Abstract
Annually, millions of people are affected by mosquito-borne Orthoflavivirus infections. These include diseases caused by the Dengue virus (DENV), Japanese encephalitis virus (JEV), and Zika virus (ZIKV), posing a formidable challenge to global public health. This research aims to explore the potential role of the Gut-Brain Axis (GBA) in Orthoflavivirus infection, particularly focusing on key metabolites involved in the process of viral invasion into the central nervous system. Given the advantages of metabolomics technology in metabolite identification. Therefore, we employed an untargeted Liquid Chromatography-Mass Spectrometry (LC-MS) metabolomics platform to examine alterations in metabolite concentrations within the feces and brain tissues of mice infected with DENV, JEV, or ZIKV, as well as uninfected controls. The results showed that 225, 240, and 252 differential metabolites were identified in the fecal metabolome of DENV, JEV, and ZIKV infections, respectively, with amino acid metabolism and lipid metabolism being significantly disrupted. In the brain metabolome, 37, 81, and 18 differential metabolites were identified for DENV, JEV, and ZIKV infections, respectively, with lipid metabolism and purine metabolism being significantly disrupted. Amino acids with low abundance in viral proteins are significantly disrupted in the amino acid metabolism pathway, suggesting that Orthoflaviviruses adapt to its needs for synthesizing viral proteins by regulating the host’s amino acid composition. The disruption of purine metabolism also implies the viral genome replication process occurring in the brain. Moreover, the disturbance of lipid metabolism is highly correlated with the biological function of the Orthoflavivirus envelope, where Sphingosine 1-phosphate (S1P) may be the key for Orthoflaviviruses to enter the human central nervous system via the GBA. This research is the first to explore the potential role of GBA in Orthoflavivirus infection through joint metabolomic analysis of fecal and brain tissue samples, providing new insights into viral invasion of the central nervous system. The findings not only elucidate the characteristics of viral infection from complementary perspectives of fecal and brain tissue samples, revealing associated metabolic changes, but also establish a foundation for subsequent identification of biomarkers to diagnose disease states—particularly for predicting central nervous system infection risks. The specific patterns revealed by fecal metabolomics analysis provide the theoretical basis for developing non-invasive predictive approaches to assess brain infection status in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-025-04192-0.
Keywords: Orthoflavivirus, GBA, Metabolomics, LC-MS, Biomarkers
Introduction
Dengue virus (DENV), Japanese encephalitis virus (JEV), and Zika virus (ZIKV) are three common mosquito-borne viruses of the genus Orthoflavivirus. These emerging mosquito-borne viruses can cause infections in millions of people every year and have become a global public health threat [1–5]. JEV and ZIKV are neurotropic, which can respectively lead to severe viral encephalitis and microcephaly in infants [6, 7]. Although DENV is generally considered non-neurotropic, emerging evidence suggests its potential neuroinvasive and neurotoxic effects [8]. The neurological damage caused by these viruses usually implies a more severe disease progression, and the initial infections often cause similar clinical symptoms [2, 9]. Therefore, it is necessary in clinical practice to rapidly and accurately diagnose the type of infecting virus and predict the disease progression.
Metabolomics is the systematic investigation of almost all organic compounds in living organisms, in addition to DNA, RNA, and proteins [10, 11]. The levels of metabolites in organisms reflect changes in their phenotypes, representing global changes in metabolic pathways and overall physiological states and this makes metabolomics analysis distinctly advantageous in characterizing the viral infection signatures of hosts [12, 13]. Therefore, the development of metabolomics technology provides unique advantages for clinical diagnosis, it can distinguish specific diseases from healthy states or predict the progression of related diseases by searching for biomarkers associated with specific disease states or conditions [14].
Although many Orthoflaviviruses can cause severe neurological diseases in humans, the exact mechanism by which they enter the human central nervous system remains poorly understood. The Gut-brain Axis (GBA) refers to a bidirectional communication network linking the digestive system and the central nervous system [15]. However, this field remains a blank in the context of Orthoflaviviruses. During the infection process of Orthoflaviviruses, the GBA may provide a pathway for the virus to enter the human central nervous system. This also potentially offers a bridge that could connect the brain metabolome and the fecal metabolome. In addition, fecal samples from patients are relatively easy to obtain in clinical settings, and it is convenient to indirectly reflect the state of brain infection through the fecal metabolome.
Therefore, this investigation aims to explore the potential role of the GBA in DENV, JEV, and ZIKV infections by jointly analyzing the brain tissue and fecal metabolome of infected mice, particularly focusing on key metabolites that may participate in the process of viral invasion into the central nervous system. Previous metabolomics studies on Orthoflavivirus infections have mostly focused on mosquito cells or serum metabolomics. However, There are usually significant differences in metabolomics between different tissue types of the same sample [16]. Therefore, this investigation innovatively conducts parallel metabolomics analyses on brain tissue and fecal samples from the same infected individual. This design not only comprehensively elucidates the characteristics of systemic metabolic disturbances induced by viral infection from complementary perspectives, but more importantly, provides a unique opportunity to directly compare correlation changes of metabolites between the central nervous system and the gut. It establishes a crucial foundation for exploring the mechanistic role of the GBA in Orthoflavivirus infections. Simultaneously, the revealed alterations in fecal metabolomic profiles offer an important theoretical basis and preliminary insights for future development of non-invasive prediction—based on fecal metabolomics—of central nervous system complication progression risks in Orthoflavivirus infections.
Materials and methods
Virus and animals
Challenge experiments were performed on specific pathogen-free, 6-week-old female BALB/c mice, utilizing DENV (serotype 2, NGC strain), JEV (P3 strain), and ZIKV (SMGC_1 strain). DENV, JEV, and ZIKV were all stored at −80℃. All female BALB/c mice were obtained from Beijing Vital River Laboratories to ensure standardized experimental conditions. The challenge experiments conducted on mice adhered strictly to the protocol sanctioned by the Institutional Animal Care and Use Committee of Capital Medical University, under approval number AEEI-2020-046.
Animal anesthesia and euthanasia protocols
Animal anesthesia protocols: Sodium pentobarbital solution (60 mg/mL) was administered via intraperitoneal injection at 50 mg/kg (e.g., 0.017 mL for a 20 g mouse). Surgical anesthesia (absence of corneal reflex and toe pinch response) was achieved within 5–10 min post-injection. Anesthesia status was assessed throughout the procedure via respiratory rate and reflex testing. Supplemental doses (25% of the initial dose, i.e., 12.5 mg/kg) were administered if necessary.
Animal euthanasia protocols: Sodium pentobarbital solution (60 mg/mL) was administered via intraperitoneal injection at a dose of 150 mg/kg (e.g., 0.05 mL for a 20 g mouse). Deep anesthesia (confirmed by loss of righting reflex and pain response) was achieved within 5–10 min post-injection. After confirming complete anesthesia, cervical dislocation was performed as the euthanasia method. This method, approved by the ethics committee, is applicable to adult mice (> 20 g body weight). It rapidly induces central nervous system shutdown and complies with the AVMA-recommended euthanasia guidelines for rodents (AVMA Guidelines, 2020).
Animal experiments
The mice (aged 6 weeks, body weight 16.5 ± 1.5 g) were divided into four groups via stratified randomization by body weight and allocated using a random number table to ensure no significant differences in initial body weight among groups. The infection groups received intraperitoneal challenge with 1 × 10⁵ PFU of JEV, DENV, or ZIKV, while the control group was treated with PBS (n = 5 per group).
Sample collection
Mice infected with the Orthoflaviviruses typically develop acute symptoms within one week, including significant weight loss, reduced activity, lethargy, and a hunched posture. They may also present neurological symptoms such as tremors or hind limb weakness. Therefore, samples were uniformly collected between 9:00–11:00 AM at 7 days post-viral challenge to minimize circadian rhythm interference. Fecal samples were collected via spontaneous defecation: mice were individually housed in sterile cages, and freshly expelled pellets were collected within 15 min. Brain tissues were fully excised via craniotomy. All samples were immediately flash-frozen in liquid nitrogen and stored at − 80 °C until analysis. Freeze-thaw cycles were strictly avoided to prevent degradation of metabolites.
