Abstract
Background
Traumatic brain injury (TBI) is one of the leading causes of disability worldwide. Clinical or imaging scales are currently used to stratify severity, but they show a limited correlation with clinical prognosis, which has raised interest in biomarkers. Extracellular vesicles (EV)-based biomarkers may be superior to soluble biomarkers because of their stability, resistance to degradation and unique signature according to tissue of origin. Identification of EV-associated TBI biomarkers remains challenging due to the significant heterogeneity in experimental design, exosome isolation methods and study populations. This systematic review aims to analyze the role of EVs as biomarkers in TBI across both animal and clinical models, with particular focus on their association with prognosis.
Methods
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Four electronic databases were searched from inception to December 31st, 2024, using the terms “traumatic brain injury”, “extracellular vesicles” and “biomarkers”. Animal studies and human cohort, case control, and case series studies were included; previous reviews, congress abstracts, and non-peer-reviewed works were excluded. Studies conducted on adult individuals or in experimental models of acute TBI, regardless of the mechanism and severity, and which studied biomarkers within the first week of injury, were included. The primary outcome was the EV-based biomarker identified by each study and its diagnostic accuracy when provided. Secondary outcomes were sample type for EV isolation, origin of EVs and methods for isolation and characterization.
Results
A total of 18 animal and 19 human studies were included. miRNAs were the most frequently identified biomarker in animal studies, while proteins were most common in human studies. The most commonly identified proteins in EVs were GFAP, UCH-L1, and Tau, with miRNA-124-3p also being repeatedly found. EVs were most frequently obtained from plasma, followed by brain tissue lysates in animals and cerebrospinal fluid or saliva in humans. Most studies used ultracentrifugation or polymer-based precipitation for EV isolation, with western blotting and electron microscopy for characterization. Few studies provided a measure of accuracy for the studied biomarkers; the highest diagnostic performance has been achieved with neural EV-based miRNA panels. While several studies explored diagnostic applications, only a limited number investigated prognostic utility, with few using scales such as GOS-E or evaluating long-term neurocognitive complications.
Conclusions
This review highlights the potential of EV-based biomarkers in TBI, emphasizing their stability and tissue-specific signatures. Standardized protocols for EV isolation and characterization are needed for consistency across studies. While diagnostic applications have been explored, more research is required on the prognostic value of EV biomarkers, particularly for neurological outcomes, with future studies incorporating performance metrics to assess their clinical relevance.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-025-05477-6.
Introduction
Traumatic brain injury (TBI) is defined as a disruption in brain function or evidence of brain damage caused by an external force, in the absence of other identifiable brain pathology, which may present with altered consciousness, memory loss, neurological deficits, or mental status changes, confirmed by clinical signs or diagnostic testing [1]. The estimated global incidence of TBI was 369 cases per 100,000 population in 2016, with a prevalence of 55.5 million cases [2]. TBI is a complex condition with consequences range from subtle short-term effects to permanent and severe neurological disability, persistent vegetative state and mortality. It is further associated an elevated risk of psychiatric disorders, such as depression, and neurodegenerative diseases, including Alzheimer’s disease [3].
Prognostication in traumatic brain injury (TBI) is challenging due to the complex and heterogeneous nature of brain injuries, which vary in severity, location, mechanism and the biological response. Clinical scales, such as the Glasgow Coma Scale [4], can be affected by various confounding factors, including pre-existing conditions and substance abuse [5]; concerns are particularly pronounced in mild TBI due to the heterogeneity in its progression [6]. On the other hand, imaging scores such as the CT scan-based Marshall and Rotterdam classifications, have limitations at both ends of the spectrum: they underestimate the severity of injury in cases with diffuse axonal injury or overestimate it when not properly correlated with the patient’s clinical status [7, 8]. Furthermore, both provide limited predictive accuracy when it comes to long-term outcomes [9]. Although other scoring systems that combine clinical, imaging, and laboratory variables, such as the IMPACT and CRASH scores, have been developed, they have not demonstrated sufficient accuracy and still present limitations, including variability in external validation and differences in patient populations [10, 11]. The dynamic progression of secondary injury processes, including inflammation, blood-brain barrier disruption and neurodegeneration, further complicates prognostication. Additionally, individual differences in age, comorbidities, and genetic factors influence recovery trajectories and make standardized prognostic models difficult to apply universally [12]. The lack of widely validated biomarkers that can reliably predict functional recovery exacerbates these challenges, highlighting the need for more precise prognostic tools [13].
Extracellular vesicles (EVs) are defined as those particles released from a cell, delimited by a lipid bilayer, which do not contain a functional nucleus and therefore lack replicative capacity [14]. EVs are fundamental mediators of intercellular communication of multicellular organisms. They act as carriers for the paracrine and endocrine communication of biologically active molecules originating from cells, including proteins, lipids, metabolites, and nucleic acids. EVs provide their cargo molecules with greater biostability and protection from degradation by extracellular enzymes compared to soluble mediators. Also, they allow their content to be targeted to a specific tissue or organ, as well as their origin to be tracked [15, 16]; due to these characteristics, they are emerging as biomarkers in a number of conditions [17–19].
EVs derived from the nervous system are essential in maintaining homeostasis and coordinating responses to injury. Under normal conditions, they mediate intercellular communication among CNS cell types, while in the context of injury, they modulate inflammation and tissue damage, thereby influencing the development and resolution of neuroinflammatory cascades [17]. Under physiological conditions, they facilitate communication between CNS cell populations. In response to CNS injury, they regulate inflammatory responses and tissue damage, impacting the pathogenesis and recovery of neuroinflammatory cascades [20]. Due to their ability to cross the blood-brain barrier and their resistance to degradation, the brain-derived EVs obtained from the peripheral system hold potential not only for the non-invasive diagnosis of TBI but also for severity classification and prognostication [20, 21]. Identification of EV-associated TBI biomarkers remains challenging due to the significant heterogeneity in experimental design, EV isolation methods and study populations [22]. No systematic reviews which address this topic comprehensively have been published to date, highlighting the need for such an effort to facilitate the synthesis of existing findings.
The goal of this systematic review is to analyze the role of EVs as biomarkers in acute TBI both in animal and clinical models. Our primary objective is to summarize the key EV-based TBI biomarkers and their association with prognosis, including reported performance metrics such as sensitivity, specificity, and area under the curve (AUC). Our secondary objective is to analyze the most frequent methods for biomarker isolation and characterization, with attention to biological sample and tissue origin of EVs. This review could help identify methodological and clinical challenges which limit the use of EVs as biomarkers and postulate strategies to facilitate their implementation in medical practice.
Methods
Search strategy and data extraction
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA-P) statement [23] (Table S1 in the Supplement) and registered in PROSPERO (CRD420250648882/CRD420250650547). Four databases (MEDLINE, EMBASE, Scopus, Cochrane) were searched for articles in English from inception to December 31 st 2024. The search strategy is based on keywords related to “traumatic brain injury”, “extracellular vesicles” and “biomarkers” (Table S2 in the Supplement). To minimize publication bias, a manual search of the reference lists of relevant reviews was performed. Abstract and full-text screening were performed by two independent reviewer groups. Any disagreements were discussed and resolved by a third reviewer. References were first retrieved into a spreadsheet and, after screening, summarized in a predefined Data Collection Form (DCF).
Eligibility criteria
Both animal and human studies (including cohort, case-control studies and case series) focusing on EV biomarkers on TBI were included in the search and analyzed in parallel. Animal studies and human cohort, case control, and case series studies were included; previous reviews, duplicates, congress abstracts, unpublished studies, preprints, studies from non-peer-reviewed sources and letters to editor were excluded. Eligible animal studies included a population of: [1] any species and gender [2] adult individuals and [3] TBI models where biomarker analysis was conducted within the first week after injury. Eligible human studies included: [1] adult patients (≥ 18 years of age) [2], with a diagnosis of TBI of any severity degree (mild, moderate, severe) [3], where the TBI had occurred within the past week [4], where biomarkers were evaluated in any biological sample (blood, urine, saliva, cerebrospinal fluid and others). Human studies involving [1] pediatric patients [2], patients with neurological comorbidities prior to injury (e.g., dementia, cerebral palsy, previous stroke) [3], cases of chronic TBI or TBI that occurred more than a week before biomarker sampling, or [4] where samples were collected post-mortem were excluded. Studies which included chronic or repetitive TBI models, or in vitro experiments in addition to all the aforementioned inclusion criteria, were included.
Data synthesis and quality assessment
The DCF included the experimental design of the study (species, sample size, mechanism of TBI, time of biomarker evaluation), sample for EVs isolation and EV tissue of origin, methods for EV isolation and characterization, key biomarkers and main findings of the study. All these variables were summarized in a table. Descriptive summaries were conducted using RStudio (Version 2024.12.0). Quality of studies was assessed in animal studies using SYRCLE’s risk of bias tool and the CAMARADES checklist for study quality, and in clinical studies though the Newcastle Ottawa Scale (NOS) for observational studies of exposures. Each analysis was conducted by two pairs of reviewers.
