Abstract
Background
To develop a nomogram for predicting overall survival (OS) and cancer-specific survival (CSS) in patients with postoperative early-stage (pT1-2N0M0) tongue squamous cell carcinoma (TSCC), and to explore the association between postoperative radiotherapy (PORT) and patient survival.
Methods
Data from 7,637 patients with pT1-2N0M0 TSCC who underwent surgery between 2000 and 2021 were extracted from the SEER database. Patients were randomly divided into a training cohort and a validation cohort in a 2:1 ratio. Prognostic factors were identified via Kaplan-Meier analysis and Cox regression, and a nomogram was constructed. To minimize confounding, propensity score matching (PSM) was used to compare outcomes between patients who received PORT and those who did not. Subgroup and interaction analyses were performed to assess potential effect modifiers.
Results
Of the 7,637 patients included, 1,336 (17.5%) received PORT. Multivariate Cox analysis identified age, race, marital status, grade, tumor size, lymph node (LN) removed status, and PORT as independent prognostic factors for OS and CSS. The nomogram demonstrated strong predictive performance based on time-dependent ROC curves, concordance indices, calibration plots, and decision curve analyses in both training and validation cohorts. After PSM, PORT remained associated with worse OS and CSS. Subgroup analyses revealed that the association between PORT and poorer OS was most evident in younger patients, married individuals, T1 stage patients, those with smaller tumors, and those without LN removal, with racial disparities also observed. For CSS, this association was more pronounced in married individuals, well-differentiated patients, T1 stage patients, those with smaller tumors, and those without LN removal.
Conclusion
The SEER-based nomogram provides survival predictions for postoperative pT1-2N0M0 TSCC patients. Although PORT was associated with worse survival in several subgroups, findings should be cautiously interpreted given the observational design and absence of key clinical variables (PNI, LVI, surgical margins). Prospective studies incorporating comprehensive clinicopathological data are warranted to confirm associations and guide individualized PORT decisions in early-stage TSCC patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-025-03883-2.
Keywords: Tongue squamous cell carcinoma (TSCC), Survival analysis, Nomogram, Postoperative radiotherapy (PORT), SEER
Introduction
Oral cancer is a common type of head and neck malignant tumor, with squamous cell carcinoma (SCC) accounting for approximately 90% of cases [1]. In 2022, there were 389,846 new cases of lip and oral cancer globally, ranking it as the 16th most common cancer, with 188,438 related deaths, ranking it 15th [2]. Tongue squamous cell carcinoma (TSCC) is one of the most prevalent forms of oral cancer, representing about 25–50% of all oral cancer cases [3, 4]. In 2020, nearly 200,000 cases of tongue cancer were diagnosed worldwide [4]. Notably, the incidence of TSCC has been shifting toward a younger demographic, with a significant rise in the number of female patients [5, 6]. Despite advances in detection and treatment, even early-stage TSCC often has a poorer prognosis compared to SCC of other oral sites [7, 8].
The primary treatment for early-stage TSCC is typically surgery [9], and many studies also recommend elective lymph node dissection to prevent or manage potential lymph node metastasis [10–13]. In view of the highly aggressive nature and recurrence risk of TSCC, the National Comprehensive Cancer Network (NCCN) guidelines suggest that some early-stage patients receive postoperative radiotherapy (PORT) [14]. However, the benefit of PORT for the prognosis of these patients remains controversial. Some studies indicate that PORT significantly improves disease outcomes in early-stage TSCC patients who present certain mildly adverse pathological features, such as poor differentiation, perineural invasion (PNI), lymphovascular invasion (LVI), or a depth of invasion (DOI) greater than 5 mm [9]. A retrospective study involving 528 patients further supports this view, demonstrating that patients with these characteristics benefited notably from PORT [3]. Conversely, other research suggests that PORT does not confer a significant survival benefit for early-stage TSCC patients, regardless of tumor DOI [15, 16]. Additionally, some data reveal no statistically significant differences in survival and local recurrence rates between early-stage TSCC patients who received PORT and those who did not [17]. Given that the prognosis of postoperative early-stage TSCC patients is influenced by multiple complex factors and remains unclear, identifying these risk factors is crucial for guiding clinical decision-making, optimizing treatment strategies, and improving patient survival prognosis [18].
Therefore, this study utilized a large-scale retrospective cohort from the SEER database to analyze survival patterns in patients with postoperative early-stage (pT1-2N0M0) TSCC and to identify key prognostic factors. We constructed a nomogram based on these factors to predict overall survival (OS) and cancer-specific survival (CSS) at 1, 3, 5, and 8 years. Using propensity score matching (PSM), we examined the association between PORT and survival outcomes and identified potential patient subgroups that may be differentially affected by PORT. Given the absence of critical clinical variables such as PNI, LVI, and surgical margin status, our findings provide observational evidence that requires cautious interpretation but may still guide individualized treatment planning in this low-risk population.
Materials and methods
Data source and data extraction
All patients in this study were sourced from the Surveillance, Epidemiology, and End Results (SEER) database (www.seer.cancer.gov). The patients were diagnosed with TSCC as their first primary malignancy, based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) and confirmed through pathological examination. Clinical and pathological data for patients from 17 registries, spanning from 2000 to 2021, were downloaded using SEER*Stat software version 8.4.3 (November 2023 submission). The collected clinical and pathological information included primary site, histologic type (ICD-O-3), age, sex, race, marital status, diagnostic confirmation, grade, TNM stage, tumor size, surgery, scope of regional lymph node removed, radiotherapy, radiation sequence with surgery, cause-specific death classification, survival status, and survival time.
