Abstract
When compared with their recently extinct relatives, living lemurs represent a mere fraction of a broad radiation that occupied unique niches in the recent past. Among living lemurs, indrids exhibit the fastest rates of dental development. This dental precocity is tightly correlated with rapid pace of postnatal dental eruption, early replacement of the deciduous teeth, high dental endowment at weaning, and relatively slow somatic growth. This pattern is in stark contrast to that seen in extant lemurids, where somatic development is highly accelerated and dental development is relatively slow. We report on the pace of dental development in one species of palaeopropithecid, the sister group to extant indrids. Like much smaller modern indrids, the chimpanzee-sized Palaeopropithecus ingens was dentally precocious at birth as evidenced by the advanced state of molar crown formation. This finding implies a pattern characteristic of Propithecus and other indrids—rapid dental development despite relatively prolonged gestation. Gestation length in this one species of subfossil lemur was likely greater than 9 months. Our results demonstrate that large body size in primates does not preclude exceedingly rapid dental development.
Keywords: body size‖Palaeopropithecus‖dental development‖incremental markings‖gestation length
The extant lemurs represent only a fraction of the lemur species that occupied Madagascar in the recent past. Skeletal remains of 17 recently extinct (or “subfossil”) lemur species are common in Quaternary deposits across most of the island (1). As a group, subfossil lemurs exhibit diverse ecological, behavioral, and anatomical adaptations, many of which are not represented in extant lemurs (2). One way in which extinct lemurs are strikingly unlike their living relatives is in their larger body size. Body masses of several of the extinct lemurs rivaled those of chimpanzees and orangutans (≈40–75 kg), and the largest, Archaeoindris fontoynontii (≈200 kg), equaled or exceeded that of adult male gorillas (≈175 kg). None of the giant lemurs was smaller than the largest-bodied of living lemurs (Propithecus diadema and Indri indri, 6–7 kg).
Life history parameters—such as growth rates, age at first reproduction, lifespan, and birth rate—generally scale allometrically with size (3–6). Presumably, the large-bodied subfossil lemurs possessed prolonged growth (and slower rates of development), relatively longer life spans, and lower reproductive rates than do their still-extant relatives. It might be posited that slow growth and development, late age at first reproduction, and poor reproductive resilience put large-bodied species at a distinct disadvantage in increasingly disturbed ecological regimes after the arrival of humans on Madagascar (see ref. 7 for a review).
For extinct species, life-history pace must be inferred indirectly from skeletal and dental correlates (8–11). Here, we examine the pace of dental development in Palaeopropithecus ingens, a chimpanzee-sized lemur (and member of the family Palaeopropithecidae, which includes the largest of the extinct lemur species). We ask two questions: First, was the pace of dental development in Palaeopropithecus slow, as one might expect of a large-bodied primate if indeed the pace of life history is strongly affected by body size? Our goal here is to quantify the dental growth rates in a giant lemur by using incremental markers preserved in developing dental hard tissues. Second, to what extent can we draw inferences regarding reproductive parameters such as gestation length in extinct taxa from their pace of dental development? We also look briefly at other indicators of the pace of the life history of Palaeopropithecus and comment on the relationships among the absolute timing of dental development, somatic growth rates, body mass, and gestation length in this remarkable radiation of primates.
Lemur Dental Development
Lemurs can be grouped into seven or eight families, at least two of which are comprised solely of extinct taxa. One of these extinct families, the Palaeopropithecidae (or “sloth lemurs”, including Mesopropithecus, Babakotia, Palaeopropithecus, and Archaeoindris) is the likely sister taxon to extant Indridae (Indri, Propithecus, and Avahi) among Lemuriformes (12, 13).
