Abstract
Cancer research is undergoing a paradigm shift from solely studying tumor cells to investigating systemic effects of cancer in the tumor macroevironment, with an emphasis on the interactions between host organs and tumors. The theory of homeostasis is an important basis for explaining biological functions from the perspective of the organism. Organic homeostasis relies on brain-body crosstalk through interception, immunoception, nociception and other supervisory processes, guaranteeing normal physiological function. Recent studies reveal that malignant tumors can hijack and exploit the brain and its central-peripheral neuronal networks to disrupt the body's homeostasis. Tumors likely disrupt normal brain-body crosstalk by establishing bidirectional brain-tumor connections. On the contrary, organism utilize these mechanisms to hinder tumorigenesis and progression. Standing at the perspective of brain-body crosstalk also promotes the conceptional evolution of cancer initiation and development, and more importantly, provides additional insight for cancer treatment. In this review, we summarize current knowledge about brain-body crosstalk under tumor-bearing contexts and propose some novel anti-cancer strategies.
Graphical Abstract
Brain-body crosstalk participates in the battle between tumors and the organism: The homeostasis of the organism is collectively maintained by interoception, nociception, neuroception, endocriception, metaboception and immunoception. However, in tumor states, tumors hijack the brain-body crosstalk system to exploit these homeostatic mechanisms, thereby constructing a macroenvironment conducive to their survival and progression. While tumors hijack the brain-body crosstalk to reestablish homeostasis, the host organism simultaneously counteracts the tumor through brain-body crosstalk, safeguarding its intrinsic homeostasis from disruption. The brain-body crosstalk between tumors and multiple organs mediated by the HPA axis-driven humoral regulation and the sympathetic and parasympathetic nerves plays a significant role in the battle between tumors and the organism. TME, tumor microenvironment; HPA, hypothalamic-pituitary-adrenal; SAS, sympatho-adrenal system. This figure was created using BioRender (https://biorender.com/).
Keywords: Brain-body, Cancer neuroscience, Drug repurposing, Homeostatic disruption, Neural circuity, Neuroimmune
Highlights
● Cancer research has experienced a conceptional evolution, from simply regarding cancer as an unorganized cell mass to a newborn organ. Therefore, standing at the perspective of interactions between organs provides novel insight for this area.
● The organic homeostasis is supervised and exerted respectively by interception, nociception, neuroception, endocriception, metaboception and immunoception, which are tightly associated with brain and other components of the nervous system.
● Tumors could hijack the brain-body crosstalk to facilitate their own survival and progression. In turn, the host could utilize this axis to constrain cancer development, and ideally promote cancer elimination.
● According to theory of brain-body crosstalk, the importance of approaches such as targeted treatment, combined therapy and comprehensive therapy is worthy of emphasis and implementation.
Introduction
Our understanding of cancer has moved beyond the intrinsic cellular alterations of tumors and changes in the local microenvironment, now placing greater emphasis on the interplay between tumors and the entire host organism. The paradigm of cancer treatment has shifted from a curative intent toward conceptualizing tumors as integral components of the organismal macroenvironment, emphasizing tumor interactions with multiple organ systems, particularly brain-body crosstalk under tumor conditions [1]. This perspective transitions from viewing tumors as"autonomous growths"to recognizing"neural-tumor communication"highlighting the systemic regulation of neural activity on tumor metabolism, immune evasion, and metastatic colonization [2].
The central nervous system (CNS) has a fundamental function in maintaining homeostasis, which ensures that functional parameters of various tissues remain within preset ranges. To achieve this, the CNS continuously receives information from both the external and internal environments. These ceaseless streams of information are centrally integrated and converted into functional output signals that are transmitted to the periphery. This bidirectional communication between the periphery and the CNS, termed the"body–brain axis"[3]. The body-brain axis is the foundation of brain-body crosstalk. Brain-body crosstalk involves dynamic dialogues mediated by neural/endocrine signaling with distant organs, primarily investigating the complex bidirectional relationship between the brain and the body. Brain-body crosstalk plays a pivotal role in preserving interoception, particularly in maintaining systemic health, enabling environmental adaptation, and responding to external challenges [4]. Numerous studies have shown that brain-body crosstalk significantly contributes to tumor initiation and progression [2, 5, 6].
It has long been hypothesized that psychosocial factors may influence cancer progression through alterations in sympathetic and parasympathetic nervous system activity [7, 8]. Significant advances in cancer neuroscience have revealed that peripheral tumors are infiltrated by autonomic and sensory nerve fibers, which interact with diverse components of the tumor microenvironment to drive tumorigenesis and metastasis [9]. Tumors establish communication with the CNS through afferent sensory neurons, inflammatory cytokines, or neurotrophic factors [10]. Under tumor conditions, the nervous system dynamically engages in bidirectional brain-body dialogues to modulate tumor progression. Conversely, tumors release tumor-derived factors that reshape neural plasticity, thereby fostering tumor heterogeneity and therapy resistance [11]. The central nervous system can remotely regulate the progression of peripheral tumors through brain-body crosstalk, involving the remodeling of the tumor microenvironment and the modulation of cancer hallmarks. This interplay between tumors and the organism is mediated by interoception, nociception, neuroception, endocriception, metaboception, and immunoception. A deeper understanding of brain-body crosstalk mechanisms will not only elucidate tumor pathogenesis but also provide a scientific foundation for developing novel therapeutic and interventional strategies.
Our approach to comprehending tumors has transcended tumor-intrinsic cellular alterations and local microenvironment modifications, now encompassing how malignancies dynamically interact with the entire host organism. The nervous system, situated at the interface between the brain and body, continuously receives and transmits signals to maintain homeostasis and respond to salient stimuli. Accumulating evidence demonstrates that tumors disrupt this brain-body dialogue through neural and humoral pathways, leading to aberrant brain activity and disease progression. These findings underscore that brain-body crosstalk must be considered a central factor in developing therapeutic strategies for cancer [12]. This review explores brain-body crosstalk under physiological conditions and in the tumor context, elucidating their roles in tumor initiation and progression, while offering novel strategies for cancer treatment.
The macroenvironment of tumor: from innervated TME to brain-body crosstalk
To survive and proliferate, cancer cells must remodel their local environment to ensure a sufficient supply of metabolic fuels and evade immune destruction [13, 14]. To achieve this, they employ a multipronged strategy, secreting a plethora of proteins and metabolites that function within paracrine, endocrine and autocrine signaling circuits in the tumor microenvironment (TME) [15]. For example, tumors secrete IL-6, GDF15 and other substances that act on the brain, leading to systemic inflammation and anorexia [16]. From this perspective, tumors are no longer mere aggregates of cancer cells and extracellular matrix (ECM) components but rather complex entities comprising multiple functional elements [17]. Like normal organs, tumors exert systemic effects and interact with the entire organism, often culminating in life-threatening conditions such as cancer cachexia [18]. Moreover, even when tumors are not located in the brain itself, many cancer-related symptoms involve brain dysfunction (e.g., sleep disturbances in primary breast cancer) [19].
In 2020, our team specialized the tumor microenvironment into six typical microenvironments: hypoxic niche, immune microenvironment, metabolism microenvironment, acidic niche, innervated niche, and mechanical microenvironment [20]. We propose that neural-derived neurotransmitters or neuropeptide-mediated communication between nerves and cancer establishes a specific local microenvironment called the"innervation niche"[20]. As research between nerves and tumors deepens, people are increasingly aware of the close relationship between neurology and cancer science. In recent years, Monje and other scientists have proposed a new field called"cancer neuroscience"to better study how the nervous system communicates with cancer [21]. Cancer neuroscience has unveiled the critical role of the nervous system in tumor initiation, progression, invasion, and metastasis [22–24]. Since the discovery that brain tumors (e.g., glioblastoma) can form functional synapses with normal neurons in the brain [25] and that autonomic nerves robustly regulate the growth and metastasis of prostate and breast cancers through norepinephrine (NE) signaling [26–28], an increasing number of researchers have engaged in cancer neuroscience studies. On the other hand, the emergence of powerful tools for monitoring and manipulating neuronal activity in freely behaving animals, such as calcium imaging and optogenetics, has enabled microscopic-level investigations into how the nervous system interacts with tumors [29–31]. Advances in this field provide fresh perspectives and opportunities for understanding tumor biology and developing innovative anti-cancer therapies. By investigating neural-tumor interactions, novel therapeutic strategies can be developed to improve outcomes and quality of life for cancer patients.
Our understanding of tumors has transcended tumor-intrinsic cellular alterations and local microenvironment modifications, now focusing more on the interactions between tumors and the entire host organism (Fig. 1). Next, we will discuss the crucial role of brain-body crosstalk in maintaining internal environmental homeostasis, and explore how brain-body crosstalk under tumor conditions influence tumorigenesis and development.
Fig. 1.
The evolution of conceptual understanding of tumors. Modern oncology research has transcended the traditional confines of tumor-intrinsic cellular alterations and local microenvironment regulation, transitioning into a profound paradigm shift from a"localized lesion-centric framework"to a"whole-organism host-oriented framework", centering on mechanistic deciphering of systemic regulatory mechanisms underlying tumor-host interactions. During tumor-host interactions, brain-body crosstalk plays a critical role. TME, tumor microenvironment. This figure was created using BioRender (https://biorender.com/)
Brain-body crosstalk: maintaining systematic homeostasis
Under physiological conditions, brain-body crosstalk plays a crucial regulatory role in interoception, nociception, neuroception, endocriception, metaboception, immunoception. Understanding how brain-body crosstalk upholds homeostasis and health represents a compelling research frontier [2, 11, 32, 33] (Fig. 2).
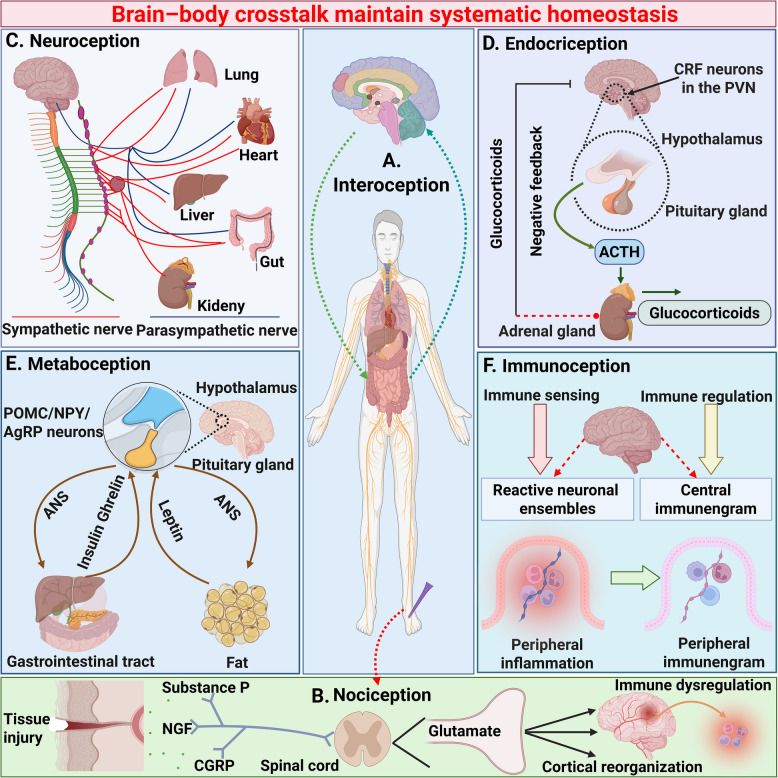
Fig. 2.
Brain-body crosstalk determinant systematic homeostasis. Under physiological conditions, as the central processor of the body's systems biology regulation, the brain-body crosstalk maintains systematic homeostasis by regulating multiple organs through the parasympathetic nerve, the spinal cord-skin nociceptive loop, and multi-interface communication from the brain to metabolism, endocrine and immune systems, achieving real-time regulation of interoception, nociception, neuroception, endocriception, metaboception and immunoception. A Interoception, defined as the physiological homeostasis process perceived by the brain and involving multiple organs, plays a vital role in maintaining systemic stability. This homeostatic regulation relies on the perception of internal bodily states mediated by neural and humoral afferent pathways, which facilitate bidirectional signaling between the body and the brain; B Nociception is the neurobiological process through which an organism detects and interprets potential or actual tissue damage. When noxious stimuli (e.g., high temperature, mechanical pressure, chemical injury) activate nociceptors in the skin, the dorsal horn of the spinal cord performs primary integration of pain signals. These signals are then transmitted via the spinothalamic tract to the thalamus and further projected to the cerebral cortex. The brain initiates a cascade of responses to such injury, which may even trigger immune dysfunction due to neuro-immune crosstalk; C Neuroception refers to the nervous system's capacity to detect and interpret internal and external environmental cues, orchestrating a multilevel regulatory cascade from molecular signaling to behavioral responses. This adaptive mechanism enables the nervous system to anticipate risks and initiate context-specific physiological adjustments, primarily through the brain's dynamic modulation of multiple organ systems via sympathetic and parasympathetic nerves to maintain systemic homeostasis; D Endocriception refers to the physiological process through which organisms continuously monitor circulating metabolites, ions, hormonal and neurochemical signals via specific receptor-mediated mechanisms, and orchestrates the dynamic adjustment of hormonal secretion through intricate regulatory mechanisms. As exemplified by the hypothalamic–pituitary–adrenal axis, which maintains endocrine homeostasis via bidirectional feedback regulation to adapt to internal and external stressors; E Metaboception in organisms involves dynamic brain-body crosstalk. The ANS governs energy homeostasis by orchestrating energy metabolism through bidirectional interactions with the gastrointestinal tract and adipose tissue, forming an integrated neuro-metabolic regulatory network; F The brain and immune system establish bidirectional communication, termed immunoception. This process enables the detection of immunological disturbances by mediating crosstalk between the immune and nervous systems, thereby mobilizing brain-body systems to restore systemic immune homeostasis. CRF, corticotropin releasing factor; PVN, paraventricular nucleus; ANS, autonomic nervous system; POMC, p-opiomelanocortin; NPY, neuropeptide Y; AgPR, agouti-related peptide. This figure was created using BioRender (https://biorender.com/)
Interoception
The biological processes that maintain homeostasis and normal bodily functions involve the coordinated actions of multiple organ systems. These systems interact through complex multidirectional communication and possess their own regulatory mechanisms. Consequently, the brain can not only exchange information with the external environment via circulating molecules (such as those from blood and cerebrospinal fluid), but also transmit signals through nerves that innervate various organs. For example, the hypothalamus regulates digestive organs by releasing neuropeptide Y (NPY) to modulate appetite, while also controlling anterior pituitary hormone secretion through the hypophyseal portal system, thereby indirectly influencing the functions of peripheral organs such as the adrenal glands [34].
Such multi-layered communication mechanisms enable the organism to precisely regulate homeostatic maintenance processes [35]. The homeostatic process of the body, perceived by the brain and involving multiple organs, is called interoception [36]. Interoception refers to the perception of the body's internal state mediated through neural and humoral afferent pathways, arising from the transmission of signals from the body to the brain [37]. Interoceptive information guides the brain's efferent physiological regulation of the body to maintain health and is integrated into the representations of sensation, emotion, cognition, and motivation [36, 37]. Interoception serves as the foundation for maintaining homeostasis and its dysfunction is associated with various physiological and psychological disorders. The organism maintains interoceptive homeostasis through brain-body crosstalk.
Other perceptions
Other perceptions, such as nociception, neuroception, endocriception, metaboception, and immunoception, also play crucial roles in maintaining organismal homeostasis.
Under physiological conditions, the brain-body crosstalk precisely maintains interoceptive homeostasis through the coordinated interplay of the nervous, metabolic, endocrine and immune systems. This equilibrium serves as the foundation for adapting to internal and external environmental changes while preserving physiological balance. However, when external stressors such as infection, trauma, or psychological distress exceed the body’s adaptive capacity, homeostatic mechanisms are disrupted. Such dysregulation can trigger pathological cascades, including chronic inflammation, metabolic dysfunction, or aberrant cellular proliferation, ultimately contributing to diseases such as cancer. Next, the discussion will focus on how brain-body crosstalk maintains organismal homeostasis under tumor conditions, and how this process impacts cancer hallmarks and the TME during the maintenance of homeostasis.
Brain-body crosstalk: participating in the battle between tumors and the organism
"Brain-body crosstalk dynamically regulates the TME through the nervous, endocrine, and immune systems, influencing biological features of tumorigenesis, metastasis, and therapy resistance (e.g., angiogenesis, immune evasion). Under tumor conditions, interoceptive homeostasis becomes dysregulated. Tumors exploit brain-body crosstalk to create a macroenvironment conducive to their survival, while the host organism attempts to counteract tumor progression through reciprocal neurobiological adaptations. This dynamic interplay establishes a complex macroenvironment characterized by reciprocal interactions between tumors and multiple organ systems. Cancer complexity spans multiple dimensions, such as tumor initiation and promotion, TME and immune macroenvironment, aging, metabolic states, cancer cachexia, circadian rhythms, and microbiome [1]. These factors collectively demonstrate that cancer is not merely a localized collection of genetic mutations but a dynamic, systemic disease shaped by and reciprocally influencing whole-body physiology. Investigating these processes through a neurobiological lens, particularly focusing on brain-body crosstalk, provides critical insights into tumor pathophysiology, revealing novel therapeutic targets (e.g., neuromodulation, chronotherapy) and redefining cancer as a disorder of systemic homeostasis. Next, we will discuss the important role of brain-body crosstalk in the battle between tumors and the organism.