Inclusion and exclusion criteria
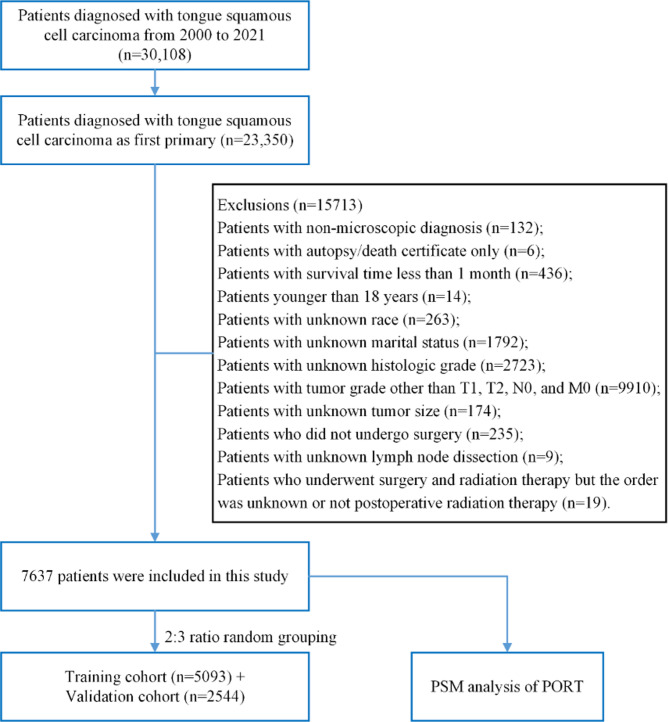
The inclusion criteria were as follows: (1) Primary sites: C02.0, C02.1, C02.2, C02.3, C02.8, C02.9; (2) Histologic type (ICD-O-3): 8050–8076, 8078, 8083, 8084, 8094; (3) Patients confirmed by microscopic examination; (4) Survival time > 1 month; (5) Age > 18 years; (6) TNM status (T: T1, T2; N: N0; M: M0); (7) Patients who underwent surgical treatment. The exclusion criteria were as follows: (1) Patients whose diagnoses were obtained solely from death certificates or autopsy reports; (2) Patients with unknown race, marital status, grade, tumor size, or scope of regional lymph node removed; (3) Patients with unknown surgery-radiation sequence or those who did not receive PORT if both surgery and radiotherapy were performed.
For patients from 2000 to 2017, histologic grade was determined using the “Grade Recode thru 2017” in the SEER database. For patients from 2018 to 2021, histologic grade was classified according to the Grade Manual (https://apps.naaccr.org/ssdi/list/). Pathological TNM status was determined based on the AJCC staging manual (6th edition) for patients from 2000 to 2016, SEER*RSA for patients from 2016 to 2017, and the most recent version of EOD (https://staging.seer.cancer.gov/) for patients from 2018 to 2021.
The primary outcomes of this study were OS and CSS. OS was defined as the time from diagnosis to death from any cause or the last follow-up, and CSS was defined as the time from diagnosis to death due to TSCC or the last follow-up.
Figure 1 illustrates the screening process of the study cohort.
Fig. 1.
Flow chart of the study cohort
Statistical analysis
Categorical variables were expressed as frequencies and percentages and analyzed using the chi-square test. For continuous variables, if the data were normally distributed with equal variance between groups, they were presented as the mean and standard deviation and analyzed using the t-test. If the data were non-normally distributed, they were reported as the median and interquartile range (IQR) and analyzed using the Mann-Whitney U test.
The main manuscript focuses on analyses based on propensity score-matched cohorts. Analyses performed prior to matching are described in detail in the Supplementary Methods section of the Supplementary Materials.
To evaluate the impact of PORT on the prognosis of pT1-2N0M0 TSCC patients, we performed PSM to balance potential confounding factors between patients who received PORT and those who did not. Propensity scores for each patient were calculated using binary logistic regression, and nearest-neighbor matching was applied with a caliper of 0.03, employing a 1:1 matching ratio without replacement. For the entire cohort, covariates included age, sex, race, marital status, grade, T stage, tumor size, and lymph node (LN) Removed. When analyzing data that included depth of invasion (DOI), we added DOI as an additional covariate. Since only patients from 2018 to 2021 had DOI data, the PSM analysis considering DOI was limited to these patients. After matching, Kaplan-Meier (K-M) survival curves were used to analyze the impact of PORT on the prognosis of pT1-2N0M0 TSCC patients before and after matching. To identify independent prognostic factors in the matched cohort, Cox regression analysis was conducted. Variables with a p-value < 0.1 in the univariate Cox regression were included in the multivariate model.
To further investigate potential reasons for unfavorable survival outcomes with PORT, we conducted stratified PSM analyses based on specific variables: age, grade, LN Removed, combined factors (grade and LN Removed), and DOI. To accurately identify patients who exhibit the most significant response to PORT, we carried out a detailed subgroup analysis, examining the interaction and association between various variables and PORT treatment.
All analyses were performed using R version 4.1.2, and a two-sided p-value < 0.05 was considered statistically significant.
Results
Detailed analyses conducted prior to propensity score matching, including baseline characteristics, K-M survival analysis, univariate and multivariate Cox regression analyses, and nomogram development and validation, are provided in the Supplementary Materials.
Baseline characteristics analysis
The SEER cohort included 7,637 pT1-2N0M0 TSCC patients (training: 5,093; validation: 2,544), with balanced demographics between cohorts (all p > 0.05; see Supplementary Materials: Table S1 for detailed characteristics).
Analysis of prognostic factors for OS and CSS in postoperative pT1-2N0M0 TSCC patients
Multivariate Cox analysis identified age, gender, race, marital status, grade, tumor size, lymph node (LN) removed status, and PORT as independent prognostic factors for OS (all p < 0.05). All except sex were also associated with CSS. PORT was associated with worse survival outcomes (OS: HR = 1.254, 95% CI:1.104–1.424; CSS: HR = 1.559, 95% CI:1.316–1.846), with detailed subgroup analyses provided in Supplementary Materials: Tables S2-S3 and Figures S1-S2.
Prognostic nomogram for postoperative pT1-2N0M0 TSCC: development and validation
Nomograms were developed to predict 1-, 3-, 5-, and 8-year survival probabilities, demonstrating good calibration and discrimination. Detailed construction and validation processes are described in Supplementary Materials: Figures S3-S5.
Characteristics of postoperative pT1-2N0M0 TSCC patients before and after PSM
To further investigate the role of PORT in postoperative pT1-2N0M0 TSCC patients, a PSM analysis was performed on the entire cohort based on PORT status. Before PSM, younger patients (< 50 years) were more likely to receive PORT, whereas patients aged ≥ 80 years were less likely to receive it (p < 0.05). Additionally, a higher proportion of Black patients received PORT (p < 0.05). Patients in the PORT group also exhibited poorer tumor differentiation, larger tumor sizes, and a higher proportion of LN removed (all p < 0.05). No significant differences were observed between the two groups regarding gender or marital status (p > 0.05).