Results
Search results
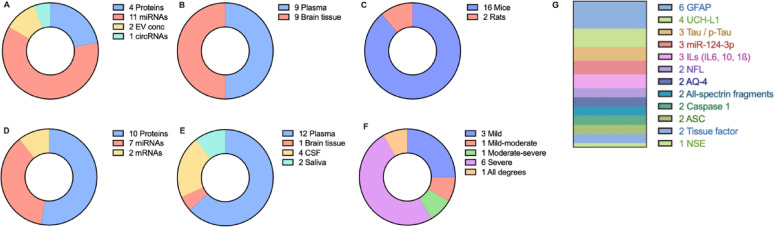
From 1,943 records identified through database screening and reference lists, the abovementioned inclusion and exclusion criteria were applied to identify 18 animal studies and 19 clinical studies directly addressing the research question (Fig. 1). Main reasons for exclusion in the abstract screening were non-animal/non-human studies or review articles, while the use of extracellular vesicles not as biomarkers but as treatment, the in vitro study design, or the use of a chronic or repetitive TBI model were reasons for exclusion in the full-text screening. Importantly, studies which included a chronic TBI group were included provided they also included a comparison between acute TBI and control groups. A descriptive summary of results is provided in Fig. 2.
Fig. 1.
PRISMA Flowchart diagram for identification and selection of studies
Fig. 2.
Summary of findings. Type of biomarkers in (A) animal and (D) human studies. Sample for EV isolation in (B) animal and (E) human studies. (C) Animal species used. (F) Severity of TBI in human studies. (G) Most frequently identified biomarkers in all studies., AQP4: Aquaporin-4, ASC: Apoptosis-associated Speck-like Protein, CSF: Cerebrospinal fluid, EV conc: Total EV concentration, GFAP: Glial Fibrillary Acidic Protein, UCH-L1: Ubiquitin C-terminal Hydrolase L1, NFL: Neurofilament Light Chain, NSE: Neuron-Specific Enolase
Animal studies
Study design
Most included studies (n = 16) were conducted in mice, and a smaller proportion in rats (n = 2). A few studies (n = 5) also included in vitro experiments, either in primary cell cultures (n = 3) or in cell lines (n = 3). EVs were primarily isolated from brain tissue (n = 10) and plasma (n = 9), as well as neuronal or microglial culture medium when in vitro models were used in paralell. Three articles determined the cellular origin of EVs as neurons, microglia or astrocytes [24–26], and two articles [27, 28] used brain-derived EVs, although cellular type was not identified. Most studies utilized the controlled cortical impact (CCI) model (n = 9) or fluid percussion injury (FPI) (n = 5); the most frequent comparator was sham surgery. Other models included blast injury, electrical injury and weight-drop methods. Two studies used also used in vitro mechanical or stretch-injury models to simulate TBI effects on cultured cells. According to SYRCLE’s scale for quality assessment, eight studies had low risk of bias, six had unclear risk of bias and four had high risk of bias (Table S3 in the Supplement). No studies had to be removed according to CAMARADES checklist. A summary of included pre-clinical studies is presented in Table 1.
Table 1.
Summary of pre-clinical studies on EVs as diagnostic biomarkers in TBI
| Reference | Species (in vivo/in vitro) | Experimental design (in vivo/in vitro) | Sample | EV isolation/Characterization method | Tissue or cellular origin of EVs | Key component | Main findings |
|---|---|---|---|---|---|---|---|
| Andrews 2016 [30] | Mice/In vitro (Primary human BMVECs) | CCI vs. sham surgery (n = 5–8 per group (2, 6, 24, 48 h after injury) | Plasma |
Polymer-based precipitation (ExoQuick) /Cryo-EM |
NA | Tight junction proteins: occludin, PECAM-1, ICAM-1 | Time-dependent increase in TJP occludin, PECAM-1 and ICAM-1 in EVs after injury in vitro. In vivo, elevated levels of EVs containing occludin were found after TBI, suggesting that the cerebral endothelium undergoes vascular remodeling through shedding of EVs after TBI. |
| Chen 2018 [39] | Rats | FPI vs. sham surgery (n = 6–7 per group, 2–8 h after trauma) | Brain tissue (cortex and hippocampus) |
No isolation/ WB for typical exosome markers (CD81, CD63) |
Brain-derived EVs | Total EV concentration | EV release was elevated in the hippocampus, but not in the cortex, following TBI. Release depends on phosphorylation of Cx43 and requires the activation of ERK signaling pathway |
| Ge 2020 [25] | Mice |
Mild concussion injury with closed skull vs. control (1–42 days post trauma, n = 6 per group) |
Brain tissue | Polymer-based precipitation and UC (1 × 100,000 × g for 70’ + immunoaffinity capture (CD11b)/TEM, NTA, WB (CD9, CD63 and CD81) | Microglial EVs | miR-124-3p | miR-124-3p level in microglial exosomes from was significantly altered after TBI in vivo. Microglial exosomes with upregulated miR-124-3p alleviate neurodegeneration in vitro and in vivo by Targeting the Rela/ApoE signaling pathway and β-amyloid changes. |
| Harrison 2016 [24] | Mice | CCI vs. sham surgery (7 days post injury, n = 15 per group) | Brain tissue | Density gradient UC (1 × 200,000 × g for 16 h)/TEM | Neuron-derived EVs | miR-21 | miR-21 is increased in neurons of the injury boundary zone near reactive microglia, and is secreted from neurons as potential EV cargo |
| Hazelton 2018 [40] | Mice | CCI vs. sham surgery (n = 5 per group). | Plasma | Serial UC (1 × 120,000 × g for 120’/TEM and WB for positive (TSG101, Alix and CD63), and negative (CD68 and H3) markers | NA | Total EV concentration | Total EV concentration in plasma is increased after TBI, and this was accompanied by an increase in CNS and hepatic leukocyte recruitment, triggering acute phase response. |
| Huang 2018 [34] | Mice/In vitro (Microglial cell line BV2) | Electric cortical injury device (n = 6 per group, 3–28 days) | Brain tissue | Serial UC (1 × 100,000 for 70’)/TEM, WB (CD9, CD63) and IF (PKH 67) | NA | miR-124-3p | miR-124-3p was increased in microglial exosomes after TBI in mice. In vitro, it exerted a protective effect by inhibiting inflammation, via supressing the activity of mTOR signaling. Increased miR-124-3p in microglia contributes to neurite outgrowth via its transfer into neurons. |
| Keane 2016 [31] | Mice | CCI vs. sham surgery (n = 6–7 per group) | Plasma | Polymer-based precipitation (ExoQuick)/NTA, WB for exosome markers (CD63) | NA | Caspase-1, ASC and IL-18 | TBI elicits EV-mediated inflammasome activation in cardiomyocytes and complement-related protein release, contributing to peripheral inflammation |
| Ko 2018 [28] | Mice | Blast injury (n = 77 test set, n = 82 blinded test set) vs. sham controls (1 h, 1 d, 4 d, 14 d) | Plasma | Immunomagnetic nanopore-based TENPO (GluR2+) | Brain-derived EVs | Panel of 7 miRNAs | An accuracy of 99% identifying the signature of TBI vs. control mice was achieved (AUC = 1). The panel was also useful in predicting the intensity of the injury, the elapsed time, and the presence of a prior injury. |
| Ko 2020 [27] | Mice | CCI and blast injury (n = 116 in total, from 1 h to 14 d) vs. sham controls | Plasma | Immunomagnetic nanopore-based TENPO (GluR2+) | Brain-derived EVs | Panel of 8 miRNAs | Panels of miRNAs accurately classified TBI versus control mice (AUC = 0.87); prediction improved depending on mechanism of injury (AUC for blast = 0.81 and for CCI = 0.89), mechanism of injury could also be differentiated (AUC = 0.87 for TBI blast vs. TBI CCI). |
| Kumar 2017 [41] | Mice/In vitro (Microglial cell line BV2) | Controlled cortical impact vs. sham surgery (24 h after trauma, n = 6 per group)/Microglial cell line treated with TBI brain extracts | Plasma | Serial UC (1 × 100,000 x g for 60’)/Flow cytometry (annexin V) and microbeads. Markers of microglial origin (P2Y12/CD45) | Brain-derived microglial EVs |
miR-155 IL-1ß |
Microglial EVs released after TBI can cause microglial activation in vitro and neuroinflammation in vivo in the cortex of naïve animals. miR-155 and IL-1ß is increased in EVs from TBI mice. |
| Li 2019 [29] | Mice/In vitro (Microglial cell line BV2) | Electric cortical injury device (n = 6 per group, 3–28 days)/Microglial cell line treated with TBI brain extracts or co-cultured with HT22 injured neurons | Brain tissue | Serial UC (2 × 100,000 × g for 60’)/TEM, WB (CD9, CD63) and IF (PKH 67) | Microglial EVs | miR-124-3p | Increased miR-124-3p in microglial exosomes following TBI may inhibit neuronal autophagy and protect against nerve injury via their transfer into neurons. Mice transfected with miR-124-3p mimic showed an improvement in neurological outcomes after TBI. |
| Long 2020 [26] | Mice/In vitro (Primary microglia and astrocyte culture from mice) | CCI (n = 20–30 per group) | Brain tissue | Serial UC (1 × 100,000 x g for 3 h)/TEM, WB (CD9, CD63) and IF (PKH 26) | Astrocyte-derived EVs | miR-873a-5p | miR-873a-5p secreted by astrocytes after TBI inhibited NF-kB signaling and reduced microglia-mediated neuroinflammation by promoting M2 phenotype, which resulted in improvement of cognitive deficits |
| Tian 2015 [38] | Mice | FPI vs. sham surgery (n = 6 per group) | Plasma | Flow cytometry (GFAP + and NSE+) and microbeads | Glial cell and neuron-derived EVs | Procoagulant phosphatidyl-serine and tissue factor | Brain-derived EVs are increased in blood after TBI versus control. These EVs migrate through the disrupted BBB and activate platelets, being involved in TBI consumptive coagulopathy. |
| Tian 2022 [32] | Mice | FPI vs. sham surgery (3 h after trauma, n = 12 per group). | Brain tissue | Polymer-based precipitation/TEM and WB for typical exosome markers (TSG101, CD63) and brain tissue markers (GFAP) | Brain-derived EVs | 50 miRNAs (5 up, 45 down) | According to miRNA predicted targets, the KEGG pathways affected after TBI include the Wnt and NF-κB pathways, which play plays a central role in astrocyte swelling and brain edema. |