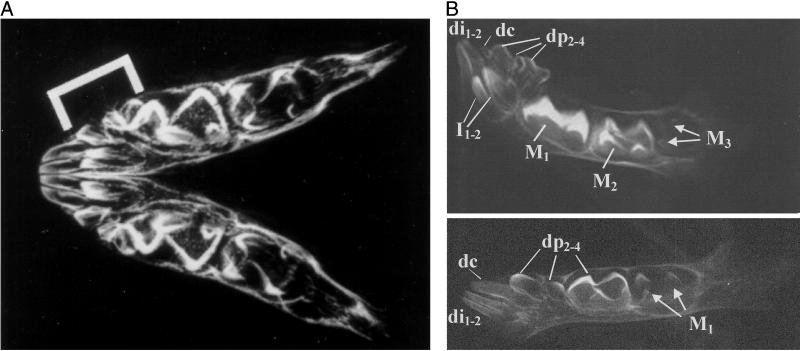
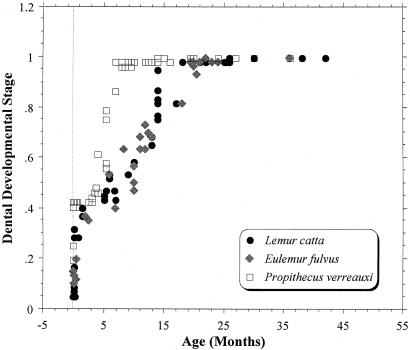
Indrids possess many craniodental features that are unique among extant lemurs, most of which are correlated with an advanced state of dental development at weaning (14, 15). In indrids, all of the deciduous teeth are very small relative to the size of the first permanent molars (ref. 15; Fig. 1A). Moreover, indrids are born with their milk dentition virtually fully erupted and with their M1 and M2 crowns in an advanced state of calcification (Fig. 1B). This early and rapid growth of molar crowns in conjunction with developmentally small jaws results in the confinement and crowding of the deciduous teeth to the front of the neonatal jaw. Thus, molar megadonty is achieved at the expense of the deciduous dentition. Accelerated tooth crown formation in indrid fetuses is associated with an accelerated postnatal dental eruption schedule (14, 15). In sifakas (Propithecus spp.), for example, the first permanent molars begin to erupt around 3 to 4 months of age, and the second molars and first replacement teeth (incisors and posterior premolars) erupt at around 5 months of age, just before weaning. In contrast, the first molars in like-sized macaques erupt at ≈1.5–1.75 years, and the first replacement teeth may erupt up to a year later (see ref. 16 for a compendium). All of the permanent teeth with the exception of the upper canine erupt in Propithecus by around 8 months, whereas a comparable stage of dental development (i.e., with the last of the permanent teeth erupting) is not attained in lemurids until 15 months or older. Without exception, indrids are far ahead of like-sized and smaller-bodied lemurids in their dental developmental and eruption schedule (Fig. 2; refs. 14 and 17; L.R.G., K.E.S., W.L.J., and M. R. Sutherland, unpublished results).
Figure 1.
(A) Radiograph of a mandible of a near-term, captive P. verreauxi from the Natural History Museum (London), specimen no. BMNH 67.1365. The bracket indicates the position of the entire set of deciduous teeth, which are confined to the anterior-most portion of the mandible. M1 and M2 crowns are clearly visible as is the developing crypt for the M3s. (B Upper) Radiograph of a mandible of a newborn (0 days) P. verreauxi coquereli male (Duke University Primate Center specimen DPC 6620M). At birth, the M1 is nearly fully formed, M2 is one-quarter to one-half formed, and the mesial cusps of M3 are visible in the crypt. The deciduous incisors (di1–2) and premolars (dp2–4) are illustrated as are the developing permanent incisors in the region of the symphysis. (Lower) Radiograph of a mandible of a three-day old male Eulemur mongoz (DPC 6537M) showing the deciduous incisors and canine (together comprising the tooth comb) and the anterior deciduous premolars only just erupting. The dp4 is only just one-half crown complete, and only the cusps of the permanent M1 have begun to mineralize.
Figure 2.
Dental developmental stage, which is an index of dental maturity and represents the percentage of teeth (deciduous and permanent) that have erupted at each age relative to the species-specific total number of teeth, vs. age in months.
