Abstract
Objectives
The purpose of this study was to evaluate the predictive value of the ABIC score in the long-term prognosis of elderly patients with acute exacerbations of chronic heart failure (CHF), and to explore whether its integration with other known prognostic variables could enhance the performance of a predictive model.
Methods
This is a retrospective cohort study of elderly patients with acute exacerbation of CHF who were hospitalized for the first time. The main clinical outcome was all-cause mortality within three years. Cox regression and Lasso regression were used to screen variables. The screened variables, along with the ABIC score, were included in the multivariate Cox regression analysis to construct a predictive nomogram model.
Results
A total of 365 patients with acute exacerbation of CHF were included. During the 3-year follow-up period, 87 patients experienced all-cause death, including 53 cardiac deaths. A total of 4 variables [NT-proBNP, serum urea nitrogen (BUN), red cell distribution width-coefficient of variation (RDW-CV) and prealbumin] were screened by univariate Cox regression analysis and Lasso regression analysis. The multivariate COX regression results showed that the risk of death increased by 33% with the increase of ABIC score by 1 point. The results of ROC curve analysis show that the area under the curve (AUC) of the ABIC score is 0.685, while the AUC of the Nomograph including the ABIC score is 0.840.
Conclusions
The ABIC score is associated with long-term adverse outcomes in elderly hospitalized patients with acute exacerbation of CHF. The integration with established prognostic variables (NT-proBNP, BUN, RDW-CV, prealbumin) significantly improves model performance, highlighting the combined value of multiple organ dysfunction and cardiac biomarkers in risk stratification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-025-05004-z.
Keywords: ABIC score, Heart failure, Prediction model, Creatinine, Bilirubin
Introduction
Advances in the diagnosis and treatment of cardiovascular disease have decreased mortality from acute cardiovascular events. However, with aging, cardiac damage can progress to heart failure (HF), leading to a higher prevalence of chronic HF and placing a heavier burden on society. According to an epidemiological survey in China in 2021, the prevalence of HF was estimated to be 3.86% in patients aged 65 to 79 years and 7.55% in patients aged 80 years, and the incidence of HF also increased with age [1]. Therefore, prognostic assessment in CHF is of utmost importance, as it not only facilitates clinical decision-making by identifying high-risk patients but also helps determine populations that may benefit most from urgent therapeutic interventions or new treatment research. Age-bilirubin-international normalized ratio (INR) -creatinine (ABIC) score was originally proposed as a tool to assess survival in alcoholic hepatitis and to identify patients who respond to corticosteroid treatment [2]. Many previous studies have shown that ABIC score is highly related to the short-term adverse prognosis of acute-on-chronic hepatitis B liver failure and alcoholic hepatitis [3, 4]. Considering that the impairment of liver and kidney function due to volume overload and hypoperfusion in patients with heart failure during acute exacerbations is unequivocal, it seems that indexes reflecting liver and kidney function can also be used to judge the prognosis of patients with HF [5]. Items in the score have been recognized in past studies as risk factors for adverse outcomes in patients with CHF exacerbation [6–8]. At present, the relationship between the prognosis of patients with acute exacerbation of chronic heart failure and ABIC score is still unclear. The purpose of this study was to evaluate the predictive value of the ABIC score in the long-term prognosis of elderly patients with acute exacerbations of CHF, and to explore whether its integration with other known prognostic variables could enhance the performance of a predictive model.
Methods and materials
Data source and study definition
Study population
This was a single center retrospective observational cohort study that included 365 patients fist admitted to the Department of Geriatrics, the Second Affiliated Hospital of Chongqing Medical University, due to acute exacerbation of CHF between February 2017 and December 2017. Chronic heart failure was defined according to the diagnostic criteria established by 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [9]: (1) Signs and symptoms of heart failure are present; (2) LVEF < 50%, or LVEF ≥ 50% with evidence of cardiac structural and/or functional abnormalities consistent with left ventricular diastolic dysfunction/elevated left ventricular filling pressure.
Exclusion criteria: (1) Age < 60 years;(2) Acute heart failure caused by acute coronary syndrome and acute cerebral stroke; (3) Patients with malignancies; (4) Severe renal insufficiency (CKD stage V) or that require dialysis and severe liver insufficiency (Child C); (5) Missing data within 24 h of admission to calculate ABIC score.
Study outcome was defined as all-cause mortality events occurring within 3 years of follow-up. Follow up of patients was be conducted through e-mail, network communication tools, telephone, outpatient follow-up, etc.
This study was approved by the ethics committee of the Second Affiliated Hospital of Chongqing Medical University, and the requirement for informed consent was waived (2022177). The investigation conformed to the principles outlined in the Declaration of Helsinki.
Measurement of indicators of interest
The measurement of indicators of interest was carried out in the hospital testing center. Biochemical indexes related to liver and kidney function were measured by Hitachi RL7600 Automatic Biochemical Analyzer. The ABIC score was calculated using the formula: ABIC = (age × 0.1) + (serum bilirubin × 0.08) + (serum creatine × 0.3) + (INR × 0.8) [10].
Statistical analysis
Enrolled patients were divided into survival and non-survival groups according to whether all-cause death occurred. The Shapiro–Wilk test (normal distribution if P > 0.05) was used to determine the normality of data. Non normally distributed continuous variables are expressed as median [25th-75th percentile]. Differences in the distribution of individual characteristics between the groups were analyzed by the Pearson’s χ 2 test and Fisher's exact test for categorical variables and the Mann Whitney U test for continuous variables. Screening of variables was performed by univariate Cox regression analysis and Lasso regression. Restricted cubic spline analysis is used to find the tangent point of continuous variables. Multivariate Cox regression analysis was used to construct the prediction model, and Normangram was plotted. The predictive abilities of the models for prognosis were compared using receiver operating characteristic (ROC) curves. Statistical significance was set at p < 0.05 (two‐sided). Data analysis was performed using R language (R for Mac version 4.2.0, R Foundation for Statistical Computing, Vienna).