Sample preparation
Fecal samples were taken and mixed with 50% methanol (100 mg: 1.5 mL), then placed in a tissue homogenizer and oscillated at 6,500 MHz for 1 min to homogenize, repeating this process three times. The homogenized samples were subjected to centrifugation at a speed of 12,000 rpm for a duration of 20 min, maintained at a temperature of 4℃. A volume of 200 µL of the resultant supernatant was carefully collected and subsequently subjected to lyophilization to facilitate further analysis. Following reconstitution with 100 µL of 50% MeOH, the samples underwent centrifugation at 12,000 rpm for a duration of 20 min at a temperature of 4℃. The clarified supernatant was carefully transferred into an autosampler vial, specifically prepared for subsequent analysis via Liquid Chromatography-Mass Spectrometry (LC-MS).
Weigh the brain tissue, add 500 µL of MeOH/H2O (1:1, v/v) per 100 mg, homogenize under ice bath conditions, vortex for 30 s, place in −20℃ refrigerator and incubate overnight. After removal in the refrigerator, vortex for 30 s, centrifuge the mixture at 12,000 rpm for 12 min at a temperature of 4℃, take the supernatant and place it in a clean EP tube, freeze-dry the solvent at low temperature. Samples were dissolved in 100 µL of a 1:1 acetonitrile-to-water solution, subjected to sonication for 5 min within an ice bath to facilitate dissolution and maintain low temperatures, followed by vortexing for 30 s. Centrifuge the mixture at 12,000 rpm for 5 min at a temperature of 4℃. Transfer the supernatant to an autosampler vial for subsequent LC-MS analysis.
Quality control (QC) sample preparation
Transfer 10 µL supernatant from each individual sample reconstitution solution into a centrifuge tube and combine. After vortexing the combined solution for 2 min, transfer the mixture to a 4 °C rotary mixer for gentle mixing at a speed of 20 rpm for 45 min. Following mixing, centrifuge the sample at 12,000 rpm for 20 min at 4 °C. After centrifugation, collect the supernatant into an autosampler vial for subsequent LC-MS analysis. The quality control (QC) samples for fecal metabolome and brain metabolome were prepared separately.
Liquid chromatography conditions
The LC analysis was conducted utilizing an ACQUITY UPLC I-Class PLUS system, supplied by Waters Corporation, located in Milford, Massachusetts, United States. To ensure optimal instrument performance and better data collection, equilibrate the chromatographic column before running actual samples. Only after the instrument is stable can the sample be injected. The chromatographic separations were achieved using an ACQUITY UPLC HSS T3 column (150 mm × 2.1 mm, 1.8 μm), supplied by Waters Corporation, Milford, Massachusetts, United States. The column temperature maintained at 40 °C, the sample temperature controlled at 10℃, the mobile phase flow rate adjusted to 0.3 mL/min, and the injection volume precisely set at 2 µL. Insert one QC sample between every 10 sample injections to ensure stability during the instrument injection process. The mobile phase for the chromatographic analysis was composed of two solvent systems: (A) 0.1% formic acid in water (v/v) and (B) 0.1% formic acid in acetonitrile (v/v). The gradient elution conditions for the chromatographic separation were programmed as follows: from 0 to 1.5 min, the solvent composition was 95% A; from 1.5 to 12 min, it was decreased to 80% A; from 12 to 13 min, it was further reduced to 10% A; from 13 to 13.1 min, it reached 0% A for a rapid transition; and from 13.1 to 16 min, the system returned to 95% A to re-equilibrate the column.
Mass spectrum conditions
Metabolite mass spectrometry detection was conducted utilizing a quadrupole time-of-flight mass spectrometer, specifically the Xevo G2-XS Q Tof model, supplied by Waters Corporation, located in Milford, Massachusetts, United States. The MassLynx software was employed to collect both primary and secondary mass spectrometry data from the samples, utilizing the MSE function to enable comprehensive and accurate data acquisition. In the positive ion acquisition mode, the mass spectrometer was configured with a capillary voltage of 0.5 kV, a cone voltage of 40 V, an ion source temperature set at 100 °C, a desolvation gas flow rate of 1000 L/h, and a cone gas flow rate of 50 L/h. The mass range scanned was from m/z 100 to 1200 Da, with a scan time of 16 min and a cycle rate of 0.2 s per cycle. In the negative ion acquisition mode, the mass spectrometer was calibrated with a capillary voltage set at 0.8 kV, a cone voltage maintained at 40 V, an ion source temperature regulated at 100 °C, a desolvation gas flow rate of 1000 L/h, and a cone gas flow rate of 50 L/h. The mass range scanned was from m/z 100 to 1200 Da, with a scan time of 16 min and a cycle rate of 0.2 s per cycle.
Metabolite identification
The raw files obtained from mass spectrometry detection were imported into Progenesis QI software (Waters, version 2.4.6911.27652) for metabolite identification. The identification process involved integrated analysis using liquid chromatography retention time (RT), high-resolution accurate mass (mass deviation tolerance ≤ 5 ppm), and MS/MS fragmentation spectra. Following extraction of features and alignment across all samples by the software, the processed LC-MS/MS data were matched and searched against the ChemSpider, Human Metabolome Database (HMDB), and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. Key database search parameters were set as follows: precursor ion mass tolerance of 0.005 Da (5 ppm), fragment ion mass tolerance of 15 ppm, and matching of experimentally acquired MS/MS spectra against theoretical fragmentation spectra within the databases. The software also considered multiple adduct ion forms, including [M + H]⁺, [M + Na]⁺, [M + K]⁺, [2 M + H]⁺ in positive ion mode, and [M-H₂O-H]⁻, [M-H]⁻, [M + FA-H]⁻ (FA: formic acid), [2 M-H]⁻ in negative ion mode. The confidence level for all identifications strictly adhered to the Metabolomics Standards Initiative confidence levels. It must be explicitly stated that, as this study is an untargeted exploratory analysis without experimental validation using chemical standards (Level 1 confirmation), all identifications are classified as either Level 2 (putatively annotated compounds) or Level 3 (putatively characterized compound classes).
Level 2 annotations, serving as the core output, required simultaneous fulfillment of stringent criteria: a mass error ≤ 5 ppm, a cosine similarity score ≥ 70% for MS/MS spectral matching against database reference spectra, reasonably consistent available RT information, and concordance with the expected isotopic pattern. While the software generated preliminary results based on preset parameters (mass tolerance ≤ 5 ppm, MS/MS match score ≥ 70%), all results underwent manual review assessing spectral match quality, RT plausibility, and biological relevance. Metabolites with insufficient structural information were categorized as Level 3. Features identified solely based on mass/retention time (Level 4) were excluded from the analysis. All identifications within the significantly different metabolite features used for downstream analysis in this study met either Level 2 or Level 3 criteria.
Data analysis process
Following compound identification, rigorous quality control analysis was first performed on the raw data. This included missing value imputation, data normalization, and necessary batch effect correction to ensure the accuracy and reliability of subsequent analytical results. Subsequently, multivariate statistical analysis was conducted on the quality-controlled data. Both Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) were performed using the MetaboAnalyst online platform (https://www.metaboanalyst.ca). Hierarchical Clustering Analysis (HCA) was also carried out on the same platform. For HCA, Euclidean distance was employed as the distance metric, and Ward’s linkage algorithm was used for clustering, enabling the grouping of metabolites and/or samples. The results were visualized as heatmaps to reveal similarities in metabolite expression patterns and relationships among samples.
To elucidate the biological significance of differential metabolites in depth, the following analyses were further conducted. The enrichment analysis was based on the union of differential metabolite lists identified through previous multivariate statistical analysis across groups in both positive and negative ion modes. This combined set of differential metabolites was submitted to the MetaboAnalyst platform by inputting Compound Name. The chemical structure-based enrichment analysis module was selected to identify significantly enriched metabolite classes or chemical subclasses among the differential metabolites (significance threshold typically set at FDR < 0.05). Pathway analysis utilized the same union of differential metabolites and was also performed on the MetaboAnalyst platform. The platform compared the input metabolites against the Mus musculus database (KEGG), employing methods such as the hypergeometric test to calculate the Enrichment and Impact values for each metabolic pathway. This enabled the identification of metabolic pathways significantly enriched with differential metabolites (significance thresholds typically set at either p < 0.05 or FDR < 0.05 with Impact > 0).
