Abstract
Background
The objective of this study was to perform a cost-utility analysis of dapagliflozin, as an add-on therapy to standard of care (SoC), compared with SoC, for patients with symptomatic chronic heart failure (HF) in Spain, including patients with reduced and preserved ejection fraction.
Methods
A Markov model was designed to simulate the progression of chronic HF over a lifetime horizon using pooled data from the DAPA-HF and DELIVER trials. Disease progression was captured by transitions between health states, defined by the Kansas City Cardiomyopathy Questionnaire Total Symptom Score. Transient events of hospitalization for HF (HHF), urgent HF visits (UHFV) and cardiovascular (CV) and non-CV death were included. The analysis was conducted from the Spanish National Health System perspective. The results were expressed as cost per quality-adjusted life year (QALY) gained. Sensitivity analyses were performed to assess the robustness of the results.
Results
Dapagliflozin + SoC showed an increase in effectiveness (0.31 QALY) and total cost per patient (€1,441) compared to SoC, yielding an incremental cost-utility ratio of €4,611/QALY. Dapagliflozin reduced the incidence of HHF by 136.4 events (752.2 vs. 886.6), UHFV by 38.8 (217.6 vs. 254.4) and CV death by 23.0 (505.8 vs. 528.8) for every 1,000 patients. Dapagliflozin + SoC was cost-effective compared to SoC in 99.9% of iterations at a willingness-to-pay (WTP) threshold of €25,000/QALY.
Conclusions
The analysis shows that dapagliflozin, as add-on therapy to SoC, would be a cost-effective option compared to SoC for the treatment of adult patients with symptomatic chronic HF in Spain at a WTP of €25,000/QALY.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-025-13089-7.
Keywords: Cost-effectiveness analysis, Cost-utility analysis, Dapagliflozin, Symptomatic chronic heart failure, Spain
Background
Heart failure (HF) is a progressive and debilitating chronic cardiovascular (CV) condition associated with high morbidity, mortality and significant economic burden [1–3]. The prevalence of HF is around 2% of the adult population in developed countries [4, 5]. However, this prevalence is expected to increase in the coming years, mainly due to the ageing population [4, 6]. In Spain, the prevalence of HF reported in recent studies was 1.9% of the population and the incidence was between 2.8 and 3.2 per 1,000 person-years [7, 8]. HF is the leading cause of hospitalization in Europe and the United States, with more than one million HF-related admissions each year and an increasing risk of death [9, 10]. In addition, symptoms associated with HF have a negative impact on patients’ health-related quality of life (HRQoL) [1, 2, 11].
The European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of HF define 3 categories according to left ventricular ejection fraction (LVEF): HF with reduced ejection fraction (HFrEF) for LVEF ≤ 40%, HF with mildly reduced ejection fraction (HFmrEF) for LVEF 41–49% and HF with preserved ejection fraction (HFpEF) for LVEF ≥ 50% [12]. The ESC guidelines 2023 recommend the use of renin-angiotensin-aldosterone system (RAAS) inhibitors, angiotensin-receptor-neprilysin (ARN) inhibitors, β-blockers, mineralocorticoid receptor antagonists (MRA) in patients with HFrEF [12]. Although these treatments reduce HF-related mortality and hospitalization, patients still face an increased risk of morbidity and mortality [13]. In addition, these therapies are not recommended for people with HFpEF since they have not improved outcomes and do not address the underlying causes of the disease [10].
The ESC guidelines also recommend sodium-glucose co-transporter-2 (SGLT2) inhibitors for the treatment of chronic symptomatic HF [12]. Dapagliflozin reduces the reabsorption of glucose from the glomerular filtrate in the proximal renal tubule, leading to urinary excretion of glucose and osmotic diuresis [14]. As a result, dapagliflozin has emerged as an important therapeutic strategy to reduce the risk of CV disease in patients with type 2 diabetes mellitus (T2DM) [15], HFrEF [16], chronic kidney disease [17] and, more recently, HFpEF and HFmrEF [18].
A pooled meta-analysis of the landmark DAPA-HF and DELIVER trials (n = 11,007) was conducted to evaluate key endpoints from both individual trials that required additional power to detect differences in the treatment of symptomatic chronic HF [19]. Dapagliflozin was associated with statistically significant reduction in total hospitalizations for heart failure (HHF) (rate ratio: 0.71; 95% confidence interval [CI]: 0.65–0.78), risk of death from CV causes (hazard ratio [HR]: 0.86; 95% CI: 0.76–0.97) and death from any cause (HR: 0.90; 95% CI: 0.82–0.99) [19].
Cost-effectiveness analyses are useful to support strategic decisions and value propositions. The objective of this study was to assess the cost-effectiveness of dapagliflozin, as an add-on therapy to standard of care (SoC) compared with SoC alone for the treatment of patients with symptomatic chronic HF in Spain, based on the pooled results of the DAPA-HF and DELIVER trials.
Methods
Patient population and alternatives evaluated
A cohort of 1,000 patients was simulated. The patient population represented patients with symptomatic chronic HF in Spain. Baseline patient characteristics were obtained from the national literature [7] and, when data were not available, patient characteristics were obtained from the pooled analysis of the DAPA-HF and DELIVER clinical trials [19]. Patients were divided into those with a LVEF > 40%, including HFpEF and HFmrEF patients, and those with LVEF ≤ 40%, including HFrEF patients [12, 19]. Patients entered the model at a mean age of 69.7 years and 53.8% of the patients were male. Overall, 44.3% of the patients had a New York Heart Association (NYHA) class III/IV, mean LVEF was 44.2% (LVEF > 40%: 41.9%; LVEF ≤ 40%: 58.1%) and 27.6% of the patients had T2DM [7, 19]. Baseline characteristics of the population are presented in Table 1.
