Abstract
UBASH3A, also known as TULA or STS-2, is a lymphoid protein that has recently emerged as a novel regulator of T-cell activation and function. It used to be discussed as a member of the UBASH3/TULA/STS family to study the unique triple domain structure and protein‒protein interactions. In this review, we focus on the multifaceted regulatory roles of UBASH3A in biological processes within T cells. Furthermore, genetic variants in UBASH3A have been linked to at least five autoimmune phenotypes. Therefore, a broader understanding of the regulatory effects of UBASH3A in innate immunity and adaptive immunity could enable further improvements in the treatment of autoimmune disorders, antimicrobial therapy, and cancer immunotherapy.
Keywords: UBASH3A, TULA, STS-2, CLIP4, Autoimmune disease, Infectious disease
Introduction
In 2001, Wattenhofer et al., to identify candidate genes associated with Down syndrome phenotypes or monogenic disorders, initially identified a 2.5-kb cDNA located in chromatin 21q22.3 as a new gene [1] whose encoded protein was characterized as the first protein containing both UBA and SH3 domains and was named UBASH3A (Ubiquitin-Associated And SH3 Domain-Containing Protein A). Since its discovery more than two decades ago, our understanding of its unique structure, intracellular functions, and effects on diseases has steadily increased. UBASH3A has a paralog called UBASH3B. Previous reports have reviewed two members of this family together, and since the dephosphorylation role of UBASH3B has been thoroughly investigated, it may have garnered more attention. After evaluating the literature, we found that the functions and mechanisms of UBASH3A differ significantly from those of UBASH3B and may play a more crucial role in immune cells. Since UBASH3A has relatively moderate phosphatase activity, its regulatory effects are most likely mediated by phosphatase-independent mechanisms. Here, we discuss the role, function, and mechanism of UBASH3A in autoimmune disorders in detail.
Biological characteristics of UBASH3A
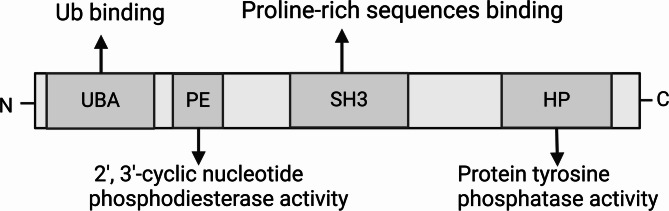
UBASH3A, also called STS-2 (Suppressor of T-cell receptor signaling 22) [2], TULA (T-cell ubiquitin ligand protein) [3], and CLIP4 (Cbl-interacting protein 4) [4], exhibits tissue distribution specificity and is exclusively expressed in the spleen, thymus, bone marrow, lung, and adult kidney, especially in T lymphocytes [1, 3]. Within cells, UBASH3A is found in the cytoplasm and nucleus. The UBASH3A locus is located on human chromosome 21 (21q22.3) and encodes a protein consisting of 661 amino acids [1]. The UBASH3A protein consists of three main functional domains (Fig. 1): the N-terminal ubiquitin-associated (UBA) domain, the central Src-homology 3 (SH3) domain and the C-terminal histidine phosphatase (HP) domain, which is also referred to as the phosphoglycerate mutase-like (PGM) domain [3]. The UBA domain binds to ubiquitin and ubiquitylated proteins; the SH3 domain is linked to Cbl, an E3 ubiquitin ligase, as well as dynamin, which is required for endocytosis; and the HP domain exhibits protein tyrosine phosphatase activity [3–7].
Fig. 1.
Structural domains and activities of UBASH3A
UBASH3A has a paralog, UBASH3B (also referred to as STS-1, TULA-2, and p70), which features identical domain compositions as UBASH3A [8]. The UBASH3B gene encodes 649 amino acids [9]. The homology of UBASH3A and UBASH3B is approximately 40%, and the similarity is approximately 75% [2, 8]. A 2-histidine (2 H) phosphoesterase motif was recently discovered between the UBA and SH3 domains of UBASH3 [10]. The 2 H phosphoesterase motif is distinguished by two invariant histidine residues and is crucial for catalytically hydrolyzing cyclic phosphate bonds on a range of substrates, including cyclic nucleotides [11, 12]. Consistent with its designation as a phosphoesterase, Yin et al. demonstrated that the UBASH3B 2 H phosphoesterase motif displays 2′,3′-cyclic nucleotide phosphodiesterase (PDE) activity [10]. They further discovered that the UBASH3B PDE domain plays a functional role in inhibiting both the T-cell receptor (TCR) pathway in T cells and the Dectin-1 signaling pathway in phagocytes [10]. However, the functional role of the UBASH3A PDE domain needs to be further studied.
There are several notable differences between UBASH3A and UBASH3B. One of the differences lies in the patterns of tissue expression. UBASH3A is expressed only in lymphocytes, whereas UBASH3B is ubiquitously expressed and has been shown to monitor receptor signaling in a variety of cell types, including mast cells, platelets, T lymphocytes, diverse phagocytes, and hematopoietic stem/progenitor cells [13–17]. Another distinction between UBASH3A and UBASH3B in T cells is that UBASH3B exhibits strong protein tyrosine phosphatase activity in vivo and in vitro and represses TCR signaling by dephosphorylating ZAP-70 and Syk via its HP domain [2, 18]. In contrast, UBASH3A has minimal, presumably acid-dependent, phosphatase activity in vitro; knocking down the murine homolog of UBASH3A in vivo leads to only a slight increase in the phosphorylation of Zap-70 [6, 19]. The ubiquitin-dependent role of the UBA and SH3 domains in UBASH3A, along with its interactions with other proteins, seems to be the principal function of UBASH3A [20]. Interestingly, despite exhibiting distinct expression patterns and functional processes, UBASH3A and UBASH3B appear to collaborate in T cells [21]. Finally, although UBASH3B has been linked to the development of leukemia and other cancers, it has not been reported to contribute to any autoimmune or immune-mediated diseases in genome-wide association studies (GWASs) [22–24]. UBASH3A has been shown to be involved in the development of a variety of autoimmune diseases, including rheumatoid arthritis and type 1 diabetes, as well as infectious diseases and malignancies [20, 25].
Mechanism of action of UBASH3A
Negative regulation of TCR signaling
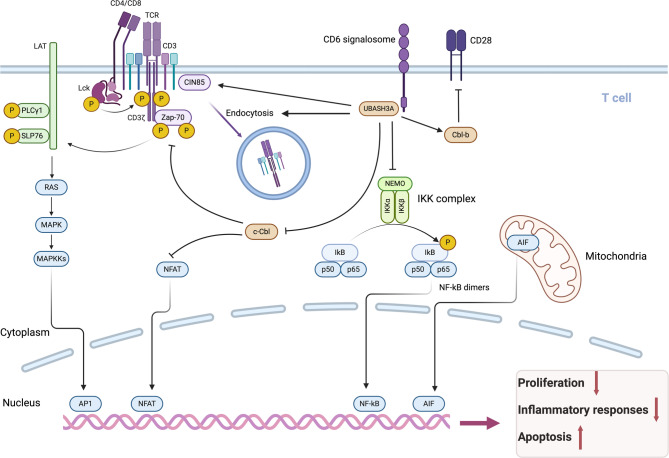
T cells play a pivotal role in the immune system, orchestrating an effective immune response to eliminate foreign pathogens by directly destroying pathogen-containing cells and secreting numerous cytokines that stimulate other cell types [26]. The T-cell receptor (TCR) is primarily responsible for regulating T-cell activity. Signals through the TCR modulate the intensity and duration of the T-cell response, as well as the development of T lymphocytes [27]. TCR signaling is a complex process that can be roughly divided into several key steps: T cells receive signals, tyrosine kinases are activated, adaptor molecules are phosphorylated, and multicomponent complexes are assembled [28–31]. These actions are essential for establishing a link between T-cell receptors and the activation of downstream signaling pathways. The duration and amplitude of signaling are strictly administered to prevent inappropriate activation, which can elicit uncontrolled cell growth, migration, transformation, or proliferation and is usually associated with developmental abnormalities or tumorigenesis [32, 33]. UBASH3A is expressed mainly in lymphocytes, particularly T cells [2]. Through a genome-wide CRISPR screen of primary human T cells, Shifrut et al. revealed that UBASH3A is a negative regulator of TCR-induced cell proliferation [34]. The negative control of TCR signaling is its main mechanism, which has been confirmed by numerous studies. The mechanisms of action are detailed below (Fig. 2).