Because of their close relationship, the Palaeopropithecidae might be expected to share with the Indridae an accelerated schedule of dental development, despite the size discrepancy between the two. Recent research on subfossil lemur cranial ontogeny hints at the presence of accelerated dental development in the Palaeopropithecidae. Godfrey et al. (15) have shown that palaeopropithecids exhibit relatively accelerated dental development vis-à-vis their craniofacial growth. In other words, very small skulls and jaws of some immature palaeopropithecids have a high percentage of their adult teeth erupted. Confirmation of dental acceleration in absolute terms has been elusive, however, because absolute age assessments in fossil specimens are difficult to obtain. Thus, it remains unknown whether Palaeopropithecus shares with like-sized anthropoids a pattern of slow dental growth and development, or with indrids a pattern of dental growth that is accelerated in absolute terms. Evidence in support of the latter would be the following: (i) deciduous P4s that are very small relative to the size of the M1s; (ii) dental precocity at birth; and (iii) short molar crown formation times.
The first criterion is easily recorded from fossils of juveniles preserving mixed deciduous and permanent dentitions. Until very recently, however, no palaeopropithecid fossils preserving deciduous and permanent teeth were known. Several isolated mandibular and maxillary teeth and a fragment of the associated mandible of a P. ingens infant were recently found at Ankilitelo Cave in Southwest Madagascar (DPC 17307). This specimen preserves a fully rooted dp4, M1 (with partial roots), M2 crown (with no roots), P4 crown (with no roots), and M1 crown (also with no roots). At death, this individual would have possessed its deciduous dentition, and an erupting mandibular (but not maxillary) first molar. As a result of this discovery, we now know that Palaeopropithecus had a low dp4/M1 length ratio (57.8)—slightly above the mean for indrids. (Indrid species' mean range = 41.2–45.2; lemurid species' mean range = 96.0–105.3; see ref. 15).
Demonstrating dental precocity at birth and an accelerated molar crown formation schedule requires histological evidence from developing dental tissues. The component hard tissues of teeth (in particular, enamel and dentine) preserve a permanent record of their growth in the form of incremental markings that are clearly seen in histological sections. These histological markers consist of short- (i.e., daily) and long-period lines that can be used to chart with unparalleled accuracy important life history variables such as the sequence and rate of dental development, the timing of birth, and the age at death in juveniles. Only attrition, abrasion, and erosion can alter teeth once they are formed so that direct evidence for the timing of important developmental events is available from even fragmentary dental remains in the fossil record. Because dental histology provides information on absolute age, it can be used to test aspects of relative dental precocity and dental endowment in subfossil lemurs. In particular, we test the following question: Is Palaeopropithecus dentally precocious at birth—i.e., is its M1 crown virtually complete and is M2 only slightly less so at birth, as in extant Propithecus? Are molar crown formation times short given body/tooth size reconstructions of P. ingens? More generally, what do these data imply about life history (e.g., gestation length) and the relationship between somatic and dental development in a giant strepsirrhine?
Materials and Methods
The molar series, M1–3, of a single juvenile P. ingens specimen from Ankazoabo Cave in southwestern Madagascar (University of Antananarivo AM-PPH1) was chosen for analysis.** Body mass of an adult P. ingens has been estimated on the basis of long bone circumferences at approximately 45 kg. The molars of UA-AM-PPH1 were carefully extracted from their maxillary alveoli. They were then cleaned, and molds were prepared by using Coltene (Mahwah, NJ) silicon medium body putty. Before sectioning, each specimen was embedded in polyester resin or coated with cyanoacrylate to reduce the risk of splintering. By using a Buehler (Lake Bluff, NY) Isomet diamond wafering blade saw, 180- to 200-μm-thick longitudinal ground sections were prepared. The sections were mounted to microscope slides, lapped to a final thickness of 100–120 μm, polished with a 3 μm aluminum powder, placed in an ultrasonic bath to remove surface debris, dehydrated through a graded series of alcohol baths, cleared in Histoclear (Fisher), and mounted with cover slips in xylene-based DPX (Sigma-Aldrich) mounting medium. A total of 11 sections were taken from planes across, and contiguous to, the principal cusps, including the entire buccal and lingual cervical walls, thereby encompassing the entire period of crown formation.