Table 1.
Baseline characteristics of the patient population
| Parameter | Mean (SE) | Distribution | Reference |
|---|---|---|---|
| Demographics | |||
| Age (years) | 69.7 (0.13) | Normal | [7] |
| Male (%) | 53.8 (10.8a) | Beta | [7] |
| BMI (kg/m2) | 29.12 (0.06) | Normal | [19] |
| Race (%) | |||
| White | 90.2 (18.0a) | Beta | [19] |
| Black/African | 4.5 (0.9a) | Beta | [19] |
| Other | 5.3 (1.1a) | Beta | [19] |
| Clinical characteristics | |||
| KCCQ-TSS (%) | |||
| KCCQ-TSS Q1 | 24.9 (0.4) | Dirichlet | [19] |
| KCCQ-TSS Q2 | 24.3 (0.4) | Dirichlet | [19] |
| KCCQ-TSS Q3 | 26.3 (0.4) | Dirichlet | [19] |
| KCCQ-TSS Q4 | 24.4 (0.4) | Dirichlet | [19] |
| NYHA Class III/IV (%) | 44.3 (8.9a) | Beta | [7] |
| LVEF (%) | 44.2 (13.0) | Normal | [19] |
| NT-proBNP (pg/ml) | 1,900.87 (23.82) | Normal | [19] |
| Creatinine (µmol/l) | 103.32 (29.0) | Normal | [19] |
| SBP (mmHg) | 125.46 (15.0) | Normal | [19] |
| Heart rate (bpm) | 71.49 (11.0) | Normal | [19] |
| Medical history | |||
| T2DM (%) | 27.6 (5.5a) | Beta | [7] |
| AFF (%) | 28.2 (5.6a) | Beta | [7] |
| Most recent HHF > 6 months (%) | 24.5 (0.4) | Beta | [19] |
| Most recent HHF ≤ 6 months (%) | 19.0 (0.4) | Beta | [19] |
| HF duration > 2 years (%) | 56.6 (0.5) | Beta | [19] |
| Prior MI (%) | 15.0 (3.0a) | Beta | [7] |
aA 20% variation was assumed when SE was not possible to calculate
Abbreviations: AFF Atrial fibrillation/flutter, BMI Body mass index, HF Heart failure, HHF Hospitalization for heart failure, KCCQ Kansas City Cardiomyopathy Questionnaire, LVEF Left ventricular ejection fraction, MI Myocardial infarction, NT-proBNP N-terminal pro-B-type natriuretic peptide, NYHA New York Heart Association, SBP Systolic blood pressure, SE Standard error, T2DM Type 2 diabetes mellitus, TSS Total symptom score
The two treatment arms evaluated were based on the pooled data from the DAPA-HF and DELIVER trials: dapagliflozin as an add-on to SoC (“Dapagliflozin + SoC”) compared to SoC alone (“SoC”). In terms of SoC, patients with LVEF > 40% were assumed to all be treated with furosemide based on the DELIVER trial [18]; and patients with LVEF ≤ 40% were assumed to be treated with angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, mineralocorticoid receptor antagonists, diuretics, If channel blockers and angiotensin receptor neprilysin inhibitors, based on Spanish literature [20] and European guidelines [21].
Analysis performed
An economic evaluation was performed to compare the consequences of two treatment alternatives in terms of health effects and costs [22]. The results of the cost-utility and cost-effectiveness analyses were expressed as an incremental cost-utility ratio (ICUR) and incremental cost-effectiveness ratio (ICER), calculated as the difference in costs divided by the difference in quality adjusted life years (QALY) and life years gained (LYG), respectively, and expressed as cost per incremental LYG saved and QALY gained [23]. All analyses assumed a willingness-to-pay (WTP) threshold of €25,000/QALY gained for Spain [24, 25].
This analysis was conducted from the Spanish National Health Service (NHS) perspective and considered a lifetime horizon to reflect the chronic and progressive nature of HF [26, 27]. For the Spanish setting, costs and QALYs were discounted at 3% each year, aligned with national technology assessment guidance [26, 27]. All inputs, structure and assumptions included in the model were validated with clinical experts to ensure that they are consistent with current clinical practice in Spain.
The model was developed using Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA). Model calculations were primarily performed using worksheet formulas, with the addition of Visual Basic macros.