After PSM, the distribution of these variables achieved a good balance (p > 0.05). In the matched cohort, 840 patients (65.2%) in the non-PORT group and 874 patients (67.8%) in the PORT group underwent LN removal. Detailed demographic and clinical characteristics before and after PSM are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the whole population before and after PSM
| Variables | Before PSM (N = 7637) | p-value | After PSM (N = 2578) | p-value | ||
|---|---|---|---|---|---|---|
| None/ Unknown |
PORT | None/ Unknown |
PORT | |||
| Number | 6301 | 1336 | 1289 | 1289 | ||
| Age(years), No. (%) | < 0.001 | 0.995 | ||||
| < 50 | 1152 (18.3) | 338 (25.3) | 312 (24.2) | 303 (23.5) | ||
| 50–59 | 1566 (24.9) | 333 (24.9) | 321 (24.9) | 322 (25.0) | ||
| 60–69 | 1720 (27.3) | 366 (27.4) | 358 (27.8) | 364 (28.2) | ||
| 70–79 | 1163 (18.5) | 216 (16.2) | 215 (16.7) | 218 (16.9) | ||
| ≥ 80 | 700 (11.1) | 83 (6.2) | 83 (6.4) | 82 (6.4) | ||
| Sex, No. (%) | 0.075 | 0.55 | ||||
| Male | 3399 (53.9) | 757 (56.7) | 731 (56.7) | 747 (58.0) | ||
| Female | 2902 (46.1) | 579 (43.3) | 558 (43.3) | 542 (42.0) | ||
| Race, No. (%) | 0.041 | 0.227 | ||||
| White | 5365 (85.1) | 1121 (83.9) | 1079 (83.7) | 1109 (86.0) | ||
| Black | 154 (2.4) | 49 (3.7) | 48 (3.7) | 45 (3.5) | ||
| Other | 782 (12.4) | 166 (12.4) | 162 (12.6) | 135 (10.5) | ||
| Marital, No. (%) | 0.724 | 0.974 | ||||
| Single | 1095 (17.4) | 229 (17.1) | 225 (17.5) | 221 (17.1) | ||
| Married | 3942 (62.6) | 826 (61.8) | 798 (61.9) | 803 (62.3) | ||
| Other | 1264 (20.1) | 281 (21.0) | 266 (20.6) | 265 (20.6) | ||
| Grade, No. (%) | < 0.001 | 0.358 | ||||
| I | 2345 (37.2) | 232 (17.4) | 231 (17.9) | 210 (16.3) | ||
| II | 3327 (52.8) | 830 (62.1) | 815 (63.2) | 849 (65.9) | ||
| III/IV | 629 (10.0) | 274 (20.5) | 243 (18.9) | 230 (17.8) | ||
| T stage, No. (%) | < 0.001 | 0.497 | ||||
| T1 | 4699 (74.6) | 526 (39.4) | 526 (40.8) | 544 (42.2) | ||
| T2 | 1602 (25.4) | 810 (60.6) | 763 (59.2) | 745 (57.8) | ||
| Tumor Size | < 0.001 | 0.887 | ||||
| Median (IQR) | 13.0 (12.0) | 21.0 (15.0) | 21.0 (13.0) | 21.0 (13.0) | ||
| >LN removed, No. (%) | < 0.001 | 0.169 | ||||
| None | 3243 (51.5) | 454 (34.0) | 449 (34.8) | 415 (32.2) | ||
| Yes | 3058 (48.5) | 882 (66.0) | 840 (65.2) | 874 (67.8) | ||
Note: LN: Lymph nodes; PORT: Postoperative Radiotherapy
Role of PORT in the full population cohort
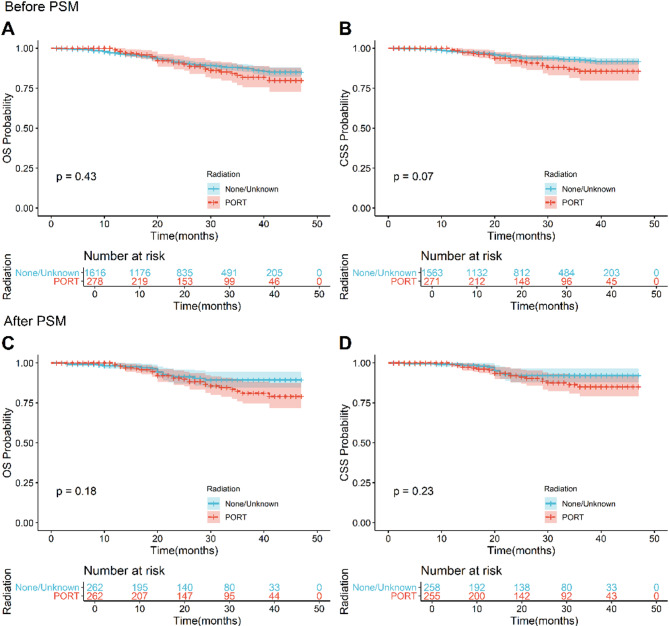
To compare the impact of PORT on the prognosis of postoperative pT1-2N0M0 TSCC patients, we analyzed the survival differences between those who received PORT and those who did not, both before and after PSM. Before PSM, the 5-year OS and CSS were significantly higher in the non-PORT group (78.3% [95% CI: 77.2-79.5%] and 85.9% [95% CI: 84.9-87.0%], respectively) compared to the PORT group (65.1% [95% CI: 62.4-67.9%] and 68.0% [95% CI: 65.1-71.0%], respectively). After PSM, this trend persisted, with the non-PORT group exhibiting superior 5-year OS and CSS (75.5% [95% CI: 73.0-78.1%] and 81.3% [95% CI: 78.8-83.9%], respectively) relative to the PORT group (64.9% [95% CI: 62.2-67.8%] and 67.7% [95% CI: 64.8-70.9%], respectively). K-M survival analysis further supported these findings, indicating that PORT was associated with decreased OS and CSS (p < 0.0001; Figs. 2A-D).
Fig. 2.
Before and after PSM, K-M survival curves compare OS and CSS between PORT patients and non-PORT patients. Before PSM: (A) OS, (B) CSS. After PSM: (C) OS, (D) CSS
In the PSM-adjusted cohort, univariate and multivariate Cox regression analyses demonstrated that PORT was independently associated with poorer OS (HR = 1.335, 95% CI: 1.173–1.519) and CSS (HR = 1.676, 95% CI: 1.411–1.989), even after adjusting for confounding factors (Tables 2 and 3).