Finally, for the key differential metabolites identified through pathway analysis, correlation analyses were performed based on the Pearson correlation coefficient and Spearman correlation coefficient. This analysis was conducted within the R programming environment. The psych package was used to calculate the Pearson correlation coefficients and their statistical significance (p-values) between all pairs of key differential metabolites. Subsequently, the pheatmap package was employed to visualize the resulting correlation matrix, generating a correlation heatmap. The heatmap intuitively displays the strength of positive (warm color scheme) and negative (cool color scheme) correlations between metabolites through color gradients, supplemented by hierarchical clustering to group metabolites. This analysis aimed to quantify the statistical associations among key metabolites, with a particular focus on exploring the relationships in metabolite expression patterns between the fecal metabolome and brain metabolome in mice.
Results
Global metabolic landscape of feces and brain samples of Orthoflavivirus-infected mice
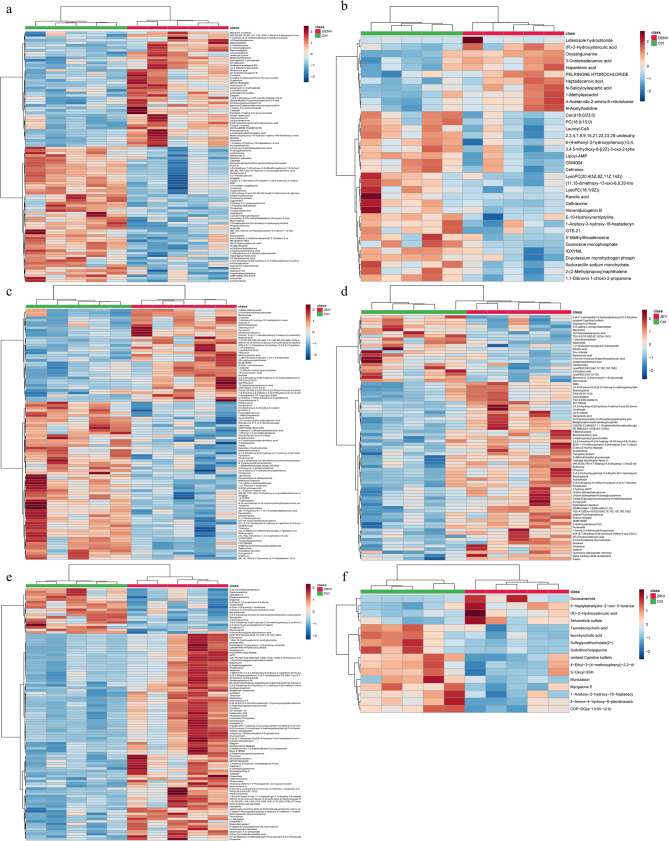
In an effort to maximize the coverage of detected metabolites, both positive and negative ion scanning modes were utilized for the quantification of metabolite peaks in fecal and brain samples from control mice and those infected with three distinct types of Orthoflaviviruses. Following this, PCA and OPLS-DA were conducted to explore and characterize the metabolic profiles. We perform quality control on the data primarily through the performance of QC samples in PCA and sample replicate correlation evaluation. The quality of the OPLS-DA model was validated using the cross-validation method. Specifically, a portion of the sample data was used to construct the grouping model, while another portion was employed to test this pre-grouped model. The resulting R2Y and Q2 values represent the explainable variance and predictive capability of the model, respectively, which can be used to evaluate the model’s performance. Meanwhile, we also used the permutation test to verify whether the OPLS-DA analysis was overfitting (Additional file 1). While the PCA results failed to demonstrate a distinct separation between the control and infected groups of mice (Additional file 2), the statistical evaluation of the OPLS-DA model revealed a significant separation between these two groups (Fig. 1). Differential metabolites were further identified based on the Variable Importance in Projection (VIP) values obtained from the OPLS-DA model. Metabolites meeting the criteria of coefficient of variation (CV) < 30% in QC samples, VIP > 1, and P-value < 0.05 were identified as differential metabolites. The statistical results of these differential metabolites are presented in Table 1. The detailed information regarding the differential metabolites is provided in Additional file 3. A hierarchical clustering heatmap was drawn for the differential metabolites selected according to the aforementioned criteria (Fig. 2). The results revealed distinct metabolic profiles in fecal and brain samples of infected mice compared to controls. At the same time, the fecal metabolomic profiles and brain metabolomic profiles of the same type of Orthoflavivirus infection also showed significant differences. Additionally, we provided loading plots and S-plots to visualize the contribution and direction of variables to the principal components, as well as to identify statistically significant variables that strongly influence inter-group separation (Additional file 4).
Fig. 1.
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) score plot under the infection conditions of three Orthoflaviviruses. a Dengue virus (DENV) fecal metabolome in negative ion mode (NEG) b DENV fecal metabolome in positive ion mode (POS) c DENV brain metabolome in NEG d DENV brain metabolome in POS e Japanese encephalitis virus (JEV) fecal metabolome in NEG f JEV fecal metabolome in POS (g) JEV brain metabolome in NEG h JEV brain metabolome in POS i Zika virus (ZIKV) fecal metabolome in NEG j ZIKV fecal metabolome in POS k. ZIKV brain metabolome in NEG l. ZIKV brain metabolome in POS
Table 1.
Statistical results of differential metabolites in fecal metabolome and brain metabolome
| Sample | Groups | Upregulated | Downregulated | Total differential metabolites |
|---|---|---|---|---|
| Feces | DENV vs. Ctrl | 123 | 102 | 225 |
| JEV vs. Ctrl | 100 | 140 | 240 | |
| ZIKV vs. Ctrl | 210 | 42 | 252 | |
| Brain | DENV vs. Ctrl | 12 | 25 | 37 |
| JEV vs. Ctrl | 59 | 22 | 81 | |
| ZIKV vs. Ctrl | 4 | 14 | 18 |
Fig. 2.
Hierarchical clustering analysis was performed on the differential metabolites identified under the infection conditions of three distinct Orthoflaviviruses. a Heatmap of fecal metabolome in Dengue virus (DENV) infection b Heatmap of brain metabolome in DENV infection c Heatmap of fecal metabolome in Japanese encephalitis virus (JEV) infection d Heatmap of brain metabolome in JEV infection e Heatmap of fecal metabolome in Zika virus (ZIKV) infection f Heatmap of brain metabolome in ZIKV infection
Enrichment analysis of fecal and brain samples from mice infected with Orthoflaviviruses
To further analyze the classification of metabolites in fecal and brain samples infected with Orthoflaviviruses, the compound names obtained from the union of differential metabolites in both positive ion mode (POS) and negative ion mode(NEG) for each group were input into MetaboAnalyst for enrichment analysis based on chemical structures. The results revealed that the metabolic patterns of different parts infected with the same type of Orthoflavivirus exhibited significant differences, and the metabolic patterns of different Orthoflavivirus infections in the same part also showed significant differences (Fig. 3). The differences in these metabolic patterns may be related to virus-specific factors and host response specificity. Among them, distinct metabolic patterns observed in different sites of the same Orthoflavivirus species show significant variations, which may be attributed to the distinct cell tropism of Orthoflaviviruses for specific cell types. This directly influences the local metabolic demands of infected cells. Meanwhile, variations in metabolic patterns between different Orthoflavivirus infections at the same site may be associated with differences in the extent and mechanisms of histopathological damage caused by different Orthoflaviviruses. These damages include neuronal death, blood-brain barrier disruption, hepatocyte necrosis, or vascular leakage, which lead to variations in metabolites released into the tissue microenvironment. Furthermore, differences in the magnitude, type, and timing of innate and adaptive immune responses mounted by the host against distinct Orthoflaviviruses contribute to significant variations in immunometabolic reprogramming.
Fig. 3.