Results
Characteristics of patients
Three (0.82%) individuals had missing relevant indicators on coagulation. Ten (2.73%) individuals had missing D-dimer. Two (0.54%) individuals had missing serum sodium and potassium. Nineteen (5.20%) individuals had missing relevant indicators on lipid. Missing data is acceptable and random, so imputation of missing values was performed by random forest interpolation using the MissForest package [11].
A total of 365 patients (median age was 78, 50.7% female) were included in the study. During the 3-year follow-up period, 87 patients experienced all-cause death, including 53 cardiac deaths.
From the Table 1, there were many differences between the survivor group and non- survivor group. Patients in the non- survivor group were older and had worse cardiac function grades. In terms of the comorbid disease, the prevalence of chronic kidney disease and peripheral atherosclerotic disease (PAD) was higher in the non-survivor group than in the survivor group. Compared to survivor group, the non-survivor group had higher D-dimer, RDW-CV, red cell distribution width standard deviation (RDW-SD), Monocyte, International normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (APTT), serum creatinine (Scr), uric acid (UA), BUN, NT-proBNP, Troponin T, total bilirubin (TBIL) and Lp(a) while red blood cell (RBC), hemoglobin (Hb), hematocrit (HCT), lymphocyte, platelet, albumin, prealbumin, triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C); high density lipoprotein cholesterol (HDL-C); and ApoA1 were lower. In terms of medication, patients in the non-survivor group had a higher use of diuretics. The baseline information of the patients is shown in Table 1.
Table 1.
Baseline characteristics of study population
| Overall Population (N = 365) | Survivors (N = 278) | Non-survivors (N = 87) | P-value | |
|---|---|---|---|---|
| Age(year) | 78.00[70.00,83.00] | 76.00[69.00,82.00] | 81.00[74.00,86.00] | < 0.001* |
| Gender(female) | 185(50.70) | 140(50.40) | 45(51.70) | 0.824 |
| IBM, kg/m2 | 23.40[21.08,25.64] | 23.47[21.21,25.74] | 22.99[19.34,25.00] | 0.092 |
| SBP, mmHg | 130.00[119.00,143.50] | 131.00[120.00,144.25] | 126.00[110.00,142.00] | 0.039* |
| DBP, mmHg | 74.00[66.00,84.00] | 75.00[67.00,85.00] | 71.00[63.00,81.00] | 0.036* |
| HFrEF, n (%) | 60(16.40) | 44(15.80) | 16(18.40) | 0.853 |
| HFmrEF, n (%) | 51(14.00) | 39(14.00) | 12(13.80) | |
| HFpEF, n (%) | 254(69.60) | 195(70.10) | 59(67.80) | |
| NYHA II, n (%) | 103(28.20) | 91(32.70) | 12(13.80) | < 0.001* |
| NYHA III, n (%) | 199(54.50) | 150(54.00) | 49(56.30) | |
| NYHA IV, n (%) | 63(17.30) | 37(13.30) | 26(29.90) | |
| ABIC score | 9.13[8.28,9.68] | 8.97[8.19,9.55] | 9.51[8.98,10.31] | < 0.001* |
| Medical History | ||||
| Hypertensive heart disease, n | 72(19.70) | 61(21.90) | 11(12.60) | 0.057 |
| Dilated cardiomyopathy, n | 29(7.90) | 19(6.80) | 10(11.50) | 0.161 |
| Rheumatic heart disease, n | 30(8.20) | 25(9.00) | 5(5.70) | 0.382 |
| Coronary heart disease, n | 222(60.80) | 163(58.60) | 59(67.80) | 0.126 |
| Chronic kidney disease, n | 69(18.90) | 38(13.70) | 31(35.60) | < 0.001* |
| Hepatopathy, n | 22(6.00) | 13(4.70) | 9(10.30) | 0.053 |
| COPD, n | 52(14.20) | 38(13.70) | 14(16.10) | 0.573 |
| Hypertension, n | 243(66.60) | 188(67.60) | 55(63.20) | 0.447 |
| Diabetes mellitus, n | 112(30.70) | 84(30.20) | 28(32.20) | 0.728 |
| Smoking history, n | 116(31.80) | 88(31.70) | 28(32.20) | 0.926 |
| Drinking history, n | 89(24.40) | 71(25.50) | 18(20.70) | 0.358 |
| Previous PCI, n | 84(23.00) | 64(23.00) | 20(23.00) | 0.995 |
| Previous stroke/TIA, n | 116(31.80) | 86(30.90) | 30(34.50) | 0.535 |
| Atrial Fibrillation, n | 180(49.30) | 130(46.80) | 50(57.50) | 0.081 |
| PAD, n | 285(78.10) | 210(75.50) | 75(86.20) | 0.036* |
| Echocardiogram | ||||
| IVST, mm | 11.00[10.00,12.00] | 11.00[10.00,12.00] | 11.00[10.00,12.00] | 0.499 |
| LAD, mm | 42.00[37.00,48.00] | 42.00[37.00,48.00] | 42.00[37.00,49.00] | 0.528 |
| RATD, mm | 40.00[34.00,45.00] | 40.00[34.00,45.00] | 41.00[34.00,48.00] | 0.169 |
| LVEDD, mm | 49.00[43.00,57.00] | 48.00[43.00,56.00] | 52.00[45.00,59.00] | 0.058 |
| RVEDD, mm | 23.00[21.00,26.00] | 23.00[21.00,26.00] | 24.00[21.00,27.00] | 0.249 |
| LVPWT, mm | 10.00[9.00,11.00] | 10.00[9.00,11.00] | 10.00[9.00,11.00] | 0.966 |
| LVEF, % | 62.00[46.50,71.50] | 63.50[47.00,72.00] | 61.00[44.00,69.00] | 0.085 |
| Laboratory Data | ||||
| D-dimer, mg/L | 0.10[0.10,0.10] | 0.10[0.10,0.10] | 0.10[0.10,0.20] | 0.024* |
| RBC, 1012/L | 4.11[3.71,4.48] | 4.16[3.75,4.52] | 4.01[3.55,4.40] | 0.041* |