Short-period (i.e., daily cross striations) and long-period (i.e., striae of Retzius) lines were clearly visible throughout the enamel (Fig. 3). Both types of incremental markings were used to measure daily enamel secretion rates (DSRs) and total crown formation times (CFTs) for each tooth. Daily enamel secretion rate was measured as the linear distance along the long axis of an enamel prism corresponding to 5 days worth of secretion; dividing by 5 yielded the average amount of enamel secreted daily. Total CFT was divided into cuspal enamel formation time plus lateral enamel formation times. Cuspal times were determined directly by counting the number of days it takes for one ameloblast to traverse the distance from the dentine horn to the cusp tip. Lateral enamel formation times were determined by counting the total number of striae of Retzius in the lateral enamel and multiplying by the periodicity, or the number of cross striations between adjacent striae. Retzius lines run obliquely across enamel prism boundaries and thus mark a discontinuity in enamel structure (each stria in the lateral enamel is continuous with one perikyma on the outer enamel surface). Determining total CFTs in this way was repeated for each molar to calculate both the rate and duration of growth for each developing tooth and the total amounts of pre- and postnatal tooth growth (see ref. 18).
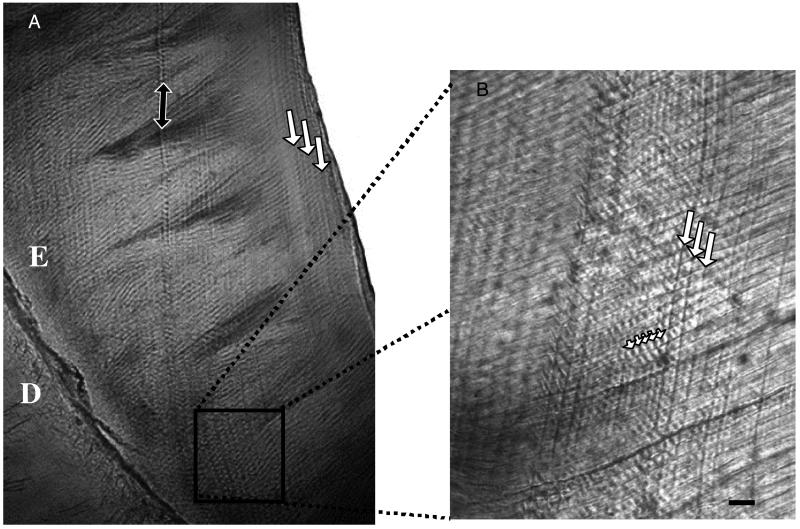
Figure 3.
(A) Long-period lines (striae of Retzius, large white arrows) in lateral enamel of a young P. ingens from the cave of Ankazoabo, southwest Madagascar, in the collections of the University of Antananarivo (AM-PPH1). E, Enamel; D, dentine. Black arrow indicates location of the neonatal line. (B) Daily lines (small white arrowheads) in between adjacent striae (large white arrows) in cervical enamel of P. ingens (AM-PPH1). Enamel prisms run from bottom left to top right. Scale bar = 10 μm.
To create a chronology (i.e., bar chart) of molar development, each molar had to be registered in time-to-zero-days development, and to one another. The former was accomplished by charting the position of the neonatal line whereas the latter required information on the timing of accentuated striae. The neonatal line is a prominent Retzius line that coincides with birth (19, 20). Accentuated striae of Retzius mark brief periods of disruption in enamel and dentine matrix secretion. As they are recorded in all teeth developing at a particular point in time, they are used to register all teeth developing at the same point in time to one another (18, 21).