Model structure
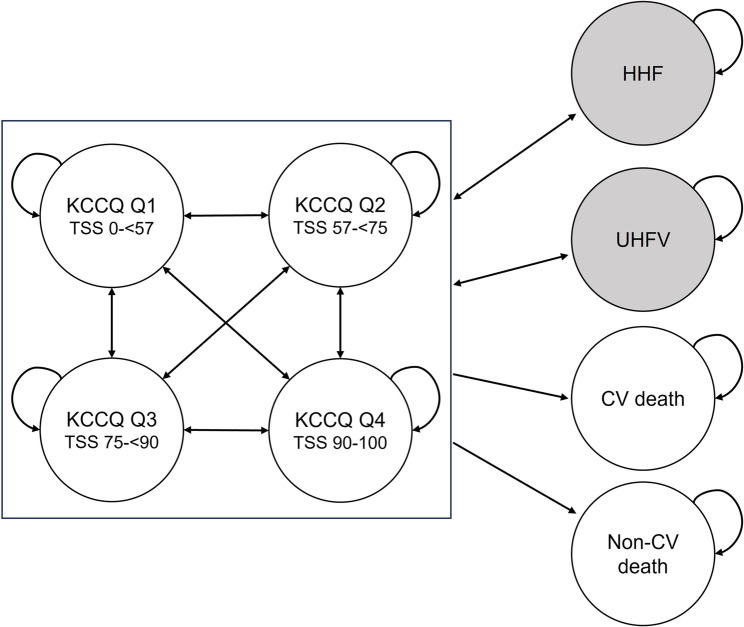
A Markov model was designed to simulate the progression of HF. Markov models assume that disease progression can be represented by a set of discrete and mutually exclusive health states, and that simulated patients can transition between these states over time [28, 29]. The model assumes that the future state of the system depends only on the current state and not on the sequence of events that preceded it. Accordingly, patients in the model can transition from one state to another or remain in the same state without transiting to other states based on transition probabilities [28]. The model consisted of four non-absorbing health states, where disease progression was captured by transitions between discrete health states defined by quartiles of the Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS Q1-Q4). The KCCQ-TSS is a well-established and extensively validated instrument for quantifying the frequency and severity of patients’ symptoms [30, 31]. The model also included the incidence of HHF and urgent heart failure visit (UHFV), defined as transient events. For transient events, patients only incur the additional event-specific direct medical costs and utility decrements in the cycle of the event [32]. Finally, the model included two absorbing health states to assess patient mortality using parametric survival equations describing CV death and non-CV death, calculated as the difference between death from any cause and CV death (Fig. 1).
Fig. 1.
Markov model structure Note: Each circle represents a Markov state; shaded circles represents non-health state transient events. Arrows indicate allowed transitions. Abbreviations: CV: cardiovascular; HHF: hospitalization for heart failure; KCCQ: Kansas City Cardiomyopathy Questionnaire; TSS: total symptom score; UHFV: urgent heart failure visit
Patients entered the model based on the initial distribution of the cohort across KCCQ-TSS health states. The model used a monthly cycle length, and no half-cycle correction was applied as a one-month cycle length was considered sufficiently granular.
Clinical parameters
Health state transitions and transient events
Time-dependent health state membership was determined by transition probabilities between KCCQ-TSS quartile health states. Treatment-specific transition probabilities were derived for each treatment arm based on the demonstrated statistically significant change in KCCQ-TSS observed in the DAPA-HF and DELIVER trials [19]. The transition probabilities by treatment arm are available in the Additional file 1.
The incidence of transient events, HHF and UHFV, was modelled using generalised estimating equations (GEE) because of their recurrence in the same patients. An advantage of a GEE over the constant hazard exponential parametric survival regression is the availability of different within-group correlation structures for events occurring within the same individual [33]. This approach ensures that the evaluation captured the full impact of treatment with dapagliflozin for both first and subsequent events observed within the DAPA-HF and DELIVER trials [19]. The GEEs were fully adjusted for influential patient characteristics. GEEs were determined using the Poisson family to represent count data in the repeated observations for each patient. The link function was log, an auto-regression working correlation structure of first order was used, and the index function is the linear combination of the included predictors and their corresponding coefficients [33]. Calculations were performed with R version 4.0, package geepack (R Core Team, Vienna, Austria). The coefficients and statistics of the adjusted GEE for predicting HHF and UHFV are available in the Additional file 2.
Survival
The median duration of follow-up was 18.2 months in the DAPA-HF trial and 2.3 years in the DELIVER trial [19]. Therefore, it was necessary to extrapolate the efficacy data to the lifetime horizon. Kaplan-Meier survival curves for CV death and death from any cause for both treatment arms from the pooled DAPA-HF and DELIVER trial data were extrapolated to match the time horizon using parametric equations. Considering AIC as a metric of goodness-of-fit, the best performing models were log-logistic, Weibull and generalised gamma. The Kaplan-Meier curves and adjusted survival model extrapolations associated with CV death and death from any cause overlayed on the trial-based Kaplan-Meier are available in the Additional file 3 and 4. External data in relevant patient populations were identified at 5-, 10- and 15-year survival and fitted to Weibull, generalised gamma and log-logistic distribution predictions. Overall, the generalised gamma distribution was considered the most appropriate for the base case, as it was the curve that most closely matched all real-world evidence survival estimates for this population and was one of the best performing in terms of statistical goodness-of-fit in the adjusted survival models [34–36]. Additionally, the age- and gender-adjusted risk of mortality for the normal Spanish population replaced the survival curve for death from any cause when it was higher than the modelled extrapolations [37].
Safety
Patients were subject to the risk of adverse events (AE) for each treatment arm observed within the DAPA-HF and DELIVER trials [19]. The AEs included were those serious AE of grade 3 + with a frequency exceeding 1% of the study population and were of special clinical interest: acute kidney injury (AKI), fracture, urinary tract infection (UTI), volume depletion and amputation. Amputation was included due to the historically suggested association between SGLT2 inhibitors and an increased risk of amputation, although there is no consistent evidence and no statistically significant increase in risk [38]. Only serious AEs were included to capture the greatest impact on healthcare resource use and patients’ HRQoL. The probabilities of AEs, stratified by treatment arm, are available in the Additional file 5.