Table 2.
Univariate and multivariate analyses of OS in the whole population cohort after PSM
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
| Age(years) | < 0.0001 | ||||
| < 50 | Reference | Reference | |||
| 50–59 | 1.569(1.264,1.948) | 1.547(1.244,1.924) | < 0.0001 | ||
| 60–69 | 2.170(1.770,2.659) | 2.010(1.630,2.477) | < 0.0001 | ||
| 70–79 | 3.197(2.581,3.961) | 2.960(2.369,3.699) | < 0.0001 | ||
| ≥ 80 | 5.252(4.080,6.761) | 4.560(3.501,5.939) | < 0.0001 | ||
| Sex | 0.478 | ||||
| Male | Reference | - | |||
| Female | 1.048(0.921,1.192) | - | - | ||
| Race | 0.350 | ||||
| White | Reference | - | |||
| Black | 1.218(0.890,1.667) | - | - | ||
| Other | 0.923(0.744,1.145) | - | |||
| Marital | < 0.0001 | ||||
| Single | Reference | Reference | |||
| Married | 0.916(0.766,1.096) | 0.773(0.644,0.928) | 0.006 | ||
| Other | 1.486(1.217,1.814) | 0.970(0.787,1.196) | 0.777 | ||
| Grade | < 0.0001 | ||||
| I | Reference | Reference | |||
| II | 1.072(0.897,1.282) | 1.095(0.914,1.311) | 0.325 | ||
| III/IV | 1.504(1.220,1.856) | 1.504(1.218,1.857) | < 0.001 | ||
| T stage | 0.030 | ||||
| T1 | Reference | Reference | |||
| T2 | 1.156(1.016,1.315) | 0.968(0.779,1.202) | 0.767 | ||
| Tumor Size | 1.013(1.006,1.020) | < 0.001 | 1.016(1.005,1.028) | 0.006 | |
| LN removed | < 0.0001 | ||||
| None | Reference | Reference | |||
| Yes | 0.624(0.549,0.710) | 0.696(0.608,0.796) | < 0.0001 | ||
| Radiation | < 0.001 | ||||
| None/Unknown | Reference | Reference | |||
| PORT | 1.380(1.213,1.569) | 1.335(1.173,1.519) | 0.0001 | ||
Note: LN: Lymph nodes; PORT: Postoperative Radiotherapy
Table 3.
Univariate and multivariate analyses of CSS in the whole population cohort after PSM
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
| Age(years) | < 0.0001 | ||||
| < 50 | Reference | Reference | |||
| 50–59 | 1.240(0.964,1.595) | 1.220(0.947,1.571) | 0.124 | ||
| 60–69 | 1.530(1.199,1.952) | 1.397(1.088,1.794) | 0.009 | ||
| 70–79 | 2.468(1.903,3.201) | 2.200(1.677,2.887) | < 0.0001 | ||
| ≥ 80 | 4.465(3.231,6.170) | 3.899(2.780,5.468) | < 0.0001 | ||
| Sex | 0.054 | ||||
| Male | Reference | - | |||
| Female | 1.178(0.998,1.390) | - | - | ||
| Race | 0.540 | ||||
| White | Reference | - | |||
| Black | 1.224(0.812,1.844) | - | - | ||
| Other | 0.930(0.710,1.219) | - | - | ||
| Marital | < 0.0001 | ||||
| Single | Reference | Reference | |||
| Married | 0.891(0.711,1.116) | 0.794(0.631,0.999) | 0.049 | ||
| Other | 1.423(1.100,1.842) | 0.970(0.739,1.273) | 0.827 | ||
| Grade | < 0.001 | ||||
| I | Reference | Reference | |||
| II | 1.116(0.881,1.413) | 1.127(0.888,1.429) | 0.326 | ||
| III/IV | 1.678(1.278,2.203) | 1.704(1.296,2.241) | < 0.001 | ||
| T stage | 0.470 | ||||
| T1 | Reference | - | |||
| T2 | 1.063(0.900,1.256) | - | - | ||
| Tumor Size | 1.014(1.004,1.023) | 0.004 | 1.018(1.008,1.027) | < 0.001 | |
| LN removed | < 0.0001 | ||||
| None | Reference | Reference | |||
| Yes | 0.548(0.464,0.647) | 0.589(0.496,0.700) | < 0.0001 | ||
| Radiation | < 0.0001 | ||||
| None/Unknown | Reference | Reference | |||
| PORT | 1.736(1.463,2.061) | 1.676(1.411,1.989) | < 0.0001 | ||
Note: LN: Lymph nodes; PORT: Postoperative Radiotherapy
Role of PORT in patients of all ages
To assess the influence of age on the efficacy of PORT, we performed a PSM analysis stratified by age groups. Due to the limited number of patients over 80 years old, this group was combined with the 70–79 age cohort to ensure sufficient statistical power. Before PSM, PORT was associated with significantly reduced OS and CSS across all age groups (Figs. 3A-D; Supplementary Materials: Figures S6A-D). After PSM analysis revealed that this association persisted in patients aged < 50, 60–69, and ≥ 70 years (Figs. 3E, G; Supplementary Materials: Figures S6E-H). Interestingly, in the 50–59 age group, no significant differences in OS or CSS were observed between the PORT and non-PORT groups (p > 0.05; Figs. 3F, H), suggesting potential age-related heterogeneity in treatment response or tumor biology.
Fig. 3.
Before and after PSM, K-M survival curves of OS and CSS between PORT patients and non-PORT patients stratified by age (< 50 years vs. 50–59 years). Before PSM: (A, C) OS and CSS in patients < 50 years old, (B, D) OS and CSS in patients 50–59 years old. After PSM: (E, G) OS and CSS in patients < 50 years old, (F, H) OS and CSS in patients aged 50–59 years old
Role of PORT in patients of various histologic grades
We examined the prognostic impact of PORT across different histologic grades. Before PSM, patients with well-differentiated (I grade) and moderately differentiated (II grade) tumors who received PORT had significantly shorter OS and CSS compared to those who did not (Figs. 4A-D). In contrast, among poorly differentiated/undifferentiated (III/IV grade) tumors, PORT did not significantly affect OS (p > 0.05; Supplementary Materials: Figure S7A) but was associated with shorter CSS (Supplementary Materials: Figure S7B). After PSM, PORT remained linked to worse OS and CSS in I grade and II grade tumors (Figs. 4E-H), while no significant survival differences were observed in III/IV grade tumors (p > 0.05; Supplementary Materials: Figures S7C, D), suggesting that the impact of PORT on survival in poorly differentiated/undifferentiated tumors is limited.