Results
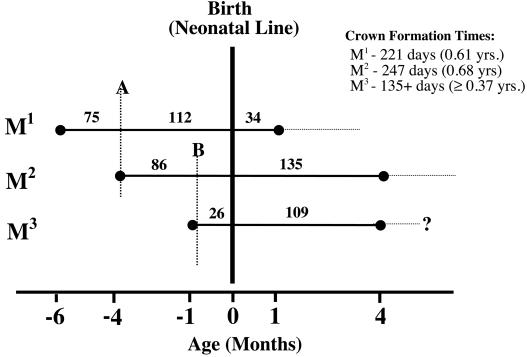
Data on daily enamel secretion rates, CFTs, and the timing of accentuated striae were collated to provide an overall chronology of molar development in Palaeopropithecus. To our initial surprise, the neonatal line was present in all three molars (see Fig. 3B). By using this line as a starting point and aided by the timing of two prominent accentuated striae (lines A and B in Fig. 4), it was determined that M1 started formation at 187 days before birth. The periodicity in P. ingens is 2; mean values for periodicity range from 6 to 11 in similarly sized extant hominoids (22) and from 2 to 4 in extant lemurs (G.T.S., unpublished data). Crown formation times are as follows: M1, 0.61 yr; M2, 0.68 yr; M3, ≥0.37 yr (Fig. 4). These data yield postnatal ages at M1–3 crown completion of 0.09, 0.37, and 0.30 yr, respectively. Molar crown development was complete in this specimen by 0.37 yr. The degree of sequential molar overlap is great, given the relatively short overall period devoted to molar crown formation. As a result, all molar teeth are initiated prenatally.
Figure 4.
Bar chart of dental development in P. ingens. Solid lines indicate crown formation times, and dashed horizontal lines are estimates of root formation times (not based on data). Vertical dashed lines, A and B, indicate the position of two prominent accentuated striae occurring at 112 days (0.31 yr) and 25 days (0.07 yr) prenatally, respectively. Cervical margin of M3 slightly broken so that the reported CFT is a slight underestimation.
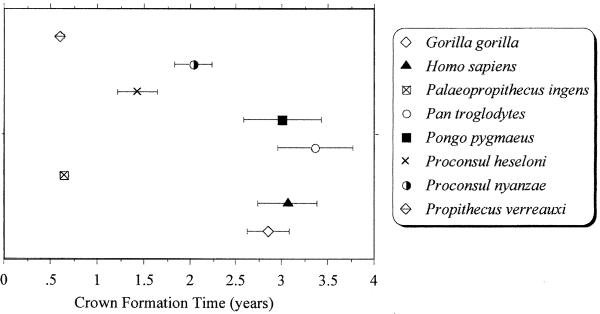
Anterior (M1–2) molar crown formation times are extremely short in P. ingens (mean = 0.65 ± 0.05 yr) compared with other primates that possess similar ranges of body and/or molar size (Fig. 5).‡‡ Anterior molar CFTs for hominoid taxa are as follows: Pan, mean = 3.36 ± 0.41 yr (range = 2.78–4.25 yr); Gorilla, mean = 2.86 ± 0.23 yr (range = 2.60–3.21 yr); Pongo, mean = 3.00 ± 0.42 yr (range = 2.72 = 3.30 yr); Homo, mean = 3.06 ± 0.33 yr (range = 2.67–3.62 yr). Despite size differences, anterior molar CFTs in P. ingens are as fast as that in Propithecus verreauxi (0.61 ± 0.04 yrs). P. ingens achieves such fast growth in such a relatively short period by possessing some of the highest daily rates of cuspal enamel secretion for all primates (e.g., Pan, mean = 4.11 ± 0.49 μm; Pongo, mean = 6.24 ± 1.02 μm; P. ingens, mean = 6.76 ± 1.48 μm). Altogether, these data support the notion that, like modern indrids, certain subfossil lemurs were dentally precocious at birth. In particular, P. ingens shares with extant Propithecus the same pattern of dental precocity as evidenced by the advanced state of crown formation of permanent molars at birth.