Patients treated with dapagliflozin were at risk of treatment discontinuation, with an annual probability of 6.73% ± 0.25% derived from the pooled DAPA-HF and DELIVER trial data [19]. After discontinuation of dapagliflozin, patients were modelled as SoC patients.
Health economic parameters
Costs
According to the perspective used, only direct healthcare costs were included in the analysis. The costs identified were updated to 2023 values based on the healthcare component of the Spanish consumer price index [39]. Patients treated with dapagliflozin incur the acquisition cost of dapagliflozin in addition to the cost of the SoC, while the other patients only incur the cost of the SoC. The acquisition cost of dapagliflozin was calculated from the ex-factory price, applying the discount of the Royal Decree Law 8/2010 (7.5%) [40, 41]. The annual cost of dapagliflozin treatment was estimated at €355.71 at the recommended dose of 10 mg/day, and the annual cost of the SoC was estimated to be €418.66 based on the weighted proportion of patients with each type of HF (LVEF > 40%: 41.9%; LVEF ≤ 40%: 58.1%) [7] (see Additional file 6).
The model included specific costs for each of the KCCQ-TSS quartile health states. HF-related healthcare resource use was derived from a study by McMurray et al. [42] that investigated the cost-effectiveness of sacubitril/valsartan for the treatment of HFrEF, and incremental resource use was assumed in KCCQ-TSS quartile health states with worse outcomes based on clinical expert opinion (see Additional file 7 and 8). Patients incurred direct medical costs associated with their health status each cycle [32]. Health state costs were calculated as annual costs and scaled to one month to match the cycle length of the model [43].
The impact of transient events on direct healthcare costs is captured using an event cost [44]. As transient events were only considered in the cycle of occurrence, a one-off event cost was applied in the same cycle. In addition to the transient events, the costs of AEs and CV death were obtained from national databases and applied as one-off event costs [43, 44]. All costs used in the model are presented in Table 2.
Table 2.
Annual Pharmacological costs and costs of the health states, transient events, mortality and adverse events (€/2023)
| Parameter | Mean cost (SEa) | Distribution | Reference |
|---|---|---|---|
| Pharmacological treatment (€, annual) | |||
| Dapagliflozin | 355.71 | - | [40, 41] |
| SoCb | 418.66 | - | [7, 40, 41] |
| Health states (€, annual) | |||
| KCCQ-TSS Q1 | 1,519.25 (303.85) | Gamma | [42, 43] |
| KCCQ-TSS Q2 | 1,387.14 (277.43) | Gamma | [42, 43] |
| KCCQ-TSS Q3 | 1,255.03 (251.01) | Gamma | [42, 43] |
| KCCQ-TSS Q4 | 1,122.92 (224.58) | Gamma | [42, 43] |
| Transient event (€, event) | |||
| HHF | 4,057.97 (811.59) | Gamma | [44] |
| UHFV | 243.21 (48.64) | Gamma | [43] |
| Mortality (€, event) | |||
| CV death | 8,785.76 (1,757.15) | Gamma | [44] |
| AEs (€, event) | |||
| AKI | 4,968.48 (993.70) | Gamma | [44] |
| Amputation | 13,945.88 (2,789.18) | Gamma | [43] |
| Fracture | 7,541.16 (1,508.23) | Gamma | [43] |
| UTI | 59.54 (11.91) | Gamma | [43] |
| Volume depletion | 59.54 (11.91) | Gamma | [43] |
aA 20% SE was assumed for all parameters
bThe weighted SoC cost assumes that 41.9% of the patients with LVEF > 40% and 58.1% with LVEF ≤ 40% [7]
Abbreviations: AE Adverse event, AKI Acute kidney injury, CV Cardiovascular, HHF Hospitalization for heart failure, KCCQ Kansas City Cardiomyopathy Questionnaire, LVEF Left ventricular ejection fraction, SE standard error, SoC Standard of care, TSS Total symptom score, UHFV Urgent heart failure visit, UTI Urinary tract infection
Utilities
The impact of HF on HRQoL was assessed using utilities and expressed as QALYs. Each of the modelled health states, including the transient states and AEs had an associated utility or disutility. Utility values were derived from the results of the EQ-5D-5 L questionnaire from the pooled DAPA-HF and DELIVER trials [19] (Table 3).
Table 3.
Utilities of health states, transient and adverse events
| Parameter | Mean (SE) | Distribution | Reference |
|---|---|---|---|
| Health states | |||
| KCCQ-TSS Q1 | 0.583 (0.002) | Beta | [19] |
| KCCQ-TSS Q2 | 0.698 (0.002) | Beta | [19] |
| KCCQ-TSS Q3 | 0.776 (0.002) | Beta | [19] |
| KCCQ-TSS Q4 | 0.856 (0.002) | Beta | [19] |
| Transient event | |||
| HHF | −0.020 (0.011) | Beta | [19] |
| UHFV | −0.060 (0.035) | Beta | [19] |
| AEs | |||
| AKI | −0.073 (0.034) | Beta | [19] |
| Amputation | −0.280 (0.056) | Beta | [49] |
| Fracture | −0.278 (0.039) | Beta | [19] |
| UTI | −0.003 (0.001) | Beta | [49] |
| Volume depletion | −0.115 (0.028) | Beta | [19] |
Abbreviations: AE Adverse event, AKI Acute kidney injury, HHF Hospitalization for heart failure, KCCQ Kansas City Cardiomyopathy Questionnaire, SE Standard error, TSS Total symptom score, UHFV Urgent heart failure visit, UTI Urinary tract infection
Sensitivity analyses
Sensitivity analyses were performed to test the robustness of the baseline results [32]. Univariate sensitivity analyses were performed using minimum and maximum individual model inputs. The estimated minimum and maximum values were defined as the lower and upper limits of the 95% confidence intervals of the parameter [32]. A 20% variation of the parameter sample estimate was used when the standard error of a certain parameter was unknown [23]. The ranges used for the discount rate (0%; 5%) and time horizon (10 and 20 years) were based on national health technology assessment recommendations [26, 27].