Fig. 4.
Before and after PSM, K-M survival curve analysis was performed on OS and CSS of PORT patients and non-PORT patients stratified by histological grade (Grade I and Grade II). Before PSM: (A, C) OS and CSS of Grade I patients, (B, D) OS and CSS of Grade II patients. After PSM: (E, G) OS and CSS of Grade I patients, (F, H) OS and CSS of Grade II patients
Role of PORT in patients with and without LN removed
We analyzed the effect of PORT based on LN removed status. Before PSM, PORT was associated with worse OS and CSS regardless of LN removal (Figs. 5A-D). After PSM, among patients who underwent LN removal, PORT continued to correlate with shorter OS and CSS (Figs. 5F, H). However, in patients without LN removal, PORT was linked to shorter CSS (Fig. 5G) but not OS (p > 0.05; Fig. 5E). These results suggest that LN removed status may influence the survival impact of PORT.
Fig. 5.
Before and after PSM, K-M survival curve analysis was performed on OS and CSS of PORT patients and non-PORT patients stratified by LN removal status. Before PSM: (A, C) OS and CSS of patients who did not undergo LN removal, (B, D) OS and CSS of patients who underwent LN removal. After PSM: (E, G) OS and CSS of patients who did not undergo LN removal, (F, H) OS and CSS of patients who underwent LN removal
Role of PORT in stratifying patients based on two factors: histological grade and LN removed status
To further analyze the prognostic impact of PORT considering both histological grade and LN removed status, we conducted a combined analysis. Before PSM, PORT was associated with significantly shorter OS and CSS in patients with I grade and II grade tumors, regardless of whether LN removal was performed (Figs. 6A-D; Supplementary Materials: Figures S8A-D). In contrast, among III/IV grade tumors, PORT did not significantly affect survival outcomes regardless of LN removed status (p > 0.05; Supplementary Materials: Figures S9A-D). After PSM, these patterns remained consistent, reinforcing the limited role of PORT in poorly differentiated/undifferentiated tumors (Figs. 6E-H; Supplementary Materials: Figures S8E-H, S9E-H).
Fig. 6.
Before and after PSM, in Grade I patients, K-M survival curve analysis was performed on OS and CSS of PORT patients and non-PORT patients stratified by LN removal status. Before PSM: (A, C) OS and CSS of patients who did not undergo LN removal, (B, D) OS and CSS of patients who underwent LN removal. After PSM: (E, G) OS and CSS of patients who did not undergo LN removal, (F, H) OS and CSS of patients who underwent LN removal
Role of PORT in patients with different DOI groups
Focusing on patients diagnosed between 2018 and 2021, we assessed the influence of DOI on PORT outcomes. Before and after PSM, PORT did not significantly affect OS or CSS in this cohort (p > 0.05; Figs. 7A-D). Based on the AJCC 8th edition [19] criteria and considering the limited sample size of patients with DOI greater than 10 mm, patients were stratified into two subgroups: DOI ≤ 5 mm and DOI > 5 mm. Before PSM, in the DOI ≤ 5 mm group, PORT was associated with worse OS and CSS (Figs. 8A, C), whereas in the DOI > 5 mm group, no significant survival differences were observed (p > 0.05; Figs. 8B, D). After PSM, PORT did not significantly impact OS or CSS in either DOI group (Figs. 8E-H), indicating no clear survival advantage from PORT regardless of DOI status.
Fig. 7.
Before and after PSM, K-M survival curves compared OS and CSS of PORT patients and non-PORT patients in the postoperative pT1-2N0M0 TSCC cohort from 2018 to 2021. Before PSM: (A) OS, (B) CSS. After PSM: (C) OS, (D) CSS
Fig. 8.
Before and after PSM, K-M survival curve analysis was performed on OS and CSS of PORT patients and non-PORT patients stratified by DOI (≤ 5 mm, > 5 mm). Before PSM: (A, C) OS and CSS of patients with DOI ≤ 5 mm, (B, D) OS and CSS of patients with DOI > 5 mm. After PSM: (E, G) OS and CSS of patients with DOI ≤ 5 mm, (F, H) OS and CSS of patients with DOI > 5 mm
Stratified analyses by potential effect modifiers
To further explore potential factors influencing the effects of PORT treatment, we conducted subgroup analyses based on age, sex, race, marital status, grade, T stage, tumor size, and LN removed status. The results of the subgroup analyses for OS and CSS are presented in Fig. 9. Overall, PORT was associated with decreased OS and CSS in the majority of subgroups. However, in terms of OS, Black patients, patients with other marital statuses, poorly differentiated/undifferentiated patients, and T2 patients were not significantly negatively affected. For CSS, only Black patients did not show significantly worse outcomes with PORT.
Fig. 9.
Subgroup analysis of the impact of PORT treatment on patient survival rates: forest plot of factors affecting (A) OS and (B) CSS
Importantly, interaction tests revealed several subgroups in which PORT was particularly associated with poorer outcomes. For OS (Fig. 9A), PORT appeared especially detrimental among younger patients (age < 50) (interaction p = 0.017), non-Black and White patients (interaction p = 0.046), married patients (interaction p = 0.001), T1 patients (interaction p < 0.001), patients with smaller tumors (tumor size ≤ 11 cm) (interaction p = 0.001), and patients who did not undergo LN removal (interaction p = 0.014). For CSS (Fig. 9B), similar patterns were observed. PORT was significantly associated with worse outcomes in married patients (interaction p = 0.002), well-differentiated patients (interaction p < 0.001), T1 patients (interaction p < 0.001), patients with smaller tumors (tumor size ≤ 11 cm) (interaction p = 0.001), and those who did not undergo LN removal (interaction p = 0.012).