| Wang 2018 [35] | Mice | CCI vs. sham surgery (n = 5 per group) | Brain tissue | Density gradient UC (1 × 100,000 × g for 70)/TEM and WB (CD63) | Brain-derived EVs | Tau and p-Tau | The levels of Tau and p-Tau in EVs after TBI were significantly elevated. TBI-derived exosomes displayed toxicity in primary neuron cultures, exacerbated TBI induced motor and cognitive impairments after TBI. |
| Wang 2020 [42] | Rats | Weight-drop (WD) method for moderate TBI vs. sham surgery (n = 7 per group) | Plasma | Membrane affinity-based isolation/TEM, NTA and WB (CD63 and HSP70, negative transferrin) | NA | 50 miRNAs (31 up, 19 down) | GO analysis revealed that most highly correlated pathways were the MAPK signaling pathway, regulation of actin cytoskeleton, Rap1 signaling pathway and Ras signaling pathway |
| Zhao 2018 [37] | Mice | FPI vs. sham surgery (3 h after trauma, n = 3 per group). | Brain tissue | Polymer-based precipitation (ExoQuick)/TEM and WB for typical exosome markers (CD63, TSG101, HSP70) from brain tissue (GFAP+) | Brain-derived EVs | 231 circRNAs (155 up, 76 down) | Differentially expressed circRNAs were related to: (1) growth and repair of neurons (2) development of the nervous system, and (3) the transmission of nerve signals |
| Zhao 2021 [43] | Mice/In vitro (BV2 microglial cell line, primary neuron culture from mice) | FPI (n = 19 per group, 24 h after trauma)/Primary neurons and microglial cell line under stretch injury (in vitro) | Plasma | Serial UC (2 × 100,000 × g for 70’)/TEM, NTA, IF (PKH26) | Microglial-derived EVs | miR-5121 | miR-5121 was decreased in EVs from mice with TBI and stretch-injured microglia. Overexpression of miR-5121 reversed the suppression of neurite outgrowth and synapse recovery of neurons both in vitro and in vivo. |
ASC: Apoptosis-associated speck-like protein, BBB: Blood-brain barrier, BMVEC: Brain microvascular endothelial cells, CCI: Controlled cortical impact, CD: Cluster of differentiation, circRNA: Circular RNA, CNS: Central nervous system, Cx43: Connexin 43, EM: Electron microscopy, ERK: Extracellular signal-regulated kinase, EV: Extracellular vesicle, GFAP: Glial fibrillary acidic protein, HSP: Heat shock protein, ICAM-1: Intercellular adhesion molecule-1, IF: immunofluorescence, IL-1ß: Interleukin-1 beta, IL-18: Interleukin-18, MAPK: Mitogen-activated protein kinase, miR: MicroRNA abbreviation, miRNA: MicroRNA, mTOR: Mechanistic target of rapamycin, MPs: Microparticles, NSE: Neuron-specific enolase, NTA: Nanoparticle tracking analysis, p-Tau: Phosphorylated tau, PECAM-1: Platelet endothelial cell adhesion molecule-1, PKH: Fluorescent dye for labeling membranes, Ras: Rat sarcoma virus (signaling pathway), Rap1: Ras-related protein 1 (signaling pathway), TBI: Traumatic brain injury, TEM: Transmission electron microscopy, TGS101: Tumor susceptibility gene 101, TJP: Tight junction protein, UC: Ultracentrifugation WB: Western blot, Wnt: Wingless-related integration site (signaling pathway), NF-kB: Nuclear factor kappa B (signaling pathway).
EV isolation and characterization
Ultracentrifugation (UC) was the most widely used method for EV isolation (n = 8) including serial ultracentrifugation alone or combined with density gradient-based techniques. Most authors apply one or two rounds at a speed ranging from 100,000 to 200,000 × g for one to three hours at 4 ºC [29]. There was only one protocol with a duration of 16 hours [24]. Ge et al. employed a hybrid approach, combining polymer-based precipitation using the Total Exosome Isolation Reagent with a single ultracentrifugation step at 100,000 × g for 70’ [25]. Methods relying on commercial kits, such as the ExoQuick polymer-precipitation based method, tend to show less heterogeneity [30, 31]. Immunoaffinity-based methods targeting surface markers, such as CD11b for microglial EVs [25] or GluR2 + for neuronal EVs [27] were also used. Flow cytometry, using markers such as GFAP for glial cells and NSE for neurons, was used in one study to enrich cell-type-specific EV populations [32].
Characterization methods were consistent across studies, with transmission electron microscopy (TEM) used in almost every study (n = 16) to provide ultrastructural confirmation of EV morphology. Western blotting (WB) was also used for typical exosome markers such as CD9, CD63, and TSG101 (n = 17). Advanced techniques like nanoparticle tracking analysis (NTA) to assess EV size distribution and immunolabeling to confirm cellular uptake or trafficking of EVs were less frequently used.
Biomarkers
Biological functions
The biomolecules identified as potential EV-associated biomarkers in animal models of TBI were predominantly miRNAs, identified in 11 studies, followed by proteins (n = 4), total EV concentration (n = 2), and circular RNAs (circRNAs) (n = 1). Notably, nine studies proposed multiple molecules, either as a miRNA panel or a combination of several proteins, highlighting the complexity and diversity of potential biomarkers. Among the miRNAs, miR-124-3p was consistently found to be significantly upregulated in TBI models (n = 3) [25, 33, 34]; showing neuroprotective roles, both in neuronal and microglial EVs, by reducing neuroinflammation [33, 34]. Another miRNA, miR-21, is secreted by neurons in the perilesional area [24], reflecting neuronal stress. Wang found miRNAs were associated with actin cytoskeleton organization, which is key to neuronal plasticity [35]. Several proteins have also been identified. Tight junction proteins such as occludin and PECAM-1 [30] are indicative of blood-brain barrier disruption; inflammasome-associated proteins like caspase-1 and IL-18 [36], reflect systemic inflammatory responses. Tau, a microtubule-associated protein involved in maintaining neuronal structure, and its phosphorylated form (p-Tau), both classically linked to neurodegeneration, were also detected in EVs following TBI [35]. CircRNAs, identified in a single study [37], are of interest because of its structure of covalently closed loops, which makes them highly stable compared to linear RNAs. Additionally, procoagulant phosphatidylserine and tissue factor were identified as biomarkers for TBI-induced consumptive coagulopathy [38].
Temporal dynamics
Few animal studies have characterized changes in EV cargo over time following injury. Tian et al. showed time-dependent increases in systemic coagulation markers associated with microparticle release, which concentration reached a peak six hours after injury [38]. Andrews et al. studied the increase of endothelial microvesicles containing ICAM-1, PECAM and TGP occluding after trauma, which reached a maximum 24 to 48 h after trauma in vitro, but did not analyze this evolution in vivo [30]. Ge et al. (2020) demonstrated that microglial exosomal miR-124-3p levels increased following TBI, with a maximum between seven and 14 days post-injury and baseline values at 42 days [25]; Huang et al. found a similar time trend for this biomarker, with a maximum at 14 days both in microglia and microglial EVs [34].
Association with outcomes
None of the included animal studies establish a direct association between EV-based biomarkers neurological outcomes or mortality after TBI. In contrast, some of them find an improvement in outcome when one of the studied miRNAs is transfected to animal models of TBI, suggesting a potential neuroregenerative role [25, 26, 29]. Ge et al. showed that increased levels of miR-124-3p in microglial-derived exosomes after mild TBI in mice led to improved cognitive outcomes and alleviated neurodegeneration by promoting neuroprotective mechanisms [25]. Li et al. found that exosomal miR-124-3p was associated with decreases in the modified neurological severity score and improvements in Morris water maze test results in TBI mice [29]. Similarly, Long et al. found that astrocyte-derived exosomes enriched with miR-873a-5p modulated microglial phenotype and reduced neuroinflammation, reducing lesion area and cerebral edema, resulting in improved cognitive function on the 7 th day [26].
Diagnostic and prognostic performance
Among animal studies, only two provided actual measurements of diagnostic performance. Ko et al. demonstrated that a panel seven of EV-derived miRNAs could distinguish TBI from control mice with an extremely high accuracy of 99% [28]. Stratified analyses revealed AUCs of 0.81 for blast injury and 0.89 for controlled cortical impact (CCI), with improved performance (AUCs of 0.88 and 0.91, respectively) when using a reduced panel of five or three miRNAs specific to each mechanism [28]. The same group expanded these findings by showing that a similar panel of eight miRNAs accurately classified TBI versus control mice with an AUC of 0.87, with an improved performance when the mechanism of injury was taken into account. In addition, they demonstrated that the mechanism of injury itself could be differentiated, achieving an AUC of 0.87 when distinguishing blast TBI from CCI TBI [27]. These studies represent the most detailed evaluations of EV-based diagnostic performance in animal models to date.