| Hb, g/L | 124.00[112.00,136.00] | 126.00[115.00,137.25] | 119.00[108.00,131.00] | 0.002* |
| HCT | 38.00[34.15,41.00] | 38.60[34.70,41.43] | 35.10[32.40,39.20] | < 0.001* |
| MCV, fL | 92.70[89.60,96.00] | 92.80[89.80,96.02] | 92.50[87.90,95.80] | 0.347 |
| MCH, pg | 30.70[29.40,31.80] | 30.80[29.50,31.80] | 30.40[28.50,32.10] | 0.421 |
| MCHC, g/L | 329.00[320.00,338.00] | 329.50[320.00,337.00] | 328.00[317.00,341.00] | 0.861 |
| RDW-CV | 14.10[13.20,14.90] | 13.90[13.20,14.80] | 14.50[13.60,15.60] | < 0.001* |
| RDW-SD, fL | 46.20[43.80,49.60] | 45.95[43.70,49.23] | 47.10[45.00,52.60] | 0.021* |
| WBC, 109/L | 6.53[5.07,7.99] | 6.51[5.04,7.97] | 6.61[5.10,8.40] | 0.629 |
| Neutrophil, 109/L | 4.75[3.54,6.42] | 4.68[3.52,6.32] | 5.07[3.76,6.64] | 0.171 |
| Lymphocyte, 109/L | 1.16[0.86,1.52] | 1.21[0.90,1.53] | 1.06[0.73,1.49] | 0.028* |
| Monocyte, 109/L | 0.41[0.28,0.56] | 0.40[0.28,0.53] | 0.45[0.33,0.69] | 0.004* |
| Platelet, 109/L | 156.00[123.50,192.50] | 158.00[126.75,195.25] | 146.00[114.00,179.00] | 0.034* |
| PTA, % | 87.00[72.00,97.00] | 89.00[75.00,99.00] | 78.00[68.00,95.00] | 0.007* |
| INR | 1.09[1.02,1.24] | 1.08[1.01,1.20] | 1.18[1.05,1.28] | 0.002* |
| PT, s | 14.10[13.30,15.50] | 13.90[13.20,15.13] | 14.90[13.50,16.00] | 0.007* |
| APTT, s | 37.10[33.50,40.90] | 36.15[33.30,40.52] | 38.70[34.90,43.30] | 0.005* |
| Fibrinogen, g/L | 3.34[2.81,4.29] | 3.31[2.83,4.18] | 3.48[2.75,4.69] | 0.491 |
| TT, s | 17.40[16.50,18.45] | 17.30[16.40,18.43] | 17.60[16.70,18.60] | 0.294 |
| K+mmol/L | 3.98[3.71,4.36] | 3.97[3.71,4.30] | 4.05[3.73,4.56] | 0.131 |
| Na+ mmol/L | 140.60[137.70,143.15] | 140.90[138.28,143.35] | 139.30[135.30,142.30] | 0.002* |
| Scr, mg/L | 0.94[0.76,1.24] | 0.90[0.72,1.11] | 1.21[0.93,1.52] | < 0.001* |
| UA, umol/L | 408.00[324.25,515.20] | 398.85[323.18,505.72] | 429.50[357.30,534.80] | 0.094 |
| BUN, mmol/L | 7.47[5.93,9.89] | 7.18[5.58,9.25] | 9.25[6.84,12.20] | < 0.001* |
| lactic acid, mmol/L | 2.31[1.72,2.97] | 2.39[1.70,3.00] | 2.20[1.80,2.85] | 0.582 |
| NT-proBNP, pg/mL | 2046.00[587.95,5301.50] | 1522.00[482.60,4573.00] | 3609.00[1217.00,10,784.00] | < 0.001* |
| Troponin T, ug/L | 0.02[0.01,0.05] | 0.02[0.01,0.04] | 0.04[0.02,0.07] | < 0.001* |
| CK-MB, ug/L | 2.17[1.50,3.15] | 2.09[1.50,3.05] | 2.43[1.50,3.92] | 0.067 |
| Albumin, g/L | 37.10[34.60,39.85] | 37.85[35.20,40.60] | 35.20[32.80,37.20] | < 0.001* |
| Prealbumin, g/dL | 1.91[1.42,2.58] | 2.02[1.55,2.41] | 1.60 [1.16,1.94] | < 0.001* |
| ALT, U/L | 17.00[11.00,26.50] | 17.00[11.00,27.00] | 16.00[11.00,25.00] | 0.322 |
| AST, U/L | 22.00[17.00,31.00] | 21.00[17.00,31.00] | 22.00[17.00,31.00] | 0.573 |
| ALP, U/L | 75.00[60.00,93.50] | 74.00[59.00,93.50] | 79.00[63.00,94.00] | 0.282 |
| GGT, U/L | 36.00[22.50,71.00] | 34.00[21.00,74.00] | 41.00[25.00,60.00] | 0.315 |
| TBIL, mg/dl | 0.68[0.49,0.95] | 0.64[0.45,0.91] | 0.78[0.57,1.15] | 0.008* |
| TBA, umol/L | 4.50[2.60,7.75] | 4.35[2.50,7.00] | 5.10[2.80,10.20] | 0.060 |
| TG, mmol/L | 1.04[0.79,1.42] | 1.07[0.81,1.46] | 0.96[0.71,1.37] | 0.030* |
| TC, mmol/L | 3.54[2.98,4.29] | 3.63[3.05,4.35] | 3.31[2.68,4.10] | 0.012* |
| HDL-C, mmol/L | 1.12[0.94,1.34] | 1.14[0.96,1.36] | 1.04[0.88,1.23] | 0.005* |
| LDL-C, mmol/L | 1.87[1.43,2.29] | 1.91[1.45,2.33] | 1.69[1.41,2.21] | 0.071 |
| ApoA1, g/L | 1.35[1.14,1.59] | 1.36[1.18,1.63] | 1.26[1.02,1.42] | < 0.001* |
| ApoB, g/L | 0.78[0.63,0.95] | 0.80[0.65,0.96] | 0.74[0.58,0.94] | 0.202 |
| ApoE, mg/L | 34.10[28.00,39.40] | 34.20[27.70,39.10] | 33.70[28.80,40.87] | 0.818 |
| Lp(a), mg/L | 144.90[80.00,239.50] | 139.15[76.55,221.52] | 165.80[92.50,308.20] | 0.019* |
| HbA1C, % | 6.48[6.18,6.97] | 6.44[6.14,6.98] | 6.51[6.30,6.90] | 0.338 |
| Medication Use | ||||
| β-blocker, n (%) | 238(59.50) | 158(61.72) | 80(55.56) | 0.228 |
| AECI, n (%) | 120(30.00) | 71(27.73) | 49(34.03) | 0.187 |
| ARB, n (%) | 131(32.75) | 90(35.15) | 41(28.47) | 0.172 |
| Aspirin, n (%) | 104(26.00) | 73(28.52) | 31(21.53) | 0.126 |
| Statin drug, n (%) | 248(62.00) | 165(64.45) | 83(57.64) | 0.178 |
| Clopidogrel, n (%) | 189(47.25) | 126(49.22) | 63(43.75) | 0.293 |
| NOAC, n (%) | 38(9.50) | 28(10.94) | 10(6.94) | 0.191 |
| Warfarin, n (%) | 52(13.00) | 33(12.89) | 19(13.19) | 0.931 |
| CCB, n (%) | 123(30.75) | 78(30.47) | 45(31.25) | 0.871 |
| Diuretic, n (%) | 322(80.50) | 196(76.56) | 126(87.50) | 0.008* |
| Hypoglycemic agent, n (%) | 98(24.50) | 65(25.39) | 33(22.92) | 0.581 |
| ARNI, n (%) | 17(4.25) | 10(3.91) | 7(4.86) | 0.650 |
| Digoxin, n (%) | 133(33.25) | 78(30.46) | 55(38.19) | 0.115 |
Data are n/N (%) or median [25th–75th percentile].
