediate cognitive function through interactions with acetylcholine.
This study also identified a positive correlation between serum GDNF levels and Block Design Test performance in patients, implying a possible link between GDNF and spatial cognition. Animal studies report that GDNF heterozygous mice exhibit reduced GDNF mRNA expression in the hippocampus and caudate nucleus, demonstrating impaired learning in the Morris water maze [49]. Naumenko [50] observed significant improvements in spatial learning in GDNF-pretreated depressive model mice. Clinical studies in late-onset depression patients found that decreased GDNF levels were associated with attention and executive dysfunction [51]. However, Niitsu [18] reported divergent findings: while healthy controls with higher serum GDNF performed better on the Digit Span Test, schizophrenia patients with elevated GDNF levels showed more pronounced attentional deficits. These discrepancies may be attributed to variations in study populations or cognitive assessment tools. Notably, GDNF has been shown to inhibit apoptosis of hippocampal dopaminergic neurons and participate in hippocampal neuroplasticity and memory processes. Astrocyte-derived GDNF may enhance hippocampal neuronal function by modulating the synthesis of acetylcholine, serotonin, and dopamine [52].
Research indicates a link between cognitive function and P300 in schizophrenia patients, but the exact cognitive aspects related to P300 remain incompletely understood. Mathis [53] found that schizophrenia patients exhibited deficits in both early sensory processing and later attention-mediated stages of information processing compared to controls, and these deficits coincided with reduced P300 amplitude. The study found a positive correlation between P300 latency and performance on the Digit Cancellation Test and TMT-part A in the patient group, whereas P300 amplitude showed a negative correlation with the Digit Cancellation Test. These tests are widely used to assess attentional function. Schizophrenia patients often struggle to filter out irrelevant stimuli, leading to attentional lapses and impaired shifting of focus. The amplitude and latency of P300 may serve as electrophysiological markers of these attentional dysfunctions.
Nevertheless, the limitations of the present study should be acknowledged. First, the cohort consisted exclusively of male, long-term hospitalization patients, most of whom were clinically stable; however, a subset had more severe symptoms and poorer treatment response. Second, potential confounding factors, including antipsychotic medication use and illness duration, may have influenced serum BDNF/GDNF levels and cognitive test results. Regarding antipsychotic medication use, we were unable to standardize treatment regimens due to the relatively small overall sample size and particularly small subsamples for specific agents. Third, the exclusion of female patients prevents examination of potential gender differences in neurotrophic factor levels and cognitive performance. Finally, the study only employed auditory P300 paradigms; other modalities (visual/somatosensory stimuli) or ERP components (P50, N400, CNV) might show stronger correlations with cognitive dysfunction. Future studies should conduct comprehensive analyses of relationships between neurotrophic factors, cognitive function, and electrophysiological measures.
Acknowledgements
We would like to thank all patients who participated in the study. We are grateful to all the psychiatrists and nurses who participated in our current study and those research staff that contributed to the subjects’ diagnosis and clinical assessments.
Clinical trial number
Not applicable.
Authors’ contributions
PJ: conceptualization, methodology, writing of the original draft, visualization, and formal analysis. XY, HY, YF, FW, XM, QZ: data collection, writing of the original draft, review and editing. QT, XZ: conception and design, assistance in drafting the article and revising it critically for important intellectual content. All authors contributed to the article, agreed to submit it to the current journal, and approved the submitted version.
Funding
Zhejiang Medical and Health Science and Technology Project (2023KY1126), Zhejiang Traditional Chinese Medicine Science and Technology Project (2023ZL668), Suzhou Clinical Medical Center for Mood Disorders (Szlcyxzx202109), Suzhou Key Laboratory (SZS2024016), and Multicenter Clinical Research on Major Diseases in Suzhou (DZXYJ202413).
Data availability
The data supporting the results of this study are available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
We declare that all experiments on human subjects were conducted in accordance with the Declaration of Helsinki and that all procedures were carried out with the adequate understanding and written consent of the subjects. All experimental protocols were approved by the Ethics Committee of WuTaiShan Hospital of Yangzhou University. Informed consent was obtained from all the participants and/or their legal guardians. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qing Tian, Email: sunnytien@126.com.
Xiaobin Zhang, Email: zhangxiaobim@163.com.