Fig. 2.
Biological functions and effects of UBASH3A in T cells
Weak dephosphorylation
The phosphorylation of proteins represents a major posttranslational modification that plays an essential role in controlling protein function [35]. Membrane-bound Lck and Fyn phosphorylate key residues in the cytoplasmic portions of TCR subunits, generating docking sites for the cytoplasmic kinase Zap-70 [28]. The recruitment of Zap-70 to the TCR complex results in its activation through tyrosine phosphorylation, enabling it to phosphorylate a multitude of downstream targets, including a wide spectrum of enzymes and adaptor proteins [36]. Malfunctions in signaling pathways mediated by protein tyrosine phosphorylation cause a wide range of human diseases [32, 36, 37]. To maintain cellular homeostasis, the dephosphorylation of activated tyrosine kinases by phosphatases represents the fastest and most efficient method for transient signal termination.
The construction and analysis of a mouse model devoid of UBASH3A and UBASH3B expression provided a preliminary understanding of how UBASH3 regulates T-cell activation cascades following TCR engagement [2]. Compared with T cells from wild-type (WT) mice, T cells derived from Ubash3a−/−Ubash3b−/− double knockout (dKO) mice exhibit greater responsiveness to TCR stimulation; significantly greater proliferation; the phosphorylation and activation of ZAP70, SLP76 and MAPK; and the production of cytokines, including interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-10 (IL-10), and interferon-gamma (IFNγ) [2, 18, 38, 39]. These in vitro investigations identified UBA3SH3A and UBASH3B as pivotal regulators of T-cell activation in TCR proximal signal transduction. However, following in vitro stimulation, compared with wild-type T cells, neither Ubash3a−/− nor Ubash3b−/− T cells displayed significant increases in T-cell proliferation or cytokine production. These results indicate that UBASH3A and UBASH3B act as overlapping, possibly redundant, negative modulators of T-cell activation triggered by TCR stimulation.
In line with the T-cell hypersensitivity phenotype of Ubash3−/− T cells observed in vitro, Newman et al. reported that Ubash3−/− mice have a greater proportion of splenic CD4 + T lymphocytes displaying a mature memory/effector phenotype, which is predominantly attributable to the absence of UBASH3B [39]. A comparable hierarchical impact is observed in the trinitrobenzene sulfonic acid (TNBS)-induced colitis model, in which the deletion of either Ubash3a or Ubash3b facilitates inflammatory responses and T-cell activities; however, the effects in the Ubash3a−/−Ubash3b−/− dKO mice are more intense than those in the single knockout (sKO) mice [2]. Furthermore, using an alternative autoimmune model, collagen-induced arthritis, Okabe et al. reported that Ubash3a−/− mice are markedly more prone to autoimmune induction than either wild-type or Ubash3b−/− animals are, which is consistent with the finding of increased numbers of IL-2-producing splenic CD4 + T cells in Ubash3a−/− than in WT mice [40]. These investigations provide evidence of the potential independent roles of UBASH3A and UBASH3B in modulating T-cell responses in vivo.
In the UBASH3 family, the HP domain exhibits His-based phosphatase activity toward phosphotyrosine (pY)-containing proteins and is thus considered atypical protein tyrosine phosphatase (PTP) activity, given that the structure of UBASH3-family PTPs differs from that of traditional PTPs, in which cysteine is an essential catalytic residue [41]. UBASH3BHP has been demonstrated to possess potent protein tyrosine phosphatase activity in vitro and is also capable of targeting the T-cell tyrosine kinases Zap-70 and Src for dephosphorylation and inactivation in vivo, thereby suppressing T-cell receptor signaling [18]. Additional tyrosine-phosphorylated proteins identified as substrates of UBASH3B include epidermal growth factor receptor (EGFR), Flt/c-Kit, Cbl, and the Zap-70 kinase homolog Syk [17, 42–44]. However, the in vitro phosphatase catalytic activity of UBASH3A is negligible in comparison to that of UBASH3B, and it lacks detectable phosphatase activity toward intracellular tyrosine-phosphorylated proteins [43]. The structural disparity between UBASH3A and UBASH3B, which accounts for the noticeably different phosphatase activities, is not well understood. Interestingly, following TCR stimulation, both naïve and activated T cells derived from genetically altered mice lacking the Ubash3 protein exhibit remarkably and transiently increased amounts of tyrosine-phosphorylated, ubiquitinated proteins [38]. Agrawal et al. reported that UBASH3A may act as a negative regulator of UBASH3B-dependent dephosphorylation, thus exerting opposite effects on protein tyrosine kinase (PTK)-mediated signaling [43].
Ubiquitylation-dependent degradation
Ubiquitination represents a principal mechanism by which cells inhibit receptor signaling and preserve normal cellular homeostasis [45]. Cbl proteins are E3 ubiquitin ligases that play critical roles in the attenuation of protein tyrosine kinase signaling [46, 47]. Cbl proteins constitute a tyrosine kinase-binding (TKB) domain, a ring finger domain, a proline-rich region, and a C-terminal segment that includes a ubiquitylation-associated domain (UBA) and a leucine zipper (LZ). It is generally recognized that Cbl utilizes its ring finger to connect with the E2 ubiquitin-conjugating enzyme and employs its TKB domain to attach to activated receptors [48]. By binding Cbl, E2, and activated RTK, Cbl targets those receptors for ubiquitylation and degradation [49]. The proline-rich region offers docking sites for proteins that contain the SH3 domain, which helps Cbl target specific proteins for internalization and destruction. Since the initial characterization of UBASH3A, its interaction with the Cbl family has been regarded as a crucial component of its mechanism [3, 4].
The impact of UBASH3A on the Cbl-dependent internalization and degradation of various receptors has been investigated. Experiments indicate that the UBASH3A SH3 domain mediates the interaction with the proline-rich region of Cbl, which is essential for the recruitment of UBASH3A to Cbl/EGFR complexes, and that the oligomerization of UBASH3A via its HP domain may increase the avidity of the UBA domain of UBASH3A binding to monoubiquitin attached to EGFR, thereby promoting interactions between UBASH3A and ubiquitinated receptors within cells [4]. Kowanetz et al. demonstrated that in 293T cells, UBASH3A is capable of impairing Cbl-mediated downregulation of epidermal growth factor receptor (EGFR) in a UBA- and SH3- domain-dependent manner, leading to a reduction in the number of EGFR-containing trafficking vesicles and subsequent blockade of receptor degradation followed by the mitogenic signaling pathway [4]. Similarly, in the presence of Cbl, the overexpression of UBASH3A blocks the degradation of the platelet-derived growth factor (PDGF) receptor in 293T cells, resulting in an increase in the ERK signaling pathway. Moreover, overexpression of UBASH3A in fibroblasts increases PDGF-induced cell proliferation and oncogenic transformation [4]. Interestingly, the reduction in the effects of c-Cbl on protein tyrosine kinases by UBASH3A appears to result from the induction of ubiquitylation and degradation of c-Cbl [3]. Although the EGFR-based experimental system may not accurately mimic the functions of UBASH3A in lymphocytes, where UBASH3A is expressed, the interactions among EGFR, c-Cbl, and UBASH3A could still hold physiological significance because UBASH3A might be present in some epithelial cells, albeit at a lower level than lymphocytes.
In the context of Jurkat T lymphoblastoid cells, Feshchenko et al. reported that UBASH3A upregulates TCR/CD3-mediated Zap kinase activity and NF-AT transcription activity by countering the suppressive effect of c-Cbl on protein tyrosine kinases [3]. These findings underscore the potential importance of Cbl-UBASH3A complexes in regulating T-cell receptor-mediated functions.
The UBA domain of UBASH3A plays a crucial role in ubiquitination-dependent regulation, as it mediates interactions with monoubiquitinated chains and polyubiquitinated chains/complexes [50]. UBASH3A has been found to have four specific ubiquitination sites located at lysine residues 15, 202, 309 and 358. Hoeller et al. reported that monoubiquitination at Lys202 leads UBASH3A to adopt a closed conformation, preventing the UBA domain from binding to ubiquitinated targets in trans [51]. They also demonstrated that monoubiquitylation of UBASH3A restricts its capacity to block ligand-triggered degradation of EGFRs.