Discussion
The etiology of TSCC remains unclear and is generally considered to be associated with multiple factors [20–22]. Therefore, accurately identifying and quantifying the factors that influence the prognosis of postoperative pT1-2N0M0 TSCC patients is critically important. This study, based on clinical prognosis data from 7,637 postoperative pT1-2N0M0 TSCC patients in the SEER database between 2000 and 2021, found that age, race, marital status, grade, tumor size, LN removed status, and PORT were all independent prognostic factors for OS and CSS. Additionally, sex was identified as an independent prognostic factor for OS only. Moreover, based on these prognostic factors, we developed and internally validated a novel nomogram model to predict individualized survival probabilities for OS and CSS in postoperative pT1-2N0M0 TSCC patients. The model demonstrated good discriminative ability and calibration, offering a potentially valuable tool to support clinical decision-making due to its visual interpretability and quantitative precision. Notably, both the K-M survival curves and the nomogram indicated that PORT was associated with poorer OS and CSS. To further explore this unexpected finding, we performed PSM to minimize baseline differences between patients who received PORT and those who did not. After matching, survival analysis confirmed that PORT remained significantly associated with worse OS and CSS. Although subgroup analyses showed that the prognostic effect of PORT was not consistent across all subgroups, no survival benefit from PORT was observed in any subgroup examined. These findings suggest that PORT may not provide a prognostic benefit in postoperative pT1-2N0M0 TSCC patients and could potentially be associated with adverse outcomes in selected subgroups.
Previous studies have suggested that age, sex, race, marital status, grade, TNM stage, tumor size, LN removed status, surgery, and whether the patient received chemoradiotherapy are factors influencing the prognosis of TSCC patients [18, 23, 24]. Given the small number of patients who received chemotherapy in our cohort (only 264), this variable was excluded from the current study. We conducted univariate and multivariate analyses on the remaining variables and developed a robust and well-performing nomogram model. The nomogram provided a visual and quantitative estimation of the prognostic impact of each factor on postoperative survival. Consistent with prior literature [18, 23, 24], we found that older age, male gender, Black race, unmarried status, poorly differentiated/undifferentiated histology, larger tumor size, and lack of LN removal were all associated with worse postoperative OS and CSS. However, our findings regarding PORT differed from most previous studies. While earlier research has reported either a beneficial or neutral effect of PORT in patients with early-stage TSCC, our analysis indicated that PORT was associated with poorer survival outcomes in certain subgroups of pT1-2N0M0 patients, even after adjusting for baseline characteristics using propensity score matching. For example, Tian et al. [3] found that PORT was associated with improved survival outcomes in pT1-2N0M0 TSCC patients. Gokavarapu et al. [17] concluded that PORT did not affect survival rates in patients with tumor DOI ≥ 4 mm and pT1-T2 deep tongue cancer. Similarly, Damazo et al. [25] also reported that T1-T2 TSCC patients who received postoperative radiotherapy and/or chemotherapy had similar survival rates compared to those who did not receive it. In contrast, our study observed a negative association between PORT and survival, particularly in subgroups characterized by lower clinical risk. Critically, the absence of PNI, LVI, and margin status data in SEER precluded adjustment for these well-established high-risk features. This limitation may have led to residual confounding, as patients selected for PORT likely harbored occult adverse pathological factors (e.g., close margins) not captured in the database. Consequently, our observed association between PORT and poorer survival may reflect unmeasured clinical risk rather than a true treatment effect. Huang et al. [18], through an in-depth analysis of the SEER database, further supported our view, indicating that the benefits of PORT may be limited to high-risk patient groups, while in intermediate-risk patients, other factors need to be considered. For low-risk patients, surgery alone may be more appropriate. These findings underscore the imperative to refine PORT selection beyond anatomical staging alone. While our nomogram provides a pragmatic tool for survival prediction in resource-limited settings, its clinical utility must be augmented by histopathological assessment of margins, PNI, and LVI—cornerstones of current PORT guidelines [14]. For low-risk patients (e.g., T1 tumors with clear margins), our data strongly caution against routine PORT use given the potential for harm.
Before conducting subgroup analyses, we first addressed potential confounding by performing PSM on the entire cohort. Even after adjusting for baseline characteristics, patients who received PORT had worse OS and CSS compared to those who did not, suggesting a possible association between PORT and poorer prognosis in postoperative pT1-2N0M0 TSCC patients. Notably, this finding contrasts with the results of Tian et al.‘s study [3].
To explore this further, we conducted stratified PSM analyses across age, grade, LN removed status, dual-factor stratification (grade and LN removed status), and DOI subgroups. While age is widely recognized as a prognostic factor in TSCC [22], few studies have examined whether the impact of PORT varies across age groups. Our results suggest that PORT was associated with worse survival in patients aged ≤ 50, 60–69, and ≥ 70 years, both before and after PSM. Interestingly, in patients aged 50–59, the effect of PORT was attenuated after matching, indicating potential age-related heterogeneity in treatment outcomes. Regarding histological grade, PORT was consistently associated with shorter survival in patients with well- and moderately differentiated tumors, regardless of adjustment for confounders. In contrast, for patients with poorly differentiated or undifferentiated tumors, PORT was not significantly associated with survival, which differs from findings by Tian et al. [3], who suggested potential benefit in this subgroup. Concerning LN removed status, many studies have focused on its role in pT1-2N0M0 TSCC patients but have not specifically examined the efficacy of PORT [11, 26]. Our study is among the first to assess the prognostic value of PORT in relation to lymph node dissection. After PSM, PORT remained associated with worse OS and CSS in patients who had undergone LN removal, and with worse CSS (but not OS) in those who had not. Dual-factor stratification further revealed that, after PSM, the negative association between PORT and CSS persisted in patients with Grade I/II tumors, regardless of LN removal status. For patients with Grade III/IV tumors, no significant association was found between PORT and survival in any LN removed subgroup. We also assessed the potential role of DOI using cases from 2018 to 2021, the only period for which DOI data were available in SEER. Patients with both DOI ≤ 5 mm and DOI > 5 mm did not demonstrate survival benefit from PORT, aligning with results from Alterio et al. [27], Rajappa et al. [15], and Gokavarapu et al. [17], but contrasting with Tian et al. [3]. This inconsistency highlights the ongoing controversy surrounding the utility of DOI in guiding PORT decisions and underscores the need for further investigation.