Clinical studies
Study design
The studies included were predominantly case control (n = 13), although retrospective (n = 2) and prospective (n = 2) cohort studies were also included, as well as time-sequential case series (n = 2). The studies included patients with TBI of varying severity, evaluated through GCS; severe TBI was the most studied (n = 6)) [26, 36, 44–47], followed by mild (n = 3) [27, 48, 49], mild-moderate (n = 1) [34], moderate-severe (n = 1) [50], and all degrees [51] (n = 1). Control groups were constituted by healthy patients, except for Guedes et al. [52], who used polytrauma patients with no head injury as comparators [52]. Most studies isolated EVs from plasma (n = 12), as well as from cerebrospinal fluid (n = 4) [36, 44, 47, 53], saliva (n = 1) [54], or brain tissue (n = 1) [26]. Notably, neuron-derived EVs (NDEs) were enriched in four studies, either using immunomagnetic nanopore techniques for GluR2 [27, 48] or immunoabsorption (CD171) [49], while other studies used astrocyte-derived EVs [26, 55] and endothelial and platelet EVs [45]. Risk of bias assessment returned the lowest risk of bias (NOS 8–9) for five studies, intermediate (NOS 6–7) for 12 studies, and high risk of bias (NOS < 6) for two studies, mainly due to the absence of a non-exposed cohort in the last group (Table S4 in the Supplement). A summary of included clinical studies is presented in Table 2. Figure 2 summarizes the main outcomes from both animal and clinical studies.
Table 2.
Summary of clinical studies on EVs as diagnostic biomarkers in TBI
| Reference | Experimental design | Sample | EV isolation/Characterization | Tissue or cellular origin of EVs | Key component | Main findings |
|---|---|---|---|---|---|---|
| Beard 2021 [48] |
Retrospective cohort. Mild TBI compared to orthopaedic patients (first 24 h, n = 46–47 per group) |
Plasma | Immunomagnetic nanopore-based TENPO (GluR2+) for neural EVs | Brain-derived EVs | GFAP and IL6, NFL, Tau and UCH-L1, IL10, TNFα | Mild TBI is associated with elevations in brain-derived proteins and cytokines in plasma and GluR2 + EVs. GFAP and IL6 were significantly elevated in both plasma and GluR2 + EVs in TBI. NFL, Tau, UCHL1, IL-10 and TNF were elevated in plasma only. AUC = 0.7 for GFAP in EVs for classification of mild TBI vs. healthy controls. |
| Cheng 2019 [56] |
Case control. Patients with TBI from concussion clinics and Eds (first 24 h, n = 31 TBI, 23 healthy controls) |
Saliva | Serial UC (2 × 120,000 x g for 60’)/TEM, Nanosight, WB (CD63) | Brain-derived EVs | CDC2, CSNK1 A1, and CTSD | 57/56 genes were differentially expressed genes in salivary EVs from patients in concussion clinics/emergency department versus healthy controls. 3 genes were upregulated in both patients with TBI vs. controls: CDC2, CSNK1 A1, and CTSD. |
| De Rivero 2016 [36] | Case series. Severe TBI or SCI patients (n = 3–6 per group) | CSF | Polymer-based precipitation (ExoQuick)/WB (CD9) | Brain-derived EVs | Inflammasome proteins: ASC, NLRP1, and caspase 1 | CSF-derived exosomes show high content in inflammasome proteins in patients with TBI. When neural-derived EVs are loaded with siRNA and delivered to CSF, inflammasome activity is decreased. |
| Goetzl 2019 [49] | Case control. Sports related mTBI (1 week after as acute mTBI, n = 18; >3 months as chronic TBI, n = 14; no TBI, n = 21). | Plasma |
Polymer-based precipitation and immunoabsorption (CD171) /ELISA for NDEs (CD81) |
Neuron-derived EVs (NDEs) |
Upregulated: Annexin VII, UCH-L1, spectrin fragments, claudin-5, NKCC-1, AQP4, synaptogyrin-3 Downregulated: Ras-related small GTPAse |
NDE levels of a range of functional brain proteins were abnormal relative to controls in acute but not chronic mTBI, delineating phase-specificity. |
| Goetzl 2020 [55] | Case control. Sports-related (sTBI, acute if < 7 days and chronic if months, n = 12 per group) and military veterans (early and late chronic) versus controls (no TBI). | Plasma | Polymer-based precipitation (ExoQuick)/TEM, WB (CD9, CD63) and immunofluorescence (PKH 67) | Astrocyte derived EVs (ADEs) | Complement proteins C4b, factor D and Bb, MBL, C3b and C5-9 | Plasma levels of ADEs were decreased in acute sTBI and returned to normal within months. Complement proteins C4b, factor D and Bb, MBL, C3b and C5-9 were increased in acute sTBI versus control, and normalized within months except for factor D, Bb and MBL. Complement inhibitors could be useful in the treatment of post-concussion syndrome. |
| Guedes 2022 [52] |
Prospective cohort (FAINT) Patients with multiple injuries (TBI, n = 41) and isolated body injuries (control, n = 67) |
Plasma | Polymer-based precipitation (ExoQuick)/Flow cytometry and MRPS | NA | Glial (GFAP), and neuronal proteins (UCH-L1, NFL) | GFAP, UCH-L1 and NFL in EVs were higher in those with multiple injuries versus isolated body injuries. There was a moderate correlation between the Head Abbreviated Injury Score and EV-GFAP. |
| Huang 2023 [58] | Case control. Patients with TBI (n = 14, 7 mild and 7 moderate), healthy controls (n = 6). | Plasma | Polymer-based precipitation + SEC/TEM, NTA, WB (CD63, TSG101) | NA | 10 miRNAs (8 upregulated, 2 downregulated) | ExomiRs expression profiles were associated with neurovascular remodeling, the integrity of the blood-brain barrier, neuroinflammation, and a cascade of secondary injury. |
| Ko 2018 [28] | Retrospective case control. Training set (n = 5 TBI, n = 10 healthy control patients)/Blinded test set (n = 60 patients) | Plasma | Immunomagnetic nanopore-based TENPO (GluR2+) | Brain-derived EVs | Panel of 7 miRNAs (miR-129, miR-152, miR-21, miR-212, miR-374, miR-664, miR9) | Machine-learning based linear discriminant analysis was used to detect EV miRNA signatures in patients with TBI vs. healthy controls (AUC = 0.9), as well as patients with TBI divided in “TBI with systemic injuries” (AUC = 0.94) and “TBI with no systemic injuries” (AUC = 0.996) vs. healthy controls. This performance was better than any of the protein-based models. |
| Ko 2020 [27] | Prospective case control. Adult patients with non-penetrating mild (GCS 13–15) TBI admitted in Level 1 Centre (< 24 h post trauma, n = 16–20 per group) | Plasma | Immunomagnetic nanopore-based TENPO (GluR2+) | Brain-derived EVs |
Panel of 4 miRNAs (miR-203b-5p, miR-203a-3p, miR-206, miR-185-5p) |
The panel of biomarkers to classify TBI vs. control patients based on GluR2 + enriched EV miRNA, achieved an AUC = 0.84 |
| Kuharic 2019 [44] | Case control. Up to 7 days after severe TBI (n = 17), no TBI (control) n = 18 | CSF | Serial UC/TEM, WB (membrane associated Flotillin-1 and Flotillin-2), NTA | NA |
Flotilin 1 Arf6 Rab7 |
Flotilin1+-EV concentration is increased in CBF after TBI, as well as EV size. Unfavorable outcomes are associated with decreasing Arf6 concentrations and a delayed Rab7a concentration increase. This suggests that brain response within several days after severe TBI includes shedding of EVs associated with neuroplasticity |
| Li 2023 [57] | Case control. Adult patients with TBI (6 h post trauma) vs. healthy control (n = 32 per group) | Plasma | Serial UC/TEM, NTA and WB for typical exosome markers (positive CD9, CD81, TSG101, and negative calnexin) | NA |
HMGB1 VWF |
Plasma EVs from patients with TBI are enriched in HMGB1 and the number of HMGB1 + EVs correlated with injury severity. TBI EVs induced endothelial dysfunction in mice via NRLP3 inflammasome activation. VWF is present in the surface of HMGB1 + EVs and mediates endotheliopathy. |
| Long 2020 [26] | Case series. Severe patients with TBI who underwent neurosurgery (n = 15) | Brain tissue | Serial UC (1 × 100,000 x g for 3 h)/TEM, WB (CD9, CD63) and immunofluorescence (PKH 26) |
Astrocyte-derived EVs |
miR-873a-5p | miR-873a-5p secreted by astrocytes after TBI was significantly higher in the lesion area versus the edema area. |
| Manek 2018 [53] | Retrospective case control. 12 h after TBI (n = 18 TBI, n = 6 healthy control). | CSF | Serial UC (2 × 100,000 for 70’)/TEM, NTA | NA |
All-spectrin BDPs, GFAP and its BDPs and UCH-L1. Proteomic analysis: 91 proteins in control, 466 in TBI |
Following severe TBI, the injured human brain released increased number of EVs CSF. All-spectrin breakdown products (BDPs), GFAP, and its BDPs and UCH-L1 are found in higher concentration in EVs from patients with TBI. Proteomic analysis identified cytoskeletal, neurite-outgrowth related and synaptic, and extracellular matrix proteins. |