References
- 1.Huang KY, Huang YJ, Chen SJ, et al. The associations between cognitive functions and TSNAX genetic variations in patients with schizophrenia. Pharmacol Biochem Behav. 2023. 10.1016/j.pbb.2023.173554. [DOI] [PubMed] [Google Scholar]
- 2.Marder SR, Cannon TD, Schizophrenia. N ENGL J MED. 2019;381(18):1753–61. [DOI] [PubMed] [Google Scholar]
- 3.McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023. 10.1038/s41380-023-01949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanchanatawan B, Thika S, Anderson G, et al. Affective symptoms in schizophrenia are strongly associated with neurocognitive deficits indicating disorders in executive functions, visual memory, attention and social cognition. Progress Neuropsychopharmacol Biol Psychiatry. 201710.1016/ j.pnpbp.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Chu AOK, Chang WC, Chan SKW, et al. Comparison of cognitive functions between first-episode schizophrenia patients, their unaffected siblings and individuals at clinical high-risk for psychosis. Psychol Med. 2018;1–8. 10.1017/S0033291718002726. [DOI] [PubMed]
- 6.Fagerlund B, Pantelis C, Jepsen JRM, et al. Differential effects of age at illness onset on verbal memory functions in antipsychoticnaïve schizophrenia patients aged 12–43 years. Psychol Med. 2020;1–11. 10.1017/S0033291720000409. [DOI] [PubMed]
- 7.Li Z, Kang Z, Xia X, et al. Associations of resilience, white matter topological organization, and cognitive functions in first-episode, drug-naïve schizophrenia patients: A moderated mediation analysis. Progress Neuropsychopharmacol Biol Psychiatry. 2024;128:110867. [DOI] [PubMed] [Google Scholar]
- 8.Katsumi A, Hoshino H, Fujimoto S, et al. Effects of cognitive remediation on cognitive and social functions in individuals with schizophrenia. Neuropsychological Rehabilitation. 2017. 10.1080/09602011.2017.1409639. [DOI] [PubMed] [Google Scholar]
- 9.MacCabe JH, Wicks S, Löfving S, et al. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70:261–70. [DOI] [PubMed] [Google Scholar]
- 10.Kitchen H, Rofail D, Heron L, et al. Cognitive impairment associated with schizophrenia: a review of the humanistic burden. Adv Ther. 2012;29:148–62. [DOI] [PubMed] [Google Scholar]
- 11.Kadakia A, Fan Q, Shepherd J, et al. Healthcare resource utilization and quality of life by cognitive impairment in patients with schizophrenia. Schizophr Res Cogn. 2022;28:100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fett AK, Viechtbauer W, Dominguez MD, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–88. [DOI] [PubMed] [Google Scholar]
- 13.Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill SK, Bishop JR, Palumbo D, et al. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother. 2010;10(1):43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10(2):86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillai A, Kale A, Joshi S, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13(4):535–9. [DOI] [PubMed] [Google Scholar]
- 17.Serra-Millàs M. Are the changes in the peripheral Brain-Derived neurotrophic factor levels due to platelet activation?? World J Psychiatry. 2016;6:84–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niitsu T, Shirayama Y, Matsuzawa D, et al. Association between serum levels of glial cell-line derived neurotrophic factor and attention deficits in schizophrenia. Neurosci Lett. 2014;575:37–41. [DOI] [PubMed] [Google Scholar]
- 19.Turkmen BA, Yazici E, Erdogan DG, et al. BDNF, GDNF, NGF and Klotho levels and neurocognitive functions in acute term of schizophrenia. BMC Psychiatry. 2021;21:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton HK, Woods SW, Roach BJ, et al. Auditory and visual oddball stimulus processing Deffcits in schizophrenia and the psychosis risk syndrome: forecasting psychosis risk with P300. Schizophr Bull. 2018. 10.1093/schbul/sby167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton HK, Boos AK, Mathalon DH. Electroencephalography and Event-Related potential biomarkers in individuals at clinical high risk for psychosis. Biol Psychiatry. 2020;88:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green MF, Barnes TR, Danion JM, et al. The FOCIS international survey on psychiatrists’ opinions on cognition in schizophrenia. Schizophr Res. 2005;74:253–61. [DOI] [PubMed] [Google Scholar]
- 23.Belgaied W, Samp J, Vimont A, et al. Routine clinical assessment of cognitive functioning in schizophrenia, major depressive disorder, and bipolar disorder. Eur Neuropsychopharmacol. 2014;24:133–41. [DOI] [PubMed] [Google Scholar]
- 24.Raffard S, Lebrun C, Bayard S, et al. Self-awareness deficits of cognitive impairment in individuals with schizophrenia. Really? Frontier Psychiatry. 2020;11:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Association AP. Diagnostic and Statistical Manual of Mental Disorders, fifth ed. DSM-5. 2013.