In addition, a 1000-iteration Monte Carlo simulation supported the probabilistic sensitivity analysis (PSA). The PSA investigated the joint uncertainty of all the parameters which were assigned a theoretical probability distribution. In each iteration, a random value of the parameters included in the PSA was drawn from the corresponding theoretical probability distribution [29].
Additional scenario sensitivity analyses included the impact of different relevant clinical assumptions to be explored based on clinical practice for HF in Spain: (1) the same healthcare resource use for all KCCQ-TSS health states based on McMurray et al. [42] (€1,321.09); (2) patient baseline characteristics from the intention-to-treat population of the DAPA-HF and DELIVER trials [19]; (3) mean patient age of 75 years, targeting only older patients; and (4) higher probability of AKI (5% vs. 3.5%) and volume depletion (2% vs. 1.85%) for the SoC arm and Dapagliflozin + SoC arm, respectively. The proportional reduction for Dapagliflozin + SoC arm were based on the results from DAPA-HF and DELIVER trials [19].
Results
Base case
Over a lifetime horizon, treatment with Dapagliflozin + SoC was more effective than SoC alone, resulting in a total of 0.31 additional QALYs per patient (5.26 vs. 4.95). Patients treated with dapagliflozin remained in better health for longer, resulting in improved HRQoL for patients. Dapagliflozin reduced the incidence of HHF by 136.4 events (752.2 vs. 886.6), UHFV by 38.8 (217.6 vs. 254.4) and CV death by 23.0 (505.8 vs. 528.8) per 1,000 patients treated.
The mean total cost per patient was €21,019 for Dapagliflozin + SoC and €19,578 for SoC, resulting in an increase of €1,441 per patient. The main drivers of the differences between the treatment arms were the incremental pharmacological costs and the reduction in mortality in the Dapagliflozin + SoC arm compared to SoC. Thus, the results of the cost-effectiveness analysis showed that Dapagliflozin + SoC was a cost-effective treatment compared to SoC at a WTP threshold of €25,000/QALY [24, 25], with an ICUR of €4,611/QALY (Table 4).
Table 4.
Base case results (/patient)
| Dapagliflozin + SoC | SoC | Incremental | |
|---|---|---|---|
| Total costs (€) | 21019 | 19578 | 1441 |
| Drug acquisition | 4665 | 2781 | 1884 |
| Health states | 8865 | 8512 | 353 |
| Transient events | 2536 | 3046 | −511 |
| CV death | 3609 | 3817 | −207 |
| AEs | 1344 | 1422 | −78 |
| LYG | 8.45 | 8.00 | 0.45 |
| QALY | 5.26 | 4.95 | 0.31 |
| ICER (€/LYG) | 3182 | ||
| ICUR (€/QALY) | 4611 |
Abbreviations: AE Adverse event, CV Cardiovascular, ICER Incremental cost-effectiveness ratio, ICUR Incremental cost-utility ratio, LYG Life years gained, QALY Quality Adjusted Life Years, SoC Standard of care
Sensitivity analyses
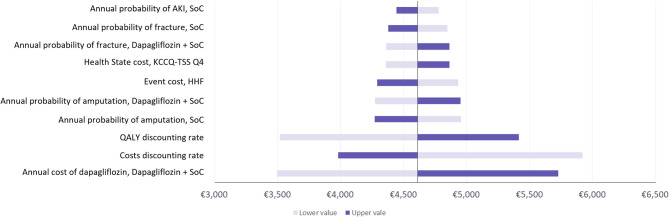
The univariate sensitivity analysis confirmed the robustness of the base case findings. In all scenarios, Dapagliflozin + SoC was a cost-effective therapeutic option compared to SoC for the treatment of HF, considering a WTP threshold of €25,000/QALY [24, 25]. The analysis showed that the annual cost of dapagliflozin was the most sensitive variable (ICUR range: €3,495–5,727/QALY). The 10 most sensitive variables are shown in Fig. 2.
Fig. 2.
Tornado diagram: Dapagliflozin + SoC vs. SoC. Abbreviations: AKI: acute kidney injury; HHF: hospitalization for heart failure; KCCQ: Kansas City Cardiomyopathy Questionnaire; QALY: Quality Adjusted Life Years; SoC: standard of care; TSS: total symptom score
Additional clinically relevant scenario analyses are shown in Fig. 3. All scenarios confirmed the robustness of the base case analysis. The variation in the probability of AKI based on clinical practice (annual probability of AKI, SoC 5%; Dapagliflozin + SoC 3,5%) had the largest impact on the baseline results, reducing the ICUR to €3,854/QALY due to the higher cost of the event and the favourable outcomes for dapagliflozin. Other scenario sensitivity analyses had a smaller impact on the results, including shorter time horizons (10 and 20 years).