Figure 5.
Anterior molar crown formation times for selected extant and extinct lemurs and hominoids. P. verreauxi specimen, BMOC 88, from the Beza Mahafaly Osteological Collection.
Our data also demonstrate that gestation length in Palaeopropithecus was greater than 187 days (6.23 months or 0.50 yr). In anthropoid primates, the onset of mineralization of deciduous teeth does not occur until the late stages of embryological development, and M1 crown mineralization normally begins late in the third trimester of gestation. Late mineralization of deciduous and permanent teeth does not characterize extant Propithecus. Gestation length in P. verreauxi is 158 days, and 179 days in P. diadema (cf. 102–136 days for the largest-bodied lemurids), and the state of molar crown formation in both species is advanced at birth (see Fig. 1B; refs. 23–29). In P. verreauxi, M1 erupts at 108–124 days (3.5–4.0 months) of postnatal age. An M1 CFT of 0.61 yr (221 days) thereby implies an M1 crown initiation time of at least 98 to 113 days before birth (this result is corroborated by the identification of a neonatal line ≈98–104 days after the initiation of M1; G.T.S., unpublished observations); i.e., molar crown formation begins at the end of the first or beginning of the second trimester in extant P. verreauxi. Even if, in Palaeopropithecus, M1 crown mineralization began at the end of the first trimester (as it does in Propithecus), gestation length would have had to have been greater than 9 months. This number exceeds gestation length in chimpanzees, orangutans, gorillas, and even humans. Again, Palaeopropithecus shows a pattern characteristic of Propithecus—rapid dental development despite relatively prolonged gestation.
Discussion
On the basis of their large body size, one might predict that giant extinct lemurs should exhibit slow dental development. One might also expect anthropoid scaling relationships between dental development and reproductive maturation to hold for giant lemurs, thus allowing paleontologists to use the pace of dental development inferred for extinct lemurs to predict other aspects of their life histories (i.e., reproductive characteristics).
Our data support neither supposition. We have shown that P. ingens exhibited extremely rapid and early molar crown formation—a pattern that parallels its living indrid relatives and not like-sized anthropoids. Like living indrids, Palaeopropithecus exhibited molar megadonty, small deciduous teeth and low dp4/M1 occlusal length ratios, a decoupling of the pace of dental and somatic growth with acceleration of dental development relative to somatic growth, and prolonged gestation. Recent field research on living indrids verifies that they reproduce exceedingly slowly (30, 31)—in stark contrast to lemurids. Lemurids tend to grow faster and reach reproductive maturity earlier than indrids, despite their far slower dental development (L.R.G., K.E.S., W.L.J., and M. R. Sutherland, unpublished results). For instance, some lemurids achieve nearly 70% of adult weight by the age of weaning, as opposed to the mere 20% of adult weight attained at weaning for certain indrids (30). Dental precocity in extant indrids is associated with relatively slow somatic growth. This result is in stark contrast to extant lemurids, where somatic development is highly accelerated and dental development is relatively slow.
Eaglen (17) was the first to explore the ecological correlates of variation in the timing of dental eruption among extant lemurs. Because most lemur species grow in cohorts (because of reproductive synchrony), selection can fine-tune the timing of the eruption of particular teeth to coincide with particular phenological events. Weaning itself is timed to correspond to the season of greatest availability of young leaves and other foods (30–32). Selection operates on dental development independently of the development of the rest of the skeleton so as to guarantee masticatory proficiency at weaning and during the first postweaning dry season (refs. 14 and 15; L.R.G., K.E.S., W.L.J., and M. R. Sutherland, unpublished results). At weaning, indrids are equipped with their first and (often) second molars, as well as their adult incisors (including the toothcomb), and, sometimes, their adult posterior premolars. In every indrid species, the posterior premolars erupt shortly after weaning, if not before. Lemurid weanlings, in contrast, may lack their first molars, and have not yet shed any of their deciduous teeth. The summed postcanine occlusal area (expressed as a proportion of adult postcanine occlusal area) is therefore much higher at weaning in indrids than in lemurids (33). The small size of indrid deciduous teeth does not compromise food processing at weaning because these teeth are shed during or just before the process of weaning. Given its extreme dental precocity, it seems probable that the diminutive deciduous premolars of Palaeopropithecus were shed early, as in extant indrids.