To identify subgroups of patients potentially more affected by PORT, we conducted a comprehensive subgroup analysis. The results showed that PORT was associated with poorer outcomes in the majority of subgroups, which was generally consistent with the stratified PSM analysis. Interaction analyses further suggested that, for OS, age, race, marital status, T stage, tumor size, and LN removed status may modify the association between PORT and survival. For CSS, marital status, grade, T stage, tumor size, and LN removed status demonstrated similar potential effect modification. These findings imply that the prognostic impact of PORT may vary across different clinical and pathological contexts. Further research is needed to elucidate the underlying biological and treatment-related mechanisms, which may help explain the heterogeneous effects of PORT across patient subgroups and support more personalized treatment strategies in the future.
Given the widespread use of PORT in advanced cancers, our findings highlight the need for a nuanced approach in postoperative pT1-2N0M0 TSCC. While PORT may not offer survival benefits in this low-risk group and could even be harmful in specific subgroups, these conclusions must be tempered by the study’s retrospective design and the SEER database’s limitations, particularly the lack of PNI, LVI, and surgical margin status. The absence of these critical variables could introduce residual confounding, as unrecorded adverse pathological features might have influenced PORT administration and survival outcomes. Consequently, we advocate for a personalized, risk-adapted approach to PORT in early-stage TSCC, emphasizing the importance of comprehensive clinical and pathological data in future prospective studies to clarify PORT’s role and inform individualized treatment decisions.
The limitations of this study are mainly reflected in the following aspects. First, as a retrospective analysis using the SEER database, the results may be subject to selection bias and unmeasured confounding. Although PSM was used to balance baseline characteristics, key clinical variables such as PNI, LVI, surgical margin status, recurrence, and comorbidity profiles are not recorded in SEER, limiting our ability to fully adjust for the indications for PORT. Second, the study population was predominantly White (84.9%), which may limit generalizability to other racial or ethnic groups. Third, as the cohort spanned multiple years, three different editions of the TNM staging system were used, which may have introduced inconsistencies in disease classification and staging accuracy. Fourth, the lack of molecular data (e.g., HPV status, TP53 mutations, immune markers) is a key limitation. While histological features guide current PORT decisions, the absence of molecular information limits our ability to interpret survival differences, including the paradoxical association between PORT and poorer outcomes. Recent studies highlight the prognostic value of TP53 mutations [28] and HPV-negative tumor stemness [29], suggesting that molecular subtypes could improve risk stratification. Future studies should integrate molecular and clinicopathological data to support more personalized treatment strategies in early TSCC. Finally, our nomogram model was only internally validated; external validation using independent cohorts is necessary to confirm its clinical utility.
Conclusions
In summary, we developed and internally validated a SEER-based nomogram that predicts OS and CSS in postoperative pT1-2N0M0 TSCC patients. Our analysis found that PORT was not associated with survival benefits and was linked to poorer outcomes in certain subgroups. However, due to the retrospective design and inherent limitations of the SEER database (such as the lack of critical variables including PNI, LVI, surgical margin status) these findings should be interpreted with caution, as unmeasured adverse pathology may confound results. Therefore, while our findings suggest that routine PORT use in low-risk TSCC patients should be carefully evaluated, further prospective studies with comprehensive clinical and pathological data are essential to clarify PORT’s role and guide individualized treatment strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge the SEER databases for the making these datasets publicly available to promote continuous research.
Abbreviations
- OS
Overall survival
- CSS
Cancer-specific survival
- pT1-2N0M0
Early-stage
- TSCC
Tongue squamous cell carcinoma
- PORT
Postoperative radiotherapy
- PSM
Propensity score matching
- LN
Lymph node
- SCC
Squamous cell carcinoma
- PNI
Perineural invasion
- LVI
Lymphovascular invasion
- DOI
Depth of invasion
- K-M
Kaplan-meier
- ROC
Receiver operator characteristic
- C-index
Concordance index
- DCA
Decision curve analysis
Author contributions
K.W. and D.T. conceived and designed the study. D.L., H.L. and X.F. analyzed, interpreted the data, and drafted the manuscript. D.T. contributed to data analysis. Y.Z. contributed to writing the manuscript. K.W. reviewed and revised the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the grants from the Youth science and technology innovation talent of Tianshan Talent Training Program in Xinjiang (Grant No.: 2022TSYCCX0099), the Technology Innovation Team (Tianshan Innovation Team) Project (Grant No.: 2022TSYCTD0018), the National High-Level Talent Development Program (Grant No.: XYD2024GR04), and the National Natural Science Foundation of China (Grant No.: 12461101).
Data availability
Any data and R script in this study can be obtained from the corresponding author upon reasonable request. The final manuscript was read and approved by all authors. In this study, publicly available datasets were analyzed. These are available on the SEER database https://www.seer.cancer.gov.
Declarations
Ethics approval and consent to participate
Ethics approval was not required for this SEER-based study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dandan Lin, Huling Li and Xing Feng contributed equally to this work.
Contributor Information
Dandan Tang, Email: 18299062172@163.com.
Kai Wang, Email: wangkaimath@sina.com.
References
- 1.Warnakulasuriya S, Kerr AR. Oral Cancer screening: past, present, and future. J Dent Res. 2021;100:1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today, Accessed [29 09 2021]. International Agency for Research on Cancer. 2021.