The NF-κB family plays a vital role in both innate and adaptive immunity by controlling the transcriptional programs of proinflammatory and antiapoptotic genes, including IL-2 [52, 53]. Ge et al. reported that UBASH3A attenuates the TCR-induced NF-κB signaling pathway by specifically curbing the activation of the Iκ-B kinase (IKK) complex, thereby inhibiting the nuclear translocation of NF-κB dimers and the subsequent initiation of targeted gene transcription [54]. They further discovered novel binding reactions of UBASH3A with nondegradative polyubiquitin chains, namely, TAK1 and NEMO, via the SH3 domain, implying that UBASH3A modulates the NF-κB signaling cascade via a ubiquitin-dependent mechanism [54] (Fig. 2).
UBASH3A can also engage with other proteins to form a complex to jointly regulate signal transduction. CIN85 (Cbl-interacting protein of 85 kDa) is an adaptor protein that participates in signal transduction, receptor endocytosis, and cytoskeletal remodeling [55]. Kong et al. reported that CIN85 colocalizes within TCR microclusters and enhances the recruitment of UBASH3A to dampen early TCR activation signals [56]. Further confirmation of the inhibitory role of UBASH3A in initial T-cell activation was recently provided by the discovery of UBASH3A within the TCR-induced CD6 signalosome and the CD5–CBLB–UBASH3A inhibitory complex [57].
Endocytosis of the TCR-CD3 complex
Dynamins are cytosolic proteins that transiently bind to membranes where they are involved in the process of vesicle pinching, which is an integral step of endocytosis and plays a central role in intracellular vesicle trafficking [58, 59]. Bertelsen et al. reported that in addition to stabilizing EGFR, the overexpression of UBASH3A prevents the uptake of transferrin (Tf) and low-density lipoprotein (LDL) [60]. Furthermore, UBASH3A overexpression reduces the internalization of CD59 and major histocompatibility complex class I (MHC-I), two other proteins located at the cell surface [60]. The internalization of Tf, LDL, CD59, and MHC-1 necessitates the formation of endocytic vesicles and relies on the involvement of dynamin. They also demonstrated that UBASH3A uses its SH3 domain to attach to the proline-rich sequences of dynamin and that the UBASH3A protein hinders endocytosis by functionally sequestering dynamin, since the blocking effect of UBASH3A on endocytosis is counteracted by the overexpression of wild-type dynamin [60]. Thus, UBASH3A seems to regulate a wide range of endocytic activities.
Another molecular mechanism by which UBASH3A influences T-cell activation is the downregulatory effect of UBASH3A on the overall amounts of TCR components and the surface expression of TCR complexes (Fig. 2). Ge et al. demonstrated that UBASH3A limits the cell-surface expression of TCR-CD3 complexes in resting Jurkat T cells and facilitates the endocytosis of cell-surface TCR-CD3 after TCR stimulation but does not affect the membrane surface levels of CD28, CD45 and HLA-B7 [61]. Mass spectrometry and protein–protein interaction studies have validated intriguing interactions of UBASH3A with several key cellular processes that regulate the synthesis and dynamics of TCR–CD3 complexes, including endoplasmic reticulum-associated protein degradation (ERAD), HSP70, 26 S proteasome regulatory subunit 6B (PSMC4), and dynamin, suggesting that UBASH3A plays a role in the production and turnover of TCR–CD3 within the cytoplasm, most likely through the ERAD machinery, as well as the endocytosis and recycling processes that regulate the dynamics and equilibrium of TCR–CD3 on the cell surface [61]. In addition, via its SH3 domain, UBASH3A binds to CBL-B, an E3 ubiquitin ligase that exerts a negative regulatory effect on CD28-mediated signaling, consequently influencing T-cell activation [61–63]. This mechanism may represent a potential target for intervention in the overactivation of T cells. Further studies are needed to elucidate the molecular basis of the endocytosis effect of UBASH3A.
Effect on apoptosis
Apoptosis, a fundamental cellular process of programmed cell death, is characterized by a series of morphological alterations, such as nuclear condensation (pyknosis) and fragmentation (karyorrhexis), along with plasma membrane blebbing, which culminates in the generation of apoptotic bodies and plays important roles in cellular homeostasis, development, and disease pathogenesis [64–66]. Under stress conditions, apoptosis-inducing factor (AIF) is released from the mitochondria and subsequently translocated to the nucleus, where it initiates caspase-independent apoptotic processes through its binding to DNA [67–71]. Using mass spectrometry and RNA interference analysis, Collingwood et al. reported that UBASH3A promotes T-cell apoptosis by interacting with AIF, and this effect is independent of either the influence of TCR signaling or caspase activity [72] (Fig. 2). The N-terminal region of UBASH3A is essential for AIF binding, but neither the UBA domain nor the SH3 domain is critical for this interaction. However, it remains to be determined whether UBASH3A exerts an AIF-induced apoptotic effect by promoting the interaction between AIF and its apoptotic cofactors.
The significance of UBASH3A in autoimmune diseases
As a negative regulator of T-cell activation and function, UBASH3A is a susceptibility gene for multiple autoimmune diseases. Diseases associated with specific UBASH3A single nucleotide polymorphisms (SNPs) include rheumatoid arthritis [73], type 1 diabetes [74], systemic lupus erythematosus [75–77], vitiligo [78], Graves’ disease [74], celiac disease [73], atopic dermatitis [79], and Addison’s disease [80]. These findings highlight the broad role of UBASH3A in the development and progression of autoimmune disorders, as well as its potential as a therapeutic target.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by synovitis and progressive joint destruction [81]. Cytokines generated by T cells following the activation of TCR signaling are significant contributors to the progression of rheumatoid arthritis (RA). A genome-wide meta-analysis identified UBASH3A as a susceptibility gene associated with an increased risk of rheumatoid arthritis in Korean and European populations [82]. The common variants in UBASH3A are also reported to be significantly associated with RA activity and severity in a Han Chinese population (p <.05) [83]. In addition to rs1893592, the rs3788013 polymorphism is also considered to be a locus for RA susceptibility in the Chinese Han population [84] (Table 1). Rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies are promising disease indicators for the early diagnosis and prognosis of RA. The rs1893592 genotype and allele frequency were shown to be substantially linked with the RF phenotype (χ2 = 6.786, p =.034; χ2 = 4.534, p =.033; respectively), whereas rs2277798 was significantly correlated with the anti-CCP phenotype in RA patients(χ2 = 7.873, p =.020; χ2 = 4.473, p =.034; respectively) [84]. Yamagata et al. demonstrated that the mRNA and protein levels of UBASH3A in CD4 + T cells from patients with rheumatoid arthritis are lower than those in healthy controls. The epigenetic regulation of super enhancers(SEs) inhibits UBASH3A transcription in CD4 + T cells, and low levels of UBASH3A lead to excessive activation of TCR signaling with subsequent IL-6 production [85]. A study by Li et al. revealed that the rs1893592 SNP in UBASH3A is significantly associated with the disease status of RA and is related to DAS28, CRP levels and bone erosion [83]. Similarly, Guderud et al. reported reduced DNA methylation at two distinct differentially methylated positions (DMPs) in the UBASH3A gene in CD4 + memory T cells from RA patients treated with methotrexate (MTX) compared with the control group, presumably resulting in higher expression levels of UBASH3A [86]. These studies provide evidence of the potential independent role of UBASH3A in the etiology of rheumatoid arthritis; furthermore, they establish a scientific foundation for considering UBASH3A activity as a potential predictive marker for disease activity in rheumatoid arthritis and for evaluating the efficacy of methotrexate (MTX) treatment.
Table 1.