The differential metabolites in the metabolome associated with three different Orthoflaviviruses were enriched according to their chemical structures. a Enrichment bubble chart of fecal metabolome in Dengue virus (DENV) infection b Enrichment bubble chart of brain metabolome in DENV infection c Enrichment bubble chart of fecal metabolome in Japanese encephalitis virus (JEV) infection d Enrichment bubble chart of brain metabolome in JEV infection e Enrichment bubble chart of fecal metabolome in Zika virus (ZIKV) infection f Enrichment bubble chart of brain metabolome in ZIKV infection
In fecal samples from mice infected with the three types of Orthoflaviviruses, multiple products of protein degradation, carbohydrates, and fatty acids and their derivatives were found to be perturbed due to Orthoflavivirus infection (Fig. 3a, c, e). This may be to meet the needs of viral replication. During the replication of Orthoflaviviruses, there is a need for viral protein translation, synthesis of viral envelopes, and a large amount of energy supply. Therefore, Orthoflaviviruses may alter the metabolic patterns of the gut during infection to absorb the substances required for viral replication.
In brain tissues infected with DENV and JEV, perturbations in the products of protein degradation, carbohydrates, and fatty acids and their derivatives were also observed. In addition, unlike the fecal metabolome, perturbations in nucleotides and their derivatives were also observed in the brain metabolome of DENV and JEV infections (Fig. 3b, d). This disturbance may be caused by the demand for Orthoflavivirus genome replication. However, possibly due to the low number of differential metabolites in the brain metabolome of ZIKV-infected mice, the aforementioned phenomena were not observed (Fig. 3f). In addition, many other bioactive substances in the fecal and brain metabolomes of Orthoflaviviruses are also disturbed, but the roles these bioactive substances play in Orthoflavivirus infection are not yet clear.
Metabolic pathway analysis of fecal and brain samples from mice infected with Orthoflaviviruses
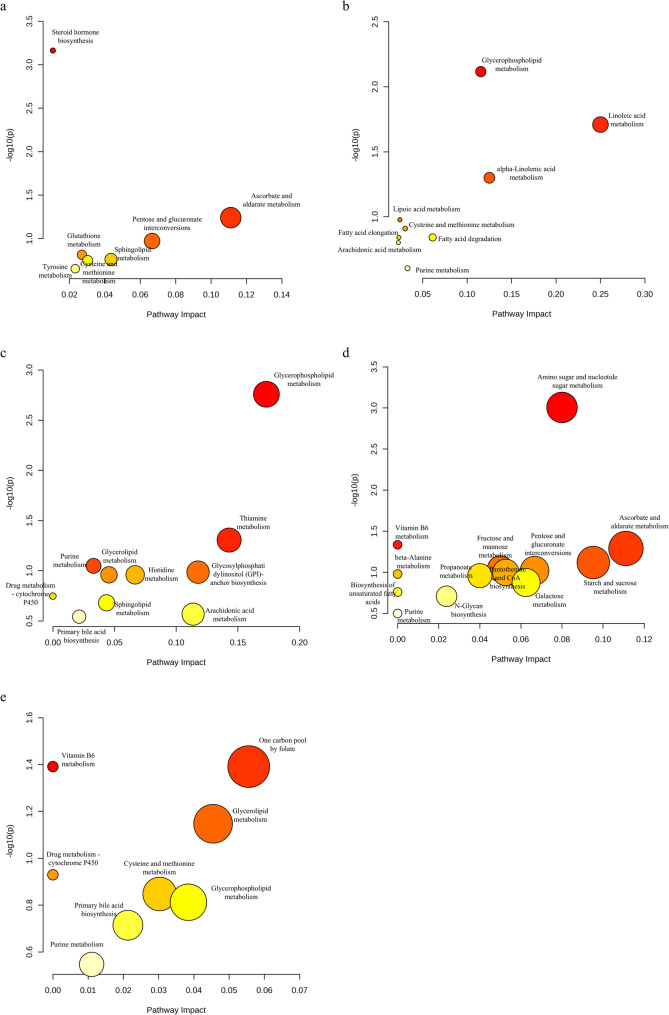
To obtain the distribution of differential metabolites in different pathways in fecal and brain samples infected with Orthoflaviviruses, and to explore the relationship between differential metabolites and pathway alterations. The compound names obtained from the union of differential metabolites in both POS and NEG for each group were input into MetaboAnalyst. These differential metabolites were cross-referenced with the Mus musculus database (KEGG) to ascertain the metabolic pathways in which they are implicated. By comparing the pathway distribution of fecal and brain metabolites infected with the same type of Orthoflavivirus, it was demonstrated that there are differences in metabolic patterns between different parts under the same infection background (Fig. 4). In addition, the differential metabolites classified in the pathway analysis are provided in Additional file 5.
Fig. 4.
Pathway analysis of differential metabolites in the metabolomes of three Orthoflaviviruses. a Metabolic pathway bubble chart of fecal metabolome in Dengue virus (DENV) infection b Metabolic pathway bubble chart of brain metabolome in DENV infection c Metabolic pathway bubble chart of fecal metabolome in Japanese encephalitis virus (JEV) infection d Metabolic pathway bubble chart of brain metabolome in JEV infection e Metabolic pathway bubble chart of fecal metabolome in Zika virus (ZIKV) infection
Although the specific metabolic pathways classified in the fecal metabolomes of the three types of Orthoflaviviruses have certain differences, there is a certain similarity in the major categories. Lipid metabolism and amino acid metabolism were perturbed in the fecal metabolomes of all three Orthoflaviviruses (Fig. 4a, c, e). The specific metabolic pathways of amino acid metabolism that are disturbed may be related to the differences in amino acid composition of the polyproteins of the three types of Orthoflaviviruses. In DENV, the disturbance in lipid metabolism is focused on sphingolipid metabolism, while in JEV and ZIKV, it is focused on glycerophospholipid metabolism and glycerolipid metabolism. The fecal metabolomes of JEV- and ZIKV-infected mice exhibited higher similarity compared to those of DENV-infected mice. Glycerophospholipid metabolism, glycerolipid metabolism, purine metabolism, primary bile acid biosynthesis, and drug metabolism - cytochrome P450 were all classified in JEV and ZIKV. This suggests that the substances required for the replication of JEV and ZIKV have higher similarity. On the other hand, the significant differences between the fecal metabolome of DENV and the other two types of Orthoflaviviruses may have the potential to provide biomarkers for clinically distinguishing the types of Orthoflavivirus infections. Notably, in the fecal metabolomics of the three types of Orthoflaviviruses, metabolites classified into the metabolic pathways of tyrosine, histidine, cysteine, and methionine were disturbed. An analysis of the amino acid composition within the viral polyproteins indicated that these amino acids are present at relatively low abundances in these polyproteins (Additional file 6).
Owing to the limited number of differential metabolites identified in the brain metabolome of mice infected with ZIKV, none were classified into any metabolic pathways. In the brain metabolomes of DENV and JEV, perturbations in lipid metabolism and purine metabolism were observed (Fig. 4b, d). In DENV, the interference in lipid metabolism is focused on fatty acid extension and degradation, linoleic acid metabolism, alpha-linolenic acid metabolism, glycerophospholipid metabolism, and arachidonic acid metabolism. In JEV, the focus is on the biosynthesis of unsaturated fatty acids. The interference in purine metabolism may be due to the need for viral genome replication. Unlike DENV, in the brain metabolome of JEV, multiple carbohydrate metabolisms were disturbed. The presence of Uridine Diphosphate N-Acetyl-alpha-D-Glucosamine (UDP-GlcNAc) and Uridine Diphosphate Glucose (UDP-Glc) in the classified pathways suggests that this metabolic pathway may be related to the glycosylation process of viral proteins [17, 18].