- 3.Tian Q, Jiang L, Dai D, Liu L, Shi X, Guo Y, et al. Impact of postoperative radiotherapy on the prognosis of Early-Stage (pT1-2N0M0) oral tongue squamous cell carcinoma. J Clin Oncol. 2024;42:1754–65. [DOI] [PubMed] [Google Scholar]
- 4.Peltonen J, Nikkilä R, Al-Samadi A, Mäkitie A, Martinsen JI, Kjaerheim K, et al. Occupation and tongue cancer in nordic countries. BMC Oral Health. 2024;24:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno K, Takeuchi M, Kikuchi M, Omori K, Kawakami K. Outcomes in patients diagnosed with tongue cancer before and after the age of 45 years. Oral Oncol. 2020;110:105010. [DOI] [PubMed] [Google Scholar]
- 6.da Silva Souto AC, Vieira Heimlich F, Lima de Oliveira L, Bergmann A, Dias FL, Spíndola Antunes H, et al. Epidemiology of tongue squamous cell carcinoma: A retrospective cohort study. Oral Dis. 2023;29:402–10. [DOI] [PubMed] [Google Scholar]
- 7.Almangush A, Bello IO, Heikkinen I, Hagström J, Haglund C, Kowalski LP, et al. Stromal categorization in early oral tongue cancer. Virchows Arch. 2021;478:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. 2008;112:345–51. [DOI] [PubMed] [Google Scholar]
- 9.Katz O, Nachalon Y, Hilly O, Shpitzer T, Bachar G, Limon D, et al. Radiotherapy in early-stage tongue squamous cell carcinoma with minor adverse features. Head Neck. 2017;39:147–50. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Wang T, Yu C, Guo X, Li C, Li L. Elective neck dissection versus Wait-and-Watch policy for oral cavity squamous cell carcinoma in early stage: A systematic review and Meta-Analysis based on survival data. J Oral Maxillofac Surg. 2019;77:2154–67. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Ghanem S, Yehuda M, Carmel NN, Leshno M, Abergel A, Gutfeld O, et al. Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with no clinically apparent lymph node metastasis in the neck a systematic review and meta-Analysis. JAMA Otolaryngol Head Neck Surg. 2016;142:857–65. [DOI] [PubMed] [Google Scholar]
- 12.Yang W, Sun M, Jie Q, Zhou H, Zhang P, Zhu J. Lingual lymph node metastasis in cT1-2N0 tongue squamous cell carcinoma: is it an Indicator for elective neck dissection. Front Oncol. 2020;10:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Wang Y, Zeng W, Xie X, Li C, Zhou Q, et al. Prognostic factors in surgically treated tongue squamous cell carcinoma in stage T1-2N0-1M0: A retrospective analysis. Cancer Med. 2024;13:e7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National comprehensive cancer network. NCCN Clinical Practice Guidelines in oncology(NCCN Guidelines): Head and neck cancers v.4.2024. [Internet]. 2024 [cited 2024 Oct 11]. Available from: https://www.nccn.org
- 15.Rajappa SK, Ram D, Bhakuni YS, Jain A, Kumar R, Dewan AK. Survival benefits of adjuvant radiation in the management of early tongue cancer with depth of invasion as the indication. Head Neck. 2018;40:2263–70. [DOI] [PubMed] [Google Scholar]
- 16.Rubin SJ, Gurary EB, Qureshi MM, Salama AR, Ezzat WH, Jalisi S, et al. Stage II oral tongue cancer: survival impact of adjuvant radiation based on depth of invasion. Otolaryngol - Head Neck Surg (United States). 2019;160:77–84. [DOI] [PubMed] [Google Scholar]
- 17.Gokavarapu S, Parvataneni N, Rao SLMC, Reddy R, Raju KVVN, Chander R. Role of postoperative radiation therapy (PORT) in pT1-T2 N0 deep tongue cancers. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e227–31. [DOI] [PubMed] [Google Scholar]
- 18.Huang F, Xu G, Du H. A new nomogram for predicting overall survival and assisting postoperative adjuvant treatment Decision-Making in stage II oral tongue squamous cell carcinoma: A surveillance, epidemiology and end results (SEER) database analysis. J Oral Maxillofac Surg. 2021;79:2147–54. [DOI] [PubMed] [Google Scholar]
- 19.Baba A, Hashimoto K, Kayama R, Yamauchi H, Ikeda K, Ojiri H. Radiological approach for the newly incorporated T staging factor, depth of invasion (DOI), of the oral tongue cancer in the 8th edition of American joint committee on Cancer (AJCC) staging manual: assessment of the necessity for elective neck dissection. Jpn J Radiol. 2020;38:821–32. [DOI] [PubMed] [Google Scholar]
- 20.Chuang ST, Chen CC, Yang SF, Chan LP, Kao YH, Huang MY, et al. Tumor histologic grade as a risk factor for neck recurrence in patients with T1-2N0 early tongue cancer. Oral Oncol. 2020;106:104706. [DOI] [PubMed] [Google Scholar]
- 21.Cui M, Du W, Fang Q, Dai L, Qi J, Luo R. Prognostic value of a family history of oral tongue squamous cell carcinoma: A Matched-Pair study. Laryngoscope. 2020;130:E605–10. [DOI] [PubMed] [Google Scholar]
- 22.Tagliabue M, Belloni P, De Berardinis R, Gandini S, Chu F, Zorzi S, et al. A systematic review and meta-analysis of the prognostic role of age in oral tongue cancer. Cancer Med. 2021;10:2566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Y, Qian C, Yu L, Fang H, Wang J, Wu P, et al. Prognostic nomogram for patients with tongue squamous cell carcinoma: A SEER-based study. Oral Dis. 2024;30:292–306. [DOI] [PubMed] [Google Scholar]
- 24.Ye Z, Tan G, Wang L, Shangguan G, Yao H, Xu X, et al. Comparison of survival between palliative surgery and no surgery for advanced tongue squamous cell carcinoma: an analysis of SEER data. Int J Oral Maxillofac Surg. 2024;S0901–5027:00212–1. [DOI] [PubMed] [Google Scholar]
- 25.Damazo BJ, Punjabi NA, Liu YF, Inman JC. Histopathologic predictors of recurrence and survival in early T stage oral tongue squamous cell carcinoma. Front Oral Health. 2024;5:1426709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsini AY, Alsubaie HM, Marzouki HZ, Abu-Zaid A, Al-Qahtani K. Elective node dissection versus observation for management of patients with early-stage cT1/T2N0 tongue carcinoma: A systematic review and meta-analysis of prospective studies. Clin Otolaryngol. 2021;46:720–8. [DOI] [PubMed] [Google Scholar]
- 27.Alterio D, D’Urso P, Volpe S, Tagliabue M, De Berardinis R, Augugliaro M et al. The impact of post-operative radiotherapy in early stage (Pt1-pT2N0M0) oral tongue squamous cell carcinoma in era of doi.cancers (Basel). 2021;13:4851. [DOI] [PMC free article] [PubMed]
- 28.Campbell BR, Chen Z, Faden DL, Agrawal N, Li RJ, Hanna GJ, et al. The mutational landscape of early- and typical-onset oral tongue squamous cell carcinoma. Cancer. 2021;127:544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Kumar P, Das BC. HPV + ve/–ve oral-tongue cancer stem cells: A potential target for relapse-free therapy. Transl Oncol. 2021;14:100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data and R script in this study can be obtained from the corresponding author upon reasonable request. The final manuscript was read and approved by all authors. In this study, publicly available datasets were analyzed. These are available on the SEER database https://www.seer.cancer.gov.