Relationships between ubash3a gene polymorphisms and susceptibility to autoimmune diseases
| Disease susceptibility | Ethnicity | Polymorphism | References |
|---|---|---|---|
| RA | Korean and European populations, Chinese Han population | rs1893592 | [82–84] |
| Chinese Han population | rs3788013 | [84] | |
| T1D | European population | rs11203203 | [54, 92, 95] |
| European population | rs80054410 | [54] | |
| European population | rs11203202 | [92] | |
| European population | rs1893592 | [92] | |
| SLE | Chinese Han population | rs2277798 | [76] |
| AD | Chinese Han population | rs1893592 | [79] |
| AAD | UK population | rs11203203 | [80] |
| PSC | rs1893592 | [103] |
RA, rheumatoid arthritis; T1D, type 1 diabetes; SLE, systemic lupus erythematosus; AD, atopic dermatitis; AAD, autoimmune Addison’s disease; PSC, primary sclerosing cholangitis
Type 1 diabetes
Type 1 diabetes (T1D) is characterized by the autoimmune destruction of pancreatic β cells, leading to metabolic failure [87]. Many studies have identified UBASH3A as a causal gene for T1D [88–91]. The minor allele of rs1893592 is associated with a reduction in UBASH3A expression due to its impact on splicing, which correlates with a decreased risk of T1D (p = 2.6 × 10⁻⁸). Three single-nucleotide polymorphisms (SNPs) in the UBASH3A gene—specifically, the risk alleles at rs11203203, rs80054410, and rs11203203—associated with an increased T1D risk (p = 2.7 × 10− 3, p = 4.3 × 10− 4, and p = 4.0 × 10− 5, respectively) -have been shown by Ge et al. to increase UBASH3A expression in activated human primary CD4 + T cells, which blocks NF-κB signaling by affecting the IκB kinase complex, leading to a decrease in IL-2 production [54, 92] (Table 1). Consequently, UBASH3A might be involved in influencing the susceptibility to type 1 diabetes through the inhibition of T-cell receptor-induced NF-κB signaling pathways. Chen et al. also showed that UBASH3A deficiency accelerates the progression of T1D and exacerbates inflammation in the salivary glands, further emphasizing its broad impact on autoimmunity [93]. Mordes et al. further reported that UBASH3A determines susceptibility to diabetes in rat models, since Ubash3a knockout rats are more susceptible to diabetes [94]. UBASH3A (rs11203203) has been identified as an independent predictor of T1D in pediatric populations [95]. Clinical trials are necessary to establish its utility in predicting the risk of T1D and in prescreening children within the general population.
Prior research has clarified the distinct impacts of UBASH3A and its genetic variants on T1D risk, and delineating the relationship between UBASH3A and other risk factors associated with T1D may provide novel perspectives on the molecular networks underlying autoimmunity. Newman et al. reported that UBASH3A, through its SH3 domain, directly interacts with PTPN22, a well-known risk factor for T1D that concurrently impedes T-cell activation and IL-2 production [96–99]. This interaction exerts a cooperative effect on IL-2 expression in human primary CD8 + T cells and jointly affects the risk for T1D [96]. Notably, UBASH3A interacts with another independent inhibitor of NF-kB signaling and IL-2 production, CBL-B, whose mutation has been implicated in the development of autoimmune diabetes in the Komeda diabetes-prone rat model [61, 100, 101]. These compounds may have synergistic effects on T-cell activation and function, as well as on the pathogenesis of T1D, may become a combined therapeutic target for T1D.
Other autoimmune diseases
In addition to its role in RA and T1D, research has suggested that the UBASH3A gene might influence the susceptibility to and clinical phenotypes of other autoimmune diseases. Liu et al. reported that the rs2277798 polymorphism in the UBASH3A gene is correlated with an increased risk of systemic lupus erythematosus (SLE) in a Chinese Han cohort [76]. Additionally, Li et al. demonstrated that the rs1893592 variant in the UBASH3A gene is linked to increased susceptibility to atopic dermatitis (AD) in individuals from the Chinese Han population (p = 1.29 × 10− 3, OR = 1.16) [79]. Howarth et al. reported in a GWAS study that variation in the UBASH3A locus increased susceptibility to autoimmune Addison’s disease (AAD) in the British population cohort of 420 patients[OR 1.40(1.16–1.68)] [80]. The rs11203203 variant may affect the autoimmune response by regulating T cell function. A meta-analysis found notable disparities in the phenotypic presentation of inflammatory bowel disease (IBD) across genders, while the rs1893592 locus within the UBASH3A gene exhibits a genome-wide significant gender dimorphic association with IBD (p < 5 × 10⁻⁸) [102]. A fine-mapping study of primary sclerosing cholangitis (PSC) found that the non-coding variant rs1893592 of the UBASH3A gene increases the risk of PSC [103]. Further functional research will be necessary to elucidate the exact role of the UBASH3A gene in the pathogenesis of these diseases, and UBASH3A could be a target worthy of exploration for the development of therapeutic agents.
The significance of UBASH3A in infectious diseases
The involvement of UBASH3 proteins in modulating host immune responses to microbial pathogens was initially investigated through an assessment of the susceptibility of Ubash3−/− model mice to systemic infection by the dimorphic fungal pathogen Candida albicans. It causes invasive candidiasis, which is characterized by lethal organ failure as a result of disseminated fungal proliferation and inflammatory damage [104]. Ubash3−/− mice exhibit pronounced resistance to C. albicans bloodstream infection, which is evidenced by a substantial increase in survival rates, increased clearance of the pathogen from the renal system, and the establishment of a unique immunological milieu that restricts the growth of C. albicans, further supported by the early induction of the proinflammatory cytokine CXL10 [105]. In addition, mice with an individual deletion of either UBASH3A or UBASH3B display an intermediate lethality phenotype, and these two homologs appear to synergistically participate in regulating the host response to C. albicans infection. To identify the underlying cellular and molecular factors contributing to the augmented antifungal immune response observed in Ubash3−/− mice, Frank et al. reported that myeloid monocytes and myeloid-derived dendritic cells (BMDCs) derived from Ubash3−/− mice present a stronger ability to suppress the growth of Candida albicans in vitro, a phenotype associated with a marked increase in the production of reactive oxygen species (ROS) downstream of Dectin-1 signaling, as well as heightened Syk kinase activity [106]. Additional research has shown that UBASH3 proteins play a role in modulating host immunity to the gram-negative bacterium Francisella tularensis. Parashar et al. reported that mice lacking Ubash3 expression (Ubash3−/−) are resistant to infection by the live vaccine strain (LVS) Francisella tularensis [107]. In the context of infection with F. tularensis, Ubash3−/− marrow-derived monocytes and neutrophils display greater bactericidal activity that is accompanied by elevated IFNγ signaling and subsequently increased nitric oxide production [108]. These reports indicate a previously unrecognized function of UBASH3 enzymes in regulating innate immune response pathways. Further analysis is needed to dissect the individual functions of UBASH3A and UBASH3B in the immune response to infection.
Using mass spectrometry, Smirnova et al. reported that UBASH3A binds to ATP-binding cassette protein family E member 1 (ABCE-1), a host factor for HIV-1 assembly [109]. They also revealed that UBASH3A interferes with HIV-1 biogenesis and affects the late stages of the HIV-1 life cycle in a UBA-dependent manner [109].
Significance of UBASH3A in tumors
Despite its crucial role in autoimmunity, the connection between UBASH3A and cancers has not yet been comprehensively established. Wang et al. reported that UBASH3A knockdown in erythroleukemic cells increases the proliferation of leukemic cells, indicating that UBASH3A functions as a tumor suppressor in erythroleukemia and that this effect is associated with activation of the HSP70 gene HSPA1B [110]. The HSP70 (Heat Shock Protein 70) family, which acts as molecular chaperones involved in protein folding and repair within cells, is closely related to the occurrence, development and metastasis of tumors [111, 112]. The expression of HSP70 is modulated by various factors. Under standard physiological conditions, the levels of HSP70 expression are typically low. However, in response to stimuli such as heat shock, oxidative stress, and hypoxia, the heat shock factor (HSF) is activated within the cells. This activation leads to the binding of HSF to the promoter region of the HSP70 gene, which subsequently enhances the transcription and translation processes of HSP70, resulting in a significant increase in its expression levels. HSP70 inhibitors, such as VER155008, have demonstrated anti-proliferative properties in acute lymphoblastic leukemia (ALL) and oral squamous cell carcinoma [113, 114]. Consequently, targeting the UBASH3A-HSP70 pathway, which plays a key role in cell proliferation, may represent a novel therapeutic approach in the treatment of cancer. Further molecular interaction and functional phenotype experiments are needed to explore whether UBASH3A activates HSPA1B by regulating HSF, thereby achieving its tumor suppressive effect.