Correlation analysis of fecal and brain samples from mice infected with Orthoflaviviruses
To explore the correlation between the fecal and brain metabolome of mice, the differential metabolites classified in the pathway analysis were analyzed using the Pearson correlation coefficient and Spearman correlation coefficient. This statistical method was employed to quantify the degree of correlation between metabolites. Pearson correlation analysis revealed significant negative correlations between Lysophosphatidylcholine (LysoPC) (18:1(9Z)) in the DENV brain metabolome and fecal metabolites including 15-Hydroxynorandrostene-3,17-dione glucuronide, L-Cystine, 16-Glucuronide-estriol, and Estradiol-17beta 3-glucuronide (Fig. 5a). In DENV infection, LysoPC can be incorporated into the membrane to increase permeability, thereby promoting the exchange of components, genome replication, and viral assembly of the DENV replication complex encapsulated within the membrane barrier [19]. The decrease in LysoPC levels in the brain metabolome (fold change, FC = 0.71) reflects an increase in DENV replication in the brain, and the levels of L-Cystine in the feces show an opposite trend to the decrease in LysoPC in the brain. However, the decrease in L-Cystine levels in the fecal metabolome (FC = 0.02) may be due to its conversion to downstream threonine. In the fecal metabolome of DENV, L-Cystine is an amino acid with low abundance in the polyprotein of Orthoflaviviruses, while its downstream threonine is an amino acid with high abundance in the polyprotein of Orthoflaviviruses. This suggests that Orthoflaviviruses may regulate the composition of amino acids by altering host amino acid metabolic pathways to meet their own synthetic needs. However, the functions of 15-Hydroxynorandrostene-3,17-dione glucuronide, 16-Glucuronide-estriol, and Estradiol-17beta 3-glucuronide in fecal metabolome in DENV are still unclear. They show significant negative correlation with LysoPC(18:1(9Z)) in brain metabolome, but it is impossible to infer the implied causal relationship. Additionally, 5’-Methylthioadenosine (MTA) in the DENV brain demonstrated consistently significant positive correlations with Sphingosine 1-phosphate (S1P) in fecal samples across both analytical approaches (Fig. 5a, c). MTA is metabolized by MTA phosphorylase, leading to the generation of 5-methylthioribose-1-phosphate and adenine, and these products play a role in the cellular purine cycle [20]. This could be attributed to DENV requires a large amount of purines to participate in the synthesis of viral genomes. The decrease in the level of MTA (FC = 0.71) in the brain metabolome implies its conversion to adenine to participate in the replication of DENV in the brain. As a bioactive lipid, S1P can regulate vascular tension and the integrity of the vascular barrier by binding to S1P receptors (S1PR1-S1PR5) [21]. Disruption of endothelial barrier integrity by overexpressing the S1PR2 receptor in DENV infection [22]. MTA is significantly positively correlated with S1P, which indicates that as DENV replicates extensively in the brain, leading to a further decrease in the level of MTA in the brain, the level of S1P in feces will maintain the same trend of decline as the decrease in MTA in the brain. This suggests that DENV infection enhances host absorption of S1P. However, compared with the control, the level of S1P (FC = 1.36) in the fecal metabolome of DENV-infected individuals increased. This could be attributed to the fecal metabolome is not only related to the ability of infected cells to absorb, retain, or excrete metabolites, but also to the metabolism of intestinal flora [14]. The intestinal microbiota may have significantly contributed to the elevation of S1P levels.
Fig. 5.
Correlation analysis shows the correlation between the differential metabolites classified by pathway analysis in the brain metabolome and the fecal metabolome. a Heatmap of Pearson correlation analysis for Dengue virus (DENV) infection b Heatmap of Pearson correlation analysis for Japanese encephalitis virus (JEV) infection. c Heatmap of Spearman correlation analysis for DENV infection d Heatmap of Spearman correlation analysis for JEV infection. The x-axis represents the fecal metabolome, and the y-axis represents the brain metabolome.* p < 0.05, ** p < 0.01
In the JEV metabolome, both Pearson and Spearman correlation analyses jointly demonstrated significant positive correlations between fecal PE(20:0/18:4(6Z,9Z,12Z,15Z)) and cerebral UDP-GlcNAc as well as UDP-Glc, while revealing a significant negative correlation with Phosphopantothenic Acid (Fig. 5b, d). UDP-GlcNAc (FC = 1.65) and UDP-Glc (FC = 1.45) in the brain may be upregulated due to their involvement in the glycosylation of viral proteins during the JEV replication process [17, 18]. PE can mediate viral infection of phagocytes by signaling to them [23, 24]. Therefore, in the context of JEV infection, PE in the feces (FC = 0.33) may be downregulated due to increased intestinal absorption. However, PE exhibits a significant positive correlation with UDP-GlcNAc and UDP-Glc. This suggests that as JEV infection progresses and leads to increased levels of UDP-GlcNAc and UDP-Glc, PE levels are likely to follow a similar upward trend. This contradicts the downregulation of PE levels in the feces in the context of JEV infection. This may be related to the metabolism of intestinal flora. Phosphopantothenic Acid (FC = 0.53), which is a precursor of Coenzyme A, may be downregulated as it is converted into Coenzyme A to participate in various life processes during JEV replication [25]. Therefore, it is significantly negatively correlated with PE. Additionally, both analytical approaches demonstrated significant negative correlations between fecal Deoxyinosine and cerebral Acrylyl-Coenzyme A, while revealing significant positive correlations with 4-Pyridoxic acid (Fig. 5b, d). Deoxyinosine (FC = 0.77) in the fecal metabolome and its downstream hypoxanthine levels are both downregulated in the feces, possibly due to their involvement in the synthesis of purine nucleotides for JEV genome replication. However, the functions of Acrylyl-Coenzyme A and 4-Pyridoxic acid in the brain metabolome in the context of JEV are not yet clear. Therefore, we can only know their correlation with Deoxyinosine and cannot infer the underlying causal relationships. Furthermore, Pearson correlation analysis revealed a significant negative correlation between fecal LysoPC(16:0) and cerebral UDP-Glc (Fig. 5b). Separately, Spearman analysis indicated significant negative correlations between fecal LysoPC(16:0) and cerebral UDP-GlcNAc, as well as between fecal Lysophosphatidic Acid (LysoPA) (0:0/18:0) and cerebral UDP-GlcNAc, UDP-Glc, and Acrylyl-Coenzyme A (Fig. 5d). Unlike LysoPC in DENV brain metabolism, which decreases in level due to the needs of DENV, the increase of LysoPC (FC = 1.64) in the fecal metabolome of JEV reflects the downregulation of LysoPC absorption in the intestine in the context of JEV infection. This may be related to the saturation degree of LysoPC. The upregulated LysoPC in the fecal metabolome of JEV is saturated, while LysoPC in the brain of DENV is unsaturated. Unsaturated phospholipids can induce membrane curvature, which is important for maintaining the highly curved membranes in JEV-infected cells [26]. LysoPC in the fecal metabolome is significantly negatively correlated with UDP-Glc and UDP-GlcNAc in the brain metabolome. As UDP-Glc and UDP-GlcNAc increases, LysoPC will maintain an opposite trend of change. This may be related to the metabolism of the intestinal flora. Meanwhile, LysoPA may possess functions in JEV similar to those of LysoPC. Therefore, LysoPA levels were also elevated (FC = 1.68) in the fecal metabolome, and similarly exhibited a statistically significant negative correlation with UDP-Glc and UDP-GlcNAc in the brain metabolome. Since the functions of Acrylyl-Coenzyme A in the brain during JEV infection remain unclear, it is likewise impossible to infer any implied causal relationship with fecal LysoPA.
Discussion
During the replication of Orthoflavivirus, a polyprotein composed of three structural proteins (capsid protein, precursor membrane protein, envelope protein) and seven non-structural proteins, namely NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5, and this polyprotein translation utilizes the virus’s positive-sense RNA as a template [27]. In this investigation, the metabolites classified into the metabolic pathways of tyrosine, histidine, cysteine, and methionine in the fecal metabolomics of the three types of Orthoflavivirus were disturbed. Previously, Cui et al. [28] observed a downward trend in tryptophan in the serum metabolomics analysis of DF patients. The metabolomics investigation by EI-Bacha et al. [29] also showed that the concentrations of plasma glutamine and histidine decreased with DENV infection, while valine and tyrosine exhibited a longitudinal increase in dengue fever subjects. Similarly, Pang et al. [30] reported a significant increase in the levels of tryptophan and kynurenine in the brains of mice infected with ZIKV. Owing to the paucity of metabolomics studies focusing on JEV, there is currently a lack of relevant reports in this area. It is noteworthy that through the analysis of the amino acid composition of the Orthoflavivirus polyprotein, it was found that cysteine, tyrosine, histidine, tryptophan, and glutamine are all amino acids with low abundance in the polyproteins of the three types of Orthoflavivirus, while valine is an amino acid with high abundance. Therefore, in Orthoflavivirus infection, it may be possible to regulate the host’s amino acid composition to meet the needs of synthesizing viral proteins. However, tyrosine and tryptophan showed an increasing trend in the brains of dengue fever patients and mice infected with ZIKV, which implies that tyrosine and tryptophan may play crucial roles in other processes of viral infection in addition to participating in the synthesis of viral proteins. Tyrosine may be involved in the recognition process of the TYRO3/AXL/MER (TAM) receptors. The TAM receptor tyrosine kinase family promotes viral invasion and replication by binding to PS on the surface of enveloped viruses [31]. In addition, it is known that enhanced tryptophan-kynurenine metabolism contributes to immune suppression and neuronal excitotoxicity [32]. Therefore, ZIKV infection may lead to neuronal excitotoxicity and/or immune suppression by disrupting the tryptophan-kynurenine metabolism.