Abbreviation: IVST Interventricular septal thickness, LAD Left atrial diameter, RATD Right atrial transverse diameter; LVEDD Left ventricular end diastolic diameter, RVEDD Right ventricular end diastolic diameter, LVPWT Late diastolic left ventricular posterior wall thickness, HCT Hematocrit, MCV Mean corpuscular volume, MCH Mean RBC hemoglobin content, MCHC Mean corpuscular hemoglobin concentration, RDW-CV Coefficient of variation of red cell distribution width, RDW-SD Red cell distribution width standard deviation, PTA Plasma prothrombin activity, INR International normalized ratio, APTT Activated partial thromboplastin time, PT Prothrombin time, TT Thrombin time, UA Uric acid, BUN Blood urea nitrogen, TBA Total bile acid, TBIL Total bilirubin, Scr Serum creatinine, TG Triglyceride, TC Total cholesterol, LDL-C Low density lipoprotein cholesterol, HDL-C High density lipoprotein cholesterol, Apo Apolipoprotein, SBP Systolic pressure, DBP Diastolic blood pressure, NOAC Non-vitamin K antagonists oral anticoagulants, CCB Calcium channel blocker, ARNI Angiotensin receptor neprilysin inhibitor, PAD Peripheral arterial disease, PCI Percutaneous transluminal coronary Intervention, TIA Transient ischemic attack, ACEI Angiotensin-converting enzyme inhibitor, ARB Angiotensin receptor blocker
*P < 0.05
Variable screen
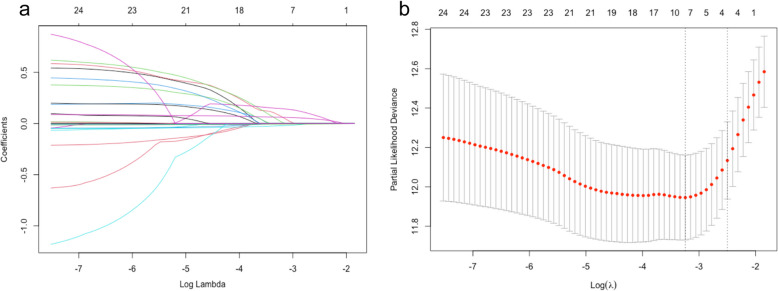
The variables with significant differences in Table 1 were considered as potential risk factors for all-cause death events and were included in univariate Cox regression analysis. The results of univariate Cox regression analysis are shown in Table 2. Variables with P < 0.1 in Table 2 were further considered as risk factors. Because an excess of variables with significance can destabilize the multivariate Cox regression model, further variable screening was performed using lasso regression fitted with a Cox equal proportional hazards model after excluding variables (age, Scr, Chronic kidney disease, INR, TBIL) associated with ABIC score. After tenfold cross-validation, the model with the first standard error of the λ value was selected as the final model, which incorporated NT-proBNP, BUN, RDW-CV and prealbumin, as shown in Fig. 1.
Table 2.