Brain-body crosstalk: reestablishing tumor homeostasis
Tumors hijack brain-body crosstalk, enhance tumor characteristics, reprogram TME and even impact the entire organism's rhythms to establish a homeostasis conducive to its survival and proliferation (Fig. 3).
Fig. 3.
Brain-body crosstalk drives TME reprogramming and cancer hallmark. In physiological states, systematic homeostasis is governed by the regulation of interoception, nociception, neuroception, endocriception, metaboception, and immunoception. Whereas neoplastic entities subvert this paradigm through hijacking brain-body crosstalk. This oncological coup manifests as: enhancing tumor characteristics, reprogramming of the TME, and remodeling of systemic biorhythmic networks, culminating in the establishment of a macroenvironment conducive to its survival and proliferation. TME, tumor microenvironment. This figure was created using BioRender (https://biorender.com/)
Enhancing cancer hallmarked abilities
Emerging research highlights 14 hallmark features of cancer [38]. The interplay between the nervous system and tumor evolution at cellular/molecular levels may drive hallmark acquisition [14]. For instance, in subsequent discussions we will elucidate how brain-body interactions under neoplastic conditions facilitate the acquisition of the following tumor hallmarks: proliferative signaling, evasion of cell death, invasion and metastasis, and tumor-promoting inflammation. Investigating the extent to which brain-body crosstalk contributes to the acquisition of these cancer hallmarks represents a critical research frontier.
Proliferative signaling
Tumor development and growth depend on the dynamic equilibrium within the microenvironment of a balance between pro-tumorigenic factors (e.g., growth promotion, angiogenesis, cell survival) and tumor-suppressive signals (e.g., proliferation inhibition, apoptosis induction) [39]. The brain promotes proliferative signaling through neural pathways.
In brain tumors, sensory experiences can drive tumorigenesis and progression. For example, photostimulation activates retinal ganglion cell neurons, triggering optic nerve activity that enhances shedding of neuroligin-3. This process regulates the initiation, growth, and maintenance of low-grade gliomas (LGGs) by fostering neuron-glioma synaptic connectivity [40]. In addition, odorant stimulation activates olfactory receptor neurons, which signal to mitral/tufted cells in the olfactory bulb. These neurons secrete insulin-like growth factor-1 in an experience and activity-dependent manner, promoting the initiation and growth of high-grade gliomas in murine models [41]. These studies demonstrate that sensory stimuli can modulate tumor progression within their respective neural circuits. Electrochemical interplay extends beyond primary brain tumors. A study found that breast cancer brain metastases co-opt astrocyte-like signaling in pseudo-tripartite synapses, activating glutamatergic NMDA receptors on metastatic cells to enhance growth [42]. In addition, gliomas increase neuronal excitability, creating a feedforward loop. Hyperexcitable neurons release more glutamate and growth factors, further fueling tumor progression [25, 43, 44].
The interaction between the nervous system and cancers outside the CNS can also promote the transmission of proliferative signals. In peripheral solid tumors, the TME is infiltrated by autonomic and sensory nerve fibers, which interact with stromal, immune, and neoplastic components to drive proliferative [9]. For example, in murine breast cancer models, corticotropin-releasing hormone neurons in the central medial amygdala project to peripheral tumors, activating intratumoral sympathetic nerves to drive proliferation [8]. In murine pancreatitis models, capsaicin-induced sensory neuron ablation eliminated central neuroinflammation and suppressed pancreatic ductal adenocarcinoma (PDAC) initiation/progression, demonstrating how peripheral inflammatory signals are relayed to the CNS via afferent nerves [45]. In addition, the role of the sympathetic and parasympathetic nervous systems in pancreatic cancer has been demonstrated. Stress-induced catecholamines (e.g., NE from sympathetic nerves or adrenal glands) promote PDAC progression via β-adrenergic receptor (β-AR) signaling, enhancing tumor cell proliferation [46–48]. Parasympathetic nerves activate the WNT signaling pathway in gastrointestinal cancer cells through cholinergic signaling, thereby driving the proliferation and growth of gastrointestinal cancers [49].
Cell death
The sympathetic nerves infiltrating the TME enable tumor cells to resist cell death. In xenograft models, both sympathetic denervation of the prostate and genetic knockout of the Adrb2 and Adrb3 genes encoding β2- and β3-adrenergic receptors significantly suppressed prostate cancer progression [26]. Genetic knockout studies in mice targeting Adrb2, Adrb3, or both genes revealed that loss of a single β-AR delayed xenograft tumor development, while combined deficiency of β2- and β3-adrenergic receptors profoundly inhibited tumor colonization. Increased numbers of apoptotic epithelial cells were observed in prostatic intraepithelial neoplasia regions of sympathectomized mice [26], indicating that sympathetic innervation of the prostate may promote tumor expansion by facilitating cancer cells to evade programmed cell death.
Invasion and metastasis
Tumor innervation is closely related to poor prognosis in many cancer patients, and multiple studies suggest that tumor innervation may be involved in the regulation of the process of invasion and metastasis [50, 51]. In animal models, cholinergic agonists promote the proliferation of prostate cancer cells and accelerate their metastatic spread to pelvic draining lymph nodes, while cholinergic antagonists inhibit lymph node metastasis [26]. Furthermore, pharmacological interventions or genetic approaches blocking the muscarinic signaling pathway in the prostate tumor microenvironment significantly suppress tumor cell proliferation, invasion, and distant metastasis [52]. In addition, studies have found that compared with low-metastatic mouse breast tumors, high-metastatic tumor models exhibit a more significant phenomenon of neural innervation. This enhanced effect of neural infiltration is attributed to the axon guidance molecule SLIT2 specifically expressed in the tumor vascular system [53]. Further mechanism analysis reveals that breast cancer cells can activate the spontaneous calcium signal activity of sensory neurons and induce the release of the neuropeptide substance P (SP). Neuron-derived SP can significantly promote the growth, invasion and metastasis of breast tumors [53]. The bidirectional interaction between the brain-body crosstalk and tumor metastasis involves a mechanism known as perineural invasion (PNI), which is particularly prominent in PDAC and prostate cancer [54–57]. This phenomenon manifests as cancer cells infiltrating adjacent tissues along nerve pathways. Recent studies reveal that the mutual crosstalk between neural innervation and cancer cells may actively drive PNI progression. For instance, PDAC cells utilize paracrine signaling to induce the c-Jun/AP-1 transcriptional network, reprogramming neighboring Schwann cells into a"tumor-activated"state [58]. This reprogramming exhibits dual effects: Firstly, activated Schwann cells form microtracks ("tracks") that envelop cancer cells, facilitating their migration along nerve pathways remodeled by PDAC cells. Secondly, these tumor-activated Schwann cells secrete the chemokine CCL2, recruiting inflammatory monocytes that differentiate into macrophages expressing the extracellular protease cathepsin B. Together, these components synergistically promote PNI progression [59]. These functional studies in PDAC highlight the critical role of the nervous system in driving clinically significant invasive phenotypes.
Beyond peripheral nervous system tumors, brain-body crosstalk plays a critical role in the metastasis and invasion of central nervous system tumors. Research indicates that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor)-mediated synaptic signaling inputs significantly enhance the invasive capacity of glioma cell subpopulations at the tumor periphery. This pro-invasive effect relies on calcium signaling pathways activated by synaptic communication, which can be blocked through calcium chelators or inhibition of cAMP response element-binding protein activity [60]. Real-time in vivo imaging techniques reveal that some invasive tumor cells gradually transition into a quiescent proliferative phenotype, while others persistently migrate to distal brain regions, collectively driving the expansion of the tumor's invasive range within the brain [60].
Tumor-promoting inflammation
Tumor-promoting inflammation represents a distinct pathological state characterized by persistent immuno-inflammatory responses. At its core, this chronic inflammatory process drives tumor initiation, invasion and metastasis through dynamic modulation of immune equilibrium, cellular signaling networks and metabolic reprogramming within the tumor microenvironment. Mechanistically, sustained inflammatory mediators (e.g., IL-6, TNF-α, CCL5) activate oncogenic signaling pathways including NF-κB and STAT3, thereby fueling malignant processes encompassing tumor cell proliferation, angiogenesis and immune evasion [61, 62]. Brain-body crosstalk orchestrates tumor-promoting inflammation through neuro-immune-tumor circuits. For example, in neurofibromatosis type 1-associated LGGs, studies have revealed that CCL5 secreted by microglia and bone marrow-derived myeloid cells promotes tumor growth. The secretion of CCL5 by these cells depends on paracrine signals from tumor-infiltrating CD8 + T lymphocytes. Consequently, CD8 + T cells form the foundation of a unique pro-tumor inflammatory microenvironment, which mediates three critical hallmarks of cancer: sustained proliferative signaling, resistance to cell death, and immune evasion [63].
The crosstalk between the brain and body plays a significant role in the formation of cancer hallmarks. With increasing reports of tumor mechanisms, future neural-tumor interactions may be viewed as another cancer hallmark for tumorigenesis.
Reprograming TME
Tumor initiation and progression are governed by the dynamic equilibrium between pro-growth factors (e.g., angiogenesis induction, cell survival) and anti-proliferative signals (e.g., apoptosis promotion) in TME [38]. Tumors actively subvert cellular activities within their local microenvironment to evade immune destruction and fulfill their metabolic demands for growth, proliferation, and metastasis. In reshaping the TME, tumors corrupt nearly all signals detectable by sensory/vagal nerves, including cytokines [64], metabolites [65], hormones[66], hypoxia [67] and tissue architecture [68]. Previous research in our laboratory specialized the tumor microenvironment into six typical microenvironments: hypoxic niche, immune microenvironment, metabolism microenvironment, acidic niche, innervated niche and mechanical microenvironment. However, our understanding of how specific TME-derived signals activate sensory/vagal nerves, how these signals propagate to the brain, and how such neural inputs interact with circulating humoral factors remains poorly understood. Next, we will discuss the effects of brain-body crosstalk on different dimensions of the TME.
Hypoxic niche
The hypoxic tumor microenvironment and brain-body crosstalk are closely interconnected, primarily mediated through hypoxia-induced molecular mechanisms and neural signaling pathways that regulate tumor progression. Rapid tumor growth leads to abnormal vasculature, creating hypoxic regions (oxygen partial pressure < 1%). Hypoxia activates the hypoxia-inducible factor-1α (HIF-1α) pathway, promoting angiogenesis (via vascular endothelial growth factor [VEGF]), metabolic reprogramming (Warburg effect), and immune suppression, thereby enhancing tumor aggressiveness [69]. There exists bidirectional regulation between hypoxia and neural signaling: Hypoxia facilitates neural infiltration by upregulating molecules like VEGF and Slit2 through HIF-1α, promoting axon guidance and tumor innervation. Conversely, neural signals exacerbate hypoxia, as NE released by sympathetic nerves activates HIF-1α via β-adrenergic receptors, forming a positive feedback loop [70]. Under hypoxic conditions, tumors influence brain function through brain-body interactions. Hypoxia-induced factors (e.g., hepatocyte growth factor, IL-6) promote neuroinflammation and alter blood–brain barrier (BBB) permeability, potentially facilitating brain metastasis or cognitive impairment [71]. The hypoxic tumor microenvironment and neural signals form a dynamic interaction network through HIF pathways, jointly driving tumor invasion, immune evasion and distant metastasis. Future research should focus on elucidating specific molecular mechanisms of the neuro-hypoxia axis and developing combined therapeutic interventions [72].
Immune microenvironment
A bidirectional regulatory network exists between the immune and nervous systems [73–75]. Neuro-effector molecules and immune mediators participate in this interaction, which aims to maintain tissue homeostasis and minimize or prevent pathological conditions [76]. When the brain detects inflammatory deviations from physiological set points via vagal afferent signaling, brainstem neural networks generate coordinated efferent corrective signals propagated through the vagus nerve, other autonomic pathways and neuroendocrine routes [76]. The brain modulates immunity via brainstem neurons and circuits. Studies reveal that cytokines produced during tissue inflammation initiate afferent reflex arcs to balance pro- and anti-inflammatory responses. Ablation of vagal afferent fibers projecting to the nucleus tractus solitaries (NTS) results in uncontrolled inflammation, whereas experimental activation of specific vagal fibers suppresses inflammation and enhances anti-inflammatory responses. These findings confirm that NTS neurons play a pivotal role in establishing the inflammatory set point required for immune homeostasis [4]. This neuroimmune reflex architecture enables dynamic, context-dependent regulation of inflammation, positioning the brainstem as a critical hub for integrating neural and immune signals to preserve systemic equilibrium.
The interplay among immune cells, tumor cells, and neurons profoundly influences tumor progression [77, 78]. Autonomic neural signals regulate the tumor immune microenvironment. Norepinephrine primarily mediates immunomodulatory functions via the β2-adrenergic receptor, which is widely expressed by immune cells. Sympathetic nerve-released norepinephrine remodels tumor-associated macrophage (TAM) functions by inducing immunosuppressive signals (e.g., IL-10, arginase 1 [ARG1]) while suppressing proinflammatory cytokines such as TNFα, IL-1β, and IL-12 [79]. Norepinephrine upregulates immunosuppressive molecules like IL-10 and programmed death-ligand 1 [80, 81], enhances the immunosuppressive activity of regulatory T cells by elevating IL-10 and cytotoxic T-lymphocyte antigen 4 levels [82].
Norepinephrine impairs natural killer (NK) cell cytotoxicity by reducing perforin and granzyme production [83]. Preclinical studies further confirm that norepinephrine accelerates tumor growth and metastasis by inhibiting CD8 + T and NK cell cytotoxicity while promoting macrophage infiltration and ARG1 expression [83, 84]. Acetylcholine, released by parasympathetic nerves, mediates diverse immunomodulatory effects through nicotinic acetylcholine receptors (nAChRs) and muscarinic acetylcholine receptors broadly expressed on immune cells [85]. Activation of nAChRs suppresses proinflammatory cytokines (e.g., TNFα, IFN-γ, IL-1β, IL-6) [86, 87], promotes IL-10 secretion [88], and dampens NK cell cytotoxicity by inhibiting granzyme and IFN-γ production [89, 90].
While autonomic neural signals regulate the tumor immune microenvironment, tumor cells actively exploit neural mechanisms to enhance survival. For example,
murine PDAC secrete trigger sympathetic nerve fiber ingrowth into the microenvironment, facilitating immune evasion [91]. Human pancreatic, colorectal, and prostate cancer cells release BDNF precursors to recruit autonomic innervation, accelerating disease progression [92].
Moreover, emerging research in cancer neuroscience has revealed that peripheral tumors may activate specific neurocircuits in brain regions such as the hypothalamus or brainstem, thereby modulating efferent autonomic neural signals. For example, murine pancreatic ductal or colorectal adenocarcinomas activate catecholaminergic neurons in the brainstem’s ventrolateral medulla (a key hub for sympathetic signaling), suppressing CD8 + T cells to promote tumor growth [93]. Murine lung carcinoma, colorectal adenocarcinoma, fibrosarcoma, or melanoma activates hypothalamic neurons, driving pituitary release of α-melanocyte-stimulating hormone (α-MSH) into circulation. This tumor-induced α-MSH promotes myeloid-derived suppressor cell (MDSC) generation in the bone marrow, suppressing antitumor immunity [94]. This adaptive mimicry highlights tumors’ capacity to co-opt neural pathways, fostering immunosuppressive microenvironments conducive to survival and metastasis. These findings underscore the nervous system’s dual role as both a regulator and target in cancer biology, revealing novel therapeutic opportunities to disrupt neuro-immune-tumor triads.
Metabolism microenvironment
Tumor cells adapt to hypoxic and nutrient-deprived microenvironments by reprogramming metabolic pathways (e.g., glycolysis, glutaminolysis), while influencing immune cell and stromal cell functions through the release of metabolites such as lactate and ketone bodies [95–99]. Research has uncovered neuro-metabolic disruption mechanisms within the context of brain-tumor interactions. For instance, in oral squamous cell carcinoma, nociceptors secrete calcitonin gene-related peptide (CGRP), which promotes tumor growth by reprogramming tumor cell metabolism. This effect is potentially mediated through the induction of cytoprotective autophagy under the low-glucose microenvironment of the oral cavity, enhancing tumor survival [100]. Additionally, breast tumors alter the activity of orexigenic neurons in the lateral hypothalamus, leading to systemic metabolic abnormalities [101]. This finding underscores the pivotal role of central neuromodulators in driving metabolic perturbations caused by peripheral tumors [102]. Together, these studies highlight how tumors exploit neural-metabolic axes via peripheral nociceptive signaling or central appetite-regulating networks to hijack host physiology, expanding our understanding of brain-body crosstalk in cancer-associated metabolic adaptation.
Acidic niche
The interplay between brain-body interactions and the acidic tumor microenvironment involves complex mechanisms encompassing neural regulation, metabolic reprogramming, immune suppression, and tumor progression. The formation of the acidic tumor microenvironment is associated with the Warburg effect, lactate accumulation, and HIF-1α [65, 103]. A recent study has shown that chronic mental stress can increase the levels of serum and tumor epinephrine, thereby activating lactate dehydrogenase A-mediated glycolysis. The resulting acidic microenvironment rich in lactic acid enhances the occurrence of breast cancer [104]. More research is needed in the future to explore whether the nervous system can regulate the acidic microenvironment of tumors, especially whether chronic stress can promote tumor glycolysis and acidification by releasing cortisol through the hypothalamic–pituitary–adrenal (HPA) axis. Brain-body crosstalk may modulate tumor microenvironment acidification via neural signaling and metabolic regulation, while the acidic microenvironment reciprocally accelerates tumor progression and neural invasion. Future research should focus on elucidating the neuro-metabolic-immune network interactions to develop combinatorial therapeutic strategies.