| Matuk 2021 [54] | Case control and time-sequential evaluation. Mixed martial arts pre and post- fight versus healthy controls (n = 8 for fighters, n = 7 controls). | Saliva | Serial UC (2 × 120,000 for 60’)/Nanosight, WB (CD63, CD81, CD9, and HSP70). | NA | ALOX5, HRH1, PDE4B, ADRB2, ANXA1, ITGB2, and MAPK8 genes | The most upregulated genes during a fight were ALOX5, ITGB2, and MAPK8, and notably, all three baseline values were downregulated when comparing pre-fight samples to control data. The genes that were found to downregulate the most during a fight were ADRB2, HRH1, ANXA1 and PDE4B. Salivary EVs are potential biomarkers in TBI. |
| Mondello 2020 [50] |
Retrospective cohort. Patients diagnosed with nonpenetrating moderate-to-severe TBI (GCS < 12) (n = 61) |
Plasma | Polymer-based precipitation ExoQuick/NTA | NA | GFAP, UCH-L1, NFL, t-tau proteins | Patients with diffuse injury had higher NFL and GFAP levels than patients with focal injury. EV UCH-L1 profile was associated with early mortality. EV biomarker concentrations did not correlate with age or GCS, and were not associated with the mechanism of injury, ISS score, brain edema, or the need for decompressive craniectomy. |
| Nekludov 2017 [46] | Case control. Patients with severe isolated TBI, from admission up to 72 h (n = 15 per group) | Arterial and cerebrovenous blood |
Not isolated/ flow cytometry |
NA | GFAP, NSE, AQP4 |
When compared to healthy controls, the severe TBI group had larger concentrations of MPs expressing GFP and AQP4. Levels of NSE-expressing EVs were also higher in the TBI group compared with healthy controls. |
| Nekdulov 2014 [45] | Prospective cohort. Patients with severe isolated TBI (n = 16) | Plasma | Not isolated/Flow cytometry and microbeads. Specific population markers: CD42a (platelet), CD144 (endothelial) CD45(leukocyte) | Endothelial-, platelet-, and leukocyte-derived microparticles (MPs) |
Origin of EVs Tissue factor (TF) in MPs |
The pattern of circulating MPs is altered after TBI. PMPs exposing P-selection and EMPs exposing TF seem to be generated in the injured brain, whereas LMPs exposing TF are accumulated. |
| Patz 2013 [47] | Case control. Patients with severe TBI (n = 11) vs. control (no TBI, n = 26) | CSF | Serial UC (1 × 170,000 for 40’)/Flow cytometry and microbeads | NA | miR-9 upregulated, miR-451 downregulated | EVs from patients with TBI were more abundant than in non-injured subjects and contained distinct genetic information suggesting that they play a role in the adaptive response to injury. |
| Puffer 2021 [51] | Case control. Patients with TBI (n = 15, mild, moderate and severe) versus healthy controls (n = 5) | Plasma | Density-gradient UC/NTA and WB (CD63, CD9, Alix, HSP90) | NA |
GFAP 11 miRNAs |
Plasma EVs are physically similar but contained approximately 10 times more GFAP in patients with TBI with altered consciousness. 11 miRNAs were identified between groups related to organismal injury and development. |
ADEs: Astrocyte-Derived Extracellular Vesicles, AII: All-Spectrin, ANXA1: Annexin A1, AQP4: Aquaporin-4, ASC: Apoptosis-associated Speck-like Protein Containing a CARD, AUC: Area Under the Curve, BDPs: Breakdown Products, CD: Cluster of Differentiation, CDC2: Cell Division Cycle 2, CFB: Cerebrospinal Fluid, CSF: Cerebrospinal Fluid, CSNK1 A1: Casein Kinase 1 Alpha 1, CTSD: Cathepsin D, EDs: Emergency departments, ELISA: Enzyme-Linked Immunosorbent Assay, EMPs: Endothelial Microparticles, EVs: Extracellular Vesicles, GFAP: Glial Fibrillary Acidic Protein, HMBG1: High Mobility Group Box 1, HSP70: Heat Shock Protein 70, IL-6: Interleukin-6, IL-10: Interleukin-10, IL-63: Interleukin-63, ITGB2: Integrin Beta 2, LMPs: Leukocyte Microparticles, MBL: Mannose Binding Lectin, miR: MicroRNA, MPs: Microparticles, MRPS: Microparticle-Specific Assay, mTBI: Mild Traumatic Brain Injury, NA: Not available, NDEs: Neuron-Derived Extracellular Vesicles, NFL: Neurofilament Light Chain, NKCC-1: Sodium-potassium-chloride cotransporter-1 NLRP1: NOD-Like Receptor Protein 1, NRL: Nucleotide Receptor Ligand, NSE: Neuron-Specific Enolase, NTA: Nanoparticle Tracking Analysis, SEC: Size Exclusion Chromatography, siRNA: Small Interfering RNA, t-tau: Total Tau Protein, TBI: Traumatic Brain Injury, TEM: Transmission Electron Microscopy, TNF: Tumor Necrosis Factor, TSG101: Tumor Susceptibility Gene 101, UCH-L1: Ubiquitin C-terminal Hydrolase L1, UC: Ultracentrifugation, VWF: Von Willebrand Factor, WB: Western Blotting.
EV isolation and characterization
Serial and density-gradient UC were the most common isolation methods, followed by polymer-based precipitation methods and immunomagnetic capture. Most studies using UC reported two rounds at 4ºC but differed in speed (from 100,000 to 170,000 × g) and time (from one to three hours), with lower heterogeneity than animal studies [53, 54, 56]. Most protocols include prior centrifugation steps for cell debris removal, typically one to three cycles at 10,000–15,000 × g for 30–60 min [26, 53, 54, 56]; another variation is the use of an ultracentrifugal filter between spins [53]. Regarding precipitation-based methods, most studies used ExoQuick, whith incubation and centrifugation times varying from 15 to 60 min [49, 52, 55], with varying incubation and centrifugation times (from 30 min to several hours). Characterization of EVs was frequently done using TEM and WB (n = 13), frequently for CD63, CD81, and CD9; flow cytometry and NTA were also used (n = 3).
Biomarkers
Biological functions
Proteins were the most common biomarker type identified in clinical studies (n = 10). Notably, GFAP and UCH-L1, well-established serum biomarkers for TBI, were also detected in EVs and found to be elevated in patients with TBI across multiple studies [46, 48, 50–52]. This finding aligns with the work by Mondello et al. [50], which showed that these proteins, in their EV-associated forms, are increased in diffuse versus focal injury, suggesting a potential role in damage progression to perilesional areas and secondary injury [50]. NFL and Tau proteins, commonly associated with axonal damage, were identified in EVs as markers of neuronal injury reinforcing their potential role in assessing neurodegeneration post-TBI [48, 50]. Complement proteins (C4b, factor D, Bb, C5-9) were found in astrocyte-derived EVs after TBI [55], indicating potential contributions to neuroinflammation, while HMGB1 and Von Willebrand factor (VWF) in EVs were associated with endothelial dysfunction [57], a key component of secondary injury mechanisms.
EV-associated miRNAs (n = 7) and mRNAs (n = 2) were identified in several studies, and their expected targets were analyzed to provide further insight into TBI pathophysiology. Huang reported ten miRNAs associated with neurovascular remodeling and neuroinflammation, which could provide insight into TBI pathophysiology beyond conventional biomarkers [58]. Patz observed miR-9 upregulation and miR-451 downregulation after severe TBI, supporting their relevance in injury response [47]. Puffer et al. found 11 miRNAs overexpressed miRNAs which were related to organismal injury and development [51]. Cheng et al. [56] and Matuk et al. [54] identified mRNAs corresponding to upregulated genes involved in cell cycle regulation (CDC2), inflammation and leukotriene synthesis (ALOX5), cell adhesion and immune response (ITGB2), and stress-activated MAP kinase signaling (MAPK8). De Rivero et al. [34] reported that inflammasome proteins (ASC, NLRP1, and caspase-1) were enriched in CSF-derived EVs, consistent with findings from previous studies on inflammatory responses in TBI.
Temporal dynamics
In clinical studies, only a few works have examined how EV-based biomarkers vary over time. The most detailed temporal analysis comes from Mondello et al. [50] who conducted a longitudinal study measuring EV-associated GFAP, UCH-L1, Tau, and APP at multiple post-injury timepoints (24 h, three days, seven days, and 30 days) in moderate-to-severe patients with TBI. They observed that persistently elevated levels of GFAP and UCH-L1 over time were linked to worse neurological outcomes and higher mortality, while declining levels predicted better recovery. Goetzl et al. provided a cross-sectional comparison between acute-phase (less than seven days) and chronic-phase (more than six months) mild patients with TBI, showing that phosphorylated Tau increased and neurogranin decreased over time, suggesting progressive neurodegeneration after mTBI; however, this study did not track the same individuals longitudinally [49].