- 26.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. [DOI] [PubMed] [Google Scholar]
- 27.Oral E, Canpolat S, Yildirim S, et al. Cognitive functions and serum levels of brain-derived neurotrophic factor in patients with major depressive disorder. Brain Res Bull. 2012;88:454–9. [DOI] [PubMed] [Google Scholar]
- 28.Jauhar S, Johnscone M, Mckenna P. Schizophrenia Lancet. 2022;399:473–86. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak P, Kus K, Murawiecka P, et al. Biochemical and cognitive impairments observed in animal models of schizophrenia induced by prenatal stress paradigm or Methylazoxymethanol acetate administration. Acta Neurobiol Exp. 2015;75:314–25. [PubMed] [Google Scholar]
- 30.Reilly JL, Sweeney JA. Generalized and specific neurocognitive deficits in psychotic disorders: utility for evaluating Pharmacological treatment effects and as intermediate phenotypes for gene discovery. Schizophr Bull. 2014;40:516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang YZ, Wei T, Mei HX, et al. Interleukin 18 and cognitive impairment in first episode and drug Naïve schizophrenia versus healthy controls. Brain Behav Immun. 2013;32:105–11. [DOI] [PubMed] [Google Scholar]
- 32.Xiao W, Ye F, Liu C, et al. Cognitive impairment in first-episode drug-naïve patients with schizophrenia: relationships with serum concentrations of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. Progress Neuropsychopharmacol Biol Psychiatry. 2017;76:163–8. [DOI] [PubMed] [Google Scholar]
- 33.Sheffeld JM, Gold JM, Strauss ME, et al. Common and specific cognitive deficits in schizophrenia: relationships to function. Cogn Affect Behav Neurosci. 2014;14:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheuk NKW, Tse W, Tsui HKH, et al. A systematic review and meta-analysis of the effect of clozapine on cognitive functions in patients with treatment-resistant schizophrenia. Schizophr Res. 2023. 10.1016/j.schres.2023.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients,paradigms, andpractical implications. Psychophysiology. 2003;40(5):684–701. [DOI] [PubMed] [Google Scholar]
- 36.Higuchi Y, Sumiyoshi T, Kawasaki Y, et al. Electrophysiological basis for the ability of olanzapine to improve verbal memory and functional outcome inpatients with schizophrenia: a LORETA analysis of P300. Schizophr Res. 2008;101(1–3):320–30. [DOI] [PubMed] [Google Scholar]
- 37.Jindal RD, Pillai AK, Mahadik SP, et al. Decreased BDNF in patients with antipsychotic Naive first episode schizophrenia. Schizophr Res. 2010;119(1–3):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SL, Lee SY, Chang YH, et al. The BDNF, Val66Met polymorphism and plasma brain-derived neurotrophic factor levels in Han Chinese patients with bipolar disorder and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51(9):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizos E, Rontos IE, Arsenis G, et al. Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1308–11. [DOI] [PubMed] [Google Scholar]
- 40.Lee AH, Lange C, Ricken R, et al. Reduced brain-derived neurotrophic factor serum concentrations in acute schizophrenic patients increase during antipsychotic treatment. J Clin Psychopharmacol. 2011;31(3):334–6. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes BS, Steiner J, Berk M, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry. 2014;20(9):1108–19. [DOI] [PubMed] [Google Scholar]
- 42.Xiao W, Ye F, Ma L, et al. Atypical antipsychotic treatment increases glial cell line-derived neurotrophic factor serum levels in drug-free schizophrenic patients along with improvement of psychotic symptoms and therapeutic effects. Psychiatry Res. 2016. 10.1016/j.psychres.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Tunca Z, Kivircik Akdede B, Ozerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30:198–204. [DOI] [PubMed] [Google Scholar]
- 44.Chao CC, Lee EH. Neuroprotective mechanism of glial cell line-derived neurotrophic factor on dopamine neurons: role of antioxidation. Neuropharmacology. 1999;38(6):913–6. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XY, Liang J, Chen da C, et al. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology. 2012;222(2):277–84. [DOI] [PubMed] [Google Scholar]
- 46.Weickert CS, Hyde TM, Lipska BK, et al. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610. [DOI] [PubMed] [Google Scholar]
- 47.Durany N, Michel T, Zöchling R, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52(1):79–86. [DOI] [PubMed] [Google Scholar]
- 48.Knipper M, Da PBM, Blöchl A, et al. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6(4):668–71. [DOI] [PubMed] [Google Scholar]
- 49.Gerlai R, Mcnamara A, Choilundberg DL, et al. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur J Neurosci. 2001;14(7):1153–63. [DOI] [PubMed] [Google Scholar]
- 50.Naumenko VS, Kondaurova EM, Bazovkina DV, et al. Effect of GDNF on depressive-like behavior, Spatial learning and key genes of the brain dopamine system in genetically predisposed to behavioral disorders mouse strains. Behav Brain Res. 2014;274:1–9. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Hou Z, Yuan Y, et al. Association study between plasma GDNF and cognitive function in late-onset depression. J Affect Disord. 2011;132(3):418–21. [DOI] [PubMed] [Google Scholar]
- 52.Pertusa M, Garcia-Matas S, Mammeri H, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29(9):1366–79. [DOI] [PubMed] [Google Scholar]
- 53.Mathis KI, Wynn JK, Jahshan C. An electrophysiological investigation of attentional Blink in schizophrenia: separating perceptual and attentional processes. Int J Psychophysiol Official J Int Organ Psychophysiol. 2012;86(1):108–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the results of this study are available upon request from the corresponding author.