Wang et al. suggested that UBASH3A can be used as a prognostic marker for head and neck squamous cell carcinoma (HNSCC), as high levels of UBASH3A are significantly associated with a favorable prognosis in the TCGA database [115]. They further confirmed that the UBASH3A mRNA expression of tumor-infiltrating lymphocytes (TILs) in 10 patients with HNSCC was significantly greater than that of matched peripheral blood lymphocytes (PBLs). In most tumors, higher expression of UBASH3A is associated with a more favorable prognosis. However, overexpression of UBASH3A is associated with poor prognosis in metastatic breast cancer [116]. Liu et al. conducted whole-exome sequencing and identified UBASH3A as a potential breast cancer susceptibility gene in a Swedish population, with the variant UBASH3A-rs201756769 being classified as a high-risk genetic variant. Nevertheless, subsequent validation through protein expression analysis did not demonstrate any functional alterations in pathways associated with cancer [117]. Considering that UBASH3A plays a role in the suppression of autoimmune responses through the negative regulation of the T cell receptor (TCR) signaling pathway, in addition to its function in predicting tumor prognosis, the expression levels of UBASH3A may also serve as indicators of chemosensitivity and the likelihood of response to immunotherapy.
UBASH3A has also been implicated in the regulation of human kidney development [118]. Studies have shown that UBASH3A, together with other developmental control genes, such as transcription factors of the PAX family, the odd-skipped related 1 (OSR1), and the iroquois homeobox genes IRX3/IRX5, is involved in the formation of nephrons, and its abnormal function is associated with congenital anomalies of the kidney and urinary tract (CAKUT) [119–121]. Jinwoo Ahn et al. reported that UBASH3A is commonly mutated in individuals with clear cell renal cell carcinoma and identified it as a metastasis-associated candidate gene [122]. In vitro UBASH3A gene knockdown in a renal cell carcinoma cell line markedly decreased cell motility and viability, suggesting a potential role for UBASH3A in the induction of metastasis in clear cell renal cell carcinoma [122]. UBASH3A plays a crucial role in kidney development, potentially affecting nephron formation by modulating the mesenchymal-epithelial transition (MET) process or by contributing to the maintenance of homeostasis in renal tubular epithelial cells through the Wnt/β-catenin and Hippo-Yap signaling pathways. This regulatory function in development may be closely associated with its role as a tumor suppressor in renal cell carcinoma (RCC). Intriguingly, a gene coexpression network (GCN) analysis of gene expression data from RCC patients in the TCGA database revealed that UBASH3A is significantly implicated in clear cell renal cell carcinoma (KIRC), particularly in conjunction with the co-occurring mutations of the driver genes VHL and PBRM1 [123]. Therefore, UBASH3A-based gene therapy or immunomodulatory therapy may hold potential applications for high-risk renal cancer patients, such as transplant recipients. Given that UBASH3A serves as a crucial regulator in kidney development and is posited to function as a potential tumor suppressor gene, investigating its mechanisms is of considerable importance for elucidating the pathogenesis of kidney cancer and for the advancement of targeted therapeutic strategies.
Future perspectives
UBASH3A is expressed predominantly in T lymphocytes and other lymphoid cells, where it plays multifaceted roles in signal transduction, immune regulation, and the maintenance of cellular homeostasis. The ability of UBASH3A to attenuate TCR signaling underlies its biological functions, which include weak dephosphorylation, TCR-CD3 complex endocytosis, and the induction of AIF-dependent T-cell apoptosis. UBASH3A is also able to counter the effects of Cbl-mediated ubiquitylation and degradation of PTK. In this review, we summarize the biological properties and functions of UBASH3A, along with its role in various human diseases, which not only provides new insights into the molecular mechanisms underlying immune-mediated disorders but also makes UBASH3A a promising target for disease prediction and therapeutic strategies.
Prior research has demonstrated that T cell exhaustion arises when T cells experience prolonged activation due to infection or inflammation [124, 125]. This chronic activation leads to a gradual decline in T cell functionality, which is attributed to the upregulation of inhibitory receptors. A recent study highlighted the involvement of PSGL-1 (P-selectin glycoprotein-1), which serves as a T-cell intrinsic checkpoint regulator of CD8 + T-cell exhaustion, in sustaining elevated levels of UBASH3B expression as a possible mechanism for attenuating TCR signaling in tumor and chronic infection models, indicating that UBASH3B could play a role in T-cell exhaustion [126]. The interaction between UBASH3A and CBL-B (an E3 ubiquitin ligase) may affect the long-term activation and exhaustion of T cells by regulating the endocytosis and degradation of the TCR-CD3 complex. A gene coexpression network (GCN) analysis found that UBASH3A is co-expressed with the inhibitory immune checkpoint receptor TIGIT on the surface of T cells, suggesting its potential involvement in CD8 + T-cell exhaustion [123].
Genetic variants of UBASH3A have been demonstrated to be associated with numerous autoimmune disorders, infectious diseases, and neoplastic illnesses, suggesting a significant role for UBASH3A in the regulation of innate and adaptive immunity. The predictive value of UBASH3A encompasses the risk of autoimmune diseases, tumor prognosis, and the response to chemotherapy or immunotherapy, making its gene polymorphisms or protein expression levels important candidate biomarkers across different disease areas. Future research is required to further validate its predictive accuracy in clinical cohorts. UBASH3A also holds significant potential as a therapeutic target in various diseases, particularly in the fields of autoimmune diseases and oncology. Although there are currently no off-the-shelf drugs targeting UBASH3A, this gene plays a crucial role in the attenuation of the NF-κB pathway, making it a potentially significant drug target. Proteasome inhibitors (PIs) are a group of existing drugs that target the NF-κB pathway, capable of releasing other pro-inflammatory cytokines and inducing apoptosis in activated immune cells [127]. PIs are currently used in the treatment of multiple myeloma and graft-versus-host disease. Therefore, the development of small molecule agonists/inhibitors or gene therapy strategies targeting UBASH3A could be employed to treat autoimmune diseases or enhance anti-tumor and anti-pathogen immune responses, while avoiding the side effects of systemic immunosuppression.
Currently, the research on UBASH3A focuses on its role in TCR signaling regulation and autoimmune diseases. However, its specific molecular mechanisms and pathophysiological significance still need to be further explored. UBASH3A exhibits a bidirectional effect in the disease process; it can not only play an anti-cancer role by inhibiting the excessive activation of T cells but may also promote the inflammatory response through the activation of NF-κB. Future research can focus on the specific functions of UBASH3A in different immune cell subsets such as regulatory T cells (Treg) and T helper (Th) cells and analyze the regulatory mechanisms of non-coding region variations on its expression. With the help of single-cell sequencing and spatial transcriptomics technologies, the dynamic expression patterns of UBASH3A in diseased tissues can be revealed, and organoid models can be used to verify its pathological functions. Through prospective cohort studies, the correlation between UBASH3A variations and treatment responses can be clarified, providing a crucial basis for clinical translation.
Conclusion
UBASH3A has emerged as a critical immune regulator with pleiotropic roles in maintaining immune homeostasis. While its involvement in autoimmune diseases is supported by genetic and functional studies, the molecular mechanisms remain partially elusive. Further exploration of UBASH3A’s interactions and translational potential may unlock novel therapeutic strategies for autoimmune disorders, bridging the gap between basic immunology and clinical applications.
Acknowledgements
This work was supported by the Beijing Natural Science Foundation, the Capital’s Funds for Health Improvement and Research, and the National Natural Science Foundation of China.
Abbreviations
- UBASH3A
Ubiquitin associated and SH3 domain containing A
- TULA
T-Cell ubiquitin ligand protein
- STS-2
T-Cell ubiquitin ligand protein
- CLIP4
Cbl-interacting protein 4
- GWAS
Genome-wide association study
- TCR
T cell receptor
- WT
Wild-type
- dKO
Double knockout
- sKO
Single knockout
- PTK
Protein tyrosine kinase
- EGFR
Epidermal growth factor receptor
- PDGF
Platelet-derived growth factor
- CIN85
Cbl-interacting protein of 85 kDa
- AIF
Apoptosis-inducing factor
- SNP
Single nucleotide polymorphism
- RA
Rheumatoid arthritis
- T1D
Type 1 diabetes
- SLE
Systemic lupus erythematosus
- OR
Odds ratio
Author contributions
Meng Zhou: Conceptualization, Writing– original draft, Visualization, Writing– review & editing. Fangzhou Sun: Data curation, methodology, writing– review & editing. Zhenguo Zhao: Methodology, Software, Validation. Haili Qian: Funding acquisition, investigation, project administration, supervision. Yan Song: conceptualization, methodology, software, and validation. Ying Zhang: Conceptualization, Funding acquisition, Investigation, Project administration.