Association with outcomes
Several human studies have explored the prognostic value of EV-based biomarkers in association to patient mortality and neurological outcomes. Puffer et al. found that plasma EV protein profiles correlated with both initial injury severity (GCS) and functional neurological outcomes (GOS-E) in patients with TBI, reinforcing the role of EV biomarkers in outcome prediction, although mortality was not a primary endpoint [51]. Similarly, Guedes et al. reported that elevated EV-contained NSE and GFAP levels in critically ill trauma patients were associated with lower GCS scores, reflecting more severe initial neurological injury, as has previously been described in soluble biomarkers [52]. Mondello et al. demonstrated that persistently elevated levels of GFAP and UCH-L1 at 7- and 30-days post-injury in moderate-to-severe patients with TBI strongly predicted higher mortality and worse neurological recovery as measured by the GOS-E scale [50]. Goetzl et al. identified that elevated levels of exosomal phosphorylated tau at threonine 181 combined with reduced levels of neurogranin in NDEs were associated with long-term cognitive decline in patients with chronic mild TBI [49].
Diagnostic and prognostic performance
In clinical studies, few studies provide measures of diagnostic performance for the studied biomarkers. Beard et al. [48] found that GluR2 + EV-derived GFAP had a moderate AUC of 0.70 to differentiate mild TBI from controls. Interestingly, their study compared EV-based versus soluble plasma biomarkers, and found that plasma GFAP alone achieved a higher AUC of 0.91 for this comparison, similar to other biomarkers (AUC = 0.6 for EV NFL versus 0.86 for plasma NFL, AUC = 0.61 for EV Tau versus 0.64 for plasma Tau, AUC = 0.59 for EV UCH-L1 versus 0.73 for plasma UCH-L1). Although EVs did not improve diagnostic performance compared to plasma biomarkers, they appeared to offer greater specificity; for example, plasma GFAP was elevated in non-TBI conditions such as orthopedic surgery, whereas EV-associated GFAP was not. Combining EV and plasma biomarkers yielded an AUC of 0.913 and an accuracy of 82.5% [48]. Ko et al. achieved an AUC of 0.9 for TBI versus control, 0.94 for TBI with systemic injuries versus controls, and 0.996 for TBI without systemic injuries versus controls, by using an EV-based miRNA panel and linear discriminant analysis (LDA) [28]. Notably, this panel outperformed conventional EV-based protein panels, mainly due to high variability in protein measurements. In comparison, conventional protein biomarkers exhibited lower AUCs (0.66 to 0.88) and combining them via machine learning did not improve classification (AUC ~ 0.5). In their 2020 study, Ko et al. also reported an AUC of 0.84 using a panel of 4 EV-associated miRNAs to differentiate patients with TBI from controls [27].
Discussion
Compared to traditional biomarkers, EV-based biomarkers may offer advantages due to their stability, protection from enzymatic degradation, and potential to reflect the cellular origin of injury more precisely [59]. Most molecules identified in our review had previously been described as soluble biomarkers, such as GFAP, UCH-L1, and NSE [60]. GFAP, an astrocytic intermediate filament protein, and UCH-L1, a neuronal enzyme involved in proteasomal degradation, were consistently detected in EVs and associated with injury severity and outcome, aligning with existing literature in TBI [61]. NSE, an enzyme involved in glycolysis and primarily expressed in neurons, serves as a biomarker of neuronal damage due to its release into the bloodstream following neuronal injury. Although only found in one study in EVs, its presence within EVs may offer improved specificity in distinguishing neuronal injury from systemic metabolic changes, which is a limitation of soluble NSE [62, 63]. S100 is a calcium-binding family of proteins found predominantly in astrocytes, which are some of the few validated biomarkers for TBI in the soluble compartment. Surprisingly, no S100 family proteins were identified in any EV-related studies, which may suggest that their release occurs mainly as free, soluble proteins rather than via EVs.
Despite these overlaps, studies comparing soluble and EV-contained biomarkers remain scarce. Notably, Beard et al. found that EV-associated biomarkers did not outperform any of the soluble plasma markers (GFAP, NSE, NFL, Tau, interleukins, among others); however, when combined, EV and plasma biomarkers achieved the highest diagnostic performance (AUC 0.913, 82.5% accuracy), suggesting that integration of both compartments may provide the most robust diagnostic strategies [48]. These findings underscore the necessity for studies directly measuring both soluble and EV-associated biomarkers for the same molecules in parallel, to fully understand the advantages EVs may confer in clinical biomarker development.
Interleukins, frequently found in the included studies, are nonspecific markers that can be elevated in a wide range of injuries. Their role in TBI is particularly relevant due to their involvement in the neuroinflammatory cascade; pro-inflammatory interleukins such as IL-6 and IL-1β have been associated with secondary injury mechanisms, while anti-inflammatory cytokines like IL-10 may modulate repair processes [64, 65]. Tau and UCH-L1 also emerged as important biomarkers, reflecting neuronal damage and degeneration. Notably, EV-associated Tau levels have been linked to long-term neurological outcomes [49], suggesting that these biomarkers could contribute to patient stratification and prognosis, helping predict the risk of dementia and other neurological conditions [66, 67].
Another strength of EVs as biomarkers is their reflection of underlying pathophysiological processes. For example, Goetzl et al. [55] reported an increase in complement-associated proteins within EVs derived from patients with TBI. The complement system plays a central role in the inflammatory response following TBI, contributing to tissue damage and neuronal injury. Notably, complement inhibitors have shown promise in reducing post-concussion syndrome by potentially modulating neuroinflammation and limiting secondary injury [68]. Likewise, alterations in tight-junction proteins in EVs may point to mechanisms of endothelial dysfunction, which could serve as both a biomarker and a therapeutic target [30]. Furthermore, EV-based biomarkers may also aid in the early detection of systemic complications following TBI. Tian and colleagues [38] highlighted the role of EVs in TBI-related consumption coagulopathy, underscoring their potential for identifying extracerebral complications that impact patient outcomes.
Although miRNAs have been less extensively studied as biomarkers in clinical settings compared to experimental models, preliminary findings are encouraging, demonstrating potential both for individual miRNAs and for diagnostic panels [27, 28]. As a single biomarker, miR-124-3p has been repeatedly identified [25, 33, 34]; its known neuroprotective roles support its use as a biomarker and underline the need for more in-depth studies of its effects. circRNAs [37], which were found to regulate neuronal growth and repair, also deserve more attention in future works; however, the high volume of EVs needed for accurate detection of circRNAs and miRNAs poses a challenge to its clinical implementation. In preclinical studies, Ko et al. demonstrated that panels of EV-derived miRNAs could reliably distinguish TBI from control animals [27, 28]. Importantly, their work showed that diagnostic accuracy was not fixed: it improved substantially when additional information, such as the mechanism of injury, was incorporated into the analysis [28]. This highlights the importance of clinical information to improve diagnostic performance of EV-based biomarker panels. Furthermore, in their 2020 study, Ko and colleagues applied machine learning through linear discriminant analysis, which further enhanced diagnostic classification, underscoring the power of computational techniques to refine EV biomarker performance [27]. Collectively, these findings suggest that EVs, particularly EV-associated miRNAs, could serve as specific and versatile biomarkers for TBI diagnosis.
However, most existing studies have focused exclusively on distinguishing patients with TBI from healthy controls. Few have tackled more clinically relevant questions, such as stratifying injury severity, predicting functional outcomes, or monitoring responses to therapy, and among those that have, performance metrics have rarely been reported. Given that increased expression of EV-contained miR-124-3p and miR-873a-5p in animal models has been associated with improved neurological outcomes, such as reduced lesion volume, attenuated neuroinflammation, and enhanced cognitive recovery, these microRNAs may serve as promising biomarkers in studies aiming to predict positive neurological outcomes following TBI; nevertheless, their role in humans remains to be further explored [25, 26, 30]. In clinical studies, persistently elevated levels of EV-associated GFAP and UCH-L1 were predictive of worse neurological outcomes and higher mortality at 7 and 30 days post-injury [50], while GFAP was associated with both initial GCS scores and long-term functional outcomes measured by the GOS-E, supporting their role in early outcome prediction [51]. Increased phosphorylated tau and decreased neurogranin in neuronal-derived EVs have been associated with long-term cognitive decline in chronic mild TBI, suggesting that EV-contained biomarkers may aid in predicting late neurodegenerative outcomes, though further longitudinal studies are needed to confirm their prognostic value across TBI severities and stages [49, 55].
To our knowledge, our review represents the first systematic synthesis of EV-based biomarkers in TBI up to date. Although previous reviews on the topic had been conducted, they were not systematic reviews [21, 69] and they focused on both soluble and EV-based biomarkers [61, 70], or on EV-based biomarkers for several CNS diseases [20], or considered EVs as therapeutic tools [20]. Systematic reviews have already been conducted on soluble and saliva biomarkers for TBI [59, 70], emphasizing the need for similar studies focused on EVs. The inclusion of both animal and human studies broadens the scope of our findings, allowing to establish a correlation between biomarkers throughout all stages of research. Furthermore, a detailed assessment of risk of bias was conducted using validated tools, enhancing the reliability of our results.