The capsid of Orthoflavivirus is enveloped by a membrane with a specific lipid composition, which can assist the virus in mutual recognition with host cells and enable it to infect host cells, and the replication and assembly of Orthoflavivirus also depend on the replication complex formed by remodeling the lipid composition of the endoplasmic reticulum membrane [23, 24, 33–37]. In our investigation, it was found that in the three types of Orthoflavivirus infections, differential metabolites were classified into lipid metabolism pathways. However, the specific lipid metabolism pathways infected under different infection backgrounds vary. In the fecal metabolome, the disruption of DENV lipid metabolism focuses on sphingolipid metabolism, while JEV and ZIKV focus on glycerophospholipid metabolism and glycerolipid metabolism; In the brain metabolome, the disruption of DENV lipid metabolism focuses on fatty acid elongation and degradation, linoleic acid metabolism, alpha-linolenic acid metabolism, glycerophospholipid metabolism, and arachidonic acid metabolism, while JEV focuses on the biosynthesis of unsaturated fatty acids. This also reflects the differences between the fecal metabolome and the brain metabolome under the same infection background. The fecal metabolome is related to the ability of infected cells to absorb, retain, or excrete metabolites, while the brain metabolome is more capable of reflecting the abundance of metabolites within infected cells [14].
In the lipid metabolism pathway, S1P in the fecal metabolome of DENV and LysoPC, Phosphatidylcholine (PC) (18:0/15:0) in the brain metabolome are classified; In the fecal metabolome of JEV, PE(20:0/18:4(6Z,9Z,12Z,15Z)), LysoPC(16:0), LysoPA(0:0/18:0), Prostaglandin H2 (PGH2), S1P, and in the brain metabolome, Stearic Acid are classified; In the fecal metabolome of ZIKV, LysoPA(0:0/18:0) is classified. The upregulation of PC in the DENV brain metabolism showed the same trend in the DENV-infected mosquito cell metabolism data of Perera et al. [38]. The upregulation of PC may be due to the fact that the assembly of DENV is located on the replication complex reshaped from the endoplasmic reticulum, and the membranes similar to or derived from the endoplasmic reticulum are rich in PC [39, 40]. In addition, the brain metabolome of DENV also showed a downregulation of LysoPC, LysoPC can increase endothelial permeability, and plasma leakage is a major pathological feature of dengue hemorrhagic fever [41, 42]. Due to the scarcity of literature regarding the metabonomics of JEV, this investigation represents the first documentation of level perturbations for specific metabolites—namely PE, PGH2, LysoPA, and S1P—within the lipid metabolic pathways associated with JEV infection. PE is an anionic lipid typically situated on the inner leaflet of the plasma membrane. Under apoptotic conditions, PE can be translocated to the outer leaflet of the membrane, serving as an external signal for cell clearance [43–45]. PE may be similar to Phosphatidylserine, mediating viral infection of phagocytes by signaling to phagocytic cells [23, 24]. PGH2 is an effective inflammatory mediator that can participate in regulating the intensity and duration of inflammatory responses [46, 47]. Although the interference of PGH2 and PE in JEV has not been observed before, an increase in the content of PGH2 was found in Aedes albopictus cells infected with ZIKV, and an increase in the content of PE was found in C6/36 mosquito cells infected with ZIKA [48, 49]. The perturbation of LysoPA in the fecal metabolome is observed for the first time in both JEV and ZIKV. LysoPA is usually present in low amounts in bacteria, but it can significantly increase under certain environmental conditions and may act as a signaling molecule involved in bacterial adaptation and survival [50]. Therefore, the increase of LysoPA in the fecal metabolome may be due to the changes in the intestinal environment caused by the infection of the Orthoflavivirus, which in turn prompts the secretion by intestinal bacteria. However, its specific role in the context of Orthoflavivirus infections remains to be elucidated. It is noteworthy that an elevation in S1P levels was detected for the first time in the fecal metabolome associated with infections by both DENV and JEV. S1P can regulate the barrier function of blood vessels by binding to S1P receptors (S1PR1-S1PR5) [21]. It is reported that in DENV infection, overexpression of the S1PR2 receptor disrupts the integrity of the endothelial barrier [22]. The role of S1P was not mentioned in previous studies on JEV, but the role of the S1P-S1PR axis in neurodegenerative diseases, neuroinflammation, and cerebrovascular diseases is widely recognized [51]. The findings of this investigation suggest that S1P may play a significant role in the infection process of JEV.
Further establish the possible connections between brain metabolome and fecal metabolome through correlation analysis. It is noteworthy that in the fecal metabolome of DENV-infected mice, S1P is significantly positively correlated with MTA in the brain metabolome. This implies that S1P may be the key through which Orthoflavivirus enter the human central nervous system via the GBA. The decrease in the level of MTA in the brain metabolome may be due to its conversion to adenine, which participates in the synthesis of the DENV viral genome [20]. In addition, studies have shown that in the intracerebral hemorrhage (ICH) model, the binding of S1P to S1PR2 was found to be positively correlated with the degree of blood-brain barrier leakage [52]. Therefore, the mechanism by which DENV enters the human central nervous system may involve increasing the host’s absorption of S1P. After S1P is absorbed through the gut and enters the bloodstream, it is transported to the brain, where it binds to the S1PR2 receptor, thereby disrupting the blood-brain barrier and facilitating DENV infection of the brain. The decrease in the level of MTA reflects the extensive replication of DENV in the brain.
Conclusion
This research employed a untargeted LC-MS metabolomics platform to assess alterations in metabolite concentrations within fecal and brain tissues of mice infected with DENV, JEV, ZIKV, and a cohort of uninfected control mice. Our research makes two key contributions to understanding Orthoflavivirus infection-associated metabolic reprogramming and its potential link to the GBA: (i) First combined brain-fecal metabolomic analysis: We pioneered simultaneous metabolomic profiling of brain tissue and fecal samples from the same Orthoflavivirus-infected host. This design systematically reveals systemic metabolic dysregulation induced by viral infection from complementary perspectives, providing unique insights into metabolic crosstalk between the central nervous system and intestinal microenvironment. (ii) First exploration of GBA involvement: We present the inaugural investigation into the potential role of the GBA during Orthoflavivirus infection, identifying key metabolites potentially mediating neuroinvasion. Key metabolic alterations include: Significant disruption of low-abundance amino acids from viral proteins in metabolic pathways, indicating viral manipulation of host amino acid composition for virion synthesis; Purine metabolism dysregulation in brains associated with viral replication; Lipid metabolic changes, with particularly significant S1P alterations, strongly suggest their role as key mediators of neuroinvasion via the GBA. Collectively, these findings provide preliminary metabolomic evidence supporting the hypothesis of Orthoflavivirus neuroinvasion through the GBA. Furthermore, the predictive linkage model established between brain infection status and specific fecal metabolic signatures lays crucial theoretical groundwork for developing non-invasive fecal metabolome-based approaches to assess neuroinvasion risk in Orthoflavivirus infections.
However, this research has certain limitations. The primary conclusions are based on metabolomics data and lack experimental validation. Future research should design experiments to provide supplementary verification. Additionally, JEV-infected mice exhibited neurological symptoms such as tremors and hindlimb weakness, whereas no neurological impairment symptoms were observed in DENV- or ZIKV-infected mice. This phenomenon was further supported by brain metabolomics results, which revealed significantly fewer differentially expressed metabolites in the brains of DENV- and ZIKV-infected mice compared to JEV-infected mice. Notably, the number of altered metabolites in ZIKV-infected mouse brains was exceptionally low, failing to enrich any significantly altered metabolic pathways; thus, these data were excluded from subsequent analyses. This may be attributed to DENV’s non-neurotropic properties and the inherent resistance of BALB/c mice to ZIKV infection. Finally, as these conclusions are derived from mouse models, caution should be exercised when extrapolating them to clinical samples. Further validation in clinical specimens is warranted in future research.