Funding
This work was supported by the Beijing Natural Science Foundation [L234039], the Capital’s Funds for Health Improvement and Research [No. 2022-2-2115], and the National Natural Science Foundation of China [No. 82472633].
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meng Zhou and Fangzhou Sun contributed equally to this work.
Contributor Information
Ying Zhang, Email: zyandzm@ccmu.edu.cn.
Yan Song, Email: songyan@cicams.ac.cn.
References
- 1.Wattenhofer M, Shibuya K, Kudoh J, Lyle R, Michaud J, Rossier C, et al. Isolation and characterization of the UBASH3A gene on 21q22.3 encoding a potential nuclear protein with a novel combination of domains. Hum Genet. 2001;108:140–7. [DOI] [PubMed] [Google Scholar]
- 2.Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, Geiger TL, et al. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20:37–46. [DOI] [PubMed] [Google Scholar]
- 3.Feshchenko EA, Smirnova EV, Swaminathan G, Teckchandani AM, Agrawal R, Band H, et al. TULA: an SH3- and UBA-containing protein that binds to c-Cbl and ubiquitin. Oncogene. 2004;23:4690–706. [DOI] [PubMed] [Google Scholar]
- 4.Kowanetz K, Crosetto N, Haglund K, Schmidt M, Heldin C-H, Dikic I. Suppressors of T-cell receptor signaling Sts-1 and Sts-2 bind to Cbl and inhibit endocytosis of receptor tyrosine kinases. J Biol Chem. 2004;279:32786–95. [DOI] [PubMed] [Google Scholar]
- 5.Mehrabipour M, Jasemi NSK, Dvorsky R, Ahmadian MR. A systematic compilation of human SH3 domains: a versatile superfamily in cellular signaling. Cells. 2023;12:2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Jakoncic J, Carpino N, Nassar N. Structural and functional characterization of the 2H-phosphatase domain of Sts-2 reveals an acid-dependent phosphatase activity. Biochemistry. 2009;48:1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Jakoncic J, Parker KA, Carpino N, Nassar N. Structures of the phosphorylated and VO(3)-bound 2H-phosphatase domain of Sts-2. Biochemistry. 2009;48:8129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpino N, Kobayashi R, Zang H, Takahashi Y, Jou S-T, Feng J, et al. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol Cell Biol. 2002;22:7491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UniProt Consortium. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 2023;51:D523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y, Frank D, Zhou W, Kaur N, French JB, Carpino N. An unexpected 2-histidine phosphoesterase activity of suppressor of T-cell receptor signaling protein 1 contributes to the suppression of cell signaling. J Biol Chem. 2020;295:8514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazumder R, Iyer LM, Vasudevan S, Aravind L. Detection of novel members, structure-function analysis and evolutionary classification of the 2H phosphoesterase superfamily. Nucleic Acids Res. 2002;30:5229–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raasakka A, Myllykoski M, Laulumaa S, Lehtimäki M, Härtlein M, Moulin M, et al. Determinants of ligand binding and catalytic activity in the myelin enzyme 2’,3’-cyclic nucleotide 3’-phosphodiesterase. Sci Rep. 2015;5:16520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reppschläger K, Gosselin J, Dangelmaier CA, Thomas DH, Carpino N, McKenzie SE, et al. TULA-2 protein phosphatase suppresses activation of Syk through the GPVI platelet receptor for collagen by dephosphorylating Tyr(P)346, a regulatory site of Syk. J Biol Chem. 2016;291:22427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DH, Getz TM, Newman TN, Dangelmaier CA, Carpino N, Kunapuli SP, et al. A novel histidine tyrosine phosphatase, TULA-2, associates with Syk and negatively regulates GPVI signaling in platelets. Blood. 2010;116:2570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Castro RO, Zhang J, Groves JR, Barbu EA, Siraganian RP. Once phosphorylated, tyrosines in carboxyl terminus of protein-tyrosine kinase Syk interact with signaling proteins, including TULA-2, a negative regulator of mast cell degranulation. J Biol Chem. 2012;287:8194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Back SH, Adapala NS, Barbe MF, Carpino NC, Tsygankov AY, Sanjay A. TULA-2, a novel histidine phosphatase, regulates bone remodeling by modulating osteoclast function. Cell Mol Life Sci CMLS. 2013;70:1269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Vakhrusheva O, Bandi SR, Demirel Ö, Kazi JU, Fernandes RG, et al. The phosphatases STS1 and STS2 regulate hematopoietic stem and progenitor cell fitness. Stem Cell Rep. 2015;5:633–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikhailik A, Ford B, Keller J, Chen Y, Nassar N, Carpino N. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol Cell. 2007;27:486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.San Luis B, Sondgeroth B, Nassar N, Carpino N. Sts-2 is a phosphatase that negatively regulates zeta-associated protein (ZAP)-70 and T cell receptor signaling pathways. J Biol Chem. 2011;286:15943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vukojević K, Šoljić V, Martinović V, Raguž F, Filipović N. The Ubiquitin-Associated and SH3 Domain-Containing proteins (UBASH3) family in mammalian development and immune response. Int J Mol Sci. 2024;25:1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsygankov AY. TULA proteins in men, mice, hens, and lice: welcome to the family. Int J Mol Sci. 2023;24:9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cutler JA, Udainiya S, Madugundu AK, Renuse S, Xu Y, Jung J, et al. Integrative phosphoproteome and interactome analysis of the role of Ubash3b in BCR-ABL signaling. Leukemia. 2020;34:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krupina K, Kleiss C, Awal S, Rodriguez-Hernandez I, Sanz-Moreno V, Sumara I. UBASH3B-mediated silencing of the mitotic checkpoint: therapeutic perspectives in cancer. Mol Cell Oncol. 2018;5:e1271494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes B, van der Geer P. STS-1 and STS-2, multi-enzyme proteins equipped to mediate protein-protein interactions. Int J Mol Sci. 2023;24:9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaman A, French JB, Carpino N. The Sts proteins: modulators of host immunity. Int J Mol Sci. 2023;24:8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaud G, Lesourne R, Love PE. Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol. 2018;18:485–97. [DOI] [PubMed] [Google Scholar]
- 28.Courtney AH, Lo W-L, Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem Sci. 2018;43:108–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voisinne G, Kersse K, Chaoui K, Lu L, Chaix J, Zhang L, et al. Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics. Nat Immunol. 2019;20:1530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swamy M. ZAP70 holds the key to kinetic proofreading for TCR ligand discrimination. Nat Immunol. 2022;23:1293–4. [DOI] [PubMed] [Google Scholar]
- 31.Lo W-L, Kuhlmann M, Rizzuto G, Ekiz HA, Kolawole EM, Revelo MP, et al. A single-amino acid substitution in the adaptor LAT accelerates TCR proofreading kinetics and alters T-cell selection, maintenance and function. Nat Immunol. 2023;24:676–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashouri JF, Lo W-L, Nguyen TTT, Shen L, Weiss A. ZAP70, too little, too much can lead to autoimmunity. Immunol Rev. 2022;307:145–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dikic I, Giordano S. Negative receptor signalling. Curr Opin Cell Biol. 2003;15:128–35. [DOI] [PubMed] [Google Scholar]
- 34.Shifrut E, Carnevale J, Tobin V, Roth TL, Woo JM, Bui CT, et al. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell. 2018;175:1958–71.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z-H, Zhang Z-Y. Regulatory mechanisms and novel therapeutic targeting strategies for protein tyrosine phosphatases. Chem Rev. 2018;118:1069–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Au-Yeung BB, Shah NH, Shen L, Weiss A. ZAP-70 in signaling, biology, and disease. Annu Rev Immunol. 2018;36:127–56. [DOI] [PubMed] [Google Scholar]
- 37.Leveille E, Chan LN, Mirza A-S, Kume K, Müschen M. SYK and ZAP70 kinases in autoimmunity and lymphoid malignancies. Cell Signal. 2022;94:110331. [DOI] [PubMed] [Google Scholar]