Innervated niche
The interplay between the brain and TME operates through direct neural regulation and remote signaling mediated by neurotransmitters and metabolites, shaping tumor progression and systemic adaptations [105–107]. Developing breast and prostate adenocarcinomas recruit doublecortin-expressing neural progenitor cells from the subventricular zone (a neurogenic niche in the brain) [108]. These progenitors migrate through locally permeabilized BBB into circulation, home to tumors, and differentiate into adrenergic neurons that drive tumor progression [109, 110]. Similarly, lung adenocarcinomas are infiltrated by doublecortin neural cells exhibiting adrenergic properties linked to tumor progression and chemoresistance [111]. In addition to peripheral tumors, neurotransmitters and metabolites released through brain-body interactions also regulate the brain tumor microenvironment. Similar to the synapses formed between neurons and oligodendrocyte precursor cells in the healthy brain, neuron-glioma synaptic interactions are mediated by calcium-permeable AMPA receptors [25, 112]. Whole-cell patch-clamp electrophysiological studies in glioma cells reveal that a subset (~ 5–10%) of tumor cells within specific regions exhibit glutamatergic, AMPA receptor-mediated synaptic activity [112]. A preclinical model found that Pharmacological inhibition of glioma AMPA receptors or disruption of neuron-glioma gap junctions significantly impedes tumor growth, and enhancing AMPA receptor signaling (e.g., via glutamate reuptake inhibitors) accelerates glioma invasion and angiogenesis [112]. This mechanism recapitulates the adaptive plasticity observed in healthy synapses, underscoring that neuron-glioma interactions can be reinforced through activity-dependent signaling pathways [113]. Targeting these neural mechanisms such as blocking β-adrenergic signaling or intercepting neurotrophic factor activity may disrupt tumor-brain crosstalk, offering novel strategies to combat cancer progression and systemic comorbidities [114–116].
Mechanical microenvironment
The bidirectional regulatory relationship between brain-body crosstalk (i.e., the interplay between the nervous system and systemic bodily systems) and the tumor mechanical microenvironment manifests in two key aspects: 1) neural regulation of mechanical signals within the tumor microenvironment, and 2) biomechanical modulation of neuro-tumor crosstalk [68, 117]. The tumor mechanical microenvironment, comprising ECM, interstitial fluid pressure, and mechanical stresses (e.g., compression, shear forces), directly influences tumor cell behavior through its stiffness and mechanical properties [118]. ECM stiffening activates pro-oncogenic pathways (e.g., integrin-FAK signaling) to upregulate YAP/TAZ expression, promoting tumor invasion and metastasis [119, 120]. The nervous system modulates the tumor mechanical microenvironment through neurotransmitters, hormones, and immune regulation. Sympathetic activation (e.g., under stress) releases NE, which enhances ECM remodeling via β-adrenergic receptors, increasing matrix stiffness (e.g., collagen deposition) and tumor aggressiveness. Conversely, parasympathetic nerves may inhibit matrix metalloproteinases through acetylcholine (ACh), reducing ECM degradation and indirectly altering mechanical properties [26, 70]. Mechanistically, tumor mechanical properties regulate neural innervation and neurotransmitter release via mechanotransduction. ECM stiffening or elevated local pressure activates mechanosensitive ion channels (e.g., Piezo1) in sensory nerve terminals, transmitting pain signals to the CNS and potentially triggering systemic inflammation [121, 122]. Future investigations should employ single-cell sequencing, organoid models and in vivo mechano-imaging to dissect its molecular architecture and therapeutic implications.
Disrupting circadian rhythms
Circadian rhythms, also known as the biological clock, are endogenous, evolutionarily conserved physiological processes that temporally coordinate external environmental changes with internal physiological functions [123–125]. Circadian disruption can promote tumor growth and progression by altering cancer cell biology [126, 127]. Many cancers disrupt circadian rhythms [128, 129]. For example, hepatocellular carcinoma perturbs the circadian system at the suprachiasmatic nucleus (SCN) level, involving alterations in SCN neuropeptides, neuronal activity, Bmal1 expression, glial activation, and oxidative stress [130]. Sympathetic nervous system (SNS) activation via NE and/or epinephrine release is a key regulator of circadian timing [131]. Furthermore, the brain dynamically modulates tumor metastasis through circadian-dependent neural and humoral signaling. Studies reveal that circadian fluctuations in hormones such as testosterone, insulin, and melatonin significantly influence the dynamics of circulating tumor cell (CTC) shedding from primary tumors [132]. In both mice and humans, CTC shedding peaks during rest phases (e.g., nighttime in nocturnal mice or sleep in humans), highlighting circadian regulation of metastatic spread [132]. Understanding circadian-tumor interactions offers novel strategies to optimize chronotherapy (timed treatment delivery) and mitigate cancer-related circadian disruptions, potentially improving therapeutic outcomes and patient quality of life [133].
Brain-body crosstalk facilitates tumor initiation and progression by modulating cancer hallmarks and the TME Investigating tumors through the lens of brain-body crosstalk offers novel insights into uncovering tumor mechanisms and advancing targeted therapeutic strategies.
Brain-body crosstalk: a powerful anti-tumor weapon for the organism
While tumors hijack brain-body crosstalk to establish a homeostasis conducive to their survival and proliferation [134], the host organism simultaneously counteracts tumors through brain-body crosstalk to preserve its own homeostasis from disruption. The crosstalk between the brain and body serves as a powerful weapon for the organism to combat tumors. The brain-body crosstalk is primarily manifested in combating tumors and regulating the body's homeostasis through axes such as the HPA, Hypothalamic-Sympathetic-Adrenal (HSA), etc. (Fig. 4).
Fig. 4.
Brain-body crosstalk is a powerful anti-tumor weapon for the organism. When a tumor hijacks brain-body crosstalk to establish a homeostasis conducive to its survival and proliferation, the host organism simultaneously combats the tumor through brain-body crosstalk to protect its own homeostasis from being disrupted. The crosstalk between the brain and the body is a powerful weapon anti-tumor weapon for the organism. Brain-body crosstalk is mainly manifested as combating tumors and regulating the homeostasis of the body through axes such as HPA and HSA, etc. A HPA/HPG-Tumor axis, upon detecting tumor-derived factors such as IL-6, the brain activates the HPA axis to secrete glucocorticoids, which suppress the NF-κB signaling pathway within tumors, thereby exerting antitumor effects. HPG-Tumor axis, upon detecting tumor-secreted factors such as IL-6, the brain downregulates estrogen ER and AR expression via the HPG axis, thereby exerting antitumor effects; B Neuro-Immune-Tumor Axis, Neural circuits coordinate systemic immune responses to execute multimodal antitumor strategies through neurotransmitter-mediated immunomodulation and neural activity-dependent checkpoint regulation., C Brain-Gut-Tumor Axis, the vagus nerve, functioning as the central conduit of the gut-brain axis, mediates antitumor effects via the cholinergic anti-inflammatory pathway, while gut microbial metabolites such as SCFAs exert direct oncosuppressive activity; D Neuro-Metabolic-Tumor Axis, upon detecting tumor-derived factors such as IL-6, the liver, gut, and pancreas secrete hormones including ghrelin that signal to the brain. This neural interface subsequently mobilizes the ANS and ACh-mediated pathways to execute multimodal antitumor actions; E HAS/SAM-Tumor axis, under tumor conditions, the HSA axis exerts antitumor effects through coordinated neuro-endocrine remodeling, mechanistically linked to elevated BDNF in the central nervous system and suppressed leptin signaling in adipose tissue. Under acute stress, activation of the SMA axis induces adrenaline release that exerts antitumor effects through β2-adrenergic receptor -mediated immune and metabolic reprogramming. F Neuro-Endocrine-Tumor Axis, the pituitary gland secretes GH to stimulate muscle synthesis, while skeletal muscle reciprocally releases myokines such as irisin that exhibit multimodal antitumor activity through endocrine-paracrine signaling networks. HPA, hypothalamic–pituitary–adrenal; ACTH, adrenocorticotropic hormone; HPG, hypothalamic-pituitary–gonadal; GnRH, gonadotropin-releasing hormone; ER, estrogen receptor; AR, androgen receptor; HAS, hypothalamic-sympathetic-adrenal; BDNF, brain-derived neurotrophic factor; SCFAs, short-chain fatty acids; ANS, autonomic nervous system; POMC, p-opiomelanocortin; NPY, neuropeptide Y; AgPR, agouti-related peptide; ACh, acetylcholine; GH, growth hormone; PRL, prolactin; SAM, sympathetic-adreno-medullary. This figure was created using BioRender (https://biorender.com/)
HPA-tumor axis
HPA axis plays a role in antitumor effects through complex regulation of stress hormones on the tumor microenvironment, immune system, and tumor cell behavior. Under acute stress conditions, glucocorticoids (e.g., cortisol) released by the HPA axis can suppress the progress of tumor cells via glucocorticoid-mediated inhibition of the NF-κB pathway [135]. This is associated with the HPA axis reducing the release of pro-inflammatory factors (e.g., IL-6, TNF-α), thereby suppressing inflammation-driven tumor progression [136]. Furthermore, glucocorticoids released by the HPA axis may directly regulate tumor cell proliferation and apoptosis by binding to glucocorticoid receptors within tumor cells, thereby modulating the expression of apoptosis-related genes (e.g., Bcl-2, Bax) [137].
HPG-tumor axis
The hypothalamic-pituitary–gonadal (HPG) Axis modulates tumor microenvironment and immune responses through direct and indirect regulation of sex hormone (estrogen/androgen) secretion. Research reveals that in breast cancer, the HPG axis inhibits estrogen receptor (ER) signaling via activation of gonadotropin-releasing hormone (GnRH), thereby suppressing tumor cell proliferation [138]. In prostate cancer, GnRH analogs (e.g., leuprolide) downregulate androgen receptor signaling pathways to induce tumor cell apoptosis [139]. Furthermore, the HPG axis potentiates antitumor immunity, a study found estrogen enhances CD8 + T cell glycolytic capacity through ER-β signaling (augmenting IFN-γ secretion), which amplifies T cell-mediated antitumor activity [140].
HSA-tumor axis
There exists a complex regulatory relationship between brain-body crosstalk and the tumor metabolic microenvironment, which is intricately linked to the fine-tuned regulation by the hypothalamus and SNS [141]. The hypothalamic sympathoneural-adipocyte (HSA) axis links physical/social environments to the regulation of adipose phenotypes, thereby modulating multiple organ systems and ultimately influencing cancer progression. Activation of the HSA axis has been shown to suppress tumor development [142]. In both obese and non-obese animals, HSA axis activation induces significant white adipose tissue atrophy, potentially limiting the pool of functional stromal cells available for tumor recruitment [143]. Furthermore, HSA activation reduces cancer proliferation through sympathetic innervation of adipose tissue, leptin suppression, and induction of hypothalamic brain-derived neurotrophic factor (BDNF) expression. Notably, genetic activation of the HSA axis via adeno-associated virus (AAV)-mediated hypothalamic BDNF overexpression resolves hepatic steatosis and demonstrates potential protective effects against liver cancer in preclinical models, suggesting therapeutic promise for metabolic dysfunction-associated malignancies [143]. This integrative axis underscores the brain’s capacity to coordinate systemic metabolic and neural adaptations that counteract oncogenic processes.
SAM-tumor axis
The antitumor effects of the sympathetic-adreno-medullary (SAM) axis exhibit significant context dependency. Short-term stress-induced activation of the SAM axis can inhibit tumor cell proliferation and survival directly while mobilizing immune responses to exert antitumor effects, whereas chronic activation is more commonly associated with tumor promotion [9]. Here, we focus on its antitumor effects. Acute activation of the SAM axis directly suppresses tumor cell proliferation and survival. Studies demonstrate that epinephrine activates the β2-AR/cAMP/PKA pathway, inhibiting Cyclin D1 and upregulating p21, thereby blocking the G1/S phase transition in prostate cancer cells [9]. NE induces ROS generation in hepatocellular carcinoma cells via β3-AR, activating BAX/BAK-dependent mitochondrial membrane permeability transition and apoptosis [144]. Short-term SAM axis activation enhances immune-mediated tumor killing. Epinephrine upregulates natural killer (NK) cell surface activating receptors (e.g., NKG2D) and promotes granzyme B release via β2-AR, amplifying tumor cell cytotoxicity [145]. SAM axis activation improves chemotherapy sensitivity. NE enhances cisplatin-induced DNA double-strand breaks in ovarian cancer cells by increasing ROS levels via β-AR signaling [146]. Research on the antitumor effects of the SAM axis remains in its early stages. Future studies should explore targeted interventions to modulate SAM axis activity for tumor suppression, emphasizing strategies that harness its acute immunostimulatory and pro-apoptotic effects while avoiding chronic activation-related protumorigenic outcomes.
Brain-gut-tumor axis
Current research on the role of the"brain-gut-tumor axis"in anti-tumor effects primarily focuses on the interactions among the gut microbiota, CNS, and tumor microenvironment. The gut microbiota influences systemic immune responses in the host through metabolites (such as short-chain fatty acids [SCFAs] and tryptophan metabolites) and immune-modulating molecules, particularly enhancing the activity of CD8 + T cells and natural NK cells, thereby improving the efficacy of immune checkpoint inhibitors like PD-1/PD-L1 inhibitors [147]. The vagus nerve plays a critical role in the brain-gut-tumor axis by suppressing tumor-associated inflammation via the cholinergic anti-inflammatory pathway (CAP), which reduces the release of pro-tumor cytokines such as TNF-α and IL-6 [148]. Studies demonstrate that vagus nerve stimulation (VNS) reduces tumor growth in colorectal cancer mouse models and its activation inhibits gut inflammation-related tumors through the α7 nicotinic ACh receptor [148]. Additionally, gut microbiota-derived metabolites exhibit direct anti-tumor effects. For instance, short-chain fatty acids (SCFAs, e.g., butyrate) enhance anti-tumor immunity via epigenetic regulation (e.g., histone deacetylase inhibition) and directly induce tumor cell apoptosis [149]. The brain-gut-tumor axis exerts anti-tumor effects through multiple pathways, including immune modulation, neural signaling, metabolites, and stress responses. Future research should further unravel the molecular mechanisms underlying microbial-host interactions and explore therapeutic strategies targeting this axis, such as probiotics, VNS, or psychological interventions.
Neuro-immune-tumor axis
The neuro-immune-tumor axis is a cutting-edge field in cancer research, revealing complex interactions among the nervous system, immune system, and tumor microenvironment [150, 151]. The parasympathetic nervous system enhances anti-tumor immunity through neurotransmitter secretion. Studies have shown that parasympathetic activation releases ACh, which binds to the α7 nicotinic ACh receptor (α7nAChR) to promote anti-tumor immune responses, such as increasing CD8 + T cell infiltration and suppressing the pro-tumorigenic phenotype of tumor-associated macrophages (TAMs) [152]. Additionally, studies have shown that electroacupuncture enhances anti-tumor immunity in mice with breast tumors by activating the vagus nerve to regulate inflammatory cytokines [153]. The neuro-immune-tumor axis influences anti-tumor immunity through multifaceted regulation of immune cell function, tumor microenvironment remodeling, and metabolic reprogramming. Targeting this axis with interventions such as β-blockers or neuromodulation holds promise as a novel direction for cancer immunotherapy.
Neuro-endocrine-tumor axis
The neuro-endocrine-tumor axis regulates tumor initiation, progression, and immune evasion through synergistic interactions between the nervous and endocrine systems. Its core mechanisms include neurotransmitter-hormone signaling and stress pathway modulation. Neurotransmitters and hormones exhibit direct anti-tumor effects. Studies have shown that Ach suppresses tumor cell proliferation via the α7 nicotinic α7nAChR and reduces the release of pro-inflammatory cytokines (e.g., IL-6, TNF-α) in lung cancer [154]. In addition, as the largest secretory organ in the human body, muscles secrete irisin, which has anti-tumor effects [155]. The neuro-endocrine-tumor axis exerts a double-edged sword effect on tumor progression—either promoting or suppressing tumors depending on the microenvironmental context and intervention timing.
Neuro-metabolic-tumor axis
The neuro-metabolic-tumor axis influences tumor cell energy acquisition, proliferation, and immune evasion through neural regulation of metabolic pathways. Its core mechanisms include neurotransmitter-driven metabolic reprogramming, metabolite-neural signaling feedback, and metabolic-immune crosstalk. Neurotransmitters can regulate tumor metabolism, a study found that parasympathetic neurotransmitter acetylcholine suppresses tumor glutamine metabolism by inhibiting the PI3K/Akt/mTOR pathway via α7nAChR, thereby blocking nucleotide synthesis.