Supplementary Information
Additional file 1. Data quality control analysis and evaluation of the Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) model.
Additional file 2. Principal component analysis (PCA) score plot under the infection conditions of three Orthoflaviviruses. (a) Dengue virus (DENV) fecal metabolome in negative ion mode (NEG) (b) DENV fecal metabolome in positive ion mode (POS) (c) DENV brain metabolome in NEG (d) DENV brain metabolome in POS (e) Japanese encephalitis virus (JEV) fecal metabolome in NEG (f) JEV fecal metabolome in POS (g) JEV brain metabolome in NEG (h) JEV brain metabolome in POS (i) Zika virus (ZIKV) fecal metabolome in NEG (j) ZIKV fecal metabolome in POS k. ZIKV brain metabolome in NEG l. ZIKV brain metabolome in POS.
Additional file 3. Detailed information about differential metabolites screened in metabolomics.
Additional file 4. Loading plots and S-plots of the OPLS-DA model.
Additional file 5. The differential metabolites classified in the pathway analysis.
Additional file 6. Amino acid composition of the polyproteins of three Orthoflaviviruses.
Acknowledgements
Not applicable.
Authors' contributions
ZS: conceived and designed the experiment, completed the experiment, analyzed the data, drafted the initial version of the manuscript. NS: completed the experiment, analyzed the data.CY: provided critical advice to the experiment. XZ: conceived and designed the experimental protocol, provided critical advice to the experiment, and participated in the final editing process. All authors have reviewed and approved the final version of the manuscript for submission.
Funding
This research was financially supported by the National Natural Science Foundation of China under Grant Number 82001749.
Data availability
Metabolomics data to support the findings are included in the article. The amino acid sequence information of DENV, JEV, and ZIKV polyproteins is obtained from the Protein Database, which can be accessed at the following URL: https://www.ncbi.nlm.nih.gov/protein/XER78274.1; https://www.ncbi.nlm.nih.gov/protein/AAA64561.1;https://www.ncbi.nlm.nih.gov/protein/AMZ03557.1.
Declarations
Ethics approval and consent to participate
All procedures were approved by the Institutional Animal Care and Use Committee of Beijing Friendship Hospital, Capital Medical University, and the Institute of Biophysics, Chinese Academy of Sciences (approval numbers: 20-2013, ABSL-2-2022015).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X. Emerging arboviruses: why today?? One Health. 2017;4:1–13. 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braack L, de Gouveia AP, Cornel AJ, Swanepoel R, de Jager C. Mosquito-Borne arboviruses of African origin: review of key viruses and vectors. Parasit Vectors. 2018;11(1):29. 10.1186/s13071-017-2559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, chikungunya, and other emerging Vector-Borne viral diseases. Annu Rev Med. 2018;69:395–408. 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuwata R, Torii S, Shimoda H, Supriyono S, Phichitraslip T, Prasertsincharoen N, Takemae H, Bautista RCJT, Ebora VDBM, Abella JAC, Dargantes AP, Hadi UK, Setiyono A, Baltazar ET, Simborio LT, Agungpriyono S, Jittapalapong S, Rerkamnuaychoke W, Hondo E, Maeda K. Distribution of Japanese encephalitis virus, Japan and Southeast asia, 2016–2018. Emerg Infect Dis. 2020;26(1):125–8. 10.3201/eid2601.190235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Cheng X, Luo M, Shi J. Japanese encephalitis virus: an overview. J Vector Borne Dis. 2024. 10.4103/JVBD.JVBD_49_24. [DOI] [PubMed] [Google Scholar]
- 7.Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Rus KR, Vipotnik TV, Vodušek VF, Vizjak A, Pižem J, Petrovec M, Županc TA. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–8. 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 8.Li G-H, Ning Z-J, Liu Y-M, Li X-H. Neurological manifestations of dengue infection. Front Cell Infect Microbiol. 2017;7. 10.3389/fcimb.2017.00449. [DOI] [PMC free article] [PubMed]
- 9.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–63. 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 11.Roberts LD, Souza AL, Gerszten RE, Clish CB. Targeted Metabolomics. Curr Protoc Mol Biol 2012, Chap. 30, Unit 30.2.1–24. 10.1002/0471142727.mb3002s98 [DOI] [PMC free article] [PubMed]
- 12.van Oort PMP, de Bruin S, Weda H, Knobel HH, Schultz MJ, Bos LD. On behalf of the Mars consortium, null. Exhaled breath metabolomics for the diagnosis of pneumonia in intubated and Mechanically-Ventilated intensive care unit (ICU)-Patients. Int J Mol Sci. 2017;18(2):449. 10.3390/ijms18020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–43. 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakaki AK, Skolnick J, McDonald JF. Marker metabolites can be therapeutic targets as well. Nature. 2008;456(7221):443. 10.1038/456443c. [DOI] [PubMed] [Google Scholar]
- 15.Byers N, Fleshman A, Perera R, Molins C. Metabolomic insights into human arboviral infections: dengue, chikungunya, and Zika viruses. Viruses. 2019;11(3):225. 10.3390/v11030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer EA, Nance K, Chen S. The Gut-Brain Axis. Annu Rev Med. 2022;73:439–53. 10.1146/annurev-med-042320-014032. [DOI] [PubMed] [Google Scholar]
- 17.Doğan HO, Metabolomics. A review of liquid chromatography mass Spectrometry-Based methods and clinical applications. Turkish J Biochem. 2024;49(1):1–14. 10.1515/tjb-2023-0095. [Google Scholar]
- 18.Elling L, Zervosen A, Gutiérrez Gallego R, Nieder V, Malissard M, Berger EG, Vliegenthart JFG, Kamerling JP. UDP-N-Acetyl-α-D-Glucosamine as acceptor substrate of β-1,4-Galactosyltransferase. Enzymatic synthesis of UDP-N-Acetyllactosamine. Glycoconj J. 1999;16(7):327–36. 10.1023/A:1007039825505. [DOI] [PubMed] [Google Scholar]
- 19.The Trypanosome UDP-Glucose Pyrophosphorylase Is Imported by Piggybacking into Glycosomes, Where Unconventional Sugar Nucleotide Synthesis Takes Place. mBio. 2021, 12 (3). 10.1128/mbio.00375-21 [DOI] [PMC free article] [PubMed]
- 20.Davidsen J, Mouritsen OG, Jørgensen K. Synergistic permeability enhancing effect of lysophospholipids and fatty acids on lipid membranes. Biochim Et Biophys Acta (BBA) - Biomembr. 2002;1564(1):256–62. 10.1016/S0005-2736(02)00461-3. [DOI] [PubMed] [Google Scholar]
- 21.Kamatani N, Kubota M, Willis EH, Frincke LA, Carson DA. 5′-Methylthioadenosine is the major source of adenine in human cells. In: De Bruyn CH, M. M., Simmonds HA, Müller MM, editors. Purine metabolism in Man-IV. Advances in Experimental Medicine and Biology. Volume 165. Boston, MA: Springer US; 1984. pp. 83–8. 10.1007/978-1-4757-0390-0_18. [DOI] [PubMed] [Google Scholar]
- 22.Weigel C, Bellaci J, Spiegel S. Sphingosine-1-Phosphate and its receptors in vascular endothelial and lymphatic barrier function. J Biol Chem. 2023;299(6):104775. 10.1016/j.jbc.2023.104775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modak A, Mishra SR, Awasthi M, Aravind A, Singh S, Sreekumar E. Fingolimod (FTY720), an FDA-Approved sphingosine 1-Phosphate (S1P) receptor agonist, restores endothelial hyperpermeability in cellular and animal models of dengue virus serotype 2 infection. IUBMB Life. 2024;76(5):267–85. 10.1002/iub.2795. [DOI] [PubMed] [Google Scholar]
- 24.Carnec X, Meertens L, Dejarnac O, Perera-Lecoin M, Hafirassou ML, Kitaura J, Ramdasi R, Schwartz O, Amara A. The phosphatidylserine and phosphatidylethanolamine receptor CD300a binds dengue virus and enhances infection. J Virol. 2016;90(1):92–102. 10.1128/JVI.01849-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morizono K, Chen ISY. Role of phosphatidylserine receptors in enveloped virus infection. J Virol. 2014;88(8):4275–90. 10.1128/jvi.03287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonardi R, Jackowski S. Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus. 2007;2(2). 10.1128/ecosalplus.3.6.3.4. [DOI] [PMC free article] [PubMed]