The evaluation of EV isolation and characterization methods, in accordance with MISEV guidelines, is critical not only for assessing study quality and ensuring methodological consistency [14], but also for understanding the feasibility of clinical implementation. Since EV isolation and analysis remain technically complex and time-consuming, their integration into real-time clinical decision-making is currently limited. For example, traditional ultracentrifugation protocols may take several hours, while polymer-based precipitation methods are faster but may yield less pure samples; newer immunomagnetic approaches like TENPO have demonstrated isolation times under one hour with higher specificity, at a higher cost. The type of biomarker analyzed also influences turnaround time; while EV protein markers can be quantified within hours using ELISA or WB, miRNA profiling typically requires at least one day due to RNA extraction, reverse transcription, and qPCR or sequencing workflows. Future studies should aim to streamline these processes to reduce processing time and enhance clinical applicability.
Our work has several limitations. As a systematic review, it is inherently dependent on the quality and completeness of the included studies; although quality of studies was evaluated through the appropriate scores, careful interpretation of results is needed. Many studies did not report time-dependent biomarker changes, making it difficult to assess the temporal dynamics of EV-based biomarkers following TBI, which could stand as a research line in the future. Additionally, only a few studies provided measures of diagnostic performance, such as sensitivity/specificity, AUC or accuracy, limiting robust comparisons [27, 28, 48]. Considerable heterogeneity was found in both experimental models of TBI and EV isolation methods. Although most studies used controlled cortical impact or blast injury, some used electrical injury [29, 34], which is questionable as a representative model of TBI due to its distinct pathophysiological mechanisms. A wide range of EV isolation techniques were used, including ultracentrifugation, polymer-based precipitation, or immunomagnetic capture, often with varying protocols regarding centrifugation speed and temperature [49, 52]. Immunomagnetic nanopore-based TENPO for GluR2 + EVs has emerged as a new efficient and highly specific method and allows for more precise biomarker identification in a specific subpopulation of EVs [27, 28, 48]. Standardizing protocols for EV isolation and characterization is key to improve the reproducibility and comparability of future studies conducted on the topic. This review highlights the potential of EV-based biomarkers in TBI and summarizes the main candidate molecules which deserve further research, but more studies are needed to address the limitations and progress toward clinical implementation. While promising candidates such as GFAP, UCH-L1, Tau, or miR-124-3p were repeatedly identified, validation in independent, prospective cohorts remains crucial before clinical translation can be considered. In this line, although commercially available assays for soluble biomarkers already offer rapid and sensitive detection, EV-based biomarkers may provide added value through higher specificity, better reflection of CNS-derived signals, and improved performance when analyzed using advanced statistical methods [48, 28, 27]. Therefore, further research is needed to directly compare EV-based approaches with conventional plasma assays to clarify their relative clinical utility and potential for integration into diagnostic workflows.
Conclusions
This systematic review highlights the potential of EV-based biomarkers in TBI, emphasizing their stability, tissue-specific signatures, and alignment with known pathophysiological processes. Both EV-associated proteins and miRNAs were frequently identified as biomarkers in human and animal studies, consistent with previously described soluble biomarkers. However, there is a need for standardized protocols in EV isolation and characterization to ensure consistency across studies. While diagnostic applications have been explored, more research is needed on the prognostic utility of EV biomarkers, particularly for neurological outcomes. Future studies should incorporate performance metrics to better assess the clinical relevance and accuracy of these biomarkers in TBI.
Electronic supplementary material
Acknowledgements
None.
Author contributions
NRG and CR initially conceived the study. BM, ARP, AV and GA performed abstract and full-text screening. JHA, JSR, CMB and FT performed data collection. MGP, AG and JC assessed risk of bias of the included studies. NRG and ARP produced the first draft of the study which was consecutively discussed with CB, CR and RB. The definitive manuscript was approved by all authors.
Funding
This project was conducted without receiving any specific funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable (Systematic review).
Consent for publication
Not applicable (Systematic review).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chiara Robba and Rafael Badenes contributed equally.
References
- 1.Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–40. [DOI] [PubMed] [Google Scholar]
- 2.James SL, Theadom A, Ellenbogen RG, Bannick MS, Montjoy-Venning W, Lucchesi LR, et al. Global, regional, and National burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(1):56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson L, Stewart W, Dams-O’Connor K, Diaz-Arrastia R, Horton L, Menon DK, et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16(10):813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow coma scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844–54. [DOI] [PubMed] [Google Scholar]
- 5.Hawryluk GWJ, Manley GT. Classification of traumatic brain injury. In: Handbook of clinical neurology 127. 2015. pp. 15–21. [DOI] [PubMed]
- 6.Joseph B, Pandit V, Aziz H, Kulvatunyou N, Zangbar B, Green DJ, et al. Mild traumatic brain injury defined by Glasgow coma scale: is it really mild? Brain Inj. 2015;29(1):11–6. [DOI] [PubMed] [Google Scholar]
- 7.Elkbuli A, Shaikh S, McKenney K, Shanahan H, McKenney M, McKenney K. Utility of the Marshall & Rotterdam classification scores in predicting outcomes in trauma patients. J Surg Res. 2021;264:194–8. [DOI] [PubMed] [Google Scholar]
- 8.Amyot F, Arciniegas DB, Brazaitis MP, Curley KC, Diaz-Arrastia R, Gandjbakhche A, et al. A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. J Neurotrauma. 2015;32(22):1693–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974;304(7872):81–4. [DOI] [PubMed] [Google Scholar]
- 10.Egea-Guerrero JJ, Rodríguez-Rodríguez A, Gordillo-Escobar E, Fernández-Delgado E, Martínez-Roldán Á, Roldán-Reina Á, et al. IMPACT score for traumatic brain injury: validation of the prognostic tool in a Spanish cohort. J Head Trauma Rehabilitation. 2018;33(1):46–52. [DOI] [PubMed] [Google Scholar]
- 11.Dullaert M, Oerlemans J, De Paepe P, Kalala Okito JP, Hallaert G. Comparison of the CRASH Score–Predicted and real outcome of traumatic brain injury in a retrospective analysis of 417 patients. World Neurosurg. 2020;137:e159–65. [DOI] [PubMed] [Google Scholar]
- 12.Yue JK, Lee YM, Sun X, van Essen TA, Elguindy MM, Belton PJ, et al. Performance of the IMPACT and CRASH prognostic models for traumatic brain injury in a contemporary multicenter cohort: a TRACK-TBI study. J Neurosurg. 2024;141(2):417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondello S, Sorinola A, Czeiter E, Vámos Z, Amrein K, Synnot A, et al. Blood-Based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: A living systematic review and Meta-Analysis. J Neurotrauma. 2021;38(8):1086–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1), 1535750. [DOI] [PMC free article] [PubMed]
- 15.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65(8):783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Iqbal Z, Xu L, Wen C, Duan L, Xia J, et al. Brain-derived extracellular vesicles: potential diagnostic biomarkers for central nervous system diseases. Psychiatry Clin Neurosci. 2024;78(2):83–96. [DOI] [PubMed] [Google Scholar]
- 18.Sanz-Ros J, Mas-Bargues C, Romero-García N, Huete-Acevedo J, Dromant M, Borrás C. Therapeutic potential of extracellular vesicles in aging and Age-Related diseases. Int J Mol Sci. 2022;23(23):14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Tian L, Lu J, Ng IOL. Exosomes and cancer - Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis. 2022;11(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan NA, Asim M, El-Menyar A, Biswas KH, Rizoli S, Al-Thani H. The evolving role of extracellular vesicles (exosomes) as biomarkers in traumatic brain injury: clinical perspectives and therapeutic implications. Front Aging Neurosci. 2022;14, 933434. [DOI] [PMC free article] [PubMed]
- 21.Guedes VA, Devoto C, Leete J, Sass D, Acott JD, Mithani S, et al. Extracellular vesicle proteins and MicroRNAs as biomarkers for traumatic brain injury. Front Neurol. 2020;11:663–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allelein S, Medina-Perez P, Lopes ALH, Rau S, Hause G, Kölsch A, et al. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Sci Rep. 2021;11(1):11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 24.Harrison EB, Hochfelder CG, Lamberty BG, Meays BM, Morsey BM, Kelso ML, et al. Traumatic brain injury increases levels of miR-21 in extracellular vesicles: implications for neuroinflammation. FEBS Open Bio. 2016;6(8):835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge X, Guo M, Hu T, Li W, Huang S, Yin Z, et al. Increased microglial Exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after RmTBI. Mol Ther. 2020;28(2):503–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long X, Yao X, Jiang Q, Yang Y, He X, Tian W, et al. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation. 2020;17(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko J, Hemphill M, Yang Z, Beard K, Sewell E, Shallcross J, et al. Multi-Dimensional mapping of brain-Derived extracellular vesicle MicroRNA biomarker for traumatic brain injury diagnostics. J Neurotrauma. 2020;37(22):2424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko J, Hemphill M, Yang Z, Sewell E, Na YJ, Sandsmark DK, et al. Diagnosis of traumatic brain injury using MiRNA signatures in nanomagnetically isolated brain-derived extracellular vesicles. Lab Chip. 2018;18(23):3617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Huang S, Yin Z, Zhu J, Ge X, Han Z, et al. Increases in miR-124-3p in microglial exosomes confer neuroprotective effects by targeting FIP200-Mediated neuronal autophagy following traumatic brain injury. Neurochem Res. 2019;44(8):1903–23. [DOI] [PubMed] [Google Scholar]