It is likely that Palaeopropithecus and other giant lemurs of Madagascar did reproduce slowly; this is certainly the case for the largest-bodied living lemurs (Indri and Propithecus). However, this finding does not imply that all other aspects of their development and life histories parallel those seen in large-bodied anthropoids. Rather, our data demonstrate that large body size in primates does not preclude exceedingly rapid dental development (even if somatic development was slow and gestation was long). Our data reaffirm the importance of extracting real developmental time in extinct primates via incremental lines in dental hard tissues. They show how dental microstructure can provide clues to dissociated aspects of the life histories of extinct species (prolonged gestation, rapid crown formation). Finally, they provide important developmental support for a phylogenetic hypothesis (sister-taxon status for indrids and sloth lemurs) developed on the basis of other anatomical data and ancient DNA.
Acknowledgments
The authors would like to thank Chris Dean, Mitchell Irwin, Jay Kelley, Lawrence Martin, Stephen Nash, Don Reid, Berthe Rakotosamimanana, Gisèle Francine Noro Randria, and two anonymous reviewers. The University of Antananarivo kindly provided access to the P. ingens specimen UA-AM-PPH1, and Alison Richard kindly loaned us the P. verreauxi specimen, BMOC 88. Fig. 1 was produced with the generous assistance of Deryck Jones (Museum of Natural History, London), David Haring (Duke University), Terry Button (Radiology Department, Stony Brook University), Lawrence Martin (Anthropology Department, Stony Brook University), and Luci Betti-Nash (Anatomical Sciences, Stony Brook University). Fred Grady (National Museum of Natural History, Washington, DC) provided assistance with molding and casting. Funding was provided by a Smithsonian Institution Postdoctoral Fellowship (G.T.S.), National Science Foundation (NSF) Grant GER 9450175 (L.R.G.), and NSF Grant SBR-9630350 (E.L.S.). This paper is Duke University Primate Center publication no. 740.
Abbreviation
- CFT
crown formation time
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
The southwestern variety of this genus is typically somewhat smaller than its congener found on the high plateau (1).
Values for M3 not used in determining mean molar CFTs as this molar is very reduced in size in P. ingens, as it is in all members of the palaeopropithecid-indrid clade.
References
- 1.Godfrey L R, Jungers W L. In: The Primate Fossil Record. Hartwig W, editor. Cambridge, U.K.: Cambridge Univ. Press; 2002. pp. 97–121. [Google Scholar]
- 2.Jungers W L, Godfrey L R, Simons E L, Wunderlich R E, Richmond B G, Chatrath P S. In: Reconstructing Behavior in the Primate Fossil Record. Plavcan J M, Kay R F, Jungers W L, van Schaik C P, editors. New York: Kluwer; 2002. pp. 371–411. [Google Scholar]
- 3.Jarman P J. Behaviour. 1974;48:215–267. [Google Scholar]
- 4.Clutton-Brock T H, Harvey P H. J Zool London. 1977;183:1–39. [Google Scholar]
- 5.Western D. Afr J Ecol. 1975;17:185–204. [Google Scholar]
- 6.Harvey P H, Clutton-Brock T H. Evolution. 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 7.Burney D A. In: Extinctions in Near Time: Causes, Contexts, and Consequences. MacPhee R, editor. New York: Plenum; 1999. pp. 145–164. [Google Scholar]