Results of univariate COX regression analysis
| HR | 95%CI (Low) | 95%CI (Up) | p-value | |
|---|---|---|---|---|
| Age | 1.06 | 1.03 | 1.09 | < 0.001* |
| SBP | 9.04 | 1.00 | 0.00 | 0.003* |
| DBP | 0.98 | 0.97 | 1.00 | 0.024* |
| NYHA II | 1.00 | |||
| NYHA III | 2.22 | 1.18 | 4.17 | 0.013* |
| NYHA IV | 4.19 | 2.11 | 8.32 | < 0.001* |
| Chronic kidney disease | 2.75 | 1.77 | 4.26 | < 0.001* |
| PAD | 1.71 | 0.93 | 3.32 | 0.086 |
| D-dimer | 1.94 | 1.27 | 2.95 | 0.002* |
| RBC | 0.66 | 0.46 | 0.95 | 0.023* |
| Hb | 0.98 | 0.97 | 0.99 | 0.002* |
| HCT | 0.97 | 0.95 | 0.99 | 0.006* |
| MCV | 0.97 | 0.94 | 1.00 | 0.048* |
| RDW-CV | 1.38 | 1.23 | 1.56 | < 0.001* |
| RDW-SD | 1.08 | 1.04 | 1.13 | < 0.001* |
| Lymphocyte | 0.61 | 0.40 | 0.93 | 0.023* |
| Monocyte | 1.29 | 0.97 | 1.72 | 0.086 |
| Platelet | 1.00 | 0.99 | 1.00 | 0.196 |
| INR | 1.24 | 0.75 | 2.07 | 0.396 |
| PTA | 0.99 | 0.98 | 1.00 | 0.036* |
| PT | 1.02 | 0.97 | 1.07 | 0.532 |
| APTT | 1.02 | 1.00 | 1.05 | 0.063 |
| Na+ | 0.93 | 0.89 | 0.97 | < 0.001* |
| Scr | 1.53 | 1.22 | 1.91 | < 0.001* |
| BUN | 1.11 | 1.07 | 1.15 | < 0.001* |
| NTproBNP | 1.00 | 1.00 | 1.00 | < 0.001* |
| Troponin T | 2.62 | 0.70 | 9.84 | 0.154 |
| Albumin | 0.88 | 0.84 | 0.93 | < 0.001* |
| Prealbumin | 0.99 | 0.99 | 0.99 | < 0.001* |
| TBIL | 1.69 | 1.20 | 2.38 | 0.003* |
| TG | 0.71 | 0.47 | 1.09 | 0.119 |
| TC | 0.79 | 0.63 | 1.00 | 0.054 |
| HDL-C | 0.34 | 0.16 | 0.71 | 0.004* |
| ApoA1 | 0.25 | 0.13 | 0.49 | < 0.001* |
| Lp(a) | 1.00 | 1.00 | 1.00 | 0.113 |
| Diuretic | 2.77 | 1.34 | 5.74 | 0.006* |
Abbreviation: SBP Systolic pressure, DBP Diastolic blood pressure, HCT Hematocrit, MCV Mean corpuscular volume, RDW-CV Coefficient of variation of red cell distribution width, RDW-SD RBC distribution width standard deviation, PTA Plasma prothrombin activity, APTT Activated partial thromboplastin time, PT Prothrombin time, Scr Serum creatinine, BUN Blood urea nitrogen, TBIL Total bilirubin, TG Triglyceride, TC Total cholesterol, HDL-C High density lipoprotein cholesterol, Apo Apolipoprotein, PAD Peripheral arterial disease
*P < 0.05
Fig. 1.
a Lasso regression fitting Cox regression model; b Tenfold cross validation
To optimize the model, restricted cubic spline analysis fitted with univariate Cox regression was used to assess the relationship between each variable and the hazard ratio (HR) for the event of all-cause death. The results of the nonlinear test suggested that the relationship between BUN and NT-proBNP and HR was nonlinearly related, and the cut-off points were 7.50 mmol/L and 2000 pg/ml, respectively (see Additional file 1).
Prediction model construction and evaluation
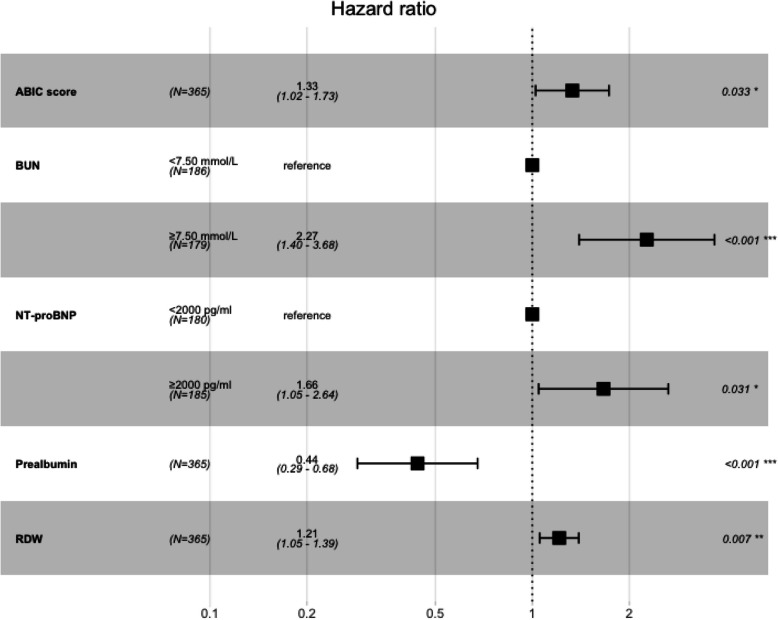
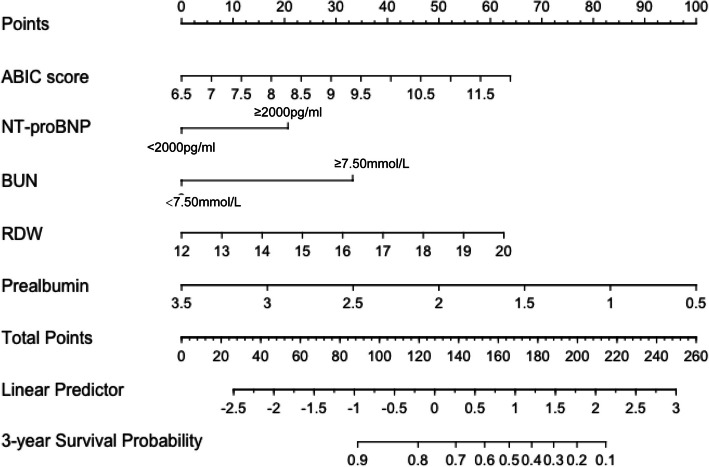
The multivariate Cox regression analysis incorporated NT-proBNP, BUN, RDW-CV, prealbumin and ABIC score, and the results are shown in Fig. 2. It can be found that after correction of NT-proBNP, BUN, RDW CV, prealbumin, the ABIC score is a significant risk factor in patients with acute exacerbation of chronic heart failure (HRadjusted 1.33, 95%CI: 1.02–1.73, p = 0.033). This means that for every 1 increase in ABIC score, the risk of death in elderly patients with acute exacerbation of CHF increases by 33%. In addition, the variables selected by Lasso regression analysis improved the C-index and AIC of the model after combining the ABIC score. Therefore, a model that included NT-proBNP, BUN, RDW-CV, prealbumin, and ABIC scores was considered the best predictive model (Table 3). The new model demonstrated a significant Net Reclassification Improvement (NRI = 0.31, 95% CI: 0.05–0.59), indicating improved accuracy in stratifying mortality risk. However, the Integrated Discrimination Improvement (IDI = 0.01, 95% CI: −0.003–0.03) suggested that the added variables may have limited impact on adjusting overall predicted probabilities. The newly established model is further demonstrated by Nomogram (Fig. 3).