- 38.Carpino N, Chen Y, Nassar N, Oh H-W. The Sts proteins target tyrosine phosphorylated, ubiquitinated proteins within TCR signaling pathways. Mol Immunol. 2009;46:3224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman TN, Liverani E, Ivanova E, Russo GL, Carpino N, Ganea D, et al. Members of the novel UBASH3/STS/TULA family of cellular regulators suppress T-cell-driven inflammatory responses in vivo. Immunol Cell Biol. 2014;92:837–50. [DOI] [PubMed] [Google Scholar]
- 40.Okabe N, Ohmura K, Katayama M, Akizuki S, Carpino N, Murakami K, et al. Suppressor of TCR signaling-2 (STS-2) suppresses arthritis development in mice. Mod Rheumatol. 2018;28:626–36. [DOI] [PubMed] [Google Scholar]
- 41.Sadatomi D, Tanimura S, Ozaki K-I, Takeda K. Atypical protein phosphatases: emerging players in cellular signaling. Int J Mol Sci. 2013;14:4596–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raguz J, Wagner S, Dikic I, Hoeller D. Suppressor of T-cell receptor signalling 1 and 2 differentially regulate endocytosis and signalling of receptor tyrosine kinases. FEBS Lett. 2007;581:4767–72. [DOI] [PubMed] [Google Scholar]
- 43.Agrawal R, Carpino N, Tsygankov A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J Cell Biochem. 2008;104:953–64. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Ren L, Kim S, Carpino N, Daniel JL, Kunapuli SP, et al. Determination of the substrate specificity of protein-tyrosine phosphatase TULA-2 and identification of Syk as a TULA-2 substrate. J Biol Chem. 2010;285:31268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu H, Sun S-C. Ubiquitin signaling in immune responses. Cell Res. 2016;26:457–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao N, Dodge I, Band H. The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J Leukoc Biol. 2002;71:753–63. [PubMed] [Google Scholar]
- 47.Thien CBF, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. [DOI] [PubMed] [Google Scholar]
- 49.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–70. [DOI] [PubMed] [Google Scholar]
- 50.Tsygankov AY. TULA proteins as signaling regulators. Cell Signal. 2020;65:109424. [DOI] [PubMed] [Google Scholar]
- 51.Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S, et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol. 2006;8:163–9. [DOI] [PubMed] [Google Scholar]
- 52.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–86. [DOI] [PubMed] [Google Scholar]
- 54.Ge Y, Paisie TK, Newman JRB, McIntyre LM, Concannon P. UBASH3A mediates risk for type 1 diabetes through inhibition of T-cell receptor-induced NF-κB signaling. Diabetes. 2017;66:2033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Havrylov S, Redowicz MJ, Buchman VL. Emerging roles of Ruk/CIN85 in vesicle-mediated transport, adhesion, migration and malignancy. Traffic Cph Den. 2010;11:721–31. [DOI] [PubMed] [Google Scholar]
- 56.Kong MS, Hashimoto-Tane A, Kawashima Y, Sakuma M, Yokosuka T, Kometani K, et al. Inhibition of T cell activation and function by the adaptor protein CIN85. Sci Signal. 2019;12:eaav4373. [DOI] [PubMed] [Google Scholar]
- 57.Mori D, Grégoire C, Voisinne G, Celis-Gutierrez J, Aussel R, Girard L, et al. The T cell CD6 receptor operates a multitask signalosome with opposite functions in T cell activation. J Exp Med. 2021;218:e20201011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antonny B, Burd C, De Camilli P, Chen E, Daumke O, Faelber K, et al. Membrane fission by dynamin: what we know and what we need to know. EMBO J. 2016;35:2270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.González-Jamett AM, Momboisse F, Haro-Acuña V, Bevilacqua JA, Caviedes P, Cárdenas AM. Dynamin-2 function and dysfunction along the secretory pathway. Front Endocrinol. 2013;4:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bertelsen V, Breen K, Sandvig K, Stang E, Madshus IH. The Cbl-interacting protein TULA inhibits dynamin-dependent endocytosis. Exp Cell Res. 2007;313:1696–709. [DOI] [PubMed] [Google Scholar]
- 61.Ge Y, Paisie TK, Chen S, Concannon P. UBASH3A regulates the synthesis and dynamics of TCR-CD3 complexes. J Immunol Baltim Md. 1950. 2019;203:2827–36. [DOI] [PMC free article] [PubMed]
- 62.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–6. [DOI] [PubMed] [Google Scholar]
- 63.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–20. [DOI] [PubMed] [Google Scholar]
- 64.Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res CR. 2011;30:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.G M, M N-S, Eh B, G K.. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014 [cited 2024 Aug 28];15. Available from: https://pubmed.ncbi.nlm.nih.gov/24401948/. [DOI] [PMC free article] [PubMed]
- 67.H O, S O, Z N, N I, K M. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005 [cited 2024 Aug 28];24. Available from: https://pubmed.ncbi.nlm.nih.gov/15775970/. [DOI] [PMC free article] [PubMed]
- 68.Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J Biol Chem. 2005;280:2266–74. [DOI] [PubMed] [Google Scholar]
- 69.Polster BM, Basañez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–54. [DOI] [PubMed] [Google Scholar]
- 70.Yuste VJ, Moubarak RS, Delettre C, Bras M, Sancho P, Robert N, et al. Cysteine protease inhibition prevents mitochondrial apoptosis-inducing factor (AIF) release. Cell Death Differ. 2005;12:1445–8. [DOI] [PubMed] [Google Scholar]
- 71.Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, et al. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol. 2002;9:680–4. [DOI] [PubMed] [Google Scholar]
- 72.Collingwood TS, Smirnova EV, Bogush M, Carpino N, Annan RS, Tsygankov AY. T-cell ubiquitin ligand affects cell death through a functional interaction with apoptosis-inducing factor, a key factor of caspase-independent apoptosis. J Biol Chem. 2007;282:30920–8. [DOI] [PubMed] [Google Scholar]
- 73.Zhernakova A, Stahl EA, Trynka G, Raychaudhuri S, Festen EA, Franke L, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011;7:e1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plagnol V, Howson JMM, Smyth DJ, Walker N, Hafler JP, Wallace C, et al. Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet. 2011;7:e1002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diaz-Gallo L-M, Sánchez E, Ortego-Centeno N, Sabio JM, García-Hernández FJ, de Ramón E, et al. Evidence of new risk genetic factor to systemic lupus erythematosus: the UBASH3A gene. PLoS ONE. 2013;8:e60646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J, Liu J, Ni J, Leng RX, Pan HF, Ye DQ. Association of UBASH3A gene polymorphisms and systemic lupus erythematosus in a Chinese population. Gene. 2015;565:116–21. [DOI] [PubMed] [Google Scholar]
- 77.Liu J, Ni J, Li L-J, Leng R-X, Pan H-F, Ye D-Q. Decreased UBASH3A mRNA expression levels in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Inflammation. 2015;38:1903–10. [DOI] [PubMed] [Google Scholar]
- 78.Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Cheng H, Xiao F-L, Liang B, Zhou F-S, Li P, et al. Association of UBASH3A gene polymorphism and atopic dermatitis in the Chinese Han population. Genes Immun. 2017;18:158–62. [DOI] [PubMed] [Google Scholar]
- 80.Howarth S, Sneddon G, Allinson KR, Razvi S, Mitchell AL, Pearce SHS. Replication of association at the LPP and UBASH3A loci in a UK autoimmune addison’s disease cohort. Eur J Endocrinol. 2023;188:lvac010. [DOI] [PubMed] [Google Scholar]
- 81.Di Matteo A, Bathon JM, Emery P. Rheumatoid arthritis. Lancet Lond Engl. 2023;402:2019–33. [DOI] [PubMed] [Google Scholar]
- 82.Kim K, Bang S-Y, Lee H-S, Cho S-K, Choi C-B, Sung Y-K, et al. High-density genotyping of immune loci in Koreans and Europeans identifies eight new rheumatoid arthritis risk loci. Ann Rheum Dis. 2015;74:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu D, Liu J, Cui G, Yang H, Cao T, Wang L. Evaluation of the association of UBASH3A and SYNGR1 with rheumatoid arthritis and disease activity and severity in Han Chinese. Oncotarget. 2017;8:103385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang X-K, Liu J, Chen S-Y, Li M, Zhang M-M, Leng R-X, et al. UBASH3A gene polymorphisms and expression profile in rheumatoid arthritis. Autoimmunity. 2019;52:21–6. [DOI] [PubMed] [Google Scholar]