Fig. 3.
Scenario analyses. Abbreviations: AKI: acute kidney injury; ICUR: incremental cost-utility ratio; KCCQ: Kansas City Cardiomyopathy Questionnaire; QALY: Quality Adjusted Life Years; TSS: total symptom score
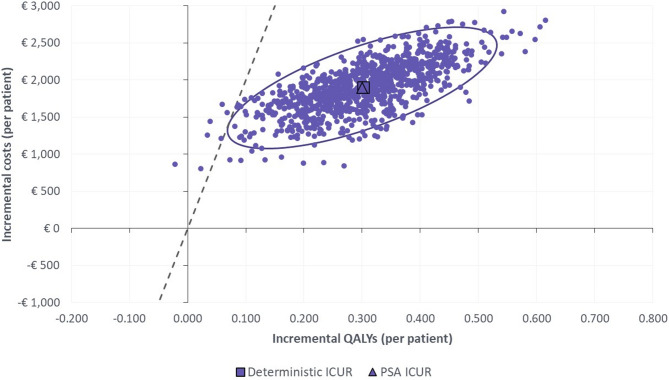
The PSA showed that Dapagliflozin + SoC was a cost-effective option compared to SoC in 99.9% of the simulations at a WTP threshold of €25,000/QALY [24, 25]. The cost-effectiveness plane resulting from the PSA is shown in Fig. 4.
Fig. 4.
Cost-effectiveness plane: Dapagliflozin + SoC vs. SoC Note: A threshold of €25,000/QALY gained is considered. Abbreviations: ICUR: incremental cost-utility ratio; PSA: Probabilistic sensitivity analysis; QALY: Quality Adjusted Life Years; SoC: standard of care
Discussion
The present cost-effectiveness analysis of dapagliflozin based on the pooled data from the DAPA-HF and DELIVER trials showed that Dapagliflozin + SoC is a cost-effective option compared to SoC in patients with symptomatic chronic HF in Spain at a WTP threshold of €25,000/QALY [24, 25]. Dapagliflozin had a beneficial effect on reducing transient events, HHF and UHFV, CV death and adverse events, leading to an increased effectiveness (0.31 QALY/patient) and cost (€1,441/patient).
To our knowledge, this is the first cost-effectiveness analysis in HF patients across the full spectrum of LVEF. A systematic review of cost-effectiveness analyses of SGLT2 inhibitors for the treatment of adults with chronic HF published by Tan et al., [45] identified 39 economic evaluations of dapagliflozin and empagliflozin (32 with LVEF ≤ 40%; 7 with LVEF > 40%). Overall, SGLT2 inhibitors were cost-effective compared with various SoC treatments in most of the economic evaluations, with use in patients with LVEF ≤ 40% favoured over those with LVEF > 40%.
We identified two studies that evaluated dapagliflozin in patients with HF from the perspective of the Spanish National Health System, based on the results of the DAPA-HF and DELIVER trials, separately [46, 47]. McEwan et al. [47] and Booth et al. [46] concluded that dapagliflozin, as an add-on therapy to SoC compared to SoC in Spain, would be cost-effective option for the treatment of patients with HFrEF with an ICUR of €9,406/QALY at a WTP threshold of €20,000 and, for patients with HFpEF and HFmrEF, with an ICUR of €5,343/QALY at a WTP threshold of €15,000, respectively. These results are consistent with the findings of our study.
A potential limitation of the present study is the long-term extrapolation of data from the DAPA-HF and DELIVER clinical trials to model disease progression over a patient’s lifetime, although this approach is common and widely accepted in most cost-effectiveness models [48]. In addition, the mortality approximation was also based on the data from the DAPA-HF and DELIVER trials and replaced with Spanish age- and gender-adjusted mortality rates when these were higher. However, another approach could be to use real-world data registries for the SoC mortality in the HF population in Spain and adjust using the relative reduction from the DAPA-HF and DELIVER trials for the Dapagliflozin + SoC treatment arm.
Another limitation of the analysis is the use of Spain-adjusted patient characteristics when possible to better reflect the population with chronic HF in Spain. However, the clinical data for dapagliflozin used in the model are from the pooled DAPA-HF and DELIVER trials with different patient characteristics. To evaluate the magnitude of this change, a scenario analysis was performed using the pooled baseline characteristics of patients in the DAPA-HF and DELIVER trials. Its results confirmed that changes in patients’ baseline characteristics had a small impact on the results.
No previous economic evaluation of health-care programmes was identified to inform the healthcare resource use associated with patients across the broad spectrum of LVEF in Spain. Therefore, resource use was based on that of patients with HFrEF in the United Kingdom. We adjusted the resource use experienced in the UK by applying arbitrary proportional variations from baseline, because patients with higher and lower disease severity were expected to have different resource use. The proportional variations were based on expert opinion. Their robustness was tested in sensitivity analyses, which showed a low impact on the overall baseline findings.
Conclusions
In conclusion, this analysis suggests that dapagliflozin, as add-on therapy to SoC, is a cost-effective alternative compared to SoC for the treatment of patients with symptomatic chronic HF for the Spanish National Health System.
Supplementary Information
Additional file 1. Transition matrices derived from the DAPA-HF and DELIVER trials.
Additional file 2. Adjusted GEE coefficients derived from the intention-to-treat population from DAPA-HF and DELIVER trials.
Additional file 3. Kaplan-Meier for CV death (A) and death from any cause (B) stratified by treatment.