Fig. 2.
Results of multivariate Cox regression analysis
Table 3.
Comparison of different prognostic models on CHF patients
| Model predictors | Likelihood ratio test χ2 | AIC | P | C-index | Adjusted C-index |
|---|---|---|---|---|---|
| Model + ABIC score | 80.38 | 888.59 | < 0.001 | 0.775 | 0.761 |
| Model | 75.67 | 891.30 | < 0.001 | 0.753 | 0.775 |
Model: NT-proBNP, bun, RDW CV, prealbumin
Abbreviation: AIC Akaike Information Criterion
Fig. 3.
Nomogram based on multivariate Cox regression model: Each variable can be projected on the points line to read the points, and then the total points are calculated by adding the points read. Find the corresponding position on the total points line and project it onto the 3-year survival probability line. The point read is the 3-year survival probability of the patient
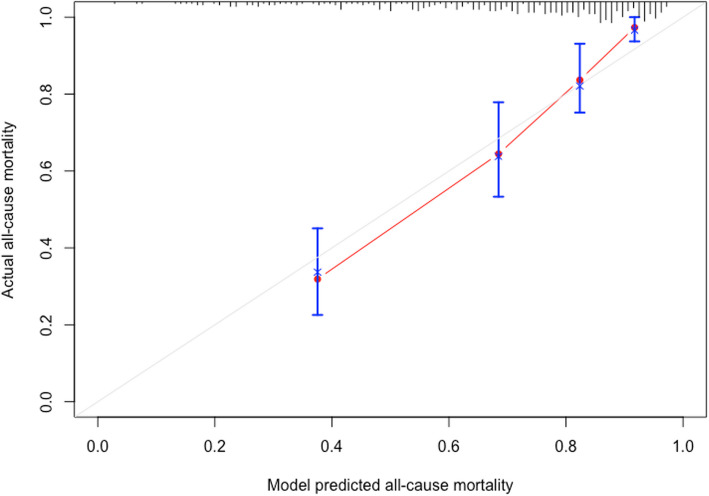
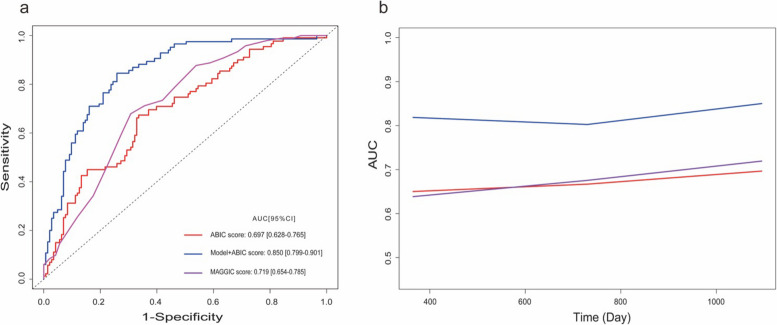
The newly established model was used to evaluate the constructed model accuracy using a calibration curve after ten-fold cross validation. The calibration curve shows that the prediction of the model is accurate (Fig. 4). Receiver operating characteristic (ROC) curves were used to contrast the prognostic ability of the single ABIC score with the newly established model for patients with acute exacerbations of CHF. At the 3-year follow-up, the newly established model (AUC:0.850, 95%CI 0.799–0.901) was more effective in predicting all-cause mortality events than a single ABIC score (AUC:0.697, 95%CI 0.628–0.765), as shown in (Fig. 5A). The MAGGIC risk score is a clinical tool used to evaluate the prognosis of patients with CHF and is primarily employed to predict 1-year and 3-year survival rates [12]. To further validate the performance of our newly established model, we conducted an additional comparison of its predictive ability against the MAGGIC risk score regarding all-cause mortality risk. The results demonstrated that the new model may exhibit superior performance, as evidenced by a higher AUC (0.850 vs 0.719, P < 0.001), along with favorable results for the NRI (0.68, 95% CI: 0.34–1.02) and IDI (0.15, 95% CI: 0.07–0.26).In addition, time-dependent AUC curve analysis showed that the predictive ability of the model for all-cause mortality events of patients was stable over time (Fig. 5B).
Fig. 4.
Calibration curve; Calibration curves demonstrated good accuracy of the newly established model (model + ABIC socre) in predicting 3-year all-cause mortality
Fig. 5.
Receiver operating characteristic curve: a Comparison of the time-dependent ROC curves at 3-year; b Time dependent AUC curves
Discussion
Timely identification of high-risk groups is a critical component of clinical management in HF, as it informs targeted interventions and personalized care strategies. This study demonstrates that the ABIC score is significantly associated with long-term all-cause mortality (HR adjusted = 1.33, P = 0.033), supporting its relevance as a prognostic indicator. The ABIC score reflects liver and kidney function to some extent, and it is important to note that impairment of liver and kidney function is often observed in patients with HF. Meanwhile, many studies have also confirmed that relevant indicators of liver and kidney function are also powerful predictors in patients with HF [13–16]. Athough the ABIC score alone exhibited a moderate ability to predict long-term outcomes (AUC = 0.697), its combination with Lasso-selected variables (NT-proBNP, blood urea nitrogen, red cell distribution width-coefficient of variation, and prealbumin) substantially improved prognostic accuracy, with the integrated model achieving an AUC of 0.850. This indicates that the ABIC score, in addition to its potential as a standalone measure, may offer supplementary value for risk stratification when incorporated into a comprehensive predictive model. By capturing multi-organ dysfunction alongside established cardiac and nutritional biomarkers, the model provides a more holistic assessment of patient prognosis, underscoring the importance of integrating systemic indices with traditional cardiac markers in HF management.