- 85.Yamagata K, Nakayamada S, Zhang T, Nguyen AP, Ohkubo N, Iwata S, et al. IL-6 production through repression of UBASH3A gene via epigenetic dysregulation of super-enhancer in CD4 + T cells in rheumatoid arthritis. Inflamm Regen. 2022;42:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guderud K, Sunde LH, Flåm ST, Mæhlen MT, Mjaavatten MD, Lillegraven S, et al. Rheumatoid arthritis patients, both newly diagnosed and methotrexate treated, show more DNA methylation differences in CD4 + Memory than in CD4 + Naïve T cells. Front Immunol. 2020;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herold KC, Delong T, Perdigoto AL, Biru N, Brusko TM, Walker LSK. The immunology of type 1 diabetes. Nat Rev Immunol. 2024;24:435–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Concannon P, Onengut-Gumuscu S, Todd JA, Smyth DJ, Pociot F, Bergholdt R, et al. A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes. 2008;57:2858–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grant SFA, Qu H-Q, Bradfield JP, Marchand L, Kim CE, Glessner JT, et al. Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes. 2009;58:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Onengut-Gumuscu S, Chen W-M, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ge Y, Concannon P. Molecular-genetic characterization of common, noncoding UBASH3A variants associated with type 1 diabetes. Eur J Hum Genet EJHG. 2018;26:1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Y-G, Ciecko AE, Khaja S, Grzybowski M, Geurts AM, Lieberman SM. UBASH3A deficiency accelerates type 1 diabetes development and enhances salivary gland inflammation in NOD mice. Sci Rep. 2020;10:12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mordes JP, Cort L, Liu Z, Eberwine R, Blankenhorn EP, Pierce BG. T cell receptor genotype and Ubash3a determine susceptibility to rat autoimmune diabetes. Genes. 2021;12:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson K, Wong R, Barriga KJ, Klingensmith G, Ziegler A-G, Rewers MJ, et al. rs11203203 is associated with type 1 diabetes risk in population pre-screened for high-risk HLA-DR,DQ genotypes. Pediatr Diabetes. 2012;13:611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jrb N, P C, Y G. UBASH3A interacts with PTPN22 to regulate IL2 expression and risk for Type 1 diabetes. Int J Mol Sci. 2023;24. Available from: https://pubmed.ncbi.nlm.nih.gov/37240014/. [DOI] [PMC free article] [PubMed]
- 97.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8. [DOI] [PubMed] [Google Scholar]
- 98.Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol. 2014;32:83–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Armitage LH, Wallet MA, Mathews CE. Influence of PTPN22 allotypes on innate and adaptive immune function in health and disease. Front Immunol. 2021;12:636618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qiao G, Li Z, Molinero L, Alegre M-L, Ying H, Sun Z, et al. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol. 2008;28:2470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yokoi N, Komeda K, Wang H-Y, Yano H, Kitada K, Saitoh Y, et al. Cblb is a major susceptibility gene for rat type 1 diabetes mellitus. Nat Genet. 2002;31:391–4. [DOI] [PubMed] [Google Scholar]
- 102.Khrom M, Li D, Naito T, Lee H-S, Botwin GJ, Potdar AA, et al. Sex-dimorphic analyses identify novel and sex-specific genetic associations in inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goode EC, Fachal L, Panousis N, Moutsianas L, McIntyre RE, Bai BYH, et al. Fine-mapping and molecular characterisation of primary sclerosing cholangitis genetic risk loci. Nat Commun. 2024;15:9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nobile CJ, Johnson AD. Candida albicans biofilms and human disease. Annu Rev Microbiol. 2015;69:71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naseem S, Frank D, Konopka JB, Carpino N. Protection from systemic Candida albicans infection by inactivation of the Sts phosphatases. Infect Immun. 2015;83:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frank D, Naseem S, Russo GL, Li C, Parashar K, Konopka JB, et al. Phagocytes from mice lacking the Sts phosphatases have an enhanced antifungal response to Candida albicans. mBio. 2018;9:e00782–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parashar K, Kopping E, Frank D, Sampath V, Thanassi DG, Carpino N. Increased resistance to intradermal Francisella tularensis LVS infection by inactivation of the Sts phosphatases. Infect Immun. 2017;85:e00406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parashar K, Carpino N. A role for the Sts phosphatases in negatively regulating IFNγ-mediated production of nitric oxide in monocytes. Immun Inflamm Dis. 2020;8:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smirnova EV, Collingwood TS, Bisbal C, Tsygankova OM, Bogush M, Meinkoth JL, et al. TULA proteins bind to ABCE-1, a host factor of HIV-1 assembly, and inhibit HIV-1 biogenesis in a UBA-dependent fashion. Virology. 2008;372:10–23. [DOI] [PubMed] [Google Scholar]
- 110.Wang J, Wang C, Hu A, Yu K, Kuang Y, Gajendran B, et al. FLI1 induces erythroleukemia through opposing effects on UBASH3A and UBASH3B expression. BMC Cancer. 2024;24:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019;20:665–80. [DOI] [PubMed] [Google Scholar]
- 112.Albakova Z, Armeev GA, Kanevskiy LM, Kovalenko EI, Sapozhnikov AM. HSP70 multi-functionality in cancer. Cells. 2020;9:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guo D, Zhang A, Huang J, Suo M, Zhong Y, Liang Y. Suppression of HSP70 inhibits the development of acute lymphoblastic leukemia via TAK1/Egr-1. Biomed Pharmacother Biomedecine Pharmacother. 2019;119:109399. [DOI] [PubMed] [Google Scholar]
- 114.Ding L-X, Zhang J, Yang S-S, Wu J, Su T, Wang W-M. Heat shock proteins 70 regulate cell motility and invadopodia-associated proteins expression in oral squamous cell carcinoma. Front Endocrinol. 2022;13:890218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Tian Y, Zhu G, Li Z, Wu Z, Wei G, et al. Establishment and validation of immune microenvironmental gene signatures for predicting prognosis in patients with head and neck squamous cell carcinoma. Int Immunopharmacol. 2021;97:107817. [DOI] [PubMed] [Google Scholar]
- 116.Rui X, Li Y, Jin F, Li F. TMPRSS3 is a novel poor prognostic factor for breast cancer. Int J Clin Exp Pathol. 2015;8:5435–42. [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Helgadottir HT, Kharaziha P, Choi J, López-Giráldez F, Mane SM, et al. Whole-exome sequencing of germline variants in non-BRCA families with hereditary breast cancer. Biomedicines. 2022;10:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lozic M, Minarik L, Racetin A, Filipovic N, Saraga Babic M, Vukojevic K. CRKL, AIFM3, AIF, BCL2, and UBASH3A during human kidney development. Int J Mol Sci. 2021;22:9183. [DOI] [PMC free article] [PubMed]
- 119.Lozic M, Vukojevic K. Altered UBASH3A expression may be involved in congenital anomalies of the kidney and urinary tract (CAKUT) phenotypes of down syndrome: SA-PO620. J Am Soc Nephrol. 2022;33:774. [Google Scholar]
- 120.Li M, Li Y, Weeks O, Mijatovic V, Teumer A, Huffman JE, et al. SOS2 and ACP1 loci identified through large-scale exome chip analysis regulate kidney development and function. J Am Soc Nephrol JASN. 2017;28:981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu J, Liu H, Chai OH, Lan Y, Jiang R. Osr1 interacts synergistically with Wt1 to regulate kidney organogenesis. PLoS ONE. 2016;11:e0159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahn J, Han KS, Heo JH, Bang D, Kang YH, Jin HA, et al. FOXC2 and CLIP4: a potential biomarker for synchronous metastasis of ≤ 7-cm clear cell renal cell carcinomas. Oncotarget. 2016;7:51423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poehlman WL, Hsieh JJ, Feltus FA. Linking binary gene relationships to drivers of renal cell carcinoma reveals convergent function in alternate tumor progression paths. Sci Rep. 2019;9:2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baessler A, Vignali DAA. T cell exhaustion. Annu Rev Immunol. 2024;42:179–206. [DOI] [PubMed] [Google Scholar]
- 125.Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19:775–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hope JL, Otero DC, Bae E-A, Stairiker CJ, Palete AB, Faso HA, et al. PSGL-1 attenuates early TCR signaling to suppress CD8 + T cell progenitor differentiation and elicit terminal CD8 + T cell exhaustion. Cell Rep. 2023;42:112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fricker LD. Proteasome inhibitor drugs. Annu Rev Pharmacol Toxicol. 2020;60:457–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.