Additional file 4. Adjusted survival model extrapolations associated with CV death (A) and death from any cause (B) for dapagliflozin and placebo treatment arms.
Additional file 5. Annual probability of AEs derived from the intention-to-treat population from DAPA-HF and DELIVER trials.
Additional file 6. Posology, presentations, daily cost and distribution of use of active ingredients for the treatment of HFrEF.
Additional file 7. Adaptation of the resource use items from McMurray et al. used to derive health state costs.
Additional file 8. Unit costs used to derive health state costs.
Acknowledgements
Not applicable.
Abbreviations
- AE
Adverse events
- AKI
Acute kidney injury
- ARN
Angiotensin-receptor-neprilysin
- CI
Confidence interval
- CV
Cardiovascular
- ESC
European Society of Cardiology
- GEE
Generalised estimating equations
- HF
Heart failure
- HFrEF
Heart failure with reduced ejection fraction
- HFmrEF
Heart failure with mildly reduced ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- HR
Hazard ratio
- HRQoL
Health-related quality of life
- ICER
Incremental cost-effectiveness ratio
- ICUR
Incremental cost-utility ratio
- LVEF
Left ventricular ejection fraction
- LYG
Life years gained
- MRA
Mineralocorticoid receptor antagonists
- NHS
National Health Service
- NYHA
New York Heart Association
- PSA
Probabilistic sensitivity analysis
- QALY
Quality-adjusted life year
- RAAS
Renin-angiotensin-aldosterone system
- SGLT2
Sodium-glucose co-transporter-2
- SoC
Standard of care
- T2DM
Type 2 diabetes mellitus
- UHFV
Urgent heart failure visits
- UTI
Urinary tract infection
- WTP
Willingness-to-pay
Authors’ contributions
MC, EPM and CC analyzed and interpreted the data. EPM and CC were major contributors in writing the manuscript. CE, DPF and JMGS assessed the validity of the model and inputs for the Spanish setting. All authors read and approved the final manuscript.
Funding
This work was supported by AstraZeneca Spain.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
CE received consultancy and/or lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis and Servier. DPF received consultancy and educational fees from AstraZeneca, Novartis, Pfizer, Roche, Rovi and Vifor. JMGS declares personal financial interests in the form of speaking/travel grants or accommodation from Amgen, AstraZeneca, Bayer, Beigene, Biogen, Boehringer Ingelheim, CSL Behring, F. Hoffmann–La Roche Ltd., Ever Pharma, GlaxoSmithKline, Gilead, Merck Sharp & Dohme, Novartis, Novo Nordisk, Laphysan, Pfizer, Samsung Bioepis, Sanofi and Takeda. MC is employed by AstraZeneca Spain. EPM and CC are employed by PharmaLex Spain, a consulting company specialized in economic evaluations of health interventions.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2017;136:e137.28455343 [Google Scholar]
- 2.Taylor CJ, Moore J, O’Flynn N. Diagnosis and management of chronic heart failure: NICE guideline update 2018. Br J Gen Pract. 2019;69:265–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–200.27206819 [Google Scholar]
- 4.van Riet EES, Hoes AW, Wagenaar KP, Limburg A, Landman MAJ, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18:242–52. [DOI] [PubMed] [Google Scholar]
- 5.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and National incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018;18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escobar C, Palacios B, Gonzalez V, Gutiérrez M, Duong M, Chen H, et al. Burden of illness beyond mortality and heart failure hospitalizations in patients newly diagnosed with heart failure in Spain according to ejection fraction. J Clin Med. 2023;12:2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escobar C, Varela L, Palacios B, Capel M, Sicras-Mainar A, Sicras-Navarro A, et al. Características clínicas, Manejo y Riesgo de complicaciones a Un Año En Pacientes Con insuficiencia cardíaca Con y sin diabetes Tipo 2 En España. Rev Clin Esp. 2022;222:195–204. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJV, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. [DOI] [PubMed] [Google Scholar]
- 10.Nassif ME, Kosiborod M. Effects of sodium glucose cotransporter type 2 inhibitors on heart failure. Diabetes Obes Metab. 2019;21:19–23. [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627–39. [DOI] [PubMed] [Google Scholar]
- 13.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose Cotransporter-2 Inhibition in heart failure. Circulation. 2017;136:1643–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Medicines Agency. Forxiga. Summary of product characteristics. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga. Accessed 9 Feb 2024.
- 15.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 16.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 17.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–98. [DOI] [PubMed] [Google Scholar]
- 19.Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sicras-Mainar A, Sicras-Navarro A, Palacios B, Varela L, Delgado JF. Epidemiología y Tratamiento de La insuficiencia cardiaca En españa: estudio PATHWAYS-HF. Rev Esp Cardiol. 2022;75:31–8. [DOI] [PubMed] [Google Scholar]
- 21.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. Guía ESC 2021 sobre El Diagnóstico y Tratamiento de La insuficiencia cardiaca Aguda y crónica. Rev Esp Cardiol. 2022;75:e5231–523114. [Google Scholar]
- 22.Neumann P, Ganiats T, Russel L, Sanders G, Siegel J. Cost-effectiveness in health and medicine. 2nd edition. New York: Oxford University Press; 2016.
- 23.Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Healthcare (Handbooks in Health Economic Evaluation). Oxford: Oxford University Press; 2010.