This study is the first application of the ABIC score across domains. During acute exacerbations of chronic HF, renal impairment is sensitive and increases in creatinine and urea nitrogen can be observed, indicating that the kidneys are in a state of hypoperfusion, which is the pathophysiological process of type I cardio-renal syndrome. Although there is no evidence that liver function damage occurs later, previous studies have found that patients with HF with elevated bilirubin are more likely to have adverse outcomes [17]. There are two main reasons for the impairment of liver function. On the one hand, hepatic ischemia–reperfusion injury, characterized by early activation of Kupffer cells, intracellular calcium overload, cytokines and chemokines, oxidative stress, mitochondrial damage, and disruption of hepatic microcirculation, occurs in HF [18, 19]. On the other hand, the elevation of right heart pressure resulted in the elevation of liver venous sinusoidal pressure, and the hepatocytes appeared hypoxic. Hepatocytes compensate for impaired blood flow by increasing oxygen uptake. However, in the acute exacerbation of HF, this compensatory mechanism is exhausted because of hepatic hypoperfusion, leading to hepatocyte hypoxia and necrosis [20]. Thus, the ABIC score provides a comprehensive assessment of multi-organ involvement and systemic congestion, complementing traditional cardiac markers and offering a more holistic view of patient prognosis.
From the components of the ABIC score, it seems that it is a difficult task to want to improve the prognosis of patients with HF by targeting the intervention for the ABIC score. Because in addition to age being an uncontrollable risk factor, it appears that only creatinine can be reduced through diuresis to make score values lower. However, there seems to be no way to improve bilirubin and INR, which limits the practical application of ABIC score. We are not pessimistic about this, because the hepatic function lesion is persistent during the progress of CHF. The liver continuously compensates for the ischemia and hypoxia caused by HF. The increase of bilirubin may indicate the failure of this compensatory mechanism. It can be speculated that the ABIC score at baseline may reflect the liver reserve function of elderly patients with acute decompensated chronic HF to some extent. However, this point remains to be confirmed by further research.
It is worth mentioning that in this study, univariate Cox regression analysis did not show a relationship between INR and prognosis. This finding contrasts with some previous studies that have reported associations between INR variability and mortality risk, particularly in patients on vitamin K antagonists [7]. I However, it is recognized that these prior studies often focused on different patient populations and clinical scenarios, such as those with atrial fibrillation or mechanical heart valves, which may not be directly comparable to the cohort in our study. The lack of association in our study could be attributed to several factors. First, the overall INR values in our cohort were relatively low and stable, potentially limiting our ability to detect a significant relationship. Second, the pathophysiology of INR elevation in HF patients is multifactorial and may not solely reflect liver dysfunction. Factors such as malnutrition, polypharmacy, and infections can independently influence INR stability, complicating its interpretation as a standalone prognostic marker. While we acknowledge the limitations of INR as a prognostic indicator in our specific study population, we emphasize that this does not negate the importance of INR monitoring in clinical practice, particularly for patients requiring anticoagulation therapy. For patients with comorbid atrial fibrillation, the balance between thrombotic and bleeding risks remains a critical clinical consideration, especially during acute exacerbations of HF.
From the ROC curves, the AUC of ABIC score is not very ideal. Therefore, to increase the predictive power of the ABIC score, this study used Lasso regression analysis to screen several indexes, including prealbumin, NT-proBNP, RDW-CV and BUN to compensate for the lack of predictive power of the ABIC score. The prognostic value of these selected indicators for HF patients has been confirmed in the past [21–24]. When comparing our model with the MAGGIC risk score, which is commonly used to evaluate the prognosis of CHF patients, our model demonstrated a higher AUC (0.840 vs 0.719, P < 0.001), along with favorable results for the NRI (0.68, 95% CI: 0.34—1.02) and IDI (0.15, 95% CI: 0.07—0.26). This comparison indicated that our newly established model might have better performance in predicting all—cause mortality risk. This is likely because the MAGGIC risk score did not include NT-proBNP or BNP, which may not fully capture the acute changes in cardiac function during acute attacks, thereby limiting its ability to accurately predict short-term and long-term mortality in such acute exacerbations. But the validation method of the final established model is cross validation, which falls within the scope of internal validation. Due to the lack of external validation, the accuracy of this model remains to be further debated. Therefore, we emphasize the need for external validation in future multicenter studies to confirm applicability in different populations.
We believe that this study is of practical significance. In the period of HF deterioration, the damage of liver and kidney function is more common, which makes us no longer regard HF as a single system disease, but a clinical syndrome involving multiple organs. The introduction of ABIC score can not only provide clinicians with the situation of other system organs after HF aggravation but also facilitate and quickly provide objective information on the prognosis of patients. For patients with high risk of liver and kidney function damage, clinicians can carefully choose drugs with less impact on liver and kidney, optimize the use of diuretics to improve hemodynamics, reduce liver and kidney perfusion insufficiency, and actively correct the factors that may lead to liver and kidney function deterioration, so as to improve the prognosis of patients. In addition, it can also assist clinicians to communicate with patients and their families, so that they can understand the severity and potential risks of patients'conditions and jointly formulate more appropriate treatment plans. While the ABIC score shows promise as a supplementary prognostic indicator, its clinical utility is most robust when integrated into comprehensive risk models. Larger multicenter studies are warranted to validate these findings and explore dynamic changes in ABIC score during treatment.
Some non-negligible limitations need to be pointed out. Firstly, the limitations of retrospective observational studies are difficult to avoid, such as the possibility of potential selection bias. Secondly, although lasso regression was used to screen the variables, there were some potential confounders that remained unidentified. Moreover, subgroup analysis was difficult to do further because of the small sample size, so further in-depth study might be necessary in terms of applicability of the model. Finally, the present study only assessed the ABIC score at admission, and after subsequent treatment, the improvement or deterioration of the ABIC score with patients'survival remains to be explored.
Conclusions
The ABIC score, reflecting liver and kidney function, demonstrates potential in predicting adverse outcomes for elderly patients hospitalized with acute exacerbations of chronic heart failure. However, further validation is needed before it can be widely applied in clinical settings. Larger prospective cohort studies should be conducted to confirm its predictive accuracy across diverse patient populations. Additionally, future research should investigate the ABIC score's potential to predict patient response to diuretic therapy and its effectiveness in guiding therapeutic interventions, particularly in optimizing diuretic use and protecting renal function. Until these studies are completed, the ABIC score remains a promising but not yet fully validated tool for risk assessment in this patient population.