Metabolite can mediate neuro-tumor interaction, studies found that ketogenic diet and ketone bodies enhance the anti-cancer efficacy of PD-1 blockade [156]. Vagal nerve signaling modulates the levels of metabolic intermediates (e.g., succinate, lactate), thereby altering the energy metabolism patterns of tumor cells. Studies have revealed that VNS inhibits the activity of mitochondrial succinate dehydrogenase, reduces hypoxia-induced succinate accumulation and consequently suppresses HIF-1α-mediated VEGF-driven angiogenesis and immunosuppression [157]. Additionally, metabolic reprogramming also contributes to anti-tumor effects, a study found leptin secreted by adipocytes promotes colorectal carcinoma migration via the JAK2/STAT3 pathway, highlighting leptin as a potential therapeutic target [158]. The neuro-metabolic-tumor axis bidirectionally modulates tumor progression through metabolic reprogramming and immune-metabolic interactions, with mechanisms highly dependent on microenvironmental context. Future studies should integrate metabolomics and single-cell neural tracing technologies to resolve spatial heterogeneity in intratumoral neuro-metabolic networks; while developing metabolic-neural combination therapies (e.g., lactate inhibitors combined with VNS).
Brain-body crosstalk addresses unresolved questions in tumor research
There are still many unresolved questions in cancer research and exploring from the perspective of brain-body crosstalk may offer some directions for addressing these issues. Clinical observations show that tumors can recur for years or even decades after primary tumor resection. This is largely attributed to cellular dormancy, where residual cancer cells enter a metabolically quiescent state due to ECM remodeling, tissue oxidative stress, and endoplasmic reticulum stress [159]. These dormant cells are typically resistant to chemotherapy, radiotherapy, and targeted therapies, making them a major obstacle in cancer eradication [160]. Whether this dormancy is controlled by the brain is a question worth studying. For example, is dormancy dynamically controlled by the central nervous system (e.g., via the hypothalamus-autonomic axis)? Do stress hormones like NE reactivate dormant cells through β-AR signaling? Does the brain influence dormant cell energy sensing and reactivation by modulating systemic metabolism (e.g., insulin/leptin signaling)? Research in this field will unravel the molecular mechanisms of brain-dormant cell interactions, offering novel therapeutic targets to prevent tumor recurrence. A critical question arises after primary tumor resection or treatment: do aberrantly activated brain circuits revert to a"pre-cancer"state? If so, how long does this recovery take, and what factors influence the process? In breast cancer, fatigue, and sleep disturbances may persist long after tumor removal and cessation of therapy [161]. However, the mechanisms underlying these sustained neural alterations remain unknown. Future studies leveraging animal models of cancer survivorship will be invaluable for addressing these questions, offering insights into neuroplasticity, stress axis recalibration, and the long-term interplay between residual tumor biology and CNS function [162].
There exists a sophisticated bidirectional interplay between tumors and the brain (and the systemic nervous system). The nervous system not only regulates tumor growth, metastasis, and drug resistance, but tumors themselves also influence brain function and systemic neural activity through multiple pathways. Brain-body crosstalk in the tumor state reveals that cancer is not merely a localized disease but a systemic disorder. Targeting this brain-body crosstalk offers novel therapeutic avenues for cancer treatment.
Brain-body crosstalk: novel anti-tumor strategies
Cancer neuroscience is a rapidly advancing discipline marked by emerging and exciting discoveries that hold potential to influence or even fundamentally transform cancer therapeutics [163–165]. Within the framework of brain-body crosstalk, targeting bidirectional neuro-tumor crosstalk may slow tumor growth or even reverse it while preserving or restoring quality of life and neural function. Recent studies highlight the clinical relevance of early histological documentation of intratumoral nerve fibers, demonstrating that increased innervation correlates with poorer patient prognosis [166, 167]. A growing body of preclinical and clinical research now shows that modulating the nervous system impacts the growth and spread of solid tumors in vivo as well as their response to therapy [27, 168–170]. Researchers are focusing on the tumor neural microenvironment, investigating how brain-body interactions become dysregulated in cancer and exploring strategies to control these pathways to block tumor progression (Table 1) (Fig. 5).
Table 1.
Tools for studying and manipulating neural innervation in brain-body-tumor crosstalk
| Tool | Mechanism of action | Application | Ref |
|---|---|---|---|
| Gene-edited mice | |||
| Advillin-Cre transgenic mouse | Peripheral sensory nerve-specific Cre recombinase (not expressed in CNS) | Enables specific deletion of genes in sensory nerves, or nerve depletion when crossed with DTA or DTR knock-in mice | [244, 245] |
| Ngf knock-in mouse | Ngf gene preceded by a stop codon inserted into the ROSA26 locus | When crossed to a Cre-expressing mouse strain (e.g. epithelial or stromal expressed Cre), increases NGF production and tissue innervation | [49, 246] |
| Nav1.8 Cre knock-in mouse | Express Cre recombinase from the endogenous sodium channel Scn10a locus | Enables selective deletion of genes in nociceptive nerves, or nociceptive neuron depletion when crossed with DTA or DTR knock-in mice | [247] |
| TrkAF592A or TrkBF616A knock-in mice | Trk knock-in alleles with modified kinase domains allowing pharmacological inhibition with nanomolar concentrations of the ligands 1NMPP1 or 1NaPP1 | Temporal pharmacological inhibition of nerve recruitment in the TME | [110, 248] |
| Trkb-CreERT2 knock-in mouse | Tamoxifen-inducible sensory nerve-specific Cre recombinase | Enables temporal control over gene expression specifically in sensory nerves or nerve depletion when crossed with DTA or DTR knock-in mice | [249] |
| Neurotoxin | |||
| 6-hydroxy dopamine (6OHDA) |
Polar monoamine mimetic that is taken up in the periphery by post-synaptic adrenergic nerves causing free radical destruction of nerve terminals |
When given neonatally, it induces permanent global sympathectomy (as well as off-target CNS effects), whereas in the adult, it only causes temporary peripheral sympathectomy (as it is unable to cross the BBB) | [26, 70, 250, 251] |
| RTX | A super agonist of sensory neuron-expressed calcium channel TRPV1, causing calciummediated neuronal death | When given neonatally, it induces permanent global sensory denervation, whereas in the adult, it causes temporary peripheral sensory denervation | [252, 253] |
| Capsaicin | TRPV1 agonist causing cytotoxicity at high doses | Less potent than RTX requiring repeated injections or incorporation into daily mouse feeds to induce lasting sensory denervation | [254] |
| Botulinum toxin |
Bacterium-derived protease that is taken up by endocytosis in the axonal terminal, and which selectively cleaves synaptic proteins preventing acetylcholine release |
When injected into a tissue of interest causes temporary denervation of cholinergic synapses | [255] |
| Novel neural-targeting nanocarrier Lar@NP-OMVs | Capable of modulating neuro-tumor crosstalk through both direct and indirect mechanisms | On the one hand, utilizes the neuron-binding peptide NP41 to precisely target neural structures in the tumor microenvironment (TME) and deliver the Trk inhibitor larotrectinib, blocking nerve growth and perineural invasion. On the other hand, leverages bacterial outer membrane vesicle (OMV) structures to polarize tumor-associated macrophages (TAMs) toward the anti-tumor M1 phenotype, enhancing phagocytic activity and cytokine secretion. In murine pancreatic cancer models, combination therapy with Lar@NP-OMVs and gemcitabine significantly enhances chemotherapeutic efficacy, reducing tumor volume by 70% and extending median survival by 40% compared to gemcitabine alone | [256] |
| Surgical denervation | |||
| Mechanical neurectomy | Mechanical transection of axonal process, or removal of ganglionic cell body | Physical disruption of tissue innervation | [257–259] |
| Genetic vector | |||
| AAV | Replication inactivated viral vector that stably and predictably integrates desired engineered genetic information into neurons with minimal neurotoxicity | When injected peripherally in a tissue of interest, it travels in a retrograde manner to an innervating ganglion with the neuronal cell type selectivity determined by an engineered promoter (e.g. tyrosine hydroxylase for adrenergic specific expression) | [260, 261] |
| Neuro modulation | |||
| Optogenetic ion channels |
Light activated microbial ion channels with high fidelity spatial and temporal activation and inactivation |
When expressed in peripheral nerves in vivo, it enables precise spatiotemporal control of nerve firing and neurotransmitter release | [262] |
| Chemogeneticreceptors | Engineered G-protein coupled receptors activated by biologically inert drugs that modulate neuronal firing via altering intracellular levels of second messengers such as cAMP and calcium | When expressed in nerves in vivo, it enables temporal (and to a degree spatial) control of nerve firing and neurotransmitter release | [261, 263] |
| Long-acting ion channels (NaChBac) | Voltage-gated prokaryotic sodium ion channel with prolonged inactivation kinetics | When expressed in nerves in vivo, it enables increased nerve firing and neurotransmitter release | [260, 264] |
| EMF | Coordinate and regulate the neural activity in the peripheral nervous system and other tissues (including the neural activity in the stomach and heart) to synchronize them with the brain's rhythmic patterns | Inhibits the growth of multiple malignant cell lines while not affecting non-malignant cells | [201, 202] |
| Vagus Nerve Stimulation (VNS) | Activation of the cholinergic anti-inflammatory pathway suppresses pro-inflammatory factors (such as IL-6, TNF-α) in the tumor microenvironment (TME) and enhances the efficacy of immune checkpoint inhibitors | [265] | |
| Neural tracing | |||
| Single neuron—single-cell resolution (Trace-n-seq) | Retrograde axonal tracing from the tissue to their respective ganglia was performed, followed by single-cell isolation and transcriptomic analysis | Molecular characterization of neurons innervating the pancreas and PDAC at single-cell resolution | [266] |
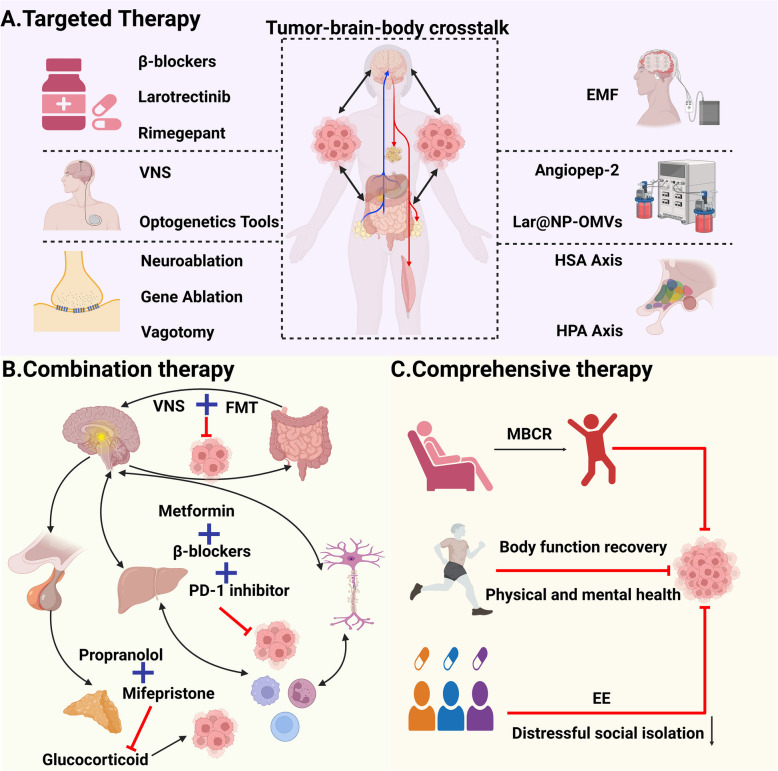
Fig. 5.
Targeting brain-body crosstalk in cancer therapy. Emerging preclinical and clinical evidence has established that neuromodulation critically governs the proliferation, metastatic dissemination, and therapeutic vulnerability of solid tumors. At present, many tumor-targeted treatments start from the tumor-associated neural microenvironment, exploring how the brain-body crosstalk in cancer are dysregulated and exploring strategies to control these pathways to prevent tumor progression, with particular emphasis on: A targeted therapy, B combination therapy, and (C) comprehensive therapy. VNS, vagus nerve stimulation; EMF, electromagnetic field; HAS, hypothalamic-sympathetic-adrenal; HPA, hypothalamic–pituitary–adrenal; FMT, fecal microbiota transplantation; MBCR, mindfulness-based cancer recovery; EE, environmental enrichment. This figure was created using BioRender (https://biorender.com/)
Targeted therapy
From the perspective of brain-body crosstalk in targeting tumors, several repurposed drugs have demonstrated therapeutic efficacy and circadian rhythm-based treatment regimens have shown clinical benefits. However, emerging therapeutic strategies including neuromodulation, neural blockade, neurometabolic interventions, bioelectric therapies, and intelligent delivery systems remain at the stage of animal experimentation or preclinical research.
Drug repurposing
Given that over 100 drugs in neurology, psychiatry, and internal medicine modulate neurotransmitters and other neural signaling components, repurposing one or several of these agents for specific tumor types represents a clinically expedient approach for rapid therapeutic translation [171]. Additionally, novel drugs targeting neuro-tumor signaling pathways are currently under development (Table 2).
Table 2.
Targeting brain-body-tumor crosstalk: current therapeutics and research landscape
| Tumor type | Therapy | Target | Phase | Actual or target accrual | Primary endpoint | Status | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| H3K27M spinal cord glioma | ONC201 | DRD2/DRD3 antagonist | Phase 2 | 84 | Progression-free survival | Terminated | 8 of 12 patients alive at < 12 months median follow-up | NCT02525692 |
| H3K27M diffuse midline glioma | ONC201 | DRD2/DRD3 antagonist | Phase 2 | 95 | Objective response rate | Ongoing | Not reported | NCT03295396 |
| Neuroendocrine tumours | ONC201 | DRD2/DRD3 antagonist | Phase 2 | 30 | Tumour response | Completed | 56% progressed | NCT03034200 |
| Paediatric diffuse intrinsic pontine glioma | ONC201 | DRD2/DRD3 antagonist | Phase 1 | 134 | Recommended phase 2 dose | Terminated | Not reported | NCT03416530 |
| Endometrial cancer | ONC201 | DRD2/DRD3 antagonist | Phase 2 | 27 | Progression-free survival | Terminated | Not reported | NCT03485729 |
| Ovarian, fallopian tube, or primary peritoneal cancer | ONC201 | DRD2/DRD3 antagonist | Phase 2 | 62 | Dose-limiting toxicity, adverse events, objective response rate, progression-free survival | Ongoing | Not reported | NCT04055649 |
| Recurrent and rare CNS tumours | ONC206 | DRD2 antagonist | Phase 1 | 102 | Dose-limiting toxicity | Ongoing | Not reported | NCT04541082 |
| Paediatric high-grade glioma | INCB7839 | ADAM10/17 inhibitor | Phase 1 | 13 | Adverse events | Completed | Not reported | NCT04295759 |
| Glioblastoma | Talampanel | AMPAR regulator | Phase 2 | 72 | Overall survival | Completed | Improved overall survival compared with historical controls | |
| Glioblastoma | Memantine | NMDAR regulator | Phase 2 | 4 | Overall survival | Terminated | Not reported | NCT01260467 |
| Glioma | Talampanel | AMPAR inhibitor | Phase 2 | 30 | Progression-free survival | Terminated | Early termination due to futility | NCT00062504 |
| Ovarian, primary peritoneal, or fallopian tube cancer | Propranolol, chemotherapy | β-adrenergic antagonist | Phase 1 | 32 | Feasibility | Completed | Not reported | NCT01504126 |
| Ovarian cancer | Propranolol | β-adrenergic antagonist | Phase 1 | 24 | Feasibility | Completed | Improved quality of life | NCT01308944 |
| Pancreatic cancer | Propranolol etodolac | β-adrenergic antagonist/COX-2 Inhibitor | Phase 2 | 210 | Recurrence | Ongoing | Not reported | NCT03838029 |
| Pancreatic cancer | Bethanechol | Muscarinic agonist | Phase 1 | 17 | Change in Ki-67 | Ongoing | Not reported | NCT03572283 |
| Cancer pain | Tanezumab | Antibody against NGF | Phase 3 | 156 | Change in pain | Completed | Not reported | |
| Cancer pain | Resiniferatoxin | Capsaicin analogue | Phase 1 | 45 | Safety | Ongoing | Not reported | NCT00804154 |
| Melanoma | Propranolol, pembrolizumab | β-adrenergic antagonist | Phase 1 | 47 | Safety | Completed | No dose-limiting toxicity; objective response rate 78% | NCT03384836 |
| Bladder cancer | Propranolol, pembrolizumab | β-adrenergic antagonist | Phase 2 | 24 | Objective response rate | Ongoing | Not reported | NCT04848519 |
| Gastrointestinal Neuroendocrine Neoplasms | Somatostatin Analogs | Somatostatin Analogs | unknown | 156 | Adverse events | Completed | Not reported | NCT02788565 |
| Prostate cancer | Carvedilol | β-adrenergic antagonist | Phase 2 | 22 | Changes in serum PSA | Ongoing | Not reported | NCT02944201 |
Beta-blockers can inhibit the adrenal gland's tumor-promoting effects [172]. Numerous studies have demonstrated that β-adrenergic blockade downregulates biomarkers of tumor cell invasion and reshapes the tumor immune response [173, 174]. For instance, propranolol has been shown to reduce breast cancer recurrence risk, correlating with increased immune cell infiltration in the tumor microenvironment and decreased metastasis-related biomarkers [174]. Moreover, β-blocker-mediated modulation of sympathetic signaling is safe in breast cancer patients and demonstrates good tolerability when combined with neoadjuvant chemotherapy [174]. Additionally, there are still some drugs currently undergoing preclinical research or animal studies. For example, tropomyosin receptor kinase A (TrkA) inhibitors targeting the nerve growth factor (NGF) pathway (e.g., larotrectinib) can block the NGF/TrkA axis, thereby reducing neural invasion and pain in cancer [175, 176]. Preclinical research revealed that rimegepant, a CGRP-blocking migraine medication, reduces cancer cell autophagy and enhances the efficacy of anti-angiogenic agents and other nutrient-deprivation therapies [100]. Notably, certain antipsychotic agents have demonstrated antitumor efficacy through mechanisms involving brain-body crosstalk. A study on olanzapine revealed that this antipsychotic agent mitigates chronic stress-induced tumorigenesis and chemoresistance. Mechanistic investigations further demonstrated that olanzapine suppresses the medial prefrontal cortex-NE-CLOCK neuroendocrine axis, effectively reversing stress-associated anxiety-like behaviors and attenuating lung cancer stemness characteristics [177].