- 24.Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27:746–61. [DOI] [PubMed] [Google Scholar]
- 25.Sacristán JA, Oliva J, Campillo-Artero C, Puig-Junoy J, Pinto-Prades JL, Dilla T, et al. What is an efficient healthcare intervention in Spain in 2020?. Gac Sanit. 2020;34:189–93. [DOI] [PubMed] [Google Scholar]
- 26.Puig Junoy J, Oliva Moreno J, Trapero Bertran M, Abellán Perpiñán J, Brosa Riestra M. Servei Català de la Salut (CatSalut). [Guidelines And Recommendations For The Conduct And Presentation Of Economic Evaluations And Budget And Budget Impact Analysis Of Medicines In The Scope Of Catsalut]. 2014. https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/medicaments_farmacia/farmaeconomica/caeip/gaeip_publica_castellano_octubre2014_catsalut.pdf
- 27.López Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, et al. Guideline proposal to economic evaluation applied to health technologies. Gac Sanit. 2010;24:154–70. [DOI] [PubMed] [Google Scholar]
- 28.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Mak. 1993;13:322–38. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell D, Briggs A, Sculpher M, Claxton K. Int J Epidemiol. 2007;36:476–7. [Google Scholar]
- 30.Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, et al. Comparable performance of the Kansas City cardiomyopathy questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6:1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 32.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 4th edition. London: Oxford University Press; 2015.
- 33.Hardin JW, Hilbe JM. Generalized Estimating Equations. 2nd edition. New York: Chapman and Hall/CRC; 2013.
- 34.Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. 2019;21:1306–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, et al. Trends in survival after a diagnosis of heart failure in the united Kingdom 2000–2017: population based cohort study. BMJ. 2019;364:l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahim A, Hourqueig M, Donal E, Oger E, Venkateshvaran A, Daubert J, et al. Predictors of long-term outcome in heart failure with preserved ejection fraction: a follow‐up from the KaRen study. ESC Heart Fail. 2021;8:4243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Statistics Insitute. Mortality tables. 2020. Accessed 14 Feb 2024. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177004&menu=ultiDatos&idp=1254735573002.
- 38.Heyward J, Mansour O, Olson L, Singh S, Caleb Alexander G. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0234065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Statistics Institute (INE). Consumer price index. Latest data. 2023. Accessed 14 Feb 2024. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176802&menu=ultiDatos&idp=1254735976607.
- 40.General Council of the Official College of Pharmacists. Pharmacy Portal. BotPLUS. 2024. Accessed 14 Feb 2024. https://botplusweb.farmaceuticos.com/.
- 41.Ministry of Health. List of medicines affected by the deductions of Royal Decree-Law 8/2010 - January 2024. 2024. Accessed 23 Jan 2024. https://www.sanidad.gob.es/areas/farmacia/infoIndustria/infoDeducciones/ley8_2010/home.htm.
- 42.McMurray JJV, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, et al. Cost-effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart. 2018;104:1006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gisbert R, Brosa M. [Spanish health costs and cost-effectiveness ratios database: eSalud]. Oblikue Consulting, S.L. 2022. http://www.oblikue.com/bddcostes/. Accessed 27 Jul 2022.
- 44.Ministry of Health. Specialized Care Activity Register of the Minimum Basic Data Set (RAE-CMBD). Health Information System. 2022. Accessed 14 Feb 2024. https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbdhome.htm.
- 45.Tan YJ, Ong SC, Kan YM. Is using Sodium-Glucose Cotransporter-2 inhibitors to treat adults with chronic heart failure Cost-Effective? A systematic review of Cost-Effectiveness studies. Appl Health Econ Health Policy. 2023;21:857–75. [DOI] [PubMed] [Google Scholar]
- 46.Booth D, Davis JA, McEwan P, Solomon SD, McMurray JJV, De Boer RA, et al. The cost-effectiveness of Dapagliflozin in heart failure with preserved or mildly reduced ejection fraction: A European health‐economic analysis of the DELIVER trial. Eur J Heart Fail. 2023;25:1386–95. [DOI] [PubMed] [Google Scholar]
- 47.McEwan P, Darlington O, McMurray JJV, Jhund PS, Docherty KF, Böhm M, et al. Cost-effectiveness of Dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health‐economic analysis of DAPA‐HF. Eur J Heart Fail. 2020;22:2147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaduganathan M, Claggett BL, Jhund P, de Boer RA, Hernandez AF, Inzucchi SE, et al. Estimated Long-Term benefit of Dapagliflozin in patients with heart failure. J Am Coll Cardiol. 2022;80:1775–84. [DOI] [PubMed] [Google Scholar]
- 49.Beaudet A, Clegg J, Thuresson P-O, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17:462–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Transition matrices derived from the DAPA-HF and DELIVER trials.
Additional file 2. Adjusted GEE coefficients derived from the intention-to-treat population from DAPA-HF and DELIVER trials.
Additional file 3. Kaplan-Meier for CV death (A) and death from any cause (B) stratified by treatment.
Additional file 4. Adjusted survival model extrapolations associated with CV death (A) and death from any cause (B) for dapagliflozin and placebo treatment arms.
Additional file 5. Annual probability of AEs derived from the intention-to-treat population from DAPA-HF and DELIVER trials.
Additional file 6. Posology, presentations, daily cost and distribution of use of active ingredients for the treatment of HFrEF.
Additional file 7. Adaptation of the resource use items from McMurray et al. used to derive health state costs.
Additional file 8. Unit costs used to derive health state costs.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].