Supplementary Information
Acknowledgements
Gratefully acknowledges the support provided by Prof. Chen during data collection.
Authors’ contributions
ZBL provided ideas for the study and wrote the manuscript. MXD participated in data analysis and interpretation. LX collected the data. YPZ participated in the study design and reviewed the final manuscript. All authors reviewed the manuscript.
Funding
YPZ is supported by the key medical science and technology project of Henan Province (No. LHGJ20240150) and the key medical science and technology project of Henan Province (No.252102310242).
Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Second Affiliated Hospital of Chongqing Medical University and exempt from the written informed consent requirement (approval number:2022177). The investigation conformed to the principles outlined in the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zebin Lin and Manxiang Deng contributed equally to this work.
References
- 1.Wang H, Chai K, Du M, Wang S, Cai JP, Li Y, Zeng P, Zhu W, Zhan S, Yang J. Prevalence and incidence of heart failure among urban patients in China: a national population-based analysis. Circ Heart Fail. 2021;14(10):e008406. [DOI] [PubMed] [Google Scholar]
- 2.Forrest EH, Atkinson SR, Richardson P, Masson S, Ryder S, Thursz MR, Allison M. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J Hepatol. 2018;68(3):511–8. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Zheng J, Cai J, Jie Y, Zhang Y, Li H, Lu T, He L, Xiao C, Zeng K, et al. Predictive value of age-bilirubin-international normalized ratio-creatinine score in short-term survival of acute-on-chronic hepatitis B liver failure. Cell Physiol Biochem. 2018;51(5):2484–95. [DOI] [PubMed] [Google Scholar]
- 4.Rana R, Wang SL, Li J, Xia L, Song MY, Yang CQ. A prognostic evaluation and management of alcoholic hepatitis. Minerva Med. 2017;108(6):554–67. [DOI] [PubMed] [Google Scholar]
- 5.Tanai E, Frantz S. Pathophysiology of Heart Failure. Compr Physiol. 2015;6(1):187–214. [DOI] [PubMed] [Google Scholar]
- 6.Okano T, Motoki H, Minamisawa M, Kimura K, Kanai M, Yoshie K, Higuchi S, Saigusa T, Ebisawa S, Okada A, et al. Cardio-renal and cardio-hepatic interactions predict cardiovascular events in elderly patients with heart failure. PLoS ONE. 2020;15(10):e0241003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santas E, Miñana G, Gummel J, Farcasan R, Payá A, Heredia R, Bodí V, Mollar A, Bertomeu-González V, Chorro FJ, et al. International Normalized Ratio and Mortality Risk in Acute Heart Failure and Nonvalvular Atrial Fibrillation Patients Receiving Vitamin K Antagonists. Rev Esp Cardiol (Engl Ed). 2019;72(8):616–24. [DOI] [PubMed] [Google Scholar]
- 8.Correale M, Tarantino N, Petrucci R, Tricarico L, Laonigro I, Di Biase M, Brunetti ND. Liver disease and heart failure: back and forth. Eur J Intern Med. 2018;48:25–34. [DOI] [PubMed] [Google Scholar]
- 9.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103(11):2747–56. [DOI] [PubMed] [Google Scholar]
- 11.Stekhoven DJ, Bühlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–8. [DOI] [PubMed] [Google Scholar]
- 12.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404–13. [DOI] [PubMed] [Google Scholar]
- 13.Lin Z, Zhao Y, Xiao L, Qi C, Chen Q, Li Y. Blood urea nitrogen to serum albumin ratio as a new prognostic indicator in critical patients with chronic heart failure. ESC Heart Fail. 2022;9(2):1360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Veldhuisen DJ, Ruilope LM, Maisel AS, Damman K. Biomarkers of renal injury and function: diagnostic, prognostic and therapeutic implications in heart failure. Eur Heart J. 2016;37(33):2577–85. [DOI] [PubMed] [Google Scholar]
- 15.Samsky MD, Dunning A, DeVore AD, Schulte PJ, Starling RC, Tang WH, Armstrong PW, Ezekowitz JA, Butler J, McMurray JJ, et al. Liver function tests in patients with acute heart failure and associated outcomes: insights from ASCEND-HF. Eur J Heart Fail. 2016;18(4):424–32. [DOI] [PubMed] [Google Scholar]
- 16.van Deursen VM, Edwards C, Cotter G, Davison BA, Damman K, Teerlink JR, Metra M, Felker GM, Ponikowski P, Unemori E, et al. Liver function, in-hospital, and post-discharge clinical outcome in patients with acute heart failure-results from the relaxin for the treatment of patients with acute heart failure study. J Card Fail. 2014;20(6):407–13. [DOI] [PubMed] [Google Scholar]
- 17.Biegus J, Zymliński R, Sokolski M, Siwołowski P, Gajewski P, Nawrocka-Millward S, Poniewierka E, Jankowska EA, Banasiak W, Ponikowski P. Impaired hepato-renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail. 2016;18(12):1518–21. [DOI] [PubMed] [Google Scholar]
- 18.Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg. 2000;63(4):336–47. [PubMed] [Google Scholar]
- 19.Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin MG, Li W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6(7):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019;7(2):87–97. [DOI] [PubMed] [Google Scholar]
- 21.Lourenço P, Silva S, Friões F, Alvelos M, Amorim M, Couto M, Torres-Ramalho P, Guimarães JT, Araújo JP, Bettencourt P. Low prealbumin is strongly associated with adverse outcome in heart failure. Heart (British Cardiac Society). 2014;100(22):1780–5. [DOI] [PubMed] [Google Scholar]
- 22.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–31. [DOI] [PubMed] [Google Scholar]
- 23.Haybar H, Pezeshki SMS, Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Exp Mol Pathol. 2019;110:104267. [DOI] [PubMed] [Google Scholar]
- 24.Miura M, Sakata Y, Nochioka K, Takahashi J, Takada T, Miyata S, Hiramoto T, Inoue K, Tamaki K, Shiba N, et al. Prognostic impact of blood urea nitrogen changes during hospitalization in patients with acute heart failure syndrome. Circ J. 2013;77(5):1221–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sets generated during the current study are available from the corresponding author on reasonable request.