Circadian rhythm regulation
Current research explores circadian rhythm-based optimization strategies for cancer therapy [126, 178]. Chronotherapy is a treatment approach that leverages circadian rhythms to optimize therapeutic effects on tumors while minimizing adverse impacts on healthy tissues [179]. For example, timing chemotherapy and immunotherapy to specific circadian phases improves efficacy. A study found anti-PD-1 antibodies administered in the evening demonstrate enhanced efficacy due to minimal PD-1 expression and peak functional activity of CD8 + T cells at this time [180]. Similarly, evening infusion of CAR-T cells reduces tumor burden by over 50% in mice [180]. Chronomodulated administration of conventional drugs (e.g., platinum-based agents) also reduces toxicity to normal cells. In colorectal cancer patients, nighttime oxaliplatin delivery significantly decreases neurotoxicity and improves tolerability [180]. Light therapy and photoperiod adjustment can restore rhythmic expression of clock genes (e.g., Bmal1, Per). In liver cancer models, normalized light exposure suppresses metastasis and reverses oncogene expression [181]. Some scholars tried to use melatonin to interfere with the biological clock [182]. Research has demonstrated that melatonin exerts antitumor effects in various types of tumors, suggesting that melatonin interventions targeting circadian rhythms represent a potential anticancer strategy [183–185]. Recent research has focused on circadian clock-targeted therapies. Studies have revealed that REV-ERB agonists can remodel the tumor microenvironment, inhibit cell cycle progression, and induce apoptosis [186]. These advances highlight the need for personalized cancer therapies tailored to patients’ chronotypes (e.g.,"morning"or"evening"types).
Neuromodulation
Neuromodulation technologies employ pulsed pharmacological stimulation, optogenetics, and acoustic methods to reset target states and regulate organ-level stress responses [187]. A prospective, randomized, phase II clinical trial study found activation of the CAP via VNS can suppress pro-inflammatory factors (e.g., IL-6, TNF-α) within the TME, enhancing the efficacy of immune checkpoint inhibitors [188]. Additionally, an animal study has shown that VNS enhances the expression of trefoil factor 2 (TFF2). TFF2, an anti-inflammatory peptide released by the spleen, has been demonstrated to suppress the proliferation and expansion of MDSC-associated bone marrow progenitor cells when overexpressed [189]. Furthermore, some emerging studies are exploring interventions targeting the brain-tumor neural circuits. For example, optogenetic activation of brain regions such as the paraventricular nucleus of the hypothalamus may influence tumor angiogenesis [190]. Future advancements hold promise for manipulating neurotransmitter signaling such as glutamate, glycine, ACh, gamma-aminobutyric acid, and dopamine to reprogram the TME, thereby inhibiting tumor growth or even reversing malignancy by converting cancer cells into normal phenotypes [191–195]. While this emerging field shows immense potential, future work must focus on elucidating the role of neurotransmitters in mediating long-term growth control signals. Which better understand the limitations of current"microenvironment-centric"approaches and develop strategies to manipulate endogenous morphogenetic cues for cancer prevention and normalization.
Nerve blockade
Neural blockade in cancer treatment primarily involves attenuating tumor-induced pain or controlling tumor progression by interrupting neural activity. A study found chemical or surgical ablation of adrenergic (sympathetic) nerves in the mouse prostate halted the growth of patient-derived orthotopic prostate cancer xenografts [26]. Moreover, in carcinogen-induced gastric cancer rat models, vagotomy (surgical severing of the vagus nerve) reduced gastric tumor number and size [196]. These findings underscore the therapeutic potential of targeting tumor-associated neural circuits.
Neurometabolic intervention
Within the framework of brain-tumor crosstalk, neuro-metabolic dysregulation emerges as a critical driver of tumor progression. Correcting these disturbances can suppress tumor growth by targeting metabolic reprogramming and neural signaling. A study found that blocking monocarboxylate transporter 4 (MCT4) inhibits lactate shuttling between tumors and neurons, disrupting their energy symbiosis. For example, syrosingopine, an MCT4 inhibitor, effectively suppresses glioma progression by accumulating intracellular lactate, lowering pH, and inducing apoptosis in preclinical models [197]. In addition, a study reported a close connection between high-fat diet (HFD) intake and the β-adrenergic signaling pathway and the growth of colorectal cancer. This study found that HFD feeding increased the expression of β2AR in xenograft tissues of colorectal cancer mouse models, and β2AR plays an important role in promoting the proliferation of colorectal cancer cells. Crucially, the study also found that knocking out β2AR eliminated the effect of palmitic acid on increasing the proliferation of colorectal cancer cells [198]. Emerging research reveals intricate mechanisms by which tumors exploit neural and metabolic pathways to support their progression. A study found adrenergic signaling promotes a metabolic shift toward aerobic glycolysis (Warburg effect) to fuel tumor angiogenesis [199]. These findings underscore the brain-tumor axis as a dynamic regulator of cancer metabolism, offering novel strategies to intercept neural-metabolic vulnerabilities in therapy-resistant tumors.
Bioelectric therapy
The human body generates endogenous electromagnetic fields (EMFs) through organs such as the heart, brain, muscles, and bones [200]. These endogenous EMFs are hypothesized to coordinate and synchronize neural activity in the peripheral nervous system and other tissues (including gastric and cardiac systems) with central brain rhythms, facilitating systemic physiological harmony [201]. Attempts to apply EMF for cancer treatment are underway. Extremely low frequency-EMF exposure has been shown to hinder the proliferation of various malignant cell lines (e.g., glioblastoma, breast cancer) while sparing non-malignant cells, suggesting tumor-specific bioeffects [202–204]. Studies show that targeted EMF exposure induces sustained objective radiological tumor responses, improves quality of life and may prolong survival in cancer patients, with no significant adverse effects reported [202, 205–207]. In the future, adopting a network physiology perspective could unravel the regulatory mechanisms governing inter-organ communication and homeostasis, advancing our understanding of EMF-mediated brain-body interactions in cancer states [208]. This integrative approach positions EMF as a non-invasive modality to disrupt tumor-neural symbiosis while preserving physiological integrity. There are other bioelectric therapy strategies under development.
Intelligent delivery system
Recent studies have developed innovative nanotherapeutic strategies to disrupt brain-tumor crosstalk through dual targeting of neural and immune pathways. A study found drug-loaded nanoparticles surface-modified with angiopep-2 peptide target the low-density lipoprotein receptor-related protein 1 on brain capillary endothelial cells. These nanoparticles deliver siRNA to silence HIF-1α in tumors, suppressing angiogenesis and chemoresistance [209]. In addition, one study found a novel nanocarrier Lar@NP-OMVs intervenes in neuro-tumor interactions via dual mechanisms. On the one hand, utilizes the neuron-binding peptide NP41 to precisely target neural structures in the TME and deliver the Trk inhibitor larotrectinib, blocking nerve growth and perineural invasion. On the other hand, leverages bacterial outer membrane vesicle structures to polarize TAMs toward the anti-tumor M1 phenotype, enhancing phagocytic activity and cytokine secretion. In murine pancreatic cancer models, combination therapy with Lar@NP-OMVs and gemcitabine significantly enhances chemotherapeutic efficacy, reducing tumor volume by 70% and extending median survival by 40% compared to gemcitabine alone [210]. Furthermore, a recent study developed a capsaicin-loaded manganese dioxide/bovine serum albumin/polydopamine composite nanoplatform with dual functionality for cancer pain alleviation and tumor therapy, aiming to achieve dual objectives of pain management and immunotherapy [211]. This approach exemplifies the potential of nanotechnology to dismantle the brain-tumor axis while leveraging immune modulation for synergistic anti-cancer effects.
Combined therapy
Multi-drug combination
Therapeutic approaches targeting neuro-tumor crosstalk may serve as monotherapies; however, they are more likely to be employed as radiosensitizers or chemosensitizers to enhance the efficacy of conventional treatments [165, 212, 213]. Disrupting neuro-tumor interactions represents an essential component of effective combinatorial therapeutic strategies. Combination therapy can increase radiosensitization, a study found β-blockers inhibit radiation-induced HIF-1α stabilization, sensitizing hypoxic tumor regions to radiotherapy [214]. Combination therapy can increase chemosensitization, preclinical studies have shown that tricyclic antidepressants can control the growth and spread of glioblastoma or breast tumors when used in combination with conventional chemotherapy [215, 216]. Combination therapy can increase immune synergy, neurotransmitter blockade (e.g., adrenergic or cholinergic inhibitors) reprogram immunosuppressive myeloid cells and enhances dendritic cell antigen presentation [217]. In addition, in metastatic melanoma patients receiving immunotherapy (e.g., anti-PD-1), adjunctive use of non-selective β-blockers (e.g., propranolol) was associated with reduced recurrence rates and improved overall survival [218]. Furthermore, preclinical investigations have revealed that the combination of propranolol-encapsulated liposomes with chemotherapy demonstrates superior therapeutic synergy over monotherapies, achieving 92% volumetric regression of primary tumors and 87% reduction in pulmonary metastases, concomitant with a 4.1-fold increase in tumor-infiltrating memory T lymphocytes [219]. Although combined treatment has many advantages, possible side effects shoud be pay attention. Studies found systemic β-blockade may exacerbate hypotension or bradycardia and localized delivery (e.g., nanoparticle-encapsulated agents) may mitigate off-target effects.
Multi-organ axis
Tumor initiation, progression, and metastasis are influenced not only by the local microenvironment but also by systemic neural, endocrine, and metabolic networks. Brain-organ axes including the HPA axis, SAM axis, and brain-gut axis play pivotal roles in tumor progression by integrating interactions between the central nervous system and peripheral organs [1]. Targeting these multi-axis synergistic regulatory mechanisms has emerged as a novel therapeutic strategy. A study found that neuro-immune-metabolic combined intervention strategies, such as beta-blockers (inhibiting SAM axis) combined with PD-1 inhibitors (enhanced immunity) and metformin (regulating metabolism) showed synergistic antitumor in melanoma models effect [220]. However, the treatment of multi-organ axis presents certain challenges. For instance, cross-axis compensatory pathways may emerge (e.g., HPA axis inhibition may activate SAM axis), necessitating the development of dynamic monitoring technologies (e.g., real-time biosensing) to guide precise interventions. Long-term suppression of sympathetic activity or HPA axis function may induce cardiovascular or metabolic side effects, underscoring the need for rigorous safety evaluation in therapeutic design. Advancing multi-axis targeting therapies from bench to bedside requires integrating systems biology and precision medicine approaches.
Comprehensive therapy
Psychotherapy
Mounting evidence indicates that mental health significantly impacts physical health, particularly within the context of cancer [221]. But much of cancer research focuses on molecular and cellular mechanisms, the impact of social interactions and psychological states remains underexplored. Stress modulation interventions, such as reducing chronic psychological distress, may achieve synergistic therapeutic outcomes in cancer care. Mindfulness-based cancer recovery (MBCR), developed by Linda Carlson and colleagues in 1995, is a specialized adaptation of mindfulness-based stress reduction (MBSR) for cancer patients. MBCR helps patients develop a mindset of challenge and openness to cope with cancer-related difficulties, fostering physical and psychological healing. Research has found that MBCR can effectively reduce the levels of anxiety, depression, and posttraumatic stress disorder in breast tumor patients receiving chemotherapy, reduce cancer-related fatigue, and promote patients'physical and mental health [222–224]. In the future, a controlled study on mindfulness meditation in tumor patients can be carried out to quantitatively analyze the epigenetic mechanism of psychological stress affecting tumor genome instability through the HPA axis. This integrative approach positions psychological resilience as a therapeutic pillar in oncology, bridging mind–body medicine with precision cancer care [225].
Exercise therapy
Physical exercise plays a vital auxiliary role in cancer treatment, exerting benefits across physiological, psychological, and quality-of-life domains [226]. Regular exercise improves neurofunctional impairments in cancer patients and mitigates tumor-associated neurocognitive disorders [227, 228]. For example, exercise protects neurocognition, exercise enhances hippocampal neurogenesis and synaptic plasticity, counteracting chemotherapy-induced cognitive decline ("chemo-brain") [229–232]. Aerobic and resistance training reduce systemic inflammation (e.g., IL-6, TNF-α) and insulin resistance, disrupting tumor-promoting metabolic pathways [233, 234]. Under physician and rehabilitation specialist guidance, cancer patients should incorporate evidence-based exercise into their treatment plans to achieve holistic benefits. Current guidelines recommend that 150 min/week of moderate aerobic activity (e.g., brisk walking) for solid tumors, resistance training 2–3 times/week to combat cachexia in advanced cancers [235]. In the future, need to elucidate the biological interplay between exercise dosage (intensity, frequency, duration) and tumor response mechanisms (e.g., angiogenesis, immune infiltration). As well as develop AI-driven algorithms integrating tumor genomics, treatment regimens, and patient biomarkers to personalize exercise protocols.
Individualized therapy
Notably, unlike distress-inducing stressors such as restraint stress, social isolation, and social conflict, eustress (beneficial stress) exerts inhibitory effects on tumorigenesis and cancer progression [236]. This concept underscores the importance of personalized medicine, advocating for patient-centric approaches that integrate all environmental and psychosocial factors. A study found environmental enrichment (EE) stimulates BDNF in the hypothalamus, activates the hypothalamic HSA axis, thereby suppressing leptin production while enhancing adiponectin secretion, ultimately exerting antitumor effects [237]. Conversely, chronic social isolation primarily activates the SAM axis, elevating circulating NE levels and potentially increasing tumor-released catecholamines, which drive angiogenesis and immune evasion [236]. These findings highlight the necessity of context-dependent therapeutic strategies in brain-tumor crosstalk. Interventions must be tailored to tumor type (e.g., adrenergic receptor expression profiles) and individual psychosocial contexts (e.g., social support networks, stress resilience). However, current research on the tumor-inhibitory effects of eustress remains in the early stages. In the future, the therapeutic effect of combined methods (e.g. EE + immunotherapy) can be tested in models of stress-associated malignancies (e.g., pancreatic, lung cancer).
Other strategies
Emerging gene therapies are being explored within the framework of brain-body interactions to combat cancer. In breast cancer model, NMDAR-knockout mice exhibit significantly prolonged brain metastasis-free survival and reduced metastatic burden [42]. Additionally, preclinical studies are exploring AAV-mediated gene delivery to knock down transient receptor potential vanilloid 1 (TRPV1) channel in dorsal root ganglia, thereby blocking nociceptive signaling that fuels tumor progression.
From an integrative neurobiological and oncological perspective, therapeutic strategies targeting brain-body interaction present intricate multidimensional challenges: Firstly, they require precise elucidation of the dynamic regulatory mechanisms through which central-peripheral neural circuits modulate the tumor microenvironment (e.g., pro-angiogenic effects mediated by sympathetic tone, immune reprogramming induced by vagus nerve activation). Secondly, they demand the development of cross-scale intervention technologies encompassing optogenetics-based spatiotemporal activation of specific neural nuclei, design of closed-loop spinal cord stimulation systems, and construction of HPA axis-targeted drug delivery platforms. Concurrently, the establishment of real-time monitoring frameworks integrating neural signaling, immune responses, and metabolic reprogramming is essential to address the dynamic adaptability of tumor niches.
Targeting brain-body interactions offers novel perspectives for cancer therapy. However, current therapeutic approaches remain limited. Future research must further unravel the molecular mechanisms underlying neuro-tumor crosstalk and pioneer the development of more precise, safe, and effective interventions.
Brain-body crosstalk: exploring the uncharted frontiers of tumors
The initiation, progression, and metastatic spread of tumors induce systemic physiological alterations. As such, tumors represent a novel homeostatic challenge to the host organism. The brain participates in homeostatic sensing, integrating and responding to tumor-driven physiological disruptions through brain-body crosstalk pathways to address these challenges. Current research in this field still faces several critical challenges that demand urgent resolution.
Current limitations
Current research on brain-body crosstalk in the tumor-bearing state still faces numerous unresolved challenges. In terms of mechanism research, the mechanisms underlying brain-body crosstalk in tumor states remain poorly understood. Future studies could employ single-cell sequencing to unravel the spatiotemporal dynamics of the"tumor-neuro-immune triad"and utilize brain-tumor organoid co-culture models to simulate in vivo microenvironmental interactions. As brain-body crosstalk research deepens, cancer may be redefined as a neurological disorder, potentially unlocking novel therapeutic paradigms [9].
In terms of researching models, current models of brain-tumor crosstalk are limited. Now can try to build multi-omics integration platforms, construct databases integrating neuroelectrophysiological, metabolomic, and microbiomic data, leveraging artificial neural networks to identify key molecular modules in neuro-tumor interactions. Preclinical model innovation is also the focus of research. For example, develop humanized neuro-tumor organoid co-cultures to mimic specific pathways (e.g., brain-gut axis) in colorectal cancer metastasis. In addition, hopes to attract interest from computing neuroscientists to develop models of systemic physiological and behavioral changes caused by tumors in the future.