- 27.Martinez-Seara H, Róg T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M, Reigada R. Interplay of unsaturated phospholipids and cholesterol in membranes: effect of the Double-Bond position. Biophys J. 2008;95(7):3295–305. 10.1529/biophysj.108.138123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peinado RDS, Eberle RJ, Pacca CC, Arni RK, Coronado MA. Review of -Omics studies on Mosquito-Borne viruses of the flavivirus genus. Virus Res. 2022;307:198610. 10.1016/j.virusres.2021.198610. [DOI] [PubMed] [Google Scholar]
- 29.Cui L, Lee YH, Kumar Y, Xu F, Lu K, Ooi EE, Tannenbaum SR, Ong CN. Serum metabolome and lipidome changes in adult patients with primary dengue infection. PLoS Negl Trop Dis. 2013;7(8):e2373. 10.1371/journal.pntd.0002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Bacha T, Struchiner CJ, Cordeiro MT, Almeida FCL, Marques ET. Da poian, A. T. 1 H nuclear magnetic resonance metabolomics of plasma unveils liver dysfunction in dengue patients. J Virol. 2016;90(16):7429–43. 10.1128/JVI.00187-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang H, Jiang Y, Li J, Wang Y, Nie M, Xiao N, Wang S, Song Z, Ji F, Chang Y, Zheng Y, Yao K, Yao L, Li S, Li P, Song L, Lan X, Xu Z, Hu Z. Aberrant NAD + Metabolism underlies Zika Virus–Induced microcephaly. Nat Metab. 2021;3(8):1109–24. 10.1038/s42255-021-00437-0. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh Roy S. Chapter Five - TAM Receptors: A Phosphatidylserine Receptor Family and Its Implications in Viral Infections. In International Review of Cell and Molecular Biology; Davra, V., Galluzzi, L., Eds.; TAM Receptors in Health and Disease; Academic Press, 2020; Vol. 357, pp 81–122. 10.1016/bs.ircmb.2020.09.003 [DOI] [PubMed]
- 33.Cervenka I, Agudelo LZ, Ruas JL, Kynurenines. Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017. 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 34.Cortese M, Goellner S, Acosta EG, Neufeldt CJ, Oleksiuk O, Lampe M, Haselmann U, Funaya C, Schieber N, Ronchi P, Schorb M, Pruunsild P, Schwab Y, Chatel-Chaix L, Ruggieri A, Bartenschlager R. Ultrastructural characterization of Zika virus replication factories. Cell Rep. 2017;18(9):2113–23. 10.1016/j.celrep.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12(4):544–57. 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. Role of the phosphatidylserine receptor TIM-1 in Enveloped-Virus entry. J Virol. 2013;87(15):8327–41. 10.1128/jvi.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morizono K, Xie Y, Olafsen T, Lee B, Dasgupta A, Wu AM, Chen ISY. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 2011;9(4):286–98. 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richard AS, Zhang A, Park S-J, Farzan M, Zong M, Choe H. Virion-Associated phosphatidylethanolamine promotes TIM1-Mediated infection by ebola, dengue, and West nile viruses. Proc Natl Acad Sci U S A. 2015;112(47):14682–7. 10.1073/pnas.1508095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012;8(3):e1002584. 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Meer G. Lipid traffic in animal cells. Annu Rev Cell Dev Biol. 1989;5(1):247–75. 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]
- 41.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CKE, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and Three-Dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5(4):365–75. 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization, editor. Comprehensive guidelines for prevention And control of dengue And dengue haemorrhagic Fever, rev. And expanded. Ed. SEARO Technical publication series. New Delhi, India: World Health Organization Regional Office for South-East Asia; 2011. [Google Scholar]
- 43.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCα and RhoA cross talk. Am J Physiology-Lung Cell Mol Physiol. 2005;289(2):L176–85. 10.1152/ajplung.00003.2005. [DOI] [PubMed] [Google Scholar]
- 44.Balasubramanian K, Schroit AJ, Aminophospholipid Asymmetry. A matter of life and death. Annu Rev Physiol. 2003;65:701–34. 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 45.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89(4):1121–32. [PubMed] [Google Scholar]
- 46.Maulik N, Kagan VE, Tyurin VA, Das DK. Redistribution of phosphatidylethanolamine and phosphatidylserine precedes Reperfusion-Induced apoptosis. Am J Physiol Heart Circ Physiol. 1998;274(1):H242–8. 10.1152/ajpheart.1998.274.1.H242. [DOI] [PubMed] [Google Scholar]
- 47.Lewis Robert A, Austen K, Frank; Soberman Roy J. Leukotrienes and other products of the 5-Lipoxygenase pathway. N Engl J Med. 1990;323(10):645–55. 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 48.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25(5):1441–8. 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melo CFOR, De Oliveira DN, Lima EDO, Guerreiro TM, Esteves CZ, Beck RM, Padilla MA, Milanez GP, Arns CW, Proença-Modena JL, Souza-Neto JA, Catharino RR. A lipidomics approach in the characterization of Zika-Infected mosquito cells: potential targets for breaking the transmission cycle. PLoS ONE. 2016;11(10):e0164377. 10.1371/journal.pone.0164377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onyango MG, Attardo GM, Kelly ET, Bialosuknia SM, Stout J, Banker E, Kuo L, Ciota AT, Kramer LD. Zika virus infection results in biochemical changes associated with RNA editing, inflammatory and antiviral responses in Aedes Albopictus. Front Microbiol. 2020;11:559035. 10.3389/fmicb.2020.559035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao X, van Putten JPM, Wösten MMSM. Biological functions of bacterial lysophospholipids. Adv Microb Physiol. 2023;82:129–54. 10.1016/bs.ampbs.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Lucaciu A, Brunkhorst R, Pfeilschifter JM, Pfeilschifter W, Subburayalu J. The S1P-S1PR Axis in neurological Disorders-Insights into current and future therapeutic perspectives. Cells. 2020;9(6):1515. 10.3390/cells9061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiang P, Chew WS, Seow WL, Lam BWS, Ong W-Y, Herr DR. The S1P2 receptor regulates Blood-Brain barrier integrity and leukocyte extravasation with implications for neurodegenerative disease. Neurochem Int. 2021;146:105018. 10.1016/j.neuint.2021.105018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Data quality control analysis and evaluation of the Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) model.
Additional file 2. Principal component analysis (PCA) score plot under the infection conditions of three Orthoflaviviruses. (a) Dengue virus (DENV) fecal metabolome in negative ion mode (NEG) (b) DENV fecal metabolome in positive ion mode (POS) (c) DENV brain metabolome in NEG (d) DENV brain metabolome in POS (e) Japanese encephalitis virus (JEV) fecal metabolome in NEG (f) JEV fecal metabolome in POS (g) JEV brain metabolome in NEG (h) JEV brain metabolome in POS (i) Zika virus (ZIKV) fecal metabolome in NEG (j) ZIKV fecal metabolome in POS k. ZIKV brain metabolome in NEG l. ZIKV brain metabolome in POS.
Additional file 3. Detailed information about differential metabolites screened in metabolomics.
Additional file 4. Loading plots and S-plots of the OPLS-DA model.
Additional file 5. The differential metabolites classified in the pathway analysis.
Additional file 6. Amino acid composition of the polyproteins of three Orthoflaviviruses.
Data Availability Statement
Metabolomics data to support the findings are included in the article. The amino acid sequence information of DENV, JEV, and ZIKV polyproteins is obtained from the Protein Database, which can be accessed at the following URL: https://www.ncbi.nlm.nih.gov/protein/XER78274.1; https://www.ncbi.nlm.nih.gov/protein/AAA64561.1;https://www.ncbi.nlm.nih.gov/protein/AMZ03557.1.