- 8.Smith BH. Evolution. 1989;4:683–688. doi: 10.1111/j.1558-5646.1989.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith B H. Am J Phys Anthropology. 1991;86:157–174. [Google Scholar]
- 10.Conroy G C, Kuykendall K L. Am J Phys Anthropol. 1995;98:121–131. doi: 10.1002/ajpa.1330980203. [DOI] [PubMed] [Google Scholar]
- 11.Beynon A D, Dean M C, Leakey M G, Reid D J, Walker A C. J Hum Evol. 1998;35:163–209. doi: 10.1006/jhev.1998.0230. [DOI] [PubMed] [Google Scholar]
- 12.Jungers W L, Godfrey L R, Simons E L, Chatrath P S, Rakotosamimanana B. Proc Natl Acad Sci USA. 1991;88:9082–9086. doi: 10.1073/pnas.88.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoder A D, Rakotosamimanana B, Parsons T. In: New Directions in Lemur Studies. Rakotosamimanana B, Rasamimanana H, Ganzhorn J U, Goodman S M, editors. New York: Plenum; 1999. pp. 1–17. [Google Scholar]
- 14.Godfrey L R, Samonds K E, Jungers W L, Sutherland M R. Am J Phys Anthropol. 2001;114:192–214. doi: 10.1002/1096-8644(200103)114:3<192::AID-AJPA1020>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Godfrey L R, Petto A J, Sutherland M R. In: Reconstructing Behavior in the Primate Fossil Record. Plavcan J M, Kay R F, Jungers W L, van Schaik C P, editors. New York: Kluwer; 2002. pp. 113–157. [Google Scholar]
- 16.Smith B H, Crummett T L, Brandt K L. Ybk Phys Anthropol. 1994;37:177–231. [Google Scholar]
- 17.Eaglen R H. Am J Phys Anthropol. 1985;66:307–315. [Google Scholar]
- 18.Reid D J, Schwartz G T, Dean M C, Chandrasekera M S. J Hum Evol. 1998;35:427–448. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- 19.Rushton M A. Dental Record. 1933;53:170–171. [Google Scholar]
- 20.Schour I. J Am Dent Assoc. 1936;23:1946–1955. [Google Scholar]
- 21.Hillson S. Dental Anthropology. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 22.Schwartz G T, Reid D J, Dean M C. Int J Primatol. 2001;22:837–860. [Google Scholar]
- 23.Brockman D K, Willis M S, Karesh W B. Zoo Biol. 1987;6:343–369. [Google Scholar]
- 24.Colquhoun I C. In: Lemur Social Systems and Their Ecological Basis. Kappeler P M, Ganzhorn J U, editors. New York: Plenum; 1993. pp. 11–24. [Google Scholar]
- 25.Doyle G A. In: The Study of Prosimian Behavior. Doyle G A, Martin R D, editors. New York: Academic; 1979. pp. 158–206. [Google Scholar]
- 26.Foerg R. Folia Primatol. 1982;38:108–121. doi: 10.1159/000156047. [DOI] [PubMed] [Google Scholar]
- 27.Kappeler P M, Ganzhorn J U. Evol Anthropol. 1993;2:159–171. [Google Scholar]
- 28. Mittermeier, R. A., Tattersall, I., Konstant, B., Meyers, D. M. & Mast, R. B., eds. Lemurs of Madagascar (Conservation International, Washington, DC).
- 29.Wright P C. Int J Primatol. 1996;16:835–854. [Google Scholar]
- 30.Wright P C. Ybk Phys Anthropol. 1999;42:31–72. doi: 10.1002/(sici)1096-8644(1999)110:29+<31::aid-ajpa3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Richard A F, Dewar R E, Schwartz M, Ratsirarson J. J Zool London. 2002;256:421–436. [Google Scholar]
- 32.Meyers D M, Wright P C. In: Lemur Social Systems and Their Ecological Basis. Kappeler P M, Ganzhorn J U, editors. New York: Plenum; 1993. pp. 179–192. [Google Scholar]
- 33.Samonds K E, Godfrey L R, Jungers W L, Martin L B. Am J Phys Anthropol. 1999;28,Suppl.:238–239. [Google Scholar]