In terms of research technology, early neurobiological research relied on neurosurgeons manually stimulating brain tissue with electrodes to correlate anatomy with behavior. With the innovation of research technology, modern tools like chemogenetics and optogenetics now enable precise manipulation of specific neuronal populations in mice to dissect immune-regulatory neural circuits [238]. Selectively manipulating specific neural types using chemical genetics and optogenetics tools makes it possible to study the effects of neural activity on cancer progression [239, 240]. However, these approaches remain insufficient. In the future, in tumor neurobiology research, it is also necessary to combine dynamic monitoring systems for neural activity, molecular engineering targeted neurotransmitters, neural circuit editing technology, and AI-driven predictive modeling.
In terms of treatment, pay attention to the central side effects of neurotargeted drugs (such as blood pressure fluctuations, cognitive effects) and develop brain-specific delivery technologies (e.g., nanoparticle carriers, focused ultrasound) to minimize systemic toxicity. In addition, there are few effective treatments available at present. In the future, scholars can try to intersect neuroscience with tumor immunology, explore anti-cancer potential of neuromodulation techniques like deep brain stimulation, and identify critical nodes in brain-body interaction networks for precision targeting. Furthermore, most biomedical interventions (e.g., antibiotics, antiparasitics, chemotherapy) target"invaders"(microbes, parasites, cancer cells) and achieve cures by eliminating the threat. However, few therapies induce permanent structural/functional repair in the host. In the tumor state, from the perspective of brain-body crosstalk, how to push the physiological circuit into a stable and healthy state and overcome the limitations of drug resistance and side effects is an issue that needs to be considered in future treatment.
Critical dilemma requiring urgent future resolution
Research on brain-body interaction under tumor conditions remains constrained by several critical dilemma issues that hinder its advancement. The following critical unresolved questions persist in understanding brain-body crosstalk under tumor conditions: (i) whether tumor-induced systemic physiological and behavioral alterations can be detected at every hierarchical level of homeostatic regulation—receptors, central integrators, and effector systems; (ii) whether normalization of homeostatic feedback loops (or malignant tumor elimination) can alleviate physiological and behavioral comorbidities; (iii) whether technological approaches can decode the transmission of peripheral physiological signals into hypothalamic neural coding. Addressing these questions holds profound significance for unraveling how tumors co-opt neurobiological pathways to disrupt systemic homeostasis and developing targeted strategies to restore physiological equilibrium while mitigating cancer-related neurological sequelae.
Transformative paradigm integrating into clinical oncology practice
The precision treatment strategy targeting brain-body interactions in oncology is intrinsically linked to the multidisciplinary team (MDT) model. At the mechanistic level, MDT facilitates the decoding of quantitative mapping relationships between specific central nervous system nuclei (e.g., locus coeruleus and nucleus tractus solitarius) and the spatiotemporal heterogeneity of the tumor immune microenvironment through the integration of neuroimaging genomics and advanced molecular profiling techniques such as spatial transcriptome sequencing [241]. In terms of technological convergence, the MDT framework provides a robust dosimetric optimization platform for spatiotemporally coordinated interventions involving neuromodulatory devices (e.g., closed-loop vagus nerve stimulators) and targeted therapies (e.g., PD-1/PD-L1 inhibitors). At the translational clinical level, MDT enables the establishment of a multimodal decision support system incorporating neurofunctional assessments (e.g., resting-state fMRI connectivity analysis), tumor evolution monitoring (e.g., ctDNA dynamic tracking), and artificial intelligence-driven prognostic modeling [242]. The paradigm of brain-body crosstalk will be profoundly integrated into contemporary clinical oncology practice [243].
Conclusion
The brain-body crosstalk in tumor states reveals the active role of the nervous system in tumor progression, transcending the traditional"local lesion"paradigm. Targeting this neural-tumor network not only offers novel avenues to suppress tumor growth and metastasis but also alleviates cancer-related neurological comorbidities. Furthermore, studying brain-body interactions in cancer extends beyond direct therapeutic outcomes. It deepens our understanding of how tumor-derived signals disrupt systemic physiology and behavior by hijacking brain function. The mechanisms driving these changes are likely not exclusive to cancer, they may also operate in other pathologies (e.g., autoimmune diseases) and environmental contexts. In the future, cross-disciplinary convergence is needed. Through combining precision medicine, neuroengineering technology, and artificial intelligence technology to develop dynamic regulatory strategies in time and space, and ultimately achieve a paradigm transformation from"treating the tumor"to"treating the patient".
Acknowledgements
We apologize to colleagues whose important work could not be cited due to space constraints.
Abbreviations
- CNS
Central nervous system
- TME
Tumor microenvironment
- ECM
Extracellular matrix
- NE
Norepinephrine
- HPA
Hypothalamic–pituitary–adrenal
- ARG1
Arginase 1
- nAChRs
Nicotinic acetylcholine receptors
- α-MSH
α-Melanocyte-stimulating hormone
- CGRP
Calcitonin gene-related peptide
- NTS
Nucleus tractus solitaries
- SCFAs
Short-chain fatty acids
- NPY
Neuropeptide Y
- LGGs
Low-grade gliomas
- PDAC
Pancreatic ductal adenocarcinoma
- β-AR
β-Adrenergic receptor
- PNI
Perineural invasion
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- HIF-1α
Hypoxia-inducible factor-1α
- VEGF
Vascular endothelial growth factor
- BBB
Blood–brain barrier
- MDSC
Myeloid-derived suppressor cell
- TRPV1
Transient receptor potential vanilloid 1
- ACh
Acetylcholine
- TAMs
Tumor-associated macrophages
- SCN
Suprachiasmatic nucleus
- SNS
Sympathetic nervous system
- CTC
Circulating tumor cell
- HSA
Hypothalamic-sympathetic-adrenal
- HPG
Hypothalamic-pituitary–gonadal
- GnRH
Gonadotropin-releasing hormone
- ER
Estrogen receptor
- BDNF
Brain-derived neurotrophic factor
- AAV
Adeno-associated virus
- SAM
Sympathetic-adreno-medullary
- NK
Natural killer
- CAP
Cholinergic anti-inflammatory pathway
- VNS
Vagus nerve stimulation
- α7nAChR
Acetylcholine receptor
- NGF
Nerve growth factor
- TFF2
Trefoil factor 2
- MCT4
Monocarboxylate transporter 4
- HFD
High-fat diet
- EMFs
Electromagnetic fields
- MBCR
Mindfulness-based cancer recovery
- MBSR
Mindfulness-based stress reduction
- EE
Environmental enrichment
- NMDARs
N-methyl-D-aspartate receptor
- MDT
Multidisciplinary team
Authors’ contributions
All authors reviewed the manuscript.WLJ and YFW contributed to the conception and design of the review. YFW and ZKD wrote the initial draft. WLJ and YFW reviewed the manuscript and provided suggestions for revision. All authors read and approved the final manuscript.
Funding
This work was financially supported by the High-Level Talent Introduction Funds from the First Hospital of Lanzhou University.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swanton C, Bernard E, Abbosh C, et al. Embracing cancer complexity: hallmarks of systemic disease. Cell. 2024;187(7):1589–616. 10.1016/j.cell.2024.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borniger JC. Cancer neuroscience at the brain-body interface. Genes Dev. 2024;38(17–20):787–92. 10.1101/gad.352288.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosang L, Flügel A, Odoardi F. Body-brain axis: orchestrating immune responses. Cell Res. 2024;34(11):757–8. 10.1038/s41422-024-01004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin H, Li M, Jeong E, Castro-Martinez F, Zuker CS. A body-brain circuit that regulates body inflammatory responses. Nature. 2024;630(8017):695–703. 10.1038/s41586-024-07469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloan EK. Harnessing brain-body communication to understand cancer. Genes Dev. 2024;38(17–20):820–2. 10.1101/gad.352292.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna S, Choudhury A, Keough MB, et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature. 2023;617(7961):599–607. 10.1038/s41586-023-06036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25. 10.1016/s1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 8.Xiong SY, Wen HZ, Dai LM, et al. A brain-tumor neural circuit controls breast cancer progression in mice. J Clin Invest. 2023;133(24). 10.1172/jci167725. [DOI] [PMC free article] [PubMed]
- 9.Magnon C, Hondermarck H. The neural addiction of cancer. Nat Rev Cancer. 2023;23(5):317–34. 10.1038/s41568-023-00556-8. [DOI] [PubMed] [Google Scholar]
- 10.Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31(3):342–54. 10.1016/j.ccell.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Cross M, Dillin A, Papagiannakopoulos T. Bridging brain and body in cancer. Genes Dev. 2024;38(17–20):814–6. 10.1101/gad.352300.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojo de la Vega M. A holistic view of cancer. Cancer Cell. 2023;41(3):373. 10.1016/j.ccell.2023.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Monje M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell. 2023;41(3):573–80. 10.1016/j.ccell.2023.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921-r925. 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol. 2018;15(1):9–20. 10.1038/s41574-018-0123-0. [DOI] [PubMed] [Google Scholar]
- 17.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901. 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer M, Anthony TG, Ayres JS, et al. Cachexia: a systemic consequence of progressive, unresolved disease. Cell. 2023;186(9):1824–45. 10.1016/j.cell.2023.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–42. 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5(1):166. 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monje M, Borniger JC, D’Silva NJ, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181(2):219–22. 10.1016/j.cell.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379–91. 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 23.Amit M, Na’ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. 2016;16(6):399–408. 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 24.Winkler F, Venkatesh HS, Amit M, et al. Cancer neuroscience: state of the field, emerging directions. Cell. 2023;186(8):1689–707. 10.1016/j.cell.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–45. 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 27.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–52. 10.1158/0008-5472.Can-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McVary KT, Razzaq A, Lee C, Venegas MF, Rademaker A, McKenna KE. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol Reprod. 1994;51(1):99–107. 10.1095/biolreprod51.1.99. [DOI] [PubMed] [Google Scholar]
- 29.Amit M, Anastasaki C, Dantzer R, et al. Next directions in the neuroscience of cancers arising outside the CNS. Cancer Discov. 2024;14(4):669–73. 10.1158/2159-8290.Cd-23-1495. [DOI] [PubMed] [Google Scholar]
- 30.Flores ER, Sawyer WG. Engineering cancer’s end: an interdisciplinary approach to confront the complexities of cancer. Cancer Cell. 2024;42(7):1133–7. 10.1016/j.ccell.2024.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Tetzlaff SK, Reyhan E, Layer N, et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. Cell. 2025;188(2):390-411.e336. 10.1016/j.cell.2024.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang QR, Wang SX, Chen R. Integrated bioelectronic and optogenetic methods to study brain-body circuits. ACS Nano. 2024;18(44):30117–22. 10.1021/acsnano.4c07256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui SX, Pasco JA. Obesity and brain function: the brain-body crosstalk. Medicina (Kaunas). 2020;56(10). 10.3390/medicina56100499. [DOI] [PMC free article] [PubMed]
- 34.Saper CB, Lowell BB. The hypothalamus. Curr Biol. 2014;24(23):R1111-1116. 10.1016/j.cub.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Le Thuc O, García-Cáceres C. Obesity-induced inflammation: connecting the periphery to the brain. Nat Metab. 2024;6(7):1237–52. 10.1038/s42255-024-01079-8. [DOI] [PubMed] [Google Scholar]
- 36.Ceunen E, Vlaeyen JW, Van Diest I. On the origin of interoception. Front Psychol. 2016;7:743. 10.3389/fpsyg.2016.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalsa SS, Adolphs R, Cameron OG, et al. Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(6):501–13. 10.1016/j.bpsc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. 10.1158/2159-8290.Cd-21-1059. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 40.Pan Y, Hysinger JD, Barron T, et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature. 2021;594(7862):277–82. 10.1038/s41586-021-03580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen P, Wang W, Liu R, et al. Olfactory sensory experience regulates gliomagenesis via neuronal IGF1. Nature. 2022;606(7914):550–6. 10.1038/s41586-022-04719-9. [DOI] [PubMed] [Google Scholar]
- 42.Zeng Q, Michael IP, Zhang P, et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573(7775):526–31. 10.1038/s41586-019-1576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269–74. 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell SL, Buckingham SC, Sontheimer H. Human glioma cells induce hyperexcitability in cortical networks. Epilepsia. 2012;53(8):1360–70. 10.1111/j.1528-1167.2012.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saloman JL, Albers KM, Li D, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113(11):3078–83. 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang D, Ma QY, Hu HT, Zhang M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 2010;10(1):19–29. 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- 47.Guo K, Ma Q, Li J, et al. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol Cancer Ther. 2013;12(3):264–73. 10.1158/1535-7163.Mct-12-0809. [DOI] [PubMed] [Google Scholar]
- 48.Kim-Fuchs C, Le CP, Pimentel MA, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40–7. 10.1016/j.bbi.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31(1):21–34. 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayala GE, Dai H, Powell M, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14(23):7593–603. 10.1158/1078-0432.Ccr-08-1164. [DOI] [PubMed] [Google Scholar]
- 51.Huang D, Su S, Cui X, et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine (Baltimore). 2014;93(27):e172. 10.1097/md.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9(6):702–10. 10.1158/2159-8290.Cd-18-1398. [DOI] [PubMed] [Google Scholar]
- 53.Padmanaban V, Keller I, Seltzer ES, Ostendorf BN, Kerner Z, Tavazoie SF. Neuronal substance P drives metastasis through an extracellular RNA-TLR7 axis. Nature. 2024;633(8028):207–15. 10.1038/s41586-024-07767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Kang R, Tang D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun (Lond). 2021;41(8):642–60. 10.1002/cac2.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B, Guo X, Huang L, et al. Tumour-associated macrophages and Schwann cells promote perineural invasion via paracrine loop in pancreatic ductal adenocarcinoma. Br J Cancer. 2024;130(4):542–54. 10.1038/s41416-023-02539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayala GE, Dai H, Ittmann M, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64(17):6082–90. 10.1158/0008-5472.Can-04-0838. [DOI] [PubMed] [Google Scholar]
- 57.Niu Y, Förster S, Muders M. The role of perineural invasion in prostate cancer and its prognostic significance. Cancers (Basel). 2022;14(17). 10.3390/cancers14174065. [DOI] [PMC free article] [PubMed]
- 58.Deborde S, Gusain L, Powers A, et al. Reprogrammed Schwann cells organize into dynamic tracks that promote pancreatic cancer invasion. Cancer Discov. 2022;12(10):2454–73. 10.1158/2159-8290.Cd-21-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bakst RL, Xiong H, Chen CH, et al. Inflammatory monocytes promote perineural invasion via CCL2-mediated recruitment and cathepsin B expression. Cancer Res. 2017;77(22):6400–14. 10.1158/0008-5472.Can-17-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkataramani V, Yang Y, Schubert MC, et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell. 2022;185(16):2899-2917.e2831. 10.1016/j.cell.2022.06.054. [DOI] [PubMed] [Google Scholar]
- 61.Bi Q, Wu JY, Qiu XM, Zhang JD, Sun ZJ, Wang W. Tumor-associated inflammation: the tumor-promoting immunity in the early stages of tumorigenesis. J Immunol Res. 2022;2022:3128933. 10.1155/2022/3128933. [DOI] [PMC free article] [PubMed]
- 62.Parodi A, Kostyushev D, Brezgin S, et al. Biomimetic approaches for targeting tumor-promoting inflammation. Semin Cancer Biol. 2022;86(Pt 2):555–67. 10.1016/j.semcancer.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Guo X, Pan Y, Xiong M, et al. Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat Commun. 2020;11(1):2177. 10.1038/s41467-020-15770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King J, Mir H, Singh S. Association of cytokines and chemokines in pathogenesis of breast cancer. Prog Mol Biol Transl Sci. 2017;151:113–136. 10.1016/bs.pmbts.2017.07.003. [DOI] [PubMed]
- 65.Apostolova P, Pearce EL. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 2022;43(12):969–77. 10.1016/j.it.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Artac M, Altundag K. Leptin and breast cancer: an overview. Med Oncol. 2012;29(3):1510–4. 10.1007/s12032-011-0056-0. [DOI] [PubMed] [Google Scholar]
- 67.Gysler SM, Drapkin R. Tumor innervation: peripheral nerves take control of the tumor microenvironment. J Clin Invest. 2021;131(11). 10.1172/jci147276. [DOI] [PMC free article] [PubMed]
- 68.Makale M. Cellular mechanobiology and cancer metastasis. Birth Defects Res C Embryo Today. 2007;81(4):329–43. 10.1002/bdrc.20110. [DOI] [PubMed] [Google Scholar]
- 69.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zahalka AH, Arnal-Estapé A, Maryanovich M, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358(6361):321–6. 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baratali L, Major K, Rouaud O, Draganski B. Cancer-related cognitive impairment in older adults. Rev Med Suisse. 2020;16(714):2172–5. [PubMed] [Google Scholar]