- 30.Andrews AM, Lutton EM, Merkel SF, Razmpour R, Ramirez SH. Mechanical injury induces brain Endothelial-Derived microvesicle release: implications for cerebral vascular injury during traumatic brain injury. Front Cell Neurosci. 2016;10, 43. [DOI] [PMC free article] [PubMed]
- 31.Keane RW, Hadad R, Scott XO, Cabrera Ranaldi E. d. L. RM, Pérez-Bárcena J, de Rivero Vaccari JP. Neural–Cardiac inflammasome Axis after traumatic brain injury. Pharmaceuticals. 2023;16(10):1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian Y, Zhao R, Li X, Zhou J, Zhan D, Wang Y, et al. Alterations of MicroRNAs expression profiles in small extracellular vesicle after traumatic brain injury in mice. Exp Anim. 2022;71(3):21–0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Xue H, Li T, Chu X, Xin D, Xiong Y, et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE–/- mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510(4):565–72. [DOI] [PubMed] [Google Scholar]
- 34.Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, et al. Increased miR-124‐3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32(1):512–28. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Han S. Exosome-associated Tau exacerbates brain functional impairments induced by traumatic brain injury in mice. Mol Cell Neurosci. 2018;88:158–66. [DOI] [PubMed] [Google Scholar]
- 36.de Rivero Vaccari JP, Brand F, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, et al. Exosome‐mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(S1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Rting, Zhou J, Dong X, long, Bi C wen, Jiang Rcai, Dong J et al. fei,. Circular Ribonucleic Acid Expression Alteration in Exosomes from the Brain Extracellular Space after Traumatic Brain Injury in Mice. J Neurotrauma. 2018;35(17):2056–66. [DOI] [PubMed]
- 38.Tian Y, Salsbery B, Wang M, Yuan H, Yang J, Zhao Z, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125(13):2151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Guo Y, Yang W, Chen L, Ren D, Wu C, et al. Phosphorylation of connexin 43 induced by traumatic brain injury promotes exosome release. J Neurophysiol. 2018;119(1):305–11. [DOI] [PubMed] [Google Scholar]
- 40.Hazelton I, Yates A, Dale A, Roodselaar J, Akbar N, Ruitenberg MJ, et al. Exacerbation of acute traumatic brain injury by Circulating extracellular vesicles. J Neurotrauma. 2018;35(4):639–51. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, et al. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation. 2017;14(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang P, Ma H, Zhang Y, Zeng R, Yu J, Liu R, et al. Plasma Exosome-derived MicroRNAs as novel biomarkers of traumatic brain injury in rats. Int J Med Sci. 2020;17(4):437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C, Deng Y, He Y, Huang X, Wang C, Li W. Decreased level of Exosomal miR-5121 released from microglia suppresses neurite outgrowth and synapse recovery of neurons following traumatic brain injury. Neurotherapeutics. 2021;18(2):1273–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuharić J, Grabušić K, Tokmadžić VS, Štifter S, Tulić K, Shevchuk O, et al. Severe traumatic brain injury induces early changes in the physical properties and protein composition of intracranial extracellular vesicles. J Neurotrauma. 2019;36(2):190–200. [DOI] [PubMed] [Google Scholar]
- 45.Nekludov M, Mobarrez F, Gryth D, Bellander BM, Wallen H. Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J Neurotrauma. 2014;31(23):1927–33. [DOI] [PubMed] [Google Scholar]
- 46.Nekludov M, Bellander BM, Gryth D, Wallen H, Mobarrez F. Brain-Derived microparticles in patients with severe isolated TBI. Brain Inj. 2017;31(13–14):1856–62. [DOI] [PubMed] [Google Scholar]
- 47.Patz S, Trattnig C, Grünbacher G, Ebner B, Gülly C, Novak A, et al. More than cell dust: microparticles isolated from cerebrospinal fluid of brain injured patients are messengers carrying mRNAs, mirnas, and proteins. J Neurotrauma. 2013;30(14):1232–42. [DOI] [PubMed] [Google Scholar]
- 48.Beard K, Yang Z, Haber M, Flamholz M, Diaz-Arrastia R, Sandsmark D et al. Extracellular vesicles as distinct biomarker reservoirs for mild traumatic brain injury diagnosis. Brain Commun. 2021;3(3), fcab151. [DOI] [PMC free article] [PubMed]
- 49.Goetzl EJ, Elahi FM, Mustapic M, Kapogiannis D, Pryhoda M, Gilmore A, et al. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019;33(4):5082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mondello S, Guedes VA, Lai C, Czeiter E, Amrein K, Kobeissy F, et al. Circulating brain injury Exosomal proteins following Moderate-to-Severe traumatic brain injury: Temporal profile, outcome prediction and therapy implications. Cells. 2020;9(4):977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puffer RC, Cumba Garcia LM, Himes BT, Jung MY, Meyer FB, Okonkwo DO, et al. Plasma extracellular vesicles as a source of biomarkers in traumatic brain injury. J Neurosurg. 2021;134(6):1921–8. [DOI] [PubMed] [Google Scholar]
- 52.Guedes VA, Mithani S, Williams C, Sass D, Smith EG, Vorn R, et al. Extracellular vesicle levels of nervous system injury biomarkers in critically ill trauma patients with and without traumatic brain injury. Neurotrauma Rep. 2022;3(1):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manek R, Moghieb A, Yang Z, Kumar D, Kobessiy F, Sarkis GA, et al. Protein biomarkers and neuroproteomics characterization of microvesicles/exosomes from human cerebrospinal fluid following traumatic brain injury. Mol Neurobiol. 2018;55(7):6112–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matuk R, Pereira M, Baird J, Dooner M, Cheng Y, Wen S, et al. The role of salivary vesicles as a potential inflammatory biomarker to detect traumatic brain injury in mixed martial artists. Sci Rep. 2021;11(1):8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goetzl EJ, Yaffe K, Peltz CB, Ledreux A, Gorgens K, Davidson B, et al. Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins. FASEB J. 2020;34(2):3359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Y, Pereira M, Raukar N, Reagan JL, Queseneberry M, Goldberg L, et al. Potential biomarkers to detect traumatic brain injury by the profiling of salivary extracellular vesicles. J Cell Physiol. 2019;234(8):14377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Li F, Bai X, Jia H, Wang C, Li P, et al. Circulating extracellular vesicles from patients with traumatic brain injury induce cerebrovascular endothelial dysfunction. Pharmacol Res. 2023;192:106791. [DOI] [PubMed] [Google Scholar]
- 58.Huang X, Xu X, Wang C, Wang Y, Yang Y, Yao T, et al. Using bioinformatics technology to mine the expression of serum Exosomal MiRNA in patients with traumatic brain injury. Front Neurosci. 2023;17:1145307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edalatfar M, Piri SM, Mehrabinejad MM, Mousavi MS, Meknatkhah S, Fattahi MR, et al. Biofluid biomarkers in traumatic brain injury: A systematic scoping review. Neurocrit Care. 2021;35(2):559–72. [DOI] [PubMed] [Google Scholar]
- 60.Gardner RC, Puccio AM, Korley FK, Wang KKW, Diaz-Arrastia R, Okonkwo DO et al. Effects of age and time since injury on traumatic brain injury blood biomarkers: a TRACK-TBI study. Brain Commun. 2022;5(1), fcac316. [DOI] [PMC free article] [PubMed]
- 61.Bravo-Miana R, del Arizaga-Echebarria C, Otaegui JK. Central nervous system-derived extracellular vesicles: the next generation of neural Circulating biomarkers? Transl Neurodegener. 2024;13(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mercier E, Tardif PA, Cameron PA, Émond M, Moore L, Mitra B, et al. Prognostic value of neuron-specific enolase (NSE) for prediction of post-concussion symptoms following a mild traumatic brain injury: a systematic review. Brain Inj. 2018;32(1):29–40. [DOI] [PubMed] [Google Scholar]
- 63.Cheng F, Yuan Q, Yang J, Wang W, Liu H. The prognostic value of serum Neuron-Specific enolase in traumatic brain injury: systematic review and Meta-Analysis. PLoS ONE. 2014;9(9):e106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Q, Zhu W, Xu F, Dai X, Shi L, Cai W, et al. The interleukin-4/PPARγ signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 2019;17(6):e3000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vincent JC, Garnett CN, Watson JB, Higgins EK, Macheda T, Sanders L, et al. Correction: IL-1R1 signaling in TBI: assessing chronic impacts and neuroinflammatory dynamics in a mouse model of mild closed-head injury. J Neuroinflammation. 2023;20(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Z, Zlokovic BV. Acetylated tau: A missing link between head injury and dementia. Med. 2021;2(6):637–9. [DOI] [PubMed] [Google Scholar]
- 67.Albayram O, Kondo A, Mannix R, Smith C, Tsai CY, Li C, et al. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat Commun. 2017;8(1):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hammad A, Westacott L, Zaben M. The role of the complement system in traumatic brain injury: a review. J Neuroinflammation. 2018;15(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dey A, Ghosh S, Bhuniya T, Koley M, Bera A, Guha S, et al. Clinical theragnostic signature of extracellular vesicles in traumatic brain injury (TBI). ACS Chem Neurosci. 2023;14(17):2981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Porteny J, Tovar E, Lin S, Anwar A, Osier N. Salivary biomarkers as indicators of TBI diagnosis and prognosis: A systematic review. Mol Diagn Ther. 2022;26(2):169–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.