- 72.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–22. 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Veiga-Fernandes H, Artis D. Neuronal-immune system cross-talk in homeostasis. Science. 2018;359(6383):1465–6. 10.1126/science.aap9598. [DOI] [PubMed] [Google Scholar]
- 74.Godinho-Silva C, Cardoso F, Veiga-Fernandes H. Neuro-Immune cell units: a new paradigm in physiology. Annu Rev Immunol. 2019;37:19–46. 10.1146/annurev-immunol-042718-041812. [DOI] [PubMed] [Google Scholar]
- 75.Huang S, Ziegler CGK, Austin J, et al. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell. 2021;184(2):441-459.e425. 10.1016/j.cell.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang A, Luan HH, Medzhitov R. An evolutionary perspective on immunometabolism. Science. 2019;363(6423). 10.1126/science.aar3932. [DOI] [PMC free article] [PubMed]
- 77.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50. 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hernandez S, Serrano AG, Solis Soto LM. The role of nerve fibers in the tumor immune microenvironment of solid tumors. Adv Biol (Weinh). 2022;6(9):e2200046. 10.1002/adbi.202200046. [DOI] [PubMed] [Google Scholar]
- 79.Qin JF, Jin FJ, Li N, et al. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015;48(5):295–300. 10.5483/bmbrep.2015.48.5.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohammadpour H, MacDonald CR, Qiao G, et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest. 2019;129(12):5537–52. 10.1172/jci129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Q, Cao Y, Kong F, et al. Multiple cancer cell types release LIF and Gal3 to hijack neural signals. Cell Res. 2024;34(5):345–54. 10.1038/s41422-024-00946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guereschi MG, Araujo LP, Maricato JT, et al. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur J Immunol. 2013;43(4):1001–1012. https://doi.org/10.1002/eji.201243005. [DOI] [PubMed] [Google Scholar]
- 83.Capellino S, Claus M, Watzl C. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cell Mol Immunol. 2020;17(7):705–11. 10.1038/s41423-020-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253(4):798–810. 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 85.Fujii T, Mashimo M, Moriwaki Y, et al. Expression and function of the cholinergic system in immune cells. Front Immunol. 2017;8:1085. 10.3389/fimmu.2017.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pfitzinger PL, Fangmann L, Wang K, et al. Indirect cholinergic activation slows down pancreatic cancer growth and tumor-associated inflammation. J Exp Clin Cancer Res. 2020;39(1):289. 10.1186/s13046-020-01796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de la Torre E, Genaro AM, Ribeiro ML, Pagotto R, Pignataro OP, Sales ME. Proliferative actions of muscarinic receptors expressed in macrophages derived from normal and tumor bearing mice. Biochim Biophys Acta. 2008;1782(2):82–9. 10.1016/j.bbadis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Zheng W, Song H, Luo Z, et al. Acetylcholine ameliorates colitis by promoting IL-10 secretion of monocytic myeloid-derived suppressor cells through the nAChR/ERK pathway. Proc Natl Acad Sci U S A. 2021;118(11). 10.1073/pnas.2017762118. [DOI] [PMC free article] [PubMed]
- 89.Zanetti SR, Ziblat A, Torres NI, Zwirner NW, Bouzat C. Expression and functional role of α7 nicotinic receptor in human cytokine-stimulated natural killer (NK) cells. J Biol Chem. 2016;291(32):16541–52. 10.1074/jbc.M115.710574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimring JC, Kapp LM, Yamada M, Wess J, Kapp JA. Regulation of CD8+ cytolytic T lymphocyte differentiation by a cholinergic pathway. J Neuroimmunol. 2005;164(1–2):66–75. 10.1016/j.jneuroim.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 91.Renz BW, Takahashi R, Tanaka T, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;34(5):863–7. 10.1016/j.ccell.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang CC, Marsland M, Wang Y, et al. Tumor innervation is triggered by endoplasmic reticulum stress. Oncogene. 2022;41(4):586–99. 10.1038/s41388-021-02108-6. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Z, Li Y, Lv X, Zhao L, Wang X. VLM catecholaminergic neurons control tumor growth by regulating CD8(+) T cells. Proc Natl Acad Sci U S A. 2021;118(28). 10.1073/pnas.2103505118. [DOI] [PMC free article] [PubMed]
- 94.Xu Y, Yan J, Tao Y, et al. Pituitary hormone α-MSH promotes tumor-induced myelopoiesis and immunosuppression. Science. 2022;377(6610):1085–91. 10.1126/science.abj2674. [DOI] [PubMed] [Google Scholar]
- 95.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200. 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Banh RS, Biancur DE, Yamamoto K, et al. Neurons release serine to support mRNA translation in pancreatic cancer. Cell. 2020;183(5):1202-1218.e1225. 10.1016/j.cell.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–41. 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gu I, Gregory E, Atwood C, Lee SO, Song YH. Exploring the role of metabolites in cancer and the associated nerve crosstalk. Nutrients. 2022;14(9). 10.3390/nu14091722. [DOI] [PMC free article] [PubMed]
- 100.Zhang Y, Lin C, Liu Z, et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 2022;34(12):1999-2017.e1910. 10.1016/j.cmet.2022.10.012. [DOI] [PubMed] [Google Scholar]
- 101.Au CC, Furness JB, Brown KA. Ghrelin and breast cancer: emerging roles in obesity, estrogen regulation, and cancer. Front Oncol. 2016;6:265. 10.3389/fonc.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borniger JC, Walker Ii WH, Surbhi, et al. A role for hypocretin/orexin in metabolic and sleep abnormalities in a mouse model of non-metastatic breast cancer. Cell Metab. 2018;28(1):118–129.e115. 10.1016/j.cmet.2018.04.021. [DOI] [PMC free article] [PubMed]
- 103.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 104.Cui B, Luo Y, Tian P, et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest. 2019;129(3):1030–46. 10.1172/jci121685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fan HY, Liang XH, Tang YL. Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk. MedComm (2020). 2024;5(11):e784. 10.1002/mco2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pundavela J, Roselli S, Faulkner S, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9(8):1626–35. 10.1016/j.molonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reavis HD, Chen HI, Drapkin R. Tumor innervation: cancer has some nerve. Trends Cancer. 2020;6(12):1059–67. 10.1016/j.trecan.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ayanlaja AA, Xiong Y, Gao Y, et al. Distinct features of doublecortin as a marker of neuronal migration and its implications in cancer cell mobility. Front Mol Neurosci. 2017;10:199. 10.3389/fnmol.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mauffrey P, Tchitchek N, Barroca V, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569(7758):672–8. 10.1038/s41586-019-1219-y. [DOI] [PubMed] [Google Scholar]
- 110.Allen JK, Armaiz-Pena GN, Nagaraja AS, et al. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res. 2018;78(12):3233–42. 10.1158/0008-5472.Can-16-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Otani Y, Katayama H, Zhu Y, et al. Adrenergic microenvironment driven by cancer-associated Schwann cells contributes to chemoresistance in patients with lung cancer. Cancer Sci. 2024;115(7):2333–45. 10.1111/cas.16164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532–8. 10.1038/s41586-019-1564-x. [DOI] [PubMed] [Google Scholar]
- 113.Taylor KR, Barron T, Hui A, et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature. 2023;623(7986):366–74. 10.1038/s41586-023-06678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63(11):1115–28. 10.1007/s00262-014-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le TT, Oudin MJ. Understanding and modeling nerve-cancer interactions. Dis Model Mech. 2023;16(1). 10.1242/dmm.049729. [DOI] [PMC free article] [PubMed]
- 116.Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 2021;81(6):1431–40. 10.1158/0008-5472.Can-20-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang SH, Zhang S, Cai Z, et al. Mechanical cues of extracellular matrix determine tumor innervation. bioRxiv. 2024:2024.2003. 2025.586535.
- 118.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–82. 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 119.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–53. 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Northey JJ, Przybyla L, Weaver VM. Tissue force programs cell fate and tumor aggression. Cancer Discov. 2017;7(11):1224–37. 10.1158/2159-8290.Cd-16-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 122.Deborde S, Omelchenko T, Lyubchik A, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126(4):1538–54. 10.1172/jci82658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sancar G, Brunner M. Circadian clocks and energy metabolism. Cell Mol Life Sci. 2014;71(14):2667–80. 10.1007/s00018-014-1574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–20. 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 125.Delaunay F, Laudet V. Circadian clock and microarrays: mammalian genome gets rhythm. Trends Genet. 2002;18(12):595–7. 10.1016/s0168-9525(02)02794-4. [DOI] [PubMed] [Google Scholar]
- 126.Amidi A, Wu LM. Circadian disruption and cancer- and treatment-related symptoms. Front Oncol. 2022;12:1009064. 10.3389/fonc.2022.1009064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen P, Hsu WH, Chang A, et al. Circadian regulator CLOCK recruits immune-suppressive microglia into the GBM tumor microenvironment. Cancer Discov. 2020;10(3):371–81. 10.1158/2159-8290.Cd-19-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shilts J, Chen G, Hughey JJ. Evidence for widespread dysregulation of circadian clock progression in human cancer. PeerJ. 2018;6:e4327. 10.7717/peerj.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17(5):321–8. 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 130.Yassine M, Hassan SA, Yücel LA, et al. Hepatocellular carcinoma in mice affects neuronal activity and glia cells in the suprachiasmatic nucleus. Biomedicines. 2024;12(10). 10.3390/biomedicines12102202. [DOI] [PMC free article] [PubMed]
- 131.Terazono H, Mutoh T, Yamaguchi S, et al. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci U S A. 2003;100(11):6795–800. 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diamantopoulou Z, Castro-Giner F, Schwab FD, et al. The metastatic spread of breast cancer accelerates during sleep. Nature. 2022;607(7917):156–62. 10.1038/s41586-022-04875-y. [DOI] [PubMed] [Google Scholar]
- 133.Gomez A, Wu Y, Zhang C, et al. A brain-body feedback loop driving HPA-axis dysfunction in breast cancer. bioRxiv. 2024:2024.2009. 2013.612923.
- 134.Prillaman M. How cancer hijacks the nervous system to grow and spread. Nature. 2024;626(7997):22–4. 10.1038/d41586-024-00240-3. [DOI] [PubMed] [Google Scholar]
- 135.Pufall MA. Glucocorticoids and cancer. Adv Exp Med Biol. 2015;872:315–333. 10.1007/978-1-4939-2895-8_14. [DOI] [PMC free article] [PubMed]
- 136.Dhabhar FS. Psychological stress and immunoprotection versus immunopathology in the skin. Clin Dermatol. 2013;31(1):18–30. 10.1016/j.clindermatol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 137.Kassi E, Moutsatsou P. Glucocorticoid receptor signaling and prostate cancer. Cancer Lett. 2011;302(1):1–10. 10.1016/j.canlet.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 138.Early Breast Cancer Trialists’ Collaborative G. Postmastectomy radiotherapy in patients with breast cancer - authors’ reply. Lancet. 2014;384(9957):1846–7. 10.1016/s0140-6736(14)62240-6. [DOI] [PubMed] [Google Scholar]
- 139.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29(27):3651–8. 10.1200/jco.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 140.Rothenberger NJ, Somasundaram A, Stabile LP. The role of the estrogen pathway in the tumor microenvironment. Int J Mol Sci. 2018;19(2). 10.3390/ijms19020611. [DOI] [PMC free article] [PubMed]
- 141.Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013. 30 Suppl(Suppl):S19–25.dio: 10.1016/j.bbi.2012.06.00510.1016/j.bbi.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117(9):1788–99. 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- 143.Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15(4):447–54. 10.1038/nm.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Calvani M, Subbiani A, Vignoli M, Favre C. Spotlight on ROS and β3-adrenoreceptors fighting in cancer cells. Oxid Med Cell Longev. 2019;2019:6346529. 10.1155/2019/6346529. [DOI] [PMC free article] [PubMed]
- 145.Thapa S, Cao X. Nervous regulation: beta-2-adrenergic signaling in immune homeostasis, cancer immunotherapy, and autoimmune diseases. Cancer Immunol Immunother. 2023;72(8):2549–56. 10.1007/s00262-023-03445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lamboy-Caraballo R, Ortiz-Sanchez C, Acevedo-Santiago A, Matta J, A NAM, G NA-P. Norepinephrine-induced DNA damage in ovarian cancer cells. Int J Mol Sci. 2020;21(6). 10.3390/ijms21062250. [DOI] [PMC free article] [PubMed]
- 147.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62(8):1214–22. 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391). 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed]
- 150.Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477–504. 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Khanmammadova N, Islam S, Sharma P, Amit M. Neuro-immune interactions and immuno-oncology. Trends Cancer. 2023;9(8):636–49. 10.1016/j.trecan.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9(6):418–28. 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang Z, Yu Q, Zhang X, et al. Electroacupuncture regulates inflammatory cytokines by activating the vagus nerve to enhance antitumor immunity in mice with breast tumors. Life Sci. 2021;272:119259. 10.1016/j.lfs.2021.119259. [DOI] [PubMed] [Google Scholar]
- 154.Zhang C, Ding XP, Zhao QN, et al. Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget. 2016;7(37):59199–208. 10.18632/oncotarget.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alshanqiti KH, Alomar SF, Alzoman N, Almomen A. Irisin induces apoptosis in metastatic prostate cancer cells and inhibits tumor growth in vivo. Cancers (Basel). 2023;15(15). 10.3390/cancers15154000. [DOI] [PMC free article] [PubMed]
- 156.Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6(2). 10.1172/jci.insight.145207. [DOI] [PMC free article] [PubMed]
- 157.Mills EL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457-470.e413. 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang D, Chen J, Chen H, et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci. 2012;37(1):91–101. 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- 159.Endo H, Inoue M. Dormancy in cancer. Cancer Sci. 2019;110(2):474–80. 10.1111/cas.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–46. 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ruiz-Casado A, Álvarez-Bustos A, de Pedro CG, Méndez-Otero M, Romero-Elías M. Cancer-related fatigue in breast cancer survivors: a review. Clin Breast Cancer. 2021;21(1):10–25. 10.1016/j.clbc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 162.Otto LD, Russart KLG, Kulkarni P, McTigue DM, Ferris CF, Pyter LM. Paclitaxel chemotherapy elicits widespread brain anisotropy changes in a comprehensive mouse model of breast cancer survivorship: evidence from in vivo diffusion weighted imaging. Front Oncol. 2022;12:798704. 10.3389/fonc.2022.798704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi DD, Guo JA, Hoffman HI, et al. Therapeutic avenues for cancer neuroscience: translational frontiers and clinical opportunities. Lancet Oncol. 2022;23(2):e62–74. 10.1016/s1470-2045(21)00596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Demir IE, Mota Reyes C, Alrawashdeh W, et al. Future directions in preclinical and translational cancer neuroscience research. Nat Cancer. 2021;1:1027–31. 10.1038/s43018-020-00146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Venkataramani V, Schneider M, Giordano FA, et al. Disconnecting multicellular networks in brain tumours. Nat Rev Cancer. 2022;22(8):481–91. 10.1038/s41568-022-00475-0. [DOI] [PubMed] [Google Scholar]
- 166.Francis N, Borniger JC. Cancer as a homeostatic challenge: the role of the hypothalamus. Trends Neurosci. 2021;44(11):903–14. 10.1016/j.tins.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li D, Hu LN, Zheng SM, et al. High nerve density in breast cancer is associated with poor patient outcome. FASEB Bioadv. 2022;4(6):391–401. 10.1096/fba.2021-00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–44. 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 169.Le CP, Nowell CJ, Kim-Fuchs C, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chang A, Botteri E, Gillis RD, et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci Transl Med. 2023;15(693):eadf1147. 10.1126/scitranslmed.adf1147. [DOI] [PubMed] [Google Scholar]
- 171.Xia Y, Sun M, Huang H, Jin WL. Drug repurposing for cancer therapy. Signal Transduct Target Ther. 2024;9(1):92. 10.1038/s41392-024-01808-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dong ZK, Wang YF, Li WP, Jin WL. Neurobiology of cancer: adrenergic signaling and drug repurposing. Pharmacol Ther. 2024;264:108750. 10.1016/j.pharmthera.2024.108750. [DOI] [PubMed] [Google Scholar]
- 173.Gandhi S, Pandey MR, Attwood K, et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res. 2021;27(1):87–95. 10.1158/1078-0432.Ccr-20-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hiller JG, Cole SW, Crone EM, et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res. 2020;26(8):1803–11. 10.1158/1078-0432.Ccr-19-2641. [DOI] [PubMed] [Google Scholar]
- 175.Yin T, Wang G, Wang L, et al. Breaking NGF-TrkA immunosuppression in melanoma sensitizes immunotherapy for durable memory T cell protection. Nat Immunol. 2024;25(2):268–81. 10.1038/s41590-023-01723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hou SM, Cheng CY, Chen WL, Chang EM, Lin CY. NGF-TrkA axis enhances PDGF-C-mediated angiogenesis in osteosarcoma via miR-29b-3p suppression: a potential therapeutic strategy using larotrectinib. Life (Basel). 2025;15(1). 10.3390/life15010099. [DOI] [PMC free article] [PubMed]
- 177.Lu J, Zhang X, Su K, et al. Olanzapine suppresses mPFC activity-norepinephrine releasing to alleviate CLOCK-enhanced cancer stemness under chronic stress. Cell Commun Signal. 2024;22(1):375. 10.1186/s12964-024-01747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fu L, Kettner NM. The circadian clock in cancer development and therapy. Prog Mol Biol Transl Sci. 2013;119:221–282. 10.1016/b978-0-12-396971-2.00009-9. [DOI] [PMC free article] [PubMed]
- 179.Shuboni-Mulligan DD, Breton G, Smart D, Gilbert M, Armstrong TS. Radiation chronotherapy-clinical impact of treatment time-of-day: a systematic review. J Neurooncol. 2019;145(3):415–27. 10.1007/s11060-019-03332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang C, Zeng Q, Gül ZM, et al. Circadian tumor infiltration and function of CD8(+) T cells dictate immunotherapy efficacy. Cell. 2024;187(11):2690-2702.e2617. 10.1016/j.cell.2024.04.015. [DOI] [PubMed] [Google Scholar]
- 181.Padilla J, Osman NM, Bissig-Choisat B, et al. Circadian dysfunction induces NAFLD-related human liver cancer in a mouse model. J Hepatol. 2024;80(2):282–92. 10.1016/j.jhep.2023.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reiter RJ, Sharma R, Ma Q, Rorsales-Corral S, de Almeida Chuffa LG. Melatonin inhibits Warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: a mechanistic hypothesis. Cell Mol Life Sci. 2020;77(13):2527–42. 10.1007/s00018-019-03438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2(2):113–32. 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 184.Florido J, Martinez-Ruiz L, Rodriguez-Santana C, et al. Melatonin drives apoptosis in head and neck cancer by increasing mitochondrial ROS generated via reverse electron transport. J Pineal Res. 2022;73(3):e12824. 10.1111/jpi.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nooshinfar E, Safaroghli-Azar A, Bashash D, Akbari ME. Melatonin, an inhibitory agent in breast cancer. Breast Cancer. 2017;24(1):42–51. 10.1007/s12282-016-0690-7. [DOI] [PubMed] [Google Scholar]
- 186.Shen W, Zhang W, Ye W, et al. SR9009 induces a REV-ERB dependent anti-small-cell lung cancer effect through inhibition of autophagy. Theranostics. 2020;10(10):4466–80. 10.7150/thno.42478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mathews J, Chang AJ, Devlin L, Levin M. Cellular signaling pathways as plastic, proto-cognitive systems: Implications for biomedicine. Patterns (N Y). 2023;4(5):100737. 10.1016/j.patter.2023.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brem S. Vagus nerve stimulation: novel concept for the treatment of glioblastoma and solid cancers by cytokine (interleukin-6) reduction, attenuating the SASP, enhancing tumor immunity. Brain Behav Immun Health. 2024;42:100859. 10.1016/j.bbih.2024.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dubeykovskaya Z, Si Y, Chen X, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 2016;7:10517. 10.1038/ncomms10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen X, Geng Y, Wei G, et al. Neural circuitries between the brain and peripheral solid tumors. Cancer Res. 2024;84(21):3509–21. 10.1158/0008-5472.Can-24-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stepulak A, Rola R, Polberg K, Ikonomidou C. Glutamate and its receptors in cancer. J Neural Transm (Vienna). 2014;121(8):933–44. 10.1007/s00702-014-1182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sachlos E, Risueño RM, Laronde S, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149(6):1284–97. 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 193.Fitzgerald PJ. Beta blockers, norepinephrine, and cancer: an epidemiological viewpoint. Clin Epidemiol. 2012;4:151–6. 10.2147/clep.S33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schuller HM, Al-Wadei HA. Neurotransmitter receptors as central regulators of pancreatic cancer. Future Oncol. 2010;6(2):221–8. 10.2217/fon.09.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sontheimer H. Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26(10):543–9. 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 196.Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115. 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Polesel J, Zucchetto A, Montella M, et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2009;20(2):353–7. 10.1093/annonc/mdn565. [DOI] [PubMed] [Google Scholar]
- 198.Fatima S, Hu X, Huang C, et al. High-fat diet feeding and palmitic acid increase CRC growth in β2AR-dependent manner. Cell Death Dis. 2019;10(10):711. 10.1038/s41419-019-1958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fitzgerald PJ. Norepinephrine release may play a critical role in the Warburg effect: an integrative model of tumorigenesis. Neoplasma. 2020;67(5):947–57. 10.4149/neo_2020_200422N432. [DOI] [PubMed] [Google Scholar]
- 200.Fujiwara K, Oogane M, Kanno A, et al. Magnetocardiography and magnetoencephalography measurements at room temperature using tunnel magneto-resistance sensors. Appl Phys Express. 2018;11(2): 023001. [Google Scholar]
- 201.Hales CG. The origins of the brain’s endogenous electromagnetic field and its relationship to provision of consciousness. J Integr Neurosci. 2014;13(2):313–61. 10.1142/s0219635214400056. [DOI] [PubMed] [Google Scholar]
- 202.Buckner CA, Buckner AL, Koren SA, Persinger MA, Lafrenie RM. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS One. 2015;10(4):e0124136. 10.1371/journal.pone.0124136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jimenez H, Blackman C, Lesser G, et al. Use of non-ionizing electromagnetic fields for the treatment of cancer. Front Biosci (Landmark Ed). 2018;23(2):284–97. 10.2741/4591. [DOI] [PubMed] [Google Scholar]
- 204.Zimmerman JW, Pennison MJ, Brezovich I, et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer. 2012;106(2):307–13. 10.1038/bjc.2011.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Barbault A, Costa FP, Bottger B, et al. Amplitude-modulated electromagnetic fields for the treatment of cancer: discovery of tumor-specific frequencies and assessment of a novel therapeutic approach. J Exp Clin Cancer Res. 2009;28(1):51. 10.1186/1756-9966-28-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Costa FP, de Oliveira AC, Meirelles R, et al. Treatment of advanced hepatocellular carcinoma with very low levels of amplitude-modulated electromagnetic fields. Br J Cancer. 2011;105(5):640–8. 10.1038/bjc.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Capareli F, Costa F, Tuszynski JA, et al. Low-energy amplitude-modulated electromagnetic field exposure: Feasibility study in patients with hepatocellular carcinoma. Cancer Med. 2023;12(11):12402–12. 10.1002/cam4.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Costa FP, Wiedenmann B, Schöll E, Tuszynski J. Emerging cancer therapies: targeting physiological networks and cellular bioelectrical differences with non-thermal systemic electromagnetic fields in the human body - a comprehensive review. Front Netw Physiol. 2024;4:1483401. 10.3389/fnetp.2024.1483401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Han Z, Huang H, Li B, et al. Engineering exosome membrane disguised thermal responsive system for targeted drug delivery and controlled release across the blood-brain barrier. Mater Today Bio. 2025;32:101656. 10.1016/j.mtbio.2025.101656. [DOI] [PMC free article] [PubMed]
- 210.Qin J, Liu J, Wei Z, et al. Targeted intervention in nerve-cancer crosstalk enhances pancreatic cancer chemotherapy. Nat Nanotechnol. 2024. 10.1038/s41565-024-01803-1. [DOI] [PubMed] [Google Scholar]
- 211.Sun J, Wang D, Wei Y, et al. Capsaicin-induced Ca(2+) overload and ablation of TRPV1-expressing axonal terminals for comfortable tumor immunotherapy. Nanoscale. 2025;17(6):3288–305. 10.1039/d4nr04454a. [DOI] [PubMed] [Google Scholar]
- 212.Osswald M, Jung E, Sahm F, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–8. 10.1038/nature16071. [DOI] [PubMed] [Google Scholar]
- 213.Schneider M, Potthoff AL, Evert BO, et al. Inhibition of intercellular cytosolic traffic via gap junctions reinforces lomustine-induced toxicity in glioblastoma independent of MGMT promoter methylation status. Pharmaceuticals (Basel). 2021;14(3). 10.3390/ph14030195. [DOI] [PMC free article] [PubMed]
- 214.Chim H, Armijo BS, Miller E, Gliniak C, Serret MA, Gosain AK. Propranolol induces regression of hemangioma cells through HIF-1α-mediated inhibition of VEGF-A. Ann Surg. 2012;256(1):146–56. 10.1097/SLA.0b013e318254ce7a. [DOI] [PubMed] [Google Scholar]
- 215.Munson JM, Fried L, Rowson SA, et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci Transl Med. 2012;4(127):127ra136. 10.1126/scitranslmed.3003016. [DOI] [PubMed] [Google Scholar]
- 216.Rajamanickam S, Panneerdoss S, Gorthi A, et al. Inhibition of FoxM1-mediated DNA repair by imipramine blue suppresses breast cancer growth and metastasis. Clin Cancer Res. 2016;22(14):3524–36. 10.1158/1078-0432.Ccr-15-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang C, He Y, Chen F, Zhang F, Shao D, Wang Z. Leveraging β-adrenergic receptor signaling blockade for improved cancer immunotherapy through biomimetic nanovaccine. Small. 2023;19(14):e2207029. 10.1002/smll.202207029. [DOI] [PubMed] [Google Scholar]
- 218.Kokolus KM, Zhang Y, Sivik JM, et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. 2018;7(3):e1405205. 10.1080/2162402x.2017.1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou S, Li J, Yu J, et al. Tumor microenvironment adrenergic nerves blockade liposomes for cancer therapy. J Control Release. 2022;351:656–66. 10.1016/j.jconrel.2022.09.049. [DOI] [PubMed] [Google Scholar]
- 220.Hua Y, Vella G, Rambow F, et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1(+) T lymphocyte niches through a feed-forward loop. Cancer Cell. 2023;41(1):226. 10.1016/j.ccell.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 221.Bower JE, Shiao SL, Sullivan P, et al. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr. 2018;2(3):pky029. 10.1093/jncics/pky029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xu F, Zhang J, Xie S, Li Q. Effects of mindfulness-based cancer recovery training on anxiety, depression, post-traumatic stress disorder, and cancer-related fatigue in breast neoplasm patients undergoing chemotherapy. Medicine (Baltimore). 2024;103(23):e38460. 10.1097/md.0000000000038460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365–73. 10.1016/j.ejca.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 224.Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231–40. 10.1002/cncr.29194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cole SW. New challenges in psycho-oncology: neural regulation of the cancer genome. Psychooncology. 2018;27(10):2305–9. 10.1002/pon.4838. [DOI] [PubMed] [Google Scholar]
- 226.Wolfe KR, Madan-Swain A, Hunter GR, Reddy AT, Baños J, Kana RK. An fMRI investigation of working memory and its relationship with cardiorespiratory fitness in pediatric posterior fossa tumor survivors who received cranial radiation therapy. Pediatr Blood Cancer. 2013;60(4):669–75. 10.1002/pbc.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang Q, Zhou W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J Sport Health Sci. 2021;10(2):201–10. 10.1016/j.jshs.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fiuza-Luces C, Valenzuela PL, Gálvez BG, et al. The effect of physical exercise on anticancer immunity. Nat Rev Immunol. 2024;24(4):282–93. 10.1038/s41577-023-00943-0. [DOI] [PubMed] [Google Scholar]
- 229.Lippi G, Mattiuzzi C, Sanchis-Gomar F. Updated overview on interplay between physical exercise, neurotrophins, and cognitive function in humans. J Sport Health Sci. 2020;9(1):74–81. 10.1016/j.jshs.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Park SS, Kim SH, Kim BK, et al. Treadmill exercise ameliorates chemotherapy-induced memory impairment through Wnt/β-catenin signaling pathway. J Exerc Rehabil. 2023;19(6):314–9. 10.12965/jer.2346594.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jiang H, Kimura Y, Inoue S, et al. Effects of different exercise modes and intensities on cognitive performance, adult hippocampal neurogenesis, and synaptic plasticity in mice. Exp Brain Res. 2024;242(7):1709–19. 10.1007/s00221-024-06854-3. [DOI] [PubMed] [Google Scholar]
- 232.Park HS, Kim CJ, Kwak HB, No MH, Heo JW, Kim TW. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology. 2018;133:451–61. 10.1016/j.neuropharm.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 233.Parent-Roberge H, Fontvieille A, Maréchal R, et al. Effects of combined exercise training on the inflammatory profile of older cancer patients treated with systemic therapy. Brain Behav Immun Health. 2020;2:100016. 10.1016/j.bbih.2019.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Padilha CS, Borges FH, Costa Mendes da Silva LE, et al. Resistance exercise attenuates skeletal muscle oxidative stress, systemic pro-inflammatory state, and cachexia in walker-256 tumor-bearing rats. Appl Physiol Nutr Metab. 2017;42(9):916–23. 10.1139/apnm-2016-0436. [DOI] [PubMed] [Google Scholar]
- 235.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. 10.1249/mss.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cao L, During MJ. What is the brain-cancer connection? Annu Rev Neurosci. 2012;35:331–45. 10.1146/annurev-neuro-062111-150546. [DOI] [PubMed] [Google Scholar]
- 237.Hassan QN 2nd, Queen NJ, Cao L. Regulation of aging and cancer by enhanced environmental activation of a hypothalamic-sympathoneural-adipocyte axis. Transl Cancer Res. 2020;9(9):5687–99. 10.21037/tcr.2020.02.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96. 10.1172/jci30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ojima K, Kakegawa W, Yamasaki T, et al. Coordination chemogenetics for activation of GPCR-type glutamate receptors in brain tissue. Nat Commun. 2022;13(1):3167. 10.1038/s41467-022-30828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Costa PAC, Silva WN, Prazeres P, et al. Chemogenetic modulation of sensory neurons reveals their regulating role in melanoma progression. Acta Neuropathol Commun. 2021;9(1):183. 10.1186/s40478-021-01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li X, Zhang S, Guo G, Han J, Yu J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine. 2022;82:104163. 10.1016/j.ebiom.2022.104163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhou H, Zhou F, Zhao C, Xu Y, Luo L, Chen H. Multimodal data integration for precision oncology: challenges and future directions. arXiv preprint arXiv:2406.19611. 2024.
- 243.Stairmand J, Signal L, Sarfati D, et al. Consideration of comorbidity in treatment decision making in multidisciplinary cancer team meetings: a systematic review. Ann Oncol. 2015;26(7):1325–32. 10.1093/annonc/mdv025. [DOI] [PubMed] [Google Scholar]
- 244.Zurborg S, Piszczek A, Martínez C, et al. Generation and characterization of an Advillin-Cre driver mouse line. Mol Pain. 2011;7:66. 10.1186/1744-8069-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lau J, Minett MS, Zhao J, et al. Temporal control of gene deletion in sensory ganglia using a tamoxifen-inducible Advillin-Cre-ERT2 recombinase mouse. Mol Pain. 2011;7:100. 10.1186/1744-8069-7-100. [DOI] [PMC free article] [PubMed]
- 246.Renz BW, Takahashi R, Tanaka T, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33(1):75-90.e77. 10.1016/j.ccell.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nassar MA, Stirling LC, Forlani G, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004;101(34):12706–11. 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen X, Ye H, Kuruvilla R, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46(1):13–21. 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 249.Liu Y, Rutlin M, Huang S, et al. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science. 2012;338(6112):1357–60. 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Szpunar MJ, Belcher EK, Dawes RP, Madden KS. Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav Immun. 2016;53:223–33. 10.1016/j.bbi.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Horvathova L, Padova A, Tillinger A, Osacka J, Bizik J, Mravec B. Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress. 2016;19(5):528–34. 10.1080/10253890.2016.1213808. [DOI] [PubMed] [Google Scholar]
- 252.Sinha S, Fu YY, Grimont A, et al. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res. 2017;77(8):1868–79. 10.1158/0008-5472.Can-16-0899-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113(9):1344–52. 10.1172/jci20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Erin N, Boyer PJ, Bonneau RH, Clawson GA, Welch DR. Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res. 2004;24(2b):1003–9. [PubMed] [Google Scholar]
- 255.Vincenzi FF. Effect of botulinum toxin on autonomic nerves in a dually innervated tissue. Nature. 1967;213(5074):394–5. 10.1038/213394a0. [DOI] [PubMed] [Google Scholar]
- 256.Qin J, Liu J, Wei Z, et al. Targeted intervention in nerve-cancer crosstalk enhances pancreatic cancer chemotherapy. Nat Nanotechnol. 2025;20(2):311–324. 10.1038/s41565-024-01803-1. [DOI] [PubMed] [Google Scholar]
- 257.Raju B, Haug SR, Ibrahim SO, Heyeraas KJ. Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience. 2007;149(3):715–25. 10.1016/j.neuroscience.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 258.Partecke LI, Speerforck S, Käding A, et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology. 2016;16(3):423–33. 10.1016/j.pan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 259.Renz BW, Tanaka T, Sunagawa M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8(11):1458–73. 10.1158/2159-8290.Cd-18-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kamiya A, Hayama Y, Kato S, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22(8):1289–305. 10.1038/s41593-019-0430-3. [DOI] [PubMed] [Google Scholar]
- 261.Ben-Shaanan TL, Schiller M, Azulay-Debby H, et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat Commun. 2018;9(1):2723. 10.1038/s41467-018-05283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K, Delp SL. Beyond the brain: optogenetic control in the spinal cord and peripheral nervous system. Sci Transl Med. 2016;8(337):337rv335. 10.1126/scitranslmed.aad7577. [DOI] [PubMed] [Google Scholar]
- 263.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 264.Lin CW, Sim S, Ainsworth A, Okada M, Kelsch W, Lois C. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron. 2010;65(1):32–9. 10.1016/j.neuron.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Abdullahi A, Wong TWL, Ng SSM. Putative role of non-invasive vagus nerve stimulation in cancer pathology and immunotherapy: can this be a hidden treasure, especially for the elderly? Cancer Med. 2023;12(18):19081–90. 10.1002/cam4.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Thiel V, Renders S, Panten J, et al. Characterization of single neurons reprogrammed by pancreatic cancer. Nature. 2025. 10.1038/s41586-025-08735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.